Abstract
Context:
A close association between insulin resistance and reduced skeletal muscle oxidative capacity has been reported in adult offspring of people with type 2 diabetes (T2D), prompting a hypothesis that insulin resistance may result from mitochondrial dysfunction or vice versa.
Objective:
We determined whether 9 d of intensive exercise training ameliorates the mitochondrial dysfunction and insulin resistance in offspring of T2D.
Methods:
We compared the response to 9 d of intensive exercise training in eight (seven females, one male) healthy adult offspring of mothers with T2D with eight (six females, two males) nondiabetic controls. Skeletal muscle mitochondrial ATP production was assessed using a luciferase-based assay, and insulin sensitivity was measured using hyperinsulinemic-euglycemic clamps.
Results:
Short-term intensive training increased skeletal muscle mitochondrial ATP production and citrate synthase activity similarly in both groups (P < 0.01). In contrast, whereas short-term intensive training reduced the fasting glucose (∼5%, P = 0.035) and insulin levels (∼40%, P = 0.011) as well as increased the glucose infusion rate during the hyperinsulinemic-euglycemic clamp (∼50%, P = 0.028) among controls, no changes in these parameters were observed among offspring except for an increase in fasting glucose (∼7%, P = 0.004).
Conclusion:
A short-term intensive exercise training program was equally effective at increasing skeletal muscle oxidative capacity in nondiabetic people and in the offspring of mothers with diabetes. In contrast, the exercise improved insulin sensitivity only in nondiabetic people but not in the offspring of T2D mothers, revealing dissociation between improvements in skeletal muscle mitochondrial function and insulin sensitivity. The exercise effect on mitochondrial function and insulin sensitivity seems to be mediated by different regulatory pathways.
First-degree relatives of people with type 2 diabetes (T2D) have been reported to have reduced skeletal muscle oxidative capacity and mitochondrial content (1, 2), reduced insulin sensitivity (2), and are at increased risk for developing T2D. Moreover, mitochondrial dysfunction has been proposed as a potential mechanism for insulin resistance (3). Alternatively, insulin-resistance may cause mitochondrial dysfunction (4, 5). The degree to which these effects are attributable to genetic predisposition vs. shared lifestyle and environmental factors is not known. Reduced cardiorespiratory fitness [peak oxygen uptake (VO2peak)] has also been reported in offspring of people with T2D (6). Exercise training, particularly traditional endurance training, induces both metabolic and morphological adaptations in skeletal muscle that increase skeletal muscle oxidative capacity and insulin sensitivity (7). Exercise training has also been shown to improve insulin sensitivity, oral glucose tolerance, and skeletal muscle oxidative capacity in first-degree relatives of individuals with T2D in some (1, 8) but not all (9) studies.
Chronic, high-intensity, continuous endurance training has been shown to improve body composition (10), insulin sensitivity (11), and oxidative capacity (12) to a greater extent than low-intensity, continuous endurance training. Recent data also indicate that short-term, high-intensity interval training is an effective stimulus for increasing cardiorespiratory fitness, skeletal muscle oxidative capacity, and insulin sensitivity in healthy adults (13, 14). Short-term exercise training provides an opportunity to examine the effects of acute skeletal muscle adaptation on insulin sensitivity in the absence of changes in body composition. Therefore, we specifically chose to examine the acute effects of short-term (9 d) intensive training on exercise-induced skeletal muscle mitochondrial adaptations and insulin sensitivity in the absence of changes in body composition. Indeed, previous studies have demonstrated that 7–10 d of intensive exercise training is sufficient to improve insulin action (15) and improve skeletal muscle oxidative capacity (13). Therefore, we examined the effect of 9 d of intensive exercise training on skeletal muscle oxidative capacity (i.e. mitochondrial ATP production and citrate synthase activity) and insulin sensitivity [i.e. steady-state glucose infusion rate (GIR) during a hyperinsulinemic-euglycemic clamp] in adult offspring of mothers with T2D, because the mitochondrial genome is maternally inherited. We hypothesized that these variables would be less responsive to exercise training in the offspring of mothers with T2D than in control subjects.
