Abstract
Pasteurella multocida is responsible for a wide range of diseases in domestic animals. In rabbits, the agent is related to nasal discharge, pneumonia, otitis media, pyometra, orchitis, abscess, and septicemia. One hundred and forty rabbits with respiratory diseases from four rabbitries in São Paulo State, Brazil were evaluated for the detection of P. multocida in their nasal cavities. A total of twenty-nine animals were positive to P. multocida isolation, and 46 strains were selected and characterized by means of biochemical tests and PCR. P. multocida strains were tested for capsular type, virulence genes, and resistance profile. A total of 45.6% (21/46) of isolates belonged to capsular type A, and 54.34% (25/46) of the isolates were untypeable. None of the strains harboured toxA or pfhA genes. The frequency of the other twenty genes tested was variable, and the data generated was used to build a dendrogram, showing the relatedness of strains, which were clustered according to origin. Resistance revealed to be more common against sulfonamides and cotrimoxazole, followed by erythromycin, penicillin, and amoxicillin.
1. Introduction
Pasteurella multocida is an important pathogen that infects a wide range of animal hosts, and it is part of the respiratory tract microbiota of different animal species [1]. In rabbits, infections by P. multocida are considered one of the most frequent diseases affecting the species. Pasteurellosis in these animals may present itself as rhinitis with purulent nasal discharge, pneumonia, otitis media, pyometra, orchitis, abscesses, and septicemia [2].
In the United States, more than 50% of the adult rabbits used in the industry are killed or removed from production due to infection by P. multocida, which represents a major economic loss [3]. Currently, control measures of pasteurellosis in rabbits involve treatment with antibiotics and the slaughter of infected animals. However, the treatment of infected animals only alleviates clinical signs and/or slows the progression of the disease, but it does not eradicate their infection [3].
Brazilian rabbitry production is mainly oriented to meat, skin, pelage, carcass, and blood, totalizing around 230,000 animals in 2010; however, the importance of rabbits as pet animals is growing in Brazil as well as in other countries [4]. With this in mind, it is also important to evaluate the zoonotic potential of P. multocida strains, when these animals have contact with children or immunosuppressed individuals [5].
Two reports of human infection by P. multocida after rabbit lick or byte were recorded in the last ten years. The first describes a case of meningitis and epidural, subdural, and subgaleal empyema in a 15-year-old boy. The second case reports an endovascular stentgraft infection caused by P. multocida after a bite by the patient's household rabbit, in a 68-year-old man [5, 6].
Data on the antibiotic resistance profile and virulence factors of the agent may lead to a better understanding of pasteurellosis epidemiology in rabbits. Reports describing the characterization of P. multocida isolated from rabbits in Brazil date back to the 1980s [7, 8]. Even though the molecular basis of the pathogenicity and host specificity of P. multocida is not well understood, several studies have reported that a number of virulence factors can be correlated with pathogenic mechanisms, and these factors have not yet been described in Brazilian strains [9].
The aim of this study was to evaluate the occurrence of P. multocida in rabbits, their resistance profile, as well as to investigate the presence of virulence genes coding for outer membrane and porin proteins (oma87, ompH, plpB, psl), adesins (ptfA, fimA, hsf-1, hsf-2, pfhA, tadD), neuraminidases (nanB, nanH), iron acquisition related factors (exBD, tonB, fur, tbpA, hgbA, hgbB), superoxidee dismutases (sodA, sodC), dermonecrotoxin (toxA), and hyaluronidase (pmHAS).
2. Material and Methods
2.1. Sample Collection and Processing
The samples were collected with sterile swabs from external nares of one hundred and forty rabbits with respiratory disease from four rabbitries in São Paulo State, Brazil. The swabs were placed in Amies transport medium (Copan Diagnostics Inc., California/USA) and kept under refrigeration until analysis.
Each swab was plated on tryptic soy yeast extract agar (Difco-BBL), supplemented with 5% of defibrinated sheep blood, and they were identified as P. multocida using standard biochemical procedures, including catalase, oxidase, indol production, urease activity, ornithine decarboxylase production, and carbohydrate fermentation [10] associated with PCR, for the detection of kmt species specific gene fragment [11].
