Abstract
Cardiovascular disease (CVD), which includes coronary artery disease and stroke, is the leading cause of mortality in the nation. Excess CVD morbidity and premature mortality in the African American community is one of the most striking examples of racial/ethnic disparities in health outcomes. African Americans also suffer from increased rates of hypovitaminosis D, which has emerged as an independent risk factor for all-cause and cardiovascular mortality. This overview examines the potential role of hypovitaminosis D as a contributor to racial and ethnic disparities in cardiovascular disease (CVD). We review the epidemiology of vitamin D and CVD in African Americans and the emerging biological roles of vitamin D in key CVD signaling pathways that may contribute to the epidemiological findings and provide the foundation for future therapeutic strategies for reducing health disparities.
Keywords: VDR, CVD, PAI, Wrch-1, Rho, Fst
Cardiovascular disease (CVD), including coronary artery disease and stroke, is the leading cause of death in the African American community.1 It also affects 80 million people in the U.S. and is the leading cause of death for the entire U.S. population.2 Excess CVD morbidity and mortality in the African American community is a striking example of a racial/ethnic disparity in health outcomes. The term health disparities is often used to denote adverse health outcomes suffered by racial or ethnic minorities that occur in the context of historic and contemporary social and economic inequality.2,3,4 In addition to health care system factors and social determinants of health, biological factors are also associated with distinct racial and ethnic clinical profiles.
Hypovitaminosis D has emerged as a key biologic predictor of increased rates of CVD risk factors (hypertension, obesity, diabetes mellitus and the metabolic syndrome)2,3,4 and progression factors (e.g., inflammation and fibrosis).2–6 While it is established that hypovitaminosis D is more common and more severe in racial/ethnic minorities, the pathological mechanisms through which vitamin D may modulate CVD risk factors and progression factors, and if these differ across racial/ethnic groups are not well known. For instance, while the parathyroid (PTH)/Vitamin D axis differs across groups, with African Americans having the highest PTH levels,7 it is not clear that this is due to racial/ethnic differences in vitamin D levels or some other trait of calcium metabolism. Racial/ethnic variations in vitamin D mediated cellular activation/metabolism such as the vitamin D receptor (VDR), vitamin D binding globulin or other related factors could contribute to observed differences. Given the importance of Calcium/PTH/Vitamin D in the regulation of blood pressure, vascular tone and vascular calcification, a pursuit for a more detailed exploration of Vitamin D as a potential CV modifier is warranted. This review will examine the potential role of hypovitaminosis D as a CVD risk factor and a contributor to racial/ethnic disparities in CVD outcomes. Specifically, this review will provide a brief overview of the biology of vitamin D followed by the epidemiology of vitamin D and CVD in African Americans, and then focus on the emerging biologic role of vitamin D in key CVD-related signaling pathways. This information may provide new targets to ameliorate cardiovascular disease as well as practical recommendations for vitamin D repletion that may eliminate the epidemic of hypovitaminosis D in the African American community and reduce health disparities.
Review Criteria
We searched PubMed terms “vitamin D,” “health disparities,” “race,” “ethnicity,” and “cardiovascular disease.” We mainly selected publications from the past five years available in English, but we did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by these strategies and selected those we judged relevant. Relevant review articles and book chapters were also included.
Biochemistry
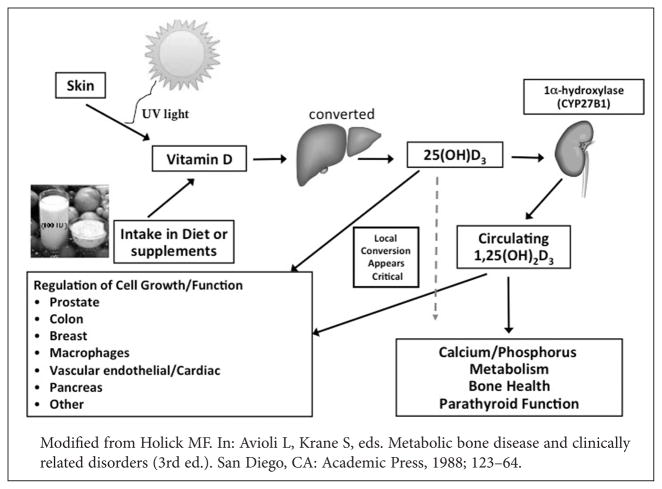
Vitamin D is a fat soluble pro-hormone obtained from dietary sources or produced from UV activation in the skin. As illustrated in Figure 1, synthesis begins in skin keratinocytes with UV conversion of 7-dehydrocholesterol to pre-vitamin D3, followed by metabolism of pre-vitamin D3 to 25(OH)D3 in the liver. In the kidney, 25(OH)D3 is converted to 1α,25(OH)2D3 which is transported to distal target organs. 1α,25(OH)2D3 binds to the vitamin D receptor in the nucleus, ultimately affecting gene transcription for cell growth and function. In dark-skinned individuals, increased melanin reduces light-induced skin synthesis of 7-dehydrocholesterol to pre-vitamin D3 for a given degree of light exposure.8 African Americans, like other at-risk groups with high rates of established or even perceived lactose intolerance, have lower intake of vitamin D fortified dairy products contributing to bone disorders and lower serum vitamin D levels.9 Religious or cultural influences, such as the Islamic tradition of women wearing burqas, may further contribute to reduce skin exposure to the sun and lesser UV conversion of pre-vitamin D.10
Figure 1.
