Abstract
There is considerable clinical interest in the risks and benefits of offering oral water intake, in the form of water protocols, to patients with thin-liquid dysphagia. We describe the design and implementation of a water protocol for patients in a rehabilitation setting with videofluoroscopically confirmed thin-liquid aspiration. The GF Strong Water Protocol (GFSWP) is an interdisciplinary initiative, with roles and accountabilities specified for different members of the interprofessional health-care team. Rules of the water protocol specify mode of water access (independent, supervised), the implementation of any safe swallowing strategies recommended on the basis of the patient’s videofluoroscopy, and procedures for evaluating and addressing oral care needs. Trial implementation of the water protocol in 15 participants showed that they remained free of adverse events, including pneumonia, over the course of an initial 14-day trial and continuing until discharge from the facility (range = 13–108 days). Seven participants were randomly assigned to a 14-day control phase in which they received standard care (without water access). Fluid intake measures taken after the oral water intake phase were increased (mean = 1,845 cc; 95% confidence interval: 1,520–2,169 cc) compared to those in the control phase (mean = 1,474 cc; 95% CI: 1,113–1,836 cc), with oral water intake measures comprising, on average, 563 cc (range = 238–888 cc) of the total post water trial fluid intake values. Fluid intake increased at least 10% of the calculated fluid requirements in 11/15 participants who received oral water access. These participants reported favorable quality-of-life outcomes, measured using the Swal-QOL. These findings support the implementation of the GFSWP, including its exclusion criteria, rules, and plans of care, for rehabilitation patients who aspirate thin liquids.
Keywords: Deglutition, Deglutition disorders, Dysphagia, Aspiration, Water, Pneumonia, Oral hygiene
Individuals with dysphagia frequently demonstrate abnormal swallowing with thin-liquid stimuli such as water; they may or may not have difficulty swallowing other food textures. Currently, there is debate among professionals who treat patients with thin-liquid dysphagia about the best approaches to managing aspiration and its consequences, while maintaining adequate hydration and nutrition [1, 2]. The major concern associated with aspiration is the risk of pneumonia that may ensue from the aspiration of pathogenic bacteria into the lungs [3, 4]. An elevated risk of pneumonia has been demonstrated in individuals with videofluoroscopically confirmed aspiration compared to individuals who do not aspirate [5, 6].
The conventional treatment for thin-liquid dysphagia is to use liquids thickened to the consistency of nectar, honey, or pudding [7–9]. The increased viscosity of a thickened liquid slows bolus flow [10] and prolongs swallowing transit times [11]. A survey of 149 speech-language pathologists (SLPs) in the United States showed that about 85% of respondents regularly recommended the use of thickened liquids for patients with thin-liquid aspiration [12]. Despite their wide usage, there is a paucity of data to support the efficacy of thickened liquids as an intervention for preventing aspiration and its sequelae [9]. In a recent trial of thin-liquid aspirators [13], aspiration was successfully resolved by at least one of three randomly sequenced interventions (nectar-thick liquids, honey-thick liquids, or the use of a chin-down posture while drinking thin liquids) in 51% of 711 patients with Parkinson’s disease and/or dementia. However, aspiration continued despite all three interventions in 49%, thus failing to demonstrate a clear benefit of using thickened liquids to reduce aspiration.
Aspiration does not always lead to pneumonia. In its 1999 report on dysphagia in stroke survivors, the United States Agency of Health Care Policy and Research (AHCPR) concluded that 43–54% of those with dysphagia are likely to aspirate, but found that only one third of these individuals developed pneumonia [14]. One of the contributing factors in this risk equation appears to be the bacterial profile of saliva and oropharyngeal secretions. When the mouth, pharynx, and oropharyngeal secretions are colonized with pathogenic bacteria, there is an increased risk of pneumonia [15–20]. The link between aspiration pneumonia and oral health has been demonstrated in several studies [3, 4, 21–23], while dysphagia, by itself, has not been found to be a sufficient independent risk factor for pneumonia.
There is some evidence to suggest that the aspiration of thickened liquids can be more harmful than aspiration of relatively thinner liquids [24, 25]. Clinical trial results reported by Robbins et al. [25] showed that patients with thin-liquid aspiration who were randomized to honey-thick liquids were twice as likely as those randomized to nectar-thick liquids to develop pneumonia over a 3-month period. These findings have been interpreted to mean that thickened liquids are more difficult to clear from the respiratory system than thinner liquids [25].
Dehydration is another risk associated with the use of thickened fluids in patients with dysphagia. Stroke patients receiving a dysphagia diet with thickened liquids have been documented to have inadequate fluid intake [1]. Robbins et al. [25] found that 5 and 7% of participants randomized to nectar-thick and honey-thick liquids, respectively, became clinically dehydrated within 3 months. Dehydration can lead to multiple complications, including orthostatic hypotension, infection, constipation, an exacerbated state of confusion, prolonged hospital stays, client suffering and discomfort, increased dependency, and increased nursing care requirements [1].
