Abstract
Objective
Atherosclerosis developed during premenopausal years predicts postmenopausal atherosclerosis burden. The objective of this study was to determine the effects of dietary soy protein isolate (SPI) and social status on atherogenesis and arterial gene expression in a premenopausal monkey model.
Design
Socially housed premenopausal cynomolgus macaques (n = 84) were fed an atherogenic diet deriving protein from casein/lactalbumin or SPI (containing 1.88 mg isoflavones/g). After 36 months of diet consumption, iliac artery biopsies were assessed for atherosclerosis and expression of mRNA transcripts related to inflammation, macrophage and T-cell content, and estrogen receptors (ERs).
Results
SPI reduced plaque size (P < 0.05), total plasma cholesterol, non–high-density lipoprotein cholesterol (HDLc), and the total plasma cholesterol/HDLc ratio (all P < 0.003), while increasing triglycerides (P < 0.006) and HDLc (P < 0.0001). Arterial mRNA for CD68 (P < 0.001), CD3 (P < 0.02), and CD4 (P < 0.001) and inflammatory markers monocyte chemotactic protein-1, intercellular adhesion molecule-1, and interleukin-6 (all P < 0.0001) were also lower in the group receiving SPI. For most outcomes, this effect remained even after adjustments for plaque size and plasma lipid concentrations. Arterial ER-α was inversely associated with atherosclerosis (P < 0.02) and increased with SPI (P < 0.001). Subordinate monkeys had lower ER-β (P < 0.02) and higher interleukin-6 (P < 0.05) transcripts but did not differ from dominant monkeys in extent of atherosclerosis (P > 0.9).
Conclusions
Premenopausal consumption of SPI had plasma lipid–independent beneficial effects on the pathobiological processes involved in atherosclerotic plaque development, thus potentially establishing the basis for reduced postmenopausal complications. Dominant social status provided similar, albeit less extensive, benefits in risk markers.
Keywords: Isoflavones, Inflammation, Nuclear factor-κB, Social status, Cynomolgus macaques
Coronary heart disease (CHD) remains the largest cause of death among women older than 55 years of age, with CHD mortality exceeding by severalfold combined deaths due to cancers of the lung, breast, colon, and endometrium. Coronary artery atherosclerosis has its origin in childhood and progresses with age. The multicenter Pathologic Determinants of Atherosclerosis in Youth study reported that by 35 years of age, about 70% of women have coronary artery fatty streaks and approximately one third have the early stages of atherosclerotic plaques.1 Such observations have made it increasingly clear that the premenopausal years are critical determinants of the post-menopausal atherosclerosis burden.2-4 This notion is supported by more than a decade of research using the cynomolgus monkey model, which has shown that the relative risk for progression of coronary artery atherosclerosis during the premenopausal years determines the extent of plaque development at the time of the menopausal transition, which in turn establishes postmenopausal plaque extent. Particularly high pre- and postmenopausal risk is carried by approximately half of the monkeys in each social group that are stressed because they are subordinate in social status; these monkeys are characterized by ovarian dysfunction, estrogen deficiency, low plasma high-density lipoprotein cholesterol (HDLc) concentrations and premature acceleration of coronary artery atherosclerosis.2,5,6 This research suggests that aggressively promoting premenopausal prevention could comprise an important strategy for maintaining cardiovascular health.
Diet represents a modifiable lifestyle contributor to global and cardiovascular health, and the type of dietary protein may be important. With respect to the potential cardiovascular effects of dietary soy protein for women, some reports suggest modest benefits,7-9 whereas others have been equivocal or negative in their recommendations based on changes in plasma lipid concentrations.10-14 In contrast with studies in women, those in nonhuman primates have demonstrated that dietary soy protein has uniformly beneficial effects on plasma lipid concentrations of both male and female animals15,16 and inhibits atherogenesis in male animals.16,17 Soy containing isoflavones (IFs) also reduced carotid artery atherosclerosis15 and soluble vascular cell adhesion molecule-1 (VCAM-1) levels in postmenopausal female monkeys.15,18 Studies of the effects of soy in women have relied on plasma lipid concentrations and other surrogate measures to estimate potential cardiovascular benefits. However, soy protein and its components may affect atherosclerosis and other aspects of arterial biology independent of plasma lipids. Soy protein contains IFs, naturally occurring estrogen-like compounds that interact with estrogen receptors (ERs) as either estrogen agonists or antagonists, depending on the level of circulating estrogens19 and the ER subtype.20 Although IF treatment of postmenopausal women has little or no effect on plasma lipid concentrations, IFs have been reported to improve arterial compliance and stiffness, measurements closely associated with degree of atherosclerosis.21 Importantly, there are no published randomized trials describing the effect of dietary soy or IFs on the extent of atherosclerosis in humans.
Here we report on the results of a study designed to determine the effects of dietary soy on the extent of premenopausal atherosclerosis and arterial inflammatory gene expression of low (dominant) and high (subordinate) risk in premenopausal cynomolgus monkeys. Plasma lipid concentrations were measured as risk factors, inflammatory gene and cell type specific markers were assessed as likely modulators or indicators of early atherogenesis, and ER expression was assessed because of its critical role in transmitting estrogen and perhaps IF activity. Further, because the effects of dietary soy and IFs on plasma lipid concentrations of women are, at best, minimal, we investigated the extent to which the arterial effects may be independent of plasma lipid lowering.
