Abstract
Background
Chronic renal failure (CRF) is associated with hypertriglyceridemia and impaired clearance of very low density lipoprotein (VLDL) and chylomicrons which are largely due to lipoprotein lipase (LPL) deficiency/dysfunction. After its release from myocytes and adipocytes, LPL binds to the endothelium in the adjacent capillaries where it catalyzes hydrolysis of triglycerides in VLDL and chylomicrons. The novel endothelium-derived molecule, glycosylphosphatidylinositol-anchored binding protein 1 (GPIHBP1), plays a critical role in LPL metabolism and function by anchoring LPL to the endothelium and binding chylomicrons. GPIHBP1-deficient mice and humans exhibit severe hypertriglyceridemia and diminished heparin-releasable LPL, pointing to the critical role of GPIHBP1 in regulation of LPL activity. Given its central role in regulation of LPL activity and triglyceride metabolism, we explored the effect of chronic kidney disease (CKD) on GPIHBP1 expression.
Methods
Expression of GPIHBP1 and LPL were determined by reverse transcriptase-polymerase chain reaction, Western blot and immunohistochemical analyses in the adipose tissue, skeletal muscle and myocardium of rats 12 weeks after 5/6 nephrectomy (CRF) or sham-operation (control).
Results
Compared to the controls, the CRF group exhibited severe hypertriglyceridemia, significant reduction of the skeletal muscle, myocardium and adipose tissue LPL mRNA and protein abundance. This was accompanied by parallel reductions of GPIHBP1 mRNA abundance and immunostaining in the tested tissues.
Conclusions
LPL deficiency in CKD is associated with and compounded by GPIHBP1 deficiency. Together these abnormalities contribute to impaired clearance of triglyceride-rich lipoproteins, diminished availability of lipid fuel for energy storage in adipocytes and energy production in myocytes and consequent hypertriglyceridemia, cachexia, muscle weakness and atherosclerosis.
Keywords: Lipid metabolism, Triglyceride metabolism, Atherosclerosis, Impaired exercise capacity, Malnutrition syndrome, Cardiovascular disease, End-stage renal disease, Muscle and fat tissues
Introduction
Chronic renal failure (CRF) is associated with hypertriglyceridemia, impaired clearance of very low density lipoprotein (VLDL) and chylomicrons and triglyceride enrichment of low density lipoproteins (LDL) and high density lipoproteins (HDL) [1–9]. These abnormalities are associated with, and largely due to, hepatic lipase [10], LDL receptor-related protein (LRP) [11] and lipoprotein lipase (LPL) deficiencies [12–16]. LPL is primarily produced and secreted by myocytes and adipocytes. The secreted LPL initially binds to the surface of the secreting cell and subsequently relocates to the adjacent capillaries where it binds to the endothelial surface. Within the capillary lumens LPL catalyzes hydrolysis of triglycerides in VLDL and chylomicrons leading to the release of free fatty acids for uptake by the adjacent myocytes for energy production and by adipocytes for re-esterification and storage as triglycerides.
LPL has been thought to bind to the capillaries via interaction of its positively charged heparin-binding domains [17] with the negatively charged heparan sulfate proteoglycans on the surface of endothelial cells [18, 19]. However, until recently the precise nature of the endothelium-derived molecules involved in the lipolytic processing of chylomicrons was unknown [18]. Recent studies have revealed the critical role of a 28-kDa endothelium-derived molecule, glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1), in the LPL-mediated lipolytic processing of triglyceride-rich lipoproteins [20].
GPIHPB1 plays a critical part in the transport and binding of LPL to the endothelial surface of the capillaries in the skeletal muscle, myocardium and adipose tissue [21, 22]. In addition, GPIHPB1 binds chylomicrons and thereby facilitates LPL-mediated lipolysis of their triglyceride contents. GPIHBP1 contains three distinct domains: (1) an acidic aspartate/glutamate-rich domain which is essential for binding LPL and chylomicrons, (2) a cysteine-rich lymphocyte antigen 6 (Ly6) domain which is also involved in binding LPL, and (3) a hydrophobic carboxyl-terminal motif that is removed and replaced with a glycosylphosphatidylinositol (GPI) anchor in the endoplasmic reticulum [21–23]. The GPIHBP1 binding to the endothelial surface is mediated by insertion of the GPI moiety in the cell membrane [22]. The role of GPIHBP1 in regulation of LPL activity is supported by the following observations: (1) the pattern of tissue GPIHBP1 expression is similar to that of LPL (high levels in heart, adipose and skeletal muscle), (2) GPIHBP1-deficient mice and humans show severe hypertriglyceridemia and diminished heparin-releasable LPL [21], and (3) GPIHBP1-expressing Chinese hamster ovary (CHO) cells avidly bind large lipoproteins harvested from GPIHBP1-deficient mice and exhibit 10- to 20-fold greater LPL binding capacity than control cells [22].
