Abstract
The purpose of this study was to examine the effects of vitamin D supplementation on inflammatory biomarkers in overweight and obese adults participating in a progressive resistance exercise training program. Twenty-three (26.1±4.7 y) overweight and obese (BMI: 31.3±3.2 kg·m−2) adults were randomized into a double-blind vitamin D supplementation (VitD, 4000 IU/d, female=5; male=5) or placebo (PL, female=7; male=6) intervention trial. Both groups performed 12 wk (three d/wk) of progressive resistance exercise training (three sets of eight exercises) at 70 – 80% of one repetition maximum. Whole blood lipopolysaccharide (LPS)-stimulated tumor necrosis factor (TNF) α production as well as circulating C-reactive protein (CRP), TNFα, interleukin 6 (IL-6), and alanine aminotransferase (ALT) were assessed at baseline and after the 12 wk intervention. No main effects of group or time were detected for circulating CRP, TNFα, IL-6 and ALT. As expected, when PL and VitD groups were combined, there was a significant correlation between percent body fat and CRP at baseline (r=0.45, P=0.04), and between serum 25OHD and CRP at 12 weeks (r=0.49, P=0.03). The PL group had a significant increase in 25 μg/ml LPS+polymixin B-stimulated TNFα production (P=0.04), and both groups had a significant reduction in unstimulated TNFα production (P<0.05) after the 12 week intervention. Vitamin D supplementation in healthy, overweight and obese adults participating in a resistance training intervention did not augment exercise-induced changes in inflammatory biomarkers.
Keywords: 25-hydroxyvitamin D, resistance training, inflammation, tumor necrosis factor α, C-reactive protein, cytokine
Introduction
Obesity disrupts human biological processes and creates an environment susceptible to diseases such as type 2 diabetes and atherosclerosis (Shah et al. 2008). The development of obesity-associated disease is in part attributed to pro-inflammatory cytokine production from adipocytes (Meshkani and Adeli 2009). Further, adipokines released in obese animals induce the secretion of cytokines [e.g. tumor necrosis factor (TNF) α and interleukin 6 (IL-6)] from circulating immune cells, which promote additional cytokine release following immune cell infiltration into adipose tissue (Gutierrez et al. 2009). Inflammatory cytokines (i.e. TNFα and IL-6) induce the production of C-reactive protein (CRP); an acute-phase protein that, along with TNFα and IL-6, is considered a reliable biomarker of systemic inflammation, and a significant contributor to the pathogenesis of disease (Dandona et al. 2004; Hirschfield and Pepys 2003). Thus, adiposity may lead to a sustained elevation in pro-inflammatory cytokines that characterize systemic inflammatory stress.
Evidence suggests a link among vitamin D status [serum 25-hydroxyvitamin D (25OHD)], inflammation, and obesity (Cigolini et al. 2006; Timms et al. 2002). For example, vitamin D deficiency associated with high levels of adipose tissue is proposed to be due to the sequestration of 25OHD in adipocytes (Blum et al. 2008; Goldner et al. 2008; Wortsman et al. 2000). There is also data to support that vitamin D deficiency is associated with the development of certain diseases that are characterized by low-grade systemic inflammation, such as type 2 diabetes (Dandona et al. 2004; Holick 2005). The number of various cells in the immune system shown to express the vitamin D receptor is rising (Mauricio et al. 1996), and there is mounting evidence to suggest that vitamin D exerts anti-inflammatory effects (May et al. 2004). For example, one year of vitamin D supplementation (3320 IU/day) during weight loss induced a more pronounced decrease in circulating TNFα compared to weight loss alone (Zittermann et al. 2009). Thus, vitamin D anti-inflammatory activity may play a role in alleviating inflammation associated with obesity, which has significant implications given that inflammatory biomarkers such as CRP, TNFα, and IL-6 contribute to obesity-associated disease progression (Lechleitner et al. 2002; Romero-Corral et al. 2008).
