Abstract
Oligodendrocyte progenitor cells (OPCs) in primary culture can give rise to mature oligodendrocytes and type-2, but not type-1 astrocytes depending on the culture conditions. The OPCs thus are called oligodendrocyte-type-2 astrocyte (O2-A) progenitor cells. Mouse embryonic stem cells (mESCs) have been efficiently differentiated into OPCs; however, the fate plasticity of mESC-derived OPCs is not well characterized. In the present study, using GFP-Olig2 mESC line, we showed that the Olig2+/GFP+/A2B5+/NG2+ OPCs derived from GFP-Olig2 mESCs can mature into oligodendrocytes when co-cultured with mESC-derived neurons. Interestingly, when induced to astrocytic differentiation by bone morphogenetic protein-4, these mESC-derived OPCs can not only generate type-2 astrocytes, but also type-1 astrocytes. These results challenge the dogma that OPCs in culture can only generate type-2, but not type-1 astrocytes, and support the in vivo finding that during perinatal development, OPCs can give rise to a subset of type-1 astrocytes.
Keywords: Oligodendrocyte progenitor cells, Mouse embryonic stem cells, neural differentiation, Type-1 and type-2 astrocytes
Introduction
Oligodendrocyte progenitor cells (OPCs), identified by membrane proteoglycan NG2 and basic helix-loop-helix transcription factor Olig2, mainly generate oligodendrocytes in the developing and mature central nervous system (CNS)[16, 22]. However, accumulative evidence has shown that OPCs exhibit multipotential fates both in vivo and in vitro. Fate-mapping studies have shown that, during perinatal development, OPCs generate a subset of astrocytes [11, 33], and during adulthood OPCs can generate small numbers of neurons, although this remains controversial [1, 6, 12, 28, 31]. For astrocytic fate, in vitro studies showed that OPCs isolated from the CNS [14, 26] can give rise to only one type of astrocytes when exposed to serum or bone morphogenetic proteins (BMP) [19]. However, this type of astrocytes is observed in in vitro cultures [25], but not in in vivo 8]. To distinguish these cells from type-1 astrocytes, which exist both in vitro and in vivothis type of astrocytes is named the type-2 astrocyte. OPCs are thus called oligodendrocyte-type-2 astrocyte (O2-A) progenitor cells. Type-2 astrocytes differ from type-1 astrocytes in many aspects, such as morphology, growth characteristics, and surface gangliosides [25]. Type-1 and type-2 astrocytes can usually be identified with two antibodies [25]: antibodies against GFAP recognize both types of astrocytes, while the A2B5 monoclonal antibody binds to type 2 but not type-1 astrocytes. Taken together, in vitro studies can not fully support the in vivo observation that OPCs have multipotential fates, specifically the observation that OPCs can generate a subset of type-1 astrocytes in vivo.
Embryonic stem cells (ESCs) have been successfully differentiated into OPCs [4, 13, 23, 30], which provide potential regenerative therapies for oligodendrocyte injury-related CNS disorders, as well as useful tools for studying the function and development of the OPCs. However, the fate plasticity of ESC-derived OPCs is not well characterized. In the present study, using the reporter line GFP-Olig2 (G-Olig2) mouse (m) ESCs, in which GFP was inserted into the Olig2 locus so that GFP expression mirrored endogenous Olig2 expression in derivates of the mESC [32], we first efficiently differentiated the mESCs into Olig2+/GFP+/A2B5+/NG2+ OPCs. When co-cultured with mESC-derived neurons (mESC-Neu), the mESC-derived OPCs (mESC-OPCs) matured into oligodendrocytes. Importantly, when induced to astrocytic fate by bone morphogenetic protein-4 (BMP4), these mESC-OPCs generated both type-1 and type-2 astrocytes.