The accumulating evidence suggests that aspiration of secretions colonized with pathogenic bacteria or aspiration of thickened liquids may lead to a greater risk of pneumonia. These findings have prompted clinical questions regarding the risks and benefits of allowing oral water intake in patients who are known to aspirate thin liquids [2]. Some authors [26] argue that the aspiration of water is a benign event. Effros et al. [27] has stated that aquaporins, which are water-conducting channels in the lung epithelium, facilitate the removal of water from the air spaces after accidental aspiration (near-drowning) or after aspiration while drinking. The debate regarding the provision of oral water to patients with thin-liquid dysphagia is further motivated by quality-of-life considerations. It is widely recognized that many patients dislike thickened liquids [12, 28, 29] and, therefore, may drink less or be less compliant with thickened-liquid recommendations, contributing to their risks for dehydration [30]. Sharpe et al. [31] demonstrated that water absorption, captured through blood measures, does not differ between the ingestion of water and of fluids thickened with different thickening agents. This suggests that dehydration in patients who are prescribed thickened liquids cannot be attributed to the fluid-binding properties of the thickeners themselves but is more likely a factor of reduced fluid intake.
These considerations establish the context for studying the safety and benefits of introducing oral water intake to patients with thin-liquid dysphagia in the form of a water protocol. A water protocol is a set of guidelines that allows access to water for selected patients with thin-liquid dysphagia in order to improve hydration and to provide an adjunct to thickened liquids. It is generally accepted in clinical circles that the decision to allow oral water intake in an aspirating patient requires management of the pneumonia risk associated with the colonization of oral and oropharyngeal secretions by pathogenic bacteria. Protocol rules typically permit oral water intake between meals and after oral care. In a retrospective review of 234 patients on a water protocol in a subacute rehabilitation hospital, Panther [2] found no higher occurrence of pneumonia than in comparable facilities.
There are only two prior prospective studies of water protocols in the literature [32, 33]. In the first [32], fluid intake was reported to increase significantly in stroke rehabilitation patients receiving oral water. Pneumonia was not observed as a negative outcome in that study. In a very recent study in subacute patients in a tertiary community hospital, a 14.3% rate of lung complications was observed in patients who were allowed water intake, compared to 0% in a control group who received thickened liquids [33]. It is important to note that the patients who developed respiratory complications had neurodegenerative diseases and/or poor mobility. Other prospective studies of water protocols [34, 35] have been reported in abstract form from conference presentations. These studies concur that water protocols promote increased fluid intake in patients receiving oral water. In one study [34], there was no evidence of an increased occurrence of pneumonia as a negative outcome among skilled nursing facility patients on water protocols, while the other study [35] reported increased pneumonia rates in subacute patients on water protocols. Across this literature, small sample sizes and short study durations mean that findings remain inconclusive.
This study describes the design and results of a water protocol trial implemented at the GF Strong Rehabilitation Center in Vancouver, Canada; the protocol will henceforth be referred to as the GF Strong Water Protocol, or GFSWP. The specific goals of the study were to (1) monitor the occurrence of adverse events during implementation of the GFSWP, and (2) determine the effect of the GFSWP on fluid intake, satisfaction, and quality of life. Based on prior literature, we hypothesized that pneumonia rates would not differ between patients randomized to the experimental water protocol group and those randomized to the control group. We further hypothesized that participants receiving oral water intake according to the rules of the GFSWP would increase overall fluid intake and report improved quality of life.
Methods
GF Strong Water Protocol Design
In 2006, we convened an interprofessional working group to design a water protocol (WP) for pilot implementation. We embarked on a three-phase project using a model of interprofessional collaboration between the professions of speech-language pathology, medicine, nursing, occupational therapy (OT), and clinical nutrition. In the initial preparation phase, a literature review was conducted, identifying several rule considerations that would require clarification in a WP for our institution. These issues and the methods chosen to address them are listed in Table 1. Additional steps in the preparation phase included consultation with researchers and clinicians who had implemented water protocols elsewhere; the assessment and upgrading of staff knowledge and skills through education sessions; and the drafting of decision-making algorithms, plans of care, team roles, and documentation requirements for our proposed WP. The speech-language pathologist was responsible for (1) recommending safe swallowing strategies, including positioning (chin-down posture, head turn, or head tilt), effortful swallows, sip-size regulation, post-swallow throat clearing, or volitional double-swallows to clear residue; (2) educating the patient, family, and team regarding safe swallowing strategies; (3) posting head-of-bed posters as per standard care at GF Strong; and (4) educating the patient and the caregivers regarding the WP rules and oral care procedures and recommendations.
Table 1.
