Abstract
The accumulation of damaged or postsynthetically modified proteins and dysregulation of inflammatory responses and angiogenesis in the retina/RPE are thought be etiologically related to formation of drusen and choroidal neovascularization (CNV), hallmarks of age-related macular degeneration (AMD). The ubiquitin proteasome pathway (UPP) plays crucial roles in protein quality control, cell cycle control and signal transduction. Selective degradation of aberrant proteins by the UPP is essential for timely removal of potentially cytotoxic damaged or otherwise abnormal proteins. Proper function of the UPP is thought to be required for cellular function. In contrast, age- or stress induced- impairment the UPP or insufficient UPP capacity may contribute to the accumulation of abnormal proteins, cytotoxicity in the retina, and AMD. Crucial roles for the UPP in eye development, regulation of signal transduction, and antioxidant responses are also established. Insufficient UPP capacity in retina and RPE can result in dysregulation of signal transduction, abnormal inflammatory responses and CNV. There are also interactions between the UPP and lysosomal proteolytic pathways (LPP). Means that modulate the proteolytic capacity are making their way into new generation of pharmacotherapies for delaying age-related diseases and may augment the benefits of adequate nutrition, with regard to diminishing the burden of AMD.
Keywords: Protein quality control, signal transduction, aging, oxidative stress, inflammation, retina degeneration, macula, proteolysis
Introduction
The ubiquitin proteasome pathway (UPP) is a highly selective proteolytic system that plays crucial roles in protein quality control, cell cycle control, proliferation, development, signal transduction, transcriptional regulation, receptor down-regulation, and synaptic plasticity [1–12]. The observation that the retina, like many other tissues, accumulates damaged proteins upon aging, particularly in disease- related drusen [13–18], indicates that protein quality control is essential for retina heath. In this review we discuss the role of the UPP in retinal protein quality control and its implications in pathogenesis of age-related macular degeneration (AMD). Because of established crucial roles for the UPP in organogenesis and homeostasis, we also review the functions of the UPP in regulating signal transduction and responses to oxidative stresses in the retina/RPE as well as relationships to inflammatory response and choroidal nevasuclarization (CNV).
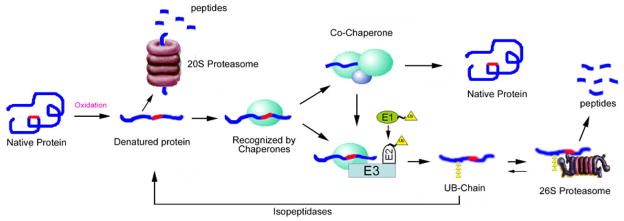
The simplest description of the UPP is as a two step process: identification of a substrate by ubiquitination and then degradation or processing of the ubiquitinated protein. The UPP begins with the attachment of a ubiquitin to a substrate protein [19, 20]. Ubiquitin is a highly conserved 76–amino acid polypeptide. Covalent attachment of ubiquitin to the protein substrate proceeds via a three-step cascade mechanism [8]. As illustrated in Figure. 1, ubiquitin is first activated by the ubiquitin-activating enzyme, E1, via formation of a high-energy thiol ester bond between C-terminal glycine residue of ubiquitin and an internal cysteine residue of E1. Next, one of dozens of ubiquitin-conjugating enzymes, E2s, transfers the activated ubiquitin, also via a ubiquitin thiol ester intermediate, to the substrate that is specifically bound to a member of the ubiquitin-protein ligase family, E3s. The E3 catalyzes the formation of an isopeptide bond between a carboxyl group at the C-terminus of ubiquitin and an ε-amine group of the substrate. In rare cases ubiquitin is transferred to the amino terminus of substrates, forming a peptide bond. There are two classes of E3s. One is called HECT, or homologous to the E6-associated protein carboxyl terminus of E6AP. Another is called RING E3, after really interesting gene. The latter often have multisubunit structures.
Figure 1. The Ubiquitin Proteasome Pathway.
Ubiquitin is activated in an ATP-requiring reaction that results in a Ub–“thiol ester”–of E1. The Ub is then transferred to E2 also as a thiol ester. In the presence of E3, substrate proteins have multiple ubiquitins attached. These conjugates are recognized and (usually) degraded in an ATP-dependent manner by the 26S proteasome. Isopeptidases and ubiquitin C-terminal hydrolases release ubiquitins. The ubiquitin is usually reused.
There are two genes in the human genome that encode different isoforms of E1 and each form has a distinct preference for E2s [21–24]. There are at least 37 genes in the human genome that encode distinctive E2s [25]. The number of the genes encoding E3s is over one thousand [26, 27]. The diversity of E2s and E3s and the combinatorial possibilities of various E2 and E3 in various cellular contexts provide the molecular basis for the exquisite substrate specificity of the UPP.
For efficient targeting of the substrate to the proteasome for degradation, multiple ubiquitins are often conjugated to the initial ubiquitin moiety to form polyubiquitin chains [12–14]. The first ubiquitin is conjugated to the ε-amino group of an internal lysine residue of the target protein. In the process of formation ubiquitin chains, additional ubiquitins are progressively conjugated to an internal lysine residue or the N-terminus of the previously conjugated ubiquitin molecule. Ubiquitin has 7 internal lysine residues. Together with the amine group at the N-terminus there are 8 possible positions for the second ubiquitin to attach. For the third ubiquitin, there are 15 possible positions of attachment. Thus, the structures of polyubiquitin chains in cells can be highly diverse. Different linkages of the polyubiquitin chains may have specific functions [28–32]. For example, K48-linked ubiquitin chains are often involved in targeting substrate for proteasomal degradation [33, 34] whereas K63-linked polyubiquitin chains are often involved in signal transduction and DNA repair [35, 36].
Whereas some ubiquitinated proteins are not degraded by the proteasome, there are also proteins that are degraded by the proteasome independent of ubiquitination. Both the 26S proteasome and the 20S proteasome can degrade some proteins in the absence of ubiquitination [37–41]. We consider the proteasome-dependent, but ubiquitin-independent protein degradation as a non-canonical UPP [42–48].
Role of the UPP in protein quality control
One of best understood functions of the UPP is for protein quality control: by selective degradation of aberrant proteins [49–52]. Both canonical and non-canonical UPP participate in the protein quality control process [9, 11, 42–48, 53–63]. Protein quality control is crucial because it prevents the accumulation of potentially toxic unfolded or misfolded proteins. Protein quality control is a post-translational process which involves folding of newly synthesized proteins and either refolding or degradation of proteins that fail to attain or maintain a native structure. Postsynthetically damaged or obsolete proteins must also be removed, most frequently by the UPP. A growing body of evidence indicates that accumulation of damaged or abnormal proteins is associated with various age-related diseases such as Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, cataract and AMD [16, 59, 64–71].
The accumulation of damaged or abnormal proteins may arise from their enhanced generation or deficiencies in repair or removal. The generation of abnormal proteins may be due to genetic mutation, failure to properly fold, or denaturation induced by various environmental stresses, including heat, oxidation, heavy metals, and light exposure. Unfolded or partially unfolded proteins are structurally unstable, and they tend to aggregate and precipitate. Accumulation of aggregation-prone proteins interferes with normal cellular functions including inhibiting or limiting access to the proteasome [16, 72–74]. To avoid disruption of cellular function by unfolded proteins, organisms have evolved multiple levels of protein quality control systems which recognize proteins with abnormal structures. These protein quality control mechanisms either refold the unfolded proteins to normal conformation or target them for degradation. Whereas molecular chaperones play central roles in the refolding of misfolded or unfolded proteins, intracellular proteolysis is responsible for the removal of unrepaired or obsolete proteins.
The majority of intracellular proteins are either degraded by the UPP or by the lysosomal proteolytic pathway (LPP). Acting independently, these two proteolytic pathways have different substrate specificities [75]. In general, short lived regulatory proteins, including damaged proteins or otherwise partially unfolded proteins are degraded by the UPP. Proteins degraded by the UPP normally contain intrinsic degradation signals (also called degrons). These signals are highly diverse, ranging from unique peptide sequences to specific domains or single amino acid residues at the N-terminus of the proteins [76]. Certain modifications of internal residues such as methionine oxidation and protein carbonyl formation also increase the susceptibility of certain proteins to UPP-mediated degradation [53, 58, 61, 77]. Many membrane-bound or organelle-associated proteins are degraded by the LPP, either through endocytosis or autophagic mechanisms. Recent studies indicate that the UPP and LPP can also work in a coordinated manner, particularly when cells are stressed [16, 75, 78]. For example, oligo/monoubiquitination of receptor and other membrane proteins is required for efficient endocytosis [79, 80].
The selective degradation of abnormal proteins has been known for about 30 years [53, 56, 81, 82], and the role of the UPP in this process has been proposed since the discovery of this pathway [9, 11, 53–59, 61]. For example, various forms of abnormal proteins, such as mutant [83–87], truncated [62], deamidated [60], denatured [88, 89] and oxidized proteins are degraded in a proteasome-dependent manner [42, 53, 58, 61, 77, 90–95]. However, the mechanism by which the UPP distinguishes abnormal from native proteins remains an unsolved mystery. Although the 19S regulatory complex of the 26S proteasome interacts directly with denatured proteins [96–99], most proteins degraded by the proteasome are first tagged by a polyubiquitin chain. In most cases the E2s and E3s are jointly responsible for substrate recognition and ubiquitination [8, 20]. Thus, these enzymes are essential in selecting proteins for degradation. Interestingly, mutations in specific E2s lead to defects in degradation of proteins with different classes of hydrophobic degradation signals, suggesting that the UPP recognizes exposed hydrophobic regions in some proteins [100].
Recent studies indicate that molecular chaperones, in particular Hsp90/Hsp70, are utilized by the UPP to recognize abnormal proteins [88, 89, 101]. As indicated above and in Figure 2, various modifications may alter protein conformation, resulting in the exposure of hydrophobic regions. These are normally buried in properly folded proteins. Such hydrophobic regions may provide a signal for the discrimination of denatured or unfolded proteins from native proteins by Hsp90 or other chaperones [102]. The C-terminus of Hsc70-interacting protein (CHIP), a co-chaperone with ubiquitin ligase activity, can target chaperone-bound substrates for degradation. CHIP contains a C-terminal U-box domain and three N-terminal tetratricopeptide repeat (TPR) domains. The C-terminal U-box domain is responsible for the ubiquitin ligase activity and the N-terminal TPR domains are responsible for the interaction with Hsp70 and Hsp90. By interacting with molecular chaperones (Hsp90 or Hsp70) and ubiquitin conjugating enzymes (Ubc4/5), CHIP catalyzes the ubiquitination of chaperone-bound proteins. Thus, CHIP functions as a bridge or molecular switch between the refolding and degradation arms of the protein quality control system. In addition to CHIP, other chaperone-interacting ubiquitin ligases, such as parkin, also participate in ubiquitinating misfolded or unfolded proteins [103].
Figure 2. Recognition and degradation of oxidatively modified proteins by the UPP.
This model predicts that most, if not all, proteins have intrinsic signals for interaction with molecular chaperones or the ubiquitination system. These signals (red) are hidden in properly folded native proteins and they are not recognized by the protein quality control systems. Upon environmental stress, such as oxidation, proteins may be unfolded with exposure of the recognition signals, such as hydrophobic patches. Some of the oxidized (unfolded) proteins can be recognized and degraded by the 20S proteasome directly whereas others are recognized by molecular chaperones. With the help of other chaperones or co-factors, molecular chaperones are capable of refolding the denatured proteins in an ATP-dependent manner. If the denatured proteins cannot be refolded rapidly, the chaperone bound substrates are ubiquitinated (yellow triangles) by chaperone-interacting ubiquitin-ligases, such as CHIP. The ubiquitinated substrates are recognized and degraded by the 26S proteasome. If the ubiquitinated proteins are deubiquitinated by isopeptidases, the denatured proteins have a second chance to be refolded by molecular chaperones. The parallel/competitive functional relationship between the UPP and molecular chaperones assures the efficiency of the protein quality control systems to remove abnormal proteins.
A common goal of the UPP and molecular chaperones is to prevent the accumulation of abnormal proteins. Both pathways do so by recognizing proteins with abnormal structures. However, to date it is not possible to predict why some denatured proteins are refolded by chaperones whereas others are degraded by the UPP. It appears that cellular protein quality control systems have a triage mechanism. The first level of triage must be identification of the proteins that are damaged and require repair or removal. The quality control system must be able to distinguish between native (properly folded, and assembled) proteins and everything else that might be considered non-native or abnormal, including partially unfolded, misfolded, incorrectly modified, unassembled subunits of complexes, or proteins in incorrect cellular compartments. Once damaged proteins have been identified, a second decision must be made: Is the protein repairable? In principle, chaperones should have the first opportunity to repair of damaged proteins. The proteins that are damaged beyond repair could then be degraded by the UPP.
