Abstract
Background: Obesity is emerging as the most significant health concern of the 21st century. Although this is attributable in part to changes in our environment—including the increased prevalence of energy-dense food—it also appears that several lifestyle factors may increase our vulnerability to this calorie-rich landscape. Epidemiologic studies have begun to show links between adiposity and behaviors such as television watching, alcohol intake, and sleep deprivation. However, these studies leave unclear the direction of this association. In addition, studies that investigated the acute impact of these factors on food intake have reported a wide variety of effect sizes, from highly positive to slightly negative.
Objective: The purpose of this article was to provide a meta-analysis of the relation between lifestyle choices and increases in acute food intake.
Design: An initial search was performed on PubMed to collect articles relating television watching, sleep deprivation, and alcohol consumption to food intake. Only articles published before February 2012 were considered. Studies that took place in a controlled, laboratory setting with healthy individuals were included. Studies were analyzed by using 3 meta-analyses with random-effects models. In addition, a 1-factor ANOVA was run to discover any main effect of lifestyle.
Results: The 3 most prominent lifestyle factors—television watching, alcohol intake, and sleep deprivation—had significant short-term effects on food intake, with alcohol being more significant (Cohen's d = 1.03) than sleep deprivation (Cohen's d = 0.49) and television watching (Cohen's d = 0.2).
Conclusions: Our results suggest that television watching, alcohol intake, and sleep deprivation are not merely correlated with obesity but likely contribute to it by encouraging excessive eating. Because these behaviors are all known to affect cognitive functions involved in reward saliency and inhibitory control, it may be that they represent common mechanisms through which this eating is facilitated.
INTRODUCTION
The risk of obesity is increasing at an alarming rate, particularly in the Western world (1). Many researchers have focused their concerns over this growing epidemic on the underlying factors that may support an “obesogenic environment” in westernized countries (2). This has led to an extensive analysis of external factors influencing weight gain, such as the density of fast-food restaurants and the prevalence of food-related marketing (3–5). These studies argued that this environment causes obesity in part through increasing the accessibility and salience of energy-dense foods.
Although the increased availability and purchasing of high-calorie food should certainly promote weight gain, it may be that this does so through an interaction with obesogenic lifestyle choices that facilitate increased consumption (6). Evidence is emerging that links prominent behavioral patterns in the Western world—such as a tendency toward sleep deprivation, television watching, and alcohol consumption—to increased sensitivity to food reward and adiposity (7, 8). A recent epidemiologic study showed that 58.9% of adults in the United States watch television for >2 h/d and that those who do have higher daily energy intakes and are more likely to be overweight (9). Similarly, sleep deprivation has been shown to enhance the brain's response to hedonic food stimuli and encourage the development of obesity (10–14). Finally, alcohol use, which is ubiquitous in the Western world, has emerged as a possible risk factor for weight gain (15–17). This cluster of behaviors may constitute an “obesogenic lifestyle” that leaves our brains vulnerable to our obesogenic environment.
However, there is some controversy with regard to the role of these behaviors in the production of increased food intake. Studies have reported a wide range of effect sizes, indicating that these factors have high variability, and showed a range from strong increases in acute consumption to mild decreases in consumption. For example, despite several prior studies that showed the opposite result, a recent study reported that short-term sleep loss produces a mild, although nonsignificant, reduction in next-day food intake (18). Similarly, some studies have found a lack of stimulatory impact for both alcohol and television on food intake (19–21). This variety of findings shows the need for a systematic analysis of the role of these lifestyle factors in the induction of spontaneous food intake.
Against this background, the purpose of this study was to evaluate the effects of these potentially obesogenic lifestyle factors—television watching, short sleep duration, and alcohol consumption—on acute food intake. To do this, controlled laboratory studies that involved the effects of these factors on consumption were assessed. The aim was to show whether or not these factors are linked to increased acute caloric consumption.
METHODS
Database searches
For each of the factors assessed, PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) searches were performed to collect primary source articles for analysis. PubMed was considered the best freely available database and was thus the exclusive search database used. In the case of alcohol consumption, searches were performed with the use of combinations of the following words: “alcohol,” “ethanol,” “food,” “intake,” and “hunger.” In the case of sleep deprivation, searches were performed with the use of the words “sleep,” “reduced,” “deprivation,” “short,” “food,” “intake,” and “hunger.” Finally, for television the words “television,” “food,” “intake,” and “hunger” were used in different combinations.
