Abstract
The aim of this study was to investigate the antiprotozoal and antiviral activities of four Argentinean Mikania species. The organic and aqueous extracts of Mikania micrantha, M. parodii, M. periplocifolia, and M. cordifolia were tested on Trypanosoma cruzi epimastigotes, Leishmania braziliensis promastigotes, and dengue virus type 2. The organic extract of M. micrantha was the most active against T. cruzi and L. braziliensis exhibiting a growth inhibition of 77.6 ± 4.5% and 84.9 ± 6.1%, respectively, at a concentration of 10 μg/ml. The bioguided fractionation of M. micrantha organic extract led to the identification of two active fractions. The chromatographic profile and infrared analysis of these fractions revealed the presence of sesquiterpene lactones. None of the tested extracts were active against dengue virus type 2.
1. Introduction
Neglected tropical diseases (NTDs) are a group of infectious diseases that cause significant morbidity and mortality in the developing world. American Trypanosomiasis or Chagas' disease, leishmaniasis, and dengue are considered NTDs [1]. According to the World Health Organization (WHO), there are more than 1 billion people that suffer one or more NTD, mostly concentrated in countries of Africa, Asia, and Latin America, where life conditions are linked to poverty. As a consequence, the development of new or better drugs to fight these diseases is not a priority for the pharmaceutical industry.
Chagas' disease, caused by the protozoan parasite Trypanosoma cruzi, affects 10 million people worldwide [2], and almost 12 million people are infected with Leishmania spp. [3]. Dengue is a viral infection caused by an RNA virus and it is estimated that 50–100 million cases occur annually while approximately half of the world's population is at risk [4].
Chagas' disease, leishmaniasis, and dengue are considered to be, among others, the most common tropical diseases in Argentina [5]. Between 1.5 and 2 million people are reported to be affected by Chagas' disease in this country [6]. American tegumentary leishmaniasis (ATL) is endemic in Northern Argentina where it is frequently associated with Chagas' disease [7] and its incidence, due in particular to Leishmania braziliensis, has increased during the last two decades [8]. On the other hand, a dengue epidemic outbreak in 2009 produced more than 25000 cases [9].
The drugs currently available to treat acute Chagas' disease infection are the nitroaromatic compounds, benznidazole and nifurtimox, both of which were discovered in the 70s'. They are effective only in the acute phase of the disease and have serious side effects [2]. The chemotherapy of leishmaniasis is based on pentavalent antimonials, amphotericin B, miltefosine and paromomycin which all have drawbacks [10]. In the case of dengue, however, currently, there are neither licensed vaccines nor any available drug to treat this viral infection [11]. In view of this situation, there is an urgent need to find new drugs to treat these NTDs.
Natural products have played an important role in the drug discovery process, since they are generally small molecules with a wide chemical diversity and more “drug-likeness” than synthetic compounds, so that they are good candidates for lead drug development [12, 13].
In the last decades, the Asteraceae family has been extensively studied due to the great number and variety of active compounds isolated from species belonging to it. Among these, the genus Mikania, which comprises nearly 450 species [14], has been reported to contain some interesting chemical substances, mostly terpenoid compounds (sesquiterpene lactones and diterpenes) and flavonoids. These secondary metabolites are known to have important biological activities, including anti-infective properties [15–18]. There are no previous reports concerning the evaluation of Mikania spp. on dengue virus, though M. micrantha has been reported to be effective against respiratory viruses [19].
The aim of the present study, thus, was to determine the in vitro antiprotozoal and antiviral activities of four Argentinean Mikania species. Organic and aqueous extracts of Mikania micrantha, M. periplocifolia, M. parodii, and M. cordifolia were tested against Trypanosoma cruzi, Leishmania braziliensis, and dengue virus.
2. Materials and Methods
2.1. Plant Material
The aerial parts of Mikania micrantha Kunth (Asteraceae) were collected in Tucumán Province, Argentina in June 2009. Botanical identification was performed by Lic. A. Slanis and Dr. B. Juarez. A voucher specimen (LIL 609699) was deposited at the Herbarium of Instituto Miguel A. Lillo.
Mikania parodii Cabrera (Asteraceae) (BAF 713) and Mikania cordifolia (L. f.) Willd. (Asteraceae) (BAF 715) were collected in May 2009 and Mikania periplocifolia Hook. & Arn. (Asteraceae) (BAF 732) in November 2011, in all cases in Entre Ríos Province. The plant material was identified by one of the authors and voucher specimens were deposited at the Museo de Farmacobotánica, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires.