How is the decision made to refold or to degrade a protein? The ability of the UPP and chaperones to interact with the same damaged or misfolded protein allows these pathways to operate in a parallel or competitive manner to either refold or degrade a given target protein (Figure. 2). We and others propose that the fate of the damaged protein depends on the kinetics and affinity of interaction between the damaged proteins and molecular chaperones or the chaperone component of the UPP as well as upon the availability of the proteolytic machinery [16, 51, 52, 89]. If the chaperone-bound nonnative protein is efficiently refolded with the assistance of other cochaperones, such Hsp40 and p23, it can be removed from the triage system and returned to cellular function. However, if the chaperone-bound nonnative protein is not efficiently refolded by chaperones, the co-chaperone CHIP, which interacts with both chaperones and Ubc4/5 or other E2s, can bring the chaperone-bound proteins to the ubiquitination machinery. If the ubiquitinated proteins are deubiquitinated by isopeptidases, including those associated with the 26S proteasome, the denatured proteins would have a second chance to be refolded by chaperones. Thus, the relative activities of ubiquitination and deubiquitination also participate in determining the fate of denatured proteins [104].
The parallel or competitive mode of action between the UPP and chaperones in the protein quality control process appears to be energy inefficient, resulting in the undesirable destruction of some repairable proteins. This may explain why ~30% of nascent proteins are degraded before they are folded properly, sometimes with dire consequences [105]. For example, premature degradation of inefficiently folded mutant cystic fibrosis transmembrane conductance regulator (CFTR) (ΔF508) causes cystic fibrosis [83]. CHIP is one of the ubiquitin ligases that target the mutant CFTR for ubiquitination and degradation [106, 107]. Blockage of the ubiquitination and degradation of CFTR by inactivation of CHIP allows a pool of CFTRΔF508 to be correctly folded and partially restores cellular function [108]. Similarly, blocking the premature degradation of mutant glucocerebrosidase (N370S or L444P) by proteasome inhibitors enhances its correct folding and partially corrects the loss of function phenotypes of mutant glucocerebrosidase [68]. From a teleological perspective, the cost of destruction of partially functional and functional proteins can be justified by efficient elimination of gain of function toxic mutants or damaged proteins.
Another interesting aspect of regulation of the UPP-chaperone axis is that if one system fails, the other system can serve as the backup. For instance, it has been shown that inhibition of the UPP results in up-regulation of heat shock proteins [109–112]. Conversely, inhibition of chaperone activity also promotes the degradation of Hsp90 client proteins by the proteasome [113]. If both arms of the UPP-chaperone axis fail, the damaged proteins, including ubiquitinated form of damaged or modified proteins, will be shuttled to lysosome for degradation or they may accumulate in the insoluble fraction of the cell [16].
The physiologic requirement for a functional UPP for cellular protein quality control and responses to various stresses is indicated by observations that disruption of UPP in cells leads to hypersensitivity to various environmental insults, such as UV light, oxidation, heavy metals and amino acid analogs [55, 61, 114–119]. All these insults, like other protein precipitation or amyloid diseases, are associated with accumulation of damaged proteins and it is believed that the increased sensitivity to these insults is related to failure to remove the damaged proteins in a timely fashion. Various forms of damaged or modified proteins in the eyes are also selective degraded by the UPP and dysfunction of the UPP may contributes to age-related diseases, such as cataract and AMD [16, 56, 60, 120–122].
Interaction between the UPP and LPP in degradation of glycated proteins
Autophagy is a highly conserved housekeeping pathway that plays a critical role in the removal of damaged intracellular organelles or aberrant proteins that were not efficiently degraded by the UPP [16, 123, 124]. Other lysosomal proteolytic capacities may also be involved in recognition and removal of damaged proteins. In this article, these are included within the abbreviation LPP. Proteins that are cleared by autophagic processes are encapsulated into autophagosomes and make their way into lysosomes for degradation [125–130]. Efficient autophagy is dependent on a balance between the formation and elimination of autophagosomes. Disruption of any part of this pathway will cause autophagic dysfunction. There are few reports regarding autophagy in retina diseases, but it appears that a decline in autophagy and lysosomal activity are associated with retinal aging and AMD [131–133].
Both the UPP and LPP are involved in selective degradation of damaged proteins. Whereas the UPP is most efficient in the degradation of soluble cytoplasmic proteins, the LPP mainly degrades damaged organelles or protein aggregates. However, there is overlap for some substrates between the UPP and LPP. It appears that the UPP and LPP complement and back-up each other in removal of damaged proteins. Such a backup mechanism was indicated in a recent study regarding the degradation of glycated proteins in cultured RPE [16].
Glycation is an adverse posttranslational modification related of eye diseases. Non enzymatic attachment of sugars, or their reactive metabolites such as methyl glyoxal, alters the activity and stability target proteins [134, 135]. Glycated proteins and advanced glycation end products (AGE) accumulate at accelerating rates upon aging in the retina, specifically in drusen [13, 14, 18], as well as in the aging lens [16], and the process is accelerated under diabetic conditions [13, 136]. Consuming high glycemic index diets, one of major risk factor for development of AMD in humans [137–141], also accelerates the accumulation of AGE in eye tissues [15, 16]. Glycation induces irreversible changes in protein structures and leads to loss activity or aggregation and toxicity [136]. We determined the effects of glycation on protein degradation and found that glycated crystallins were resistant to degradation by the UPP and that glycative stress also diminished the activity of several components of the UPP [16]. While it remains to be elucidated how glycation prevents the degradation of UPP substrates, one of the possibilities is that glycation modifies lysine residues of the substrates and blocks their ubiquitination [142].
In cultured RPE, both the UPP and LPP are involved in removal of MGO-modified proteins. Inhibition of the proteasome or lysosome alone only partially slowed the degradation of MGO-modified protein [16]. When both the proteasome and lysosome were inhibited, there is greater accumulation of MGO-modified proteins [16]. Furthermore, when the proteasome was inhibited, ubiquitin-conjugates and MGO-modified proteins were enriched in the lysosome fraction, indicating that glycated and ubiquitinated proteins were delivered to the lysosome when the proteasome is not functional [16]. The interaction between UPP and lysomome was also observed in lens epithelial cells [143]. We found the HNE-modified proteins were ubiquitinated, but degraded by the lysosome. In RPE, inhibition of the proteasome results in accumulation of perinuclear aggregates positive for Hsp70, ubiquitin-protein conjugates and the lysosomal membrane protein LAMP-2 [144]. Our work also indicates that impairment of the UPP in RPE activates the autophagic pathway as indicated by the increase in levels of LC3II upon inhibition of the proteasome, or expression of K6W-ubiquitin [16]. The interaction between UPP and LPP also provides an opportunity to manipulate the activities of these degradation pathways for AMD prevention or treatment. For example, chemical induction of autophagy may help to prevent the accumulation of ubiquitin-containing aggregates. Enhancement of UPP function may also reduce the burden of the LPP and thus promotes the degradation of lipofuscin and dysfunctional organelles, such as damaged mitochondria, by the LPP [133].
The interaction between UPP and LPP and its physiologic significance is summarized in Figure 3. This scheme proposes that under normal homeostatic conditions, the UPP and LPP functions independently or in concert to maintain the proteome homeostasis. The proper functions of the UPP and LPP prevent the accumulation of abnormal proteins or adversely modified proteins, such as AGEs. However, upon chronic stress and aging these capacities are insufficient to maintain the proteome, or are themselves compromised. This results in accumulation of damaged proteins. Degrons, or amino acid residues that encode susceptibility for degradation on substrates may be blocked by adverse modifications. Thus, upon severe chronic stress there is a vicious cycle starting with glycative insult to substrates or proteolytic components, accumulation of cytotoxic damaged proteins, altered interactions between the UPP and LPP. Together these result in compromised cellular function, possibly including the development of AMD-like lesions.
Figure 3. Scheme of interaction between the ubiquitin-proteasome pathway (UPP) and lysosomal proteolytic pathway (LPP) in protein quality control and proposed ramifications of dysfunctions of these proteolytic pathways.
Environment stress, including consuming high glycemic index diet, causes protein denaturation or unfolding, resulting in exposure of degradation signals (deg) and triggering their ubiquitination and degradation. Majority of ubiquitinated proteins are degraded by the proteasome, but some ubiquitinated proteins are degraded by lysosome via autophagy, particularly when the function of the proteasome is impaired or overburdened. The rapid degradation of damaged proteins by concerned action of the UPP and LPP prevents the accumulation of cytotoxic damaged proteins and maintains the homeostasis of the proteome, such as those observed in young and healthy tissues. When the levels of damaged proteins overwhelmed the capacity of the UPP and LPP, damaged protein will accumulate. Some of the damaged protein may cross link forming the higher mass aggregates noted in the figures above. Accumulated oligomerized damaged proteins may impair the proteolytic machinery, further compromising the proteome and resulting in diseases related to accumulation of damaged proteins, such in the retina/RPE of AMD patients.
Role of the UPP in degradation of oxidized proteins
The retina has the highest metabolic rate and oxygen consumption in the body [71, 145]. The high metabolic rate and oxygen consumption is usually accompanied by generation of reactive oxygen species. Chronic exposure to light also increases the production of reactive oxygen species [146, 147]. Age-related accumulation of lipofuscin in RPE is another source of oxidative stress (Also see reviews by Sparrow, Mettu, Handa, Boulton in this issue). Lipofuscin is a mixture of non-degradable protein-lipid aggregates derived from the ingestion of photoreceptor outer segments [148]. A2E is a major fluorophore of lipofuscin and acts as a photosensitizer to generate reactive oxygen species inside the cells upon exposure to blue light [148–150]. An increasing body of literature indicates that oxidative stress is associated with the pathogenesis of AMD [151, 152]. Thus, relationship between oxidative stress and selective degradation of oxidized proteins is of keen interest to AMD researchers.
Literature regarding the degradation of oxidized proteins in retina and RPE is scanty. Most data regarding the degradation of oxidized proteins were collected in lens or cultured lens epithelial cells. H2O2 is a natural oxidant in the eye [153–155] and is often used to model oxidative stress in the eye. Exposure of proteins or cells to H2O2 results in generation of protein carbonyls, disulfide and non-disulfide-linked aggregates [58, 61, 90, 156–158]. We found that oxidized, as compared with native, crystallins are preferentially degraded [90, 156, 157, 159]. Preferential degradation of oxidized proteins was also observed in non-ocular cells. While there is consensus that the proteasome plays a key role in selective degradation of oxidized proteins [47, 48, 61, 63, 77, 92, 93, 160–166], the extent to which ubiquitination is involved in the degradation of oxidized proteins remains controversial. Many publications suggest that suggest that oxidized proteins are degraded by the 20S proteasome independent of ubiquitin [42, 45, 46, 91–95, 165, 167–170]. However, there is also solid evidence that supports the involvement of ubiquitination in degradation of oxidized proteins [53, 58, 61, 77, 90, 104, 171].
Evidence that support the involvement of the UPP in degradation of oxidized proteins includes the preferential ubiquitination of certain oxidized proteins [53, 58, 61, 77, 171] and accumulation of ubiquitinated species of oxidized proteins upon proteasome inhibition [58, 61]. The stabilization of ubiquitinated species of oxidized proteins by proteasome inhibitors indicates that these ubiquitin conjugates are en route to proteasomal degradation. A transient increase in levels of ubiquitinated proteins that correlated with increased intracellular proteolysis during recovery from mild oxidative stress [11, 57] also implies that the UPP is involved in restoring protein homeostasis during recovery from oxidative stress. A recent study provided direct evidence for the involvement of ubiquitination in proteasomal degradation of oxidized proteins [104]. In that study, the authors demonstrated that USP14, a proteasome-associated deubiquitinating enzyme, is a negative regulator of UPP by its trimming of the ubiquitin chain of ubiquitinated substrates. They further demonstrated that inhibition of USP14 not only promoted the degradation of classic substrates of the UPP, but also promoted the clearance oxidized proteins and enhanced the resistance to oxidative stress [104], indicating that the UPP is involved in targeting oxidized proteins for degradation.
Glutathiolation is another type of oxidative damage. Glutathiolation results in formation of mixed disulfides in response to oxidative stress [172]. Glutathiolation is reversible when cellular reducing potential is restored. Glutathiolated protein was degraded more rapidly than unmodified protein by the UPP [63]. Consistent with the involvement of ubiquitination in the degradation of glutathiolated proteins, we found that addition Ubc4, a ubiquitin conjugating enzyme, to cell lysates promotes the degradation of glutathiolated γC-crystallin, whereas addition of a dominant negative ubiquitin to cell lysates inhibits the degradation of glutathiolated proteins [63].
While data regarding degradation of oxidized proteins in retina/RPE by the UPP is scanty at present, the conserved nature of functions of the UPP in most tissues examined suggests that comparable phenomena will be obtained with retina samples. Given the prominent etiologic relationship between oxidation, increase in levels of oxidized proteins in the neural retina and RPE, aggregate accumulation, and retinopathy, this should be a high priority [17, 173].
Role of the UPP in retinal development
A growing body of data shows that the UPP plays critical roles in mammalian and fly eye differentiation and development. A role for ubiquitination in neurite outgrowth was presaged by experiments in PC-12 cells [174] and developing Xenopus retinal ganglion cells [175]. The roles for the UPP in axon guidance or steering are also established in mammalian systems [7, 176].
