Abstract
Transient spinal cord ischemia in humans can lead to the development of permanent paraplegia with prominent spasticity and rigidity. Histopathological analysis of spinal cords in animals with ischemic spastic paraplegia show a selective loss of small inhibitory interneurons in previously ischemic segments but with a continuing presence of ventral α-motoneurons and descending cortico-spinal and rubro-spinal projections. The aim of the present study was to examine the effect of human spinal stem cells (hSSCs) implanted spinally in rats with fully developed ischemic paraplegia on the recovery of motor function and corresponding changes in motor evoked potentials. In addition the optimal time frame for cell grafting after ischemia and the optimal dosing of grafted cells were also studied.
Spinal cord ischemia was induced for 10 min using intra-aortic balloon and systemic hypotension. In the functional recovery study, hSSCs (10 000–30 000 cells/0.5 μl/injection) were grafted into spinal central gray matter of L2-L5 segments at 21 days after ischemia. Animals were immunosuppressed with Prograf (1mg/kg or 3mg/kg) for the duration of the study. After cell grafting the recovery of motor function was assessed periodically using BBB scoring system and correlated with the recovery of motor evoked potentials. At predetermined times after grafting (2–12 weeks), animals were perfusion-fixed and the survival, and maturation of implanted cells were analyzed using antibodies recognizing human-specific antigens: nuclear protein (hNUMA), neural cell adhesion molecule (hMOC), neuron-specific enolase (hNSE) and synapthophysin (hSYN) as well as the non-human specific antibodies TUJ1, GFAP, GABA, GAD65 and GLYT2.
After cell grafting a time-dependent improvement in motor function and suppression of spasticity and rigidity was seen and this improvement correlated with the recovery of motor evoked potentials. Immunohistochemical analysis of grafted lumbar segments at 8 and 12 weeks after grafting revealed intense hNSE immunoreactivity, an extensive axo-dendritic outgrowth as well as rostrocaudal and dorsoventral migration of implanted NUMA-positive cells. An intense hSYN immunoreactivity was identified within the grafts and in the vicinity of persisting α-motoneurons. On average, 64% of hSYN terminals were GAD65 immunoreactive which corresponded to GABA immunoreactivity identified in 40–45% of NUMA-positive grafted cells. The most robust survival of grafted cells was seen when cells were grafted 21 days after ischemia. As defined by cell survival and laminar distribution, the optimal dose of injected cells was 10 000–30 000 cells per injection. These data indicate that spinal grafting of hSSCs can represent an effective therapy for patients with spinal ischemic paraplegia.
Keywords: spinal cord ischemia, paraplegia, cell replacement, human neural stem cells, spasticity, rigidity
Ischemic spinal cord injury represents a serious complication associated with transient cross-clamping of the descending thoracic or thoracoabdominal aorta (Tarlov, 1967, Svensson et al., 1986). Depending on the duration of ischemic interval, corresponding neurological dysfunction resulting from spinal ischemic injury may be expressed as paraparesis or fully developed spastic paraplegia with or without a component of rigidity. After longer ischemic intervals flaccidity is seen (Dimitrijevic and Nathan, 1967).
The mechanism leading to ischemia-induced neuronal degeneration is only partially understood and may involve excessive release/activity of excitatory amino acids, prostaglandins and/or oxygen free radicals (Marsala et al., 1994, Saganova and Marsala, 1994, Facchinetti et al., 1998, Lombardi et al., 1998, Akins and Atkinson, 2002, Stys, 2005). In addition to a well documented sensitivity of the brain and spinal cord neurons to transient ischemia, recent evidence demonstrates a similar AMPA-receptor mediated degeneration of oligodendrocytes and astrocytes. The loss of these glial cells, even in the absence of a significant neuronal degeneration, can lead to an altered local segmental conductivity and a corresponding loss of function (for review see Stys, 2005).
Histopathological analysis of spinal cords taken from animals with rigidity and spasticity show a selective loss of small inhibitory neurons, but with persisting α-motoneurons in previously ischemic spinal segments. Similar spinal neuronal pathology has been described in patients after spinal ischemic injury (Tarlov, 1967, Taira and Marsala, 1996, Marsala et al., 2004). In contrast, in animals with flaccid paraplegia, pan-necrotic neurodegenerative changes are seen affecting both small inhibitory and excitatory interneurons as well as ventral α-motoneurons (Zivin and DeGirolami, 1980). Corresponding with the time frame of neuronal degeneration after spinal ischemia, similarly as in focal or global brain ischemia, an injury-dependent activation of local microglia and infiltration of macrophages occur. Depending on the extend of injury, the peak of inflammatory changes is typically seen between 2–3 days after spinal ischemia in the rabbit model and then shows a gradual loss of inflammatory elements over the following 2–3 weeks (Giulian and Robertson, 1990).
In the past 2–3 decades a considerable effort has been made assessing the therapeutic potential of spinal grafting of a variety of cell lines, acutely isolated spinal cord fetal tissue, as well as direct spinal gene delivery, in ameliorating neurological dysfunction using several models of spinal injury including mechanical traumatic injury (Reier et al., 1992a, Reier et al., 1992b, Mori et al., 1997, Liu et al., 1999, McDonald et al., 1999, Chow et al., 2000), chemically lesioned spinal cord (Onifer et al., 1997) or genetically manipulated animals which display progressive α-motoneuronal degeneration (e.g. ALS transgenic mice or rat), (Nagai et al., 2001, Vastag, 2001, Acsadi et al., 2002). In general these studies show long term survival and preservation of neuronal phenotype in grafts generated from fetal tissue or neuronally committed precursors (Anderson et al., 1995, Onifer et al., 1997). In some of these studies, depending on the spinal injury model, a variable degree of neurological recovery was also demonstrated and characterized by improved ambulatory functions (McDonald et al., 1999).
In contrast, in vitro expanded neural precursors grafted into previously mechanically or chemically injured spinal cord showed only limited neuronal differentiation and maturation (Onifer et al., 1997). While the mechanism of this preferential non-neuronal differentiation is not completely defined it is hypothesized that a local release of proinflammatory cytokines (such as TNFα, TGFβ) at the site of previous injury is likely involved.
As previously discussed spinal ischemia-induced spastic paraplegia is associated with a selective loss of small inhibitory interneurons but with a continuing presence of ventral α-motoneurons and descending supraspinal input including corticospinal and rubrospinal tracts (Taira and Marsala, 1996). In previous studies using a rat spinal ischemia model, we have demonstrated that spinal grafting of postmitotic human hNT neurons or committed rat spinal neuronal precursors is associated with significant recovery of motor function and suppression of otherwise exacerbated peripheral myogenic response after electrical stimulation of motor cortex. This neurological recovery was associated with a robust maturation of grafted neurons and development of a GABAergic phenotype in a subpopulation of grafted neurons (Marsala et al., 2004).
In the present study, using immunosuppressed rats with ischemia-induced spasticity and rigidity, we characterized the effect of spinal grafting of human spinal stem cells when grafted into previously ischemic spinal cord segments. The effect of such treatment was characterized by: i) correlative analysis between the degree of recovery of ambulatory function (BBB score) and motor evoked potentials (MEPs), ii) stereological quantification of grafted cells, and, iii) neurotransmitter phenotype of grafted neuronally differentiated neurons. In addition, the optimal time period for cell grafting after the ischemic episode and the most favorable dose of grafted cells was studied.
EXPERIMENTAL PROCEDURES
All procedures were approved by the Animal Care Committee at the University of California, San Diego.
Derivation of the spinal human spinal stem cells (hSSCs)
Human SSCs were prepared from the cervical-upper thoracic region of spinal cord tissue obtained from a single 8-week human fetus after an elective abortion. The fetal tissue was donated by the mother in a manner fully compliant with the guidelines of NIH and FDA and approved by an outside independent review board. The spinal cord tissue was removed of meninges and dorsal root ganglia and dissociated into a single cell suspension by mechanical trituration in serum-free, modified N2 media. The modified N2 media was composed of: 100mg/L human plasma apo-transferrin, 25mg/L recombinant human insulin, 1.56g/L glucose, 20nM progesterone, 100μM putrescine, and 30nM sodium selenite in DMEM/F12. For growth of the hSSCs, 10ng/ml bFGF as the sole mitogen was added to the modified N2 media (growth media). The initial culture was serially expanded as a monolayer culture in precoated flasks (T-175) or plates (150mm, Nunc) (Johe et al., 1996). Briefly, the precoated vessels were prepared by incubating them for 1 hour at room temperature with 100μg/ml poly-D-lysine in 10mM HEPES buffer at 0.165ml/cm2. The vessels were washed three times with water and allowed to completely dry aseptically in the hood. They were then further incubated with 100μg/ml fibronectin/PBS for 5 minutes or alternatively 25μg/ml fibronectin/PBS for 1 hour. The fibronectin solution was aspirated and the vessels were used immediately without drying.