2.2. Microorganisms
Trypanosoma cruzi epimastigotes (RA strain) were grown in a biphasic medium and Leishmania braziliensis promastigotes (2903 strain) in liver infusion tryptose medium (LIT). Cultures were routinely maintained by weekly passages at 28°C and 26°C, respectively.
The replication of dengue virus type 2 (DENV-2) (16681 strain) was performed using Vero cells (ATCC CCL-81), baby hamster kidney (BHK-21) clon 15.
2.3. Preparation of Plant Extracts
The aerial parts of Mikania micrantha, M. periplocifolia, M. parodii, and M. cordifolia (20 g each) were air dried and extracted with dichloromethane/methanol (1 : 1) (200 mL) at room temperature for 24 h and then vacuum filtered. The process was repeated twice and the extracts were combined and dried under vacuum. The marc was then extracted with water in the same conditions. Aqueous extracts were freeze-dried.
2.4. Fractionation of Mikania micrantha Organic Extract
The aerial parts of M. micrantha (400 g) were extracted with dichloromethane/methanol (1 : 1) as described above. The organic extract was subjected to open column chromatography on silica gel 60 and eluted successively with hexane, hexane: ethyl acetate (1 : 1), ethyl acetate and methanol yielding 48 fractions of 250 mL each. According to their thin-layer chromatography profile, these fractions were combined into 8 final fractions (1–8). These were subsequently tested for trypanocidal activity against T. cruzi epimastigotes.
2.5. High Performance Liquid Chromatography Analysis (HPLC)
The HPLC analysis of fractions 3 and 4 was performed on a Varian Pro Star instrument equipped with a Rheodyne injection valve (20 μl) and a photodiode array detector set at 210 nm.
A reversed-phase column Phenomenex—C18 (2) Luna (250 mm × 4.6 mm·5 μ.) was used. Samples were eluted with a gradient of water (A) and acetonitrile (B) from 0% B to 75% B in 30 min and 75% B to 100% B in 2 min. The flow rate was 1.0 mL/min and the separation was done at room temperature. Chromatograms were recorded and processed using the Varian Star Chromatography Workstation version 6.x.
Fractions 3 and 4 were dissolved in methanol and water (90 : 10) to a final concentration of 5 mg/mL.
Water employed to prepare the mobile phase was of ultrapure quality (Milliq). Acetonitrile (HPLC) J. T. Baker and methanol (HPLC) J. T. Baker were used.
2.6. Infrared Spectroscopic Analysis
The IR-spectra of the active fractions 3 and 4 were recorded on FT-IR spectrophotometer (Bruker IFS-25) in chloroform solution.
2.7. Trypanocidal and Leishmanicidal Activity Assay
Growth inhibition of T. cruzi epimastigotes and L. braziliensis promastigotes was evaluated using the previously described [3H] thymidine uptake assay [15]. Parasites were adjusted to a cell density of 1.5 × 106/mL and cultured in the presence of each extract or fraction for 72 h. Benznidazole (5 to 20 μM; Roche) and Amphotericin B (0.27–1.6 μM, ICN) were used as positive controls. The percentage of inhibition was calculated as 100 − {[(cpm of treated parasites)/(cpm of untreated parasites)] × 100} [16]. Organic and aqueous extracts of Mikania species were tested on both parasites at concentrations of 100, 10 and 1 μg/mL for 72 h. Extracts which showed an inhibition below 30% at a concentration of 10 μg/mL were no further tested. Fractions 1–8 were assayed on T. cruzi at concentrations of 100 and 10 μg/mL.
2.8. Antiviral Activity Assay
Vero cells were seeded in Minimal Essential Medium (MEM), supplemented with 10% fetal bovine serum (FBS, PAA) in microwell plates (96 wells) at a density of 2.2 × 104 cells per well. After 24 h in a 5% CO2 incubator at 37°C, the cells were infected with DENV-2 in MEM supplemented with 2% FBS that induced an 80–90% cytopathic effect (CPE) on the sixth day postinoculation in absence of the drug.
The cytotoxic concentration 50% (CC50), defined as the concentration that inhibits the proliferation of exponentially growing cells by 50%, was calculated for organic and aqueous extracts.