Controlled degradation of transcription factors or signaling molecules by the UPP is essential for proper retinal development. For example, UPP-mediated degradation of microphthalmia-associated transcription factor (Mitf) is required for retina/RPE differentiation. Mitf plays essential roles in proliferation, survival, and differentiation of neural crest-derived melanocytes during retinal development [177, 178]. The degradation of Mitf depends on a specific degron called a PEST sequence [178]
Formation of the retina has been most extensively studied in Drosophila. The fly eye is composed of ~750 unit eyes called ommatidia, arranged in a hexagonal pattern. Each ommatidium has eight photoreceptor neurons and six support cells. During larval life, the eye disc changes from an undifferentiated epithelial sheet of cells to a highly patterned neuroepithelium through a series of ordered differentiation steps. The first cells to be specified are the clusters of photoreceptors, which will each give rise to an ommatidial unit. During late larval and early pupal stages, the photoreceptors retreat below the apical surface of the eye as they recruit the next cell type, the four cone cells, each of which in turn recruit two primary pigment cells. The remaining cells will form the interommatidial lattice of secondary pigment cells, tertiary pigment cells and mechanosensory bristles that separate neighboring ommatidia and set the precise hexagonal pattern of the adult eye. In order to allow for proper alignment following primary pigment cell differentiation, approximately 30% of the secondary and tertiary pigment cells must be removed by apoptosis. If these excess cells are not eliminated, the adult eye appears roughened because ommatidial units are separated by a varying number of cells.
The apoptotic editing of retinal cells is a highly regulated process. Many mutants which cause abnormal apoptosis and abnormal eyes are found to be related to aberrations in UPP function, indicating that the UPP plays an important role in regulating these apoptotic pathways. Some members of the inhibitors of apoptosis (IAP) are members of the E3 family and provide a critical barrier to apoptosis by binding to and inactivating caspases. For example, the drosophila IAP, DIAP, is an E3 that blocks apoptosis by promoting the ubiquitination and degradation of Dronc, an upstream caspase [179]. Caspase-mediated cleavage of DIAP1, at position 20 converts the stable, longer DIAP1 into the highly unstable, N-terminal Asn-bearing, DIAP1 [180]. An SCF is involved in degradation of DIAP and the F-box for this degradative process appears to be Drosophila Morgue [181, 182]. Reaper (Rpr) and Grim, but not Hid, also promote the degradation of DIAP1 in vivo [182]. Thus, the degradation of DIAP1 is mediated through sequential caspase activity and subsequently N-end rule-dependent UPP activity. This coordinated mutual destruction of DIAP and associated active caspases is essential for suppression of apoptosis.
Another family of E3s involved in retinal development includes the widely conserved SINA (seven in absentia) protein and its mammalian orthologs (Siah, seven in absentia homolog). These E3s facilitate degradation of a wide range of cellular proteins, including β-catenin, a protein with dual roles in development. β-catenin is a cell junction protein thought to link the actin cytoskeleton to cell–cell adhesion, and also a nuclear protein that modulates transcription in response to the Wnt signaling pathway [183, 184]. Over expression of the Xenopus homolog Xsiah-2 during development results in a small eye characterized by a reduced size of the lens, the retina, and the pigmented epithelium.
As indicated above, ubiquitination can also be a signal for endocytosis. The Notch receptor is part of an intercellular signaling pathway which is required for proper eye development [185]. The only known ligand for Notch expressed in the pupal eye is Delta. Neuralized acts as an E3, triggering endocytosis of Delta, and inhibition of Drosophila neurogenesis [186, 187]. Delta is expressed at high levels in cone cells and at lower levels in primary pigment cells and in some lattice cells [188]. Whereas polyubiquitination usually results in degradation of substrates, Delta appears to be monoubiquitinated at the cell surface [186, 187]. Such monoubiquitination is consistent with Delta being endocytosed and delivered to lysosomes [79]. Planar cell polarity (PCP) is a common feature in many epithelia, reflected in cellular organization within the plane of an epithelium. In the Drosophila eye, Frizzled (Fz)/PCP signaling induces cell-fate specification of the R3/R4 photoreceptors through regulation of Notch activation in R4. Neuralized is asymmetrically expressed within the R3/R4 pair and the asymmetric expression is controlled in a Fz/PCP-dependent manner. The Fz/PCP-dependent neuralized expression in R3 ensures the proper R3/R4 specification [189].
Several additional E3s appear to be involved in signaling pathways which control eye development. Hedgehog and Decapentaplegic pathways direct the progressive differentiation of the eye imaginal disc. Levels of Hedgehog and Decapentaplegic are controlled by the transcription factor Cubitus interruptus (Ci). A HECT domain E3 regulates levels of Hedgehog and Decapentaplegic by regulating levels of Ci [190]. Levels of Ci, along with Arm, and CycE are also regulated by the SCF E3, with a Cul1 backbone [191]. In cells anterior to the morphogenetic furrow, Ci proteolytic processing requires the activity of the Nedd8-modified, Cul1-based SCFSlimb E3 complex. Nedd8 is a ubiquitin-like protein and is involved in regulating SCF E3 function by modifying SCF components or Ubc3. In cells posterior to morphogenetic furrow, Ci degradation is controlled by a mechanism that requires the activity of Cul3, another member of the Cullin family. This posterior Ci degradation mechanism is activated by Hedgehog signaling. Cul3 suppresses Hedgehog signaling through downregulating the level of Ci. High-level Hedgehog signaling promotes Cul3-dependent Ci degradation, leading to the downregulation of Hedgehog signaling. This process is manifested in controlling cell proliferation during Drosophila retinal development. Consistent with a role for Cul3 in controlling levels of Ci and eye development, Cul3 mutation resulted in an accumulation of Ci and an increase in the population of interommatidial cells. This is corroborated by the finding that the phenotype of Cul3 mutation can be suppressed by depleting endogenous Ci [192].
Deubiquitinating enzymes or isopeptidases also play critical roles in retinal development. The first deubiquitinating enzyme found in retina is a product of the Uch-L1 gene, also known as PGP 9.5 [193–195]. Uch-L1 also exhibits an ubiquitin ligase activity [196]. The two opposing enzymatic activities of ubiquitination and deubiquitination control the degradation of α-synuclein and affect Parkinson’s disease susceptibility [197]. However, the physiological role for Uch-L1 in retina remains to be defined.
Another deubiquitinating enzyme, Uch-L3, displays 52% amino acid identity to Uch-L1 [198]. A murine Uch-L3 deletion mutant displays retinal degeneration, muscular degeneration, and mild growth retardation. In the normal retina, Uch-L3 was enriched in the photoreceptor inner segment. Curiously, the retina of Uch-L3-deficient mice showed no significant morphological abnormalities during retinal development. However, prominent retinal degeneration became manifested after 3 weeks of age with massive apoptosis of photoreceptors. Retina degeneration in Uch-L3 deficient mice was associated with an increase in immunoreactivities for manganese superoxide dismutase, cytochrome c oxidase I, and apoptosis-inducing factor in the inner segment, indicating Uch-L3 deficient may trigger mitochondrial oxidative stress [199].
Deubiquitinating enzymes are also involved in establishing the number of photoreceptors as well as in guiding the photoreceptor neuron projection to different synaptic layers in the brain. During eye development in Drosophila, axons from photoreceptor neurons 1–6 terminate in the lamina, while axons from photoreceptor neurons 7 and 8 pass through the lamina and stop in the medulla. As the photoreceptor neuron axons enter the lamina, they encounter both glial cells and neurons. Mutation of a ubiquitin hydrolase, nonstop, results in altered developmental cues from glial cells and disrupts targeting of photoreceptor neurons 1–6. Particularly, this suggests that ubiquitin-hydrolase activities in glial cells, provide the initial stop signal promoting growth cone termination in the lamina [200].
Fat facets is another essential deubiquitinating enzyme that limits to 8 photoreceptor cells in each facet of the Drosophila compound eye, by preventing the proteasomal degradation of specific substrates such as Liquid facets [201–204] and beta-catenin. Liquid facets (Lqf) or epsin, the eukaryotic homolog, appears to bridge 3 components: the cell membrane, a transmembrane protein to be internalized, and the core endocytic complex during endocytosis [205, 206]. Retention of liquid facets facilitates endocytosis in order to generate an inhibitory signal that prevents ectopic photoreceptor determination. It remains to be determined if neuralized and liquid facets activities are related with respect to functions in endocytosis. The enhanced endocytosis due to deubiquitination by the Fat facets protein is in contrast to enhanced endocytosis noted upon ubiquitination of many plasma membrane proteins [207]. Interestingly, Fam [208] the mouse homolog of Fat facets, can substitute for Fat facets in all of its essential functions in Drosophila [201]. Biochemical experiments implicate Af-6 and beta-catenin as substrates for Fam [209]. Af-6 is a tight junction protein thought to mediate the effects of Ras signaling on cell-cell junctions.
Role of the UPP in visual signaling
The UPP also plays important roles in visual signaling. Light signals are transduced via GTP-binding proteins such as transducin. Transducin and other GTP-binding proteins are essential heterotrimeric (Tα, β, γ) signaling molecules, which dissociate into Tα-GTP and Tβγ upon light activation by heptahelical membrane receptors such as rhodopsin. Ubiquitination of Tγ occurs when it is dissociated from Tα. Although only Tγ is ubiquitinated, the entire Tβγ subunit complex is degraded [210, 211].
Ubiquitination of Tγ is selectively catalyzed by UbcH5 and UbcH7. Interestingly, Tβγ association with the photoreceptor-specific protein phosducin following light-induced Tα, β, γ dissociation blocks Tβγ ubiquitination and its subsequent degradation. Conversely, inhibition of phosducin-Tβγ complex formation by phosphorylation of phosducin (by Ca2+/calmodulin-dependent protein kinase II) restores Tβγ ubiquitination and degradation. Thus, it appears that Tβγ is a substrate of the UPP and that phosducin serves to protect Tβγ following the light-dependent dissociation of Tα, β, γ [211].
These observations suggest a UPP-dependent mechanism in which the cycle of phosducin phosphorylation/dephosphorylation regulates the light sensitivity of the photoreceptor by controlling the amount of transducin available for activation. Since formation of the phosducin-T complex reduces the availability of free Tβγ for re-association with Tα GDP, (a requirement for subsequent transducin activation), such a mechanism might be important for photoreceptor light adaptation, during which potentially saturating levels of light input to photoreceptor cells are countered by desensitization [212]. A potential problem with this hypothesis is that most phosducin is localized not in the outer segments of photoreceptors, where rhodopsin is present and where phototransduction occurs, but in the inner segments, which are primarily responsible for the metabolic functions of the cell. This hypothesis and these data may be reconciled by the observation that upon continuous illumination, up to 90% of both Tα and Tβγ translocate from the photoreceptor outer segment to the inner segment [213].
Role of UPP in regulating signal transduction
Efficient and proper cell signalling are essential for retina health. One of the most important functions of the UPP is to regulate intracellular signaling process by conditional degradation of regulators of signaling pathways. [122, 214–217]. The signal pathways that are controlled by the UPP include, but are not limited to, Notch pathway [218, 219], Wnt/PCP pathway [220, 221], NF-κB pathway [216, 222], STAT pathway [223, 224], MAP kinase signal pathways [225–227] and hypoxia-induced signaling pathway [228, 229]. Proper regulation of these signal pathways is essential for development and visual functions. Dysregulation of these signal pathways may leads to retinal degeneration.
Role of the UPP in regulating NF-κB signaling pathway
Inflammation appears to be involved in the pathogenesis of AMD. NF-κB is a master regulator of genes that control immune and inflammatory responses [230] (see reviews by Gorin, Handa, Sparrow). Relationships between the UPP and the NF-κB pathway have been extensively investigated. The UPP is required for both activation and termination of this signal pathway [122].
There are several NF-κB proteins and all share an approximately 300-amino-acid Rel homology domain (RHD) that regulates DNA binding, nuclear localization and dimerization [231]. NF-κB family members form homo- and heterodimers, including p65/RelA, c-Rel, RelB, p105/p50 and p100/p52. NF-κB proteins are sequestered in the cytoplasm as latent complexes by inhibitory proteins, or I-κBs, that prevent NF-κB nuclear translocation and DNA binding [232]. Members of the I-κB family, consisting of I-κBα, I-κBβ, I-κBε, NF-κB1, NF-κB2, I-κBζ (also known as MAIL) and Bcl-3, all contain a series of ankyrin repeat domains that mediate binding with NF-κB subunits [233]. The NF-κB1 and NF-κB2 proteins, also known as p105 and p100, respectively, are precursor forms of the p50 and p52 NF-κB subunits [225].
There are two unique NF-κB signaling pathways, termed canonical and noncanonical. These have distinct biological roles [234]. The canonical NF-κB pathway plays an important role in innate and adaptive immunity and cell survival. In this pathway proinflammatory cytokines such as TNF-α or IL-1β, or pathogen-derived components such as bacterial lipopolysaccharide (LPS), trigger the rapid nuclear translocation of NF-κB subunits [230, 234]. A wide variety of stimuli activate canonical NF-κB signaling, all of which converge at the IκB kinase (IKK), consisting of catalytic subunits IKKα and IKKβ and the regulatory subunit IKKγ (also known as NEMO) [235, 236]. IKK phosphorylates I-κB proteins, triggering their ubiquitination and degradation by the proteasome, thus allowing NF-κB to enter the nucleus and activate stimulus-specific gene programs [237].