Subjects and Methods
Eight healthy, sedentary adult offspring of mothers with T2D (T2D offspring) and eight healthy controls with no family history of T2D participated in this exercise training study (Table 1). The Mayo Clinic Institutional Review Board approved, and informed written and verbal consent was obtained from each participant. Participants underwent an initial screening as previously described (5). To be eligible, the offspring had to have a mother with T2D, which was determined by a detailed family history. Participants were excluded if they had any evidence of diseases such as diabetes, cardiovascular disease, thyroid dysfunction, or substance abuse. Participants were also excluded if they reported exercising greater than 60 min/wk or reported using β-blockers or insulin-sensitizing agents. Body composition was measured using dual x-ray absorptiometry (Lunar DPX-L; Lunar Radiation, Madison, WI), and VO2peak was measured using indirect calorimetry and graded-cycle ergometry (5).
Table 1.
Participant characteristics
| Control | Offspring | |
|---|---|---|
| n (female/male) | 6/2 | 7/1 |
| Age (yr) | 37.0 (26.0–38.5) | 40.5a (39.8–42.3) |
| Body mass (kg) | 81.5 (75.7–85.7) | 69.8 (65.0–79.7) |
| Body mass index (kg/m2) | 26.9 (25.1–29.7) | 25.9 (23.1–27.0) |
| Waist (cm) | 85.5 (79.0–96.5) | 85.8 (82.0–94.6) |
| Body fat (%) | 34.7 (31.8–37.3) | 35.3 (31.0–39.2) |
| Lean mass (kg) | 47.8 (42.7–55.8) | 41.1 (39.1–44.4) |
| Fat mass (kg) | 26.8 (23.3–30.6) | 26.8 (23.3–30.6) |
| VO2peak (ml/kg · min) | 26.1 (25.8–32.5) | 27.3 (21.7–30.9) |
| Fasting glucose (mg/dl) | 91 (88–95) | 95 (91–99) |
| Fasting insulin (μU/ml) | 6.1 (4.9–7.7) | 5.1 (4.5–5.5) |
| HOMA-IR | 1.27 (0.95–1.90) | 1.11 (1.00–1.34) |
Data presented as median (interquartile range). HOMA-IR, Homeostasis model assessment for insulin resistance.
P < 0.05 (Mann-Whitney U test).
Participants completed two inpatient study days (pre- and posttraining) in the Mayo Clinic's Center for Translational Science Activities, Clinical Research Unit. In brief, a standardized weight-maintaining diet was provided 3 d before each inpatient study, as previously described (5). Participants were admitted to the Clinical Research Unit for an overnight stay on the evening of the third day of their standardized meals, and the study procedures commenced the following morning under fasting conditions. The pretraining measurements were performed greater than 7 d after the VO2peak assessment, whereas the posttraining measurements were performed approximately 28 h after the last exercise bout under fasting conditions. Percutaneous skeletal muscle biopsies were obtained from the vastus lateralis (∼200 mg) using a University College Hospital skeletal muscle biopsy needle (Cadence Scientific, Staunton, VA) under local anesthesia (lidocaine, 2%). The posttraining skeletal muscle biopsy was taken approximately 4 cm proximal to the pretraining skeletal muscle biopsy site. A portion of the muscle (∼50 mg) was kept fresh on ice in saline-soaked gauze for measurement of mitochondrial ATP production rate (MAPR), with the remainder frozen in liquid nitrogen and stored at −80 C for later analyses. MAPR was measured immediately after biopsy in isolated mitochondria using a bioluminescent assay as previously described (5). The coefficient of variations (test-retest reliability) of our MAPR measurements are 10–12%, as previously reported (16). Citrate synthase activity was measured as previously described (5). Real-time PCR (7900-HT Sequence Detection System; Applied Biosystems, Foster, CA) was used to measure the mitochondrial DNA abundance in duplicate as previously described (4).