Two-fold serial dilutions of organic (12–0.75 μg/mL) and aqueous extracts (500–31.25 μg/mL) of Mikania species were tested in quadruplicate. Mock-infected cells with and without extracts and infected cells without extract were included as controls. Ribavirin (100–1 μg/mL; Sigma-Aldrich) was used as a positive control. The CPE was determined by the measurement of cell viability using the MTS/PMS method (CellTiter 96 Aqueous) (Promega, Madison, WI) as previously described [20]. All extracts were tested at concentrations below their CC50.
2.9. Statistical Analysis
The results are expressed as mean ± SEM. The level of statistical significance was determined submitting the data to one-way analysis of variance (ANOVA) using GraphPad Prism 3.0 software (GraphPad Software Inc., San Diego, CA). All data were referred to the control group. P values of <0.05 were considered significant.
3. Results
3.1. Trypanocidal Activity
The trypanocidal activity of organic and aqueous extracts of Mikania species was evaluated in vitro on T. cruzi epimastigotes. Results are presented in Table 1.
Table 1.
Trypanocidal activity of organic and aqueous extracts of Mikania micrantha, M. periplocifolia, M. parodii, and M. cordifolia.
| Species | Extract | % of growth inhibition ± SEM | ||
|---|---|---|---|---|
| 100 μg/mL | 10 μg/mL | 1 μg/mL | ||
| Mikania micrantha | Organic | 91.1 ± 3.7 | 77.6 ± 4.5 | 14.2 ± 4.6 |
| Aqueous | 40.4 ± 1.4 | 23.2 ± 2.9 | n.d. | |
| Mikania periplocifolia | Organic | 95.5 ± 0.4 | 56.7 ± 5.0 | 7.0 ± 4.2 |
| Aqueous | 40.2 ± 2.5 | 25.2 ± 1.0 | n.d. | |
| Mikania parodii | Organic | 94.9 ± 0.5 | 33.0 ± 1.3 | 2.3 ± 1.5 |
| Aqueous | 30.2 ± 2.0 | 19.0 ± 1.8 | n.d. | |
| Mikania cordifolia | Organic | 86.2 ± 1.8 | 10.5 ± 2.5 | n.d. |
| Aqueous | 13.6 ± 2.8 | 12.2 ± 5.4 | n.d. | |
Results are expressed as mean ± SEM. n.d.: not determined.
The organic extracts of the four Mikania species were found to be active against T. cruzi epimastigotes with an inhibition above 85% at 100 μg/mL. The organic extract of M. micrantha proved to be the most active of all tested species, showing an inhibition of 77.6 ± 4.5% and 14.2 ± 4.6%, when applied at concentrations of 10 and 1 μg/mL, respectively. The aqueous extracts of the four Mikania species showed inhibitions ranged between 13 and 40% at 100 μg/mL (Table 1).
According to these results, the organic extract of M. micrantha was selected for further fractionation.
3.2. Leishmanicidal Activity
The leishmanicidal effect of extracts of Mikania species was evaluated on L. braziliensis promastigotes. The results are shown in Table 2.
Table 2.
Leishmanicidal activity of organic and aqueous extracts of Mikania micrantha, M. periplocifolia, M. parodii, and M. cordifolia.
| Species | Extract | % of growth inhibition ± SEM | ||
|---|---|---|---|---|
| 100 μg/mL | 10 μg/mL | 1 μg/mL | ||
| Mikania micrantha | Organic | 90.9 ± 0.8 | 84.9 ± 6.1 | 77.8 ± 1.1 |
| Aqueous | 41.6 ± 4.1 | 29.9 ± 1.5 | n.d. | |
| Mikania periplocifolia | Organic | 73.4 ± 5.3 | 69.2 ± 2.0 | 53.5 ± 4.3 |
| Aqueous | 78.4 ± 7.2 | 11.4 ± 4.0 | n.d. | |
| Mikania parodii | Organic | 87.3 ± 1.7 | 73.0 ± 0.6 | 58.7 ± 3.9 |
| Aqueous | 48.7 ± 8.4 | 7.7 ± 1.5 | n.d. | |
| Mikania cordifolia | Organic | 69.7 ± 6.6 | 55.7 ± 7.4 | 35.3 ± 7.5 |
| Aqueous | 38.9 ± 3.5 | 5.0 ± 1.1 | n.d. | |
Results are expressed as mean ± SEM. n.d.: not determined.
