Abstract
BACKGROUND
Many assessment instruments for psychopathy are multidimensional, suggesting that distinguishable factors are needed to effectively capture variation in this personality domain. However, no prior study has examined the factor structure of the DSM-IV criteria for antisocial personality disorder (ASPD).
METHODS
Self-report questionnaire items reflecting all A criteria for DSM-IV ASPD were available from 4,291 twins (including both members of 1,647 pairs) from the Virginia Adult Study of Psychiatric and Substance Use Disorders. Exploratory factor analysis and twin model fitting were performed using, respectively, Mplus and Mx.
RESULTS
Phenotypic factor analysis produced evidence for 2 correlated factors: aggressive-disregard and disinhibition. The best-fitting multivariate twin model included two genetic and one unique environmental common factor, along with criteria-specific genetic and environmental effects. The two genetic factors closely resembled the phenotypic factors and varied in their prediction of a range of relevant criterion variables. Scores on the genetic aggressive-disregard factor score were more strongly associated with risk for conduct disorder, early and heavy alcohol use, and low educational status, whereas scores on the genetic disinhibition factor score were more strongly associated with younger age, novelty seeking, and major depression.
CONCLUSION
From a genetic perspective, the DSM-IV criteria for ASPD do not reflect a single dimension of liability but rather are influenced by two dimensions of genetic risk reflecting aggressive-disregard and disinhibition. The phenotypic structure of the ASPD criteria results largely from genetic and not from environmental influences.
Keywords: antisocial personality disorder, psychopathy, genetics, twin study, diagnosis, environment
Substantial evidence suggests that antisocial personality and the closely interrelated concept of psychopathy are multidimensional rather than unitary constructs (1;2). The influential Psychopathy Checklist-Revised (PCL-R; (3)) is widely considered to have two factors (often termed “affective-interpersonal” and “antisocial deviance”), and alternative models involving three and four factors have been proposed (1). The Psychopathic Personality Inventory (PPI) indexes psychopathy using eight subscales (4) that reflect two higher-order dimensions along with a narrower “coldheartedness” facet (5). Integrating these findings, Patrick and colleagues propose that psychopathy encompasses three phenotypic components: boldness, meanness, and disinhibition (6).
Recent work examining the structure of the DSM-IV (7) conduct disorder symptoms (8;9) points to distinctive aggression and rule-breaking dimensions, and follow-up work has evaluated the external validity of item subsets reflecting these dimensions (10;11)(12). However, we are unaware of any prior effort to evaluate directly the dimensionality of the adult criteria for -IV ASPD using structural modeling methods. Do the DSM-IV ASPD criteria index a single psychopathologic disposition or, like measures of psychopathy and conduct disorder, do they tap distinguishably different dimensions?
If distinct dimensions are detected within the criteria for ASPD, it is of interest to understand their etiology. Do subdimensions of ASPD reflect multiple genetic and/or environmental influences? For example, Burt (12) showed evidence for differential etiologic contributions to aggression and rule-breaking variants of antisocial behavior in children and adolescents. In a sample of adolescent twins, Blonigen and colleagues estimated scores on the higher-order factors of the PPI (fearless dominance and impulsive antisociality) and found evidence for separable genetic contributions to the two factors (13).
The current work addressed the issue of the dimensionality of ASPD criteria in three steps. First, we used exploratory factor analysis to delineate the dimensional structure of the seven diagnostic criteria comprising part A of DSM-IV ASPD criteria as assessed by self-report questionnaire in adult twins from the Virginia Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) (14). Second, we used multivariate twin analysis to test for the presence of distinguishable genetic and environmental factors on these diagnostic criteria. Third, we evaluated the validity of these identified dimensions by examining how the two genetic factor scores obtained from the best-fit model predicted disorders and traits of potential relevance to ASPD.
Methods
Sample
Participants in this study derived from two inter-related studies of Caucasian same-sex twin pairs who participated in the VATSPSUD (14). All VATSPSUD subjects were ascertained from the population-based Virginia Twin Registry, formed from a systematic review of birth certificates in the Commonwealth of Virginia. Female-female (FF) twin pairs, from birth years 1934-1974, became eligible if both members previously responded to a mailed questionnaire in 1987-1988, for which the response rate was approximately 64%. The data on ASPD symptoms used in this study were collected by self-report questionnaire (SRQ) at the 4th wave of interviews (FF4), conducted in 1995-1997. For this wave, we succeeded in interviewing 85% of the eligible sample and obtained questionnaires from 77% of those interviewed (n=1,497). Data for the male-male (MM) pairs came from a sample (birth years 1940-1974) initially ascertained directly from registry records, which contained all twin births. The first interview (MM1) was completed largely by phone in 1993-1996 and obtained a 72% response rate. This was followed by a 2nd wave of interviews (MM2), conducted in 1994-1998, for which the response rate was 83%. SRQs were obtained from 94% of these individuals (n=5,333, including opposite-sex pairs not included in the current analyses). The availability of the SRQ was higher for the MM2 than the FF4 subsample because assessments for the former were largely conducted face-to-face, and most MM twins completed the SRQ during the home visit. By contrast, the FF4 was conducted by telephone and questionnaires were filled out separately and returned by mail.