Similar to vitamin D supplementation, exercise training may have potent anti-inflammatory properties (Beavers and Nicklas 2011; McFarlin et al. 2004; Stewart et al. 2005; Stewart et al. 2007), particularly when the intensity of exercise is high. For example, a 12 week resistance training program that progressed to 85 – 100% of one repetition maximum (RM) significantly reduced muscle TNFα mRNA and protein content (Greiwe et al. 2001). Results supporting an anti-inflammatory effect of resistance exercise after a period of moderate-to-high intensity resistance training are accumulating (Stewart et al. 2007; Timmerman et al. 2008). One study reported a significant reduction in CRP levels in sedentary individuals following resistance training compared to no change in CRP after aerobic training (Donges et al. 2010). The authors speculate that the enhanced protein synthesis rate due to resistance training may induce the suppression of inflammatory responses that results in a more pronounced reduction in circulating CRP (Donges et al. 2010; Greiwe et al. 2001). A large adipose tissue mass, however, has been shown to interfere with the favorable responses associated with exercise training (Sitnick et al. 2009). It has not been determined whether vitamin D supplementation can provide an enhanced anti-inflammatory response to progressive resistance training in overweight and obese individuals. Thus, the purpose of this study was to investigate the impact of vitamin D supplementation on inflammatory biomarkers in overweight and obese adults during participation in a progressive resistance exercise training program. We hypothesized that vitamin D supplementation during exercise training would result in a greater reduction of circulating inflammatory biomarkers compared to exercise training alone.
Methods
Subjects
Overweight and obese (BMI: 25 – 39.9 kg·m−2) participants were recruited during winter months (October to March). Preliminary eligibility was determined following participation in a brief telephone screening interview. During the first visit to the laboratory, participants completed a health history and a physical activity assessment by questionnaire (Topolski et al. 2006). Subject’s height, weight, and estimated maximal oxygen consumption were also obtained (Armstrong et al. 2006). Individuals with physical activity scores in the “low” to “very low” category coupled with maximal oxygen consumption estimations in the “below average” or lower categories were eligible for participation (Armstrong et al. 2006). Exclusionary criteria included use of tanning booths or other artificial UV light exposure; high baseline vitamin D (>600 IU/day) and calcium intake (>1000 mg/day); plans to visit sunny/warm destinations during the study period; type 2 diabetes; history or presence of metabolic disease; history of eating disorder; presence of gastrointestinal disorders; pregnancy or lactation; use of drugs to treat obesity (last 12 weeks); use of over the counter anti-obesity agents (last 12 weeks); and recent initiation of an exercise program (last four weeks).
Eligible participants (n=34) were randomized into the trial with 23 (12 females, and 11 males) completing the requirements of the study. Of the 11 drop-outs, none were related to adverse effects due to the study interventions. All experimental procedures were approved by the Purdue University Committee on the Use of Human Research Subjects and each participant provided written informed consent.
Vitamin D supplementation
The experimental design was a double-blind, randomized, placebo controlled vitamin D supplementation trial. Participants were matched for age, weight, BMI, and sex before randomization to consume daily either 4000 IU vitamin D3 (VitD, five females, and five males) or a placebo (PL, seven females and six males) that contained microcrystalline cellulose, in identical capsules provided by Family Pharmacy, West Lafayette, IN. Participants were also asked to consume one calcium tablet (500 mg/tablet) per day and to refrain from consuming other supplements or medications during the 12 week trial period. Single use sunscreen packets were provided to all participants with instructions to apply the contents of four packets per day to all exposed skin surfaces. Compliance for pill consumption and sunscreen use was determined by random pill and sunscreen packet counts. All participants were asked to complete a three-day diet record (two weekdays, one weekend day) to assess vitamin D intakes prior to participation and following the 12 week program. Dietary records were analyzed using Nutrition Data System for Research, Version 4.04, Food and Nutrient Database 28 (Minneapolis, Minnesota) by the same trained nutritionist.
Acclimation and exercise training
Participants were acclimated to the treadmill (LifeFitness Treadmills, Schiller Park, IL) and eight resistance exercises: leg extension, leg flexion, leg press, hip adduction, hip abduction, chest press, seated row, and “lat” pull down (Keiser Equipment, Fresno, CA) during a three-session acclimation week. The acclimation protocol used in this experiment is described in detail elsewhere (Timmerman et al. 2008). Briefly, during the first acclimation session, participants were provided with detailed instruction on proper weight lifting technique and thereafter completed an eight repetition maximum (RM) for each of the eight exercises listed above. During the second acclimation session, participants completed two sets of the eight resistance exercises at 50% of their estimated 1RM. The third acclimation session required the participants to complete a 1RM muscular strength assessment for chest press, leg press, and leg curl. The sum of chest press, leg press, and leg curl 1RMs’ was calculated to assess muscular strength. Participants were required to allow at least 48 hours recovery between acclimation sessions. Muscular strength tests were conducted again at the end of the resistance training program.
