Abstract
Purpose
We evaluated predictors of progression after starting active surveillance, especially the role of prostate specific antigen and immediate confirmatory prostate biopsy.
Materials and Methods
A total of 238 men with prostate cancer met active surveillance eligibility criteria and were analyzed for progression with time. Cox proportional hazards regression was used to evaluate predictors of progression. Progression was evaluated using 2 definitions, including no longer meeting 1) full and 2) modified criteria, excluding prostate specific antigen greater than 10 ng/ml as a criterion.
Results
Using full criteria 61 patients progressed during followup. The 2 and 5-year progression-free probability was 80% and 60%, respectively. With prostate specific antigen included in progression criteria prostate specific antigen at confirmatory biopsy (HR 1.29, 95% CI 1.14–1.46, p <0.0005) and positive confir-matory biopsy (HR 1.75, 95% CI 1.01–3.04, p = 0.047) were independent predictors of progression. Of the 61 cases 34 failed due to increased prostate specific antigen, including only 5 with subsequent progression by biopsy criteria. When prostate specific antigen was excluded from progression criteria, only 32 cases progressed, and 2 and 5-year progression-free probability was 91% and 76%, respectively. Using modified criteria as an end point positive confirmatory biopsy was the only independent predictor of progression (HR 3.16, 95% CI 1.41–7.09, p = 0.005).
Conclusions
Active surveillance is feasible in patients with low risk prostate cancer and most patients show little evidence of progression within 5 years. There is no clear justification for treating patients in whom prostate specific antigen increases above 10 ng/ml in the absence of other indications of tumor progression. Patients considering active surveillance should undergo confirma-tory biopsy to better assess the risk of progression.
Keywords: prostate, prostatic neoplasms, prostate-specific antigen, biopsy, disease progression
Routine serum PSA testing for prostate cancer and more extensive biopsy strategies have led to increased diagnosis of prostate cancer at earlier stages. An estimated 23% to 42% of all prostate cancers detected by PSA testing do not pose an immediate threat to the patient.1 Thus, treatment for all screening detected cancer would result in many men receiving unnecessary therapy. To decrease the overtreatment rate AS with deferred de-finitive therapy has gained acceptance as a viable option in this patient group.
The main goal of AS is to avoid unnecessary treatment and its related morbidity in patients with low risk disease. Definitive therapy should be offered only to those who have unfavorable disease characteristics during followup while cure is still possible.2 Despite the appealing concept of AS most physicians still recommend aggressive treatment for small, screening detected prostate cancer. A recent review of management strategies suggested that AS is used to manage only 10% of low risk cancer cases, which is typically attributed to physician uncertainty in determining the biological potential of prostate cancer.3
Various eligibility criteria have been proposed to appropriately identify patients for AS and exclude those at higher risk for progression. Previously we reported that the result of immediate confirmatory biopsy can help stratify patients by progression risk.4,5 In the current study we examined predictors of progression while on AS with attention to baseline PSA and the role of immediate confirmatory biopsy. PSA and the change in PSA with time were suggested to predict progression on AS.6–10 However, PSA is normally used to define progression and trigger treatment in these studies. Thus, we evaluated the role of PSA as a predictor of progression using 2 end points, that is including and excluding PSA greater than 10 ng/ml as a progression criterion.
METHODS
After receiving institutional review board approval we identified 531 patients diagnosed with low risk prostate cancer between 1993 and 2009 who met study inclusion criteria on initial biopsy. All patients underwent confirmatory biopsy before AS was recommended. Of these patients 185 (35%) did not meet our criteria on confirmatory biopsy and 119 were ineligible due to more than 1 criterion. The most common reason was more than 3 positive cores (131 patients) and Gleason grade greater than 6 (129). Of the 346 patients confirmed to be eligible for AS on confirmatory biopsy 249 elected AS. A total of 11 patients with insufficient followup were excluded from study, leaving a final cohort of 238 available for analysis. In these 238 patients AS was initiated between September 1997 and February 2009. Study inclusion criteria were PSA less than 10 ng/ml, no prostate biopsy Gleason grade 4 or 5, clinical stage T1–T2a, 3 or fewer positive biopsy cores (minimum 10), no biopsy core containing more than 50% cancer involvement and confirmatory biopsy to reassess eligibility before starting AS. Patients with data missing on clinical stage but who met all other criteria were considered eligible for study.