2. Materials and Methods
2.1 Maintenance of mESCs
The mESC line GFP-Olig2 (G-Olig2) was purchased from the American Type Culture Collection (ATCC) and maintained using standard mESC culture methods as described [15]. In brief, the mESCs were grown at an optimal density that required routine passaging every 3 days on irradiated MEF feeder layers (GlobalStem). The culture medium was Dulbecco’s modified Eagle’s medium (DMEM; GIBCO) supplemented with 20% fetal bovine serum (GIBCO), 2 mM L-glutamine (GIBCO), 1 mM sodium pyruvate (GIBCO), 0.1 mM β-mercaptoethanol (GIBCO), 0.1 mM nonessential amino acids (NEAA; GIBCO), and 1,000 U/ml leukemia inhibitory factor (Millipore).
2.2 Differentiation of mESCs
The OPC differentiation from G-Olig2 mESCs was performed by following our published protocol [15] with modifications. Briefly, to initiate embryoid body (EB) formation, mESC colonies were trypsinized into single cells and cultured in Costar ultra-low attachment six-well plate (Corning) in EB medium that consisted of α-minimal essential medium (α-MEM) supplemented with 20% KSR, 1 mM sodium pyruvate , 0.1mM NEAA, and 0.1 mM β-mercaptoethanol (all from GIBCO). From day 4 to day 7, retinoic acid (RA, 0.2 µM; Sigma) and purmorphamine (Pur, 1 µM; Cayman Chemical) were included in EB or N2 medium. The N2 medium was α-MEM supplemented with 1× N2 (GIBCO), 1 mM sodium pyruvate (GIBCO), 0.1mM NEAA, and 0.1 mM β-mercaptoethanol. At day 8, EBs were disaggregated in 0.05% TrypLE (Invitrogen) and plated on dishes coated with poly-L-ornithine (0.002%; Sigma) and fibronectin (10 µg/ml; Millipore) in OPC medium. The OPC medium was optimized based on a recent study on OPC differentiation from mouse epiblast stem cells [21] and consisted of Dulbecco’s modified Eagle’s medium with F12 (DMEM/F12; Hyclone), 1×N2, 1×B27, Pur (1µM) and fibroblast growth factor-2 (FGF-2, 20 ng/ml; Millipore).
To differentiate mESCs into neurons, the protocol described elsewhere was used [2]. Briefly, the EBs were cultured in suspension from day 0 to day 3 in the absence of RA, and then cultured for 4 days in the presence of RA (0.2 µM). At day 8, the EBs was disaggregated into single cells and plated onto 12 mm glass coverslips (Deckglaser, Germany) coated with poly-L-ornithine (0.002%; Sigma) and laminin (10 µg/ml; Sigma) in the neuronal differentiation medium which consisted of DMEM/F12, 1× N2, 1× B27, 20 ng/ml BDNF (Peprotech), 20 ng/ml GDNF (Peprotech), 1 mM dibutyryl-cyclic AMP (Sigma) and 200 nM ascorbic acid (Sigma).
To coculture mESC-OPCs with mESC-Neu, day 30 GFP+ mESC-OPCs were plated onto each coverslip with mESC-Neu cultured in the neuronal differentiation medium for two weeks. The coculture medium consisted of DMEM/F12, 1×N2 and 1×B27. After 7 days in coculture, the coverslips with cells were processed for immunocytochemistry.
2.3 Immunocytochemistry
Cells cultured on coverslips were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. For staining of NG2 and A2B5 antigens, live cells were incubated with primary antibodies for 15 min before fixation. After washing with PBS, cells were permeablized for 10 min in 0.1% Triton X-100, and incubated for 1 h in blocking buffer (5% goat or donkey serum and 1% bovine serum albumin) to reduce nonspecific binding. Cells then were incubated in primary antibodies diluted in blocking buffer at 4°C overnight. Appropriate fluorescence-conjugated secondary antibodies were used for single and double labeling. All antibodies were tested for cross-activity and nonspecific immunoreactivity.