Zygosity was determined on the basis of discriminant function analysis utilizing standard twin questions validated against DNA genotyping in 496 pairs (15). The mean age of FF pairs at the time of assessment was 36.5 (SD = 8.4). For the MM pairs, the parallel figures were 37.2 (9.2). SRQs were obtained for both members of 1,647 complete twin pairs (346 monozygotic FF pairs, 214 dizygotic FF pairs, 650 monozygotic MM pairs, and 437 dizygotic MM pairs) and for 997 individual twins without their cotwin.
As detailed previously (16), the SRQ for both the FF4 and MM2 waves contained the same items directed at assessing all of the seven DSM-IV A criteria for ASPD (7). Subjects were instructed to indicate on a four-point scale (“never,” “1-2 times,” “3-5 times,” “more than 5 times”) their frequency of engagement in 17 behaviors “since you turned 18.” Example items (with corresponding DSM-IV A criterion numbers) are: “I was arrested or put in jail” (#1); “I got into physical fights” (#4); and “I did things which put me or other people in physical danger, such as driving very recklessly” (#5). Five of the 7 criteria (#s 1, 3, 4, 5, and 6) were assessed by multiple items, for which averages were created for each criterion variable. Scales for these criteria were organized such that there were a maximum of 4 ordered categories (3 thresholds) per criterion.
Neuroticism was measured by Eysenck’s short scale (17) and novelty seeking by an adapted version of the Cloninger’s TPQ (18). Conduct disorder symptoms, major depression, and cannabis, cocaine, and alcohol dependence were diagnosed according to DSM-IV criteria (7) using an adapted version of the SCID interview (19).
Statistical Analyses
To investigate the dimensional structure of the seven ASPD criteria in the full sample, an exploratory factor analysis (EFA) was carried out using the Mplus software package, Version Mplus version 6.0 (20) , followed by confirmatory factor analysis (CFA). Because DSM A criterion variables were ordinal, the robust, weighted least-squares mean- and variance-adjusted estimator available in Mplus was used to fit alternative one- and two-factor CFA models to the twin data. Adjustments to standard errors and fit indices were implemented using a sandwich-estimator procedure to account for the non-independence of the twin data. To be consistent with the twin modeling analyses in which orthogonal variance components were extracted (see below), an orthogonal (Varimax-rotated) solution was specified for the 2 factor EFA. We evaluated our EFA analyses using three fit-indices which reflect the model’s balance of explanatory power and parsimony: the Tucker-Lewis Index (TLI) (21), the Comparative Fit Index (CFI) (22), and the root mean square error of approximation (RMSEA) (23). For the TLI and CFI, values between 0.90 and 0.95 are considered acceptable and ≥0.95 as good. For the RMSEA, good models have values ≤ 0.05.
A series of multivariate twin models positing different combinations of additive genetic (A), shared (or common) environment (C), and unique environment (E) components were fit to the individual criterion level twin data. The various independent pathway models were fit to four-group (MZ/DZ, male/female) same-sex twin pair raw data using the full-information maximum likelihood estimation procedure available in the Mx software (24). Each observed ordinal ASPD criterion was modeled as a set of estimated ordered thresholds on a normally distributed, continuous latent liability/response variable. Parameter estimation was carried out by integrating across these latent variable continua. Differing threshold estimates were allowed for males and females. We began by fitting a “111_111” baseline model in which twin resemblance among the 7 ASPD criteria was posited to be adequately accounted for by common additive genetic, shared environmental, and unique environmental components along with specific additive genetic, shared environmental, and unique environmental effects. Sex effects were evaluated quantitatively for the 111_111 model by comparing a model in which all biometric components and thresholds were allowed to vary across sex with models forcing invariance on common ACE, specific ACE, and thresholds, respectively. Models dropping the common and specific C components, and then including additional A and E common components, were next tested. Unique environmental specific effects were not set to zero in any of the models tested because this entails the unrealistic assumption that individual responses to items were reported without error.
The goal of model fitting was to achieve an optimal balance of explanatory power and parsimony. We operationalized this goal using the Bayesian information criterion (BIC), which has been shown to perform particularly well with complex models of the kind evaluated here (25). Lower values of BIC indicate relatively better fits.
Maximum likelihood genetic factor scores were estimated by computing the conditional likelihood of the twin pairs’ item responses, weighted by the joint likelihood of the factor score estimates. This factor score model was iteratively fitted, separately for each of the four different zygosity/sex groups, to each twin pairs’ raw data to obtain an estimate of the genetic factor scores for each individual. To validate the genetic factors found in our best fit twin model, we used these genetic factor scores to predict a representative group of variables relevant to ASPD.