Approximately 6.1 × 106 total cells were obtained upon the initial dissociation of the spinal cord tissue. All of the cells were plated onto one 150mm plate in 20ml of the growth media. The growth media was changed every other day and in the alternate days, 10ng/ml of bFGF was directly added to the culture. The first passage was conducted 16 days after plating. At this point, the culture was composed mostly of post-mitotic neurons and mitotic hSSCs. The mitotic cells were harvested by brief treatment with trypsin (0.05% + 0.53mM EDTA). Trypsin was stopped by addition of soy bean trypsin inhibitor to 0.05% final concentration. The cell suspension was triturated with a pipette to obtain a single cell suspension and centrifuged at 1400 rpm for 5 minutes. The cell pellet was resuspended in growth media and the cells were replated in new pre-coated plates at 1.2 × 106 cells in 20ml of growth media per 150mm plate. The cells were harvested at approximately 75% confluence, which occurred in 5 to 6 days. This process was repeated for 20 passages. At various passages, the cells were frozen in the growth medium plus 10% DMSO at 5 × 106 – 10 × 106 cells/ml using a programmable freezer. The frozen cells were stored in liquid nitrogen. Upon thawing, the overall viability and recovery was typically 80–95%. The resulting cell line, which was produced by epigenetic means only, using bFGF as the sole mitogen, was named “566RSC”. A cell bank of passage 16 cells was prepared and used for this study.
Induction of spinal ischemic paraplegia
Transient spinal cord ischemia (10 min) was induced as previously described (Taira and Marsala, 1996). Briefly, in halothane (1.5%) anesthetized SD rats, a 2F Fogarty catheter (Am.V. Muller, CV 1035) was passed through the left femoral artery to the descending thoracic aorta to the level of the left subclavian artery. Distal arterial pressure (i.e. below the level of aortic occlusion) was monitored by cannulation of the tail artery (PE-50). Spinal cord ischemia was induced by inflation of the intra-aortic balloon catheter (0.05mL of saline) and concurrent systemic hypotension (40 mm Hg) induced by blood withdrawal (10.5–11 cc into a heated (37°C) external reservoir) via a 20-gauge polytetrafluoroethylene catheter in the left carotid artery. The efficacy of the occlusion was demonstrated by an immediate and sustained drop in distal blood pressure. After 10-min ischemia, the balloon was deflated, and the blood was reinfused. When the arterial blood pressure was stabilized (20–30 min after reflow), the arterial lines were removed and wounds were closed.
After ischemia, the recovery of motor function was assessed in 2-day intervals using a modified 21-point open field locomotor scale (Basso et al., 1996). Only animals with BBB score of 0–4 at 21 days after ischemia were selected for the transplantation study.
Experimental groups
There were three separate components in the study. The specific goals of these studies were to establish: i) the optimal post-injury time frame for spinal hSSCs grafting as defined by cell survival; ii) the optimal density of grafted cells as defined by the effect of cell grafts on spinal morphology; and, iii) the therapeutic-behavioral effect after cell grafting in animals with fully developed ischemic paraplegia as defined by the recovery of motor function and suppression of electrophysiologically-defined signs of spasticity and rigidity (see Table 1 for Experimental Groups).
Table 1.
Experimental Groups
| Exp. groups | Post-injury time Cell/medium inj. | Cell density/No. of injections | Assessment of Motor function (BBB score) | Motor Evoked Potentials | Post- Graft survival | Histology/Stereo. Quant. |
|---|---|---|---|---|---|---|
| Study I | ||||||
| A1 (n=4) | 3 days | 30, 000/10 | − | − | 7–14 days | +/− |
| A2 (n=5) | 7 days | 30, 000/10 | − | − | 7–14 days | +/− |
| A3 (n=12) | 21 days | 30, 000/10 | + | − | 1–8 weeks | +/− |
| A4 (n=7) | 21 days | Medium/10 | + | − | 8 weeks | +/− |
| Study II | ||||||
| B1 (n=4) | 21 days | 5, 000/10 | − | − | 2–7 weeks | +/− |
| B2 (n=4) | 21 days | 10, 000/10 | − | − | 2–7 weeks | +/+ |
| B3 (n=4) | 21 days | 30, 000/10 | − | − | 2–7 weeks | +/+ |
| B4 (n=4) | 21 days | 50, 000/10 | − | − | 2–7 weeks | +/− |
| Study III | ||||||
| C1 (n=13) | 21 days | 10, 000/25–30 | + | − | 3 months | +/− |
| C2 (n=6) | 21 days | Medium/25–30 | + | + | 3 months | +/− |
In the first study (Study I: Groups A1–A4) animals were grafted with hSSCs at 3 days (Group A1; n=4), 7 days (Group A2; n=5) or 21 days (Group A3; n=12) after ischemic injury (see Spinal Cord implantation for cell dosing; Table 1). In a separate control group (Group A4; n=7) medium was injected only. Animals in this study survived between 7 days-8 weeks.
In the second study (Study II: Groups B1–B4) a variable density of cells (5 000, 10 000, 30 000 or 50 000 in 0.5μl of vehicle; n=4 for each cell density) was injected spinally in animals at 21 days after ischemia. The survival, total cell density (see Stereological quantification) and differentiation profile of grafted cells was then analyzed between 2–7 weeks after grafting.
In the third functional long-term recovery study (Study III: Groups C1 and C2) the effect of spinal hSSCs on the recovery of motor function and corresponding changes in motor evoked potentials was studied in animals with defined spastic paraplegia. In the first group (Group C1; n = 13) animals were grafted with the hSSCs. In the second control group (Group C2; n=6) animals were spinally injected with medium only. In both groups, the recovery of motor function (see Assessment of motor function) and motor evoked potentials (see Recording of motor evoked potentials) were assessed/recorded in 7-day intervals for up to 12 weeks. At 3 months all animals were perfusion fixed for immunohistopathological analysis of the spinal cords (see Immunohistological processing).
Animals in all experimental groups received daily immunosuppressive treatment with FK-506 (Prograf; Fujisawa; 1mg/kg (Study I & II) or 3mg/kg (Study III); i.p.) during the entire survival period, with the treatment initiated 3 days before spinal transplantation.
Preparation of hSSCs for Implantation
One day prior to each surgery day, one cryopreserved vial of the previously prepared passage 16 cell bank was thawed, washed, concentrated in a hibernation buffer, and shipped from the cell preparation site (Neuralstem, Inc., Rockville, MD) to the surgery site (UCSD, San Diego, CA) at 2–8°C by overnight delivery. Upon receipt the following day, the cell concentration was adjusted to a final concentration of 5 000, 10 000, 30 000, or 50 000 in 0.5μl of buffer and used directly for implantation without further manipulation. Before and after implantation the viability of cells was tested using fluorescein diacetate/propiodium iodide. On average a 70–85% viability rate was recorded. At the same time, some of the cells were plated on a previously prepared rat astrocyte monolayer for in vitro differentiation (see following paragraph).
Culture of hSSCs on rat astrocyte monolayer for in vitro differentiation
Some of the cells were differentiated in vitro on rat astrocyte monolayer. Astrocytes were isolated from lumbar spinal cords of P2 rat pups using Papain Dissociation System (Worthington Biochem. Corp., NJ). After isolation astrocytes were cultured with DMEM-10% FBS on Nunclon 6-well plates. To purify astrocytes, mechanical shaking was used on day 7 after isolation. Astrocytes were then fed with fresh DMEM-10% FBS every 3 days until confluent. For co-cultivation astrocytes were passaged into Lab-Tek 4-chamber slides and cultivated for additional 5–7 days. The hSSCs, resuspended in DMEM-10% FBS into final concentration of 3–5×106neurons/ml, were then added into the astrocyte culture and cultivated for 2–6 weeks. For immunofluorescence staining cells were fixed with 4% paraformaldehyde/0.1% glutaraldehyde for 10 min at 24°C, blocked in 0.1M TRIS solution for 30 min and stained with antibodies raised against neuron specific enolase (NSE, human specific 1:200; Vector), and GABA (1:15,000; Sigma). Standard immunofluorescence technique was then used as described (see Perfusion fixation and tissue processing).