After initiating our AS program patients were generally followed semiannually with digital rectal examination, free and total PSA measurements, and a review of general health and urinary symptoms. Biopsy was routinely recommended within 12 to 18 months of starting AS and subsequently repeated every 2 to 3 years or as prompted by a change in digital rectal examination or a sustained PSA increase. Treatment was recommended when the patient no longer met study eligibility criteria during followup.
Increasing PSA was generally used to recommend repeat biopsy rather than trigger intervention. Thus, all statistical analysis was done using 2 definitions of progression, including 1 based on full inclusion and 1 based on these criteria without the criterion of PSA greater than 10 ng/ml, called modified criteria.
We analyzed predictors of progression defined by full and modified criteria at any subsequent followup. Survival time started at the date of confirmatory biopsy. Patients who received any treatment for prostate cancer before progression were censored at the time of treatment. Those without treatment or who continued to meet AS criteria were censored at the date of last followup. The Kaplan-Meier method was used to plot the overall probability of meeting AS criteria with time and patients were stratified by the confirmatory biopsy result. Univariate and multivariate Cox proportional hazards regression was used to evaluate predictors of progression. All predictors were those at confirmatory biopsy, including patient age, PSA, prostate volume, PSA density, number of positive cores (fewer than 3 or 3), result of confirmatory biopsy (positive or negative for cancer), initial biopsy extent (fewer than 10, or 10 or greater cores), time between initial and confirmatory biopsy (6 or less, or greater than 6 months), laterality (bilateral or unilateral) and clinical stage (T1a/b/c or T2a). Due to the limited number of events in our cohort we included only 5 of the original 10 predictors on multivariate analysis. These 5 predictors were prespecified before data analysis and included PSA, PSA density, number of positive cores, result of confirmatory biopsy and initial biopsy extent. Statistical analysis was done using Stata® 11.
RESULTS
Full Criteria
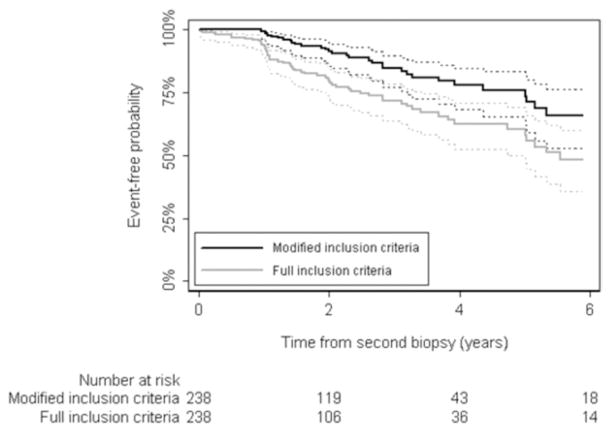
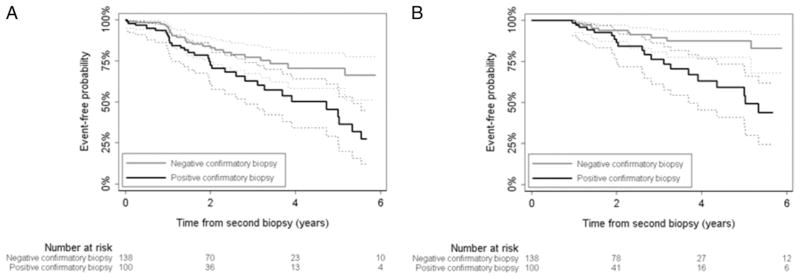