Participants in both groups completed a supervised 12-week (three days per week) resistance exercise training program previously employed to assess the impact of resistive exercise on inflammation (Phillips, 2010). Each session began with a warm-up that included walking on a treadmill for five minutes and light stretching. Thereafter, participants performed three sets, at 70 – 80% of one repetition maximum (RM), of the eight resistance exercises listed above. For the first set, participants were asked to complete eight repetitions, and for the second and third set participants completed the exercise until “momentary muscular failure”, or until 15 repetitions were performed. Each training session lasted approximately 40 min. Resistance was increased each week (5 – 10%) based on individual performance. During each exercise session participants were supervised to ensure that they exercised at the appropriate intensity and completed all of the assigned sets.
To control for the timing of post-exercise caloric intake, all participants were asked to consume a nutrition shake (Gatorade Performance Series: Nutrition Shake) during the hour following each exercise session. The composition of the drink (360 kcal) was fat 8 g, carbohydrate 54 g, milk protein isolate 20 g, vitamin D (100 IU), and calcium (300 mg).
Body composition
Fasting body composition was assessed in the morning following a 12 hour overnight fast at baseline and after the 12 week intervention, by dual energy x-ray absorptiometry (DXA, software version 4.3e Lunar Corp, Madison, WI). All participants were asked to consume the same foods and drinks during the 24 hours prior to each testing day (baseline and 12 weeks) and DXA measurements for female participants were completed during the early follicular phase. In addition, subjects were instructed to refrain from exercise, supplement intake and medications for 72 hours prior to DXA measurement. For the DXA measurements, the % CV based on 10 subjects measured twice for total body fat mass is 2%, respectively.
Biochemical analysis
A resting blood sample was collected at baseline and after the intervention for all participants. To standardize dietary intake prior to blood collection, all participants were asked to consume the same foods and drinks during the 24 hours prior to each testing day (baseline and 12 weeks). Participants were also instructed to refrain from exercise, supplement intake, and any medications for the 72 hours prior to blood sampling. To control for biological differences during the menstrual cycle, blood was collected from female participants were completed during the early follicular phase. Participants arrived at the laboratory between six and eight in the morning following an overnight fast (12 hours). A venous blood sample was collected in serum tubes which were maintained at room temperature for 30 minutes followed by separation for serum by centrifugation at 1500 × g for 15 minutes. Aliquots of serum were frozen at −80°C until further analysis. Blood drawn into sodium heparin tubes were left at room temperature and used for same day whole-blood lipopolysaccharide (LPS)-stimulation.
Serum 25OHD was measured by enzyme immunoassay (Heartland Assays Inc, Ames, IA). The inter-assay coefficient of variation was 10.5%. Serum alanine aminotransferase (ALT) was measured by kinetic UV method using the ALT (GPT) Liquid Stable Reagent according to the manufacturer’s specifications purchased from Thermo Scientific (Middletown, VA). For analysis of CRP, serum samples were diluted 1:100 in assay diluent prior to analysis. CRP was measured using a commercially available high-sensitivity ELISA kit purchased from ALPCO Diagnostics (Salem, NH) according to the manufacturer’s specifications. Baseline and 12 week samples were run on the same plate along with appropriate controls. The intra- and inter-assay coefficient of variation was 4.9% and 10.5%, respectively. TNFα and IL-6 were quantified using commercially available high-sensitivity ELISA kits purchased from ALPCO Diagnostics (Salem, NH) according to the manufacturer’s specifications. Serum samples were diluted 1:2 in assay diluent prior to analysis. Intra-assay coefficients of variation were less than 4% and inter-assay coefficients of variation were less than 9%.