Inclusion criteria
In all of the cases only controlled, laboratory studies in healthy participants were used. In studies that used alcohol, the administration of alcohol must have occurred a maximum of 30 min before an ad libitum test meal. Calorie consumption for all alcohol studies was calculated as the total energy consumed in the preload and meal (for both the alcohol and the control conditions). In studies that examined television viewing, only designs involving the co-occurrence of television watching and ad libitum eating were included (as opposed to eating before or after watching). Sleep-deprivation studies were included if they involved a maximum of 5.5 h of sleep (in the deprivation condition) and a minimum of 8 h of sleep in the sleep condition on the day before intake measurements. Because the effect of sleep deprivation is not specific to meal type, only studies involving a full day of food intake after sleep deprivation were included. Studies with multiple comparison groups were included, and each group was considered independently. By using these criteria, 8 television studies, 5 sleep studies, and 10 alcohol studies were included for analysis (12, 18–39). Only studies published before 1 February 2012 were included.
Data abstraction
Mean food intake, SD, and sample sizes were included for analysis in both the experimental and control conditions. Cases in which data were missing (eg, SDs) were either calculated from existing data (eg, the SD was calculated from the SEM) or retrieved via e-mail from corresponding authors. However, if it was not possible to recalculate the data or to contact the authors, the study was excluded from the meta-analysis.
Quantitative data synthesis
Analyses were performed by using MIX 2.0 PRO for Microsoft Excel (BioStatXL). Three meta-analyses were performed, one for each lifestyle factor (sleep deprivation, television watching, and alcohol consumption). A random-effects model was used because the studies presented with a variety of heterogeneous qualities (participant ages, food items presented, precise paradigm used, etc). Correlations between sample size and effect size were made to investigate the common problem with small studies of inflating the effect sizes in meta-analysis. A funnel plot was created to investigate publication bias (also called the drawer problem) in which null results are often “left in the drawer.” In addition, a 1-factor ANOVA was conducted on the cumulative effect sizes of the 3 studies to determine whether there was a main effect.
RESULTS
Search results
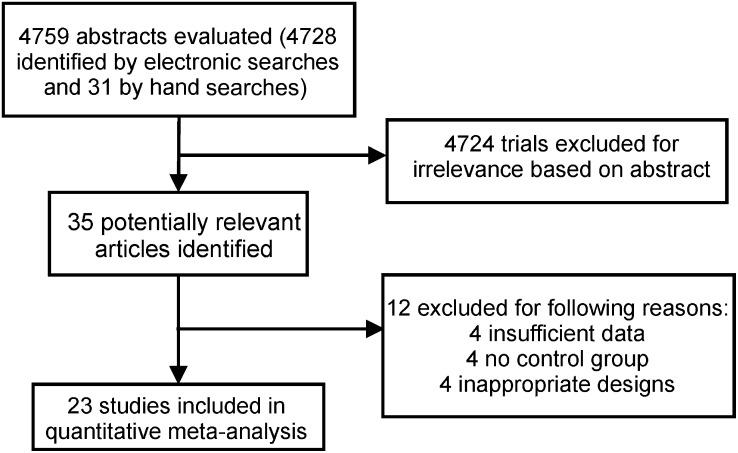
Initially, 4759 articles were screened as potential candidates. From this initial screening, 35 articles emerged for possible inclusion. The majority of exclusions at this stage were because articles were reviews, irrelevant, or correlational in nature. Of these 35 studies, 12 were excluded on the basis of our exclusion criteria, which left 23 studies for quantitative analysis (Figure 1).
FIGURE 1.
Flow chart of the literature search and study selection process.