Vitamin D is a fat soluble pro-hormone obtained from dietary sources or produced from UV activation in the skin. In the liver vitamin D is converted by the vitamin 1,25D hydroxylase (25-OHase) to 25-D3 (25-hydroxivitamin D3). 25-D3 is biologically inactive and is converted primarily in the kidney by the 25-hydroxyvitamin D-1α-hydroxylase (1-OHase) to its biologically active form 1,25-dihydroxyvitamin D (1,25-D3) or calcitriol.
Epidemiology
The optimal plasma level of vitamin D (25D) is commonly said to be 30 ng/ml or above, while levels of 21–29 ng/ml are generally considered insufficient, and levels below 20 ng/ml considered deficient.11 The term severe deficiency is commonly used in settings when serum vitamin D levels are less than 10 ng/ml.11 Recent epidemiologic studies have correlated low levels of vitamin D with increased rates of CV disease,11,12,13 endstage renal disease,14 and even death,15,16 suggesting hypovitaminosis D (<10–18 ng/ml) may be an underappreciated non-classical risk factor for CVD. Indeed, the increased rates of CV and related diseases among African Americans may be related in part to more frequent hypovitaminosis D and more overt 25D deficiency.6 Data from the 2001–04 NHANES revealed that the prevalence of sufficient 25D was present in only 30% of Whites, 10% of Hispanics and 5% of African Americans, while severe 25D deficiency (<10 ng/ml) was present in 3% of Whites, 7% of Hispanics and 30% of African Americans.14 The high rates of hypovitaminosis D as well as severe 25D deficiency (<10 ng/ml), well below the at-risk level for CV events of 10–18 ng/ml, may contribute to the excess rates of CVD and related diseases in African Americans.17 In a prospective nested case-controlled study of 18,225 men in the Health Professionals Follow-Up Study, there was a 2.4 fold adjusted increased risk for myocardial infarction for men with 25D levels <15 ng/mL.18 Although association studies point to a strong link between CVD and hypovitaminosis D, the increased rate of CV mortality for African Americans in NHANES III was attenuated by adjustment for 25D levels and fully eliminated with further adjustment for income. This suggests that the effect of 25D on mortality did not significantly differ by race/ethnicity and that racial/ethnic differences are primarily mediated by increased frequency and severity of 25D deficiency in African Americans, but not by a unique biologic response.19,20,21 However, this has yet to be confirmed.
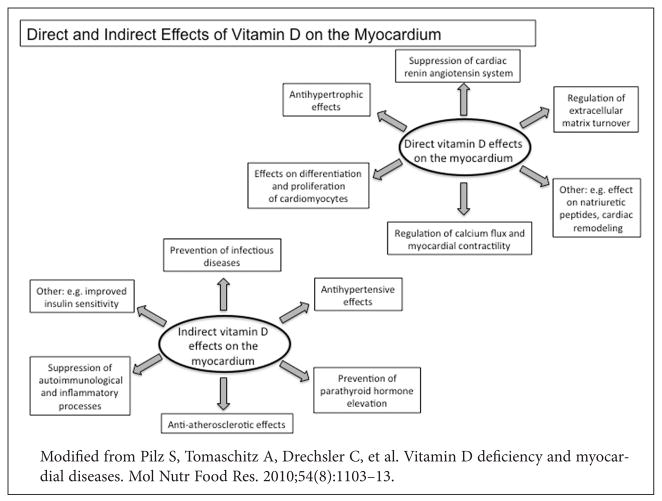
An informative review by Pilz et al. in 201022 compiled the direct and indirect effects of vitamin D on myocardial structure and function. The same authors pointed out that the indirect vitamin D effects are mostly related to disturbances of calcium homeostasis, classic cardiovascular risk factors, atherosclerosis, infections or auto immunological processes.22 Figure 2 summarizes these effects. Finally, Fiscella et al. examined the association of 25D levels and CV mortality to elevated risk among African Americans using baseline data from NHANES III and cause-specific mortality. They found the statistically significant 38% higher age- and sex-adjusted CV mortality observed in African Americans vs. Whites was attenuated to a non significant 14% higher relative risk after adjustment for 25D levels and fully eliminated with further adjustment for income, suggesting vitamin D and socioeconomic factors could account for racial/ethnic disparities in CV mortality.19
Figure 2.