METHODS
Monkeys and diets
Female cynomolgus macaques (Macaca fascicularis) were imported as adults from Indonesia (Institute Pertanian Bogor). Adult status (average age of 11.5 y; approximately equivalent to 30-35 human y) was confirmed by dentition and evidence of epiphyseal closure. Upon arrival, monkeys were individually housed during a 30-day quarantine period during which time they consumed monkey chow (Ralston Purina) and then were placed in 16 social groups comprising 5 or 6 monkeys each. Monkeys were fed an IF-free, atherogenic (0.28 mg cholesterol/cal) control diet containing casein and lactalbumin (C/L) along with wheat flour as sources of protein for 8 months (Table 1), during which time baseline characteristics were determined. After the baseline phase, social groups of monkeys were randomized to two treatment groups stratified for body weight and plasma lipids (total plasma cholesterol [TPC] and HDLc). The control group (C/L: n = 41) continued receiving the atherogenic control diet containing C/L as the major protein source, whereas the soy protein isolate (SPI) group (n = 38) was fed a diet of SPI containing 1.88 mg aglycone IF/g protein (SUPRO (R) SOY Isolated Soy Protein, Solae St. Louis, MO) (Table 1). All monkeys received 0.28 mg cholesterol/cal. Monkeys consume approximately 120 cal diet/kg body weight, and therefore the SPI group consumed approximately 8.6 mg IF/kg body weight, which approximates a woman's daily consumption of 129 mg IF/day (as aglycones). Diets were formulated to be identical in caloric content of protein (19% of calories), fat (35%), carbohydrates (46%), and cholesterol. The diets were formulated to produce atherogenic lipid profiles and elevated TPC/HDLc ratios. Plasma TPC/HDLc ratios determined 8 months into the treatment phase were found to be significantly higher than desired, and 11 months into the experimental period the cholesterol content of the diets was lowered to 0.20 mg cholesterol/cal for the remainder of the study. Monkeys were treated for 36 months premenopausally before undergoing left iliac artery biopsy.
TABLE 1.
Composition of the experimental diets
| Ingredient | Casein/lactalbumin diet, g/100 g | Soy protein isolate diet, g/100 g |
|---|---|---|
| Casein, USP | 8.5 | |
| Lactalbumin | 8.5 | |
| Soy protein isolate | 17.09 | |
| DL-Methionine | 0.30 | |
| Wheat flour, self-rising | 35.76 | 35.76 |
| Dextrin | 9.00 | 8.90 |
| Sucrose | 7.00 | 7.00 |
| Alphacel | 8.03 | 8.23 |
| Lard | 5.00 | 5.00 |
| Beef tallow | 4.00 | 4.00 |
| Butter, lightly salted | 3.10 | 3.10 |
| Safflower oil (linoleic) | 3.00 | 2.56 |
| Crystalline cholesterol | 0.09/0.06a | 0.09/0.06a |
| Complete vitamin mix | 2.50 | 2.50 |
| Ausman-Hayes mineral mix | 5.00 | 5.00 |
| Calcium carbonate | 0.40 | 0.36 |
| Calcium phosphate, monobasic | 0.15 | 0.15 |
Soy protein was derived from SUPRO (R) SOY Isolated Soy Protein (Solae, St. Louis, MO).
Animals were fed 0.28 mg cholesterol/cal during the baseline period and the first 11 months of the treatment phase, then switched to 0.20 mg cholesterol/cal for the remainder of the study period.
All procedures were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Determination of social status
The social status of each animal was determined relative to the status of others in each social group. Data were collected during weekly 30-minute observation sessions conducted after formation of the social groups and before the initiation of treatment. Dominance and subordination were determined by the outcome of competitive interactions, which are highly asymmetric, and thus offer clear winners and losers as determined by specific facial expressions, postures, and vocalizations.22,23 As in prior studies, ranks over the baseline period were averaged, and for analysis, animals ranking first and second in groups of five animals or first through third in groups of six were labeled “dominant” and the remainder of the monkeys were labeled “subordinate.” Under the experimental conditions used, ranks tend to be highly stable within social groups.2
Serum IF measurements
During baseline and experimental periods, monkeys were fed the morning of sample collection and then were sedated 4 hours after feeding for blood collection. Serum IF and IF metabolite concentrations were determined by liquid chromatographic-photodiode array mass spectrometric analysis.24 Detection limits were previously found to be 1 to 15 nM, depending on the analyte. Intra-assay coefficients were all less than 14% and varied depending on the analyte, whereas interassay coefficients of variation were 8% to 22% at levels less than 20 nM, 7% to 14% at 20 to 100 nM, and 3% to 12% at levels greater than 100 nM. Mean values for IF concentrations in the SPI group were as follows: daidzein, 129 ± 15.9 nM; genistein, 123 ± 14.6 nM; and equol, 546 ± 45.5 nM.25
Plasma lipid measurements
TPC, HDLc, and plasma triglycerides (TGs) were determined at the beginning and end of the pretreatment phase and at 6, 11, 15, 25, and 32 months of the 36-month treatment period in the Wake Forest Primate Center Clinical Chemistry Laboratory,16,18 which was fully standardized with the Centers for Disease Control and Prevention-National Institutes of Health Lipid Standardization Program specifications for accuracy and precision. Intra- and interassay coefficients of variation were less than 5% for all analytes. Non-HDLc, which approximates the sum of low-density lipoprotein (LDL) cholesterol and very-low-density lipoprotein cholesterol, was calculated by subtracting HDLc from TPC.