A total of 238 patients met full inclusion criteria at initial and confirmatory biopsy. Table 1 lists patient characteristics. Median patient age was 64 years (IQR 58, 68), median time to subsequent biopsy was 4.7 months (IQR 3.4, 7.6) and median PSA was 4.1 ng/ml (IQR 2.5, 5.6). Of the patients 25 were censored due to treatment and 61 failed to meet full criteria at a subsequent followup. Most cases were considered to have progressed due to increasing PSA, or Gleason grade 4 or 5 cancer in the followup (third or subsequent) prostate biopsy (table 2). Median followup in those without progression was 1.8 years. A total of 27 patients (11%) were followed at least 5 years without progression. The 2 and 5-year probability of meeting full inclusion criteria was 80% (95% CI 73–85) and 60% (95% CI 50–69), respectively (fig. 1). With PSA included in the progression criteria patients without evidence of cancer on confirmatory biopsy had a 2-year progression-free probability of 84% (95% CI 76–90) vs 73% (95% CI 60–82) in those with positive confirmatory biopsy who still met AS eligibility criteria (fig. 2). On univariate analysis PSA at confirmatory biopsy (HR 1.31, 95% CI 1.17–1.47, p <0.0005), PSA density (HR 1.09, 95% CI 1.04–1.15, p = 0.0005), 3 positive cores on confirmatory biopsy (HR 3.71, 95% CI 1.57–8.78, p = 0.003) and confirmatory biopsy positive for cancer (HR 2.17, 95% CI 1.30–3.63, p = 0.003) were significantly associated with progression defined by the full criteria. On multivariate analysis in 233 patients baseline PSA (HR 1.29, 95% CI 1.14–1.46, p <0.0005) and evidence of cancer on confirmatory biopsy (HR 1.75, 95% CI 1.01–3.04, p = 0.047) were the only independent predictors of progression (table 3). Five patients were excluded from multivariate analysis since the number of cores on initial biopsy was missing.
Table 1.
Patient characteristics at confirmatory biopsy
| No. Pts (%) | |
|---|---|
| Subsequent biopsy within 6 mos | 151 (63) |
| Ca on confirmatory biopsy: | |
| Yes | 100 (42) |
| No | 138 (58) |
| Clinical stage: | |
| T1 | 205 (86) |
| T2a | 31 (13) |
| Missing | 2 (1) |
| Gleason grade:* | |
| 5 | 1 (1) |
| 6 | 99 (99) |
| No. pos cores:* | |
| 1 | 60 (60) |
| 2 | 25 (25) |
| 3 | 15 (15) |
Only in patients with positive confirmatory biopsy.
Table 2.
Reason for progression in patients who progressed on AS
| No. Full Criteria* | No. Modified Criteria* | |
|---|---|---|
| PSA 10 ng/ml or greater | 34 | — |
| Gleason 7 or greater | 23 | 28 |
| Greater than 3 pos cores | 7 | 7 |
| Clinical stage greater than T2a | 1 | 1 |
| Tumor in greater than 50% of 1 biopsy core | 2 | 2 |
| No longer met criteria overall | 61 | 32 |
Four and 1 patients progressed due to 2 and 3 factors, respectively.
Figure 1.
Progression-free probability by full and modified criteria results with 95% CI.
Figure 2.
Progression-free probability by confirmatory biopsy results. A, full criteria with PSA. B, modified criteria without PSA.
Table 3.