Issues identified during the preparation phase for GFSWP implementation
| Issue | Response |
|---|---|
| Eligibility | Exclusion criteria to determine those patients for whom a water protocol would be contraindicated |
| Oral health |
(a) Procedures for evaluating and documenting oral health status in patients being considered for a water protocol (b) Procedures to determine whether suction equipment is needed during oral care activities for patients who will be on the water protocol |
| Medical approval | A physician’s order will be obtained for the water protocol for all selected patients, so that medical contraindications are considered and so that the provision of water is considered of equal importance to the administration of medications |
| Supervision/assistance | Team assessment to determine whether a patient is able to follow the rules and complete all the steps of the water protocol, including oral care, independently, or whether supervision/assistance is needed |
| Rules for water provision | Water will be delivered by a nurse in a new, labeled, graduated bottle each morning. Water will be poured from this bottle into a cup for drinking. New bottles of water will be stored at the nursing station. Water bottles will be replaced when empty, not refilled |
| Procedures for recording water intake | The water remaining in the previous bottle will be recorded on a water intake sheet, noting the bottle label number, upon delivery of a new bottle |
| Access | Patients who are determined to need assistance will be explicitly offered water by clinical staff throughout the day, between meals |
| Accountabilities | Policies to identify the roles, responsibilities, accountabilities, and documentation requirements of different members of the interdisciplinary health-care team in supporting implementation of the water protocol |
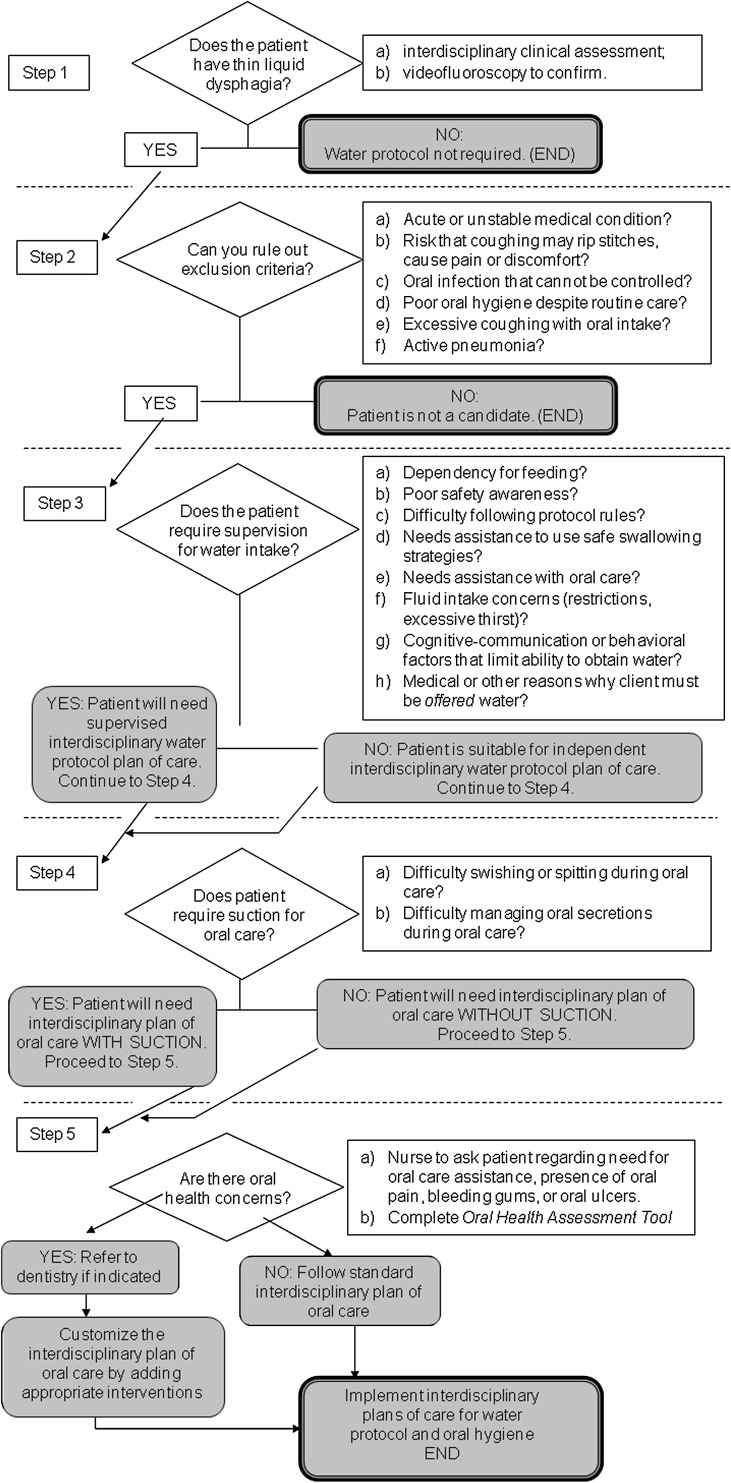
Together with the OT, the speech-language pathologist evaluated the patient’s ability to understand and comply with the WP rules and to use the water bottle as required, and determined the patient’s need for supervision and assistance. The nursing staff on the team was responsible for delivering new bottles of water to patients who were independent in their water intake, evaluating oral health, and documenting fluid intake. Together with the OT, nursing provided oral care or oral care assistance, as required. For patients who were determined to need supervision and assistance, the plan of care detailed the specific schedule that the nurse would follow for offering water, and also identified other team members who were expected to assist by offering water throughout the day. The dietitian on the team was responsible for calculating each patient’s fluid requirements, for monitoring completion of the fluid intake records, and for tallying fluid intake data. All team members encouraged water intake and were accountable for noting any signs of adverse events, such as fever or congestion, and bringing these to the immediate attention of the team and physician. Prior to the implementation phase, all documents related to the GFSWP were finalized (including a 5-step interdisciplinary decision-making algorithm, as shown in Fig. 1), further education sessions were conducted, and research procedures were developed.
Fig. 1.
Water protocol algorithm
As shown in Fig. 1, the GFSWP is similar in its rules to the water protocols described by Garon et al. [32] and Panther [2]. It differs from these other protocols in its specification that each participant requires two plans of care (PoCs) as part of the protocol: the first PoC addresses rules for water intake and the second lays out the rules for oral care of that patient. The GFSWP emphasizes patient and family education, requires a baseline swallowing assessment by a speech-language pathologist and other members of the interdisciplinary team, and encourages the use of any compensatory strategies recommended from that assessment. The oral PoC component of the protocol specifies the details of the oral hygiene routine (frequency, equipment, and process). Patients with a nil-per-oris (NPO) diet order are not permitted to drink water unless it is specifically stipulated by the physician. The term free water is not used due to the potential for this term to be misleading for patients whose water access is contingent on supervision or the use of prescribed safe swallowing strategies. The outcome evaluation phase comprised the research project described in this article as well as post-project feedback meetings with staff to evaluate the utility of the decision-making algorithms developed for the protocol. Research ethics approval was provided by the clinical research ethics board of the University of British Columbia.