The primary antibodies used in this study were listed in Table 1. 4´, 6-diamindino-2-phenylindole, dihydrochloride (DAPI, 1:100; Invitrogen) was used to identify the nuclei. Coverslips were mounted with the anti-fade Fluoromount-G medium (Southern Biotechnology). Images were captured using a Nikon Eclipse C1 confocol laser-scanning microscope.
Table 1.
List of antibodies used for immunostaining
| Antibodies | Vendor | Type | Dilution |
|---|---|---|---|
| NG2 | Millipore | Rabbit IgG | 1:200 |
| MBP | Millipore | Rat IgG | 1:100 |
| A2B5 | Millipore | Mouse IgM | 1:200 |
| Tuj1 | Millipore | Mouse IgG | 1:200 |
| GFAP | Millipore | Rabbit IgG | 1:1000 |
| FGFR3 | Santa Cruz Biotechnology | Rabbit IgG | 1:200 |
| GFP | Invitrogen | Rabbit IgG | 1:1000 |
| GFP | Rockland Immunochemicals | Goat IgG | 1:500 |
| GFP | Antibodies Incorporated | Mouse IgG | 1:200 |
The cell counting analysis was performed with ImageJ software. At least five fields of each coverslip were chosen randomly and at least three coverslips in each group were counted. All the values were expressed as means ± SEM.
3. Results
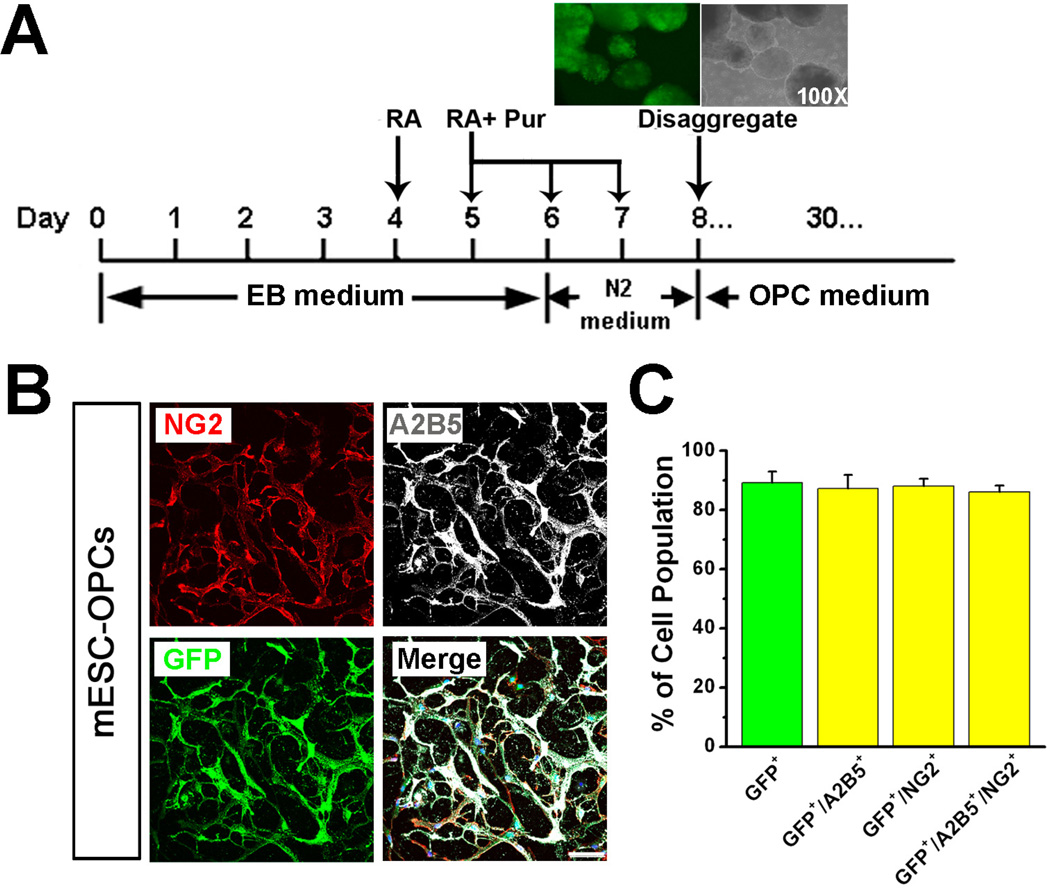
