Abstract
Development of neuroprotective strategies for peripheral neuropathies requires high-throughput drug screening assays with appropriate cell types. Currently, immortalized dorsal root ganglion (DRG) sensory neuronal cell lines that maintain nociceptive sensory neuronal properties are not available. We generated immortalized DRG neuronal lines from embryonic day 14.5 rats. Here, we show that one of the immortalized DRG neuronal lines, 50B11, has the properties of a nociceptive neuron. When differentiated in the presence of forskolin, these cells extend long neurites, express neuronal markers, and generate action potentials. They express receptors and markers of small-diameter sensory neurons and upregulate appropriate receptor populations when grown in the presence of glial cell line–derived neurotrophic factor or nerve growth factor. Furthermore, they express capsaicin receptor transient receptor potential vanilloid family-1 (TRPV-1) and respond to capsaicin with increases in intracellular calcium. In a 96-well plate format, these neurons show a decline in ATP levels when exposed to dideoxycytosine (ddC) in a proper time- and dose-dependent manner. This ddC-induced reduction in ATP levels correlates with axonal degeneration. The immortalized DRG neuronal cell line 50B11 can be used for high-throughput drug screening for neuroprotective agents for axonal degeneration and antinociceptive drugs that block TRPV-1.
Keywords: DRG, high-throughput screen, nociceptive, sensory neuronal line, TRPV-1, 50B11
Introduction
Many relatively common clinical conditions are associated with neuropathic pain (Berger et al., 2004). Traditionally, combinations of tricyclic antidepressants or antiepileptics along with analgesics have been used to treat neuropathic pain (reviewed in Mendell and Sahenk, 2003; Wolfe and Trivedi, 2004). However, treatment of neuropathic pain is often unsatisfactory; persistent neuropathic pain affects quality of life and leads to significant morbidity. In recent years with the identification of the receptor for capsaicin (Caterina et al., 1997; 2000), neuropathic pain research has been directed to identification of drugs that interfere with the transient receptor potential vanilloid family-1 (TRPV-1) physiology. Primary effort has focused on antagonists that block nociceptive pain sensation at the receptor level, but so far, no drug has reached clinical use (Szallasi and Appendino, 2004).
Previous attempts at identifying TRPV-1 antagonists have used nonneuronal cell lines expressing recombinant TRPV-1 and the calcium flux induced by capsaicin as an outcome measure for high-throughput screening (HTS) (Garcia-Martinez et al., 2002; Gunthorpe et al., 2004; Masip et al., 2004). Although these cells expressing recombinant TRPV-1 may be useful, a nociceptive sensory neuronal cell expressing TRPV-1 might be more relevant because the nonneuronal cell lines may lack the appropriate intracellular signaling pathways associated with and downstream of TRPV-1 in nociceptive sensory neurons. Similarly, drug screening efforts for peripheral neuropathies have been hampered by lack of appropriate peripheral neuronal lines suitable for HTS.
To generate tools for a more rational approach to drug screening for neuropathic pain and peripheral neuropathies, we have developed an immortalized dorsal root ganglion (DRG) sensory neuronal line with nociceptive properties. These cells extend neurites when differentiated, express TRPV-1 and other receptors characteristic of small sensory neurons, generate action potentials when depolarized and respond to capsaicin. Furthermore, they are suitable for assays for HTS in models of neuropathic pain and peripheral neuropathies. We believe, with these cells, the hits identified as blockers of capsaicin-induced toxicity in DRG neurons might be more relevant to neuropathic pain and lead to better drug development. In addition, the technology used in derivation of this neuronal line may be applicable to generation of human DRG sensory neurons as well as neurons from other regions of the nervous system and other difficult-to-immortalize cells.
Materials and Methods
Unless noted otherwise, all reagents and materials were purchased from Invitrogen. Animal studies were conducted according to the protocols approved by the institutional Animal Care and Use Committee.
Construction of SV40 largeT-antigen and human telomerase reverse transcriptase expression vectors
For cloning of the SV40 large T-antigen, plasmid construct pZipSV776-1 (a kind gift of Dr. William C. Hahn, Harvard University) was used as the template for polymerase chain reaction (PCR) amplification of the gene fragment coding for SV40 large T-antigen. PCR reaction was primed by oligonucleotides 5′CACCGCTTTGCAAAGATGGATAAAG (sense) and 5′-AATTGCATTCATTTTATGTTTCA (antisense). Amplification was performed using the Expend High Fidelity PCR System (Roche). The PCR product was cloned into the pENTR/D-TOPO vector by directional Tag polymerase-amplified (TA) cloning. After confirmation of the sequence, the target SV40 large T-antigen gene was transferred into pLenti6/V5-Dest vector using Gateway technology (Invitrogen). In the destination vector, the SV40 large T-antigen was under the control of Pcmv, and the selection marker, blasticidin resistance gene, was under the control of Psv40. The human telomerase reverse transcriptase (hTERT) expression construct pBabe-hygro-hTERT carrying hygromycin resistant gene (also a kind gift of Dr. William C. Hahn, Harvard University) was used to transfer the hTERT gene into the pcDBNA3.2/V5-DEST/Neo vector using Gateway technology. In the destination vector, the hTERT was under the control of Pcmv, and the selection marker, neomycin resistance gene, was under the control of Psv40. The expression plasmids were prepared and purified using Plasmid MIDI Kit (Qiagen). Endotoxin-free plasmid was suspended in distilled water for electroporation.