Direct and indirect effects of vitamin D on Myocardium structure and function.
Molecular Effects of Vitamin D on Cardiovascular Disease
Current evidence suggests that in the CV system the genomic mechanisms responsible for vitamin D’s non-classical effects are mediated primarily by the active form of vitamin D (1α,25(OH)2D3 or 1,25D) interacting with the intracellular VDR.23,24,25 1,25D may modulate key processes involved in the pathogenesis of CVD including, but not limited to, vascular inflammation,26 platelet aggregation/thrombogenesis,27 vascular smooth muscle cell proliferation,28 the renin-angiotensin system,29,30 and cardiomyocyte proliferation, vascular calcification, myocardial fibrosis and proliferation.13,31 Multiple genes and signaling pathways mediate the above processes, and reflect the pleiotropic action of 1,25D in different cellular processes including emerging roles in cell-cycle progression, apoptosis, cellular adhesion, and oxidative stress.32,33 A few of these 1,25D modulated CV pathways are highlighted below.
Inflammatory response
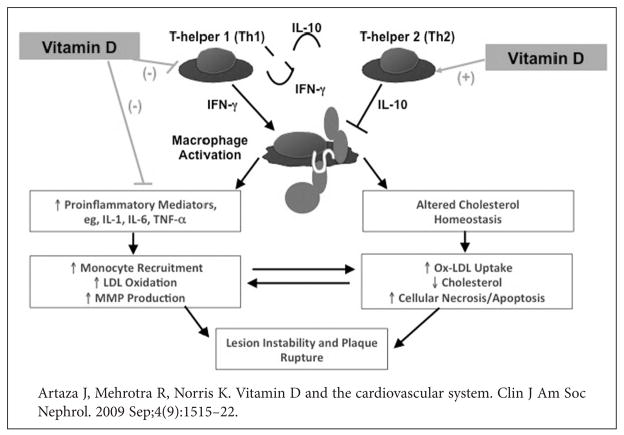
Heightened inflammatory responses may underlie the excess rates of atherosclerotic heart disease and stroke in African Americans. A key potential modifiable factor could be hypovitaminosis D, which has recently been linked to excess inflammation. Critical phases of CVD involve the initial formation of the atherosclerotic plaque followed by plaque rupture or erosion, then subsequent arterial thrombosis.34 Inflammation is a key factor driving the processes of plaque formation, progression and rupture.35 An inflammatory subset of monocytes and macrophages have been reported to selectively concentrate in the atherosclerotic plaque and produce pro-inflammatory cytokines, which include interleukin (IL)-1, IL-4, IL-6, interferon (INF)-γ, and tumor necrosis factor (TNF)-α.35,36,37 T-lymphocytes also enhance thrombosis by driving the production of collagen-degrading proteinases, such as matrix metalloproteinases38 and pro-coagulant tissue factor.35 1,25D and VDR activators may be effective for future interventions in this clinical arena. 1,25D has long been shown to possess immunoregulatory properties39 and may inhibit key steps in the inflammatory process37 (Figure 3).
Figure 3.
Vitamin D, lymphocyte, and macrophage involvement in atherosclerosis. Recent studies suggest that lymphocytes and macrophages play the initial role in the generation of atheromas. It is hypothesized that Th1 cells start producing excess IFNγ, which is a potent stimulator of macrophage activity. Activated macrophages secrete IL-1β, IL-6, and TNF-α. These cytokines recruit additional monocytes, increase LDL oxidation, and generate production of MMPs that can destabilize the plaque to cause rupture and thrombosis. In contrast, the TH2 lymphocyte subset is called the antiatherogenic phenotype, because these cells produce IL-10, an anti-inflamatory cytokine that suppresses macrophage activation and Th1 proliferation.