LPS-stimulated TNFα production
LPS-stimulated TNFα production was quantified using a modified whole-blood procedure described originally by Zheng et al. (1997). Whole blood was diluted 1:10 with RPMI (2 mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin) and treated as follows in five 2 mL wells: 1) 1 ng/ml LPS, (S. enteriditis, Sigma-Aldrich, St. Louis, MO); 2) 1 ng/ml LPS and 100 μg/ml polymixin B (PMB); 3) 25 μg/ml LPS; 4) 25 μg/ml LPS and 100 μg/ml PMB; and 5) 0 μg/ml LPS. PMB interferes with LPS-stimulated cytokine production by binding to LPS and thus, preventing its interaction with the cell-surface of monocytes. LPS and PMB were added to the wells 15 minutes prior to the addition of whole blood. Plates were incubated (5% CO , 37° 2 C) for 24 hours. Following the 24 hour incubation, the plates were centrifuged (500 × g, 10 min) and the supernatant was removed and filtered (0.2 micron). Supernatant was stored at −80°C until analysis for TNFα using ELISA (OptEIA, Becton-Dickinson, San Diego, CA). The inter-assay coefficient of variation for TNFα was 10.3%.
Statistical analysis
Descriptive and univariate statistics were assessed for all variables. Assumptions of normality (Shapiro-Wilkes) and homogeneity of variance (residual plots) were determined and outliers removed (>3 standard deviations from the mean). Non-normally distributed data were appropriately transformed following a diagnostic Box-Cox analysis. Assumptions of normality and homogeneity of variance were satisfied following log transformations of CRP, TNFα production from whole blood, and ALT. Inflammatory biomarkers (CRP, TNFα, and IL-6), TNFα production from whole blood and ALT were assessed using a two-way analysis of covariance (ANCOVA) to determine cross-sectional and longitudinal differences. Baseline values of each variable were included as a covariate in all models. The influence of sex was also tested in each model, and did not influence the results. A Tukey post hoc multiple comparison analysis was used when appropriate. Statistical analyses were performed with SAS computer software (SAS Institute, Inc., Cary, NC; Version 8.01) and SPSS 15.0. The level of P<0.05 was used as the threshold for declaring statistical significance.
Results
No significant differences were detected at baseline for age, height, body weight, BMI, or percent body fat between the drop-outs and those who completed the study. Age, height, weight, BMI and percent body fat were similar between groups at baseline (Table 1).
Table 1.
Baseline Characteristics.
| Placebo (n = 13) | Vitamin D (n = 10) | |
|---|---|---|
| Age (y) | 26.0 ± 4.5 | 26.2 ± 5.1 |
| Height (cm) | 169.4 ± 10.6 | 168.5 ± 9.2 |
| Weight (kg) | 92.3 ± 16.9 | 86.8 ± 10.9 |
| BMI (kg·m−2) | 31.9 ± 3.3 | 30.6 ± 3.1 |
| Body fat (%) | 43.7 ± 5.8 | 41.3 ± 5.1 |
| 25OHD (ng·mL−1) | 18.1 ± 6.5 | 20.8 ± 8.3 |
No differences detected between groups (P>0.05). Values are means ± SD. BMI, body mass index; 25OHD, 25–hydroxyvitamin D.
Compliance to the 12 week supplement (vitamin D or placebo) and calcium intervention for both groups was 93% and 88%, respectively. Compliance to sunscreen use was 42.5% and 40.4% in the PL and VitD groups, respectively. Vitamin D intake was similar at baseline between the groups. Total vitamin D intake was significantly higher in the VitD group (VitD: 3859 ± 445 IU/day) compared to the PL group (215 ± 213 IU/day) at the end of the 12 week intervention. There was no significant difference in serum 25OHD levels at baseline between the groups (PL = 18.1 ± 6.5 ng/mL, vitD = 20.8 ± 8.3 ng/mL), and did not increase in the PL group at 12 weeks (23.5 ± 6.0 ng/mL) to the baseline levels of the PL group. Consistent with compliance to the supplement intervention, serum levels of 25OHD at the end of the 12 week intervention were significantly higher in the vitD group (33.4 ± 7.2 ng/mL) compared to the vitD group at baseline (P=0.003), and significantly higher than the levels of the PL group at 12 weeks (P=0.002).
Further, compliance to exercise training sessions for the vitamin D and placebo group was 96% and 97%, respectively. Consistent with this, the change in muscular strength (i.e., sum of chest press, leg press, and leg curl 1RMs’) significantly increased by 24.7% and 24.1% in both the PL (baseline: 328.6 ± 91.3 kg, 12 weeks: 409.9 ± 93.3 kg) and VitD (baseline: 339.0 ± 93.7 kg, 12 weeks: 420.9 ± 101.6 kg) groups, respectively.