Participants
Eligible participants for this study included all English-speaking patients aged 19 years and older, who were admitted consecutively to the Acquired Brain Injury, Neuromusculoskeletal, Adolescent and Young Adult, or Spinal Cord Injury programs at the GF Strong Rehabilitation Centre, and who had thin-liquid dysphagia on admission. A videofluoroscopic swallowing study (VFSS) was performed to further confirm eligibility, except in cases where a report describing a VFSS performed within 5 days prior to admission was available from the referring acute-care institution. VFSS exclusion criteria included evidence of an absent pharyngeal swallow (i.e., complete absence of upper esophageal sphincter opening), which would make frank aspiration essentially inevitable during the administration of water. Additional exclusion criteria included active pneumonia, an acute or unstable medical condition, oral dental bacteria or infection that could not be controlled with oral care, and excessive or uncomfortable coughing during or after water intake. VFSS inclusion criterion was thin-liquid aspiration below the level of the true vocal folds, resulting in either a thickened liquid or a NPO diet order.
Sixteen inpatient clients between 19 and 62 years of age (10 males, mean age = 53.7 years; 6 females, mean age = 44.1 years) were enrolled between July 2008 and December 2009. Two additional patients met the eligibility criteria but declined participation in the study. Primary medical diagnoses for those enrolled in the study included cerebrovascular accident (left, right, or brainstem), spinal cord injury, and traumatic brain injury. One participant had a previous diagnosis of head and neck cancer. At the time of enrollment, seven participants had enteral feeding tubes in place, but only two of these were on a NPO diet. Nectar-thick liquids were prescribed for five participants, honey-thick for eight participants, and spoon-thick for the remaining patient. For those who were permitted oral intake, food texture recommendations were pureed for six participants and mechanical soft for eight participants.
Participants were randomly assigned to one of two groups: (1) an immediate implementation group (n = 9) or (2) a delayed implementation group (n = 7), in which standard care (i.e., no oral water intake) was provided for an initial 14-day control phase, followed by cross-over to the WP phase. One participant assigned to the control phase did not cross over, resulting in a total experimental sample of 15. Nine participants were determined to need the supervised water protocol PoC, in which water was deliberately and repeatedly offered to them by participating staff. Based on the rules of the GFSWP, all participants were determined to be suitable to follow the oral hygiene plan of care without suction. The two groups did not differ notably in their mean American Speech-Language Hearing Association National Outcome Measurement System dysphagia scores (ASHA NOMS; http://www.asha.org/members/research/noms/) at baseline (immediate: 3.20; delayed: 3.71).
Water Protocol Implementation
Due to the short-stay nature of our facility, we decided to monitor primary measures (fluid intake and quality of life) over 14 days for each phase. Adverse events were further monitored in all participants beyond the end of the 14-day WP phase, for the participant’s entire hospital stay until discharge. During each 14-day observation period, baseline fluid intake and quality-of-life measures were collected from days 1 to 3. All participants were trained in their oral care regimens at that time, and in the use of any compensatory swallowing strategies that had been recommended by the SLP as a result of their baseline VFSS. For those in the control phase, standard care continued throughout the initial 14-day observation period and oral water intake was not permitted. During the WP phase, days 1–3 were used to train participants on the rules of the protocol, which are summarized in Table 2. Water intake commenced on day 4 and continued until discharge (range = 13–108 days, mean = 54 days). Post-trial fluid intake measures were collected over a 48-h period between days 10 and 14 of each phase. The specific rules of the GFSWP are summarized in Table 2.
Table 2.
Rules of the GFSWP
| Consideration | Instructions |
|---|---|
| Oral care |
To be done first thing in the morning, prior to oral intake, and at bedtime Swab mouth or rinse-and-spit to be performed prior to any water intake |
| Oral water intake |
Water from a cup permitted between meals, after oral care Any strategies or precautions recommended based on videofluoroscopy to be used |
| Meal-time fluids |
Prescribed thickened liquid (i.e., nectar- or honey-thick liquid) to be used Oral water intake not permitted during meals or for 30 min afterwards |
| Medications | All pills to be taken with the prescribed thickened liquid or puree |
Analysis
Dependent variables in this study included (1) the occurrence of adverse events, (2) fluid intake (expressed as a % of fluid requirements), and (3) quality of life (measured using the Swal-QOL). Adverse events of interest included aspiration pneumonia, a new need to initiate intravenous fluids, a new need to initiate tube-feeding, or acute-care hospitalization. The criteria for a diagnosis of pneumonia included physician documentation in the medical chart plus one or more of the following: elevated white blood cell count (≥12,000), fever (temperature ≥ 38.0°C), or new infiltrate on chest radiograph [4].
For the purposes of measuring fluid intake, fluid was defined as a liquid at room temperature and included oral intake of thickened liquids and water as well as any fluids administered by tube. A standard 24-h fluid intake record was completed by the nursing staff according to standard procedures in our facility. Daily oral water intake was calculated based on the water remaining in the water bottle and documented on the 24-h fluid balance record. Each participant’s fluid intake requirements were calculated using the following standard: 100 ml per kg body weight for the first 10 kg, 50 ml per kg body weight for the next 10 kg, and 15 ml per kg body weight for each kg above 20 kg [36]. Actual fluid intake was divided by the calculated fluid requirement to determine the percentage of fluid requirement achieved.