To determine whether the genetic factor scores differed from each other in their prediction of the external validators, two regression analyses were performed. First, separate regressions for the two genetic factor scores were conducted to examine the pattern of differences in prediction for each validator. Second, a model constraining the two genetic regression coefficients to be equal within each validator variable was specified. Since the outcome variables were binary or ordinal (or rescaled to be ordinal), the robust weighted least-squares mean and variance adjusted estimator (WLSMV) in Mplus version 6.0 (20) was used to optimize models. In this modeling approach, probit regression coefficients are estimated for each of the genetic factor scores. Since the estimated genetic factor scores are calibrated on a uniform standard scale, the effect size units are more readily interpretable when comparing coefficients.
RESULTS
Phenotypic Factor Analysis
The polychoric correlation matrix used for the EFA yielded first and second Eigen values of 3.72 and 0.92, respectively. However, to determine the dimensionality of the ASPD criteria set, model fit indexes were used. A one-factor EFA solution exhibited marginal fit indices (χ2= 386.7, df=12, CFI=0.94, TLI=0.94, RMSEA=0.07). Allowing a correlated second factor produced a substantial improvement in these indices (CFI=0.99, TLI=0.98, RMSEA=0.04) as well as a drop in the chi-square (χ2=82.3, df=8, p <0.0001). The EFA results were verified using CFA models. Because the 1 and 2 factor models are not strictly nested, information criteria were also examined. Akaike Information Criterion (AIC=−320.9), the Bayesian Information Criterion (BIC=−280.0), and the sample size adjusted (BIC=−299.1) all point to retaining a second factor. Table 1 gives the factor loadings of individual ASPD A criteria for the orthogonally rotated EFA two factor model. Three of the ASPD criteria loaded substantially (≥ +0.55) on the first factor: #1 – not conforming, #4 irritability/aggressiveness and #5 – reckless disregard. We termed this factor aggressive-disregard. The second factor was marked by substantial loadings for the remaining four ASPD criteria: # 2 – deceitfulness, # 3 – impulsivity/failure to plan, # 6 – irresponsibility, and # 7 – lack of remorse. We termed this factor disinhibition (although, as discussed below, the loading of criterion # 7 appears anomalous). Positive cross-loadings were evident for all criteria, in particular criteria #1, 5, and 7.
Table 1.
Factor Loadings for the Phenotypic Exploratory Factor Analysis of the 7 DSM-IV Antisocial Personality disorder Criteria with Varimax Rotation
| Criterion # | Brief Description | Factor | Loading |
|---|---|---|---|
| Factor 1 | Factor 2 | ||
| 1 | Not Conforming | 0.62 | 0.46 |
| 2 | Deceitful | 0.26 | 0.71 |
| 3 | Failure to Plan | 0.23 | 0.56 |
| 4 | Irritability/Repeated Fights |
0.68 | 0.25 |
| 5 | Reckless Disregard | 0.78 | 0.30 |
| 6 | Irresponsibility | 0.28 | 0.60 |
| 7 | Lacks Remorse | 0.30 | 0.69 |
Note: Loadings >.50 are bolded.
Twin Analyses
As shown in Table 2, model I specified one genetic, one shared environmental, and one unique environmental common factor as well as criteria-specific genetic and environmental effects. This model included separate genetic and environmental parameters and thresholds for males and females. Model II, which constrained the genetic and environmental parameters to equality across males and females while permitting the thresholds to differ, fit much better than model I based on the reduction in BIC from that obtained from model I (ΔBIC=−134.8). Model III, which omitted the shared environmental common factor and specific C effects, further improved the BIC (−153.8). By contrast, the fit of model IV, which omitted the genetic common factor and specific components, was comparatively worse fit (ΔBIC=−129.6).
Table 2.
Twin Model-Fitting Results for DSM-IV Criteria for Antisocial Personality Disorder
| Model | Sex Effects |
Common Factors |
Item-Specific Effects |
Δ zχ2 | Δ df | Δ BIC |
|---|---|---|---|---|---|---|
| I# | Present | 1-1-1 | 1-1-1 | -- | -- | -- |
| II | Absent | 1-1-1 | 1-1-1 | 61.4 | 42 | −134.8 |
| III | Absent | 1-0-1 | 1-0-1 | 133.8 | 56 | −153.8 |
| IV | Absent | 0-1-1 | 0-1-1 | 182.2 | 56 | − 129.6 |
| V* | Absent | 2-0-1 | 1-0-1 | 42.9 | 50 | − 175.6 |
| VI | Absent | 1-0-2 | 1-0-1 | 55.6 | 50 | − 169.2 |
| VII | Absent | 3-0-1 | 1-0-1 | 36.1 | 45 | − 159.3 |
The change (Δ) for χ2, df and BIC are all with respect of model I.