Whole-body insulin sensitivity was measured during a hyperinsulinemic [0.25 mU/kg fat-free mass (FFM) · min] euglycemic clamp as previously described (4). Euglycemia was maintained at approximately 90 mg/dl in both groups (Supplemental Fig. 1, A and B, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Somatostatin (7 μg/kg FFM · h) was infused to suppress endogenous insulin production, and GH (5 ng/kg FFM · min) was replaced to approximate basal concentration (4).
Exercise training on a stationary bicycle was performed daily for 9 d. Training sessions consisted of alternating days of continuous (steady-state) exercise and aerobic interval training. Continuous training sessions were performed at 70–80% VO2peak for 60 min. Aerobic interval training sessions began with a 5-min warm-up and then six 5-min bouts of cycling at 85–95% VO2peak, with 3-min recovery intervals at 30–50% VO2peak. Exercise intensity was regulated using continuous heart rate monitoring (Polar, Port Washington, NY). On d 10, the subjects completed their posttraining VO2peak assessment, and on d 11, the subjects completed their posttraining inpatient assessments.
The data are presented as median (interquartile range). Mann-Whitney U tests were used to test for significant pretraining differences between groups. Two-way, mixed-effects analysis of covariance (ANCOVA) was used to examine mean differences in pre- to posttraining values (with the use of log transformation). A detailed description of the statistical methods is provided in Supplemental Methods.
Results
Participant characteristics (Table 1)
Overall, the groups were similar in their baseline demographic and clinical characteristics. However, the offspring were slightly older (P = 0.028). There were no significant changes in anthropometric characteristics or VO2peak during the 9-d training period (data not shown, all P > 0.05).
Exercise-induced changes in mitochondrial function
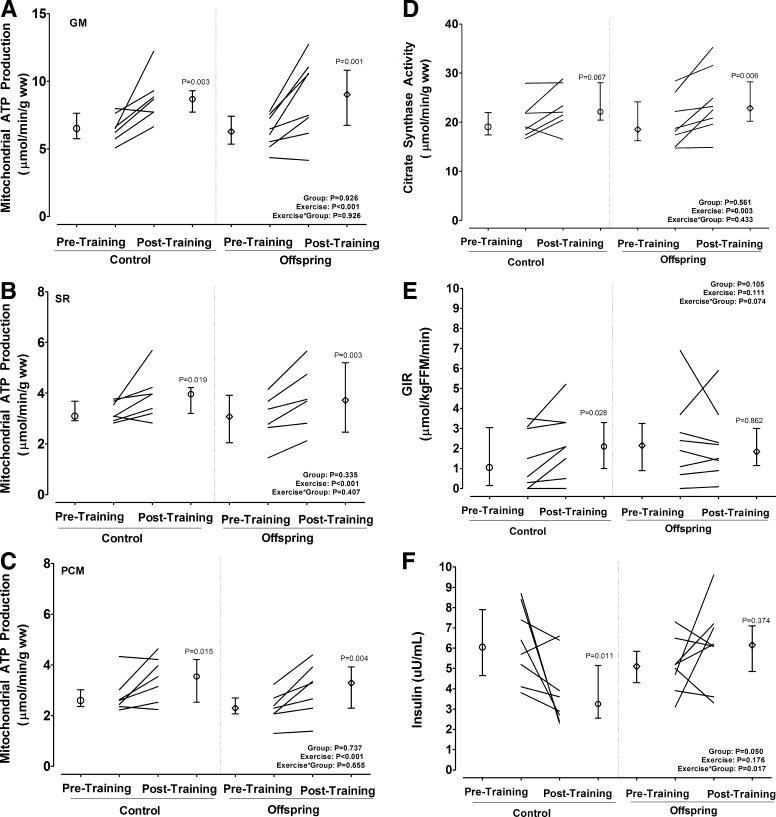
Figure 1, A–C, demonstrates that there were no significant differences between the offspring and control participants for their maximal MAPR at baseline (all P > 0.05). Likewise, 9 d of exercise increased maximal MAPR in both the offspring and control participants (P < 0.001 for exercise effect, Fig. 1, A–C). Similarly, mitochondrial citrate synthase activity was similar at baseline between the offspring and control participants (P > 0.05) and increased significantly in both groups after training (P = 0.002 for the exercise effect, Fig. 1D). Mitochondrial DNA abundance (arbitrary units) tended to be lower in control than the offspring participants [0.60 (0.51–0.91) vs. 1.30 (0.78–3.79), P = 0.058 for ND1, and 0.83 (0.47–1.21) vs. 1.53 (0.78–6.63), P = 0.232 for ND4] at baseline and did not change in response to training (data not shown).