Univariate and multivariable Cox regression analysis of progression predictors on AS using full and modified criteria
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Full criteria | ||||
| Biopsy 2 PSA | 1.31 (1.17–1.47) | <0.0005 | 1.29 (1.14–1.46) | <0.0005 |
| Ca evidence at biopsy 2 (yes vs no) | 2.17 (1.30–3.63) | 0.003 | 1.75 (1.01–3.04) | 0.047 |
| PSA density | 1.09 (1.04–1.15) | 0.0005 | 1.06 (0.99–1.12) | 0.075 |
| 3 Pos cores at biopsy 2 (yes vs no) | 3.71 (1.57–8.78) | 0.003 | 2.42 (0.97–6.05) | 0.058 |
| 10 or More cores at biopsy 1 (yes vs no) | 0.85 (0.49–1.45) | 0.5 | 0.81 (0.47–1.39) | 0.4 |
| Age at biopsy 2 | 1.01 (0.98–1.05) | 0.5 | — | |
| Prostate vol | 1.01 (1.00–1.01) | 0.1 | — | |
| Biopsy 2 within 6 mos of biopsy 1 (yes vs no) | 0.94 (0.56–1.58) | 0.8 | — | |
| Biopsy 2 lat (bilat vs unilat) | 1.19 (0.47–3.01) | 0.7 | — | |
| Biopsy 2 clinical stage: | ||||
| T1a/b/c | Referent | — | ||
| T2a | 1.22 (0.60–2.49) | 0.6 | — | |
| Modified criteria | ||||
| Biopsy 2 PSA | 1.14 (0.98–1.33) | 0.09 | 1.13 (0.95–1.35) | 0.18 |
| Ca evidence at biopsy 2 (yes vs no) | 3.22 (1.55–6.68) | 0.002 | 3.16 (1.41–7.09) | 0.005 |
| PSA density | 1.09 (1.02–1.18) | 0.018 | 1.06 (0.97–1.16) | 0.19 |
| 3 Pos cores at biopsy 2 (yes vs no) | 4.00 (1.19–13.40) | 0.025 | 2.40 (0.66–8.77) | 0.18 |
| 10 or More cores at biopsy 1 (yes vs no) | 0.78 (0.37–1.64) | 0.5 | 0.69 (0.32–1.46) | 0.3 |
| Age at biopsy 2 | 1.05 (0.99–1.10) | 0.092 | — | |
| Prostate vol | 1.00 (0.99–1.01) | 0.9 | — | |
| Biopsy 2 within 6 mos of biopsy 1 (yes vs no) | 1.21 (0.59–2.50) | 0.6 | — | |
| Biopsy 2 lat (bilat vs unilat) | 1.18 (0.28–4.98) | 0.8 | — | |
| Biopsy 2 clinical stage: | ||||
| T1a/b/c | Referent | — | ||
| T2a | 1.51 (0.61–3.69) | 0.4 | — | |
Modified Criteria
In addition to the 25 patients censored in the full criteria model, another 7 were censored since they were treated before progression by the modified criteria. About half as many patients progressed by the modified than the full criteria, that is 32 vs 61, meaning that 29 were deemed to have progression only due to a PSA increase to 10 ng/ml or greater. Only 5 of the 34 PSA failures subsequently progressed for another reason, that is a subsequent increase in biopsy Gleason grade.
Median followup in cases that who did not fail the modified criteria was 1.9 years. A total of 32 patients (13%) were followed at least 5 years. The 2 and 5-year probability of meeting the modified criteria was 91% (95% CI 86–95) and 76% (95% CI 66–83), respectively (fig. 1). When excluding PSA from progression criteria, PSA density (HR 1.09, 95% CI 1.02–1.18, p = 0.018), 3 positive cores on confirmatory biopsy (HR 4.00, 95% CI 1.19–13.40, p = 0.018) and positive confirmatory biopsy (HR 3.22, 95% CI 1.55–6.68, p = 0.002) were significantly associated with progression on univariate analysis. In the multivariate model positive confirmatory biopsy significantly increased the patient risk of progression (HR 3.16, 95% CI 1.41–7.09, p = 0.005, table 3). At 2 years patients without evidence of cancer on confirmatory biopsy had a progression-free probability of 94% (95% CI 87–97) while those with positive confirmatory biopsy had an 87% progression-free probability (95% CI 74–93, fig. 2).
DISCUSSION
This study provides further evidence that AS can be offered as an option in a highly select group of patients with low risk prostate cancer. To assess predictors of subsequent progression we used 2 definitions of progression, that is including and excluding a PSA increase of greater than 10 ng/ml. Results were more favorable when the PSA definition was excluded with a 5-year probability of 76% vs 60% to still meet the criteria when using PSA as a criterion. This difference occurred since most cases that failed due to increased PSA had no other evidence of progression. PSA at diagnosis was an independent predictor of progression only when a PSA increase was deemed to be progression. When biopsy criteria were used as the end point, baseline PSA did not distinguish patients at higher risk for failure.