-2logliklihood=29,545.9, df=29,794 BIC= −103,587.8
best-fitting model
Working from model III, we then added a second genetic common factor (model V) or a second unique environmental factor (model VI). While both of these models yielded an improvement based on ΔBIC compared to model III, the fit for model V was appreciably better (−175.6). Working from model V, we sought to add a third genetic factor (model VII), but the model fit in this case deteriorated (ΔBIC=−159.3). Thus, model V emerged as the best-fitting model.
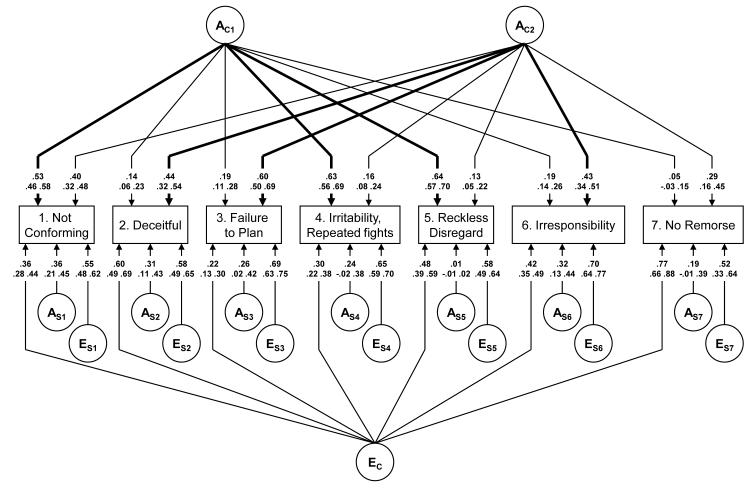
The parameter estimates from this best-fitting model, along with their 95% confidence intervals, are depicted in Figure 1. As noted above, the best fit model contains no shared environmental risk factors. That is, our modeling suggests that the tendency for ASPD criteria to aggregate within families is entirely a result of genetic factors.
Figure 1.
Parameter estimates, top line, and 95% confidence intervals, bottom line, from the best-fitting model (Model V) for the DSM-IV A Criteria for Antisocial Personality Disorder. Paths from the First and Second Common Genetic Factor (AC1 and AC2) that are greater than +0.40 in value are darkened.
Values shown are medians of the point estimates obtained via bootstrapping of the full best-fitting 201_101 model (N=500). The first genetic common factor (AC1) closely resembled the first phenotypic factor loading pattern, with prominent loadings (≥ +0.40) evident for the same three ASPD criteria: #s 1, 4, and 5. Therefore, we labeled this genetic factor aggressive-disregard. The second genetic common factor (AC2) evidenced prominent loadings (≥ +0.40) for one ASPD criterion in common with the first genetic factor (i.e., #1), and three others: #2, 3, and 6. Of note, the confidence intervals show that the loading on the predicted factor is significantly greater than the loading on the other factor for all variables except # 1.
This second genetic factor closely resembled the loadings of the second phenotypic factor, with one major difference. Whereas criterion 7 (lack of remorse) loaded very highly on the second phenotypic factor (+0.69), the loading of this criterion on the second genetic factor was much more modest (+0.29). Notably, criterion #1 evidenced moderate loadings on both the second phenotypic and second genetic factor, although in both cases this criterion nonetheless loaded more prominently on factor 1. Based on these results, even more clearly than with the second phenotypic factor, we labeled this second genetic factor disinhibition.1
The various ASPD criteria exhibited a more diverse pattern of loadings on the single unique environmental factor specified in model V, with criterion 7 loading most highly (+0.77) and three other criteria also loading quite substantially: # 2 – deceitfulness, #5 – reckless disregard for safety, and # 6 – consistent irresponsibility. This pattern of loadings does not correspond with those seen for either of the phenotypic or genetic factors.
Criterion-specific genetic effects varied from 0.36 for criterion 1, to 0.01 for criterion 5. Criterion-specific unique environmental effects (which also include most of the variance attributable to errors of measurement) were larger, ranging in value from +0.52 for criterion 7, to +0.70 for criterion 6.
A somewhat different perspective on the genetic results is shown in Table 3. This Table depicts the percent of the genetic variance in each of the ASPD criteria attributable to the two factors, along with the proportion of overall variance in each criterion that is heritable (i.e., attributable to genetic influence). The percentage figures indicate that the 7 ASPD criteria can be divided quite clearly into those with a large proportion of their genetic variance arising from factor 1 (criteria 1, 4 and 5) versus those with a large proportion of genetic variance attributable to factor 2 (criteria 2, 3, 6 and 7). However, per values in the a2 column, the heritabilities of the individual ASPD criteria varied widely and appear roughly divisible into three groups. Criteria 1, 3, 4, and 5 all had relatively high heritabilities, ranging from 0.43 to 0.57. Criterion 2 and 6 had lower heritabilities of 0.32 and 0.33, respectively. Criterion 7 was the one outlier, with an estimated heritability of only 0.12.
Table 3.