Food intake
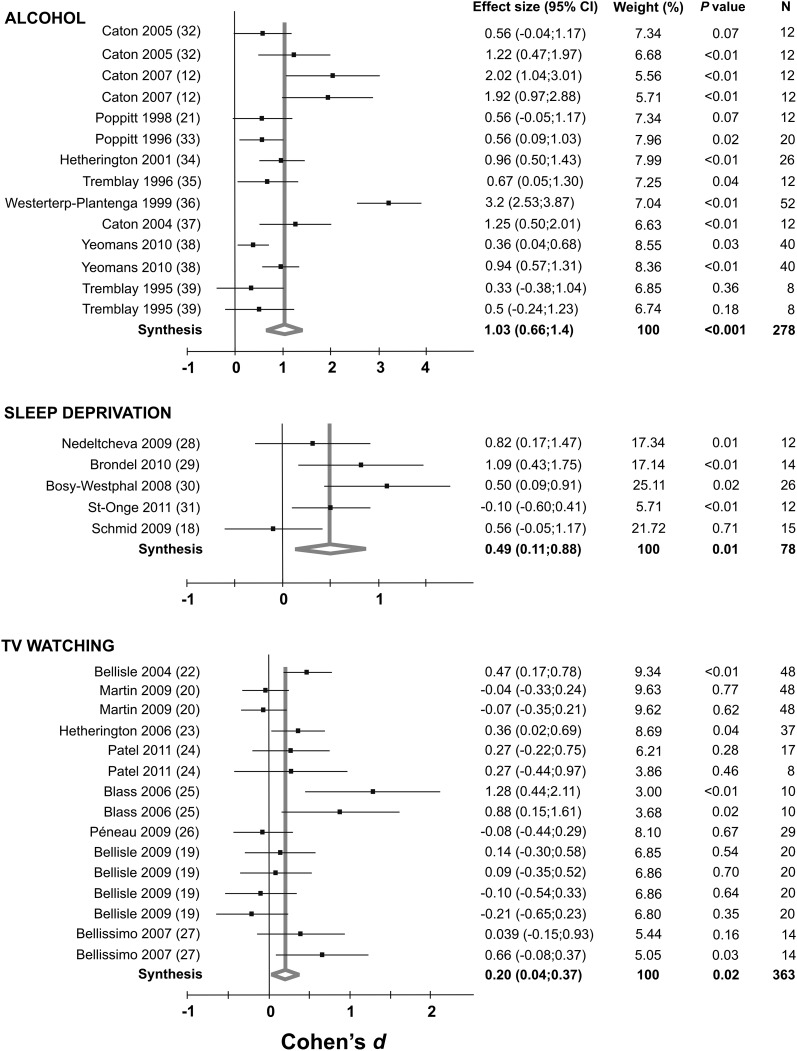
Forest plots of all of the studies included in the analysis for alcohol, sleep deprivation, and television watching are shown in Figure 2. All 3 lifestyle factors showed significant cumulative effect sizes (Cohen's d) on food intake. The effect was greatest for alcohol (Cohen's d = 1.03; 95% CI: 0.66, 1.4; P < 0.001) followed by sleep deprivation (Cohen's d = 0.49; 95% CI: 0.11, 0.88; P < 0.05) and television watching (Cohen's d = 0.2; 95% CI: 0.04, 0.37; P < 0.05). Specific study characteristics, including design and population, are included in Supplementary Tables S1–S3 under “Supplemental data” in the online issue.
FIGURE 2.
Forest plots (meta-analyses, random-effects models) indicating the cumulative effect sizes (Cohen's d) for the impact of alcohol intake, sleep deprivation, and television watching on acute food intake. Sleep-deprivation studies observed intake over the entire day, whereas alcohol and television studies observed single meals. TV, television.
Cumulative effect sizes
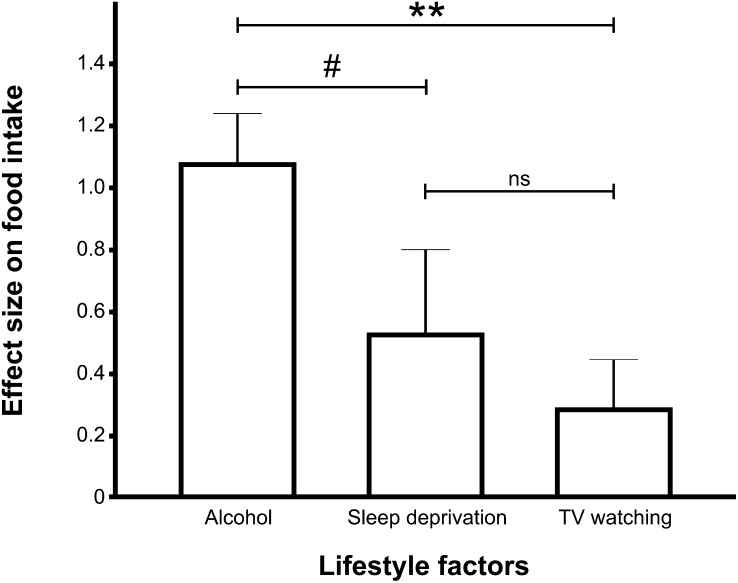
As shown in Figure 3, a 1-factor ANOVA determined that alcohol produced a significantly greater effect size (for calorie intake) than did television watching (P < 0.001) and trended toward producing a greater effect size than did sleep deprivation (P < 0.10). The effect sizes were 1.03 for alcohol consumption, 0.49 for sleep deprivation, and 0.20 for television watching.