As shown in the schematic diagram in Fig 1A, treatment with retinoic acid (RA) and purmorphamine (Pur) induced G-Olig2 mESCs to form GFP+/Olig2+ embryoid bodies (EBs) at day 8, indicated by the GFP fluorescence. By using the optimized OPC culture medium [21], the cells proliferated more robustly than the cells did in the OPC medium used in our previous protocol [15]. After 30-day differentiation, a high percentage of the cells were GFP+/Olig2+ (Fig 1B and C, 89.2 ± 3.8%, n = 4). To examine whether these GFP+ cells were OPCs, we co-stained GFP with OPC markers, A2B5 and NG2. As shown in Fig 1B and C, nearly all the GFP+ cells were labeled by A2B5 and/or NG2 (87.3 ± 4.5%, 88.1 ± 2.5% and 86.1 ± 2.1% for GFP+/A2B5+, GFP+/NG2+ and GFP+/A2B5+/NG2+ cells, respectively; n = 4).
Fig. 1.
Differentiation of GOlig2 mESCs into OPCs by using optimized OPC medium. (A) A schematic procedure for differentiating GOlig2 mESCs into OPCs. Inset, at day 8 of differentiation, many EBs start to express Olig2, indicated by the GFP fluorescence. At this time point, the EBs are ready to be disaggregated and plated as single cells. (B) Representative images showing that after 30-day differentiation in the optimized OPC medium, many cells show GFP fluorescence and are positive for A2B5 and NG2. (C) Quantitative results showing the percentage of GFP+, GFP+/A2B5+, GFP+/NG2+, and GFP+/A2B5+/NG2+ cells. Note that nearly all the GFP+ cells are positive for the OPC markers, A2B5 and NG2. Data are presented as mean ± SEM. Blue, DAPI-stained nuclei. Scale bars represent 50 µm.