At a concentration of 100 μg/mL, organic extracts of M. micrantha and M. parodii displayed leishmanicidal activity with growth inhibition rates above 85%. At the lowest concentration tested (1 μg/mL), M. micrantha was the most active extract with an inhibition of 77.8 ± 1.1%.
Aqueous extracts displayed inhibition rates below 30% at a concentration of 10 μg/mL.
3.3. Antiviral Activity
None of the organic and aqueous extracts from the four tested Mikania species was able to inhibit the replication of DENV-2. Approximately 30% reduction of the CPE effect was observed when infected cells were treated with 500 μg/mL aqueous extracts.
3.4. Bioassay-Guided Fractionation of Mikania micrantha Organic Extract
The fractionation of the organic extract of M. micrantha by column chromatography yielded eight final fractions (1–8), which were tested in vitro against T. cruzi epimastigotes. Fractions 3, 4, and 6, at a concentration of 100 μg/mL, showed trypanocidal activity with percentages of growth inhibition of 93.2 ± 1.0%, 91.8 ± 1.2%, and 91.4 ± 2.6%, respectively (Figure 1).
Figure 1.
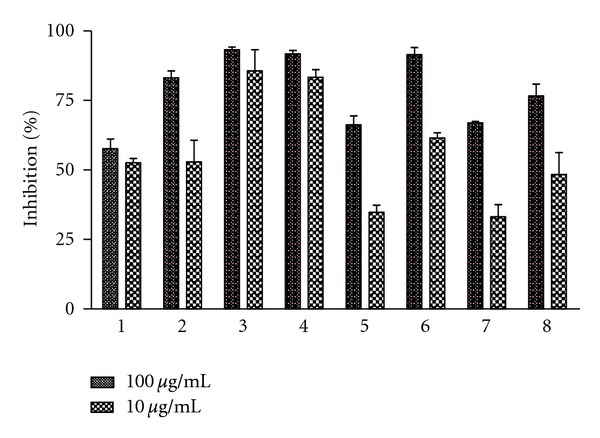
Trypanocidal activity of fractions 1–8 of Mikania micrantha on T. cruzi epimastigotes.
At the lowest tested concentration (10 μg/mL), fractions 3 and 4 were the most active against T. cruzi with inhibition rates of 85.7 ± 7.6% and 83.4 ± 2.8%, respectively (Figure 1). The analysis of the HPLC profile of these two fractions showed the presence of the same three major peaks with retention times of 16.5, 19.2, and 20.6 min and UV maximum at 219, 217, and 223 nm, respectively (Figure 2).The infrared spectroscopic analysis of these fractions showed the presence of bands between 1750–1790 cm−1, corresponding to γ-lactone carbonyl group (data not shown).
Figure 2.
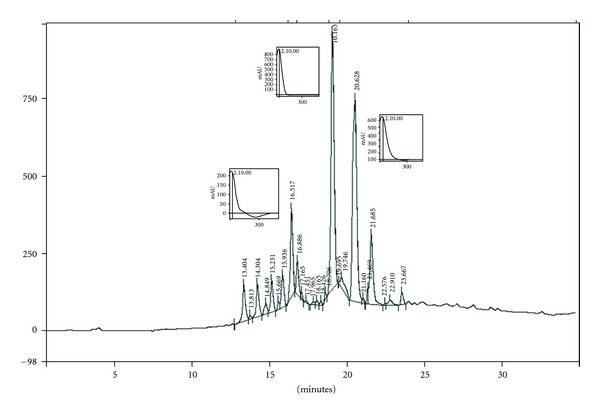
HPLC chromatographic profile of fraction 4 from Mikania micrantha.
4. Discussion
In the present study, the antiprotozoal and antiviral effects of extracts of four Argentinean Mikania species against Trypanosoma cruzi, Leishmania braziliensis, and dengue virus type 2 were evaluated.
The organic extracts of M. micrantha, M. periplocifolia, M. parodii, and M. cordifolia showed significant in vitro antiprotozoal activity against T. cruzi epimastigotes and L. braziliensis promastigotes. The M. micrantha organic extract was the most active against the two protozoans. All aqueous extracts displayed moderate to low activity against T. cruzi and L. braziliensis. This is the first time that trypanocidal and leishmanicidal activities of M. parodii are reported.
In the case of antiviral activity, neither the organic nor the aqueous extracts were able to inhibit the replication of dengue virus type 2 under the described experimental conditions.