Electroporation into dissociated DRG neurons and selection of clones
Dissociated primary DRG neuronal cells were prepared as previously described (Hoke et al., 2003; Keswani et al., 2003) and the plasmid was electroporated. Approximately 5 × 104 cells in 90 μl of Opti-MEM media were mixed with 10 μl plasmid (1 mg/ml) and transferred into a 0.2-cm Gene Pulser cuvette (Bio-Rad). After 10 min of incubation at room temperature, a single square-wave pulse (100 V, 950 mF, approximately 40 ms) was delivered by a Gene Pulser II with a Capacitance Extender Plus (Bio-Rad). Culture medium at 4°C was immediately added to the cells and the cuvette was kept on ice for 10 min. Cells were plated in T75 flasks in culture medium without antibiotics (Neurobasal medium, 10% fetal bovine serum (FBS), 0.5 mM glutamine, 1 × B-27 supplement, 0.2% glucose). To increase the efficiency of electroporation and incorporation of large T-antigen into terminally differentiated sensory neurons, the process of electroporation was repeated three to four times before addition of antibiotic selection media. About 60–70% of the cells survived the electroporation process. Twenty-four hours after the last electroporation, culture medium was replaced by selection medium containing blasticidin (5 μg/ml), and cells were maintained in this medium for 1–2 weeks until isolated colonies with 200–300 cells formed. Colonies were picked and expanded using standard culture methods when reached 80–90% confluence. For hTERT transduction, SV40 transfected and blasticidin resistant cells were trypsinized and electroporated with the hTERT plasmid as above for the large T-antigen. The electroporation was repeated three to four times. About 60–70% of the cells survived the electroporation process. Twenty-four hours after the last electroporation, culture medium was replaced by selection medium containing neomycin (50 mg/ml), and cells were maintained in this medium for 1–2 weeks until isolated colonies with 200–300 cells formed. Colonies were picked and expanded using standard culture methods when reached 80–90% confluence.
Induction of neuronal differentiation and characterization of the immortalized neuronal clone
One of the immortalized DRG neuronal cell lines (50B11) maintained self-replication capability over many cell divisions (>300), and it was used in further analysis of neuronal properties. The results described in this article were obtained with cells between 100 and 400 passages. The 50B11 cells were easy to grow in uncoated plastic dishes and had a replication rate of about 36 h. Differentiation and axonal elongation was induced in these cells by addition of forskolin (50 μM) into the culture medium. Within hours, more than 90% cells stopped dividing and extended long neurites. These cells were grown in 24-well plates on glass coverslips, fixed with 4% paraformaldehyde, and immunostained for presence of neurofilament (SMI-32 antibody from Sternberger Monoclonals Inc.), βIII-tubulin (Promega), transient receptor potential channel, vanilloid subfamily member-1 (TRPV-1) (Abcam), calcitonin gene–related protein (CGRP) (Abcam), or isolectin B4 (IB4) (Vector Laboratories) using standard methods (Keswani et al., 2003). Dilutions of the primary antibodies were all 1: 2,000 and the fluorophore-tagged secondary antibodies were used at 1: 200 dilution. Slides were counterstained with 4′,6-diamidino-2-phenylindole and mounted with Vectashield (Vector Laboratories). Specificity of all primary and secondary antibodies was confirmed using appropriate positive and negative control cultures.
The electrophysiological recording techniques employed were similar to those described by Hamill et al. (1981). The external solution contained 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose, and 10 mM HEPES (pH 7.4; 310–320 mOsm.) Cells were continuously superfused at 2–3 ml/ min. Using the whole-cell patch-clamp technique, data were obtained with borosilicate thin-walled micropipettes (BORO, BF150-110-10, Sutter) made with a Flaming-Brown Puller (P-87, Sutter Instruments). Micropipettes were filled with (in millimolar) 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 ethyl glycol-bis (β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid (EGTA), 4 MgATP adjusted to a pH of 7.3 with Tris buffer. Pipette resistances measured 3–6 MΩ. Current-clamp recordings were obtained with an Axopatch 200B amplifier (Axon Instruments Inc.), and data were filtered on-line at 2 kHz. Recordings were made at a holding potential (VH) of −60 mV. For statistical evaluation, we used ANOVA (Origin version 6, Microcal Software Inc.).