2.2. Antimicrobial Susceptibility Testing
The determination of the susceptibility profile was performed with disc diffusion test dilution technique according to the standardized protocol of the M31-A3 Document, issued by the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2008). The antimicrobial agents tested included ceftiofur, penicillin, amoxacillin, florfenicol, norfloxacin, enrofloxacin, ciprofloxacin, tetracycline, doxycycline, sulfamethazine, trimethoprim-sulphamethoxazole, and erythromycin (Oxoid Ltd. Cambridge, UK). Reference strains Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as quality control organisms in all antimicrobial susceptibility tests.
2.3. DNA Preparation
The bacteria were cultured overnight in brain hearth infusion (BHI) broth at 37°C, and 200 μL of this cell suspension was submitted to the DNA extraction procedure described by Boom et al. [12].
2.4. PCR Analyses and Gel Electrophoresis
P. multocida strains were evaluated for the presence of capsule biosynthesis genes cap A, B, D, E, and F [11] and the following virulence related genes: oma87, ompH, plpB, psl, ptfA, fimA, hsf-1, hsf-2, pfhA, tadD, nanB, nanH, exBD/tonB, fur, pmHAS, tbpA, hgbA, hgbB, sodA, sodC, toxA, and pmHAS [9, 13]. The following P. multocida strains were used as positive control: ATCC 12945, ATCC 12948, and NCTC 10323.
For all reactions, 5 μL of DNA template was added to the 45 μL mixture containing 20 pmoles of each primer pair (Invitrogen Corporation, California/USA), 1.5 mM of MgCl2, 200 mM of each dNTP, 1U of Taq DNA polymerase (Fermentas Inc. Maryland, USA), 1X PCR buffer, and ultrapure water. PCR conditions were carried out according to the respective authors' protocols. The amplified products were subjected to electrophoresis in 1.5% agarose gel, stained with BlueGreen (LGC Biotecnologia, São Paulo, Brazil), and identified by means of a 100 bp DNA ladder.
2.5. Statistical Analysis
Relatedness among P. multocida strains was determined by a comprehensive pairwise comparison of different genes combinations, using the Dice coefficient by means of the Bionumerics 6.6 software (Applied Maths NV, Sint-Martens-Latem, Belgium) to generate the dendrogram. The discriminatory index was calculated according to the method described by Hunter and Gaston [14].
3. Results
One hundred and forty animals were examined and twenty-nine were positive to P. multocida isolation (Table 1). From positive animals, 46 strains were characterized as P. multocida through biochemical tests and PCR. All 46 strains were tested for the five capsular types (A to F), and 45.6% (21/46) of isolates belonged to capsular type A and 54.34% (25/46) isolates were untypeable using the PCR described.
Table 1.
Frequency of positive animals in four rabbitries examined in São Paulo State, Brazil.
| Year | Rabbitries | Number | Positive | Frequency (%) |
|---|---|---|---|---|
| 2010 | A | 40 | 21 | 52.5 |
| B | 20 | 2 | 10 | |
| 2011 | C | 53 | 4 | 7.5 |
| D | 27 | 2 | 7.4 | |
|
| ||||
| Total | 140 | 29 | 20.7 | |
Strains were screened for the presence/absence of a total of twenty-two different genes coding for virulence factors, and the complete results are given in Table 2. Among the 46 P. multocida strains, the 22 virulence genes ranged in prevalence from 0% (toxA, pfhA) to 97.8% (SodC) (Table 2). Most of the strains presented the ptfA gene (93.4%), coding for type 4 fimbrial subunit, as well as putative nonspecific tight adherence protein D encoding gene tadD (91.3%). The gene locus exbBD- tonB, which is involved in energy-coupled transport of transferrin binding proteins through bacterial membrane spaces, was present in 60.8% of the P. multocida strains tested.
Table 2.
Frequency of protein-coding genes and virulence factors in P. multocida strains isolated from rabbits.