To our knowledge the effect of chronic kidney disease (CKD) on expression of GPIHBP1in the heart, adipose tissue and skeletal muscle has not been previously investigated. Given the critical role of GPIHBP1 in regulation of LPL activity and triglyceride-rich lipoprotein metabolism, the present study was undertaken to explore the effect of CKD on expression of this endothelium-derived protein in the skeletal muscle, adipose tissue and myocardium.
Materials and methods
Study groups
Male Sprague–Dawley rats with an average body weight of 225–250 g (Harlan Sprague–Dawley Inc., Indianapolis, IL, USA) were used in this study. Animals were housed in a climate-controlled vivarium with 12-h day and night cycles and were fed a standard laboratory diet (Purina Mills, Brentwood, MO, USA) and water ad libitum. The animals were randomly assigned to the CRF and sham-operated control groups. The CRF group underwent 5/6 nephrectomy by surgical resection of the upper and lower thirds of the left kidney, followed by right nephrectomy 7 days later. The control group underwent a sham operation. The procedures were carried out under general anesthesia with an intraperitoneal injection of ketamine/xylazine, using strict hemostasis and aseptic techniques. The animals were provided free access to regular rat chow and water and observed for 12 weeks. Six animals were included in each group. Timed urine collections were carried out using metabolic cages. Tail arterial blood pressure was determined as described previously [24]. At the conclusion of the observation period, animals were euthanized by exsanguination using cardiac puncture under general anesthesia. Blood, heart, soleus muscle, subcutaneous and visceral fat tissues were collected.
Plasma total cholesterol, triglyceride, LDL cholesterol, HDL cholesterol, urea and creatinine and urine protein and creatinine concentrations were measured as described previously [24, 25]. Creatinine clearance was calculated using a standard equation. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Western blot analyses
The tissues were homogenized on ice in modified RIPA lysis buffer containing 25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 1 mM PMSF, and protease inhibitor cocktail (Sigma-Aldrich Inc., St. Louis, MO, USA). Protein concentration in the tissue homogenates was determined by BSA assay kit (Pierce Inc., Rockford, IL, USA) and 60 μg of total protein from each sample were fractionated on 4–12% Bis–Tris gradient gel (Invitrogen Inc., Carlsbad, CA, USA) at 120 V for 2 h and transferred to a nitrocellulose membrane. The membrane was then incubated with anti-LPL (1:200 dilutions, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and anti-actin antibodies (1:10,000 dilution; Sigma-Aldrich Inc.) overnight. The appropriate horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich Inc.) were used at a 1:5,000 dilution. The membrane was visualized with SuperSignal® West Pico Substrate (Pierce Inc.) and developed by autoluminography.
Real-time absolute quantitative reverse transcriptase-polymerase chain reaction (real-time AqRT-PCR)
Total RNA was extracted from rat tissues with TRI Reagent (Sigma-Aldrich Inc.), and reverse-transcribed into cDNA in 20 μl reaction volume with a mixture of random primers and oligo dT and Superscript III (Invitrogen Inc.). The cDNAs were diluted and quantified for expression of GPIHBP1, LPL and internal reference gene β-actin with Mx 300 real-time PCR system (Stratagene, Santa Clara, CA, USA). Absolute quantification of GPIHBP1 and LPL expressions relative to reference genes (β-actin) was achieved by using the single standard for both target and reference genes provided by Ziren Research LLC (Irvine, CA, USA). The primer sequences can be obtained from Ziren Research LLC (http://www.zirenresearch.com) upon request.