For analysis of changes in gene expression, 50B11 cells were grown in media containing forskolin (50 μM), nerve growth factor (NGF) (10 ng/ml), glial cell line–derived neurotrophic factor (GDNF) (10 ng/ml), or vehicle control for 24 h. Total RNA was isolated using the TRIzol Reagent (Invitrogen) according to the manufacturer’s recommendation. Two micrograms of total RNA was reverse transcribed using Ready-To-Go You-Prime First-Strand Bead (Amersham Biosciences) according to manufacturer’s protocols. Real-time PCR was carried out in a DNA Engine Opticon Continuous Fluorescence Detection System using DyNAmo SYBR Green Polymerase (MJ Research). All primers were designed using the individual gene sequences in the GenBank/EMBL nucleotide sequence database (primer sequences are available upon request). The binding positions of all primers were chosen to produce amplicons of 150–200 bp and to achieve maximum efficiency and specificity. All primer sequences were checked for specificity by a BLAST search in the GenBank/EMBL nucleotide sequence database. The primers were synthesized by Integrated DNA Technologies Inc. The amplification of internal control housekeeping gene, GAPDH, was carried out using a commercial kit (Applied Biosystems) according to the protocol supplied by the manufacturer. The expression levels of individual genes before and after treatment were calculated using the comparative CT method as described before (Hoke et al., 2006).
Ca2+ microfluorimetry and imaging in forskolin-differentiated 50B11 cells were performed by ratio-metric imaging of the Ca2+-sensitive fluorescent dye fura-2. Cells were grown on glass coverslips in 12-well dishes, and calcium imaging was done with and without differentiation with 50-μM forskolin. Cells were loaded for 15 min at 37°C with 2 M fura-2 acetoxymethyl ester (Molecular Probes) in Krebs-HEPES buffer (100 mM NaCl, 2.0 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 1.0 mM NaH2PO4, 4.2 mM NaHCO3, 12.5 mM HEPES, and 10.0 mM glucose), then washed for three times in buffer to remove remaining fura-2 ester. The coverslip with loaded cells was then mounted on an inverted epifluorescence microscope (Zeiss, Axiovert 200) and covered with 60 μl Krebs-HEPES buffer or buffer with capsaicin (75 μM). In capsazepine pretreatment experiments, capsazepine at a concentration of 75 μM was added 20 min prior to addition of capsaicin. Images were acquired approximately every 3 s with an extended Hamamatsu Digital Camera C4742-95 (Hamamatsu Photonics) using a dual filter wheel (Sutter Instruments, Novato) equipped with 340 and 380 nm, 10-nm-bandpass filters (Omega Optics). Data were acquired using InCyt Im2 software (Intracellular Imaging Inc.). Fluorescence changes are expressed as the ratio of fluorescence at 340 and 380 nm (F340/F380).
Neuronal toxicity assays
Conditions for culturing the 50B11 cells and measuring the ATP levels were optimized for the 96-well plate format. Initially, 500 cells/well were plated in 96-well plates for 24 h and then differentiated with forskolin (50 μM) in culture medium with reduced serum (0.2%). Varying concentrations of dideoxycytosine (ddC) with or without immunophilin ligand GPI-1046 were added to the wells for another 24 h. Cellular ATP levels were measured using the ViaLight Plus kit (Cambrex) according to manufacturer’s instructions. This luciferase-based assay allows measurement of ATP levels on a luminometer with minimal manipulation of the well contents.
Measurements of axonal lengths to determine axonal degeneration induced by ddC were done as described before (Keswani et al., 2003). Briefly, 50B11 cells were plated onto collagen-coated glass coverslips and allowed to extend axons for 24 h in the presence of forskolin (50 μM). Then ddC, GPI-1046, or vehicle controls were added to the media for another 24 h. Cells were fixed and stained with anti-βIII-tubulin antibody to delineate the axons. Axon length measurements were done in multiple fields using a random sampling method as described previously. Each experiment was done in triplicates and repeated at least twice. Statistical analysis was done using ANOVA. Correction for multiple comparisons was done using Fisher’s protected least significant difference.