Total Heritability and Sources of Heritability and Individual Specific Environmental Effects for the Individual DSM-IV Criteria for Antisocial Personality Disorder
| Criterion # |
Brief Description | Genetic Influences | Unique Environmental Influences |
|||||
|---|---|---|---|---|---|---|---|---|
| a2 | Factor 1 % |
Factor 2 % |
Specific % |
e2 | Factor % |
Specific % |
||
| 1 | Not Conforming | 0.57 | 48 | 28 | 24 | 0.43 | 31 | 69 |
| 2 | Deceitful | 0.32 | 6 | 62 | 32 | 0.68 | 51 | 49 |
| 3 | Failure to Plan | 0.47 | 8 | 76 | 16 | 0.53 | 10 | 90 |
| 4 | Irritability/Repeated Fights |
0.50 | 82 | 5 | 13 | 0.50 | 18 | 82 |
| 5 | Reckless Disregard | 0.43 | 96 | 4 | 0 | 0.57 | 41 | 59 |
| 6 | Irresponsibility | 0.33 | 10 | 56 | 34 | 0.67 | 27 | 73 |
| 7 | Lacks Remorse | 0.12 | 2 | 70 | 28 | 0.88 | 69 | 31 |
Heritability or proportion of variance in liability due to genetic factors
Proportion of variance in liability due to unique environmental factors
Ability of Genetic ASPD Factors to Predict Relevant Criterion Variables
Based on results from our best-fit model and utilizing information from both the twin and the cotwin, controlling for sex, we estimated the level of genetic liability for all subjects in our sample for each of the two identified genetic factors. To evaluate the discriminant validity of these genetic factors, we then examined whether scores on the two factors differentially predicted a representative set of external criterion variables. If these two genetic factors reflect different aspects of the liability to ASPD, we reasoned that they should differ from one another in their ability to predict at least some important variables that played no role in the assignment of the DSM ASPD criteria.
The first criterion variable we examined was age (table 4). While the genetic factor score for aggressive-disregard was uncorrelated with age, the genetic factor score for disinhibition showed a strong inverse relationship. Next, we examined personality scores. Both genetic factors showed similar, weak positively correlations with neuroticism. By contrast, the disinhibition genetic factor showed a markedly stronger association with novelty seeking than did the aggressive-disregard factor.
Table 4.
Prediction of External Potential Validating Variables by the 2 Additive Genetic ASPD Factors
| Genetic Factors | Validator Variable | Estimated Effect Size |
Robust X2 (df) test for Constrained Model |
p - value |
|---|---|---|---|---|
| Aggressive-disregard | Age | − 0.255 | — | 0.394 |
| Disinhibition | Age | − 1.806 | — | 0.000 |
| Factor 1=Factor 2 | 9.62 (1)* | 0.002 | ||
| Aggressive-disregard | Neuroticism | 0.159 | — | 0.000 |
| Disinhibition | Neuroticism | 0.198 | — | 0.000 |
| Factor 1=Factor 2 | 0.56 (1) | 0.453 | ||
| Aggressive-disregard | Novelty Seeking | 0.180 | — | 0.000 |
| Disinhibition | Novelty Seeking | 0.613 | — | 0.000 |
| Factor 1=Factor 2 | 66.8 (1) | 0.000 | ||
| Aggressive-disregard | Conduct Disorder | 0.480 | — | 0.000 |
| Disinhibition | Conduct Disorder | 0.379 | — | 0.000 |
| Factor 1=Factor 2 | 4.34 (1) | 0.036 | ||
| Aggressive-disregard | Major Depression | 0.236 | — | 0.000 |
| Disinhibition | Major Depression | 0.418 | — | 0.000 |
| Factor 1=Factor 2 | 10.23 (1) | 0.001 | ||
| Aggressive-disregard | Cannabis Dependence |
0.485 | — | 0.000 |
| Disinhibition | Cannabis Dependence |
0.537 | — | 0.000 |
| Factor 1=Factor 2 | 0.29 (1) | 0.589 | ||
| Aggressive-disregard | Cocaine Dependence | 0.504 | — | 0.000 |
| Disinhibition | Cocaine Dependence | 0.695 | — | 0.000 |
| Factor 1=Factor 2 | 2.7 (1) | 0.100 | ||
| Aggressive-disregard | Alcohol Dependence | 0.609 | — | 0.000 |
| Disinhibition | Alcohol Dependence | 0.491 | — | 0.000 |
| Factor 1=Factor 2 | 3.1 (1) | 0.078 | ||
| Aggressive-disregard | Age of First Drink | − 0.360 | — | 0.000 |
| Disinhibition | Age of First Drink | − 0.243 | — | 0.000 |
| Factor 1=Factor 2 | 5.2 (1) | 0.022 | ||
| Aggressive-disregard | Maximum Drinks | 0.585 | — | 0.000 |
| Disinhibition | Maximum Drinks | 0.301 | — | 0.000 |
| Factor 1=Factor 2 | 28.6 (1) | 0.000 | ||
| Aggressive-disregard | Treatment (Alc) | 0.687 | — | 0.000 |
| Disinhibition | Treatment (Alc) | 0.427 | — | 0.000 |
| Factor 1=Factor 2 | 8.3 (1) | 0.004 | ||
| Aggressive-disregard | Educational Level | − 0.298 | — | 0.000 |
| Disinhibition | Educational Level | − 0.023 | — | 0.536 |
| Factor 1=Factor 2 | 23.4 (1) | 0.000 |
Notes: All analyses controlled for age and sex. The sample size for the “treatment (Alc)” was truncated due to missing values. All coefficient invariance tests were done in Mplus using the robust WLSMV chi-square difference test.