Spinal cord implantation of hSSCs
Animals were anesthetized with 1.5–2% halothane (in room air), placed into a spinal unit apparatus (Stoelting) and a partial Th12-L1 laminectomy was performed using a dental drill (exposing the dorsal surface of L2-L5 segments). Using a glass capillary (tip diameter 80–100μm) connected to a pressure-controlled microinjector (Stoelting), rats were injected with 0.5 μl of the hSSCs in hibernation buffer. The duration of each injection was 60 seconds followed by 30-second pause before capillary withdrawal. The center of the injection was targeted into central gray matter (laminae V–VII) (distance from the dorsal surface of the spinal cord at L3 level: 1 mm), (Kakinohana et al., 2004). The rostrocaudal distance between individual injections ranged between 200 μm (25–30 injections) to 500μm (10 injections). The distance between the right and the left injection entry point ranged between 1.6–1.8 mm.
In study I, animals received a total of 10 bilateral injections (10 injections on the left and 10 injections on the right side) of 30 000 cells/injection. In study II, animals received a total of 10 bilateral injections of 5 000, 10 000, 30 000 or 50 000 cells/injection. In study III, animals received a total of 25–30 injections (10 000 cells per injection), (see Table 1). After implantation, the incision was cleaned with 3% H2O2 and Penicillin/Streptomycin mixture and closed in 2 layers.
Evaluation of neurological function
The recovery of motor function was evaluated in 7-day intervals using a 21-point open field locomotor scale developed by Basso, Beattie and Bresnahan (BBB), (Basso et al., 1996). BBB scores categorize combinations of rat hindlimb movements, trunk position and stability, stepping, coordination, paw placement, toe clearance, and tail position, representing sequential recovery stages that rats attain after spinal injury/ischemia. The assessment of the neurological function was performed by an observer unaware of the treatment protocol. Neurological function was evaluated in Groups A3, A4, C1, and C2.
Recording of motor evoked potentials
To record motor evoked potentials (MEPs), animals were anesthetized (90mg/kg) and maintained with ketamine (100mg/kg/hr, i.m.). Selection of ketamine anesthesia was based on previous studies reported from other laboratories which demonstrate a minimal suppressive effect of ketamine anesthesia on MEPs and Hoffmann reflex. (Thompson et al., 1992, Chiba et al., 1998, Ho and Waite, 2002). Depth of anesthesia was verified periodically by the absence of corneal reflexes, whisker tremor and the pinna reflex.
MEPs were elicited by transcranial electrical stimulation (20V, 200μs; ISOSTIM™ A320, WPI) of the motor cortex using percutaneous stainless steel stimulating electrodes. Responses were recorded from the gastrocnemius muscle using silver needle (22G) electrodes (distance between recording electrodes = 1 cm) connected to a preamplifier (HS4 fiber optic BIOAMP HEADSTAGE, WPI) and amplified using DB4 fiber optic amplifier (WPI). Sampling rate was 80 kHz and filter bandwidth was set at 100–10,000 Hz. MEPs were recorded in 7-day intervals and stored on a PC for analysis. MEPs were recorded and analyzed in Group C1 and C2.
Perfusion fixation and tissue processing
At the end of the survival periods, rats were anesthetized with pentobarbital (40 mg/kg; i.p.) and transcardially perfused with heparinized saline (100 ml) followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB; 500 ml). The spinal cords were dissected and postfixed in the same fixative overnight at 4°C. After postfixation tissue was cryoprotected in a graded sucrose solutions (10, 20 and 30%). Frozen coronal, parasagittal or horizontal spinal cord sections (10–30μm) were then cut. For immunohistochemistry, free floating sections (30μm) were placed in PBS (0.1M; pH=7.4) containing 5% normal goat or donkey serum (NGS, DS), 0.2% Triton X100 (TX), for 2 hrs at room temperature to block non-specific protein activity. This was followed by overnight incubation at 4°C with primary human specific antibodies: mouse anti-nuclear protein/h-nuc (hNUMA; 1:100; Chemicon Int.); mouse anti-neural cell adhesion molecule (hMOC; 1:200; DAKO); mouse anti-neuron–specific enolase (hNSE; 1:200; Novocastra Lab); mouse anti-synaptophysin (hSYN; 1:1000; Chemicon Inc.). These antibodies were combined in multiple labeling experiments with polyclonal antibodies, rabbit anti-GFAP (1:500; Chemicon Int.), rabbit anti-GABA (1:15000, Chemicon Int.), or rabbit anti-synaptophysin (1:200; Novocastra Lab.), rabbit anti-GAD65 (1:2500; Chemicon); guinea pig anti-GLYT2 (1:2500; Chemicon); goat anti-CHAT (1:100; Chemicon). Mouse anti-ED1 monoclonal antibody (1:1000; Chemicon Int.) was used for identification of activated macrophages/microglia.
After incubation with primary antibodies, sections were washed 3x in PBS and incubated with fluorescent-conjugated secondary goat anti-rabbit, goat anti-mouse, or donkey anti-goat antibodies (Alexa 488, 594, 680; 4μl/ml; Molecular Probes). All blocking and antibody preparations were made in 0.1M PBS/0.2%TX/5%NGS or 5%DS. For multiple labeling experiments, primary antibodies from different species were applied simultaneously, followed by application of secondary antibodies conjugated to different fluorescent markers. For general nuclear staining DAPI (3μl/ml) was added to the final secondary antibody solutions. After staining, sections were mounted on slides, dried at room temperature and covered with Prolong anti-fade kit (Molecular Probes).
Stereological quantification of grafted hSSCs by confocal microscopy
The total number of NUMA-positive human cells was estimated using stereological, unbiased, and systematic sampling (West et al., 1991). Every tenth previously-stained section (30-μm thick) was used for stereological quantification after applying fractionator sampling scheme. The optical images (1-μm spacing) were obtained using a Leica DMLB microscope with a 100x oil-immersion objective (numerical aperture 1.3). The optical images were captured using digital camera (Olympus) and ImagePro software (Media Cybernetics) supplied with a StagePro (Media Cybernetics) controlled motorized Z stage. The total number of grafted NUMA-positive cells was then calculated by applying the fractionator formula N = Q×(1/hsf)×(1/asf)×(1/ssf), where N is a total number of positive nuclei, Q is sum of cells counted, hsf is the height sampling fraction, asf is area sampling fraction and ssf is slice sampling fraction. Stereological quantification was performed in Study II (Groups B2 and B3) in animals which received 10 000 or 30 000 cells/injection (n=3 for each cell dosing group).
In colocalization analysis, images were captured with an Olympus confocal microscope (Fluoview 1000). In general, optical sections spaced by 0.2–0.5 μm were taken. Digital images of blue, green, red and far red fluorescence were captured for each optical section. The intensity values for each color were kept within the linear range of the camera. Lenses included 40x and 60x oil immersion (numerical aperture 1.3).
Volume reconstruction of grafted spinal cord segments
To systematically analyze the position of the grafts with respect to their rostro-caudal as well as laminar distribution, a volume reconstruction of the individual grafting sites and spinal cords was performed. For this analysis, previously cut serial sections (average 150 sections/animal) were imaged with a 20x objective and images were then aligned using Ellipse software (Ellipse-2.0.6.1, Sk), (Kakinohana et al., 2004) and volume reconstructed using 3-D Constructor (Media Cybernetics).
Statistical analysis
For the statistical analysis of neurologic outcome non-parametric tests were used. The overall treatment and time-dependent main effect was analyzed using the Kruskal-Wallis test. Intergroup differences at specific time points after grafting were analyzed using Mann-Whitney U-test (an unpaired two-group test). A P-value of < 0.05 was considered significant.