Results
Immortalized DRG neuronal cells extend neurites, express neuronal markers, and generate action potentials after differentiation
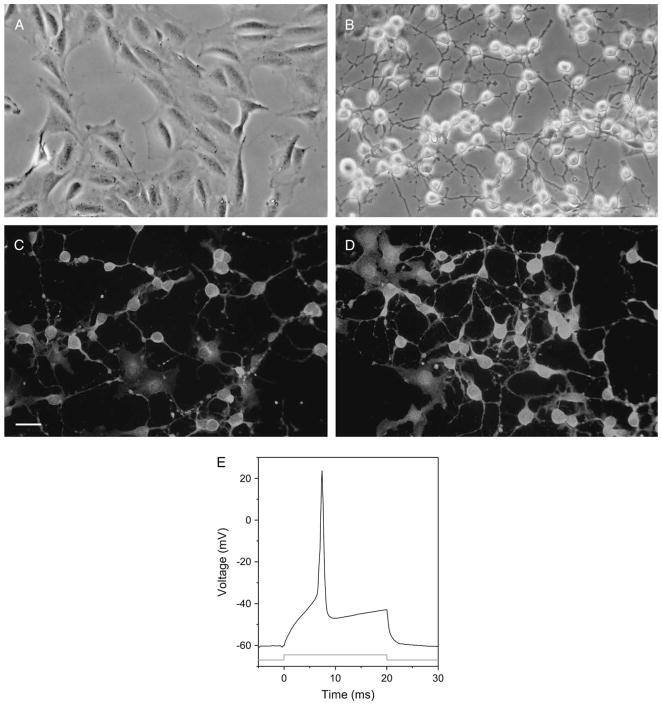
One of the clones generated after immortalization was further studied after evaluation using the initial screening of neurite extension in response to forskolin. This clone, 50B11, stopped dividing immediately after addition of forskolin and within 4 h extended neurites at least twice as long as the neuronal body diameter (Figs. 1A, 1B). Within 24 h of differentiation, the cells were positive for neuronal markers βIII-tubulin and neurofilament (Figs. 1C, 1D). Glial markers such as glial fibrillary acidic protein (GFAP) and S-100β were negative (data not shown).
Figure 1.
Immortalized DRG neuronal cells extend neurites, express neuronal markers, and generate action potentials after differentiation. Phase contrast images of (A) undifferentiated and (B) differentiated 50B11 cells show extension of axons 4 h after differentiation with forskolin. Twenty-four hours after differentiation, 50B11 cells stain with (C) antineurofilament and (D) anti-βIII-tubulin antibodies. 4′,6-diamidino-2-phenylindole counterstain shows nuclei. Scale bar = 20 μm. (E) A representative action potential is seen in a differentiated 50B11 cell after application of a depolarizing current.
We studied these cells before and after differentiation using patch clamping. Data were obtained from a total of 14 cells (8 undifferentiated cells and 6 differentiated cells). Collectively, these cells displayed a mean resting membrane potential of −57.9 ± 2.1 mV. There were no statistically significant differences between the mean resting potential values of the undifferentiated and differentiated groups (−57.4 ± 1.9 mV vs. −58.3 ± 2.7 mV, mean ± SEM; ANOVA, p > 0.05). No spontaneous activity, either synaptic or action potential discharge, was observed when differentiated or undifferentiated cells were held at their resting membrane potential for periods up to 10 min. Electrical stimulation of undifferentiated cells with depolarizing current steps did not induce an action potential (n = 0/8). In contrast, when differentiated cells were stimulated, action potentials could be elicited (n = 5/6; Fig. 1E).
The 50B11neuronal line express markers of small-diameter sensory neurons
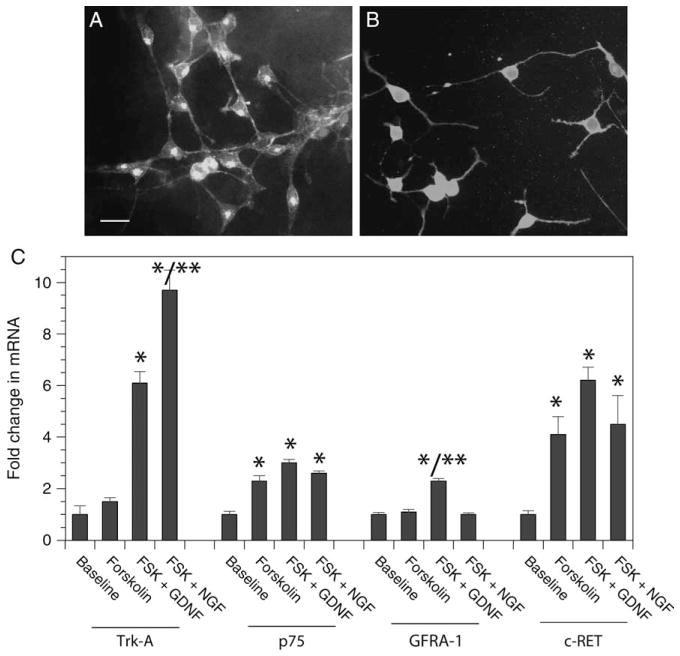
Small-diameter DRG sensory neurons are generally divided into two categories as follows: peptidergic ones with dependence on NGF and nonpeptidergic ones with dependence on GDNF (Bennett et al., 1998). Markers such as CGRP for peptidergic neurons and IB4 for nonpeptidergic neurons can identify these subgroups of small-diameter sensory neurons. The 50B11 line expressed both markers when differentiated in the presence of forskolin (Figs. 2A, 2B). Furthermore, 50B11 cells expressed receptors for NGF (low-affinity NGF receptor p75 and high-affinity Trk-A) and GDNF [c-ret and GDNF family receptor alpha-1 (GFRa-1)] and upregulated these receptors when differentiated with forskolin (Fig. 2C). Interestingly, the upregulation of receptors was neurotrophic factor specific; NGF receptor, Trk-A was more upregulated in the presence of NGF compared with GDNF and similarly GDNF receptor, GFRa-1 was more upregulated in the presence of GDNF compared with NGF. Markers for other DRG neurons, i.e., Trk-B and Trk-C were expressed at very low levels and were not upregulated upon differentiation with forskolin (data not shown).