Immunohistochemistry
Immunohistochemical analysis of the GPIHBP1 expression in the heart, skeletal muscle and adipose tissues was performed as follows. Briefly, 8-μm-thick cryosections were cut, mounted on slides, air dried and fixed in 4% paraformaldehyde/phosphate buffered saline. Endogenous peroxidase activity was removed using 3% hydrogen peroxide in water, and blocked with Protein Block Serum-Free (Dako North America, Inc., Carpinteria, CA, USA). The sections were incubated overnight at 4°C with primary antibodies (1:50 rabbit anti-GPIHBP1 antibody; Abcam Inc., Cambridge, MA, USA). Antibody binding was amplified using ImmPRESS™ Anti-Rabbit Ig Reagent Kit (Vector Laboratories, Inc., Burlingame, CA, USA) and the complex visualized using diaminobenzidine. Nuclei were lightly stained with Mayer's hematoxylin.
Statistical analysis
Student's t test was used in statistical evaluation of the data that are expressed as mean ± SEM. P values ≤ 0.05 were considered significant.
Results
General data
Data are summarized in Table 1. As expected, the CRF group exhibited significant increases in plasma urea, creatinine, triglyceride, cholesterol and LDL cholesterol concentrations, arterial blood pressure and urine protein excretion. This was associated with a significant reduction in creatinine clearance (1.7 ± 0.47 vs. 5.3 ± 1.1 ml/min, P < 0.001) and body weight when compared with the sham-operated control group. Hypercholesterolemia and elevation of plasma LDL in this model is due to heavy proteinuria which is caused by glomerulosclerosis.
Table 1.
General data in the 5/6 nephrectomized (CRF), and sham-operated control (CTL) rats
| CTL | CRF | |
|---|---|---|
| Body weight 12 weeks (g) | 459.80 ± 21 | 411.7 ± 55.3 |
| Systolic blood pressure 12 weeks (mmHg) | 123.5 ± 13 | 168.8 ± 2.8** |
| 24 h urine protein 12 weeks (g/day) | 6.7 ± 1.2 | 80.3 ± 7.3** |
| Plasma urea nitrogen (mg/dl) | 25.3 ± 2.0 | 60.0 ± 16.4*** |
| Plasma creatinine (mg/dl) | 0.50 ± 0.14 | 2.2 ± 1.5* |
| Plasma total cholesterol (mg/dl) | 73.6 ± 8.6 | 221.2 ± 2.5*** |