RESULTS
Characterization of in vitro differentiated hSSCs
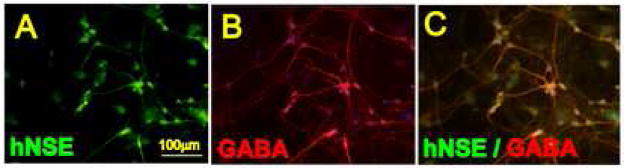
The hSSCs co-cultured on the rat astrocyte monolayers showed a pattern of a progressive neuronal maturation. This was expressed as extensive axodendritic branching and the development of neuron-like morphology. Staining of cultured cells with human specific NSE antibody confirmed an advanced degree of neuronal maturation in NSE positive neurons (Fig. 1A). Double labeling with NSE and GABA antibody showed development of clear GABA immunoreactivity in a subpopulation of NSE positive neurons (Fig. 1B, C). Quantitative analysis performed in 4 sister cultures at 4 weeks after astrocyte/hSSCs co-culture showed that on average, 45±6% of NSE-positive neurons were GABA immunoreactive.
Figure 1 A, B, C.
hSSCs co-cultured on a rat astrocyte monolayer for 6 weeks and stained with human-specific NSE antibody (A) and GABA antibody (B). Extensive axodendritic sprouting and GABA immunoreactivity in terminally differentiated neurons was noted.
Study I (variable postinjury time and cell grafting)
In animals grafted at 3 or 7 days after ischemia only occasional or no surviving cells were seen at 7–14 days after cell grafting as defined by the presence or absence of NUMA-positive cells. While the individual injections tracts were identified, these areas were heavily infiltrated with ED1 immunoreactive elements or GFAP-positive reactive astrocytes (data not shown).
In contrast animals grafted at 21 days (Group A3) after ischemia showed a consistent presence of transplanted cells. The majority of individual grafts were located in the central gray matter distributed between dorsal and ventral horn. Phenotypic examination of grafted cells was identical as was seen in study B and is described in detail in the following section (Study II).
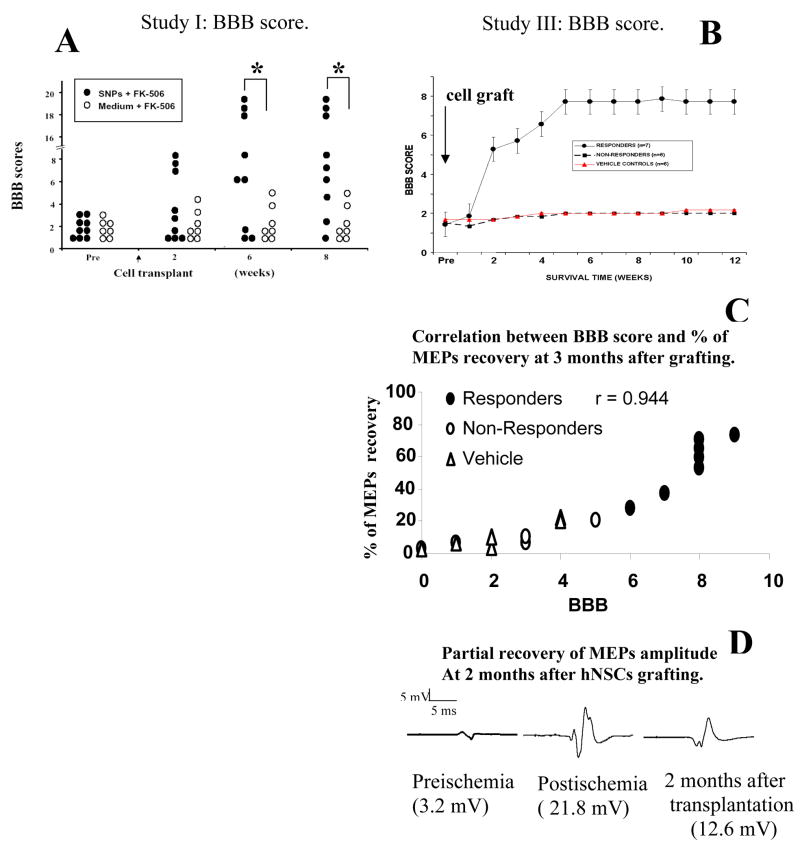
Evaluation of the recovery of motor function in Group A3 and A4 showed that there was a progressive recovery of ambulatory functions in animals grafted with hSSCs. Three of nine animals displayed a significant improvement at 2 weeks after grafting (BBB score 7–8) and showed near normal ambulatory function at 6 weeks (BBB score 18–20). Three other animals, while not able to stand, showed a clear relief of otherwise increased muscle tone and improvement in the mobility of all 3 joints in the lower extremities (Fig. 3A). Statistical analysis at 6–8 weeks after grafting revealed a significantly better motor score in animals grafted with hSSCs if compared to animals injected with medium only (Fig. 3A).
Figure 3 A – D.
Recovery of motor function and motor evoked potentials in animals grafted with hSSCs. A: Statistical analysis of the BBB score between groups A3 and A4 (Study I) revealed a significant improvement at 6 and 8 weeks (P<0.05). B: In Study III 7 of 13 animals grafted with hSSCs showed an improvement in the movement of all 3 joints of the lower extremities and relief of muscle spasticity and rigidity. Six animals showed no improvement. Correlative assessment with the degree of BBB recovery and the degree of MEPs normalization showed a significant correlation (C). D: MEPs recording in an individual animal at baseline, after ischemia and at 2 months after hSSCs grafting. Increased MEPs amplitudes were found after ischemia, followed by a partial normalization at 2 months after grafting.
Study II (cell dosing study)
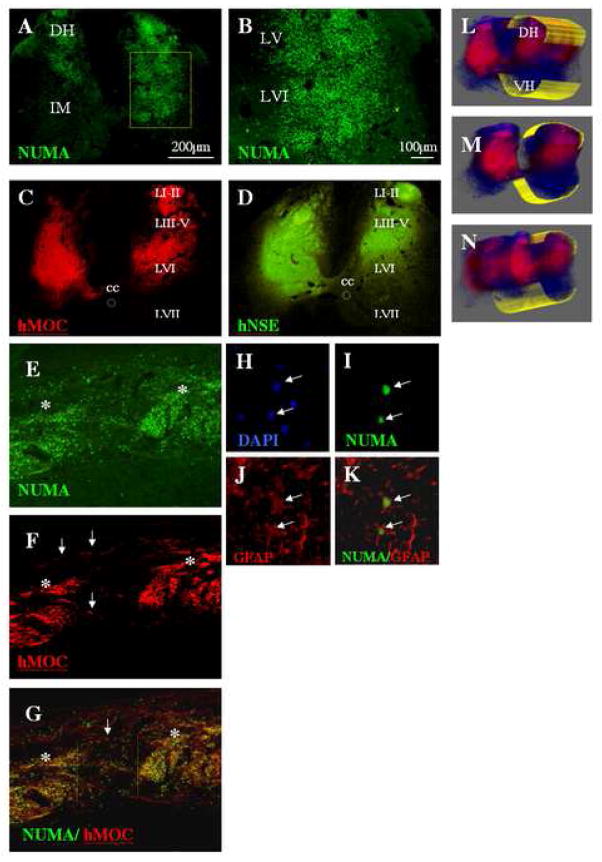
Staining of spinal cord sections taken from animals grafted at 21 days after ischemia with 5 000–50 000 cells per injection site showed a consistent presence of grafted cells. Fig. 2A, B, C show the presence of bilateral NUMA and hMOC-positive grafts in an animal that received 30 000 cells per injection. A comparable graft-specific expression of hNSE was also seen (Fig. 2D). The laminar distribution of the individual grafts was between the surface of the dorsal spinal cord (lamina I) and extended in the majority of animals to lamina VIII.
Figure 2 A – N.
A, B: Transverse lumbar spinal cord sections taken at 7 weeks after grafting from an animal injected with 30 000 cells/injection. Staining with human specific nuclear antibody (NUMA) confirmed the presence of grafted cells distributed between the superficial dorsal horn layers and lamina VIII.
C, D: Staining with human specific MOC and NSE antibody revealed intense immunoreactivity within the individual grafts.
E, F, G: Sagittal lumbar spinal cord sections stained with NUMA and MOC antibody. The core of the individual injection sites could clearly be recognized (E, F, G: asterisks). Numerous NUMA- and MOC-positive cells which migrated for distances greater then 500μm from the borders of the grafts can also be identified (white arrows).
H, I, J, K: Confocal analysis of NUMA and GFAP antibody-stained sections showed that a sub-population of NUMA-positive cells differentiated into astrocytes (I, J, K). These cells were typically localized at the periphery of the individual grafts.