| Gene | Virulence factor | No. of positives/(%) |
|---|---|---|
| toxA | Dermonecrotic toxin | 0/(0) |
| pfhA | Filamentous hemagglutinin | 0/(0) |
| hgbA | Hemoglobin-binding protein | 34/(73.9) |
| hgbB | Hemoglobin-binding protein | 14/(30.4) |
| exbBD-tonB | Iron acquisition | 28/(60.8) |
| nanH | Neuraminidase | 31/(67.3) |
| psl | Porin | 44/(95.6) |
| nanB | Neuraminidase | 44/(95.6) |
| ompH | Outer membrane protein H | 35/(76) |
| oma87 | Outer membrane protein 87 | 39/(84.7) |
| ptfA | Type 4 fimbriae | 43/(93.4) |
| sodA | Superoxide dismutase | 35/(76) |
| sodC | Superoxide dismutase | 45/(97.8) |
| tbpA | Transferrin binding protein | 4/(8.6) |
| fimA | Fimbriae | 30/(65.2) |
| hsf1 | Autotransporter adhesion | 13/(28.2) |
| hsf2 | Autotransporter adhesion | 31/(67.3) |
| tadD | Putative nonspecific tight adherence protein D | 42/(91.3) |
| fur | Ferric uptake regulation protein | 02/(4.3) |
| pmHAS | Hyaluronan synthase | 35/(76.0) |
| ompA | Outer membrane protein A | 13/(28.2) |
| plpB | Lipoprotein B | 16/(34.7) |
With respect to the genes encoding hemoglobin-binding proteins, the most frequent was hgbA (73.9%), followed by the hgbB gene (30.4%). Among genes encoding outer membrane proteins, OmpH (76%) and Oma87 (84.7%) genes were more frequent than OmpA (28.2%) and plpB (34. 7%) genes. The tbpA (8.6%) and fur (4.3%) genes presented frequencies below ten percent.
By using the virulence genes profile, it was possible to build a dendrogram (Figure 1) that distributes the strains in 38 different combinations. Considering groups with more than 80% of similarity (presence or absence of up to 4 genes), it was possible to observe that strains from rabbitries A are clustered together, and with exception of a few cases, strains from the same animal are also clustered together. The discriminatory index of the virulence gene profile was 0.99.
Figure 1.
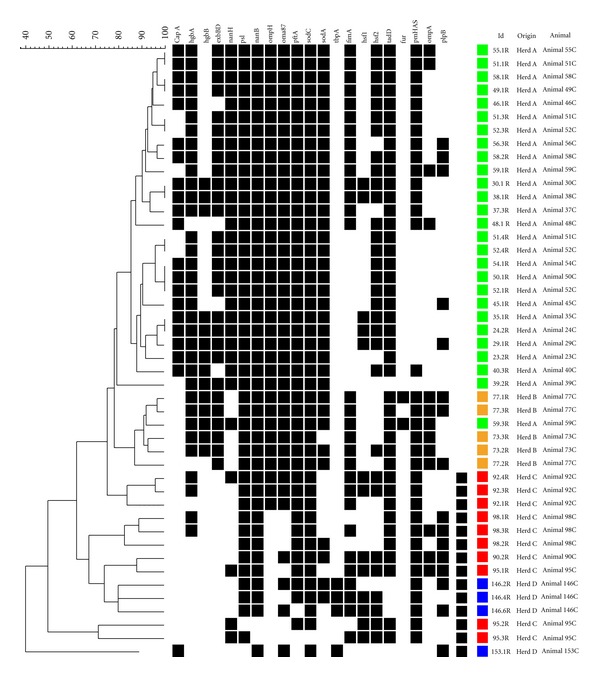
Dendrogram representing relatedness among P. multocida strains isolated from rabbits according to virulence profile.
Considering the resistance profile, 47.8% (22/46) of the strains were resistant to at least one of the tested drugs. The resistance was more common against sulfonamides and cotrimoxazole with 28.3% of strains (13/46), followed by penicillin with 10.9% (5/46), amoxicillin with 6.5% (3/46), and erythromycin with 4.3% (2/46). All tested strains were sensitive to ceftiofur, florfenicol, norfloxacin, enrofloxacin, ciprofloxacin, tetracycline, and doxycycline.
4. Discussion
Stahel et al. [15] describe a frequency ranging from seven to nearly 100% of P. multocida in the upper respiratory tract of rabbits of different origins. At present, the frequency observed was 20.7% (29/140), varying from 7.4 to 52.5% in four Brazilian rabbitries. Using PCR, less than 50% of the strains were identified as capsular type A, which is the capsular type most often described for rabbits [13]. The high frequency of untypeable strains for the gene encoding the capsule (54.3%) using PCR was an unexpected data.
Arumugam et al. [16] compared identification of capsular type in P. multocida from different hosts using conventional phenotypic methods as well as the PCR multiplex described by Townsend et al. [11]. The authors reported 19.3% (22/114) of strains untypeable using PCR, against 48.2% (55/114) of untypeable strains using the hyaluronidase test, and acriflavine flocculation for serogroup A and D identification, respectively, as well as serological test to capsular type B.