Surgical biopsy of the iliac artery
Atherosclerotic plaque size and gene expression measures were determined in iliac artery biopsy sections removed at the end of the premenopausal portion of the study. Plaque size in such sections has been shown previously to be predictive of the extent of concomitantly measured coronary artery atherosclerosis.15,17 To obtain the biopsy samples, monkeys were anesthetized with an intramuscular injection of ketamine (10 mg/kg) followed by isoflurane gas. Sections approximately 0.5 cm in length were excised from the left common iliac artery of each animal. The artery biopsy sections were cleaned of adventitial tissue, opened longitudinally, and sectioned. Distal sections were placed in RNAlater (Ambion, Inc.) for gene expression analyses and stored at 4°C for 24 hours and then were stored at –80°C. Central sections (histological evaluation) were placed into 4% paraformaldehyde for 24 hours and then were moved to 70% ethanol, dehydrated, and embedded into paraffin blocks. Proximal sections were cryopreserved in OCT embedding medium for immunohistochemical analyses.
Atherosclerosis assessment
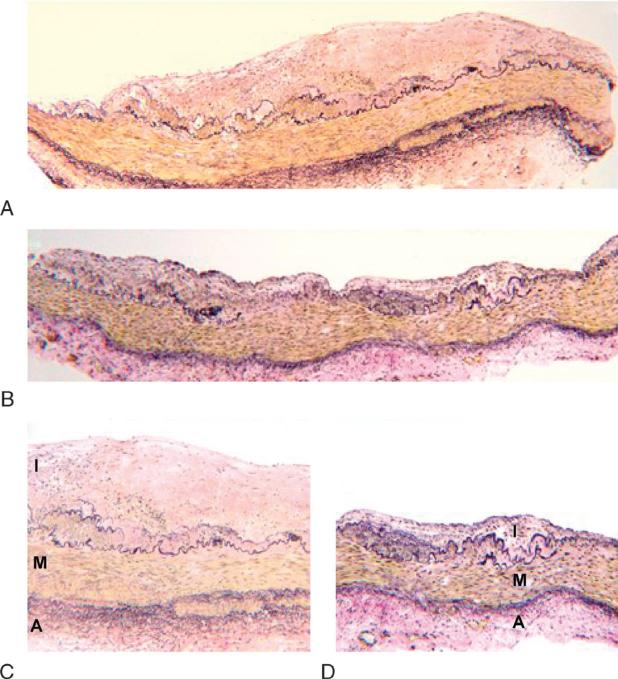
Artery blocks were cut to 5-μm thick sections that were deparaffinized and stained with Verhoeff and Van Gieson stain (Fig. 1). Plaque size (mm2) of the iliac biopsy section was determined by computer-assisted histomorphometry using Image Pro Plus software (Media Cybernetics, Inc., Silver Springs, MD). Measurements were made by an experienced technician blinded to treatment group using a well-established protocol.15 Reevaluation of a subset of these sections by a second experienced technician verified robustness of the methodology as there was a correlation between the independent assessments (r = 0.99, P = 0.001) with an interobserver variability of 5.4%.
FIG. 1.
Composite images of representative Verhoeff and Van Gieson–stained iliac arteries for each treatment group. A, C: Iliac artery from casein/lactalbumin–treated female (A: ×4 magnification; C: ×10 magnification). B, D: Iliac artery from soy protein isolateYtreated female (B: ×4 magnification; D: ×10 magnification). I, intima, M, media, A, adventitia.
RNA isolation and quantification
RNA was isolated from intima-media sections using the Tri-Reagent protocol as described previously.26,27 Samples were assessed for total RNA concentration and quality on an Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano kit (Agilent Technologies). Aliquots of RNA were reverse transcribed to generate a cDNA archive using a high-capacity cDNA archive kit (Applied Biosystems). Quantitative real-time reverse transcriptase–polymerase chain reaction (PCR) was performed on an ABI Prism 7000 system using cynomolgus macaque-specific (monocyte chemotactic protein-1 [MCP-1], VCAM-1, intercellular adhesion molecule-1 [ICAM-1], CD3 [δ chain], CD4, ER-α, and ER-β) or human-specific (microsialin/CD68, T-cell receptor-β, and interleukin-6 [IL-6]) TaqMan FAM-MGB primer-probe assays (Applied Biosystems). Individual PCR reactions were performed using cDNA generated from 36 ng total RNA. All quantitative reverse transcriptase–PCR data were normalized to the geometric mean of endogenous constitutively expressed control genes glyceraldehyde-3-phosphate dehydrogenase, B-actin, and ribosomal protein, large P028 using the 2–ΔCT procedure.17,29 Amplifications not reaching threshold by 40 cycles were designated as zero expression.
Statistical analysis
Significant effects of diet treatment and social status were determined using linear models. No significant interactions between diet and social status were determined for any outcome. Because plasma lipid and lipoprotein concentrations are traditionally strong predictors of atherosclerosis outcomes, baseline plasma lipids and lipoproteins were included in the linear model when they were significantly associated with the outcome. The effects of diet and social status on gene expression were initially tested for significance by creating a model including only diet treatment and social status. In the case of outcomes in which we observed significant effects of diet or status, we developed additional models that first included plaque size and later plasma risk factors that were significant covariates. Diet and status effects on plasma lipids were analyzed by repeated measures analysis for each animal over the entire treatment period. A bootstrapping method for testing mediation was used in an effort to estimate the extent to which plasma lipids mediated the effects of diet on plaque size and arterial gene expression.30 Spearman partial correlations were used to assess relationships among plaque size, plasma lipids, and IF concentrations. Significance was defined as P less than 0.05. All analyses were done using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Baseline measurements
No significant differences in baseline measures of body weight or plasma lipids were observed between the two diet groups (P > 0.65 for all) (Table 2), and body weight between the two diet groups did not differ at the end of the treatment period (C/L: 3.37 ± 0.13 kg; SPI: 3.48 ± 0.13 kg; P > 0.60). As indicated by previous analyses, SPI treatment had no significant effects on menstrual cyclicity or the reproductive hormone profile.31
TABLE 2.