Each participant’s perceived swallowing-related quality of life was monitored using the Swal-QOL, a 44-item validated tool that evaluates ten quality-of-life domains: swallow burden, food selection, eating desire, duration, fear, sleep, fatigue, communication, mental health, and social factors [37–39]. A higher score on the scale indicates a greater detrimental impact of dysphagia on perceived quality of life. We expected a priori that some of the Swal-QOL subscales (such as communication and social participation) might not differ as a result of the water protocol.
Results
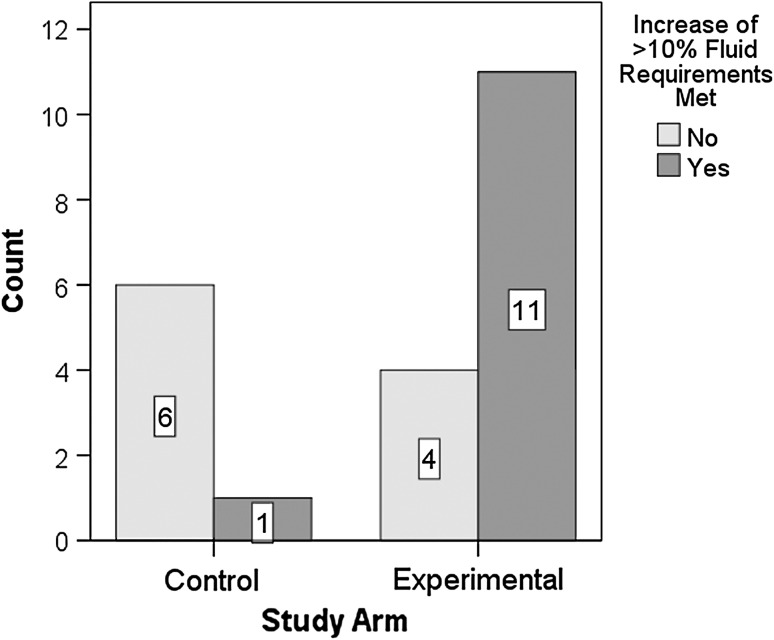
No adverse events were detected in either the control phase or the WP phase, or in the variable time period that followed until discharge (mean duration = 54 days). To put this finding in context, it should be recognized that annual pneumonia occurrence rates in this organization run at 2.25% (13 cases), with 30% of these cases attributed to aspiration. Table 3 provides descriptive statistics (means and 95% confidence intervals) for fluid intake (in cc and in % of calculated fluid requirements) at the baseline and post-trial measurements, with breakdowns by phase and water access condition. Post-trial totals (broken down into oral water and other sources of fluid intake such as thickened liquids or tube-feeds) are also given. Fluid intake measures at baseline did not differ significantly between the control (mean: 95% CI: 1,113–1,836 cc) and WP phases (mean: 95% CI: 1,180–1,740 cc; F(1,20) = 0.128, P = 0.724]. Post-trial fluid intake measures for the WP phase had a mean value of 1,845 cc (95% CI: 1,520–2,169 cc). Oral water intake comprised, on average, 563 cc of the total post-trial fluid intake measures for the WP phase (95% CI: 238–888 cc) across the 15 participants in the study. These post WP phase fluid intake measures represent a substantial increase in the percentage of calculated fluid requirements consumed (mean = 84%; 95% CI: 69–100%) when compared to the stable fluid intake measures observed at the end of the control phase (mean = 61%; 95% CI: 44–79%). However, there was substantial variation in the amount of oral water intake across participants at the post WP phase measurements, as shown by the large confidence intervals in Table 3. Closer inspection of these data revealed large standard deviations and non-normal distribution of fluid intake residuals, which violates the assumptions required to support an ANOVA of groupwise differences. Therefore, we opted to perform a nonparametric analysis of the difference between baseline and post-trial % fluid intake using a categorical binary classification of <10% and >10% increase in fluid requirements versus the baseline measure. χ2 analysis revealed a significantly higher proportion of participants (11/15 vs. 1/7) in the post WP phase who achieved an overall increase of 10% or greater of their fluid requirements (χ2 = 6.712, df = 1, 22, P = 0.01). This result is shown in Fig. 2.
Table 3.
Means (and 95% confidence intervals) for 24-h fluid intake measures at baseline and at the post-trial measurement
| Time point | Parameter | Control phase | Water protocol | ||
|---|---|---|---|---|---|
| No oral water access (N = 7) | Unsupervised oral water access (N = 6) | Supervised oral water access (N = 9) | Access conditions combined (N = 15) | ||
| Pre-trial fluid balance | Oral water intake (cc) | N/A | N/A | N/A | N/A |
| Other fluid intake (cc) | 1474 (1113–1836) | 1407 (729–2086) | 1495 (1167–1823) | 1460 (1180–1740) | |
| Total fluid intake (cc) | 1474 (1113–1836) | 1407 (729–2086) | 1495 (1167–1823) | 1460 (1180–1740) | |
| % of calculated requirements (%) | 63 (48–78) | 65 (30–100) | 68 (53–82) | 67 (53–81) | |
| Post-trial fluid balance | Oral water intake (cc) | N/A | 920 (181–1658) | 326 (43–609) | 563 (238–888) |
| Other fluid intake (cc) | 1427 (999–1855) | 1039 (682–1396) | 1423 (1009–1876) | 1281 (996–1566) | |
| Total fluid intake (cc) | 1427 (999–1855) | 1959 (1172–2745) | 1768 (1397–2140) | 1845 (1520–2169) | |
| % of calculated requirements (%) | 61 (44–79) | 90 (49–130) | 80 (64–96) | 84 (69–100) | |
Fluid: liquid consistency at room temperature. Total fluid intake: the combination of oral water intake plus other fluid intake (including oral thickened liquids and nonoral fluids)
Fig. 2.