| Plasma triglyceride (mg/dl) | 45.8 ± 18.2 | 99.7 ± 3.5** |
| Plasma LDL cholesterol (mg/dl) | 18.1 ± 5.3 | 95.0 ± 14.0*** |
N = 6 in each group. Data are mean ± SD
P<0.05
0.01
0.001
LPL and GPIHBP1 data
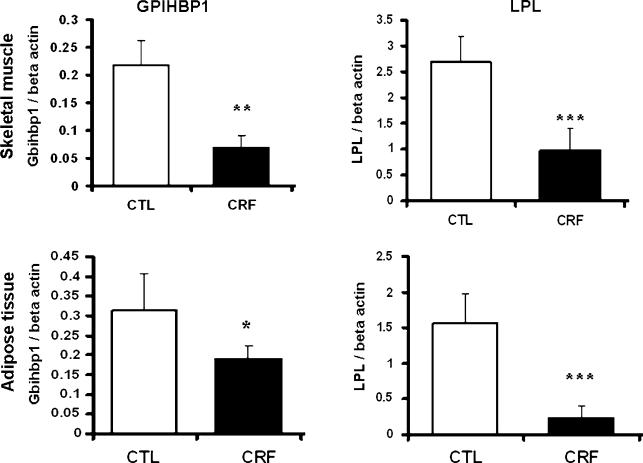
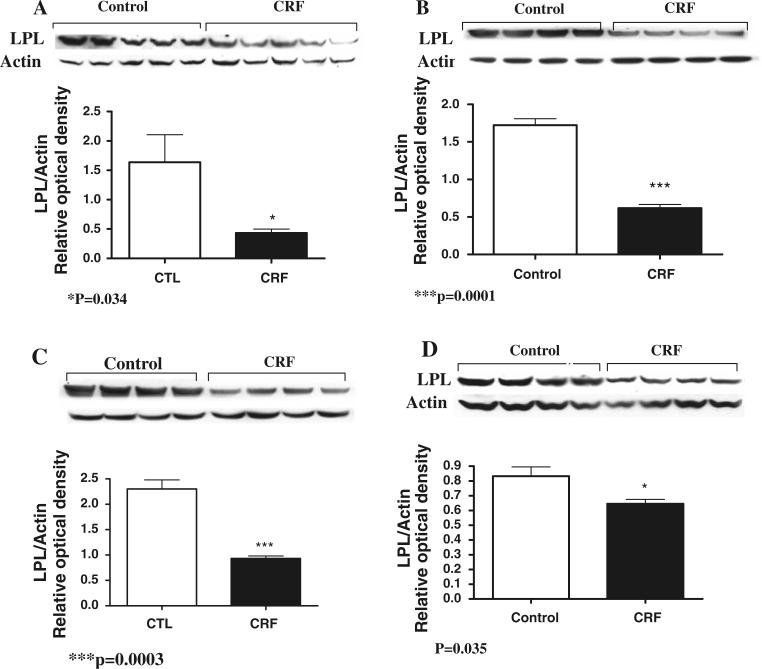
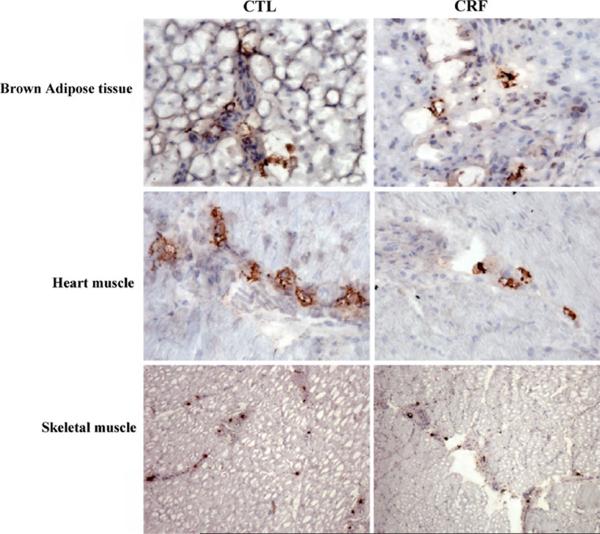
Data are depicted in Figs. 1, 2, and 3. Compared with the sham-operated control rats, the CRF group exhibited a significant reduction of LPL mRNA abundance in both skeletal muscle and adipose tissue (P < 0.001). Likewise LPL protein abundance was significantly reduced in skeletal muscle (P = 0.0003), myocardium (P = 0.035) as well as subcutaneous (P = 0.034) and visceral (P = 0.0001) adipose tissues of the CRF rats. The reductions in LPL mRNA and protein abundance in the skeletal muscle and adipose tissue in the CRF animals were accompanied by parallel reductions of GPIHBP1 mRNA abundance in the tested tissues. Histological examination of the adipose tissue revealed a significant reduction of the size of the adipocytes in the CRF compared to the control group. This observation points to diminished lipid stores in the adipose tissue which is largely mediated by CKD-induced LPL deficiency. Immunohistological examination of the tissues showed a significant reduction of the GPIHBP1 immunostaining in the endothelium of the capillaries in the skeletal muscle, adipose tissue and myocardium in the CRF animals compared to the corresponding tissues in the control group (Fig. 3).
Fig. 1.
Bar graphs depicting LPL/beta-actin mRNA ratios and GPIHBP1/beta-actin mRNA ratios in the skeletal muscle and adipose tissues of the CRF and normal control groups. N = 6 in each group, *P < 0.05, **0.01, ***0.001
Fig. 2.
Representative Western blot and group data depicting LPL and beta actin protein abundance in the subcutaneous fat (a), visceral fat (b), skeletal muscle (c), and myocardium (d) of the CRF and normal control groups. N = 6 in each group, *P < 0.05, ***0.001
Fig. 3.