indicates Robust MLR chi-square difference test was used (for continuous Novelty Seeking factors).
We next explored relations of the two genetic factor scores with psychiatric disorders known to exhibit comorbidity with ASPD. The two factors showed strong positive correlations, of comparable magnitude, with risk for cannabis, cocaine, and alcohol dependence. However, comparisons for specific features of alcohol use revealed differing relations for the two genetic factors. Specifically, the aggressive-disregard genetic factor was more strongly associated with age at first drink, maximum drinks consumed in 24 hours, and treatment seeking than the disinhibition genetic factor. In addition, the genetic aggressive-disregard factor exhibited a significantly stronger association with symptoms of conduct disorder than the genetic disinhibition factor. By contrast, the genetic disinhibition factor showed a stronger relationship with symptoms of major depression.
Finally, we examined the key demographic variable of educational attainment. The aggressive-disregard genetic factor was strongly and inversely associated with education level whereas the disinhibition genetic factor was unrelated.
DISCUSSION
This paper had three inter-related goals. First, we sought to clarify the phenotypic structure of the seven DSM-IV criteria for ASPD. We showed that the pattern of associations among the item endorsements pertaining to these seven criteria in a population-based sample of male and female twins was not well accounted for by a single underlying factor. Rather, two factors, that could be labeled aggressive-disregard and disinhibition, were needed.
Second, we attempted to clarify, using multivariate twin models, the structure of the genetic and environmental risk factors for the 7 ASPD criteria. Several noteworthy results emerged. Shared environmental factors could be omitted and no evidence was found for quantitative sex effects. Only one factor was needed in the model to account for unique environmental effects, whereas clear evidence emerged that two factors were required to adequately account for genetic effects. These findings suggest that the two-factor phenotypic structure of the ASPD criteria in the general population was largely “driven” by genetic and not environmental factors. This interpretation was supported by the finding that loadings of the differing APSD criteria on the two genetic factors paralleled observed loadings on the two phenotypic factors. The major difference was that while criterion 7 (lack of remorse) loaded strongly on the second factor in the phenotypic analysis, its loading on the second factor in the genetic analysis was much more modest. This criterion also showed the lowest heritability (a2 = .12) of any of the ASPD criteria. Notably, only a single questionnaire item (“I took advantage of someone or hurt them and I didn’t feel bad about it”) entered into the score for this criterion. Taken together, these observations suggest that this item may not have effectively indexed criterion 7, which calls for more self-evaluative inference than other items focusing more predominantly on acts/behaviors.
Third, we sought to validate the two genetic factors by examining the ability of genetic factor scores to predict a range of relevant criterion variables that played no role in the ASPD diagnosis. Findings of these analyses provided support for the discriminant validity of the two factors.
In particular, the genetic aggressive-disregard factor differentially predicted conduct disorder, age at first alcoholic drink, maximum drinks consumed in 24 hours, treatment seeking for alcohol problems, and educational level, while the disinhibition genetic factor proved more effective in predicting novelty seeking and major depression. Associations with age also differed markedly for the two factors. Notably, scores on the two genetic factors predicted risk for cannabis, cocaine, and alcohol dependence to a comparable degree, suggesting two potentially distinct genetic pathways mediating the observed association between ASPD and substance use disorders.
The two distinct factors of adult ASPD identified here can be tied to the existing literatures on the structure of conduct disorder and psychopathy in youth, and of externalizing psychopathology in adults. The current findings parallel prior work demonstrating distinctive aggressive and rule-breaking factors underlying the criteria for child conduct disorder (8), which have emerged also as distinctive themes in factor analyses of child disruptive disorder symptoms more broadly as assessed by interview (26;28;29) and questionnaire instruments (10;30). The current results can also be linked to work on child psychopathy that distinguishes between callous-unemotional traits (reflecting a lack of concern for others, and marked by proactive as well as reactive aggression) and impulsive conduct problems (entailing heightened negative affect in conjunction with impulsive, unreliable behavior)(31;32). Supporting a link to the former, items that contributed to criteria loading most strongly on the aggressive-disregard factor of ASPD in the current study included questions dealing with intentional injury of others and putting others at risk (pertinent to reckless disregard) as well as fighting with and hitting others (pertinent to irritability/aggressiveness). In contrast, the finding that the adult ASPD disinhibition factor was associated much more strongly with novelty seeking (indicative of impulsiveness and boredom susceptibility) than the aggressive-disregard factor coincides with findings for the impulsive conduct problem component of child psychopathy (32). Further, the current findings also mirror the finding of distinct impulsive-disinhibitory (“general externalizing”) and callous-aggression factors emerging from recent structural modeling work on the adult externalizing spectrum (27).