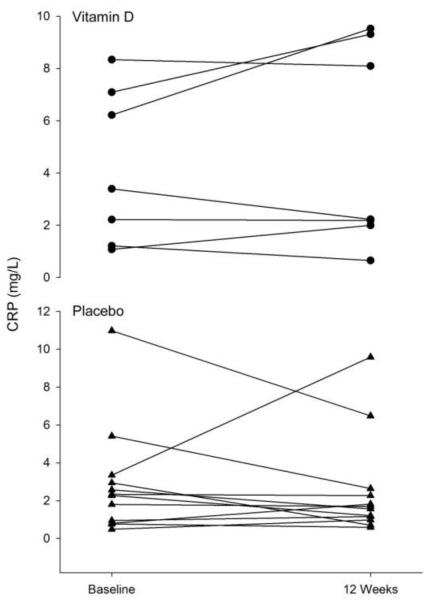
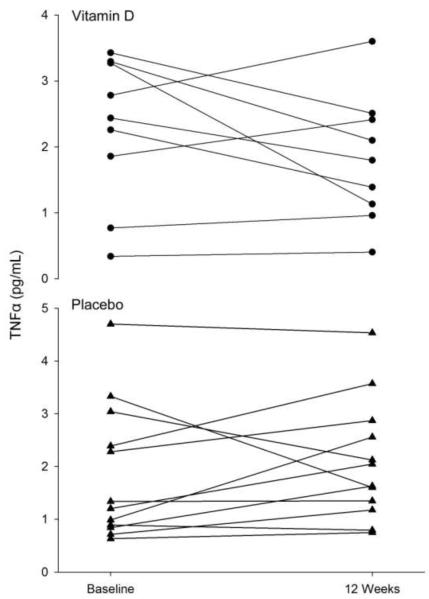
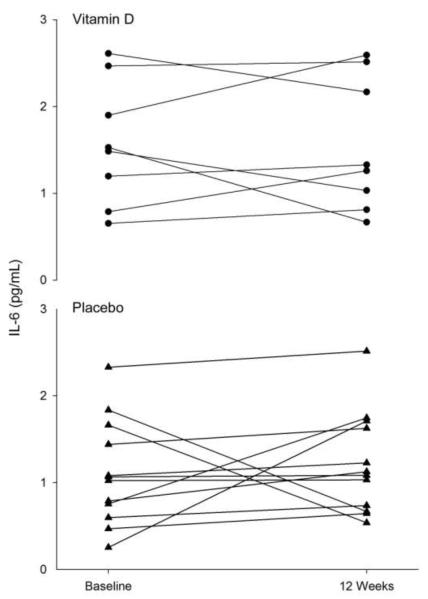
No main effects of group or time were found for serum CRP, TNFα, and IL-6 (Table 2, Figures 1, 2, and 3). For the PL and VitD groups combined, there was a significant correlation between percent body fat and CRP at baseline (r=0.45, P=0.04). Although there was no main effect of group on CRP at 12 weeks, for both groups combined there was a significant correlation detected between serum 25OHD and CRP at 12 weeks (r=0.49, P=0.03). Similar results are obtained when fat mass or group are included in the model.
Table 2.
Serum levels of CRP, TNFα, and IL-6 at Baseline and following the 12-week intervention.
| Placebo (n = 13) | Vitamin D (n = 10) | |||
|---|---|---|---|---|
|
|
||||
| Baseline | 12 weeks | Baseline | 12 weeks | |
| CRP (mg·L−1) | 3.8 ± 2.8 | 2.9 ± 2.9 | 2.8 ± 2.7 | 4.8 ± 3.6 |
| TNFα (pg·mL−1) | 1.9 ± 1.3 | 1.9 ± 1.2 | 2.3 ± 1.1 | 2.1 ± 1.2 |
| IL-6 (pg·mL−1) | 1.1 ± 0.6 | 1.2 ± 0.6 | 1.6 ± 0.7 | 1.5 ± 0.7 |
No main effects of time or group were detected (P>0.05). Values are means ± SD. CRP, C-reactive protein; TNFα, tumor necrosis factor alpha, IL-6, interleukin 6.
Figure 1.