Previous reports on the chemical composition of species of the genus Mikania (Asteraceae) describe terpenoid compounds and flavonoids as the main constituents [21–24]. There are some references about the antiprotozoal and antiviral activities of Mikania spp. [19, 25, 26] and particularly, in the case of the four studied species, there are some reports of studies performed on different strains, stages, and parasite species than the ones used herein [27–29].
The bioguided fractionation of M. micrantha organic extract resulted in the identification of the most active fractions (3, 4) against T. cruzi. The chromatographic profile of these fractions revealed the presence of three major peaks with UV spectra that could be attributed to terpenoid compounds. Besides, in the infrared spectrum of these fractions, characteristic bands of sesquiterpene lactones could be observed. Thus, the active fractions 3 and 4 contain sesquiterpene lactones.
Terpenoids, mainly sesquiterpene lactones and diterpenes, are characteristic constituents of the genus Mikania and some of these metabolites have shown trypanocidal activity [24]. Thus, the trypanocidal activity of fractions 3 and 4 could be due to the presence of sesquiterpene lactones, since some of these compounds have been previously reported in M. micrantha [30–32].
These findings reveal the importance of the Mikania genus as a rich source of antiprotozoal molecules. Isolation and purification of the bioactive compounds from the active fractions of M. micrantha and bioassay-guided fractionation of the other active extracts are under way.
Authors' Contribution
These two authors contributed equally to this work L. C. Laurella, F. M. Frank.
Acknowledgments
The authors wish to thank Dr. Monica Esteva and Estela Lammel for kindly providing Leishmania promastigotes and T. cruzi epimastigotes, respectively, and Mrs. Cristina Aguilera and Mrs. Teresa Fogal for their valuable technical assistance. Dengue virus strain was kindly provided by Dr. Andrea Gamarnik from Instituto Leloir, Argentina. This research was supported in part by PIP 01540 (Consejo Nacional de Investigaciones Científicas y Técnicas) and UBACYT 20020090300115, 20020090200478 and 20020100100201.
References
- 1.World Health Organization. First WHO report on neglected tropical diseases. 2010 http://www.who.int/neglected_diseases/2010report/en/
- 2.World Health Organization. Chagas disease (American trypanosomiasis) Fact Sheet. 2010;(340) http://www.who.int/mediacentre/factsheets/fs340/en/index.html.
- 3.World Health Organization. Leishmaniasis, http://www.who.int/Leishmaniasis/en/
- 4.World Health Organization. Dengue control, http://www.who.int/denguecontrol/en/index.html.
- 5.Instituto Nacional de Medicina Tropical. Enfermedades más frecuentes en Argentina. http://www.msal.gov.ar/inmet/frecuentes.php.
- 6.Drugs for Neglected Diseases Initiative. Argentina: more action needed. Newsletter. 2010;19, article 5 [Google Scholar]
- 7.Frank FM, Fernández MM, Taranto NJ, et al. Characterization of human infection by Leishmania spp. in the Northwest of Argentina: immune response, double infection with Trypanosoma cruzi and species of Leishmania involveds. Parasitology. 2003;126(1):31–39. doi: 10.1017/s0031182002002585. [DOI] [PubMed] [Google Scholar]
- 8.Salomón OD, Acardi SA, Liotta DJ, et al. Epidemiological aspects of cutaneous Leishmaniasis in the Iguazú falls area of Argentina. Acta Tropica. 2009;109(1):5–11. doi: 10.1016/j.actatropica.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Ministerio de Salud. Plan Nacional de control del dengue y la fiebre amarilla, http://www.msal.gov.ar/inmet/documentos.php, 2009.
- 10.Drugs for Neglected Diseases Initiative. Leishmaniasis. Current treatments, http://www.dndi.org/diseases/vl/current-treatment.html.