Fig. 1.
Effect of intensive training on mitochondrial oxidative capacity and insulin sensitivity in eight controls and eight adult offspring of mothers with T2D. Paired data are shown as individual lines, with pre- and posttraining group medians (interquartile ranges) shown as adjacent circles and vertical lines, respectively. A–C, MAPR, for which mitochondria were incubated with glutamate plus malate (GM), succinate plus rotenone (SR), and palmitoyl carnitine plus malate (PCM); D, citrate synthase activity; E, steady-state GIR achieved during a hyperinsulinemic-euglycemic clamp measured between min 300–360; F, fasting insulin concentrations. Mixed-effects analysis of covariance models were used to test the effects of group, time, and their interaction adjusted for baseline values and age. Post hoc analyses were conducted using the Fisher's restricted least significant differences criterion. ww, Wet weight.
Exercise-induced changes in insulin sensitivity
Figure 1E presents the pre- and posttraining steady-state GIR achieved during the hyperinsulinemic-euglycemic clamp (300–360 min) for the controls and offspring subjects, respectively. The main effects of group, time, or their interaction on insulin sensitivity were also not statistically significant after adjusting for age (all P > 0.05). However, within-group paired Wilcoxon-signed rank test revealed that training significantly increased insulin sensitivity by approximately 50% in the control participants (P = 0.039), whereas there was no change in the offspring. Consistent with this finding, Fig. 1F demonstrates that the fasting insulin concentration was reduced in the control participants after training (P = 0.007) but did not significantly change in the offspring (P > 0.05). Moreover, fasting blood glucose concentration was reduced in the control participants after training [93.5 (90.5–100.0) vs. 92.0 (85.5–95.5) mg/dl, P = 0.035], whereas it increased in the offspring [91.0 (87.0–95.3) vs. 97.3 (90.5–101.5) mg/dl, P = 0.004].
Discussion
The primary finding of the present study is that 9 d of vigorous exercise increased skeletal muscle mitochondrial oxidative capacity in both control and T2D offspring, but insulin sensitivity was increased only in control participants, while remaining unchanged in T2D offspring.
Our finding that short-term (7–10 d) training improves skeletal muscle mitochondrial oxidative capacity (e.g. MAPR and citrate synthase activity) is consistent with previous reports in healthy adults (13, 17). In contrast, it was reported that skeletal muscle oxidative capacity did not improve with very short-term (i.e. two training sessions) intensive training (8). Thus, there appears to be a minimal threshold of at least 3 d of training before skeletal muscle mitochondrial adaptations to exercise are detected.
In the present study, we demonstrated that short-term intensive exercise training increased insulin sensitivity in people without a known family diabetes history similar to previous studies (14). This was verified by increased posttraining GIR and decreased fasting levels of insulin and glucose. However, the training-induced increase in the GIR was attenuated after normalizing to the steady-state insulin concentration achieved during the clamp protocol (data not shown). In contrast, T2D offspring were resistant to exercise-induced improvements in insulin action. This latter finding is consistent with a recent observation that insulin-mediated glucose uptake increased in response to 12 wk of continuous endurance training in controls but not T2D offspring (9).
Our results show that improvements in skeletal muscle mitochondrial oxidative capacity and insulin action may be dissociated from one another in T2D offspring. This is also consistent with another recent report that exercise training increased mitochondrial enzyme activities but not insulin sensitivity in T2D offspring (1). Another study also reported that within the population of T2D offspring there may also be so-called responders who demonstrate improvements in both skeletal muscle oxidative capacity and insulin sensitivity (8), whereas nonresponders show no change in either skeletal muscle oxidative capacity or insulin sensitivity after short-term exercise training. Further work is warranted to determine how diabetes family history affects exercise responsiveness including in-depth studies examining changes in mitochondrial respiratory function using high-resolution respirometry.