Individual differences in C-Reactive Protein (CRP) at baseline and after 12 weeks of resistance exercise training in both the placebo (▲) and vitamin D ( • ) groups.
Figure 2.
Individual differences in tumor necrosis factor (TNF) α at baseline and after 12 weeks of resistance exercise training in both the placebo (▲) and vitamin D ( • ) groups.
Figure 3.
Individual differences in interleukin 6 (IL-6) at baseline and after 12 weeks of resistance exercise training in both the placebo (▲) and vitamin D ( • ) groups.
A main effect of time was detected for absolute TNFα production (expressed as pg.ml−1 in culture) incubated in 1 ng/ml LPS + PMB and 25 μg/ml LPS + PMB, such that the PL group had a significant increase in 25 μg/ml LPS + PMB TNFα production following the 12 week exercise training intervention (Table 3, P=0.04). A main effect of time was detected for unstimulated (no LPS), absolute TNFα production such that both the PL and VitD groups had a significant reduction in absolute TNFα production (P=0.003 and P=0.01, respectively) after the 12 week intervention (Table 3). As expected, the addition of PMB significantly blunted TNFα production at both concentrations (1 ng/ml and 25 μg/ml) of LPS stimulation (P<0.05).
Table 3.
Monocyte TNFα (pg·mL−1) production for PL and VitD groups at baseline and following the 12 weeks of resistance training.
| Placebo (n = 13) | Vitamin D (n = 10) | |||
|---|---|---|---|---|
|
|
||||
| Baseline | 12 weeks | Baseline | 12 weeks | |
| 1 ng/mL LPS | 441.1 ± 247.8 | 750.8 ± 436.8 | 603.1 ± 453.1 | 730.5 ± 544.0 |
| 1 ng/mL LPS+PMB | 1.6 ± 1.5 | 2.3 ± 1.2 | 1.4 ± 1.5 | 1.9 ± 0.9 |
| 25 μg/mL LPS | 939.4 ± 412.0 | 1255.1 ± 482.6 | 1029.5 ± 604.6 | 1276.1 ± 738.3 |
| 25 μg/mL LPS+PMB | 275.3 ± 104.9 | 398.5 ± 155.9a | 265.2 ± 99.9 | 343.2 ± 129.5 |
| No LPS | 1.6 ± 0.9 | 0.6 ± 0.5a | 1.5 ± 0.6 | 0.6 ± 0.5a |
Whole blood was treated as follows in five 2 ml wells: 1) 1 ng/ml lipopolysaccharide (LPS); 2) 1 ng/ml LPS and 100 μg/ml polymixin B (PMB); 3) 25 μg/ml LPS; 4) 25 μg/ml LPS and 100 μg/ml PMB; and 5) 0 μg/ml LPS. PMB interferes with LPS-stimulated tumor necrosis factor alpha (TNFα) production by binding to LPS and thus, preventing its interaction with the monocyte cell-surface. Plates were incubated for 24 hours. Values are means ± SD.
Significant difference from baseline (P<0.05). No differences were detected between groups (P>0.05).
There were no main effects of group or time detected for serum ALT (PL, Baseline: 19.4 ± 17.8 pg/ml, 12 weeks: 13.3 ± 15.7 pg/ml; VitD, Baseline: 13.7 ± 12.7 U/L, 12 weeks: 7.9 ± 6.2 U/L).
Discussion
The results of the current study demonstrated that, in healthy overweight and obese subjects, high dose vitamin D supplementation did not influence circulating inflammatory biomarkers during participation in a 12-week progressive resistance exercise training program.
Circulating inflammatory biomarkers (CRP, TNFα, and IL-6) were previously reported to be either decreased (Timms et al. 2002), or unchanged (Bjorkman et al. 2009; Jorde et al. 2010b; von Hurst et al. 2009) with vitamin D supplementation without exercise training. The results of the current study are consistent with previous findings that indicate no change in CRP and inflammatory cytokines after six months or one year of high dose (~4000 – 5700 IU/day) vitamin D supplementation (Jorde et al. 2010b; von Hurst et al. 2009). In one study, vitamin D supplementation (3320 IU/day) reduced inflammation in obese individuals during weight loss (Zittermann et al. 2009). In the current study, overweight and obese participants remained weight-stable during the 12 week intervention. Furthermore, there was a lower than expected increase in serum 25OHD with supplementation that may have been due to the subjects being overweight or obese (Heaney 2000; Wortsman et al. 2000). Decreased 25OHD bioavailability with high levels of adipose tissue is also consistent with evidence showing no effect of high dose vitamin D supplementation in overweight and obese subjects on changes in cardiovascular risk factors, inflammation, and bone mineral density (Jorde et al. 2010a; Jorde et al. 2010b; Jorde et al. 2010c). Thus, future work should incorporate longer supplementation with vitamin D intake and/or concomitant weight loss to test the anti-inflammatory impact in overweight and obese subjects.