Baseline characteristics of monkeys by diet
| C/L | SPI | P | |
|---|---|---|---|
| Body weight, kg | 2.94 ± 0.06 | 2.90 ± 0.06 | 0.65 |
| TPC mmol/L | 7.23 ± 0.30 | 7.21 ± 0.30 | 0.96 |
| TGs mmol/L | 0.29 ± 0.02 | 0.29 ± 0.02 | 0.96 |
| Non-HDLc mmol/L | 6.19 ± 0.32 | 6.19 ± 0.33 | 0.99 |
| HDLc mmol/L | 1.05 ± 0.07 | 1.02 ± 0.07 | 0.79 |
| TPC/HDLc ratio | 8.85 ± 0.74 | 9.21 ± 0.75 | 0.85 |
Data are mean ± SEM. C/L, casein/lactalbumin; SPI, soy protein isolate; TPC, total plasma cholesterol; TGs, triglycerides; HDLc, high-density lipoprotein cholesterol.
Plasma IF and lipid measures
Treatment with SPI significantly elevated serum IF concentrations compared with C/L (P < 0.0001 for all).25 SPI also had a beneficial effect on the mean treatment plasma lipid profiles, reducing TPC (P < 0.003), non-HDLc (P < 0.0001), and the TPC/HDLc ratio (P < 0.0001), while increasing plasma HDLc (P < 0.0001) and plasma TGs (P < 0.006) (Table 3). The TPC/HDLc ratio was significantly reduced in dominant monkeys (P < 0.001).
TABLE 3.
Plasma lipid levels by diet groups and social status
| C/L | SPI | Diet P | Dominant | Subordinate | Status P | |
|---|---|---|---|---|---|---|
| TPC, mmol/L | 8.98 ± 0.25 | 7.92 ± 0.25 | 0.003 | 8.12 ± 0.25 | 8.78 ± 0.24 | 0.060 |
| TGs, mmol/L | 0.36 ± 0.02 | 0.43 ± 0.02 | 0.006 | 0.41 ± 0.02 | 0.38 ± 0.02 | 0.162 |
| Non-HDLc, mmol/L | 8.12 ± 0.27 | 6.51 ± 0.28 | 0.0001 | 6.95 ± 0.28 | 7.68 ± 0.27 | 0.057 |
| HDLc, mmol/L | 0.85 ± 0.04 | 1.41 ± 0.04 | 0.0001 | 1.17 ± 0.04 | 1.10 ± 0.04 | 0.262 |
| TPC/HDLc ratio | 14.9 ± 0.74 | 9.22 ± 0.76 | 0.0001 | 10.4 ± 0.75 | 13.8 ± 0.73 | 0.001 |
Data are mean ± SEM. C/L, casein/lactalbumin; SPI, soy protein isolate; TPC, total plasma cholesterol; TGs, triglycerides; HDLc, high-density lipoprotein cholesterol.
Atherosclerosis and gene expression measures
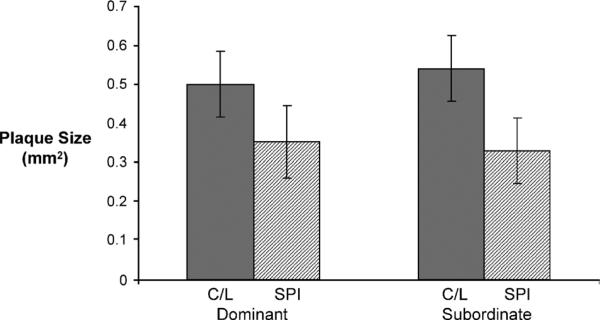
Iliac artery plaque size was significantly reduced in the SPI group (0.31 ± 0.06 mm2) compared with the C/L group (0.51 ± 0.06 mm2; P < 0.05) and was not influenced by the social status of the monkeys (dominant 0.43 ± 0.06 mm2; subordinate 0.44 ± 0.06 mm2; P > 0.92) (Fig. 2). Plaque size was found to be positively correlated with TPC (C/L: r = 0.58, P < 0.0001; SPI: r = 0.36, P < 0.05) and non-HDLc (C/L: r = 0.61, P < 0.0001; SPI: r = 0.33, P < 0.05) in both diet groups. Plaque size was not correlated with plasma IF concentrations in the SPI group.
FIG. 2.
Mean atherosclerotic plaque size (mm2) of iliac biopsy sections by dietary treatment and by social rank. C/L, casein/lactalbumin; SPI, soy protein isolate.