Ubiquitination is also involved in activation of IKK. For example, during TNFα-induced NF-κB activation, TNFα binds to TNF receptor 1 (TNFR1) and results in ubiquitination of receptor-interacting protein 1 (RIP1) [238, 239]. TNF-receptor-associated factors (TRAF) are a class of ring finger E3s that play an important role in IKK activation. Activated TRAFs function as ubiquitin ligases (E3) for RIP1 ubiquitination through K63-linked polyubiquitin chain [240, 241]. A20 acts the deubiquitinating enzyme to disassemble K63-linked polyubiquitin chain. The polyubiquitin chains of ubiquitinated RIP1 recruit IKKγ/Nemo in the IKK complex [236, 241, 242]. This ubiquitination requires the dimeric E2 enzyme complex Ubc13/Uev1a as well as the transforming growth factor receptor-β-activated kinase 1 (TAK1) and its regulatory subunits TAB1 and TAB2 [35].
TRAF6 also undergoes oligomerization and K63-linked polyubiquitination. This leads to the recruitment of the TAB1-TAB2-TAK1 complex and subsequent activation of TAK1, which in turn activates IKKβ by phosphorylation [243, 244]. The activated IKK complex phosphorylates I-κBα at two sites (32 and 36) [245], triggering its ubiquitination and degradation [246–248].
UPP activity is also required for the regulation of the noncanonical NF-κB pathway. The MAP 3 kinase MAP3K14, also known as NF-κB inducing kinase (NIK) is a central player in the noncanonical pathway [249]. A multi-subunit E3 ubiquitin ligase complex consisting of TRAF2, TRAF3, cIAP1 and cIAP2 promotes the degradation of NIK, thus, maintaining it at extremely low levels in most cell types [250]. If NIK is not degraded in time, it phosphorylates IKKα, and triggers the proteasome dependent processing of NF-κB2 [251]. NF-κB2 is processed by the proteasome to generate p52, which together with RelB regulates a distinct subset of target genes [234].
In addition to regulating immune and inflammation responses, the NF-κB pathway is essential for survival. Many pro-survival genes, such as survivin, Bcl-2, and inhibitors of apoptosis, are under the control of NF-κB [252–254]. The activation of NF-κB in cultured ocular cells also requires UPP activity [121, 122, 255, 256]. We found that sustained oxidative stress impairs UPP function and inhibits NF-κB activation [256]. Inhibition of NF-κB activation during recovery from transient oxidative stress reduces the cell viability [256]. Inhibition of the UPP in RPE also suppresses the expression of NF-κB-regulated genes, such as MCP-1 and IL-6 [121, 257].
The role of the UPP in regulating expression of angiogenic factors
While establishing and maintaining blood flow in the inner and outer retina is essential for retinal health, unscheduled angiogenesis is a major problem, particularly in the aging or diseased retina. The hypoxia inducible factor (HIF) signaling cascade controls vasculature adaptations to hypoxia, including the formation of new blood vessels. HIF is an α-heterodimer that was first recognized as a DNA-binding factor that mediates hypoxia-inducible activity of the erythropoietin 3′ enhancer [258, 259]. In addition, the HIF signaling pathway is a key regulator of many other processes, including transcription of an array of pro-angiogenic factors, such as VEGF, TGF-β3, and various components of glucose transport and glycolysis. These are generally thought to be exploited to overcome vascular insufficiency [260–262]. Both the HIF-α and HIF-α subunits exist as a series of isoforms encoded by distinct genetic loci. HIF-α subunits are constitutive nuclear proteins, whereas HIF-α subunits are inducible by hypoxia. While regulation of HIF-α can occur at the mRNA level [258], it is primarily regulated through post-translational modification and UPP-mediated degradation of HIFα. Oxygen-dependent hydroxylation at two prolyl residues (Pro402 and Pro564 in human HIF-1α) mediates its interactions with E3, von Hippel–Lindau (VHL), which targets HIF-1α for rapid proteasomal degradation [263–265]. In addition to VHL, a recent study showed that CHIP, another E3, is also involved in degradation of HIF-1α in RPE. But CHIP-mediated ubiquitination and degradation of HIF-1α is independent of prolyl hydroxylation [266].
Deubiquitinating enzymes are also involved in regulating the stability of HIF-1α by removing ubiquitin from the substrate. For example, VHL-interacting deubiquitinating enzyme 2 (VDU2) interacts with HIF-1α and specifically deubiquitinates and stabilizes HIF-1α [267]. Thus, overexpression of VDU2 increases expression of HIF-1α targeted genes, such as VEGF. The balance between the VHL-mediated ubiquitination and VDU2-mediated deubiquitination of HIF-1α provides another level of control for HIF-1α stabilization.
Failure of degradation of HIF-1α increases the expression and secretion of VEGF and other pro-angiogenesis factors and abnormal angiogenesis, such as those observed in AMD and diabetic retinopathy [121, 268, 269]. The discoveries regarding HIF-1α signaling, together with new drugs that can control ubiquitination, deubiquitination and degradation provide new avenues for AMD therapy [270].
Redox regulation of UPP activity in eye
The capacity of the UPP to degrade proteins in cells or tissues is altered in response to environmental insults, including oxidative stress. It has been demonstrated in several cell types that exposure to various forms of oxidative stress results in a transient increase in the intracellular degradation of both short-lived and long-lived cellular proteins. An increase in substrate availability is one reason for the increased intracellular protein degradation in response to oxidative stress. Enhancement of ubiquitination and degradation capacities in response to oxidative stress is another reason for the increased intracellular protein degradation [11, 57, 91, 119, 167, 271, 272]. We demonstrated that both E1 and E2 activities of the ubiquitin conjugation system increase in response to mild oxidative stress [11, 58, 173] and Pickering et al demonstrated that the levels and activity of the proteasome, including immunoproteasome, increased upon adaption to mild oxidative stress [272]. The AMD-related increase in immunoporteasome in retina might be also related to oxidative stress [273].
Whereas mild oxidative stress up-regulates the activity of the UPP, all components of the UPP (E1, E2s, some E3s, proteasome and deubiquitinating enzymes) can be targets of extensive oxidative insults because they all have sulfhydryl groups as critical features of their active sites [10, 173, 274–280]. Exposure to oxidative stress rapidly depletes reduced glutathione (GSH) and elevates the levels of oxidized glutathione (GSSG). GSSG reacts with the cysteine residues in the active site of E1 and E2, forming mixed disulfide bonds, and blocking their binding to ubiquitin [10]. In addition, other types of modifications, such as S-nitrosylation, can inactivate these enzymes [275]. When oxidants are removed from the medium and cells are allowed to recover, the GSH level is restored, and the levels of ubiquitin conjugates, together with the ability to form the conjugates are also restored [10, 57, 274]. Moreover, after several hours of recovery from oxidation, there is hyperactivation of E1 and increased ubiquitination and proteolysis relative to levels found in untreated cells [11, 57, 281]. Thus, intracellular proteolysis can show a biphasic response to oxidative stress. Mild to moderate oxidative stress increases susceptibility of proteins to degradation and enhances the proteolytic capacity, therefore, promoting intracellular protein degradation. In contrast, extensive but non lethal oxidative stress impairs the functions of the UPP reduces intracellular protein degradation [10, 11, 57, 91, 167, 256, 274] and induces intracellular accumulation and aggregation of damaged or abnormal, potentially cytotoxic, proteins.
The regulation of ubiquitin conjugation activity by redox status was corroborated by treatment of RPE cells with diamide and these experiments also led to a new definition for an oxidative stress message. Diamide specifically oxidizes sulfhydryl groups and results in an elevation of GSSG/GSH ratios. It also caused dose-dependent inhibition of ubiquitination and ubiquitin-dependent protein degradation [274]. Thus, the GSSG/GSH ratio is the molecular redox message that regulates the cellular ubiquitin conjugating activity. The down-regulation of ubiquitin conjugating activity upon oxidative stress is due to reversible S-thiolation of E1 and E2 enzymes by increased levels of GSSG. The transient inhibition of the ubiquitin conjugation system by reversible thiolation may also have a protective role: by preventing ubiquitination and degradation of essential, reversibly modified, proteins upon oxidative stress [63]. It may also be perceived that glutathiolation of ubiquitin conjugating enzymes is a protective mechanism, which blocks the cysteine residue from irreversible oxidation and allowing for recovery of activity when redox status is restored.
The proteasome is also a target of oxidative stress. Reactive oxygen species and reactive lipid peroxidation products, such as 4-hydroxynonenal (HNE), impair the proteasome [95, 276–279, 282]. It appears that that the 26S proteasome is more susceptible than the 20S proteasome to oxidative inactivation [277]. The relative susceptibility of the ubiquitination system vs the proteasome to oxidative stress was also compared in RPE [167]. The proteasome is more susceptible to oxidative inactivation than the ubiquitination enzymes in RPE [173]. Sustained exposure to as low as 50 μM H2O2 resulted in 30–50% inactivation of the proteasome, whereas these levels of oxidative stress had no detectable effect on ubiquitin conjugation enzymes and ubiquitination activity. Given that the 26S proteasome is more susceptible to oxidative stress than the 20S proteasome [283], the loss of the peptidase activity of the proteasome upon oxidative stress is most likely due to inactivation of the 26S proteasome. Oxidative inactivation of the 26S proteasome prior to inactivation of the ubiquitination system may explain why levels of endogenous ubiquitin conjugates increase in response to oxidative stress in various types of cells [11, 57, 58, 61, 281, 284–289]. Age-related inactivation or inhibition of the proteasome [170, 290] may also contribute to the increased levels of ubiquitin conjugates in old tissues [284, 285, 291]. Constant light exposure also resulted in increase in levels of ubiquitin conjugates in retina [292], probably due to impairment of the proteasome. Furthermore, extensive oxidative insults may result in non-degradable cross-linked protein aggregates and these may interfere with the functions of the proteasome indirectly [74, 282, 293–297]. This would have the result of insufficient proteasome capacity even if the proteasome per se was not inactivated by the oxidative stress.
Interaction between the UPP and cellular antioxidant systems
Cellular antioxidant systems provide the primary defence mechanisms for cells to cope with oxidative stress via directly quenching reactive oxygen species. Just as the UPP is affected by cellular redox status, the UPP also regulates cellular redox status. Regulation of intracellular redox status by the UPP is mediated by degradation of nuclear factor-E2-related factor 2 (Nrf2), an important transcription factor involved in the transcriptional activation of antioxidant enzymes [298–302](see reviews by Pennesi and Handa in this issue). Nrf2 binds to the antioxidant-response element (ARE) and regulates ARE-mediated expression of antioxidant enzymes, such as catalase, superoxide dismutase, NAD(P)H:quinone oxidoreductase, certain forms of glutathione-S-transferases, and γ-glutamate cysteine ligase regulatory subunit [303–305].
As with many other transcription factors, the abundance of Nrf2 is regulated by UPP-mediated degradation [299]. The Kelch-like ECH-associating protein (Keap1) is a cytosolic inhibitor of Nrf2 [306, 307]. The main function of Keap1 is to serve as an adapter for the cullin 3-dependent E3 [308, 309]. Keap1 binds to Cul3 via its N-terminal BTB/POZ domain and binds to Nrf2 via its C-terminal Kelch domain [310], leading to the ubiquitination and degradation of Nrf2 through the 26S proteasome [298, 307, 311, 312].
Under normal cellular conditions, Nrf2 is constantly ubiquitinated by the cytosolic Keap1/Cul3/Rbx1 complex and degraded by the proteasome. When a cell is exposed to oxidative stress, Nrf2 dissociates from the Keap1/Cul3 complex and translocates into the nucleus, leading to activation of ARE-mediated gene expression [313].
A recent study indicates that a specific ubiquitin conjugating enzyme, UbcM2, acts as a redox sensor and a regulator of Nrf2 activation [314]. Oxidation of the cys-136 residue of UbcM2 increases its interaction with Nrf2 and results in Nrf2 stabilization. When the function of the UPP was impaired or inhibited, Nrf2 accumulated and the ARE is activated [311, 315–317]. The stabilization of Nrf2 with up-regulation of antioxidant enzymes explains why partial inhibition of the UPP results in cytoprotection under certain conditions [311, 315–320]. Nrf2 activation also enhances the expression of proteasome subunits and increases proteasome activity [321]. The activation of Nrf2 may explain why pre-treatment of cultured neocortical neurons with low doses of proteasome inhibitors led to increased, rather than decreased, proteasome activity [322].
In corroboration of essential roles for this antioxidant pathway in the eye, RPE are more susceptible to environmental stress in Nrf2 knockout mice [323, 324] and development of AMD-like lesions at later stage of life [325]. Additional elucidation of the interrelationship between the UPP and antioxidant system will bring new drug targets for AMD and other oxidation-induced diseases.
Impairment or dysregulation of the UPP: implications in AMD pathogenesis
Given the essential roles of the UPP in protein quality control and signaling transduction, it is anticipated that impairment or dysregulation of the UPP in retina/RPE would have several consequences. Impairment of the UPP may contribute to accumulation and aggregation of various forms of damaged proteins, such as those observed in subretinal deposits. However, dysregulated induction of UPP activity may promote the degradation of functional proteins and impair vision functions. For example, inflammation-related degradation of rhodopsin causes deficiency of this critical phototransduction protein and abnormal retinal function [326].