Figure 2.
The 50B11 neuronal line expresses markers of small-diameter sensory neurons. Immunofluorescence images of 50B11 cells stained with (A) isolectin B4 and (B) anticalcitonin gene–related protein. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole. Scale bar = 20 μm. (C) Changes in expression of mRNA for p75, Trk-A, c-ret, and GDNF family receptor alpha-1 in the presence of forskolin (FSK), nerve growth factor, and glial cell line–derived neurotrophic factor (GDNF) compared with baseline levels of undifferentiated 50B11 cells. n = 6–8/group, error bars denote SEM, *p < 0.05 compared with baseline, **p < 0.05 FSK + GDNF vs. FSK + nerve growth factor.
The 50B11 neuronal line express nociceptive markers and respond to capsaicin
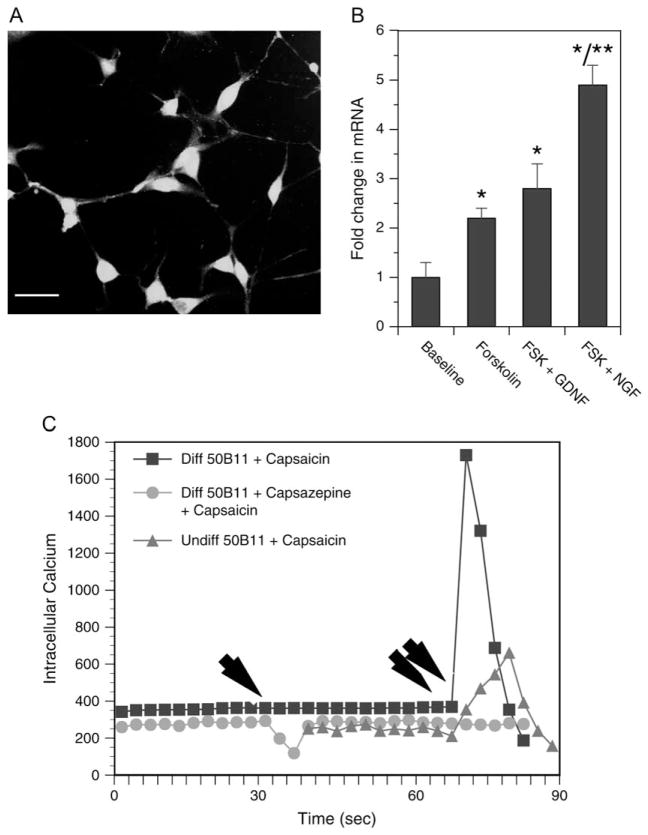
Once we determined that the 50B11 line had small-diameter sensory neuronal markers, we explored the possibility that it may be a nociceptive neuron. The cells expressed capsaicin receptor TRPV-1 and upregulated their expression when differentiated with forskolin with and without neurotrophic factor treatment (Fig. 3). Furthermore, the 50B11 cells responded to capsaicin with a rapid rise in intracellular calcium levels. This effect of capsaicin on the 50B11 cells was preventable by pretreatment of the cells with capsazepine, a specific blocker of TRPV-1, suggesting that the effect of capsaicin was mediated through TRPV-1. A graph representative of multiple intracellular calcium measurements is shown in Fig. 3C.
Figure 3.
The 50B11 neuronal line expresses nociceptive markers and respond to capsaicin. (A) Immunofluorescence image of 50B11 cells stained with anti-transient receptor potential vanilloid family-1 (TRPV-1) antibody. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole. Scale bar = 25 μm. (B) Fold change in TRPV-1 mRNA in response to differentiation and neurotrophic factor treatment is seen. n = 6–8/group, error bars denote SEM, *p < 0.05 compared with baseline, **p < 0.05 forskolin (FSK) + glial cell line–derived neurotrophic factor vs. FSK + nerve growth factor. (C) Measurements of intracellular calcium levels of undifferentiated 50B11 with vehicle control treatment (red line), after differentiation with FSK with (green), and without (blue) capsazepine pretreatment. Each line represents the average of three to five fields of cells with multiple cells in each view. Single arrow indicate the time at which capsazepine was added and double arrows indicate the time at which capsaicin was added.