- 11.Whitehorn J, Farrar J. Dengue. British Medical Bulletin. 2010;95(1):161–173. doi: 10.1093/bmb/ldq019. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Deng J, Gao Y, Zhu L, He R, Xu Y. The screening toolbox of bioactive substances from natural products: a review. Fitoterapia. 2011;82(8):1141–1151. doi: 10.1016/j.fitote.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Harvey AL. Natural products in drug discovery. Drug Discovery Today. 2008;13(19-20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Herz W. Terpenoid chemistry of Mikania species. Journal of the Indian Chemical Society. 1998;75(10–12):559–564. [Google Scholar]
- 15.Sülsen VP, Frank FM, Cazorla SI, et al. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) Antimicrobial Agents and Chemotherapy. 2008;52(7):2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sülsen VP, Frank FM, Cazorla SI, et al. Psilostachyin C: a natural compound with trypanocidal activity. International Journal of Antimicrobial Agents. 2011;37(6):536–543. doi: 10.1016/j.ijantimicag.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi D. Sesquiterpene lactones: structural diversity and their biological activities. In: Tiwari V, Mishra B, editors. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Kerala, India: Research Signpost; 2011. pp. 313–334. [Google Scholar]
- 18.Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.But PPH, He ZD, Ma SC, et al. Antiviral constituents against respiratory viruses from Mikania micrantha . Journal of Natural Products. 2009;72(5):925–928. doi: 10.1021/np800542t. [DOI] [PubMed] [Google Scholar]
- 20.Finkielsztein LM, Castro EF, Fabián LE, et al. New 1-indanone thiosemicarbazone derivatives active against BVDV. European Journal of Medicinal Chemistry. 2008;43(8):1767–1773. doi: 10.1016/j.ejmech.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Krautmann M, de Riscala EC, Burgueño-Tapia E, Mora-Pérez Y, Catalán CAN, Joseph-Nathan P. C-15-functionalized eudesmanolides from Mikania campanulata . Journal of Natural Products. 2007;70(7):1173–1179. doi: 10.1021/np070154t. [DOI] [PubMed] [Google Scholar]
- 22.Catalán CAN, Cuenca MDR, Hernández LR, Joseph-Nathan P. cis,cis-Germacranolides and melampolides from Mikania thapsoides . Journal of Natural Products. 2003;66(7):949–953. doi: 10.1021/np030055p. [DOI] [PubMed] [Google Scholar]
- 23.Ohkoshi E, Kamo S, Makino M, Fujimoto Y. Ent-Kaurenoic acids from Mikania hirsutissima (Compositae) Phytochemistry. 2004;65(7):885–890. doi: 10.1016/j.phytochem.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez XA. Eupatorieae—chemicals review. In: Heywood V, Harbone J, Turner B, editors. The Biology and Chemistry of the Compositae Vol I. London, UK: Academic Press; 1977. pp. 487–502. [Google Scholar]
- 25.Do Nascimento AM, Chaves JS, Albuquerque S, de Oliveira DCR. Trypanocidal properties of Mikania stipulacea and Mikania hoehnei . Fitoterapia. 2004;75(3-4):381–384. doi: 10.1016/j.fitote.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Luize PS, Tiuman TS, Morello LG, et al. Effects of medicinal plant extracts on growth of Leishmania (L.) amazonensis and Trypanosoma cruzi . Brazilian Journal of Pharmaceutical Sciences. 2005;41(1):85–94. [Google Scholar]
- 27.Chaves JS, Nascimento AMD, Soares AP, et al. Screening of Southeastern Brazilian Mikania species on Trypanosoma cruzi . Pharmaceutical Biology. 2007;45(10):749–752. [Google Scholar]
- 28.Muelas-Serrano S, Nogal JJ, Martínez-Díaz RA, Escario JA, Martínez-Fernández AR, Gómez-Barrio A. In vitro screening of American plant extracts on Trypanosoma cruzi and Trichomonas vaginalis . Journal of Ethnopharmacology. 2000;71(1-2):101–107. doi: 10.1016/s0378-8741(99)00185-3. [DOI] [PubMed] [Google Scholar]
- 29.Calderón ÁI, Romero LI, Ortega-Barría E, et al. Screening of Latin American plants for antiparasitic activities against malaria, Chagas disease, and Leishmaniasis. Pharmaceutical Biology. 2010;48(5):545–553. doi: 10.3109/13880200903193344. [DOI] [PubMed] [Google Scholar]
- 30.Cuenca MDR, Bardon A, Catalan CAN, Kokke WCMC. Sesquiterpene lactones from Mikania micrantha . Journal of Natural Products. 1988;51(3):625–626. doi: 10.1021/np50057a040. [DOI] [PubMed] [Google Scholar]
- 31.Nicollier G, Thompson AC. Essential oil and terpenoids of Mikania micrantha . Phytochemistry. 1981;20(11):2587–2588. [Google Scholar]
- 32.Huang H, Ye W, Wu P, Lin L, Wei X. New sesquiterpene dilactones from Mikania micrantha . Journal of Natural Products. 2004;67(4):734–736. doi: 10.1021/np034027i. [DOI] [PubMed] [Google Scholar]