Inflammatory gene expression (normalized to endogenous control genes glyceraldehyde-3-phosphate dehydrogenase, B-actin, and ribosomal protein, large P0) was significantly reduced with SPI treatment (Table 4), before and after covarying for atherosclerotic plaque size. SPI-treated monkeys had significantly reduced arterial MCP-1, ICAM-1, and IL-6 mRNA transcripts (all P < 0.0001). Cell type–specific markers to macrophages (CD68; P < 0.001) and T cells (CD3 and CD4; P < 0.015 and P < 0.001) were also significantly reduced with SPI treatment; these effects were significant before and after inclusion of plaque size in the model (all P < 0.0001). SPI-treated animals had increased ER-α transcript levels (P < 0.001), and arterial ER-α expression was inversely associated with atherosclerosis (P < 0.02), independent of treatment group. High-risk females had decreased ER-β (P < 0.017), increased IL-6 (P < 0.047), and a tendency toward increased MCP-1 transcript levels (P < 0.056). There were no significant interactions between diet and social status, although macrophage (CD68) and inflammatory transcripts (MCP-1, ICAM-1, VCAM-1, and IL-6) were lowest in dominant females treated with SPI (NS, data not shown).
TABLE 4.
Effects on iliac artery gene expression relative to the endogenous control genes, GAPDH, B-actin, and RPLP
| Diet |
Status |
||||||
|---|---|---|---|---|---|---|---|
| C/L | SPI | P | Adjusted Pa | Low-risk | High-risk | P | |
| MCP-1 | 0.0277 ± 0.0035 | 0.0085 ± 0.0036 | <0.0001 | <0.0001 | 0.0147 ± 0.0036 | 0.0216 ± 0.0035 | 0.056 |
| VCAM-1b,c | 0.5147 ± 0.1245 | 0.5423 ± 0.1275 | 0.456 | — | 0.4545 ± 0.1292 | 0.6025 ± 0.1228 | 0.408 |
| ICAM-1 | 0.0143 ± 0.0013 | 0.0072 ± 0.0013 | <0.0001 | <0.0001 | 0.0096 ± 0.0013 | 0.0118 ± 0.0013 | 0.161 |
| IL-6d | 0.0944 ± 0.0001 | 0.0470 ± 0.0001 | <0.0001 | <0.0001 | 0.0522 ± 0.0001 | 0.0892 ± 0.0001 | 0.047 |
| CD68 | 0.1477 ± 0.0197 | 0.0482 ± 0.0202 | 0.001 | <0.0001 | 0.0760 ± 0.0205 | 0.1199 ± 0.0195 | 0.183 |
| CD3d | 0.3148 ± 0.0004 | 0.1534 ± 0.0004 | 0.015 | <0.0001 | 0.2238 ± 0.0004 | 0.2445 ± 0.0004 | 0.711 |
| CD4 | 0.0308 ± 0.0039 | 0.0111 ± 0.0040 | 0.001 | <0.0001 | 0.0187 ± 0.0041 | 0.0232 ± 0.0039 | 0.689 |
| TCR-βd | 0.0119 ± 0.0001 | 0.0079 ± 0.0001 | 0.956 | — | 0.0104 ± 0.0001 | 0.0093 ± 0.0001 | 0.290 |
| ER-α | 0.0069 ± 0.0005 | 0.0094 ± 0.0005 | 0.001 | <0.0001 | 0.0086 ± 0.0005 | 0.0077 ± 0.0005 | 0.191 |
| ER-βd,e | 0.0098 ± 0.0097 | 0.0111 ± 0.0100 | 0.091 | — | 0.0122 ± 0.0100 | 0.0090 ± 0.0100 | 0.017 |
Data are mean expression ± SEM. GADPH, glyceraldehyde-3-phosphate dehydrogenase; RPLP, ribosomal protein, large P0; C/L, casein/lactalbumin; SPI, soy protein isolate; Low-risk, dominant social status; High-risk, subordinate social status; MCP-1, monocyte chemotactic protein-1; VCAM-1, cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; TCR-β, T-cell receptor-β; ER, estrogen receptor.
Adjusted P represents significance of SPI treatment on gene expression measures covarying for plaque size.
Mean × 10–5.
SE × 10–6.
Mean × 10–2
SE × 10–8.
Within the SPI group alone, serum dihydrodaidzein levels were inversely associated with arterial expression of ICAM-1 as well as macrophage (C68) and T-cell (CD4 and T-cell receptor-A) markers (all r < –0.33, P < 0.05). Serum glycitein showed a similar pattern, although values for CD4 and CD68 were not significant. Arterial ER-α expression was correlated with serum genistein (r = 0.45, P < 0.005), and ER-β expression was correlated with plasma daidzein (r = 0.39, P < 0.015) and glycitein (r = 0.37, P < 0.023).
Relationships between plasma lipids and arterial endpoints
Analysis of the data by a bootstrapping method using plasma lipids as a mediator, social status as a covariate, and plaque size as the outcome suggested that plasma lipids were important mediators of the effects of SPI on plaque size. Plasma lipid concentration–mediated effects on gene expression were also evaluated by a bootstrapping analysis that treated the TPC/HDLc ratio as a mediator and plaque size and social status as covariates. These analyses suggested that the beneficial effects of SPI on arterial expression of ICAM-1, IL-6, the cell-specific markers for macrophages (CD68) and T cells (CD3 and CD4), and ER-α were largely unexplained by effects on plasma lipid concentrations.