L, M, N: Systematic 3-D analysis of serial transverse spinal cord sections stained with the hMOC antibody confirmed a consistent presence of MOC-positive grafts across 3 individual injections sites (DH-dorsal horn; VH-ventral horn).
Analysis of sagittal sections revealed a number of NUMA and MOC-positive grafted cells which migrated for distances greater than 500μm from the borders of the grafts and can easily be identified as solitary cells localized between the individual grafting sites (Fig. 2E, F, G; white arrows; white asterisks-2 grafts). The majority of solitary cells which migrated outside of the grafts showed differentiation into astrocytes as evidenced by colocalization of NUMA staining with GFAP immunoreactivity (Fig. 2H–K).
Systematic 3-D analysis of serial transverse spinal cord sections stained with the hMOC antibody confirmed a consistent presence of MOC-positive grafts across 3 individual injection sites (Fig. 2L, M, N).
Stereological quantification of grafted hSSCs and 3D volume reconstruction
Stereological estimation of grafted cells was performed in 3 animals injected with 10 000 cells/injection and in 3 animals injected with 30 000 cells/injection. NUMA stained sections were used for stereological analysis.
In animals that received 10 000 cells/injection an average 257 800±37 867 of grafted cells was counted in 5 bilateral grafts. Since, approximately 100 000 cells were injected (5× 10 000 counted in 5 injections = 50 000 × 2/both spinal cord sides) we estimate about 1.5–2 doublings of the cell number after grafting. A similar increase in the number of NUMA-positive cells in animals injected with 30 000 cells was seen where the average was 794 000 ± 45 697 in 5 bilateral grafts.
Neuroanatomical examination of the spinal cords revealed that in 4 of 12 animals injected with ≥30 000 cells/injection there was an oversized graft extending to the surface of the dorsal horn. In these animals individual grafts were found extending to the surface of the dorsal horn and corresponded to the positions of the injection needle tract. As a result of graft outgrowth the topographical organization of the spinal cord was partially lost and the laminar borders of the dorsal horn were difficult to recognize. Such changes were not identified in animals which received ≤10 000 cells/injection. Based on these data a 10 000 cells/injection was chosen for the recovery study using an adult Sprague-Dawley rat of 350g of body weight.
Study III (functional recovery study)
Effect of spinal grafting of hSSCs on motor function and motor evoked potentials
In animals grafted with hSSCs 2 types of responses were noted. In the first group of animals (i.e. responders) a progressive improvement in motor behavior was seen, expressed as an increase in voluntary movement in all 3 joints of the lower extremities (Fig. 3B). This improved behavioral function correlated with an improvement in MEPs response (i.e. suppression of otherwise increased MEPs amplitudes; Fig. 3C, D). In the second group of grafted animals no significant recovery was observed (non-responders) and all animals continue to show extensor type of paraplegia and increased muscle tone (Fig. 3B). These animals also showed a continued increase in MEPs amplitude (Fig. 3C).
In the control group, where animals were injected with vehicle only, no significant recovery of motor function or change in MEPs amplitudes was noted for the entire 3-month survival period (Fig. 3B, C).
Immunohistological analysis of grafted cells
Examination of spinal cord sections at 12 weeks after grafting showed a consistent presence of hSSCs cells in all grafted animals. The majority of grafts were localized in the gray matter in L2-L5 segments and were distributed between the dorsal horn and the intermediate zone (lamina VII).
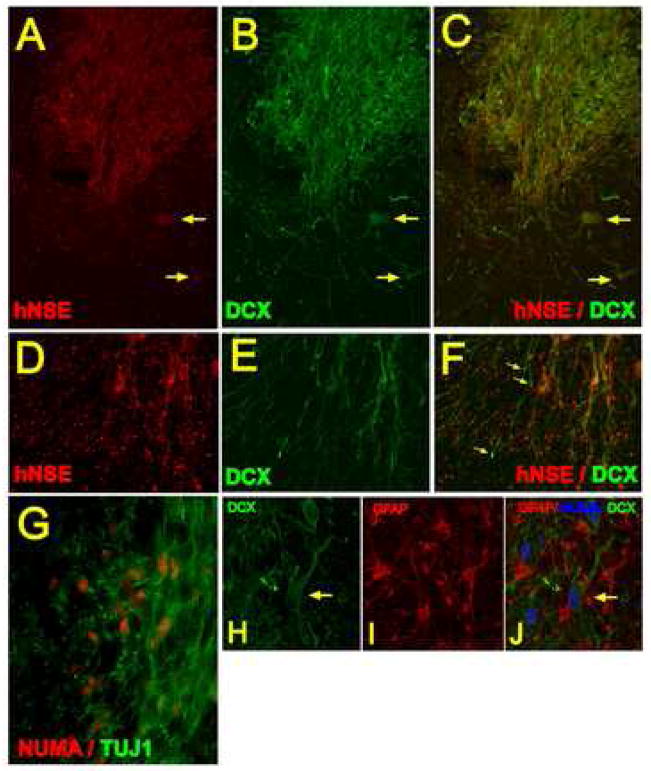
Double labeling with human-specific NSE antibody combined with DCX antibody revealed clear expression of both proteins within the grafts. While the intensity of staining varied between individual neurons, the majority of neurons appeared to express both proteins (Fig. 4A–F; arrows). In contrast, numerous DCX-positive fibers were identified extending from the border of the graft but were NSE-negative (Fig. 4D, E, F; yellow arrows). Similarly, confocal analysis of double labeled sections with TUJ1 and NUMA antibodies revealed colocalization of both antigens within the grafted cells (Fig. 4A–H). Staining with a non-human specific GFAP antibody revealed numerous host-derived GFAP-positive astrocytes in the vicinity of NUMA and DCX immunoreactive grafted neurons. (Fig. 4H, I, J).
Figure 4 A – J.
Transverse spinal cord sections taken at 3 months after grafting and double stained with human specific NSE antibody (A-red) and DCX antibody (B-green). Intense NSE and DCX staining was seen within the graft. In a number of grafted terminally differentiated neurons the colocalization of both proteins was identified (A, B, C- yellow arrows). Numerous processes were found extending from the graft which were DCX immunoreactive but NSE negative (D, E, F; yellow arrows). Double staining with NUMA and TUJ1 antibody revealed that virtually all NUMA immunoreactive cells were also TUJ1-positive (G).
H, I, J- Projected confocal images taken from sections triple stained with DCX, NUMA and non-human specific GFAP antibody. Numerous host-derived GFAP-positive astrocytes which were NUMA-negative were identified within the graft. DCX and NUMA-labeled neurons with extensive axo-dendritic sprouting can also be seen (H, I, J-yellow arrow).
Development of GABA-ergic phenotype in grafted neurons
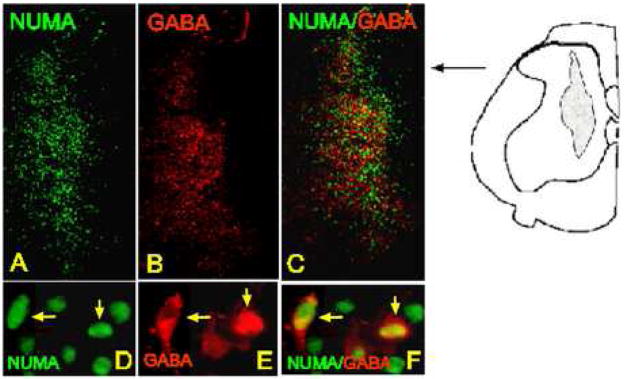
Systematic analysis of NUMA-positive cells double stained with GABA antibody revealed that on average 40–45% of grafted cells developed a GABAergic phenotype (n=4). GABA immunoreactivity was clearly identified within the grafts and in individual NUMA-positive neurons (Fig. 5A–F).
Figure 5 A – F.
Transverse spinal cord section taken after 7 weeks of survival and stained with NUMA and GABA antibody. A subpopulation of NUMA-positive cells showed colocalization with GABA immunoreactivity (D, E, F: yellow arrows).