Accumulation of ubiquitin or ubiquitin conjugates is prominent in age-related sub-RPE deposits, i.e. drusen, basal laminar deposits (BLD) and inclusion bodies in retinas in patients with Ataxia type II [292, 327, 328], as well as in ganglion cells in rats with chloroquine retinopathy [329, 330]. The presence of ubiquitin in drusen—without active forms of ubiquitin-processing enzymes—raises the possibility that certain proteins become ubiquitinated within RPE but that further degradation of the ubiquitin-protein complexes does not occur [18, 330–332]. Several “amyloid diseases” are associated with accumulation rather than the timely degradation of ubiquitin conjugates derived from a myriad of, but as yet mostly uncharacterized, protein substrates. Lewy bodies are ubiquitin-containing inclusions in many premature neurological aging syndromes. Studies show that α-synuclein, a neuron specific molecule of unknown functions is a substrate for the UPP and is found within neurofibrilary deposits in brains of patients with Parkinson-dementia and abnormal eye function [333]. This corroborates the concept that limited UPP activity is causally related to the “amyloid diseases”. How these deposits are related to physiologic dysfunction is not known, but it would appear that drusen-related AMD can be included among these age-related “amyloid diseases”.
As in many other tissues, proteasome activity in retina decreases with aging [17, 334]. The age-related decline in proteasome activity may be a consequence of oxidative stress, since the decline in proteasome activity was accompanied by increased levels of oxidatively modified proteins, including nitrotyrosine and 4-hydroxy-2-nonenal (HNE) modified proteins [17]. In a cell culture system we demonstrated that the proteasome in RPE is more susceptible to oxidative stress than the ubiquitin conjugating system. Oxidative stress, including photo-oxidation, is often associated with accumulation of ubiquitinated proteins, both in neural retina and RPE [10, 122, 173, 217, 292]. Impairment of the UPP in RPE not only resulted in accumulation of oxidatively modified proteins [173], but also increased the expression and secretion of pro-angiogenesis and pro-inflammation factors [121, 122, 217]. Impairment of the UPP in RPE resulted in accumulation of HIF-1α and subsequently increased the expression of VEGF and other HIF-regulated genes [121]. The increased VEGF expression and secretion may trigger retinal and choroidal angiogenesis, such as those observed in wet AMD and proliferative retinopathy.
Prolonged inhibition of the UPP in RPE also triggers the expression of IL-8, a potent pro-inflammation and pro-agiogenesis factor [122, 217]. Disruption or up-regulation of NF-κB, p38 MAP kinase or PI3K signaling pathways also appears to be involved in the increased expression of IL-8 upon inhibition of the UPP [122, 217]. The inhibition of NF-κB signaling by proteasome inhibition is also related to the down-regulation of MCP-1, an important monocyte chemotactic protein [121, 335]. MCP-1 deficiency in mice causes AMD-like lesions, such as accumulation of lipofuscin in RPE, photoreceptor atrophy and CNV [336]. Thus, down-regulation of MCP-1 expression upon proteasome inhibition may be a factor for AMD pathogenesis. The relationship between impairment of the UPP and enhanced inflammation is not limited in the eyes. A recent study demonstrated that a mutation in the human proteasome subunit beta type 8 gene (PSMB8) that encodes the immunoproteasome subunit β5i was detected in patients with Nakajo-Nishimura syndrome, a disease with characteristics of chronic inflammation [337]. The β5i mutant is not efficiently incorporated during immunoproteasome biogenesis, resulting in reduced proteasome activity and accumulation of ubiquitinated and oxidized proteins within cells expressing immunoproteasomes [337]. As a result, the level of IL-6 and IFN-γ inducible protein-10 in patient sera is markedly increased. Levels of phospho-p38 and the secretion of IL-6 are increased in cells that harbor this mutant.
Despite much suggestive evidence, direct evidence for UPP impairment in AMD pathogenesis is still lacking. Ethen et al compared proteasome activity in retina from donor eyes with different grades of AMD and found that the chymotrypsin-like activity of the proteasome increased in neurosensory retina with disease progression [273]. Increased proteasome activity was correlated with a dramatic increase in the inducible subunits of the immunoproteasome, suggest the proteasome was transformed in retina of AMD patients, probably due to local inflammation or oxidative stress. Compared to the standard proteasome, the immunoproteasome has higher chymotrypsin activity [338], but the trypsin-like and caspase-like activity of the proteasome are comparable [338]. The transformation of the proteasome may be a mechanism for the increase chymotrypsin-like activity in the retina of AMD patients. Induction of immonoproteasome was also observed in retina and brain upon cytotoxic T lymphocytes induced damage [339]. The up-regulation of immunoproteasome in response to retinal and brain injury implies that the immonoproteasome may have a role in neuronal protection or and/or repair of damage. Consistent with the notion, immunoproteasome deficient RRE are more susceptible to oxidative stress [338].
Taken together, these data imply that age-or stress-related impairment of the UPP may contribute to pathogenesis of AMD and other age-related eye diseases. As illustrated in Figure 4, age-or stress-related impairment of the UPP may be etiologically linked to AMD pathogenesis via multiple UPP-requiring mechanisms.
Figure 4. The hypothesized molecular mechanisms for the potential link between impairment of the UPP in RPE and the pathogenesis of AMD.
Oxidative stress may reduce UPP activity in RPE. Impairment of the UPP in RPE decreases NF-κB and STAT transcription activity by stabilization of I-κB and SOCS, respectively. Impairment of the UPP enhances HIF activity by stabilization of HIFα. Impairment of the UPP also activates p38 and JNK MAP kinases via indirect mechanisms. Changes in activities of these signaling pathways will alter the expression of many genes, such as MCP-1, complement factor H, IL-8 and VEGF. The altered expression of these genes may trigger the development of AMD-related phenotypes, such as local inflammation, activation of the complement system, accumulation of drusen and choroidal neovascularization.
Protecting the UPP from oxidative stress may be a valid strategy for prolonging retina function
Age-or stress-related impairment of the UPP appears to be a contributing factor for the age-related eye diseases, such as AMD (Fig 4). Any means that can preserve or control the functions of the UPP should be beneficial. Since oxidative stress is major cause of AMD and dysfunction of the UPP, adequate dietary intake nutritional antioxidants may protect the UPP from oxidative inactivation (see review by Weikel in this issue). Indeed, we recently found that supplementation of lutein, zeaxanthin or vitamin E to cultured RPE can protect the proteasome from photooxidative inactivation in cultured RPE (Bian et al, manuscript submitted). Lutein and zeaxanthin supplementation also attenuated photooxidation-induced accumulation of ubiquitin conjugates and alteration in inflammatory response, such as expression and secretion of MCP-1, complement factor H and IL-8 (Bian et al, manuscript submitted). The protection of the UPP from oxidative inactivation may be one of mechanisms for the protective effects of lutein, zeaxanthin and other nutritional anti-oxidants in reducing the risk of AMD. Consuming low glycemic index diets would also help to preserve the function of the UPP via decreasing glycative/oxidative stress and reducing burdens of the UPP. In addition to antioxidant nutrients, pharmaceutical activators of the UPP or LPP may also be used to promote the degradation of abnormal proteins and reduce the risk for AMD.
Conclusions and future perspectives
The UPP is involved in regulating many cellular functions, from protein quality control to signal transduction and stress responses. A fully functional UPP is essential for eye development and maintaining the normal functions of the eye. Aging or stress-related impairment of the UPP in the retina/RPE appears to result in accumulation of abnormal or damaged proteins, which contribute to drusen formation and accumulation. Impairment of the UPP also disrupts signal transduction pathways, which in turn, alters inflammation responses and angiogenesis, common features of AMD-related lesions. Any means that can protect the UPP from impairment or reduce the burden of the UPP would be a beneficial for the eye health. Sufficient intake of nutritional antioxidants, such as lutein, zeaxanthin, vitamins C, E and zinc may protect the proteasome from oxidative inactivation and preserve UPP function in the eye, and reduce the risk for AMD. Consuming low glycemic index diets would also help to preserve the function of UPP via decreasing glycative/oxidative stress and reducing burdens of abnormal proteins on the UPP. The interaction between nutrition and UPP, as well as between UPP and other protective mechanisms warrant comprehensive investigation.
Acknowledgments
This work is partially supported by NIH grants EY 011717, EY 013250, EY 021212, USDA AFRI Award 2009-35200-05014, USDA contract 1950-510000-060-01A, AHAF grant M2010038, Dennis L. Gierhart Charitable Gift Fund, and a gift from Alcon laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geng F, Wenzel S, Tansey WP. Ubiquitin and Proteasomes in Transcription. Annu Rev Biochem. doi: 10.1146/annurev-biochem-052110-120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 125(Pt 2):265–75. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 3.Mocciaro A, Rape M. Emerging regulatory mechanisms in ubiquitin-dependent cell cycle control. J Cell Sci. 125(Pt 2):255–63. doi: 10.1242/jcs.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredrickson EK, Gardner RG. Selective destruction of abnormal proteins by ubiquitin-mediated protein quality control degradation. Semin Cell Dev Biol. doi: 10.1016/j.semcdb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Chen ZJ. The role of ubiquitylation in immune defence and pathogen evasion. Nat Rev Immunol. 12(1):35–48. doi: 10.1038/nri3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendotti C, et al. Dysfunction of constitutive and inducible ubiquitin-proteasome system in amyotrophic lateral sclerosis: Implication for protein aggregation and immune response. Prog Neurobiol. doi: 10.1016/j.pneurobio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32(6):1013–26. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 8.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 9.Jahngen JH, et al. The eye lens has an active ubiquitin-protein conjugation system. J Biol chem. 1986;261(29):13760–13767. [PubMed] [Google Scholar]
- 10.Jahngen-Hodge J, et al. Regulation of ubiquitin conjugating enzymes by glutathione following oxidative stress. J Biol chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 11.Shang F, Gong X, Taylor A. Activity of ubiquitin dependent pathway in response to oxidative stress: Ubiquitin activating enzyme (E1) is transiently upregulated. J Biol chem. 1997;272:23086–23093. doi: 10.1074/jbc.272.37.23086. [DOI] [PubMed] [Google Scholar]
- 12.Taylor A. Nutritional and environmental influences on risk for cataract. In: Taylor A, editor. Nutritional and environmental influences on the eye. CRC; New York: 1999. pp. 53–93. [Google Scholar]
- 13.Ishibashi T, et al. Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol. 1998;116(12):1629–32. doi: 10.1001/archopht.116.12.1629. [DOI] [PubMed] [Google Scholar]
- 14.Howes KA, et al. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(10):3713–20. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- 15.Weikel KA, et al. Natural History of Age-Related Retinal Lesions that Precede AMD in Mice Fed High or Low Glycemic Index Diets. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchiki T, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics) Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie JL, Kapphahn RJ, Ferrington DA. Proteasome function and protein oxidation in the aged retina. Exp Eye Res. 2002;75(3):271–84. [PubMed] [Google Scholar]
- 18.Crabb JW, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(23):14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciechanover A, Orian A, Schwartz AL. The ubiquitin-mediated proteolytic pathway: mode of action and clinical implications. J Cell Biochem Suppl. 2000;34:40–51. doi: 10.1002/(sici)1097-4644(2000)77:34+<40::aid-jcb9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 21.Handley-Gearhart PM, et al. Human ubiquitin-activating enzyme, E1. Indication of potential nuclear and cytoplasmic subpopulations using epitope-tagged cDNA constructs. J Biol chem. 1994;269(52):33171–33178. [PubMed] [Google Scholar]
- 22.Handley-Gearhart PM, et al. Rescue of complex temperature-sensitive phenotype of chinese hamster ovary E36ts20 cells by expression of human ubiquitin activating enzyme cDNA. Biochem J. 1994;304:1015–1020. doi: 10.1042/bj3041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang F, et al. Ubiquitin-activating enzyme (E1) isoforms in lens epithelial cells: origin of translation, E2 specificity and cellular localization determined with novel site-specific antibodies. Exp Eye Res. 2001;73(6):827–36. doi: 10.1006/exer.2001.1091. [DOI] [PubMed] [Google Scholar]
- 24.Jin J, et al. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature. 2007;447(7148):1135–8. doi: 10.1038/nature05902. [DOI] [PubMed] [Google Scholar]
- 25.Michelle C, et al. What was the set of ubiquitin and ubiquitin-like conjugating enzymes in the eukaryote common ancestor? J Mol Evol. 2009;68(6):616–28. doi: 10.1007/s00239-009-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695(1–3):55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 28.Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8(6):610–6. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21(8):921–6. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 30.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137(1):133–45. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komander D, et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10(5):466–73. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickliffe KE, et al. K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 2011;21(11):656–63. doi: 10.1016/j.tcb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chau V, et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 34.Thrower JS, et al. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19(1):94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103(2):351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 36.Chiu RK, et al. Lysine 63-polyubiquitination guards against translesion synthesis-induced mutations. PLoS Genet. 2006;2(7):e116. doi: 10.1371/journal.pgen.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami Y, et al. Ornithine decarboxylase is degraded by the 26S proteosome without ubiquitin. Nature. 1992;360:597–599. doi: 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- 38.Tarcsa E, et al. Ca2+-free calmodulin and calmodulin damaged by in vitro aging are selectively degraded by 26 S proteasomes without ubiquitination. J Biol Chem. 2000;275(27):20295–301. doi: 10.1074/jbc.M001555200. [DOI] [PubMed] [Google Scholar]