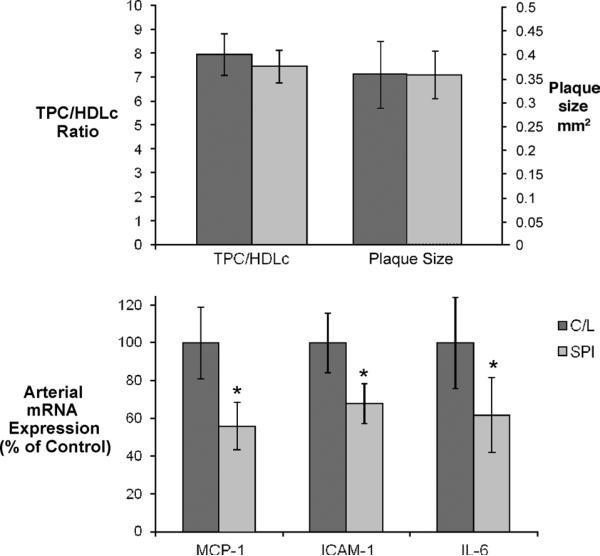
To further examine this issue, subsets of animals having similar TPC/HDLc ratios were identified in each dietary treatment condition. Consistently across all subset groups generated, MCP-1, ICAM-1, and IL-6 expression was significantly lower in the SPI treatment group (data not shown). Similar results were obtained when subsets of animals were matched for both TPC/HDLc and plaque size (C/L: n = 21; SPI: n = 32). Again, SPI significantly reduced MCP-1, ICAM-1, and IL-6 expression (P < 0.05 for all) (Fig. 3), supporting the suggestion that dietary soy affected inflammatory expression independently of plasma lipids and lesion size.
FIG. 3.
Plasma lipid–dependent and –independent effects of dietary soy. A: Characteristics of subsets of casein/lactalbumin (C/L)–treated (n = 21) and soy protein isolate (SPI)–treated (n = 32) animals selected for similar plasma total plasma cholesterol (TPC)/high-density lipoprotein cholesterol (HDLc) ratios and plaque sizes. B: Relative expression of arterial inflammatory genes in subsets described above. Despite similarities in plaque size and plasma lipid concentrations, animals consuming dietary SPI had significantly lower arterial monocyte chemotactic protein-1 (MCP-1), intercellular adhesion molecule-1 (ICAM-1), and interleukin-6 (IL-6) gene expression. *P < 0.05.
DISCUSSION
Women begin developing fatty streaks and/or early atherosclerotic plaques during young adulthood, and these lesions become larger, more complex, and inflammatory in nature, with progression of the atherosclerotic lesions occurring through the perimenopausal transition and continuing into the postmenopausal years.32 Prior studies in monkeys have shown that premenopausal atherosclerosis predicts the extent of postmenopausal atherosclerosis in the same monkeys and does so irrespective of any postmenopausal intervention.2 The current study was designed to determine whether premenopausal dietary SPI intervention affects atherosclerotic plaque progression and vascular gene expression and thereby might be expected to provide postmenopausal benefits to treated individuals in later life.
This study, the first to evaluate the effects of soy on atherosclerosis and arterial gene expression in premenopausal females, showed that dietary SPI treatment significantly improved plasma lipid profiles. In addition, SPI treatment significantly reduced atherosclerotic lesion area, and this reduction seemed to be mediated largely by changes in plasma lipid concentrations. Dietary SPI reduced atherosclerosis in both dominant and subordinate females. Arterial inflammatory gene expression and cell marker expression for macrophage and T cells were significantly reduced by SPI treatment, whereas ER-α expression was increased. These differences in gene expression did not seem to depend on changes in plasma lipid concentrations or on the resulting reduction in lesion size. Therefore, whereas soy may only modestly affect plasma lipid concentrations of women, these findings suggest that it nonetheless may provide substantial arterial benefits.
There has been controversy about the effects of dietary soy on risk factors for cardiovascular disease in women. In 1999, the Food and Drug Administration approved labeling for soy-containing foods as being potentially protective against CHD based on published effects on plasma cholesterol levels. However, in 2006 the American Heart Association assessed data from several randomized trials in mostly postmenopausal women, which showed only modest changes in plasma lipids after dietary soy interventions and concluded that these modest changes were not likely to affect CHD risk.12 The controversy continues as a recent randomized trial in postmenopausal women found that soy supplements not only significantly reduced plasma LDL cholesterol compared with a C/L control group, but also significantly decreased LDL particle number, a measure that has been shown to be a stronger predictor of CHD events and disease progression.33 Nevertheless, the plasma lipids of cynomolgus monkeys seem to be more responsive to the influence of dietary soy than observed in humans. The significant reduction in atherosclerotic lesion size in this study was largely mediated by the effects on plasma lipids. However, this result does not dismiss the possibility of lipid-independent effects of soy on other modulators of the atherosclerotic process, such as inflammation or other processes.