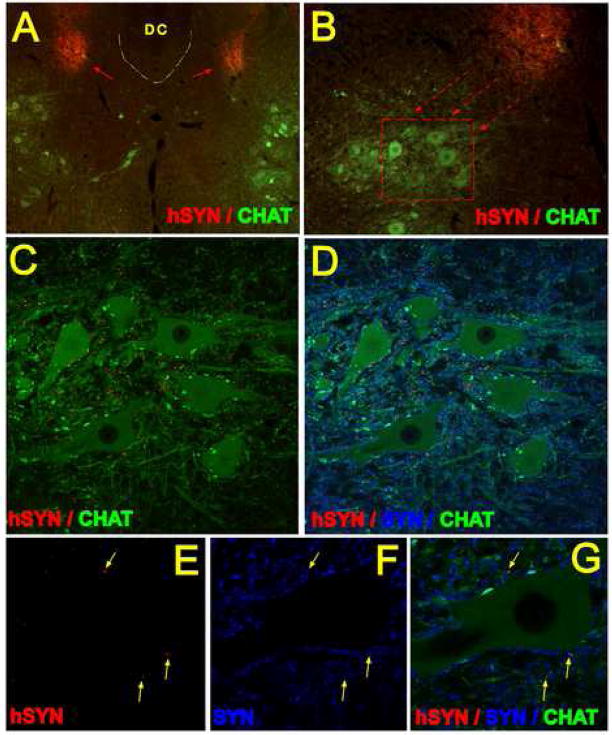
Development of human specific synaptophysin (hSYN) immunoreactivity
Staining of transverse spinal cord sections with hSYN antibody revealed a dense hSYN immunoreactivity within the grafts (Gig.6 A, B -red arrows). In addition, numerous hSYN-positive terminals were identified adjacent to α-motoneuronal membranes (Fig. 6C, D-red). The distances between the border of the individual grafts and the hSYN-positive terminals in the ventral horn ranged between 300–800 μm. Confocal analysis of sections double stained with hSYN and a synaptophysin (SYN) antibody that recognizes both the human and the rat synaptophysin (Fig. 6D, F, G-blue color) demonstrated that only a subpopulation of SYN-positive terminals were also labeled with the hSYN antibody (Fig. 6C, D, E, F, G; yellow arrows).
Figure 6 A – G.
A, B, C - Fluorescent microscopy images (A, B) and projected confocal images (C, D) of transverse spinal cord sections taken at 3 months after grafting and stained with human specific synaptophysin antibody (red), CHAT antibody (green) and synaptophysin antibody which cross-reacts with both human and rat synaptohysin (SYN; blue). Intense hSYN immunoreactivity was found within the two bilateral grafts (A; red arrows). Numerous hSYN immunoreactive terminals were localized in the base of the dorsal horn and extending into ventral α-motoneuron pools (B, C; red).
E, F, G - single optical images (0.3μm thick) demonstrating colocalization of hSYN and SYN immunoreactivity in the vicinity of persisting CHAT+ α-motoneuron (yellow arrows).
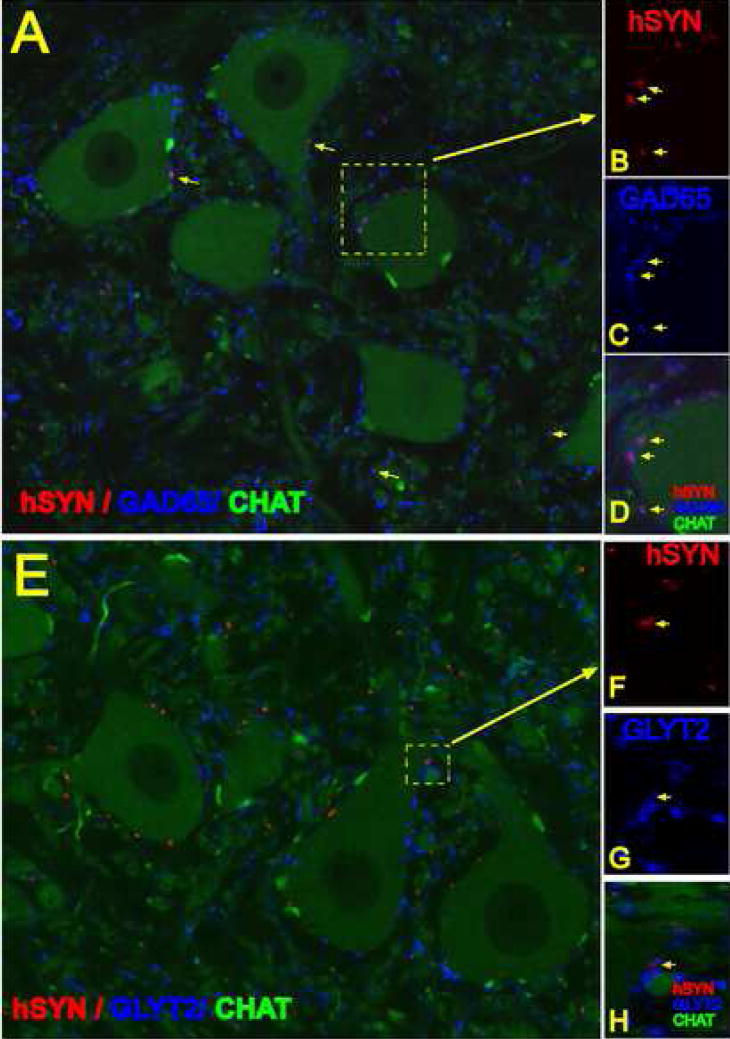
Development of GAD65 and GLYT2 phenotype in hSYN immunoreactive terminals
Confocal analysis of spinal cord sections triple labeled with GAD65, hSYN and CHAT antibody showed that numerous hSYN terminals in the ventral horn or in the vicinity of α-motoneuronal membranes were also GAD65 immunoreactive (Fig. 7A–D). Quantitative analysis showed that 64+15% of hSYN terminals were GAD65 immunoreactive. Single optical sections showed colocalization of both hSYN and GAD65 immunoreactivity (Fig. 7B, C, D). In contrast to GAD65 immunoreactive terminals, less then 2% of hSYN positive structures showed colocalization with GLYT2 immunoreactivity (Fig. 7E, F, G, H; yellow arrow).
Figure 7 A – H.
A, B, C, D- single confocal optical images taken from section at 3 months after grafting and stained with hSYN (red), CHAT (green) and GAD65 (blue) antibodies. The majority of hSYN terminals showed colocalization with GAD65 (B, C, D-yellow arrows).
E, F, G, H - single confocal optical images taken from sections at 3 months after grafting and stained with hSYN (red), CHAT (green) and GLYT2 (blue) antibodies. Only occasional colocalization of hSYN and GLYT2 antibody was noted (F, G, H - yellow arrow).
DISCUSSION
In the present study, by using a well-characterized rat model of spinal ischemic paraplegia, we demonstrate that spinal grafting of viable human spinal stem cells expanded for 16 passages leads to a progressive recovery of motor function over 2–3 months after grafting. Such an improvement in ambulatory functions correlates with improvement of motor evoked potentials recorded during the same time frame. Histological analysis of grafted cells shows long term survival, robust neuronal differentiation, and development of GABAergic phenotype in a subpopulation of grafted neurons. Similarly, in our previous study we demonstrated a comparable treatment effect and long term cell survival after spinal grafting of postmitotic human hNT neurons or committed rat spinal neuronal precursors (Marsala et al., 2004).
Region specific loss of inhibitory interneurons after spinal ischemia in rats and in humans
As outlined above, the likely mechanism of spinal ischemic paraplegia (i.e. spastic/rigid paraplegia) is primarily based on the loss of segmental inhibitory GABA- and glycin-ergic interneurons in ischemic-injured segments with a resulting increase in α-motoneuronal excitability in persisting α-motoneuronal pools. In addition, using this rat aortic occlusion model we have also demonstrated a continuing presence and functionality of spinopetal projections (corticospinal and rubrospinal tract) as well as VGLUT1-positive Ia afferents in previously ischemic spinal cord segments (Marsala et al., 2005, Kakinohana et al., 2006). Similarly, comparable qualitative and quantitative histopathological and functional deficits have been described in several other animal models of spinal ischemic injury as well as in humans (Dimitrijevic and Nathan, 1967, Tarlov, 1967, Zivin and DeGirolami, 1980, Taira and Marsala, 1996). These findings indicate that the rat model is predictive of the dysfunction resulting from spinal ischemia in human patients who undergo thoraco-abdominal aortic aneurysm repair.