The biologic mechanism by which 1,25D exerts its effect on cardiovascular inflammation appears to be via VDRs in the heart and vascular wall, as well as in immune cells.39,40 1,25D may reduce inflammation by down-regulating nuclear factor-kβ (NF-kβ) gene expression, activating anti-inflammatory cytokines such as IL-10, and inhibiting pro-inflammatory cytokines IL-6, IL-12, IFN-γ, and TNF-α (39). 1,25D also regulates expression of the collagen-degrading proteinases, which contribute to plaque rupture and thrombosis.34,38,41,42 Among these are matrix metalloproteinases produced by macrophages, which are responsible for vascular wall and myocardium remodeling.37,42,43,44
Platelet aggregation and thrombogenesis
Excess rates of hypovitaminosis D in African Americans could also exert an effect on disparities in CV outcomes from its role in platelet aggregation and thrombogenesis. 1,25D reduces platelet aggregation and thrombogenesis, likely through activation of the VDR.27,45 1,25D also appears to modulate plasminogen-activator-inhibitor (PAI) expression in endothelial cells. Incubation of mesenchymal multipotent cells with 1,25D demonstrated decreased expression of plasminogen-activator-inhibitor,42 while down-regulated expression of tissue factor, another potent coagulation factor, was reported in monocytic cell cultures incubated with 1,25D.46
Vascular smooth muscle cell proliferation
Proliferation of vascular smooth muscle cells is a key event during plaque formation.46 Plaque formation is an important contributor to the excess rates of CVD in African Americans. Incubation of a mesenchymal multipotent cell line with 1,25D showed a reduction in cell numbers suggesting an anti-proliferative effect.31 In addition, 1,25D inhibited G1 to S phase progression by down-regulating the expression of the F-box protein Skp2 (p45) that induces cell growth inhibition via G1 arrest. The anti-proliferative effect of 1,25D may also be mediated through the Rho and Rho/Wrch-1 pathways. The Wrch-1 gene and Rho, a member of the GTPase family were demonstrated to be activated concomitant with decreased cellular proliferation in the presence of 1,25D.31 Recognition of 1,25D as a modulator of vascular smooth muscle cells proliferation reinforces its potential role as a health disparities mediator and a potential CV therapeutic.
Myocyte effects
Congestive heart failure (CHF) is another key CV disparity affecting young African Americans at rates of CHF related death as much as 20 times that of young Whites.47 In the absence of expression, VDR knockout mice develop cardiomyocyte hypertrophy and display notable effects on the systemic and myocardial renin-angiotensin system (RAS). These also have an increased expression of matrix metalloproteinases and reduced expression of the tissue inhibitors of metalloproteinases. 48,49 Artaza et al. recently presented preliminary data suggesting that 1,25D promotes cardiac differentiation through negative modulation of the non-canonical Wnt signaling pathway, supporting the hypothesis that vitamin D repletion might attenuate CVD not only by down regulating excess cell proliferation but by promoting cardiac cellular differentiation.50 These key pathways may play an important role in cardiac remodeling and the subsequent development of CHF and associated mortality risk.
Fibrosis
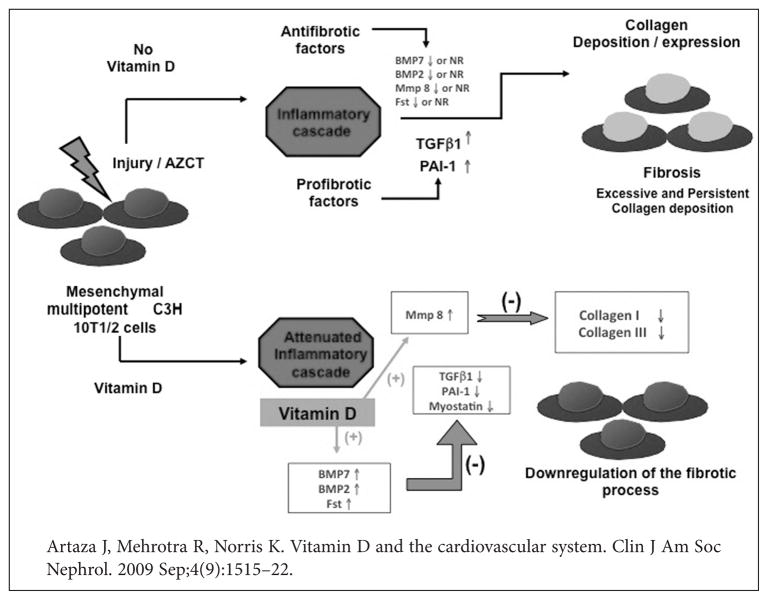
Fibrosis is another important pathway contributing to the morbidity and mortality of CVD. Indeed, hyperinflammatory and hyperfibrotic states may underlie the high rates and severity of multiple chronic disorders including CVD and related conditions in the African American community. Incubation of multipotent mesenchymal cells with 1,25D reduced expression of different collagen isoforms (the ultimate marker of fibrosis).13,42 1,25D exposure also induced an antifibrotic phenotype characterized by increase expression of BMP7 (TGFβ antagonist), and Follistatin, which blocks activin signaling51 and the pro-fibrotic factor myostatin52 and matrix metalloproteinase 8 (Figure 4). The role of 1,25D repletion as a powerful antifibrotic agent may have important clinical implications for CVD in African Americans.
Figure 4.
Vitamin D reverts the fibrotic process induced by 5′-azacytidine (AZCT) in mesenchymal multipotent cells. (A) In the absence of vitamin D, an injury triggers the inflammatory cascade, which can lead to a fibrotic process (progressive scarring). (B) 1,25D (the active form of vitamin D) induces a VDR-mediated antifibrotic signaling phenotype in multipotent mesenchymal cells.