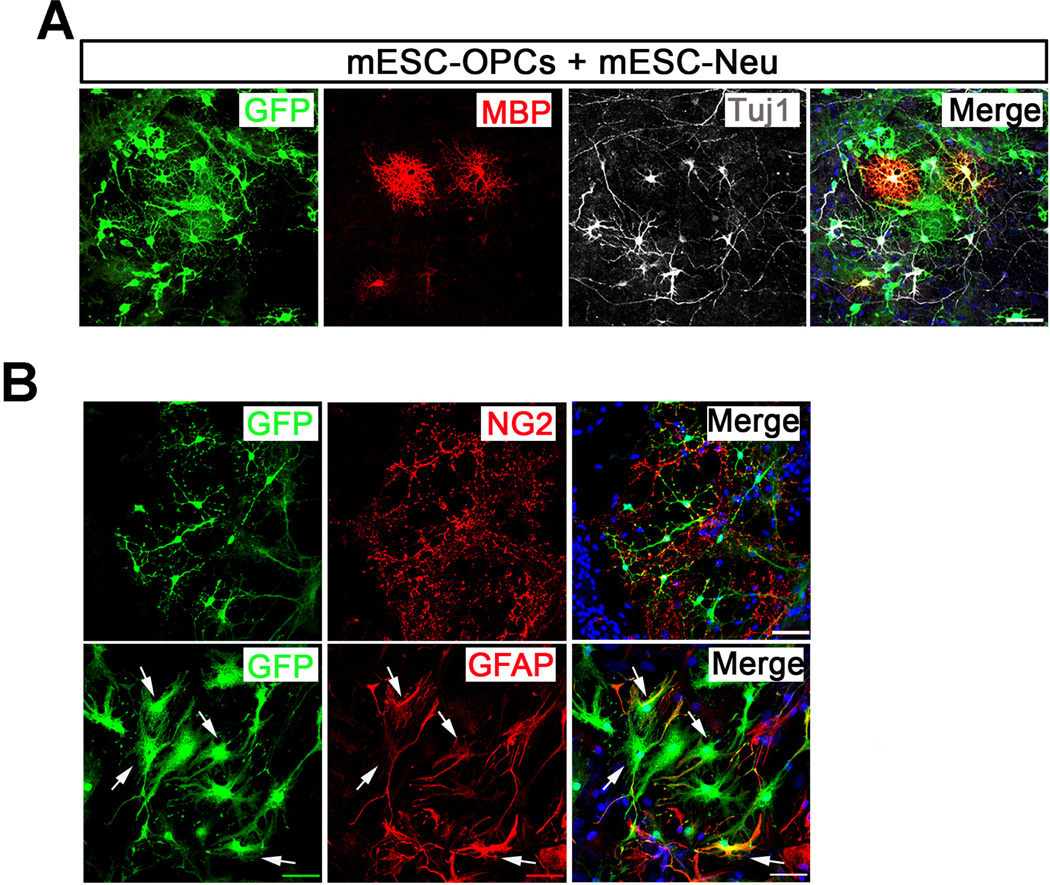
To investigate the fate of these cells in vitro, we co-cultured the mESCOPCs with mESC-Neu. As shown in Fig 2A, in the presence of Tuj1+ mESC-Neu about 10% of the GFP+ mESC-OPCs further matured into process-bearing oligodendrocytes, indicated by the co-labeling of GFP and mature oligodendrocyte marker, myelin basic protein (MBP). As expected, many of the cells were still at OPC stage, indicated by the co-staining of GFP and NG2 in the co-cultures (Fig 2B). Interestingly, there were many cells double-labeled by GFP and GFAP, suggesting that these GFP+ mESC-OPCs also gave rise to astrocytes when co-cultured with mESC-Neu.
Fig. 2.
Co-culture of mESC-OPCs with mESC-Neu. (A) Representative images showing that after 7-day co-culture with mESC-Neu, the GFP+ mESC-OPCs mature into MBP+ process-bearing oligodendrocytes. GFP in green, MBP in red and Tuj1 in white. (B) Representative images showing that after 7-day co-culture, many GFP+ mESC-OPCs are still at NG2+ OPC stage and some of them generate GFAP+ astrocytes. Arrows indicate the GFAP+ astrocytes generated by GFP+ mESC-OPCs. GFP in green, NG2 and GFAP in red. Blue, DAPI-stained nuclei. Scale bars represent 50 µm.