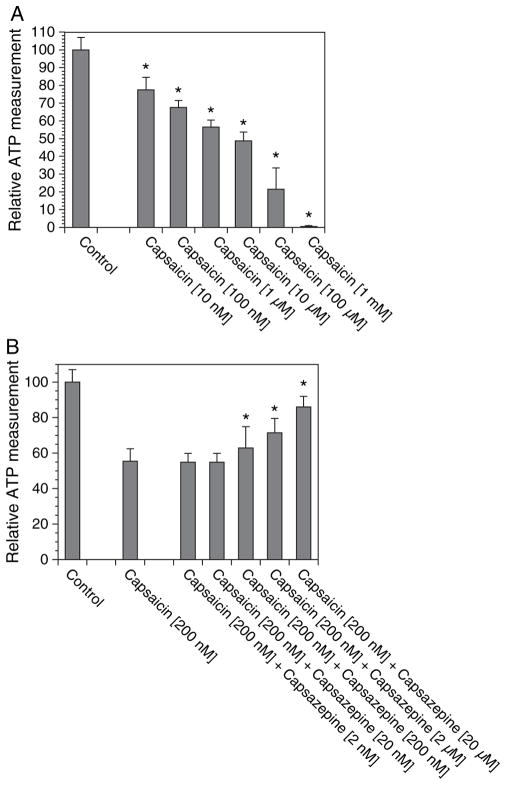
Next, we tested whether we can evaluate the neurotoxicity of capsaicin in an assay suitable for 96-well plate format. Capsaicin causes axonal degeneration and death of nociceptive DRG sensory neurons (Jancso et al., 1977). To evaluate cell survival, we used a luciferase-based assay to measure cellular ATP levels. We first optimized the assay by measuring ATP levels in different numbers of cells grown in the 96-well plates and found that the optimum number of cells for neurotoxicity assays was between 500 and 1,000 cells/well. We also optimized the culture conditions and found that low serum levels of 0.2% fetal bovine serum provided the most reliable results (data not shown). We then examined the dose-response curve of capsaicin and found that 10 μM of capsaicin caused about 50% reduction in ATP levels (Fig. 4A); similar to the dose required for neurotoxicity of capsaicin in primary DRG neurons (M. Polley and A. Höke, unpublished data, Johns Hopkins University, Baltimore). The capsaicin-induced neurotoxicity was preventable by coadministration of TRPV-1 blocker capsazepine in a dose-dependent manner (Fig. 4B).
Figure 4.
Capsaicin-induced neurotoxicity. The 50B11 cells were grown in 96-well plates and treated with varying doses of (A) capsaicin and (B) capsaicin + capsazepine for 24 h; cellular ATP levels were measured and expressed as a percentage of control cultures. n = 8/condition, error bars denote SEM, *p < 0.05 compared with controls.
The immortalized 50B11 neuronal line is suitable for high-throughput drug screening
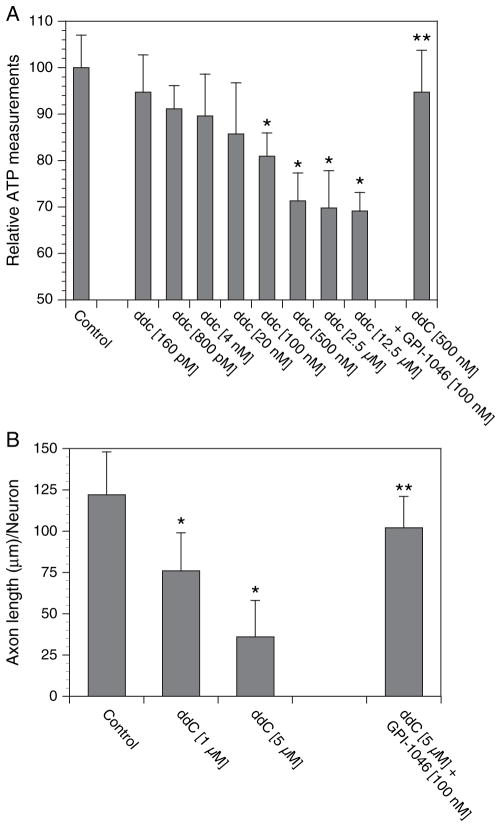
One of the potential uses of 50B11 sensory neuronal line will be their use in HTS assays. Our laboratory had developed an in vitro model of antiretroviral toxic neuropathy using primary DRG sensory neurons (Keswani et al., 2003). We had shown that ddC causes mitochondrial toxicity and reduction in ATP levels with subsequent axonal degeneration. We adapted this assay to the 50B11 cells and measured cellular ATP levels after varying concentrations of ddC (Fig. 5A). There was a dose-dependent toxicity of ddC at concentrations similar to therapeutic plasma levels in patients infected with HIV. This neurotoxicity was preventable by coadministration of a neuroprotective compound, GPI-1046, a nonimmunosuppressive immunophilin ligand (Steiner et al., 1997). We also validated this toxicity assay against a relevant measure of axonal degeneration. We first differentiated the 50B11 cells and let them extend axons for 24 h. Then, we treated them with ddC with and without GPI-1046 for 24 h and measured their total axonal lengths (Fig. 5B). Similar to the primary DRG neurons, ddC caused axonal degeneration of 50B11 cells in a dose-dependent manner and this was preventable by GPI-1046.