Vascular calcification
Vascular calcification is a common manifestation of vascular disease, especially in patients with chronic kidney disease (CKD). The underlying causes of the more rapid progression of CKD in African Americans are not well known, but vascular calcification is considered a key risk factor for CVD and CKD progression. Serum 1,25D levels were reported by Watson and colleagues to inversely correlate with vascular calcification.53 A three-year follow-up of patients without baseline coronary artery calcification from the Multi-Ethnic Study of Atherosclerosis revealed that low 1,25D levels were independently associated with an increased risk for coronary artery calcification.54 Artaza and colleagues recently presented preliminary data demonstrating that 1,25D promotes osteogenic cell differentiation by down-regulating key family members of the Wnt signaling pathway and may decrease osteoblast differentiation and bone formation by inhibiting expression of the Nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) gene.50 These findings support the delicate balance whereby excess or insufficient vitamin D may both be associated with increased vascular calcifications. 55,56 Thus, optimizing serum vitamin D levels could play an important role in attenuating vascular calcification and the associated risk for CVD and CKD progression.
Renin-angiotensin system
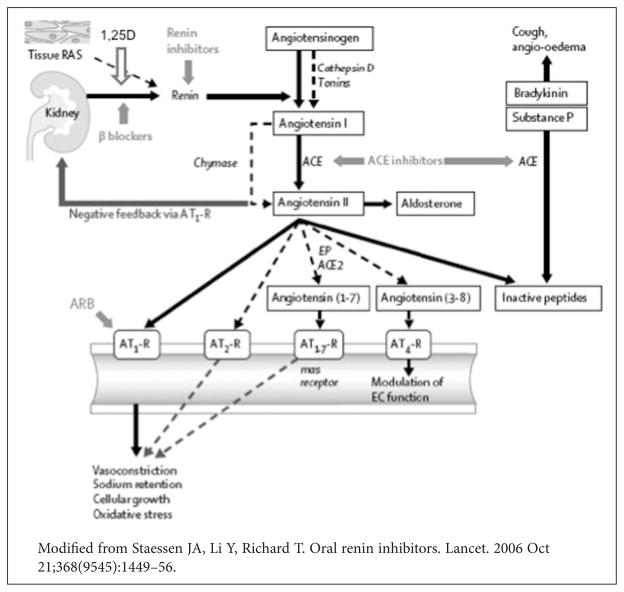
High blood pressure is a prominent risk factor for CVD. African Americans suffer from the highest incidence of hypertension in the nation. Multiple causes have been postulated, but our understanding of hypertension remains poorly defined. African Americans tend to suffer from hypertension characterized by salt sensitivity and low circulating renin levels.57 Although low renin levels would be expected to be associated with less severe target organ damage, African Americans still experience more severe hypertension-related end-organ complications (e.g., proteinuria, cardio-renal disease). This is likely due to a dissociation of circulating RAS from the intrarenal RAS commonly seen in the type of hypertension characterized by salt sensitivity and low circulating renin levels.58 Thus, the renin-angiotensin system (RAS) is not only a key regulator of blood pressure, but also an important mediator of the pathophysiology of the vascular system, including coronary artery disease, cardiac hypertrophy, and congestive heart failure.59 Renin synthesis, the first and rate-limiting component of the RAS cascade, rose significantly in the juxtaglomerular cells of vitamin D receptor (VDR) knockout mice with concomitant plasma RAS activation; and this was independent of calcium and PTH.60,61 Cumulative data from animal and clinical studies suggest that 1,25D or activated Vitamin D, via the VDR receptor, suppresses renin gene expression.29,36,62 As a result, 1,25D or novel VDR activators are emerging as potential independent negative RAS regulators (Figure 5) and may have a role in treatment of RAS-related pathologies similar to other agents that induce RAS blockade. RAS blockade has been reported to reverse endothelial dysfunction, attenuate proteinuria and reduce renal injury independent of blood pressure changes in animals,63 and improve clinical outcomes in humans.64 Thus, vitamin D repletion could contribute to the amelioration of intrarenal RAS activity and improved CV outcomes for African Americans.
Figure 5.
Vitamin D and the renin-angiotensin system. Vitamin D or novel VDR activators have emerged as potential independent negative RAS regulators.