Although it was suggested that PSA changes (PSA velocity or PSA doubling time) should be used to trigger therapy in patients on AS,11 little evidence supports such an approach. In many studies PSA changes were used to define progression, creating an artifactual relationship between predictor and outcome. For example, Klotz et al treated 450 men with prostate cancer expectantly with a median followup of 6.8 years.12 The absolute treatment rate was 30% with PSA doubling time less than 3 years the most common trigger for treatment. Serum PSA testing at baseline was investigated to characterize its potential to predict disease progression.6,8–10 Highly variable results coupled with arbitrary cutoffs limit its use in clinical practice.2,11 Results in studies analyzing PSA changes before radical treatment were also used to justify using PSA dynamics in AS cohorts.13 However, evidence to support the hypothesis that PSA dynamics have additional prognostic value over a single PSA level is lacking.14,15 While following a cohort from the Scandinavian radical prostatectomy trial, Fall et al concluded that the PSA rate of change had low accuracy to classify the disease as destined to progress, casting doubt on the accuracy of early PSA characteristics as a decision tool for therapeutic intervention in patients at low risk on AS.16 Ross et al also found that PSA velocity after diagnosis is an unreliable predictor of progression in patients on AS.17
Baseline PSA proved to be an independent predictor for AS failure only when we included PSA as a criterion for progression. This finding highlights a problem when analyzing PSA as a predictor of treatment in AS series. Usually PSA is an entry criterion as well as a trigger to define failure, creating a self-fulfilling prophecy. For example, if high PSA triggers treatment, higher PSA at entry onto AS means that this patient is more likely to meet the definition of PSA failure while on AS. Cancer was up-graded on subsequent biopsy in only 5 of the 34 men in whom failure was defined only by PSA greater than 10 ng/ml. Thus, we believe that until more accurate predictors of tumor biology become available, PSA criteria alone should not be used to define progression. Rather, PSA changes should prompt repeat biopsy. Also, stable or even decreasing PSA should not provide a false sense of security. Men on AS should undergo routine re-biopsy regardless of any PSA trends.
A critical factor for the success of an AS program is the use of appropriate entry criteria (patient selection).18 While a number of prognostic models have been developed to help identify men who are appropriate candidates for AS, their accuracy is limited and has not been validated.19,20 Most patients in whom AS fails experience failure in the initial observation period, usually within 2 years.12,21 In this short period it is unlikely that cancer actually increases substantially in grade or volume and failure more likely results from sampling error on initial biopsy.22 Berglund et al confirmed the limitations of a single biopsy by analyzing a cohort of 104 patients from our institution who met full inclusion criteria for AS based on initial biopsy but underwent confirmatory prostate biopsy within 3 months of diagnosis.5 Larger, higher grade cancer was found on confirmatory biopsy in 27% of the patients, who were then excluded from AS. Our policy has been to recommend repeat confirmatory biopsy, usually within 6 months, in all men eligible for AS to decrease the risk of substantially underestimating cancer size and grade. A multi-institutional cohort of patients on AS with the same inclusion and progression criteria as in the current study confirmed that the absolute proportion of patients treated was 16% compared with the 25% to 30% treatment rate reported in other series.12,23–25 The low progression rate by biopsy criteria in our series underscores the importance of repeat, confirmatory biopsy before recommending AS.
Absent tumor on confirmatory biopsy identifies a patient group that is unlikely to progress during the first 5 to 10 years of AS. Previous studies showed similar results.4,21,26 Despite a higher risk of progression during followup almost half of the patients with cancer in the confirmatory biopsy remain progression free at 5 years. Thus, treatment is not mandatory if cancer is seen on the confirmatory biopsy. Biopsy results should be used to counsel patients about therapy and judge the intensity of followup if the patient elects AS.