Fluid intake in control and study groups
As mentioned previously, we expected a priori that not all of the subscale domains evaluated by the Swal-QOL would be sensitive to the WP intervention. We therefore calculated a key subscale composite score comprising the symptom, burden, mental health, fear, and fatigue subscales. A univariate ANOVA revealed a significant difference with a strong effect size in key subscale composite change scores from the baseline to post-intervention measures, with a mean improvement of −2.9 points for the WP phase and a mean worsening of 13.7 points for the control phase measures [F(1, 20) = 9.55, P = 0.0006, Cohen’s d = 1.2]. Among the subscales that compose the composite score, there were no significant group differences in pre–post intervention change (P > 0.05) in the burden, mental health, or fatigue domains. The post WP measures showed a mean improvement in symptom subscale scores of −1 point, while the control phase measures showed a perceived worsening of symptoms in the order of 5 points on average [F(1, 20) = 8.58, P = 0.0008, Cohen’s d = 0.753] (strong effect size). Similarly, the post WP measures revealed a mean improvement in swallowing-associated fear of −1.5 points, while the control phase measures exhibited a mean worsening of 4 points on this subscale [F(1, 20) = 10.55, P = 0.0004, Cohen’s d = 1.23] (strong effect size).
Discussion
This study demonstrated positive outcomes, with no adverse events over an average time frame of 54 days, in participants who received oral water intake according to the rules of the GFSWP in a rehabilitation setting. Important aspects of the water protocol used in this study include the clear specification of eligibility and exclusion criteria, the option of receiving water with supervision, the involvement of an interprofessional health-care team in implementing the protocol, and specifications regarding oral care for all participants. It should be emphasized that the extensive preplanning of exclusion criteria and plans of care to support implementation of the GFSWP addressed issues that have been raised as barriers to water protocol implementation in previous reports [32]. Given such preplanning, it was our experience that implementation of the GFSWP was not an overly onerous undertaking. Our approach was interdisciplinary, involving collaboration with many different team members, with protocol documents outlining roles and accountabilities for each member of the interprofessional team. Although the assignment of specific activities to particular professionals might differ, depending on the staffing complement in an institution, we believe these roles reflect components that must be addressed and considered when planning water protocol implementation. However, we caution that our study results may not be generalizable to situations where such interdisciplinary collaboration and support cannot be provided. Copies of our detailed plans of care and oral care assessment tool can be accessed by contacting the first author.
This study provides new insights to the quality-of-life impact of allowing oral water access to patients with thin-liquid dysphagia. It should come as no surprise that restriction to thickened liquids is viewed as having a negative impact on quality of life [29]. In this study we identified a trend toward improved quality-of-life reports, particularly with respect to the impact of dysphagia symptoms and associated fear following 2 weeks on the GFSWP. The nature of our study prevented us from blinding participants to their assignment. It is, therefore, perhaps not surprising that those assigned initially to the control phase actually reported a perceived worsening in their swallowing-associated quality of life at the post control phase measures. These patients reported that they were more bothered by their swallowing symptoms; this may reflect their disappointment in being assigned to the control phase, knowing that earlier access to oral water intake might have been a possibility when they consented to participate in the study.
This study shares several limitations with previous attempts to study the outcomes of water protocols, including the small study size, a short study period for fluid intake monitoring (i.e., 14 days), and the inclusion of patients with a wide range of medical diagnoses. Our patient population was likely similar to that seen at the Frazier Rehabilitation Institute [2] but was diagnostically more heterogeneous than that studied by Garon et al. [32]. The overall rate of pneumonia in the study facility was also low (13 cases or 2.25% annually), similar to that reported by Panther [2]. We did not specifically track the number of potentially eligible individuals who were excluded on the basis of active pneumonia or unstable medical conditions at the time of their admission. Furthermore, the role of the safe-swallowing strategies that were recommended for and implemented by study participants was not closely scrutinized with respect to their impact on the participant’s aspiration status. In comparison to the subacute sample studied recently by Karagiannis et al. [33], the reduced medical acuity of our sample, paired with the exclusion criteria and oral hygiene rules of our water protocol, may have contributed to the lack of adverse events in our study.
Questions of sample size and study length are important to consider with respect to water protocol studies. Recent estimates suggest that more than 750,000 adults suffer strokes annually in the United States (www.stroke.org). Of these, between 43 and 54% are likely to be aspirators, with a 37% incidence of pneumonia among aspirators [14]. Assuming that an increase in the occurrence of pneumonia is the main adverse event of interest when introducing a water protocol, then it is reasonable to perform sample size calculations using these figures, along with upper thresholds for pneumonia incidence that would be considered appropriate in prospective experimental studies. We have undertaken this exercise using PASS 2000 sample size calculation software (Number Cruncher Statistical Systems, Kaysville, UT), and an 80% power criterion, as shown in Table 4. The table also models the sample size required if the same calculations are applied to an assumed stroke population of 250 cases annually at an institutional level. It should be recognized, however, that this modeling exercise is overly simplistic and does not take into consideration such issues as the time frame of the water protocol intervention, the duration of the subsequent adverse event monitoring window, and the relative acuity of the patients seen in a given institution; all of these factors have implications for sample size calculations.