It is becoming increasingly evident that changes in insulin sensitivity and skeletal muscle oxidative capacity are not consistently associated. Indeed, weight loss has been previously reported to improve insulin sensitivity in the absence of improvements in mitochondrial function in obese adults (18). Higher muscle mitochondrial capacity also has been reported to occur in Asian Indians despite the presence of severe insulin resistance (19). It should be emphasized that these results do not indicate that exercise is not beneficial to T2D offspring, merely that a cause and effect relationship between skeletal muscle mitochondrial function and insulin sensitivity cannot be supported by these or other data. Clearly, cardiorespiratory fitness and interventions that increase physical activity seem to benefit individuals at high risk for developing diabetes and should be encouraged (20).
In summary, short-term intensive exercise training increases skeletal muscle mitochondrial oxidative capacity in both healthy controls and T2D offspring but does not improve insulin sensitivity in T2D offspring, unlike in healthy controls, thus demonstrating that improving muscle mitochondrial function does not necessarily improves insulin sensitivity.
Supplementary Material
Acknowledgments
Current address for C.S.S.: Section of Endocrinology, Diabetes, and Metabolism, University of Arizona School of Medicine, and Southern Arizona Veterans Affairs Health Care System, Tucson, AZ.
Current address for K.R.S.: Department of Pediatrics, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
Disclosure Summary: The authors declare no conflicts of interest and financial disclosure.
Footnotes
- FFM
- Fat-free mass
- GIR
- glucose infusion rate
- MAPR
- mitochondrial ATP production rate
- T2D
- type 2 diabetes
- VO2peak
- peak oxygen uptake.
References
- 1. Østergård T, Andersen JL, Nyholm B, Lund S, Nair KS, Saltin B, Schmitz O. 2006. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 290:E998–E1005 [DOI] [PubMed] [Google Scholar]
- 2. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen KF, Dufour S, Shulman GI. 2005. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. 2006. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55:3309–3319 [DOI] [PubMed] [Google Scholar]
- 5. Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. 2003. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nyholm B, Mengel A, Nielsen S, Skjaerbaek C, Møller N, Alberti KG, Schmitz O. 1996. Insulin resistance in relatives of NIDDM patients: the role of physical fitness and muscle metabolism. Diabetologia 39:813–822 [DOI] [PubMed] [Google Scholar]
- 7. Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. 2008. Endurance exercise as a countermeasure for aging. Diabetes 57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kacerovsky-Bielesz G, Chmelik M, Ling C, Pokan R, Szendroedi J, Farukuoye M, Kacerovsky M, Schmid AI, Gruber S, Wolzt M, Moser E, Pacini G, Smekal G, Groop L, Roden M. 2009. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 58:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dela F, Stallknecht B. 2010. Effect of physical training on insulin secretion and action in skeletal muscle and adipose tissue of first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 299:E80–E91 [DOI] [PubMed] [Google Scholar]
- 10. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. 2008. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc 40:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiPietro L, Dziura J, Yeckel CW, Neufer PD. 2006. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 100:142–149 [DOI] [PubMed] [Google Scholar]
- 12. Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, Gandrakota R. 2008. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc 40:1336–1343 [DOI] [PubMed] [Google Scholar]
- 13. Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. 2005. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 98:1985–1990 [DOI] [PubMed] [Google Scholar]
- 14. Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. 2009. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. 2009. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 297:E151–E156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Short KR, Nygren J, Barazzoni R, Levine J, Nair KS. 2001. T(3) increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab 280:E761–E769 [DOI] [PubMed] [Google Scholar]
- 17. Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. 2003. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. J Physiol 546:327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE. 2008. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57:987–994 [DOI] [PubMed] [Google Scholar]
- 19. Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. 2008. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 57:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katzmarzyk PT, Church TS, Blair SN. 2004. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med 164:1092–1097 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.