Furthermore, whereas atherosclerotic lesion size depends on prior events occurring at that site over the lifetime of the lesion; this measurement provides little information on the metabolic characteristics of the lesion. Exploration of vascular gene expression provides a sensitive means to evaluate molecular events occurring in the artery at a given point in a study. Dietary SPI reduced arterial expression of mRNA transcripts of key inflammatory genes such as MCP-1 and ICAM-1 as well as markers for inflammatory cell types in these premenopausal female monkeys. MCP-1 and ICAM-1 are nuclear factor-κB (NF-κB)Ydependent inflammatory molecules critical for the adhesion and recruitment of monocytes and T lymphocytes into the arterial intima, a key process in the initiation and progression of atherosclerosis.34,35 Consistent with the findings for MCP-1 and ICAM-1, we also observed significantly reduced expression of markers CD68, CD3, and CD4 for the cell types attracted by these molecules in these arterial sections. The scavenger receptor CD68 is often used as a macrophage marker, although it is also expressed by other cells, such as dendritic cells. CD3 and CD4 are T-cell specific markers, with CD3 being present on the majority of T cells, whereas CD4 is found on T-helper cells, the majority of T cells within atherosclerotic lesions.35 Aside from the likely reduction in adhesion and recruitment of these cells owing to lower MCP-1 and ICAM-1 expression observed here, in vitro studies have shown that IF may inhibit both proliferation and activation of macrophages.36 Regardless of the mechanism, our studies suggest that there is an overall reduction in arterial macrophage and T-cell content with SPI treatment and of a reduced arterial inflammatory state that may slow atherosclerosis progression and retard development of more complex and perhaps clinically relevant lesions. More importantly, however, this reduction in arterial inflammation and potentially in macrophage and T-cell content was partially independent of changes in plasma lipid concentrations, demonstrating that dietary soy has arterial anti-inflammatory effects that could influence overall lesion progression and complexity in women, regardless of changes in plasma lipids. These anti-inflammatory effects could occur through modulation of the activity of the nuclear transcription factor NF-κB, a central regulator of inflammation that regulates the expression of adhesion molecules, cytokines, and chemokines within nascent and developing atheromas.34,37 ERs are known to interact with the NF-κB inflammatory pathway, and estrogen and IF have been shown to selectively block NF-κB transactivation, leading to decreased expression of NF-κB–dependent genes.38 ER levels have been found to be (1) reduced in atherosclerotic human arteries, (2) negatively correlated with atherosclerosis in pre- and postmenopausal women,39 and (3) negatively correlated with lesion size and arterial NF-κB–dependent inflammatory gene expression in both male17 and female monkeys (present study). Studies in transgenic mice have shown that the atheroprotective effects of both estrogen and IF are mediated primarily by ER-α.40,41 Thus, interactions occurring between IF, ERs, and NF-κB may contribute to the inhibition of arterial inflammation and plaque progression by dietary SPI.
Prior studies with socially housed monkeys suggested that subordinate monkeys experienced exacerbated atherosclerosis.5 This effect is presumably mediated by the relative ovarian impairment and estrogen deficiency that characterize such individuals as it could be prevented by treatment with an estrogen-containing oral contraceptive.5,42 Effects of social status on ovarian function and menstrual cyclicity were also observed in the present study, as subordinate female monkeys had more cycle length variability, lower luteal phase plasma progesterone concentrations, and a reduced number of ovarian cycles considered normal compared with dominant female monkeys.31 Although lesion size did not differ between dominant and subordinate animals, subordinate animals did have elevated TPC/HDLc ratios, indicating higher risk.5,43 Status also influenced gene expression, as subordinate females exhibited increased IL-6 and tended to have higher MCP-1 mRNA transcript levels compared with dominant females, suggestive of a proinflammatory and proatherogenic state in the subordinate monkeys. Dominant female monkeys also had increased arterial ERβ expression compared with high risk female monkeys.
Finally, it should be noted that in a parallel study in male monkeys,16,17 dietary soy consumption did not result in anti-inflammatory effects on arterial gene expression,17 even though beneficial effects on plasma lipid concentrations and extent of atherosclerosis comparable to that of the present study were observed. The mechanisms underlying this sex-specific outcome are unknown, although dietary soy increased insulin sensitivity in the female monkeys, while causing an insulin resistance in the male monkeys.44 Given the influence of hyperglycemia and diabetes on arterial pathological changes, it seems likely that some of the differences in arterial gene expression responses to soy may relate to this contrasting effect.
CONCLUSION
In summary, the present study suggests that premenopausal SPI consumption has plasma lipid–independent beneficial effects on the pathobiological processes involved in atherosclerotic plaque development, thus setting the stage for reduced complicated atherosclerosis and associated conditions postmenopausally. In addition, dominant social status had modest beneficial effects on atherosclerosis risk factors, as seen by a more favorable arterial inflammatory gene expression and plasma lipid profiles.
Acknowledgments
The authors gratefully acknowledge the Solae Company for their generous contributions of soy products and thank Gerald Perry, Debbie Golden, Megan Phillips, Dewayne Cairnes, Melissa Ayers, Cheryl Wilmoth, and Lou Craddock for their technical contributions.
Funding/support: This project was supported in part by National Institutes of Health Grants HL45666 and HL 79421 (J.R.K.), RR020890 (A.A.F.), AG18170 (T.C.R.), and AG28641 (T.C.R.).
Footnotes
Financial disclosure: None reported.
REFERENCES
- 1.McGill HC, Jr, McMahan CA, Zieske AW, et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth: the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 2002;20:1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan JR, Manuck SB, Anthony MS, et al. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 3.Lobo R. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Academic Press; San Diego, CA: 2007. [Google Scholar]
- 4.Speroff L. Premenopausal atherosclerosis: setting the stage for later clinical disease. [July 6, 2007];Contemp Ob/Gyn. 2007 Available at: http://www.modernmedicine.com/modernmedicine/Cardiovascular+Disease/Premenopausal-atherosclerosis-Setting-the-stage-fo/ArticleStandard/Article/detail/411949.