Figure 5.
Neurotoxicity assay suitable for high-throughput screening. The 50B11 cells were grown in 96-well plates and treated with varying doses of (A) dideoxycytosine (ddC) and ddC + GPI-1046 for 24 h; cellular ATP levels were measured and expressed as a percentage of control cultures. n = 8/ condition, error bars denote SEM, *p < 0.05 compared with controls. In validation experiments, 50B11 cells were differentiated and allowed to extend their neurites 24 h. (B) Then, they were treated with ddC or ddC + GPI-1046 for another 24 h, and total neurite lengths were measured. n = 6/condition, error bars denote SEM, *p < 0.05 compared with controls.
Discussion
Cellular tools for drug development for peripheral neuropathies and neuropathic pain are limited. We developed a novel method to immortalize nociceptive DRG neurons and show that the 50B11 immortalized DRG neuronal line cells extend neurites when differentiated, express receptors characteristic of nociceptive small-diameter sensory neurons, generate action potentials when depolarized and respond to capsaicin. Furthermore, the cells are easy to grow in large quantities and suitable for high-throughput drug screening using in vitro assays for neuropathic pain and peripheral neuropathies.
DRG neurons are terminally differentiated cells that extend long axons to their target tissues. More than half of all DRG neurons are unmyelinated, extend axons to the skin, and have nociceptive properties. In many studies on peripheral neuropathies and neuropathic pain, rat or mouse primary DRG neurons are used. However, obtaining these cells in sufficient quantities to perform high-throughput drug screening is nearly impossible. An alternative is to use neuronal cell lines derived from neuroblastomas or immortalized neural crest precursor/stem cell lines (Rao and Anderson, 1997). However, these approaches have caveats for neuropathic pain research and drug screening. These cells are often heterogeneous and retain the potential to differentiate into multiple neuronal and nonneuronal cell types in a mixed environment. Furthermore, they are not likely to respond to drugs in a consistent manner because of this heterogeneity in culture. In contrast, the nociceptive DRG neuronal cell line that we developed is clonal; therefore, all of the cells are similar to each other and in biological assays behave in a predictable manner. Furthermore, 50B11 cells can be grown in large quantities, differentiated into nociceptive neurons easily, and used in high-throughput drug screening.
The 50B11 cell line is likely to be generated from a nociceptive neuron with potential to differentiate into either a peptidergic neuron with NGF dependency or a nonpeptidergic neuron with GDNF dependency. During early development, all future nociceptive neurons are dependent on NGF and express markers for NGF-dependent neurons (p75 and Trk-A) (McMahon et al., 1994), but a subgroup switch dependency and express markers of GDNF-dependent neurons such as IB4 (Molliver et al., 1997). The 50B11 line retains this bipotentiality because in the presence of a given neurotrophic factor, it upregulated the appropriate receptors. Furthermore, the cells extended longer neurites in the presence of either NGF or GDNF (data not shown).
The 50B11 line is clearly a nociceptive neuronal line as evidenced by the expression of TRPV-1 and response to capsaicin. Furthermore, we examined the expression of sodium channels that are present in sensory neurons and implicated in generation of neuropathic pain (reviewed in Ekberg and Adams, 2006). Voltage-gated sodium channels NaV 1.4 and NaV 1.7 were expressed at high levels, but the NaV 1.8 was expressed at low levels in 50B11 cells as measured by real-time reverse transcriptase–PCR (data not shown). Interestingly, differentiation in the presence of forskolin and NGF upregulated the expression of NaV 1.4 and NaV 1.8, but differentiation in the presence of GDNF and forskolin had no significant effect. Some of these channels have been implicated in neuropathic and inflammatory pain (Akopian et al., 1999; Fjell et al., 1999; Yang et al., 2004). Presence of these channels on the 50B11 increases their utility for neuropathic pain research, but the exact utility of the expression of these channels on the 50B11 cells remains to be explored.
To immortalize rat DRG neurons, we used a two-step transformation process. Although combination of SV40 large T-antigen and hTERT had been used previously to immortalize primary airway epithelial cells (Lundberg et al., 2002), this approach had not been applied to generation of immortalized cell lines from terminally differentiated cells such as neurons. To increase our transfection efficiency and generation of transformed neurons, we used a different approach and performed multiple electroporations before adding the selection antibiotic to the media. We obtained multiple clones and characterized one in detail. We were able to grow this cell line, 50B11, through multiple doublings (well over 300) without loss of differentiation potential. Stocks of cells from early and late passages had similar properties in terms of their differentiation potential. This method of immortalization may be better suited for difficult-to-immortalize cell populations such as terminally differentiated neurons and human cells. In fact, we now have used the technique to immortalize human fetal astrocytes and Schwann cells (data not shown).