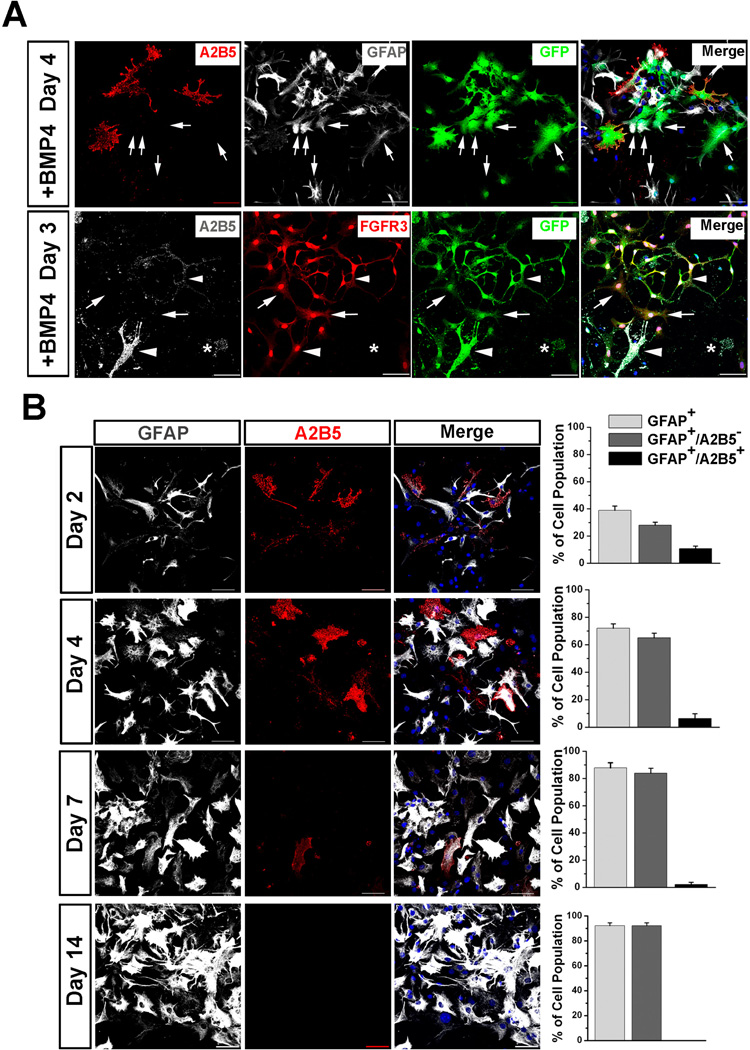
To further explore which types of astrocytes mESC-OPCs can give rise to, we attempted to induce astrocytic differentiation from GFP+ D30 mESC-OPCs using BMP4, which is known to promote astrocyte lineage commitment during the period of gliogenesis in vivo [9, 10] and induce brain-derived OPCs to become type-2 astrocytes in vitro 19]. After 4 to 7 days cultured in the presence of BMP4, GFP fluorescence became dim when observed at fluorescence microscope, suggesting that the Olig2/GFP expression was down-regulated or terminated in these cells. However, using anti-GFP staining, many cells that had expressed Olig2 /GFP could be labeled (Fig 3A), allowing us to track down the fate of GFP+ mESC-OPCs cultured in the presence of BMP4. As shown in Fig 3A upper panels, consistent with previous studies from brain-derived OPCs [14, 26], these mESC-OPCs could give rise to type-2 astrocytes, identified by staining triple positive for GFP, GFAP and A2B5. Strikingly, we observed that, there were many type-1 astrocytes in the culture, identified by positive for GFAP staining but negative for A2B5 staining. These type-1 astrocytes were also co-labeled by GFP staining, suggesting that they were differentiated from the GFP+ mESCOPCs. To further verify that mESC-OPCs can generate type-1 astrocytes, we stained the cells with fibroblast growth factor receptor 3 (FGFR3) which was found to be present in type-1, but not type-2 astrocytes [27]. As shown in Fig 3A lower panels, many type-1 astrocytes were identified by positive for FGFR3 and GFP but negative for A2B5. We also observed that some A2B5+ type-2 astrocytes were positive for FGFR3 in the culture. Interestingly, as shown in Fig 3B, the percentage of type-1 and type-2 astrocytes changed with the time cultured in the presence of BMP4. The percentage of type-1 GFAP+/A2B5− astrocytes increased from 38.9 ± 3.2% at day 2 to 72.0 ± 3.2% and 87.8 ± 3.8% at day 4 and 7, respectively (n = 4 for each time point). The percentage of type-2 GFAP+/A2B5+ astrocytes decreased from 10.8 ± 1.9% at day 2 to 6.4 ± 3.5% and 2.1 ± 1.6% at day 4 and 7, respectively. (n = 4 for each time point). After 14-day culture, the type-1 astrocytes persisted in the culture (92.3 ± 2.3%, n = 4) while type-2 astrocytes did not maintain their progeny and became undetectable.
Fig. 3.