The negative results of all samples for gene toxA may be related to the fact that the gene encoding dermonecrotic toxin is more frequent in atrophic rhinitis of swine. This gene was less frequent also in sheep and poultry strains [13]. Even in porcine toxigenic isolates, the presence of the gene proved to be low, and the gene is usually lost after a few subcultures. Some authors describe that the toxA gene encoding the toxin is not inserted into the bacterial chromosome but in a lysogenic bacteriophage that infects the agent [17].
Gene pfhA was also not found in any strain analyzed, which contrasts with that described by Ewers et al. [13], who found the gene in 25% (2/8) of isolates from dogs, 18.5% from cats (10/54), and 21.2% (11/52) from swine. Tang et al. [9] working with swine strains describe 15% of P. multocida tested positive to the pfhA gene (35/233). To date, no author describes the presence of this gene in P. multocida strains from rabbits.
The high prevalence of the ptfA gene (type 4 fimbriae) found in this study (93.4%-43/46) was expected since it is supposed to be a key element in fixing bacteria on the surface of the epithelial cells of hosts being rather common in rabbits [13].
The gene tadD has been described as a putative nonspecific tight adherence protein D in P. multocida [18] and was present in 91.3% (42/46) of rabbit strains. In swine strains this gene was described in 43.3% (100/233) of the isolates tested [9].
According to Atashpaz et al. [19] and Ewers et al. [13], the tbpA gene is closely related to ruminant strains (cattle, sheep, and buffalo), which explains their low frequency in the present study (8.6%-4/46).
Genes encoding proteins with different functions, such as iron acquisition (exbBD-tonB, fur), hyaluronidase (pmHAS), hemoglobin-binding proteins (hgbA and hgbB), superoxidee dismutase (SodA, SodC), porin (psl), neuraminidase (nanH and nanB), adesins (fimA, hsf-1, hsf-2), and membrane proteins (oma87, ompH, plpB), showed frequencies similar to those reported by Ewers et al. [13] and Tang et al. [9].
In contrast to what described by Tang et al. [9], the present study did not permit the association of a particular virulence factor to a specific capsular type, since the distribution of virulence factors was very heterogeneous among capsular type A and untypeable strains. It was observed that frequency of untypeable strains was significantly higher in strains isolated from herds B, C, and D, when compared to herd A.
The virulence genes profile generated were compared, and according to the presence or absence of tested genes the strains were clustered in 38 gene combinations with a high discrimination index (0.99). This information could not be compared to those found in the literature, but a strong association among strains from rabbitry and the animal were observed in clusters formed considering more than 80% of similarity. The virulence gene profile showed to be a good tool for the discrimination of P. multocida strains.
Infections caused by P. multocida are usually treated with a broad spectrum of antibiotics [20]. The antimicrobial susceptibility data from this study indicate that cephalosporins, florfenicol, tetracyclines, and fluoroquinolones are the most effective drugs, a fact also reported in studies conducted in France, North America, and Japan [20–22]. The high resistance of the isolates against sulfonamides and cotrimoxazole has also been previously described [9].
Rabbits have a growing role as companion animals and are traditionally used as a source of animal protein. The present study showed a relatively high frequency of agent isolation in Brazilian rabbits and a great potential of virulence and zoonotic transmission concerning the virulence gene profiles detected.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This study was supported by FAPESP—Fundação de Amparo a Pesquisa do Estado de São Paulo—(process: 2007/08592-3 and 2007/03024-7) and CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
References
- 1.Dziva F, Muhairwa AP, Bisgaard M, Christensen H. Diagnostic and typing options for investigating diseases associated with Pasteurella multocida . Veterinary Microbiology. 2008;128(1-2):1–22. doi: 10.1016/j.vetmic.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Langan GP, Lohmiller JJ, Swing SP, Wardrip CL. Respiratory diseases of rodents and rabbits. Veterinary Clinics of North America—Small Animal Practice. 2000;30(6):1309–1335. doi: 10.1016/S0195-5616(00)06009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Tayeb AB, Morishita TY, Angrick EJ. Evaluation of Pasteurella multocida isolated from rabbits by capsular typing, somatic serotyping, and restriction endonuclease analysis. Journal of Veterinary Diagnostic Investigation. 2004;16(2):121–125. doi: 10.1177/104063870401600205. [DOI] [PubMed] [Google Scholar]