Differentiation into neurons with nociceptive properties was accomplished by using forskolin. During development, DRG neurons express high levels of cAMP but downregulate it after they mature and their axons reach target tissues (Weill, 1986). Forskolin has been used to elevate intracellular cAMP levels and induce differentiation of a variety of cell types (Grega and Macdonald, 1987). Elevated cAMP levels in the DRG line could be accomplished by methods other than forskolin, but we chose forskolin mainly because of ease of use and relatively lower cost compared with other choices such as membrane permeable di-butryl-cAMP. This is an important issue to consider in designing assays for HTS. Furthermore, we chose an assay that is also easy to scale up for HTS using robotics. Measurement of cellular ATP levels using the luciferase-based assay is suitable in multiple ways. First, one can use it as a measure of cellular health and cell numbers as ATP levels in cells correlate with the number of healthy cells (Crouch et al., 1993). Second, the assay is simple to administer with no wash steps involved. It requires addition of reagents into the wells twice, once to lyse the cells and second to add reagents for the luminescence. This improves reproducibility of the assay and will reduce the number of multiplicates needed during drug screening.
In summary, we have developed an immortalized DRG sensory neuronal line with nociceptive properties suitable for high-throughput drug screening for peripheral neuropathies and neuropathic pain. The transfection and selection methods we developed can be used to generate other neuronal populations, including neurons from human tissues such as brain, spinal cord, dorsal root sensory ganglia, or autonomic ganglia.
Acknowledgments
This work was supported by National Institutes of Health grants RO1NS43991 and RO1NS47972 from National Institute of Neurological Disorders and Stroke and PO1MH70056 from NIMH. We thank Chunhua Zhou and Hong Liang for technical help.
References
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5:143–149. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- Ekberg J, Adams DJ. Neuronal voltage-gated sodium channel subtypes: key roles in inflammatory and neuropathic pain. Int J Biochem Cell Biol. 2006;38:2005–2010. doi: 10.1016/j.biocel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Fjell J, Cummins TR, Fried K, Black JA, Waxman SG. In vivo NGF deprivation reduces SNS expression and TTX-R sodium currents in IB4-negative DRG neurons. J Neuro-physiol. 1999;81:803–810. doi: 10.1152/jn.1999.81.2.803. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Humet M, Planells-Cases R, Gomis A, Caprini M, Viana F, De La Pena E, Sanchez-Baeza F, Carbonell T, De Felipe C, Perez-Paya E, Belmonte C, Messeguer A, Ferrer-Montiel A. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc Natl Acad Sci USA. 2002;99:2374–2379. doi: 10.1073/pnas.022285899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega DS, Macdonald RL. Activators of adenylate cyclase and cyclic AMP prolong calcium-dependent action potentials of mouse sensory neurons in culture by reducing a voltage-dependent potassium conductance. J Neurosci. 1987;7:700–707. doi: 10.1523/JNEUROSCI.07-03-00700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Chander B, Hasan C, Griffin JW, McArthur JC, Hoke A. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol. 2003;53:57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21:4577–4586. doi: 10.1038/sj.onc.1205550. [DOI] [PubMed] [Google Scholar]
- Masip I, Ferrandiz-Huertas C, Garcia-Martinez C, Ferragut JA, Ferrer-Montiel A, Messeguer A. Synthesis of a library of 3-oxopiperazinium and perhydro-3-oxo-1,4-diazepinium derivatives and identification of bioactive compounds. J Comb Chem. 2004;6:135–141. doi: 10.1021/cc030002q. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in sub-populations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Sahenk Z. Clinical practice. Painful sensory neuropathy. N Engl J Med. 2003;348:1243–1255. doi: 10.1056/NEJMcp022282. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Rao MS, Anderson DJ. Immortalization and controlled in vitro differentiation of murine multipotent neural crest stem cells. J Neurobiol. 1997;32:722–746. doi: 10.1002/(sici)1097-4695(19970620)32:7<722::aid-neu7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Steiner JP, Connolly MA, Valentine HL, Hamilton GS, Dawson TM, Hester L, Snyder SH. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 1997;3:421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Appendino G. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse? J Med Chem. 2004;47:2717–2723. doi: 10.1021/jm030560j. [DOI] [PubMed] [Google Scholar]
- Weill CL. Cyclic nucleotide content of ciliary and dorsal root ganglia during embryonic development in the chick. Brain Res. 1986;391:305–309. doi: 10.1016/0165-3806(86)90298-1. [DOI] [PubMed] [Google Scholar]
- Wolfe GI, Trivedi JR. Painful peripheral neuropathy and its nonsurgical treatment. Muscle Nerve. 2004;30:3–19. doi: 10.1002/mus.20057. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]