- 39.Benaroudj N, et al. The unfolding of substrates and ubiquitin-independent protein degradation by proteasomes. Biochimie. 2001;83(3–4):311–8. doi: 10.1016/s0300-9084(01)01244-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Pickart CM, Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22(7):1488–96. doi: 10.1093/emboj/cdg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuzawa S, et al. Method for targeting protein destruction by using a ubiquitin-independent, proteasome-mediated degradation pathway. Proc Natl Acad Sci U S A. 2005;102(42):14982–7. doi: 10.1073/pnas.0507512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacifici RE, Salo DC, Davies KJA. Macroxyproteinase (M.O.P.): a 670 kDa proteinase complex that degrades oxidatively denatured proteins in red blood cells. Free Radic Biol & Med. 1989;7:521–536. doi: 10.1016/0891-5849(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 43.Salo DC, et al. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265(20):11919–27. [PubMed] [Google Scholar]
- 44.Dean RT, Geiseg S, Davies MJ. Reactive species and their accumulation on radical-damaged proteins. Trends in Biological Science. 1993:437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 45.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58(10):1442–50. doi: 10.1007/PL00000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shringarpure R, et al. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278(1):311–8. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 47.Ferrington DA, et al. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2001;276(2):937–43. doi: 10.1074/jbc.M005356200. [DOI] [PubMed] [Google Scholar]
- 48.Balog EM, et al. Site-specific methionine oxidation initiates calmodulin degradation by the 20S proteasome. Biochemistry. 2009;48(13):3005–16. doi: 10.1021/bi802117k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–9. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 50.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51(1):5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27(7):368–75. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 52.McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8(4):303–8. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hershko A, et al. The protein substrate binding site of the ubiquitin-protein ligase system. The Journal of Biological Chemistry. 1986;261:11992–11999. [PubMed] [Google Scholar]
- 54.Hershko A, Eytan E, Ciechanover A. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. The Journal of Biological Chemistry. 1982;257(23):13964–13970. [PubMed] [Google Scholar]
- 55.Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperature, starvation, and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- 56.Taylor A, Davies KJA. Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens. Free Radic Biol & Med. 1987;3:371–377. doi: 10.1016/0891-5849(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 57.Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem J. 1995;307:297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang F, Nowell TR, Jr, Taylor A. Removal of oxidatively damaged proteins from lens cells by the ubiquitin-proteasome pathway. Exp Eye Res. 2001;73(2):229–38. doi: 10.1006/exer.2001.1029. [DOI] [PubMed] [Google Scholar]
- 59.Shang F, Taylor A. Function of the ubiquitin proteolytic pathway in the eye. Exp Eye Res. 2004;78(1):1–14. doi: 10.1016/j.exer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Dudek EJ, et al. Ubiquitin proteasome pathway-mediated degradation of proteins: effects due to site-specific substrate deamidation. Invest Ophthalmol Vis Sci. 2010;51(8):4164–73. doi: 10.1167/iovs.09-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dudek EJ, et al. Selectivity of the ubiquitin pathway for oxidatively modified proteins: relevance to protein precipitation diseases. FASEB J. 2005;19(12):1707–9. doi: 10.1096/fj.05-4049fje. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, et al. Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 2007;48(9):4200–8. doi: 10.1167/iovs.07-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zetterberg M, et al. Glutathiolation Enhances the Degradation of {gamma}C-crystallin in Lens and Reticulocyte Lysates, Partially via the Ubiquitin-Proteasome Pathway. Invest Ophthalmol Vis Sci. 2006;47(8):3467–73. doi: 10.1167/iovs.05-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2(11):E207–9. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 65.Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell. 2001;104(4):581–91. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 66.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 67.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22(11):1427–38. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mu TW, et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134(5):769–81. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balch WE, et al. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 70.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9(10):759–67. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7(11):860–72. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 72.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem. 2008;283(44):29639–43. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A. 2009;106(35):14745–50. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–5. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 75.Korolchuk VI, Menzies FM, Rubinsztein DC. A novel link between autophagy and the ubiquitin-proteasome system. Autophagy. 2009;5(6):862–3. doi: 10.4161/auto.8840. [DOI] [PubMed] [Google Scholar]
- 76.Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9(9):679–90. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwai K, et al. Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: implications for degradation of oxidized proteins. Proc Natl Acad Sci USA. 1998;95(9):4924–8. doi: 10.1073/pnas.95.9.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korolchuk VI, et al. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell. 2009;33(4):517–27. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hicke L. A new ticket for entry into budding vesicles-ubiquitin. Cell. 2001;106(5):527–30. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 80.Saksena S, et al. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32(12):561–73. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Goldberg AL. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin) Proc Natl Acad Sci U S A. 1972;69(2):422–6. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Etlinger JD, Goldberg AL. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977;74(1):54–8. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 84.Benharouga M, et al. COOH-terminal truncations promote proteasome-dependent degradation of mature cystic fibrosis transmembrane conductance regulator from post-Golgi compartments. J Cell Biol. 2001;153(5):957–70. doi: 10.1083/jcb.153.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gelman MS, Kannegaard ES, Kopito RR. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277(14):11709–14. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- 86.Hyun DH, et al. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem. 2003;86(2):363–73. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 87.Hoffman EK, et al. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J Neurol Sci. 1996;139(1):15–20. [PubMed] [Google Scholar]
- 88.Murata S, et al. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2(12):1133–8. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marques C, et al. The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones. FASEB J. 2006;20(6):741–3. doi: 10.1096/fj.05-5080fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang LL, et al. Degradation of differentially oxidized α-crystallins in bovine lens epithelial cells. Expl Eye Res. 1995;61:45–54. doi: 10.1016/s0014-4835(95)80057-3. [DOI] [PubMed] [Google Scholar]
- 91.Grune T, Reinheckel T, Davies KJA. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 92.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB Journal. 1997;11(7):526–34. [PubMed] [Google Scholar]
- 93.Grune T, Davies KJ. Breakdown of oxidized proteins as a part of secondary antioxidant defenses in mammalian cells. Biofactors. 1997;6(2):165–72. doi: 10.1002/biof.5520060210. [DOI] [PubMed] [Google Scholar]
- 94.Pacifici RE, Kono Y, Davies KJA. Hydrophobicity as the signal for selective degradation of hydroxyl radical modified hemoglobin by the multicatalytic protease complex, proteasome. J Biol chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 95.Grune T, et al. Protein oxidation and degradation during postmitotic senescence. Free Radic Biol Med. 2005;39(9):1208–15. doi: 10.1016/j.freeradbiomed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Braun BC, et al. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol. 1999;1(4):221–6. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- 97.Liu CW, et al. Conformational remodeling of proteasomal substrates by PA700, the 19 S regulatory complex of the 26 S proteasome. J Biol Chem. 2002;277(30):26815–20. doi: 10.1074/jbc.M201782200. [DOI] [PubMed] [Google Scholar]
- 98.Strickland E, et al. Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J Biol Chem. 2000;275(8):5565–72. doi: 10.1074/jbc.275.8.5565. [DOI] [PubMed] [Google Scholar]
- 99.Benaroudj N, Goldberg AL. PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone. Nat Cell Biol. 2000;2(11):833–9. doi: 10.1038/35041081. [DOI] [PubMed] [Google Scholar]
- 100.Sadis S, Atienza C, Jr, Finley D. Synthetic signals for ubiquitin-dependent proteolysis. Mol Cell Biol. 1995;15(8):4086–94. doi: 10.1128/mcb.15.8.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meacham GC, et al. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3(1):100–5. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 102.Feldman DE, Frydman J. Protein folding in vivo: the importance of molecular chaperones. Curr Opin Struct Biol. 2000;10(1):26–33. doi: 10.1016/s0959-440x(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 103.Morishima Y, et al. CHIP deletion reveals functional redundancy of E3 ligases in promoting degradation of both signaling proteins and expanded glutamine proteins. Hum Mol Genet. 2008;17(24):3942–52. doi: 10.1093/hmg/ddn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee BH, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467(7312):179–84. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404(6779):770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 106.Younger JM, et al. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126(3):571–82. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 107.Younger JM, et al. A foldable CFTR{Delta}F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J Cell Biol. 2004;167(6):1075–85. doi: 10.1083/jcb.200410065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grove DE, et al. Mechanisms for Rescue of Correctable Folding Defects in CFTR{Delta}F508. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18(9):5091–8. doi: 10.1128/mcb.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat shock response, induction of endoplasmic reticulum chaperones and thermotolerance. J Biol chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 111.Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18(1):30–8. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bian Q, et al. Expression of K6W-ubiquitin in lens epithelial cells leads to upregulation of a broad spectrum of molecular chaperones. Mol Vis. 2008;14:403–12. [PMC free article] [PubMed] [Google Scholar]
- 113.Mimnaugh EG, et al. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3(5):551–66. [PubMed] [Google Scholar]
- 114.Johnson ES, et al. Ubiquitin as a degradation signal. EMBO J. 1992;11:497–505. doi: 10.1002/j.1460-2075.1992.tb05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spence J, et al. A ubiquitin mutant with specific defects in DNA repair and multiubiquitination. Mol Cell Biol. 1995;15(3):1265–1273. doi: 10.1128/mcb.15.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng L, Watt R, Piper PW. Polyubiquitin gene expression contributes to oxidative stress resistance in respiratory yeast (Saccharomyces cerevisiae) Mol Gen Genet. 1994;246:358–362. doi: 10.1007/BF00301072. [DOI] [PubMed] [Google Scholar]
- 117.Jungmann J, et al. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- 118.Tsirigotis M, et al. Sensitivity of mammalian cells expressing mutant ubiquitin to protein damaging agents. J Biol Chem. 2001;11:11. doi: 10.1074/jbc.M109023200. [DOI] [PubMed] [Google Scholar]
- 119.Shang F, et al. Lys6-modified ubiquitin inhibits ubiquitin-dependent protein degradation. J Biol Chem. 2005;280(21):20365–74. doi: 10.1074/jbc.M414356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jahngen JH, et al. Aging and cellular maturation causes changes in ubiquitin-eye lens protein conjugates. Arch Biochem Biophys. 1990;276(1):32–37. doi: 10.1016/0003-9861(90)90006-k. [DOI] [PubMed] [Google Scholar]
- 121.Fernandes AF, et al. Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res. 2006 doi: 10.1016/j.exer.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fernandes AF, et al. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J Biol Chem. 2008;283(30):20745–53. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 124.Mizushima N, Hara T. Intracellular quality control by autophagy: how does autophagy prevent neurodegeneration? Autophagy. 2006;2(4):302–4. doi: 10.4161/auto.2945. [DOI] [PubMed] [Google Scholar]
- 125.Cuervo AM, et al. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 126.Mizushima N, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14(9):959–65. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124(Pt 2):161–70. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–96. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang AL, et al. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4(1):e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang AL, et al. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009;5(4):563–4. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- 133.Mitter SK, et al. Autophagy in the retina: a potential role in age-related macular degeneration. Adv Exp Med Biol. 2012;723:83–90. doi: 10.1007/978-1-4614-0631-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swamy MS, et al. Glycation mediated lens crystallin aggregation and cross-linking by various sugars and sugar phosphates in vitro. Experimental Eye Research. 1993;56:177–185. doi: 10.1006/exer.1993.1025. [DOI] [PubMed] [Google Scholar]
- 135.Cheng R, Lin B, Ortwerth BJ. Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim Biophys Acta. 2002;1587(1):65–74. doi: 10.1016/s0925-4439(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 136.Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2010 doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 137.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30(1):18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chiu CJ, et al. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86(1):180–8. doi: 10.1093/ajcn/86.1.180. [DOI] [PubMed] [Google Scholar]
- 139.Chiu CJ, et al. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007;86(4):1210–8. doi: 10.1093/ajcn/86.4.1210. [DOI] [PubMed] [Google Scholar]
- 140.Chiu CJ, et al. Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology. 2009;116(5):939–46. doi: 10.1016/j.ophtha.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaushik S, et al. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88(4):1104–10. doi: 10.1093/ajcn/88.4.1104. [DOI] [PubMed] [Google Scholar]
- 142.Takizawa N, Takada K, Ohkawa K. Inhibitory effect of nonenzymatic glycation on ubiquitination and ubiquitin-mediated degradation of lysozyme. Biochem Biophys Res Commun. 1993;192(2):700–6. doi: 10.1006/bbrc.1993.1471. [DOI] [PubMed] [Google Scholar]
- 143.Marques C, et al. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J. 2004;18(12):1424–6. doi: 10.1096/fj.04-1743fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ryhanen T, et al. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J Cell Mol Med. 2009;13(9B):3616–31. doi: 10.1111/j.1582-4934.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cringle SJ, et al. Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci. 2002;43(6):1922–7. [PubMed] [Google Scholar]