There was no individual group effect on CRP, although when the groups were combined CRP was positively correlated with 25OHD after the 12 week intervention. However, circulating levels of the liver enzyme ALT, which is elevated during hepatic stress, were unaffected by the 12 week intervention with no differences detected between groups. These results support that vitamin D supplementation in the current study did not lead to liver damage during the 12 week intervention and did not alter CRP levels.
The results of the current study did not show an effect of resistance exercise training on circulating inflammatory biomarkers (CRP, TNFα, and IL-6). These results are similar to other studies that report no change in either CRP (Timmerman et al. 2008) or circulating TNFα, and IL-6 (Stewart et al. 2007) with similar exercise training regimens. In contrast, exercise-induced reductions in CRP and inflammatory cytokines are associated with weight loss (Fisher et al. 2010; Okita et al. 2004). In fact, it was suggested that weight loss has a more profound influence on reducing inflammation compared to exercise training alone (Fisher et al. 2010), and that weight loss may be necessary to observe an exercise-induced reduction in CRP (Okita et al. 2004). Although the participants in the current study were overweight and obese, they were otherwise apparently healthy and remained weight-stable, without any changes in body composition, throughout the 12 week intervention. Thus, exercise training interventions in conjunction with weight loss may be more likely to induce responses that are more robust than observed in individuals who remain weight-stable.
Unstimulated, absolute TNFα production from whole blood was reduced in both groups after the 12 week intervention that may reflect a modest anti-inflammatory influence of exercise, independent of a vitamin D. These findings, however, are difficult to interpret given that LPS-stimulated TNFα production was not reduced in either group at 12 weeks, and that the PL group had a significant increase in 25 μg/ml LPS + PMB, absolute TNFα production following the 12 week exercise training intervention. Other investigators have implemented similar exercise training protocols to that of the current study, and report either no change (Stewart et al. 2005) or a significant reduction (Timmerman et al. 2008) in LPS-stimulated TNFα production from whole blood. One limitation of the current study is that LPS-stimulated TNFα was reported only in absolute values (pg.ml−1) rather than also including TNFα production relative to CD14+ monocyte, as is shown in other studies that report both (Timmerman et al. 2008). Yet absolute levels of LPS-stimulated TNFα production tend to reveal similar results when reported relative to CD14+ monocyte (Timmerman et al. 2008). Another possibility is that the increase in 25 μg/ml LPS-stimulated TNFα production may have occurred due to normal seasonal variation (Myrianthefs et al. 2003). Indeed, Myrianthefs et al. (2003) reported that LPS-stimulated production of TNFα is lowest during autumn, compared to other seasons. Participants in the current study were recruited during late autumn/early winter and may have experienced a seasonal increase in LPS-stimulated TNFα during participation in winter.
This is the first study in which the effects of vitamin D supplementation (4000 IU/day) on inflammatory biomarkers in healthy, overweight and obese adults participating in a resistance exercise training intervention have been examined. A limitation of the present study was the inclusion of healthy, overweight and obese participants. This subject population may have a limited possibility of attaining improvements in serum 25OHD due to a decreased 25OHD bioavailability with high levels of adipose tissue compared to thinner individuals. Further, the results of this study cannot be applied to a normal weight population. Thus, future studies investigating the impact of vitamin D supplementation in conjunction with weight loss in obese individuals or comparing the response in normal weight and overweight or obese subjects while participating in a resistance exercise training program are warranted.
Conclusions
In conclusion, vitamin D supplementation in healthy, weight-stable, overweight and obese subjects during a resistance exercise training program did not influence inflammatory biomarkers or induce liver damage.