AT-R = Angiotensin Receptor
EP = Endopeptidases
EC = Endothelial Cells
Vitamin D treatment and cardiovascular disease
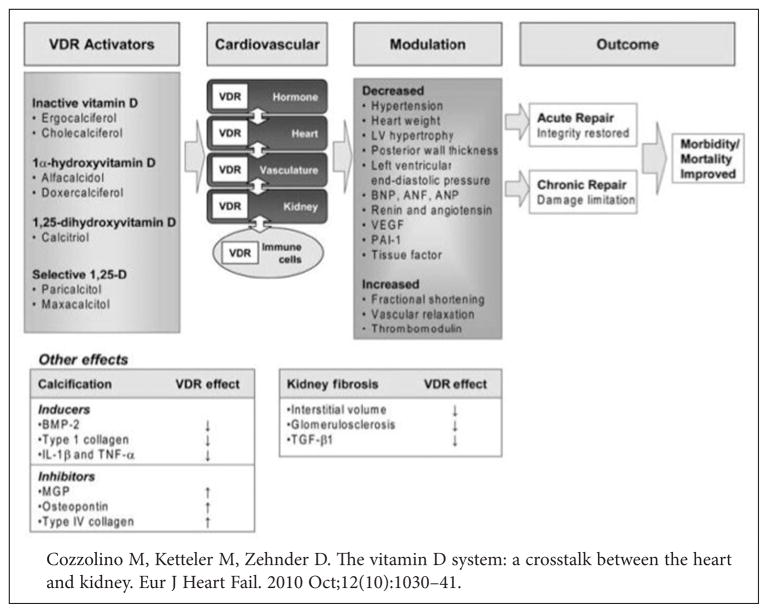
A systematic review of 17 prospective studies and randomized trials that examined vitamin D supplementation, calcium supplementation, or both, and subsequent cardiovascular events. This review found that vitamin D supplements at moderate to high doses had a trend of reducing CVD risk (pooled relative risk, 0.90 [95% CI, 0.77 to 1.05] with vitamin D supplementation at approximately 1000 IU/d), whereas calcium supplements seemed to have minimal cardiovascular effects.65 Autier and Gandini examined all cause mortality risk in subjects who participated in 18 randomized trials. They tested the effect of vitamin D supplementation (mean daily vitamin D dose was 528 IU of either 25D2 or 25D3) and found a reduced relative risk for mortality of 0.93 (95% confidence interval, 0.87–0.99)66. Exogenous administration of calcitriol (synthetic 1,25-D3) or paricalcitol (synthetic 1,25-D2, a VDR activator) in two different in vivo models of cardiac hypertrophy have shown improvements in left ventricular structure and function, myocardial collagen, and cardiac output compared with the control.67,68 In patients with advanced CKD, who are known to be deficient in both 25D and 1,25D, treatment with active vitamin D or a VDR agonist was associated with improved parameters of CV health such as reduced pulse wave velocity and arterial stiffness along with reduced LVH and cardiovascular mortality69 (Figure 6). In a multivariate analyses of over 9,000 incident hemodialysis patients treated with activated vitamin D, African Americans had reduced mortality compared with Whites (HR 0.84; 95% CI 0.72, 0.99) whereas among the untreated, African Americans had increased mortality (HR 1.34; 95% CI 1.07, 1.69).70 These data support the potential contribution of activated vitamin D on the well recognized but poorly understood survival benefit of African Americans on hemodialysis and reinforce the need to better explore the potential role of vitamin D as a modulator of outcomes and disparities in the general population. Although few of the above studies found differential racial/ethnic effects of vitamin D, the fact that African Americans are more likely to suffer from both low vitamin D levels and CVD require further evaluation using randomized prospective trials. Repletion of vitamin D levels could provide a potentially easily modifiable, natural, low cost intervention, which would be extremely relevant as a possible strategy for improving CV outcomes overall, and reducing racial/ethnic health disparities, in particular.71
Figure 6.
Impact of vitamin D receptor activators on cardiovascular endpoints and patient outcome.
ANF = Atrial Natriuretic Factor
ANP = Atrial Natriuretic Peptide
BMP-2 = Bone Morphogenetic Protein-2
BNP = Brain Natriuretic Peptide
IL-1β = Interleukin-1beta
LV = Left Ventricular
MGP = Matrix Gla Protein
PAI-1 = Plasminogen Activator Inhibitor-1
TGF-β = Tumour Growth Factor-β
TNF-α = Tumour Necrosis Factor-alpha
VEGF = Vascular Endothelial Growth Factor
Conclusion and Recommendations
Hypovitaminosis D has emerged as an independent risk factor for all-cause and cardiovascular mortality. An improved understanding of the epidemiologic associations and biologic pathways through which vitamin D may affect cardiovascular health can inform future clinical trials and ultimately evidenced-based therapeutic recommendations. A recent IOM report, reinforced attaining a baseline vitamin D serum level of ≥30 ng/mL but also recommending a supplemental dose of only 600 IU of 25D daily although noting that an upper level intake can be as high as 4,000 IU for persons age 9 and above.20 Interestingly, a report from Bischoff-Ferrari et al. estimates that 1,000 IU daily is required to bring 50% of younger and older adults to 25(OH)D levels above 30 ng/mL.72 However, to bring 85% to 90% of younger and older adults to 25(OH)D levels above 30 ng/mL, 2,000 IU daily are needed.72 Thus, a practical recommendation for achieving levels at or above 30 ng/mL in the African American community will require at least 1,000–2,000 IU/day of vitamin D. This should be directed for addressing daily needs, while it remains premature to make such recommendations for the purpose of preventing cardiovascular disease or death or improving quality of life.73 While prospective clinical interventions to define the cardioprotective effects of nutritional vitamin D repletion or VDR activators are still needed, the recognition of biochemical and transcriptional effects of 1,25D and VDR activators within the CV system may reveal new targets to treat CVD and ultimately reduce health disparities. Since African Americans are at high risk for hypovitaminosis D they should be assessed more frequently and treated when necessary to achieve a serum level of ≥30 ng/mL, recognizing that this may require doses in the upper range of the IOM recommendations. In summary, hypovitaminosis is a major epidemic with important CV implications. Vitamin D repletion is an affordable, natural and easily modifiable intervention that holds tremendous potential as a public health solution for reducing CV related health disparities, as does potential new vitamin D-related cellular targets.