These parallels in turn point to etiological implications of the current findings. The disinhibitory factor of externalizing psychopathology has been posited to reflect disturbances in anterior brain systems mediating affective and behavioral control (33;34). Consistent with findings of the current study, scores on this disinhibitory factor tend to decline substantially with age (e.g., (35)). In contrast, the callous-aggression (aka ‘meanness’) factor, considered more characteristic of psychopathy and posited to reflect low dispositional fear in conjunction with deficient affiliation/nurturance (6), tends to show more developmental stability (e.g., (36)). Consistent with this, scores on the callous-disregard factor in the current study were uncorrelated with age.
Strengths and Limitations
These results should be interpreted in the context of the strengths and limitations of this study. Two important methodologic strengths are noteworthy. First, we studied a large population-based sample of adult male and female twin pairs that is, on a variety of social and psychopathological features, broadly representative of the US White population (14). Second, all ASPD-related items were assessed in all subjects so we had no missing-data problems due to skip-outs which can substantially complicate model fitting.
Four potential limitations are also noteworthy. First, the items were assessed by self-report questionnaire and so reflect self-judgment which may be faulty in individuals with high ASPD traits. However, a range of research suggests that for socially undesirable traits, more accurate responses are obtained by more anonymous assessment methods (37). Nonetheless, one criterion in particular in the current study (“lacks remorse,” assessed via a single item) was of questionable validity. Follow-up research is needed to clarify phenotypic and genotypic relations of this criterion with the two ASPD factors, particularly in view of past work by some investigators suggesting closer coherence of lack of remorse with non-aggressive than with aggressive antisocial deviance (26;30). Second, we did not evaluate in this report responses to conduct disorder criteria. Because fulfillment of criteria for conduct disorder is a requirement for a DSM-IV diagnosis of ASPD, our assessment focused only on the “adult” symptoms of ASPD, not the entire diagnostic category. Third, relatively few subjects in our sample endorsed large numbers of these ASPD criteria. Therefore, most of the information from this sample comes from individuals with sub-syndromal levels of ASPD symptomatology. Fourth, for computational simplicity, we did not include opposite-sex dizygotic pairs in the analyses and thus cannot comment on the degree to which the same genes impact on ASPD traits in males and female.
ACKNOWLEDGEMENTS
This work was supported in part by grants AA-R37-011408 and AA-P20-017828 from the US National Institutes of Health. Dr. Carol Prescott played a central role in the design and implementation of the VATSPSUD study.
Footnotes
A potential issue is whether the label “disinhibition” is appropriate for a factor that includes deceitfulness as an indicator. In fact, available empirical data point to deceitfulness or lying as a prominent element of a broad disinhibitory/externalizing pattern in childhood and adulthood (9;11;26;27). In particular, the convergence in the current study between deceitfulness and other APSD items reflecting irresponsibility and failure to plan is highly consistent with the findings of Krueger et al (27), who reported fraudulence, along with irresponsibility and problematic impulsivity, to be strongly indicative of a general externalizing factor. In view of this, the term “disinhibition”—which connotes lack of behavioral restraint—seems apt as a label for this factor.