Acknowledgements
We would like thank our phlebotomist Douglas Maish, as well as Krysta Rickey, Elizabeth Kuhns, Lauren Wagner, and Jessica Harris for assisting with data collection, analysis, and for guiding and motivating participants during training sessions.
This research was supported by Gatorade Sports Science Institute and by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program (Yan Jiang) administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Standards All experimental procedures conformed to the standards set by the Declaration of Helsinki and were approved by the Purdue University Committee on the Use of Human Research Subjects. Each participant provided written informed consent.
References
- Armstrong L, Balady G, Berry M, Davis S, Davy B, Davy K, Franklin B, Gordon N, Lee I, McConnell T, Myers J, Pizza F, Rowland T, Stewart K, Thompson P, Wallace J. ACSM’s Guidelines for Exercise Testing and Prescription. Seventh Edition Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- Beavers KM, Nicklas BJ. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front Biosci (Schol Ed) 2011;3:168–177. doi: 10.2741/s142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman MP, Sorva AJ, Tilvis RS. C-reactive protein and fibrinogen of bedridden older patients in a six-month vitamin D supplementation trial. J Nutr Health Aging. 2009;13:435–439. doi: 10.1007/s12603-009-0080-3. [DOI] [PubMed] [Google Scholar]
- Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42:304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of Diet With and Without Exercise Training on Markers of Inflammation and Fat Distribution in Overweight Women. Obesity (Silver Spring) 2010;19:1131–1136. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep. 2009;9:26–32. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney RP. Vitamin D: how much do we need, and how much is too much? Osteoporos Int. 2000;11:553–555. doi: 10.1007/s001980070074. [DOI] [PubMed] [Google Scholar]
- Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J. 2005;98:1024–1027. doi: 10.1097/01.SMJ.0000140865.32054.DB. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010a;267:462–472. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Torjesen PA, Figenschau Y, Goransson LG, Omdal R. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010b;50:175–180. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Jorde R, Sneve M, Torjesen PA, Figenschau Y, Hansen JB, Grimnes G. No significant effect on bone mineral density by high doses of vitamin D3 given to overweight subjects for one year. Nutr J. 2010c;9:1. doi: 10.1186/1475-2891-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleitner M, Herold M, Dzien-Bischinger C, Hoppichler F, Dzien A. Tumour necrosis factor-alpha plasma levels in elderly patients with Type 2 diabetes mellitus-observations over 2 years. Diabet Med. 2002;19:949–953. doi: 10.1046/j.1464-5491.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- Mauricio D, Mandrup-Poulsen T, Nerup J. Vitamin D analogues in insulin-dependent diabetes mellitus and other autoimmune diseases: a therapeutic perspective. Diabetes Metab Rev. 1996;12:57–68. doi: 10.1002/(SICI)1099-0895(199603)12:1<57::AID-DMR155>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377–393. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. 2004;36:1876–1883. doi: 10.1249/01.mss.0000145465.71269.10. [DOI] [PubMed] [Google Scholar]
- Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Myrianthefs P, Karatzas S, Venetsanou K, Grouzi E, Evagelopoulou P, Boutzouka E, Fildissis G, Spiliotopoulou I, Baltopoulos G. Seasonal variation in whole blood cytokine production after LPS stimulation in normal individuals. Cytokine. 2003;24:286–292. doi: 10.1016/j.cyto.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Okita K, Nishijima H, Murakami T, Nagai T, Morita N, Yonezawa K, Iizuka K, Kawaguchi H, Kitabatake A. Can exercise training with weight loss lower serum C-reactive protein levels? Arterioscler Thromb Vasc Biol. 2004;24:1868–1873. doi: 10.1161/01.ATV.0000140199.14930.32. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Sierra-Johnson J, Lopez-Jimenez F, Thomas RJ, Singh P, Hoffmann M, Okcay A, Korinek J, Wolk R, Somers VK. Relationships between leptin and C-reactive protein with cardiovascular disease in the adult general population. Nat Clin Pract Cardiovasc Med. 2008;5:418–425. doi: 10.1038/ncpcardio1218. [DOI] [PubMed] [Google Scholar]
- Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638–644. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol. 2009;587:5753–5765. doi: 10.1113/jphysiol.2009.180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, McFarlin BK, Coen PM, Talbert E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2009:1–7. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, Franchimont N, Geenen V, Albert A, Reginster JY. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/s0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]