Acknowledgments
Grant Support: This work was supported in part by National Institutes of Health (NIH) grants U54RR022762, RR019234, RR003026, MD000545, MD000182, MD000103, and U54RR026138.
Notes
- 1.Keppel KG, Pearcy JN, Heron MP. Is there progress toward eliminating racial/ethnic disparities in the leading causes of death? Public Health Rep. 2010 Sep-Oct;125(5):689–97. doi: 10.1177/003335491012500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- 4.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008 Jan;11(1):7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 5.Philbin EF, McCullough PA, DiSalvo TG, et al. Underuse of invasive procedures among medicaid patients with acute myocardial infarction. Am J Public Health. 2001 Jul;91(7):1082–8. doi: 10.2105/ajph.91.7.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins D, Wolf M, Pan D, et al. The prevalence of cardiovascular risk factors and the serum levels of vitamin D in the United States: data from the NHANES III. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 7.Valiña-Tóth AL, Lai Z, Yoo W, et al. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clin Endocrinol (Oxf) 2010 May;72(5):595–603. doi: 10.1111/j.1365-2265.2009.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011 Jan;12(1):4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 9.Kull M, Kallikorm R, Lember M. Impact of molecularly defined hypolactasia, self-perceived milk intolerance and milk consumption on bone mineral density in a population sample in Northern Europe. Scand J Gastroenterol. 2009;44(4):415–21. doi: 10.1080/00365520802588117. [DOI] [PubMed] [Google Scholar]
- 10.Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12(11):931–5. doi: 10.1007/s001980170021. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008 Jan 29;117(4):503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. Epub 2008 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009 Sep;4(9):1515–22. doi: 10.2215/CJN.02260409. Epub 2009 Aug 20. [DOI] [PubMed] [Google Scholar]
- 14.Melamed ML, Astor B, Michos ED, et al. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20(12):2631–9. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrotra R, Kermah D, Salusky I, et al. Chronic kidney disease, hypovitaminosis D and mortality in the United States. Kidney Int. 2009;76(9):977–83. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the U.S population, 1988–2004. Arch Intern Med. 2009;169(6):626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E, Liu Y, Hollis BW, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008 Jun;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national U.S. sample. Ann Fam Med. 2010 Jan-Feb;8(1):11–8. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick M. Vitamin D: physiology, molecular biology, and clinical applications. 2. New York, NY: Humana Press; 2010. [Google Scholar]
- 21.Institute of Medicine of the National Academy of Sciences. Dietary reference intakes for calcium and vitamin D. Washington, DC: Institute of Medicine of the National Academy of Sciences; 2010. [Google Scholar]
- 22.Pilz S, Tomaschitz A, Drechsler C, et al. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54(8):1103–13. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 23.Merke J, Hofmann W, Goldschmidt D, et al. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int. 1987 Aug;41(2):112–4. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- 24.Somjen D, Weisman Y, Kohen F, et al. 25-Hydroxyvitamin D3-1-alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005 Apr 5;111(13):1666–71. doi: 10.1161/01.CIR.0000160353.27927.70. Epub 2005 Mar 28. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. High prevalence of vitamin D inadequacy and implication for health. Mayo Clin Proc. 2006 Mar;81(3):353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 26.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3: specific inhibition at the level of messenger RNA. J Clin Invest. 1987 Jun;79(6):1659–64. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279:35798–802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991 Jun;87(6):1889–95. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003 Feb;88(2):327–31. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 30.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in Vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–E32. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 31.Artaza JN, Sirad F, Ferrini MG, et al. 1,25-(OH)2Vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol. 2010;119(1–2):73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008 Oct;29(6):726–76. doi: 10.1210/er.2008-0004. Epub 2008 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstuyf A, Carmeliet G, Bouillon R, et al. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78(2):140–5. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 34.Chapman MJ. From pathophysiology to targeted therapy for atherothrombosis: a role for the combination of statin and aspirin therapy in secondary prevention. Pharmacol Therapy. 2007;113(1):184–96. doi: 10.1016/j.pharmthera.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Card. 2003;91(3A):3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 36.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with chronic kidney disease? Kidney Int. 2005;68(5):1973–81. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 37.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 38.Timms PM, Mannan N, Hitman GA, et al. VCirculation MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002 Dec;95(12):787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 39.