DISCLOSURES – The authors report no biomedical financial intersts or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Cooke DJ, Michie C, Hart SD. Facets of Clinical Psychopathy: Toward Clearer Measurement. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford Press; New York, NY: 2006. pp. 91–106. [Google Scholar]
- (2).Lilienfeld SO, Fowler KA. The Self-Report Assessment of Psychopathy: Problems, Pitfalls, and Promises. In: Patrick CJ, editor. Handbook of Psychopathy. Guilford Press; New York, NY: 2006. pp. 107–32. [Google Scholar]
- (3).Hare RD. The Hare Psychopathy Checklist-Revised. Second Edition Multi-Health Systems; Toronto, ON, Canada: 2003. [Google Scholar]
- (4).Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. J Person Assess. 1996 Jun;66(3):488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- (5).Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the psychopathic personality inventory: validity and implications for clinical assessment. Psychol Assess. 2003 Sep;15(3):340–50. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- (6).Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009 Aug;21(3):913–38. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- (7).American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- (8).Tackett JL, Krueger RF, Sawyer MG, Graetz BW. Subfactors of DSM-IV conduct disorder: evidence and connections with syndromes from the Child Behavior Checklist. J Abnorm Child Psychol. 2003 Dec;31(6):647–54. doi: 10.1023/a:1026214324287. [DOI] [PubMed] [Google Scholar]
- (9).Tackett JL, Krueger RF, Iacono WG, McGue M. Symptom-based subfactors of DSM-defined conduct disorder: evidence for etiologic distinctions. J Abnorm Psychology. 2005 Aug;114(3):483–7. doi: 10.1037/0021-843X.114.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Burt SA, Donnellan MB. Development and validation of the Subtypes of Antisocial Behavior Questionnaire. Aggress Behav. 2009 Sep;35(5):376–98. doi: 10.1002/ab.20314. [DOI] [PubMed] [Google Scholar]
- (11).Hopwood CJ, Burt SA, Markowitz JC, Yen S, Shea MT, Sanislow CA, et al. The construct validity of rule-breaking and aggression in an adult clinical sample. J Psychiatr Res. 2009 May;43(8):803–8. doi: 10.1016/j.jpsychires.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Burt SA. Are there meaningful etiological differences within antisocial behavior? Results of a meta-analysis. Clin Psychol Rev. 2009 Mar;29(2):163–78. doi: 10.1016/j.cpr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- (13).Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychol Med. 2005 May;35(5):637–48. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1st ed. Guilford Press; New York: Jul 26, 2006. 2006. [Google Scholar]
- (15).Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999 Jan;56(1):39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- (16).Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Developmental Psychopathology. 2002;14(2):395–416. doi: 10.1017/s0954579402002110. [DOI] [PubMed] [Google Scholar]
- (17).Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personal Individual Differences. 1985;6:21–9. [Google Scholar]
- (18).Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep. 1991 Dec;69(3 Pt 1):1047–57. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- (19).Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) Biometrics Research Department, New York State Psychiatric Institute; New York: 1985. [Google Scholar]
- (20).Muthen LK, Muthen BO. Mplus User’s Guide: Sixth Edition; version 6.0. Sixth Edition - version 6.0 Muthen & Muthen; Los Angeles, CA: 2010. [Google Scholar]
- (21).Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- (22).Bentler PM. Comparative fit indexes in structural models. PB. 1990 Mar;107(2):238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- (23).Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivar Beh Res. 1990;25:173–80. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- (24).Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th ed Dept. of Psychiatry, Virginia Commonwealth University Medical School; Box 980126, Richmond VA 23298: 2003. [Google Scholar]
- (25).Markon KE, Krueger RF. An empirical comparison of information-theoretic selection criteria for multivariate behavior genetic models. Behav Genet. 2004 Nov;34(6):593–610. doi: 10.1007/s10519-004-5587-0. [DOI] [PubMed] [Google Scholar]
- (26).Achenbach TM. The Child Behavior Profile: I. Boys aged 6--11. J Consult Clin Psychol. 1978 Jun;46(3):478–88. doi: 10.1037//0022-006x.46.3.478. [DOI] [PubMed] [Google Scholar]
- (27).Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychology. 2007 Nov;116(4):645–66. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Frick PJ, Lahey BB, Loeber R, Tannenbaum L, Vanhorn Y, Christ MAG, et al. Oppositional Defiant Disorder and Conduct Disorder - A Meta-Analytic Review of Factor-Analyses and Cross-Validation in A Clinic Sample. Clin Psychol Rev. 1993;13(4):319–40. [Google Scholar]
- (29).Loeber R, Schmaling KB. Empirical evidence for overt and covert patterns of antisocial conduct problems: a metaanalysis. J Abn Child Psychol. 1985 Jun;13(2):337–53. doi: 10.1007/BF00910652. [DOI] [PubMed] [Google Scholar]
- (30).Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. University of Vermont, Research Center for Children, Youth & Families; Burlington, VT: 2003. [Google Scholar]
- (31).Frick PJ, O’Brien BS, Wootton JM, McBurnett K. Psychopathy and conduct problems in children. J Abnorm Psychol. 1994 Nov;103(4):700–7. doi: 10.1037//0021-843x.103.4.700. [DOI] [PubMed] [Google Scholar]
- (32).Frick PJ, Marsee MA. Psychopathy and developmental pathways to antisocial behavior in youth. In: Patrick CJ, editor. Handbook of psychopathy. Guilford Press; New York, NY: 2006. pp. 353–74. [Google Scholar]
- (33).Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000 Jul 28;289(5479):591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- (34).Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007 Apr;18(4):326–33. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Continuity and change in psychopathic traits as measured via normal-range personality: a longitudinal-biometric study. J Abnorm Psychol. 2006 Feb;115(1):85–95. doi: 10.1037/0021-843X.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Loney BR, Taylor J, Butler MA, Iacono WG. Adolescent psychopathy features: 6-year temporal stability and the prediction of externalizing symptoms during the transition to adulthood. Aggress Behav. 2007 May;33(3):242–52. doi: 10.1002/ab.20184. [DOI] [PubMed] [Google Scholar]
- (37).Siemiatycki J. A comparison of mail, telephone, and home interview strategies for household health surveys. Am J Public Health. 1979 Mar;69(3):238–45. doi: 10.2105/ajph.69.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]