- 5.Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 6.Adams MR, Kaplan JR, Clarkson TB, et al. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Johnstone BM, Cook-Newell ML. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 8.Erdman JW. Soy protein and cardiovascular disease: a statement for healthcare professionals from the nutrition committee of the AHA. Circulation. 2000;102:2555–2559. doi: 10.1161/01.cir.102.20.2555. [DOI] [PubMed] [Google Scholar]
- 9.Crouse JR, Morgan T, Terry JG, et al. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999;159:2070–2076. doi: 10.1001/archinte.159.17.2070. [DOI] [PubMed] [Google Scholar]
- 10.Merz CN, Johnson BD, Braunstein GD, et al. Phytoestrogens and lipoproteins in women. J Clin Endocrinol Metab. 2006;91:2209–2213. doi: 10.1210/jc.2005-1853. [DOI] [PubMed] [Google Scholar]
- 11.Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462–1469. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Lichtenstein A, Horn LV, et al. Soy protein, isoflavones, and cardiovascular health: an American Heart Association science advisory for professionals from the nutrition committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 13.Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein AH, Jalbert SM, Adlercreutz H, et al. Lipoprotein responses to diets high in soy or animal protein with and without isoflavones in moderately hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2002;22:1852–1858. doi: 10.1161/01.atv.0000033513.18431.a1. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effect of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 16.Adams MR, Golden DL, Williams JK, et al. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr. 2005;135:2852–2856. doi: 10.1093/jn/135.12.2852. [DOI] [PubMed] [Google Scholar]
- 17.Walker SE, Adams MR, Franke AA, et al. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008;196:106–113. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Register TC, Cann JA, Kaplan JR, et al. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–1740. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 19.Hwang CS, Kwak HS, Lim HJ, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Henley DV, Korach KS. Endocrine-disrupting chemicals use distinct mechanisms of action to modulate endocrine system function. Endocrinology. 2006;147(6 Suppl):S25–S32. doi: 10.1210/en.2005-1117. [DOI] [PubMed] [Google Scholar]
- 21.Nestel PJ, Yamashita T, Sasahara T, et al. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:3392–3398. doi: 10.1161/01.atv.17.12.3392. [DOI] [PubMed] [Google Scholar]
- 22.Sade DS. Determinants of dominance in a group of free-ranging rhesus monkey. In: Altmann S, editor. Social Communication Among Primates. University of Chicago Press; Chicago, IL: 1967. pp. 99–114. [Google Scholar]
- 23.Sade DS. An ethogram for rhesus monkeys, I. Antithetical contrasts in posture and movement. Am J Phys Anthropol. 1973;38:537–542. doi: 10.1002/ajpa.1330380263. [DOI] [PubMed] [Google Scholar]
- 24.Franke AA, Custer LJ, Wilkens LR, et al. Liquid chromatographic analysis of dietary phytoestrogen from human urine and blood. J Chromatogr B. 2002;777:43–57. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 25.Lees CJ, Kaplan JR, Chen H, et al. Bone mass and soy isoflavones in premenopausal female macaques. J Nutr. 2007;86:245–250. doi: 10.1093/ajcn/86.1.245. [DOI] [PubMed] [Google Scholar]
- 26.Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both classical estrogen receptor and the newly described estrogen receptor β. J Steroid Biochem Mol Biol. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- 28.Vandesompele J, DePreter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Bollen KA, Setin R. Direct and indirect effects: classical and bootstrap estimates of variability. Sociol Methodol. 1990;20:115–140. [Google Scholar]
- 31.Kaplan JR, Berga SL, Wilson ME, et al. High isoflavone soy protein does not alter menstrual cyclicity or ovarian function in fully mature, premenopausal monkeys [Abstract]. Fertil Steril. 2004;82(Suppl 2):S269–S270. [Google Scholar]
- 32.Mikkola TS, Clarkson TB. Coronary heart disease and postmenopausal hormone therapy: conundrum explained by timing? J Womens Health. 2006;15:51–53. doi: 10.1089/jwh.2006.15.51. [DOI] [PubMed] [Google Scholar]
- 33.Allen JK, Becker DM, Kwiterovich PO, et al. Effect of soy protein-containing isoflavones on lipoproteins in postmenopausal women. Menopause. 2007;14:106–114. doi: 10.1097/01.gme.0000229572.21635.49. [DOI] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Getz GS. Immune function in atherogenesis. J Lipid Res. 2005;46:1–10. doi: 10.1194/jlr.R400013-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Comalada M, Ballester I, Bailon E, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Evans MJ, Eckert A, Lai KD, et al. Reciprocal antagonism between estrogen receptor and NF-κB activity in vivo. Circ Res. 2001;89:823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 38.Vanden Berghe W, Dijsselbloem N, Vermeulen L, et al. Attenuation of mitogen- and stress-activated protein kinase-1-driven nuclear factor-κB gene expression by soy isoflavones does not require estrogen activity. Cancer Res. 2006;66:4852–4862. doi: 10.1158/0008-5472.CAN-05-2957. [DOI] [PubMed] [Google Scholar]
- 39.Losordo DW, Kearney M, Kim EA, et al. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 40.Hodgin JB, Krege JH, Reddick RL, et al. Estrogen receptor α is a major mediator of 17β-estradiol`s atheroprotective effects on lesion size in Apoe-/- mice. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams MR, Golden DL, Register TC, et al. The atheroprotective effect of dietary soy isoflavones in apolipoprotein E-/- mice requires the presence of estrogen receptor-α. Arterioscler Thromb Vasc Biol. 2002;22:1859–1864. doi: 10.1161/01.atv.0000042202.42136.d0. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan JR, Adams MR, Anthony MS, et al. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;12:2094–2100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 43.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related disease. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 44.Ingram K, Kaplan J, Kavanagh K, Wagner JD. The effect of dietary soy on adipose tissue, adipocytokines, and insulin sensitivity.. Paper presented at: 6th International Symposium on the Role of Soy in Preventing and Treating Chronic Disease; Chicago, IL. October 30-November 2, 2005. [Google Scholar]