- 146.Beatty S, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 147.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76(4):397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 148.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 149.Rozanowska M, et al. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270(32):18825–30. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 150.Rozanowska M, et al. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24(7–8):1107–12. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 151.Boulton M, Roanowska M, Wess T. Ageing of the retinal pigment epithelium: implications for transplantation. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):76–84. doi: 10.1007/s00417-003-0812-8. [DOI] [PubMed] [Google Scholar]
- 152.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 153.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 154.Giblin FJ, et al. A direct correlation between the levels of ascorbic acid and H2O2 in aqueous humor. Exp Eye Res. 1984;38(1):87–93. doi: 10.1016/0014-4835(84)90142-8. [DOI] [PubMed] [Google Scholar]
- 155.Ramachandran S, et al. Radio-isotopic determination of hydrogen peroxide in aqueous humor and urine. Exp Eye Res. 1991;53(4):503–6. doi: 10.1016/0014-4835(91)90167-d. [DOI] [PubMed] [Google Scholar]
- 156.Jahngen-Hodge J, et al. Oxidative stress to lens crystallins. Methods in Enzymology. 1994;233:512–522. doi: 10.1016/s0076-6879(94)33057-3. [DOI] [PubMed] [Google Scholar]
- 157.Shang F, Huang L, Taylor A. Degradation of native and oxidized beta- and gamma-crystallins using bovine lens epithelial cell and rabbit reticulocyte extracts. Curr Eye Res. 1994;13:423–431. doi: 10.3109/02713689408999870. [DOI] [PubMed] [Google Scholar]
- 158.Taylor A, Hobbs M. The 2001 Assessment of Nutritional Influences on risk for cataract in Nutrition and Aging. In: Rosenberg I, Sastre A, editors. Nutrition and Aging. Karger; 2002. pp. 163–192. [DOI] [PubMed] [Google Scholar]
- 159.Murakami K, et al. Lens proteosome shows enhanced rates of degradation of hydroxyl radical modified alpha-crystallin. Free Radic Biol & Med. 1990;8:217–222. doi: 10.1016/0891-5849(90)90066-r. [DOI] [PubMed] [Google Scholar]
- 160.Blazquez H, Fominaya JM, Hofsteenge J. Oxidation of sulfhydryl group of ribonuclease inhibitor in epithelial cells is sufficient for its intracellular degradation. J Biol chem. 1996;271:18638–18642. doi: 10.1074/jbc.271.31.18638. [DOI] [PubMed] [Google Scholar]
- 161.Shang F, Taylor A. Oxidation and protein degradation in the lens. Mol Aspects Med. 1997;18:339–351. [Google Scholar]
- 162.Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998;440(3):399–402. doi: 10.1016/s0014-5793(98)01495-1. [DOI] [PubMed] [Google Scholar]
- 163.Ullrich O, Grune T. Proteasomal degradation of oxidatively damaged endogenous histones in K562 human leukemic cells. Free Radic Biol Med. 2001;31(7):887–93. doi: 10.1016/s0891-5849(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 164.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580(12):2910–6. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 165.Sitte N, et al. Protein oxidation and degradation during cellular senescence of human BJ fibroblasts: part I--effects of proliferative senescence. FASEB J. 2000;14(15):2495–502. doi: 10.1096/fj.00-0209com. [DOI] [PubMed] [Google Scholar]
- 166.Sitte N, et al. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic Biol Med. 2000;28(5):701–8. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 167.Grune T, et al. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of multicatalytic proteinase complex, proteasome. J Biol chem. 1995;270(5):2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- 168.Grune T, et al. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273(18):10857–62. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- 169.Giulivi C, Pacifici RE, Davies KJ. Exposure of hydrophobic moieties promotes the selective degradation of hydrogen peroxide-modified hemoglobin by the multicatalytic proteinase complex, proteasome. Arch Biochem Biophys. 1994;311(2):329–41. doi: 10.1006/abbi.1994.1245. [DOI] [PubMed] [Google Scholar]
- 170.Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology. 2006;66(2 Suppl 1):S93–6. doi: 10.1212/01.wnl.0000192308.43151.63. [DOI] [PubMed] [Google Scholar]
- 171.Yamanaka K, et al. Identification of the ubiquitin-protein ligase that recognizes oxidized IRP2. Nat Cell Biol. 2003;5(4):336–40. doi: 10.1038/ncb952. [DOI] [PubMed] [Google Scholar]
- 172.Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Expl Eye Res. 1992;55(6):889–96. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- 173.Zhang X, et al. The proteasome: a target of oxidative damage in cultured human retina pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49(8):3622–30. doi: 10.1167/iovs.07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Obin M, et al. Neurite outgrowth in PC12 cells. Distinguishing the roles of ubiquitylation and ubiquitin-dependent proteolysis. J Biol Chem. 1999;274(17):11789–95. doi: 10.1074/jbc.274.17.11789. [DOI] [PubMed] [Google Scholar]
- 175.Drinjakovic J, et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65(3):341–57. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neves DD, Rehen SK, Linden R. Differentiation-dependent sensitivity to cell death induced in the developing retina by inhibitors of the ubiquitin-proteasome proteolytic pathway. Eur J Neurosci. 2001;13(10):1938–44. doi: 10.1046/j.0953-816x.2001.01571.x. [DOI] [PubMed] [Google Scholar]
- 177.Galy A, et al. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev Biol. 2002;248(2):251–64. doi: 10.1006/dbio.2002.0736. [DOI] [PubMed] [Google Scholar]
- 178.Xu W, et al. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp Cell Res. 2000;255(2):135–43. doi: 10.1006/excr.2000.4803. [DOI] [PubMed] [Google Scholar]
- 179.Wilson R, et al. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat Cell Biol. 2002;4(6):445–50. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 180.Ditzel M, et al. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat Cell Biol. 2003;5(5):467–73. doi: 10.1038/ncb984. [DOI] [PubMed] [Google Scholar]
- 181.Wing JP, et al. Drosophila Morgue is an F box/ubiquitin conjugase domain protein important for grim-reaper mediated apoptosis. Nat Cell Biol. 2002;4(6):451–6. doi: 10.1038/ncb800. [DOI] [PubMed] [Google Scholar]
- 182.Hays R, Wickline L, Cagan R. Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nat Cell Biol. 2002;4(6):425–31. doi: 10.1038/ncb794. [DOI] [PubMed] [Google Scholar]
- 183.House CM, et al. A binding motif for Siah ubiquitin ligase. Proc Natl Acad Sci U S A. 2003;100(6):3101–6. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bogdan S, et al. Misexpression of Xsiah-2 induces a small eye phenotype in Xenopus. Mech Dev. 2001;103(1–2):61–9. doi: 10.1016/s0925-4773(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 185.Li Y, Baker NE. The roles of cis-inactivation by Notch ligands and of neuralized during eye and bristle patterning in Drosophila. BMC Dev Biol. 2004;4:5. doi: 10.1186/1471-213X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev Cell. 2001;1(6):795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- 187.Lai EC, et al. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1(6):783–94. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- 188.Parks AL, Turner FR, Muskavitch MA. Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech Dev. 1995;50(2–3):201–16. doi: 10.1016/0925-4773(94)00336-l. [DOI] [PubMed] [Google Scholar]
- 189.del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Dev Cell. 2006;11(6):887–94. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 190.Lee JD, et al. The ubiquitin ligase Hyperplastic discs negatively regulates hedgehog and decapentaplegic expression by independent mechanisms. Development. 2002;129(24):5697–706. doi: 10.1242/dev.00159. [DOI] [PubMed] [Google Scholar]
- 191.Ou CY, et al. Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 2002;16(18):2403–14. doi: 10.1101/gad.1011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ou CY, et al. Suppression of Hedgehog signaling by Cul3 ligases in proliferation control of retinal precursors. Dev Biol. 2007;308(1):106–19. doi: 10.1016/j.ydbio.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 193.Bizzi A, et al. Axonal transport of two major components of the ubiquitin system: free ubiquitin and ubiquitin carboxyl-terminal hydrolase PGP 9.5. Brain Res. 1991;548(1–2):292–9. doi: 10.1016/0006-8993(91)91135-n. [DOI] [PubMed] [Google Scholar]
- 194.Chen ST, et al. Protein gene product 9.5-immunoreactive retinal neurons in normal developing rats and rats with optic nerve or tract lesion. Brain Res Dev Brain Res. 1994;78(2):265–72. doi: 10.1016/0165-3806(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 195.Piccinini M, et al. Affinity purification and characterization of protein gene product 9.5 (PGP9.5) from retina. Biochem J. 1996;318(Pt 2):711–6. doi: 10.1042/bj3180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu Y, et al. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111(2):209–18. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 197.Proctor CJ, Tangeman PJ, Ardley HC. Modelling the role of UCH-L1 on protein aggregation in age-related neurodegeneration. PLoS One. 2010;5(10):e13175. doi: 10.1371/journal.pone.0013175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wilkinson KD, Deshpande S, Larsen CN. Comparisons of neuronal (PGP 9.5) and non-neuronal ubiquitin C-terminal hydrolases. Biochem Soc Trans. 1992;20(3):631–7. doi: 10.1042/bst0200631. [DOI] [PubMed] [Google Scholar]
- 199.Sano Y, et al. Photoreceptor cell apoptosis in the retinal degeneration of Uchl3-deficient mice. Am J Pathol. 2006;169(1):132–41. doi: 10.2353/ajpath.2006.060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poeck B, et al. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001;29(1):99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 201.Chen X, et al. On the conservation of function of the Drosophila fat facets deubiquitinating enzyme and Fam, its mouse homolog. Dev Genes Evol. 2000;210(12):603–10. doi: 10.1007/s004270000109. [DOI] [PubMed] [Google Scholar]
- 202.Fischer-Vize JA, Rubin GM, Lehmann R. The fat facets gene is required for Drosophila eye and embryo development. Development. 1992;116(4):985–1000. doi: 10.1242/dev.116.4.985. [DOI] [PubMed] [Google Scholar]
- 203.Huang Y, Baker RT, Fischer-Vize JA. Control of cell fate by a deubiquitinating enzyme encoded by the fat facets gene. Science. 1995;270(5243):1828–31. doi: 10.1126/science.270.5243.1828. [DOI] [PubMed] [Google Scholar]
- 204.Fischer JA, Leavell SK, Li Q. Mutagenesis screens for interacting genes reveal three roles for fat facets during Drosophila eye development. Dev Genet. 1997;21(2):167–74. doi: 10.1002/(SICI)1520-6408(1997)21:2<167::AID-DVG6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 205.Overstreet E, et al. Either Part of a Drosophila Epsin Protein, Divided after the ENTH Domain, Functions in Endocytosis of Delta in the Developing Eye. Curr Biol. 2003;13(10):854–60. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 206.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394(6695):793–7. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 207.Carthew RW, Xu C. Endocytosis: why not wait to deubiquitinate? Curr Biol. 2000;10(14):R532–4. doi: 10.1016/s0960-9822(00)00587-x. [DOI] [PubMed] [Google Scholar]
- 208.Wood SA, et al. Cloning and expression analysis of a novel mouse gene with sequence similarity to the Drosophila fat facets gene. Mech Dev. 1997;63(1):29–38. doi: 10.1016/s0925-4773(97)00672-2. [DOI] [PubMed] [Google Scholar]
- 209.Taya S, et al. The deubiquitinating enzyme Fam interacts with and stabilizes beta-catenin. Genes Cells. 1999;4(12):757–67. doi: 10.1046/j.1365-2443.1999.00297.x. [DOI] [PubMed] [Google Scholar]
- 210.Obin MS, et al. Ubiquitinylation and ubiquitin-dependent proteolysis in vertebrate photoreceptors (rod outer segments). Evidence for ubiquitinylation of Gt and rhodopsin. J Biol Chem. 1996;271(24):14473–84. doi: 10.1074/jbc.271.24.14473. [DOI] [PubMed] [Google Scholar]
- 211.Obin M, et al. Ubiquitylation of the transducin betagamma subunit complex. Regulation by phosducin. J Biol Chem. 2002;277(46):44566–75. doi: 10.1074/jbc.M205308200. [DOI] [PubMed] [Google Scholar]
- 212.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 213.Sokolov M, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34(1):95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 214.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–49. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wickliffe K, et al. The multiple layers of ubiquitin-dependent cell cycle control. Chem Rev. 2009;109(4):1537–48. doi: 10.1021/cr800414e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fernandes AF, et al. Proteasome inactivation promotes p38 mitogen-activated protein kinase-dependent phosphatidylinositol 3-kinase activation and increases interleukin-8 production in retinal pigment epithelial cells. Mol Biol Cell. 2009;20(16):3690–9. doi: 10.1091/mbc.E08-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Le Bras S, Loyer N, Le Borgne R. The multiple facets of ubiquitination in the regulation of notch signaling pathway. Traffic. 2010;12(2):149–61. doi: 10.1111/j.1600-0854.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 219.Weinmaster G, Fischer JA. Notch ligand ubiquitylation: what is it good for? Dev Cell. 2011;21(1):134–44. doi: 10.1016/j.devcel.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9(18):3700–9. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Narimatsu M, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137(2):295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 222.Harhaj EW, V, Dixit M. Deubiquitinases in the regulation of NF-kappaB signaling. Cell Res. 2011;21(1):22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ungureanu D, Silvennoinen O. SLIM trims STATs: ubiquitin E3 ligases provide insights for specificity in the regulation of cytokine signaling. Sci STKE. 2005;2005(304):pe49. doi: 10.1126/stke.3042005pe49. [DOI] [PubMed] [Google Scholar]
- 224.Carbia-Nagashima A, Arzt E. Intracellular proteins and mechanisms involved in the control of gp130/JAK/STAT cytokine signaling. IUBMB Life. 2004;56(2):83–8. doi: 10.1080/15216540410001668064. [DOI] [PubMed] [Google Scholar]
- 225.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382(Pt 2):393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26(22):3214–26. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 227.Yamashita M, et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31(6):918–24. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kallio PJ, et al. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem. 1999;274(10):6519–25. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 229.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272(36):22642–7. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 230.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 231.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 232.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25(51):6685–705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 233.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 234.Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29(10):469–78. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zandi E, et al. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91(2):243–52. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 236.Chen F, et al. Finding NEMO by K63-linked polyubiquitin chain. Cell Death Differ. 2006;13(11):1835–8. doi: 10.1038/sj.cdd.4402014. [DOI] [PubMed] [Google Scholar]
- 237.Brown K, et al. Control of IκB proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1491. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 238.Harper N, et al. Protein kinase C modulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by targeting the apical events of death receptor signaling. J Biol Chem. 2003;278(45):44338–47. doi: 10.1074/jbc.M307376200. [DOI] [PubMed] [Google Scholar]