Consistent with the proposed mechanism of ischemia-induced spasticity and rigidity, we have shown that spinal delivery of baclofen (GABA B receptor agonist) or nipecotic acid (GABA uptake inhibitor) as well as dorsal L2-L5 rhizotomy, is effective in suppressing behavioral and electrophysiological indices of rigidity and spasticity (Marsala et al., 2005, Kakinohana et al., 2006). Human patients also display clinical relief from spasticity and rigidity with administration of baclofen or other GABA-mimetic drugs (Penn et al, 1988). Thus, given the similar pathophysiology of the spinal ischemic injury in the rat model and human patients, a donor cell population having the potential to develop an inhibitory phenotype (e.g. GABA- or glycin-ergic) may have viable therapeutic potential when transplanted into human patients to replace the lost segmental inhibitory neurons in the injured spinal segments.
In this respect it should be emphasized that spinal ischemic paraplegia represents a unique and well-defined pathological state in which region-specific grafting of neuronal precursors is aimed at restoring local inhibitory circuits and/or provide increased release of inhibitory neurotransmitters such as GABA and/or glycine. This is in contrast to spinal trauma which typically results in a loss of descending motor tracts as well as ascending sensory systems, and depending on the severity and segmental level of the injury, leads to variable degrees of motor/sensory dysfunctions (Vanicky et al., 2001, Young, 2002).
Cell survival and neuronal differentiation of spinally grafted hSSCs
In the present study the majority of grafted cells developed a neuronal phenotype as validated by staining with human-neuron-specific antibodies such as NSE or MOC, or colocalization of staining with human neuronal marker (NUMA) and TUJ1. The capability of these cells to differentiate into neurons when grafted into a previously ischemic (injured) spinal cord during their Nestin-positive “stage” appears to be unique. Previous studies using in vitro expanded neural precursors have reported only limited neuronal differentiation and maturation when grafted into a previously mechanically or chemically injured spinal cord (Onifer et al., 1997). Contrary to the conclusion of the previous studies, it is unlikely that the host spinal tissue is specifically inhibitory to neuronal differentiation of multipotent neural stem cells. Also, similar neurogenic potential of these cells has been observed when transplanted spinally in SOD1 G93A mice (Yan et al., 2006). Rather, the simplest explanation is that the neurogenic potential of this stem cell line has been better maintained than the previously studied cell population. In that regard, it may be of significance that this cell line, unlike others reported, was initiated and maintained as an adherent culture as opposed to a neurosphere culture (Johe et al., 1996).
Variable stages of neuronal maturation in hSSCs –derived neurons
As the current results demonstrate, a mixed population of grafted neurons expressing hNSE, DCX (doublecortin) or both markers was seen 3 months after grafting. In addition a number of DCX-immunoreactive fibers were identified extending from the grafts but which were NSE negative. Previous data show that DCX is only expressed during early stages of spinal cord development and is absent in the adult rat spinal cord (Gleeson et al., 1999, Couillard-Despres et al., 2005). In contrast, the expression of NSE coincides with the morphological and functional maturation of brain neurons (Marangos et al., 1980). These data indicate that with respect to the developmental stage of grafted neurons, different subpopulations of grafted cells are still present 3 months after grafting. Considering the developmental stage of cells when they were grafted (i.e. nestin-positive) this maturation profile is not unexpected and suggests that a much longer post-grafting period is required to achieve full maturity in all grafted cells. This hypothesis is in line with our previously published data showing a more advanced stage of hNT neuron maturation at 3 months after grafting when they were grafted as fully postmitotic neurons (Marsala et al., 2004). In addition, it should be emphasized, that the maturation profile of grafted neurons can be significantly influenced by the animal species of the recipient. For example in contrast to the current rat data, grafting of the same cells into the spinal cord of an uninjured minipig lead to only modest NSE expression at 6–7 weeks after grafting but with robust DCX expression (Motlik and Marsala, unpublished observation). These observations are in line with a recently published report demonstrating that the time frame of neuronal differentiation of human embryonic stem cells implanted in the brain ventricles of embryonic mice is consistent with the maturation profile of the recipient (Muotri et al., 2005).
Also in contrast with other reported donor cells, the cell survival appeared to be quantitative. While a detailed time-course study is needed to quantitatively document the fate of individual cells, in general the current results indicated a 1.5–2 fold increase in the number of cells after injection. This increase in cell number is readily accounted for by the fact that the cells were mostly undifferentiated at the time of implantation. Based on their in vitro behavior, it is expected that the undifferentiated neural stem cells gradually differentiate into neurons and glia during their last cell division over the first three days after transplantation. The doubling time of the undifferentiated hSSCs in vitro is approximately 39 hours in the presence of optimal bFGF concentrations. Most likely, the glial progenitors continue to proliferate slowly over the next 2 weeks. Labeling of mitotic cells in vivo by BrdU (bromodeoxyuridine) injection or by staining with the endogenous proliferation marker, ki67 at 2 months post implantation showed only occasional NUMA-positive human cells (data not shown). Thus, the donor cells, although they initially increase in cell number, are not tumorogenic and do not appear to show uncontrolled cell growth. An extensitive tumorgenicity study is under progress to further demonstrate this point.
Mechanism of cell graft-mediated therapeutic effect
As demonstrated in the present study, numerous hSSCs-derived neurons expressed NSE, suggesting an advanced stage of maturation. Staining with a human-specific synaptophysin antibody revealed a relatively dense population of hSYN-positive terminals particularly in the ventral horn in the vicinity of persisting α-motoneurons. More then 60% of these terminals were GAD65 immunoreactive which was consistent with robust GABA expression in grafted cells. While electron microscopic analysis will be required to provide definitive evidence of synapse formation, previous studies have reported the development of putative synapses between human brain-derived neuronal precursors and mice host neurons (NOD-scid/shi mice) at 16 weeks post-grafting when grafted into traumatically injured spinal cord (Cummings et al., 2005).
Based on these data we believe that the mechanism involved in the observed therapeutic effect during the first 2–3 months after grafting is likely mediated by: i) diffusion of tonically-released GABA from grafted GABA-positive neurons and an improvement in local tonic inhibitory tone; ii) development of GABA- and/or glycine-ergic inhibitory synapses on persisting α-motoneurons or dysinhibited Ia afferents.
In addition, recent evidence suggests that ischemia may lead to AMPA receptor -mediated degeneration of astrocytes and oligodendrocytes (see Stys PK, 2005 for review). The loss of these glial elements can lead to the loss of local conductivity and a resulting loss of neurological function. We speculate that the release of neurotrophic factors from grafted cells can a) promote local remyelinization due to its stimulatory effect on the host oligodendrocyte precursors, b) increase astrocyte proliferation, as well as c) stimulate long tract and segmental fiber sprouting. Such an effect can also contribute to the observed improvement in neurological function. Consistent with this hypothesis a GDNF and BDNF expression in spinally grafted hSSCs in SOD1 G93A mice was recently reported (Yan et al., 2006). An alternative or additional protective mechanism associated with the local release of trophic factors from the graft may be related to the suppression of progressive neuronal/glial apoptosis as well as axonal sparing in persisting host neurons residing in the vicinity of grafted cells.
Functional recovery after grafting
With respect to the degree of behavioral recovery observed in the present study we have seen three principal groups after grafting. First, animals which displayed the most robust recovery and the ability to walk (BBB >16), second, animals which showed improvement in the active mobility of all 3 joints in the lower extremities but were not able to stand (BBB ~8), and the third group in which animals displayed little or no recovery (i.e. non-responders). The most robust recovery was seen in animals grafted with 30 000 cells/injection (total of 10 bilateral injections). However in some animals grafted with this density a graft outgrowth in the superficial regions of the dorsal horn was found and therefore 10 000 cells/injection was used in the second recovery study. While the degree of functional recovery was not so robust in this group, 7 of 13 animals showed significant improvement in the voluntary movement of the lower extremities and this improvement positively correlated with the degree of recovery of motor evoked potentials. Based on these behavioral data we speculate that the optimal cell delivery regimen in the adult rat in this particular model will likely include multiple (10–20) bilateral injections with the density of cells per injection being in the vicinity of 15 000cells/injection.
The reason for the differences in responsiveness to the grafting in both recovery studies and among individual animals is not clear but we speculate that subtle differences playing a role can be graft positions with respect to the dysinhibited primary afferents and/or α-motoneurons as well as the magnitude of GABA release, GABA-ergic and glycine-ergic synapse formation and/or neurotrophic activity mediated by the graft.