Table 4.
Sample-size calculations modeling the number of participants required in a prospective trial in order to detect an increase in the incidence of pneumonia as a negative outcome
| Stroke incidence figures | Range of probable aspirators (ACHPR) | Range of probable pneumonia incidence (ACHPR) | Threshold for detecting increased pneumonia occurrence (%) | No. of participants needed over 12 months (80% power) | |||
|---|---|---|---|---|---|---|---|
| Lower bound (43%) | Upper bound (54%) | Lower bound (16%) | Upper bound (20%) | ||||
| National | 745,781 | 320,686 | 402,722 | 119,325 | 149,850 | 45 | 238 |
| 42 | 592 | ||||||
| 40 | 1,628 | ||||||
| Local/institutional | 250 | 108 | 135 | 40 | 50 | 45 | 86 |
| 42 | 110 | ||||||
| 40 | 127 | ||||||
Based on reported national annual incidence rates for stroke (www.stroke.org) and pneumonia (ACHPR [14]) and hypothetical estimates of equivalent incidence at a local level
Conclusion
The goals of our project were to develop, implement, and determine the impact of the GFSWP on fluid intake, quality of life, and adverse event rates. The results suggest that a trial of oral water access can be safely introduced for rehabilitation patients with documented thin-liquid aspiration, provided they meet certain exclusion criteria, and that considerations regarding the need for supervision and an appropriate oral care regimen are addressed. Fluid intake can be expected to increase over 2-week trials. Most importantly, our data suggest that the risk of adverse events, including pneumonia, is low when careful exclusion criteria and plans of care for water access and oral care are implemented, although this conclusion requires validation through further studies with sufficient statistical power. We believe that these findings form an adequate basis for justifying water protocols for patients with thin-liquid dysphagia in the rehabilitation population. Importantly, our study included individuals who were considered inappropriate to access oral water intake without supervision and/or those who were unable to access water independently. Such individuals have typically been excluded from prior water protocol studies, but our results suggest that they should be given equal opportunity to access oral water, with appropriate supervision. We are pleased to share the algorithm and rules of the GFSWP in the hope that these will be useful resources for others who may wish to introduce water protocols in their facilities.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Biographies
Caren Carlaw
MA
Heather Finlayson
MD, FRCPC
Kathleen Beggs
BSc
Tiffany Visser
MS
Caroline Marcoux
MS
Dawn Coney
BA
Catriona M. Steele
PhD
References
- 1.Finestone H, Foley NC, Woodbury MG, Greene-Finestone L. Quantifying fluid intake in dysphagic stroke patients: a preliminary comparison of oral and nonoral strategies. Arch Phys Med Rehabil. 2001;82:1744–1746. doi: 10.1053/apmr.2001.27379. [DOI] [PubMed] [Google Scholar]
- 2.Panther K. The Frazier free water protocol. Perspectives. 2005;14:4–9. [Google Scholar]
- 3.Langmore SE. Risk factors for aspiration pneumonia. Nutr Clin Pract. 1999;14:S41–S44. doi: 10.1177/088453369901400110. [DOI] [Google Scholar]
- 4.Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, Loesche W. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 5.Pikus L, Levine MS, Yang YX, Rubesin SE, Katzka DA, Laufer I, Gefter WB. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol. 2003;180:1613–1616. doi: 10.2214/ajr.180.6.1801613. [DOI] [PubMed] [Google Scholar]
- 6.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 7.Felt P. The national dysphagia diet: the science and practice. Nutr Clin Pract. 1999;14:S60–S63. [Google Scholar]
- 8.Cichero JA, Atherton M, Bellis-Smith N, Suter M. Texture-modified foods and thickened fluids as used for individuals with dysphagia: Australian standardised labels and definitions. Nutr Diet. 2007;64:S53–S76. doi: 10.1111/j.1747-0080.2007.00153.x. [DOI] [Google Scholar]
- 9.Robbins J, Nicosia MA, Hind JA, Gill GD, Blanco R, Logemann JA. Defining physical properties of fluids for dysphagia evaluation and treatment. Perspectives. 2002;11(2):16–19. [Google Scholar]
- 10.Ku DN, Ma PP, McConnel FM, Cerenko D. A kinematic study of the oropharyngeal swallowing of a liquid. Ann Biomed Eng. 1990;18:655–669. doi: 10.1007/BF02368453. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus CL, Logemann JA, Rademaker AW, Kahrilas PJ, Pajak T, Lazar R, Halper A. Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Arch Phys Med Rehabil. 1993;74:1066–1070. doi: 10.1016/0003-9993(93)90063-G. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JM, Chambers Et, Molander M. Thickened liquids: practice patterns of speech-language pathologists. Am J Speech Lang Pathol. 2005;14:4–13. doi: 10.1044/1058-0360(2005/003). [DOI] [PubMed] [Google Scholar]