- 239.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 240.Lee TH, et al. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279(32):33185–91. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 241.Ea CK, et al. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 242.Wu CJ, et al. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8(4):398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 243.Chen ZJ, Bhoj V, Seth RB. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13(5):687–92. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- 244.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 245.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 246.Alkalay I, et al. Stimulation-dependent IκB phosporylkation marks NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gonen H, et al. Identification of the ubiquitin carrier proteins, E2s, involved in signal-induced conjugation and subsequent degradation of IkappaBalpha. J Biol Chem. 1999;274(21):14823–30. doi: 10.1074/jbc.274.21.14823. [DOI] [PubMed] [Google Scholar]
- 248.Yaron A, et al. Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B-ubiquitin ligase. EMBO J. 1997;16(21):6486–94. doi: 10.1093/emboj/16.21.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7(2):401–9. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 250.Vallabhapurapu S, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9(12):1364–70. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Senftleben U, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293(5534):1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 252.Rogers PR, et al. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15(3):445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 253.Angileri FF, et al. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112(10):2258–66. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 254.Matsuzawa A, Ichijo H. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J Biochem (Tokyo) 2001;130(1):1–8. doi: 10.1093/oxfordjournals.jbchem.a002947. [DOI] [PubMed] [Google Scholar]
- 255.Dudek EJ, Shang F, Taylor A. H(2)O(2)-mediated oxidative stress activates NF-kappaB in lens epithelial cells. Free Radic Biol Med. 2001;31(5):651–8. doi: 10.1016/s0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 256.Wu M, et al. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46(1):62–9. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Parry GC, et al. IL-1beta-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol. 1998;18(6):934–40. doi: 10.1161/01.atv.18.6.934. [DOI] [PubMed] [Google Scholar]
- 258.Wang GL, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–54. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13(1):29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- 261.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16(10):1151–62. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 262.Manalo DJ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105(2):659–69. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 263.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 264.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 265.Masson N, et al. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20(18):5197–206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bento CF, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1alpha for degradation in the presence of methylglyoxal. PLoS One. 2010;5(11):e15062. doi: 10.1371/journal.pone.0015062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li Z, et al. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6(4):373–8. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–83. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 269.Arjamaa O, et al. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD) Ageing Res Rev. 2009;8(4):349–58. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 270.DeNiro M, Alsmadi O, Al-Mohanna F. Modulating the hypoxia-inducible factor signaling pathway as a therapeutic modality to regulate retinal angiogenesis. Exp Eye Res. 2009;89(5):700–17. doi: 10.1016/j.exer.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 271.Pirlich M, et al. Increased proteolysis after single-dose exposure with hepatotoxins in HepG2 cells. Free Radic Biol Med. 2002;33(2):283–91. doi: 10.1016/s0891-5849(02)00880-8. [DOI] [PubMed] [Google Scholar]
- 272.Pickering AM, et al. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432(3):585–94. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ethen CM, et al. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581(5):885–90. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Obin M, et al. Redox regulation of ubiquitin-conjugating enzymes: mechanistic insights using the thiol-specific oxidant diamide. FASEB Journal. 1998;12(7):561–9. doi: 10.1096/fasebj.12.7.561. [DOI] [PubMed] [Google Scholar]
- 275.Yao D, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101(29):10810–4. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ishii T, et al. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 s proteasome. Biochemistry. 2005;44(42):13893–901. doi: 10.1021/bi051336u. [DOI] [PubMed] [Google Scholar]
- 277.Caballero M, et al. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308(2):346–52. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 278.Okada K, et al. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274(34):23787–93. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- 279.Conconi M, et al. Protection from oxidative inactivation of the 20S proteasome by heat-shock protein 90. Biochem J. 1998;333(Pt 2):407–15. doi: 10.1042/bj3330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77(4):1010–7. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 281.Shang F, et al. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Experimental Eye Research. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- 282.Shringarpure R, et al. 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: possible importance in Alzheimer’s disease. Cell Mol Life Sci. 2000;57(12):1802–9. doi: 10.1007/PL00000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Reinheckel T, et al. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377(1):65–8. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- 284.Scrofano MM, et al. Calorie restriction, stress and the ubiquitin-dependent pathway in mouse livers. Mech Ageing Dev. 1998;105(3):273–90. doi: 10.1016/s0047-6374(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 285.Scrofano MM, et al. Aging, calorie restriction and ubiquitin-dependent proteolysis in the livers of Emory mice. Mech Ageing Dev. 1998;101(3):277–96. doi: 10.1016/s0047-6374(97)00178-4. [DOI] [PubMed] [Google Scholar]
- 286.Adamo AM, et al. Effect of oxidant systems on the ubiquitylation of proteins in the central nervous system. J Neurosci Res. 1999;55(4):523–31. doi: 10.1002/(SICI)1097-4547(19990215)55:4<523::AID-JNR12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 287.Ramanathan M, et al. Oxidative stress increases ubiquitin--protein conjugates in synaptosomes. Neuroreport. 1999;10(18):3797–802. doi: 10.1097/00001756-199912160-00014. [DOI] [PubMed] [Google Scholar]
- 288.Figueiredo-Pereira ME, Yakushin S, Cohen G. Accumulation of ubiquitinated proteins in mouse neuronal cells induced by oxidative stress. Molecular Biology Reports. 1997;24:25–38. doi: 10.1023/a:1006848405975. [DOI] [PubMed] [Google Scholar]
- 289.Shang F, et al. Protein quality control by the ubiquitin proteolytic pathway: Roles in resistance to oxidative stress and disease. Israel J Chem. 2006;46:145–158. [Google Scholar]
- 290.Vernace VA, et al. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 2007;21(11):2672–82. doi: 10.1096/fj.06-6751com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ohtsuka H, Takahashi R, Goto S. Age-related accumulation of high-molecular-weight ubiquitin protein conjugates in mouse brains. J Gerontol A Biol Sci Med Sci. 1995;50(5):B277–81. doi: 10.1093/gerona/50a.5.b277. [DOI] [PubMed] [Google Scholar]
- 292.Naash MI, Al-Ubaidi MR, Anderson RE. Light exposure induces ubiquitin conjugation and degradation activities in the rat retina. Invest Ophthalmol Vis Sci. 1997;38(11):2344–54. [PubMed] [Google Scholar]
- 293.Bennett EJ, et al. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell. 2005;17(3):351–65. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 294.Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405(1):21–5. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 295.Bulteau AL, et al. Impairment of proteasome function upon UVA- and UVB-irradiation of human keratinocytes. Free Radic Biol Med. 2002;32(11):1157–70. doi: 10.1016/s0891-5849(02)00816-x. [DOI] [PubMed] [Google Scholar]
- 296.Grune T, et al. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36(12):2519–30. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 297.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389(3):203–9. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 298.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 Antioxidant Response by the Ubiquitin Proteasome System: An Insight into Cullin-Ring Ubiquitin Ligases. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nguyen T, et al. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278(7):4536–41. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 300.Ha KN, et al. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: implication for protection against oxidative stress. Invest Ophthalmol Vis Sci. 2006;47(6):2709–15. doi: 10.1167/iovs.05-1322. [DOI] [PubMed] [Google Scholar]
- 301.Mandal MN, et al. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46(5):672–9. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pitha-Rowe I, et al. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Invest Ophthalmol Vis Sci. 2009;50(11):5339–47. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- 303.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93(25):14960–5. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274(47):33627–36. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 305.Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J Biol Chem. 2000;275(20):15466–73. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 306.Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20(29):3906–17. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- 307.Itoh K, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cullinan SB, et al. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24(19):8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang DD, et al. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24(24):10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25(1):162–71. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sekhar KR, Yan XX, Freeman ML. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene. 2002;21(44):6829–34. doi: 10.1038/sj.onc.1205905. [DOI] [PubMed] [Google Scholar]
- 312.Stewart D, et al. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem. 2003;278(4):2396–402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 313.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–9. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Plafker KS, et al. The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J Biol Chem. 2010;285(30):23064–74. doi: 10.1074/jbc.M110.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Dreger H, et al. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83(2):354–61. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- 316.Dreger H, et al. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc Res. 2010;85(2):395–403. doi: 10.1093/cvr/cvp279. [DOI] [PubMed] [Google Scholar]
- 317.Westphal K, et al. Human-specific induction of glutathione peroxidase-3 by proteasome inhibition in cardiovascular cells. Free Radic Biol Med. 2009;47(11):1652–60. doi: 10.1016/j.freeradbiomed.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 318.Chen J, Regan RF. Increasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injury. Curr Neurovasc Res. 2005;2(3):189–96. doi: 10.2174/1567202054368344. [DOI] [PubMed] [Google Scholar]
- 319.Meiners S, et al. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006;40(12):2232–41. doi: 10.1016/j.freeradbiomed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 320.Yamamoto N, et al. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem. 2007;282(7):4364–72. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- 321.Kapeta S, Chondrogianni N, Gonos ES. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J Biol Chem. 2010;285(11):8171–84. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lee CS, et al. A proteasomal stress response: pre-treatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. J Neurochem. 2004;91(4):996–1006. doi: 10.1111/j.1471-4159.2004.02813.x. [DOI] [PubMed] [Google Scholar]
- 323.Cano M, et al. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50(7):652–64. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wei Y, et al. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51(1):216–24. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhao Z, et al. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011;6(4):e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ozawa Y, et al. Roles of STAT3/SOCS3 pathway in regulating the visual function and ubiquitin-proteasome-dependent degradation of rhodopsin during retinal inflammation. J Biol Chem. 2008;283(36):24561–70. doi: 10.1074/jbc.M802238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mauger C, et al. Identification and localization of ataxin-7 in brain and retina of a patient with cerebellar ataxia type II using anti-peptide antibody. Brain Res Mol Brain Res. 1999;74(1–2):35–43. doi: 10.1016/s0169-328x(99)00256-9. [DOI] [PubMed] [Google Scholar]
- 328.Naash M, Izbicka E, Anderson RE. Rat retina has an active and stable ubiquitin-protein conjugating system. J Neurosci Res. 1991;30(2):433–41. doi: 10.1002/jnr.490300220. [DOI] [PubMed] [Google Scholar]
- 329.Yoshida T, et al. Amyloid precursor protein, A beta and amyloid-associated proteins involved in chloroquine retinopathy in rats--immunopathological studies. Brain Res. 1997;764(1–2):283–8. doi: 10.1016/s0006-8993(97)00600-8. [DOI] [PubMed] [Google Scholar]
- 330.Loeffler KU, Mangini NJ. Immunolocalization of ubiquitin and related enzymes in human retina and retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 1997;235(4):248–54. doi: 10.1007/BF00941767. [DOI] [PubMed] [Google Scholar]
- 331.Anderson DH, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78(2):243–56. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 332.Leger F, et al. Protein aggregation in the aging retina. J Neuropathol Exp Neurol. 2011;70(1):63–8. doi: 10.1097/NEN.0b013e31820376cc. [DOI] [PubMed] [Google Scholar]
- 333.Brett FM, Henson C, Staunton H. Familial diffuse Lewy body disease, eye movement abnormalities, and distribution of pathology. Arch Neurol. 2002;59(3):464–7. doi: 10.1001/archneur.59.3.464. [DOI] [PubMed] [Google Scholar]
- 334.Kapphahn RJ, Bigelow EJ, Ferrington DA. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp Eye Res. 2007;84(4):646–54. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Uetama T, et al. Phenotypic change regulates monocyte chemoattractant protein-1 (MCP-1) gene expression in human retinal pigment epithelial cells. J Cell Physiol. 2003;197(1):77–85. doi: 10.1002/jcp.10342. [DOI] [PubMed] [Google Scholar]
- 336.Ambati J, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9(11):1390–7. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 337.Arima K, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108(36):14914–9. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hussong SA, et al. Immunoproteasome deficiency alters retinal proteasome’s response to stress. J Neurochem. 2010;113(6):1481–90. doi: 10.1111/j.1471-4159.2010.06688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ferrington DA, et al. Immunoproteasome responds to injury in the retina and brain. J Neurochem. 2008;106(1):158–69. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]