Furthermore it should be noted that the maximum survival time was only 3 months in the present study and that numerous DCX immunoreactive neurons were still seen at this time point. Based on these data we speculate that a long term (6–12 months) post-grafting survival period and physical rehabilitation would likely be associated with a higher degree of functional recovery. Nonetheless, in contrast to the grafted groups, no significant recovery was seen in any animals injected with medium only.
Endogenous neuronal proliferation after global cerebral ischemia vs. spinal cord
In previous experimental studies significant neuronal proliferation was noted in the hippocampal CA1 region and dentate gyrus after injurious intervals of ischemia. In these studies it was shown that there is up to 40%–60% of neuronal repopulation at 26–90 days after global cerebral ischemia and that such neuronal repopulation correlates with recovery of memory function (Liu et al., 1998, Bendel et al., 2005). In contrast, the spinal ischemia model used in the present study exhibits a continued presence of spastic paraplegia up to 14 months after ischemic injury and this continued deficit correlates with no detectable increase in the population of small interneurons in previously ischemic spinal cord segments (Kakinohana et al., 2006). While enhancement/modulation of intrinsic neuronal proliferative capacity would be preferable, these data indicate that in the case of spinal ischemic injury the only currently available therapeutic approach to achieve sufficient neuronal re-population in previously neuron-depleted segments will likely be region specific grafting of neuronal precursors. In addition to spinal ischemic injury which primarily affects the interneuronal population in the spinal lumbar region, selective neuronal loss resulting from a localized trauma in the same regions can also represent a potential candidate for cell replacement therapy.
CONCLUSION
In the present study by using a well-defined rat model of ischemic spastic paraplegia we demonstrated that spinal, region-specific grafting of human neural stem cells leads to a progressive recovery of motor function and correlative improvement in motor evoked potentials over 2–3 months after grafting. The improvement in motor function was associated with long term survival of grafted neurons, neuronal differentiation and development of GABAergic phenotype in a sub-population of grafted cells. These data indicate that the use of human spinal stem cells may be an effective therapy for patients suffering from spinal ischemia-induced paraplegia.
Acknowledgments
This research was supported by NIH grant NS 40386 (M.M.), Neuralstem Inc., MD and Centrum of Excellence APVV 51-002105 grant (D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA, Shy ME. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- Akins PT, Atkinson RP. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin. 2002;18(Suppl 2):s9–13. doi: 10.1185/030079902125000660. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Howland DR, Reier PJ. Fetal neural grafts and repair of the injured spinal cord. Brain Pathol. 1995;5:451–457. doi: 10.1111/j.1750-3639.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. Journal of Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Bendel O, Bueters T, von Euler M, Ove Ogren S, Sandin J, von Euler G. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- Chiba A, Nakanishi H, Hiruma S, Satou T, Hashimoto S, Chichibu S. Magnetically induced motor evoked potentials and H-reflex during nembutal and ketamine anesthesia administration in rats. Research communications in molecular pathology and pharmacology. 1998;101:43–57. [PubMed] [Google Scholar]
- Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, Hodge L, Tessler A, Fischer I. Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryonic rat spinal cord. Brain Res. 2000;874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW. Studies of spasticity in man. I. Some features of spasticity. Brain. 1967;90:1–30. doi: 10.1093/brain/90.1.1. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Robertson C. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Annals of Neurology. 1990;27:33–42. doi: 10.1002/ana.410270107. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Ho SM, Waite PM. Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol. 2002;177:494–502. doi: 10.1006/exnr.2002.8013. [DOI] [PubMed] [Google Scholar]
- Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- Kakinohana O, Cizkova D, Tomori Z, Hedlund E, Marsala S, Isacson O, Marsala M. Region-specific cell grafting into cervical and lumbar spinal cord in rat: a qualitative and quantitative stereological study. Exp Neurol. 2004;190:122–132. doi: 10.1016/j.expneurol.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Kakinohana O, Hefferan MP, Nakamura S, Kakinohana M, Galik J, Tomori Z, Marsala J, Yaksh TL, Marsala M. Development of gaba-sensitive spasticity and rigidity in rats after transient spinal cord ischemia: A qualitative and quantitative electrophysiological and histopathological study. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.04.083. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. Journal of Neuroscience. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. Journal of Neuroscience. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V, Valko L, Stolc S, Valko M, Ondrejickova O, Horakova L, Placek J, Troncone A. Free radicals in rabbit spinal cord ischemia: electron spin resonance spectroscopy and correlation with SOD activity. Cell Mol Neurobiol. 1998;18:399–412. doi: 10.1023/A:1022597431593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos PJ, Schmechel DE, Parma AM, Goodwin FK. Developmental profile of neuron-specific (NSE) and non-neuronal (NNE) enolase. Brain Res. 1980;190:185–193. doi: 10.1016/0006-8993(80)91168-3. [DOI] [PubMed] [Google Scholar]
- Marsala M, Hefferan MP, Kakinohana O, Nakamura S, Marsala J, Tomori Z. Measurement of peripheral muscle resistance in rats with chronic ischemia-induced paraplegia or morphine-induced rigidity using a semi-automated computer-controlled muscle resistance meter. J Neurotrauma. 2005;22:1348–1361. doi: 10.1089/neu.2005.22.1348. [DOI] [PubMed] [Google Scholar]
- Marsala M, Kakinohana O, Yaksh TL, Tomori Z, Marsala S, Cizkova D. Spinal implantation of hNT neurons and neuronal precursors: graft survival and functional effects in rats with ischemic spastic paraplegia. Eur J Neurosci. 2004;20:2401–2414. doi: 10.1111/j.1460-9568.2004.03702.x. [DOI] [PubMed] [Google Scholar]
- Marsala M, Sorkin LS, Yaksh TL. Transient spinal ischemia in rat: characterization of spinal cord blood flow, extracellular amino acid release, and concurrent histopathological damage. Journal of Cerebral Blood Flow and Metabolism. 1994;14:604–614. doi: 10.1038/jcbfm.1994.75. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nature Medicine. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- Mori F, Himes BT, Kowada M, Murray M, Tessler A. Fetal spinal cord transplants rescue some axotomized rubrospinal neurons from retrograde cell death in adult rats. Exp Neurol. 1997;143:45–60. doi: 10.1006/exnr.1996.6318. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Cannon AB, Whittemore SR. Altered differentiation of CNS neural progenitor cells after transplantation into the injured adult rat spinal cord. Cell Transplantation. 1997;6:327–338. doi: 10.1177/096368979700600315. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Anderson DK, Thompson FJ, Stokes BT. Neural tissue transplantation and CNS trauma: anatomical and functional repair of the injured spinal cord. J Neurotrauma. 1992a;9(Suppl 1):S223–248. [PubMed] [Google Scholar]
- Reier PJ, Stokes BT, Thompson FJ, Anderson DK. Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord. Exp Neurol. 1992b;115:177–188. doi: 10.1016/0014-4886(92)90245-l. [DOI] [PubMed] [Google Scholar]
- Saganova K, Marsala M. Intrathecal administration of dizocilpine maleate (MK-801) attenuates ischemic damage in the rabbit spinal cord. Exp Neurol. 1994;130:337–343. doi: 10.1006/exnr.1994.1212. [DOI] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. Journal of the neurological sciences. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Svensson LG, Von Ritter CM, Groeneveld HT, Rickards ES, Hunter SJ, Robinson MF, Hinder RA. Cross-clamping of the thoracic aorta. Influence of aortic shunts, laminectomy, papaverine, calcium channel blocker, allopurinol, and superoxide dismutase on spinal cord blood flow and paraplegia in baboons. Annals of Surgery. 1986;204:38–47. doi: 10.1097/00000658-198607000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat [see comments] Stroke. 1996;27:1850–1858. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- Tarlov IM. Rigidity in man due to spinal interneuron loss. Arch Neurol. 1967;16:536–543. doi: 10.1001/archneur.1967.00470230088012. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- Vanicky I, Urdzikova L, Saganova K, Cizkova D, Galik J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma. 2001;18:1399–1407. doi: 10.1089/08977150152725687. [DOI] [PubMed] [Google Scholar]
- Vastag B. Stem cells step closer to the clinic: paralysis partially reversed in rats with ALS-like disease. Jama. 2001;285:1691–1693. doi: 10.1001/jama.285.13.1691. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem cells (Dayton, Ohio) 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- Zivin JA, DeGirolami U. Spinal cord infarction: a highly reproducible stroke model. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]