- 4.de Souza ARM, Arthur V, Canniatti-Brazaca SG, Couto MAL. Efeito da irradiação em carne de coelho congelada. Ciência e Tecnologia de Alimentos. 2010;30(1):30–34. [Google Scholar]
- 5.Per H, Kumandaş S, Gümüş H, Öztürk MK, Çoşkun A. Meningitis and subgaleal, subdural, epidural empyema due to Pasteurella multocida . The Journal of Emergency Medicine. 2010;39(1):35–38. doi: 10.1016/j.jemermed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Silberfein EJ, Lin PH, Bush RL, Zhou W, Lumsden AB. Aortic endograft infection due to Pasteurella multocida following a rabbit bite. Journal of Vascular Surgery. 2006;43(2):393–395. doi: 10.1016/j.jvs.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 7.Brogden KA. Physiological and serological characteristics of 48 Pasteurella multocida cultures from rabbits. Journal of Clinical Microbiology. 1980;11(6):646–649. doi: 10.1128/jcm.11.6.646-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning PJ, Naasz MA, DeLong D, Leary SL. Pasteurellosis in laboratory rabbits: characterization of lipopolysaccharides of Pasteurella multocida by polyacrylamide gel electrophoresis, immunoblot techniques, and enzyme-linked immunosorbent assay. Infection and Immunity. 1986;53(3):460–463. doi: 10.1128/iai.53.3.460-463.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang X, Zhao Z, Hu J, et al. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. Journal of Clinical Microbiology. 2009;47(4):951–958. doi: 10.1128/JCM.02029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutters R, Mannheim W, Bisgaard M. Taxonomy of the group. In: Adlan C, Rutter JM, editors. Pasteurella and Pasteurellosis. London, UK: Academic Press; 1989. pp. 3–34. [Google Scholar]
- 11.Townsend KM, Frost AJ, Lee CW, Papadimitriou JM, Dawkins HJS. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. Journal of Clinical Microbiology. 1998;36(4):1096–1100. doi: 10.1128/jcm.36.4.1096-1100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-van Dillen PME, van der Noordaa J. Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology. 1990;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewers C, Lübke-Becker A, Bethe A, Kießling S, Filter M, Wieler LH. Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status. Veterinary Microbiology. 2006;114(3-4):304–317. doi: 10.1016/j.vetmic.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. Journal of Clinical Microbiology. 1988;26(11):2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahel ABJ, Hoop RK, Kuhnert P, Korczak BM. Phenotypic and genetic characterization of Pasteurella multocida and related isolates from rabbits in Switzerland. Journal of Veterinary Diagnostic Investigation. 2009;21(6):793–802. doi: 10.1177/104063870902100605. [DOI] [PubMed] [Google Scholar]
- 16.Arumugam ND, Ajam N, Blackall PJ, et al. Capsular serotyping of Pasteurella multocida from various animal hosts—a comparison of phenotypic and genotypic methods. Tropical Biomedicine. 2011;28(1):55–63. [PubMed] [Google Scholar]
- 17.Pullinger GD, Bevir T, Lax AJ. The Pasteurella multocida toxin is encoded within a lysogenic bacteriophage. Molecular Microbiology. 2004;51(1):255–269. doi: 10.1046/j.1365-2958.2003.03829.x. [DOI] [PubMed] [Google Scholar]
- 18.May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3460–3465. doi: 10.1073/pnas.051634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atashpaz S, Shayegh J, Hejazi MS. Rapid virulence typing of Pasteurella multocida by multiplex PCR. Research in Veterinary Science. 2009;87(3):355–357. doi: 10.1016/j.rvsc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Kehrenberg C, Schulze-Tanzil G, Martel JL, Chaslus-Dancla E, Schwarz S. Antimicrobial resistance in Pasteurella multocida and Manheimia: epidemiology and genetic basis. Veterinary Research. 2001;32(3-4):323–339. doi: 10.1051/vetres:2001128. [DOI] [PubMed] [Google Scholar]
- 21.Salmon SA, Watts JL, Case CA, Hoffman LJ, Wegener HC, Yancey RJ., Jr. Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United States, Canada, and Denmark. Journal of Clinical Microbiology. 1995;33(9):2435–2444. doi: 10.1128/jcm.33.9.2435-2444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimura H, Ishimaru M, Endoh YS, Kojima A. Antimicrobial susceptibility of Pasteurella multocida isolated from cattle and pigs. Journal of Veterinary Medicine B. 2001;48(7):555–560. doi: 10.1046/j.1439-0450.2001.00468.x. [DOI] [PubMed] [Google Scholar]