Representative photomicrographs depicting GPIHBP1 immunostaining of the skeletal muscle, myocardium, and adipose tissue of a CRF and a normal control rat
Discussion
Until recently the lipolytic process was thought to be primarily controlled by myocytes and adipocytes with the adjacent capillary endothelial cells playing a passive part by hosting this process. However recent discovery of GPIHBP1 has unraveled the critical role of endothelial cells in regulation of the LPL-mediated lipolytic process [20–23]. The role of GPIHBP1 in regulation of LPL activity is supported by the observations that the pattern of tissue GPIHBP1 expression is similar to that of LPL (high levels in heart, adipose and skeletal muscle), and both GPIHBP1-deficient mice and humans show severe hypertriglyceridemia and diminished heparin-releasable LPL [21]. Moreover, GPIHBP1-expressing CHO cells avidly bind large lipoproteins(d < 1.006 g/ml) from GPIHBP1-deficient mice and exhibit 10- to 20-fold greater LPL binding capacity than control cells [22].
In a series of earlier studies we found a significant reduction of gene expression, protein abundance and enzymatic activity of LPL, and heparin releasable LPL in adipose tissue, skeletal muscle and myocardium of rats with CKD [14, 15]. In confirmation of the earlier studies, CRF rats employed in the present study exhibited a significant down-regulation of LPL mRNA and protein expressions in the skeletal muscle, myocardium and visceral as well as subcutaneous fat tissues. Down-regulation of LPL in skeletal muscle and adipose tissue in the CRF animals was accompanied by a significant reduction of GPIHBP1 mRNA abundance in these tissues. This observation suggests that CKD can simultaneously reduce LPL and GPIHBP1 transcript abundance by either suppressing their gene expression of or lowering their mRNA stability. The reduction of mRNA abundance was accompanied by a parallel reduction of immunostaining for GPIHBP1 protein in the corresponding tissues of the CRF animals. Thus acquired LPL deficiency is compounded by GPIHBP1 deficiency in CKD. LPL and GPIHBP1 deficiencies in CKD result in impaired clearance of triglyceride-rich lipoproteins and diminished availability of lipid fuel to adipocytes for energy storage and to myocytes for energy production. Together these defects contribute to the CKD-associated hypertriglyceridemia, cachexia, reduced exercise capacity and atherogenic diathesis.
The authors wish to note that the mechanism by which CRF down-regulates GPIHBP1 is presently unclear and awaits future investigations. Moreover, while demonstrating a direct association, the data presented are not sufficient to prove causality between LPL and GPIHBP1 deficiencies in CRF animals. Further studies are needed to determine the contribution of down-regulation of GPIHBP1 to LPL deficiency in CRF. Longitudinal studies employing animals with different types and severities of renal insufficiency can help to further define the course and consequences of the CRF-induced GPIHBP1 deficiency.
In conclusion, LPL deficiency in CKD is associated with and compounded by GPIHBP1 deficiency. Together these abnormalities contribute to impaired clearance of triglyceride-rich lipoproteins, diminished availability of lipid fuel for energy storage in adipocytes and energy production in myocytes and consequent hypertriglyceridemia, cachexia, muscle weakness and atherosclerosis.
Acknowledgment
This study was in part funded by the NIH Accelerating Excellence in Translational Science (AXIS) grant 5U54 RR026138-02 (10-11-KN-G0811C00-UCI).