Our study has several limitations. Results are based on modest followup, which is related to the higher number of patients who elected AS in recent years. Long-term AS results are lacking and patients should be appropriately counseled regarding this limitation. Our study includes a long period, which led to variations in followup intensity, diagnostic biopsy strategies and the frequency of surveillance biopsy. Lastly, the only definitive evidence for progression of AS is metastasis or death from prostate cancer that could have been prevented by immediate treatment. To our knowledge there is no evidence that our progression criteria are appropriate surrogates for metastasis or death from prostate cancer. However, until better selection criteria are defined our bias is to be conservative, including routine confirmatory biopsy, when identifying appropriate AS candidates.
CONCLUSIONS
Our data provides further short-term evidence of the feasibility of AS in men with low risk prostate cancer. Most patients show little evidence of progression within 5 years. In men on AS there is little justification for treatment just because PSA increases above 10 ng/ml in the absence of other indications of tumor progression since most such men show no significant change in cancer by biopsy criteria. Patients with no cancer detected on confirmatory biopsy are less likely to progress during followup. Men considering AS should undergo confirmatory biopsy to confirm eligibility and better assess the cancer risk.
Acknowledgments
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations and Acronyms
- AS
active surveillance
- DRE
digital rectal examination
- PSA
prostate specific antigen
References
- 1.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastham JA. Active surveillance for prostate cancer with selective delayed definitive therapy. Clin Prostate Cancer. 2005;4:45. doi: 10.3816/cgc.2005.n.011. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Broering JM, Kantoff PW, et al. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 5.Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180:1964. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54:1297. doi: 10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Khatami A, Aus G, Damber JE, et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007;120:170. doi: 10.1002/ijc.22161. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Aprikian AG, Souhami L, et al. Utility of PSA doubling time in follow-up of untreated patients with localized prostate cancer. Urology. 2002;59:652. doi: 10.1016/s0090-4295(02)01526-1. [DOI] [PubMed] [Google Scholar]
- 9.Choo R, Klotz L, Danjoux C, et al. Feasibility study. watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664. [PubMed] [Google Scholar]
- 10.Khan MA, Carter HB, Epstein JI, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170:2274. doi: 10.1097/01.ju.0000097124.21878.6b. [DOI] [PubMed] [Google Scholar]
- 11.van den Bergh RC, Roemeling S, Roobol MJ, et al. Prostate-specific antigen kinetics in clinical decision-making during active surveillance for early prostate cancer—a review. Eur Urol. 2008;54:505. doi: 10.1016/j.eururo.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, Savage C, O’Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398. doi: 10.1200/JCO.2008.18.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien MF, Cronin AM, Fearn PA, et al. Pretreatment prostate-specific antigen (PSA) velocity and doubling time are associated with outcome but neither improves prediction of outcome beyond pretreatment PSA alone in patients treated with radical prostatectomy. J Clin Oncol. 2009;27:3591. doi: 10.1200/JCO.2008.19.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fall K, Garmo H, Andren O, et al. Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst. 2007;99:526. doi: 10.1093/jnci/djk110. [DOI] [PubMed] [Google Scholar]
- 17.Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810. doi: 10.1200/JCO.2009.25.7311. [DOI] [PubMed] [Google Scholar]
- 18.Bastian PJ, Carter BH, Bjartell A, et al. Insignificant prostate cancer and active surveillance: from definition to clinical implications. Eur Urol. 2009;55:1321. doi: 10.1016/j.eururo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 20.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368. [PubMed] [Google Scholar]
- 21.Al Otaibi M, Ross P, Fahmy N, et al. Role of repeated biopsy of the prostate in predicting disease progression in patients with prostate cancer on active surveillance. Cancer. 2008;113:286. doi: 10.1002/cncr.23575. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JI, Walsh PC, Carter HB. Dedifferentiation of prostate cancer grade with time in men followed expectantly for stage T1c disease. J Urol. 2001;166:1688. [PubMed] [Google Scholar]
- 23.Eggener SE, Mueller A, Berglund RK, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181:1635. doi: 10.1016/j.juro.2008.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 25.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359. doi: 10.1016/j.juro.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231. [PubMed] [Google Scholar]