Astrocytic differentiation of GFP+ mESC-OPCs in the presence of BMP4. (A) Upper panels after culture in the presence of BMP4, GFP+ mESC-OPCs generate not only GFAP+/A2B5+ type-2 astrocytes, but also GFAP+/− type-1 astrocytes that are indicated by arrows. A2B5 in red, GFAP in white and GFP in green. Lower panelstype-1 astrocytes (indicated by arrows) were also identified by positive for FGFR3 and GFP, but negative for A2B5 staining. A2B5+/FGFR3+ (indicated by arrowheads) and A2B5+/FGFR3− (indicated by star) type-2 astrocytes were identified in the culture. A2B5 in white, FGFR3 in red and GFP in green. (B) Representative images and quantitative analyses showing that the percentage of type-1 astrocytes increases and the percentage of type-2 astrocytes decreases from day 2 to day 7 cultured in the presence of BMP4. A2B5 in red and GFAP in white. Data are presented as mean ± SEM. Blue, DAPI-stained nuclei. Scale bars represent 50 µm.
Discussion
In the present study, we first establish the co-culture system of mESC-OPCs and mESC-Neu. We demonstrate that in the presence of mESC-Neu, mESC-OPCs can mature into process-bearing MBP+ oligodendrocyte. Interestingly, the mESC-OPCs also generate astrocyte in the co-culture. To further study the astrocytic fate, we culture mESC-OPCs in the presence of BMP4 to induce astrocytic differentiation. Importantly, we find that mESC-OPCs can generate both type-1 and type-2 astrocytes.
It is well established that mESCs can be efficiently differentiated into OPCs [4, 15, 30]. In this study, taking advantage of the genetically labeled G-Olig2 mESCs [32], we examined the fate of mESC-OPCs in in vitro differentiation by monitoring the GFP fluorescence. During the embryonic development in the spinal cord, Olig2+ progenitors sequentially generate motoneurons and OPCs [3, 7, 29, 30]. Recent studies demonstrated that the Olig2+ progenitor in the spinal cord can also give rise to a subset of astrocytes [20] [5]. However, in our study, after 30-day differentiation, nearly all the GFP+/Olig2+ cells are OPCs but not Olig2+ progenitors, indicated by the co-expression of GFP and OPC marker NG2. Considering the high percentage of type-1 astrocytes generated from the day 30 GFP+/Olig2+ cells cultured in the presence of BMP4 for 7 days, it is unlikely that these astrocytes are generated from the Olig2+ progenitor, if any in the culture. Thus, we demonstrate here that the mESC-OPCs can not only generate type-2 astrocytes but also type-1 astrocytes in vitro.
Previous studies showed that OPCs isolated from the CNS can only give rise to type-2 astrocytes [26] [14], which does not fully support the in vivo observation that OPCs generate a subset of type-1 astrocytes [11, 33]. In this study, by co-staining of A2B5 with GFAP or FGFR3, we demonstrated that mESC-OPCs generated a subpopulation of type-1 astrocytes. One possible explanation to the different fate plasticity between our mESC-OPCs and brain-derived OPCs is that mESC-OPCs represent a more immature stage than the brain-derived OPCs do because ESC progeny are shown to be at a more immature stage compared to cells taken from tissue [24]. Unexpectedly, we identified that some A2B5+ type-2 astrocytes were also positive for FGFR3. This discrepancy between the present study and the previous report [27] could be due to the different starting cells and different culture conditions. We induced the astrocytic differentiation from mESC-OPCs using BMP4, while the previous study induced astrocytic differentiation from rat glial-restricted precursor cells using fetal calf serum [27]. Under the present culture conditions, the added BMP4 or secreted BMPs in the coculture system from the neural cells derived from mESCs by neuronal differentiation procedure [18] may work through BMP receptor-mediated specific signal transduction pathways [19] to direct the mESC-OPCs to become type-1 and type-2 astrocytes. Interestingly, after long-term culture in the presence of BMP4, type-1 astrocytes were enriched in the culture while type-2 astrocytes became undetectable. Therefore, results from the present study on mESC-OPCs fate in vitro fully support the in vivo observation that OPCs can generate a subset of typ-1 astrocytes [11, 33].