Footnotes
Conflict of interest The authors have no conflict of interest to declare and warrant that the results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 2011;31(1–3):189–96. doi: 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms and potential consequences. Am J Physiol Renal Physiol. 2006;290:262–72. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 3.Attman PO, Samuelsson O, Alaupovic P. Lipoprotein metabolism and renal failure. Am J Kidney Dis. 1993;21:573–92. doi: 10.1016/s0272-6386(12)80030-8. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri ND. Causes of dysregulation of lipid metabolism in chronic renal failure. Semin Dial. 2009;22(6):644–51. doi: 10.1111/j.1525-139X.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010;6(5):287–96. doi: 10.1038/nrneph.2010.36. [DOI] [PubMed] [Google Scholar]
- 6.Shoji T, Nishizawa Y, Nishitani H, Yamakawa M, Morii H. Impaired metabolism of high density lipoprotein in uremic patients. Kidney Int. 1992;41:1653–61. doi: 10.1038/ki.1992.238. [DOI] [PubMed] [Google Scholar]
- 7.Catrran DC, Fenton SS, Wilson DR, Steiner G. Defective triglyceride removal in lipemia associated with peritoneal dialysis and haemodialysis. Ann Intern Med. 1976;85:29–33. doi: 10.7326/0003-4819-85-1-29. [DOI] [PubMed] [Google Scholar]
- 8.Horkko S, Huttunen K, Korhonen T, Kesaniemi YA. Decreased clearance of low-density lipoprotein in patients with chronic renal failure. Kidney Int. 1994;45:561–70. doi: 10.1038/ki.1994.73. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub M, Burstein A, Rassin T, Liron M, Ringel Y, Cabili S, Blum M, Peer G, Laina A. Severe defect in clearing postprandial chylomicron remnants in dialysis patients. Kidney Int. 1992;42:1247–52. doi: 10.1038/ki.1992.411. [DOI] [PubMed] [Google Scholar]
- 10.Klin M, Smogorzewski M, Ni Z, Zhang G, Massry SG. Abnormalities in hepatic lipase in chronic renal failure: role of excess parathyroid hormone. J Clin Invest. 1996;97:2167–73. doi: 10.1172/JCI118657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaziri ND, Liang K. Down regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 1997;51:913–9. doi: 10.1038/ki.1997.129. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Vaziri ND. Downregulation of hepatic LDL receptor-related protein (LRP) in chronic renal failure. Kidney Int. 2005;67:1028–32. doi: 10.1111/j.1523-1755.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 13.Akmal M, Kasim SE, Soliman AR, Massry SG. Excess parathyroid hormone adversely affects lipid metabolism in chronic renal failure. Kidney Int. 1990;37:854–8. doi: 10.1038/ki.1990.58. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri ND, Liang K. Down-regulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int. 1996;50:1928–35. doi: 10.1038/ki.1996.515. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri ND, Wang XQ, Liang K. Secondary hyperparathyroidism downregulates lipoprotein lipase expression in chronic renal failure. Am J Physiol (Renal Physiol) 1997;273(42):F925–30. doi: 10.1152/ajprenal.1997.273.6.F925. [DOI] [PubMed] [Google Scholar]
- 16.Sendak RA, Bensadoun A. Identification of a heparin-binding domain in the distal carboxyl-terminal region of lipoprotein lipase by site-directed mutagenesis. J Lipid Res. 1998;39:1310–5. [PubMed] [Google Scholar]
- 17.Chan MK, Persaud J, Varghese Z, Moorhead JF. Pathogenic role of post-heparin lipases in lipid abnormalities in hemodialysis patients. Kidney Int. 1984;25:812–8. doi: 10.1038/ki.1984.94. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 19.Parthasarathy N, Goldberg IJ, Sivaram P, Mulloy B, Flory DM, Wagner WD. Oligosaccharide sequences of endothelial cell surface heparan sulfate proteoglycan with affinity for lipoprotein lipase. J Biol Chem. 1994;269:22391–6. [PubMed] [Google Scholar]
- 20.Young SB, Davies SJ, Fong LG, Gin P, Weinstein MM, Bensadoun A, Beigneux AP. GPIHBP1—an endothelial cell molecule required for the lipolytic processing of chylomicrons. Curr Opin Lipidol. 2007;18:389–96. doi: 10.1097/MOL.0b013e3281527914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale P, Bunting S, Walzem RL, Wong JS, et al. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–91. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigneux AP, Davies BS, Bensadoun A, Fong LG, Young SG. GPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins. J Lipid Res. 2009;50(Suppl):S57–62. doi: 10.1194/jlr.R800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Véniant MM, Beigneux AP, Bensadoun A, Fong LG, Young SG. Lipoprotein size and susceptibility to atherosclerosis—insights from genetically modified mouse models. Curr Drug Targets. 2008;9:174–89. doi: 10.2174/138945008783755629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HJ, Moradi H, Yuan J, Norris K, Vaziri ND. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296(6):F1297–306. doi: 10.1152/ajprenal.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, Vaziri ND, Norris K, An WS, Quiroz Y, Rodriguez-Iturbe B. High-calorie diet with moderate protein restriction prevents cachexia and ameliorates oxidative stress, inflammation and proteinuria in experimental chronic kidney disease. Clin Exp Nephrol. 2010;14(6):536–47. doi: 10.1007/s10157-010-0340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]