- 13.Logemann JA, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, Kosek S, Dikeman K, Kazandjian M, Gramigna GD, Lundy D, McGarvey-Toler S, Miller Gardner PJ. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J Speech Lang Hear Res. 2008;51:173–183. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AHRQ . Diagnosis and treatment of swallowing disorders (dysphagia) in acute-care patients. Rockville: Agency for Health Care Policy and Research; 1999. [Google Scholar]
- 15.Scannapieco FA. Pneumonia in nonambulatory patients. The role of oral bacteria and oral hygiene. J Am Dent Assoc. 2006;137(Suppl):21S–25S. doi: 10.14219/jada.archive.2006.0400. [DOI] [PubMed] [Google Scholar]
- 16.Shay K, Scannapieco FA, Terpenning MS, Smith BJ, Taylor GW. Nosocomial pneumonia and oral health. Spec Care Dentist. 2005;25:179–187. doi: 10.1111/j.1754-4505.2005.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 17.Scannapieco FA, Rethman MP. The relationship between periodontal diseases and respiratory diseases. Dent Today. 2003;22:79–83. [PubMed] [Google Scholar]
- 18.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 19.Scannapieco FA, Mylotte JM. Relationships between periodontal disease and bacterial pneumonia. J Periodontol. 1996;67:1114–1122. doi: 10.1902/jop.1996.67.10s.1114. [DOI] [PubMed] [Google Scholar]
- 20.Yoon MN, Steele C. The oral care imperative: the link between oral hygiene and aspiration pneumonia. Top Geriatr Rehabil. 2007;23:280–288. [Google Scholar]
- 21.Terpenning M. Geriatric oral health and pneumonia risk. Clin Infect Dis. 2005;40:1807–1810. doi: 10.1086/430603. [DOI] [PubMed] [Google Scholar]
- 22.Terpenning M. Prevention of aspiration pneumonia in nursing home patients. Clin Infect Dis. 2005;40:7–8. doi: 10.1086/426030. [DOI] [PubMed] [Google Scholar]
- 23.Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T, Mizuno Y, Ohsawa T, Akagawa Y, Hashimoto K, Sasaki H, Oral Care Working Group Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50:430–433. doi: 10.1046/j.1532-5415.2002.50106.x. [DOI] [PubMed] [Google Scholar]
- 24.Holas MA, De Pippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Arch Neurol. 1994;51:1051–1053. doi: 10.1001/archneur.1994.00540220099020. [DOI] [PubMed] [Google Scholar]
- 25.Robbins J, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D, Baum H, Lilienfeld D, Kosek S, Lundy D, Dikeman K, Kazandian M, Gramigna GD, McGarvey-Toler S, Miller Gardner PJ. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148:509–518. doi: 10.7326/0003-4819-148-7-200804010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinberg MJ, Knebl J, Tully J, Segall L. Aspiration and the elderly. Dysphagia. 1990;5:61–71. doi: 10.1007/BF02412646. [DOI] [PubMed] [Google Scholar]
- 27.Effros RM, Jacobs ER, Schapira RM, Biller J. Response of the lungs to aspiration. Am J Med. 2000;108(Suppl 4a):15S–19S. doi: 10.1016/S0002-9343(99)00290-9. [DOI] [PubMed] [Google Scholar]
- 28.Macqueen C, Taubert S, Cotter D, Stevens S, Frost G. Which commercial thickening agent do patients prefer? Dysphagia. 2003;18:46–52. doi: 10.1007/s00455-002-0084-1. [DOI] [PubMed] [Google Scholar]
- 29.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. Am J Speech Lang Pathol. 2005;14:61–70. doi: 10.1044/1058-0360(2005/008). [DOI] [PubMed] [Google Scholar]
- 30.Goulding R, Bakheit AM. Evaluation of the benefits of monitoring fluid thickness in the dietary management of dysphagic stroke patients. Clin Rehabil. 2000;14:119–124. doi: 10.1191/026921500667340586. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe K, Ward L, Cichero J, Sopade P, Halley P. Thickened fluids and water absorption in rats and humans. Dysphagia. 2007;22:193–203. doi: 10.1007/s00455-006-9072-1. [DOI] [PubMed] [Google Scholar]
- 32.Garon B, Engle M, Ormiston C. A randomized control study to determine the effects of unlimited oral intake of water in patients with identified aspiration. J Neurol Rehabil. 1997;11:139–148. [Google Scholar]
- 33.Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronson-Lowe C, Leising K, Bronson-Lowe D, Lanham S, Hayes S, Ronquillo A, Blake P. Effects of a free water protocol for patients with dysphagia. Dysphagia. 2008;23(4):430. [Google Scholar]
- 35.Becker DL, Tews LK, Lemke JH. An oral water protocol for rehabilitation patients with dysphagia for liquids. Chicago: American Speech-Language Hearing Association Convention; 2008. [Google Scholar]
- 36.Chidester JC, Spangler AA. Fluid intake in the institutionalized elderly. J Am Diet Assoc. 1997;97:23–28. doi: 10.1016/S0002-8223(97)00011-4. [DOI] [PubMed] [Google Scholar]
- 37.McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Bricker DE. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 38.McHorney CA, Bricker DE, Robbins J, Kramer AE, Rosenbek JC, Chignell KA. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. Item reduction and preliminary scaling. Dysphagia. 2000;15:122–133. doi: 10.1007/s004550010013. [DOI] [PubMed] [Google Scholar]
- 39.McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins J, Chignell KA, Logemann JA, Clarke C. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15:115–121. doi: 10.1007/s004550010012. [DOI] [PubMed] [Google Scholar]