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002 Apr;8(4):174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell TD, Simpson RU. Immunochemical identification of the 1,25-dihydroxyvitamin D3 receptor in the male rat. Cell Biol Int. 1996;20:621–4. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 41.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 42.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key pro-fibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200(2):207–21. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001 Feb 23;104(4):503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999 Nov;138(5 Pt 2):S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 45.Ohsawa M, Koyama T, Yamamoto K, et al. 1alpha, 25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000 Dec 5;102(23):2867–72. doi: 10.1161/01.cir.102.23.2867. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009 Mar 19;360(12):1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of systemic and cardiac renin–angiotensin systems. Am J Physiol Endocrinol Metab. 2005 Jan;288(1):E125–32. doi: 10.1152/ajpendo.00224.2004. Epub 2004 Sep 14. [DOI] [PubMed] [Google Scholar]
- 49.Rahman A, Hershey S, Ahmed S, et al. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007 Mar;103(3–5):416–9. doi: 10.1016/j.jsbmb.2006.12.081. Epub 2006 Dec 22. [DOI] [PubMed] [Google Scholar]
- 50.Artaza JN, Garcia LA, Gibbons G, et al. 1,25D induces cardiac differentiation through Wnt signaling pathway. RCMI International Symposium; Nashville (TN). Dec 6–9, 2010. [Google Scholar]
- 51.Sulyok S, Wankell M, Alzheimer C, et al. Activin: an important regulator of wound repair, fibrosis, and neuroprotection. Mol Cell Endocrinol. 2004;225(1–2):127–32. doi: 10.1016/j.mce.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Artaza JN, Singh R, Ferrini MG, et al. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196(2):235–49. doi: 10.1677/JOE-07-0408. [DOI] [PubMed] [Google Scholar]
- 53.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997 Sep 16;96(6):1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 54.de Boer IH, Kestenbaum B, Shoben AB, et al. 25 hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009 Aug;20(8):1805–12. doi: 10.1681/ASN.2008111157. Epub 2009 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizobuchi M, Finch JL, Martin DR, et al. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007 Sep;72(6):709–15. doi: 10.1038/sj.ki.5002406. Epub 2007 Jun 27. [DOI] [PubMed] [Google Scholar]
- 56.Shroff R, Egerton M, Bridel M, et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008 Jun;19(6):1239–46. doi: 10.1681/ASN.2007090993. Epub 2008 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luft FC, Grim CE, Fineberg N, et al. Effects of volume expansion and contraction in normotensive Whites, Blacks, and subjects of different ages. Circulation. 1979 Apr;59(4):643–50. doi: 10.1161/01.cir.59.4.643. [DOI] [PubMed] [Google Scholar]
- 58.Chandramohan G, Bai Y, Norris K, et al. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H Oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2007 Oct 19;28(1):158–67. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 59.Miura S, Saku K. Do angiotensin II type I receptor blockers have molecular effects? Hypertens Rev. 2010;33(2):105–6. doi: 10.1038/hr.2009.202. [DOI] [PubMed] [Google Scholar]
- 60.Zhou C, Lu F, Cao K, et al. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin–angiotensin system in 1-alphahydroxylase knockout mice. Kidney Int. 2008 Jul;74(2):170–9. doi: 10.1038/ki.2008.101. Epub 2008 Apr 2. [DOI] [PubMed] [Google Scholar]
- 61.Kong J, Qiao G, Zhang Z, et al. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008 Dec;74(12):1577–81. doi: 10.1038/ki.2008.452. Epub 2008 Oct 1. [DOI] [PubMed] [Google Scholar]
- 62.Li YC, Kong J, Wei M, et al. 1,25-dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002 Jul;110(2):229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997 Oct 7;96(7):2407–13. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 64.Agodoa LY, Appel L, Bakris GL, et al. Effect of ramipril vs. amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001 Jun 6;285(21):2719–28. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Manson JE, Song Y, et al. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010 Mar 2;152(5):315–23. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 66.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Sep 10;167(16):1730–7. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 67.Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):16810–5. doi: 10.1073/pnas.0611202104. Epub 2007 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mancuso P, Rahman A, Hershey SD, et al. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008 Jun;51(6):559–64. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 69.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007 Oct;72(8):1004–13. doi: 10.1038/sj.ki.5002451. Epub 2007 Aug 8. [DOI] [PubMed] [Google Scholar]
- 70.Wolf M, Betancourt J, Chang Y, et al. Impact of activated Vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008 Jul;19(7):1379–88. doi: 10.1681/ASN.2007091002. Epub 2008 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in Black-White health disparities in the United States. J Am Med Dir Assoc. 2010 Nov;11(9):617–28. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 73.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. [DOI] [PubMed] [Google Scholar]