Studies in vivo also have shown that OPCs can generate small numbers of neurons [1, 6, 12, 28, 31]. However, the observation is still controversial because the current in vivo fate-mapping studies are confounded by many factors, for example the activity and specificity of promoter-driven Cre expression at different cell types and brain regions [16]. Although an in vitro study showed that brain-derived OPCs can be reprogrammed to multipotential CNS stem cells that can give rise to neurons [17], it is unclear whether OPCs can be directly converted to neurons by physiological cues without experiencing a type 2-astrocyte stage. In the future, it would be interesting to test whether the OPCs can directly generate neurons in in vitro cultures, by using the mESC-OPCs that represents the OPCs at very immature development stage.
Highlights.
We optimize our previous protocol for differentiating mESCs into OPCs
We establish the co-culture system of mESC-derived OPCs and neurons
The mESC-derived OPCs generate oligodendrocytes and astrocytes in coculture
Induced by BMP4, mESC-derived OPCs generate both type-1 and type-2 astrocytes
Acknowledgements
This work was supported by a postdoctoral fellowship from Shriners Hospitals for Children to Dr. Peng Jiang , and in part supported by grants from National Institutes of Health (R01NS061983 and R01ES015988 to W. D.), the National Multiple Sclerosis Society (to W. D.), and Shriners Hospitals for Children (to W. D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 3.Billon N, Jolicoeur C, Ying QL, Smith A, Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineageselectable mouse ES cells. J Cell Sci. 2002;115:3657–3665. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- 4.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- 6.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du ZW, Li XJ, Nguyen GD, Zhang SC. Induced expression of Olig2 is sufficient for oligodendrocyte specification but not for motoneuron specification and astrocyte repression. Mol Cell Neurosci. 2006;33:371–380. doi: 10.1016/j.mcn.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 10.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 11.Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito S, Sugama K, Inagaki N, Fukui H, Giles H, Wada H, Hayaishi O. Type-1 and type-2 astrocytes are distinct targets for prostaglandins D2, E2, and F2 alpha. Glia. 1992;6:67–74. doi: 10.1002/glia.440060109. [DOI] [PubMed] [Google Scholar]
- 15.Jiang P, Selvaraj V, Deng W. Differentiation of embryonic stem cells into oligodendrocyte precursors. J Vis Exp. 2010;19 doi: 10.3791/1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 18.Lu HZ, Hu JG. Expression of bone morphogenetic proteins-2/4 in neural stem cells and their lineages. Acta Neurobiol Exp (Wars) 2009;69:441–447. doi: 10.55782/ane-2009-1755. [DOI] [PubMed] [Google Scholar]
- 19.Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Najm FJ, Zaremba A, Caprariello AV, Nayak S, Freundt EC, Scacheri PC, Miller RH, Tesar PJ. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nat Methods. 2011;8:957–962. doi: 10.1038/nmeth.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Colocalization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 24.Patterson M, Chan DN, Ha I, Case D, Cui Y, Van Handel B, Mikkola HK, Lowry WE. Defining the nature of human pluripotent stem cell progeny. Cell Res. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 27.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 30.Shin S, Xue H, Mattson MP, Rao MS. Stage-dependent Olig2 expression in motor neurons and oligodendrocytes differentiated from embryonic stem cells. Stem Cells Dev. 2007;16:131–141. doi: 10.1089/scd.2006.0023. [DOI] [PubMed] [Google Scholar]
- 31.Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 32.Xian HQ, McNichols E, St Clair A, Gottlieb DI. A subset of ES-cellderived neural cells marked by gene targeting. Stem Cells. 2003;21:41–49. doi: 10.1634/stemcells.21-1-41. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]