FIGURE 3.
Graph indicating the relative cumulative mean (±SEM) effect size on food intake for alcohol consumption, sleep deprivation, and television watching. A 1-factor ANOVA showed a significant main effect, such that alcohol's effect size was greater than television's effect size (P < 0.001) and trended toward being larger than that of sleep deprivation (P < 0.10). There was no difference between television watching and sleep deprivation. The effect size was greatest for alcohol (Cohen's d = 1.03, n = 278, P < 0.001), followed by sleep deprivation (Cohen's d = 0.49, n = 78, P < 0.05) and television watching (Cohen's d = 0.2, n = 363, P < 0.05). **P < 0.001, #P < 0.10. ns, nonsignificant; TV, television.
Correlations between sample and effect size
No significant correlations were found between effect and sample size for any of the lifestyle factors analyzed.
Funnel plot
Visual inspection of the funnel plot showed relative symmetry along the treatment-effects axis, which suggests little publication bias (see Figure S1 under “Supplemental data” in the online issue).
DISCUSSION
These results show that lifestyle factors, such as television watching, sleep deprivation, and alcohol consumption, stimulate spontaneous food intake, with alcohol causing the most significant increases. This finding supports the notion that these behavioral patterns are not merely linked to weight gain but that they likely contribute to it by promoting less restricted food consumption. This is a serious concern because of the increasingly obesogenic environment of the Western world in which calorie-dense foods are a seemingly permanent part of the landscape. In the following paragraphs, we discuss potential mechanisms by which television watching, sleep deprivation, and alcohol consumption increase food intake.
From food reward to hedonic experience
Obesity results, in part, from the reward saliency of food becoming abnormally enhanced to the point that it overwhelms the brain's homeostatic control mechanisms (40). The reward value of food can be amplified in genetically vulnerable individuals, but it can also be increased by environmental factors (40). There are several lines of evidence that suggest that the lifestyle factors analyzed in this study increase food intake, partly through enhancing this reward value, and this may explain their association with obesity. Alcohol is known to induce alterations in circulating ghrelin, a peptide implicated in food reward (41, 42). In addition, alcohol affects γ-aminobutyric acid and opioid systems. The alteration of γ-aminobutyric acid signaling in reward centers of the brain stimulates appetite, and opioid signaling has been implicated in regulating the orosensory reward components of eating (43, 44). These pharmacologic findings are consistent with human studies that showed a greater increase in hunger during the early phase of a test meal after an alcohol preload compared with an energy-matched carbohydrate preload (45). This mimics the pattern of response shown when the palatability of food is enhanced through flavor manipulation (46).
There is similar evidence that links sleep deprivation to an increase in the hedonic value of food. Sleep loss causes a constellation of metabolic and endocrine changes, including an increase in circulating ghrelin (47). Interestingly, recent studies on sleep deprivation have found that it increases overall brain response to palatable food images (11, 48). In particular, short sleep increased activation in brain areas involved in reward processing, such as the putamen, nucleus accumbens, thalamus, insula, and anterior cingulate cortex. This strongly suggests that sleep deprivation, like alcohol, leads to deregulation of reward system activation in response to food.
Finally, there is evidence that suggests that television watching also increases the saliency of food reward. Several of the studies included in the meta-analysis found that the effect of television viewing on food intake was most pronounced with high-calorie foods, which suggests that television viewing alters the saliency of food reward (25, 27). Epidemiologic studies have shown a similar trend, in that those who watch more television tend to snack more while watching and to consume more energy-dense snacks (49). Additional evidence suggests that watching images of palatable food increases plasma ghrelin concentrations (50). This shows that visual stimuli associated with food evoke changes in circulating ghrelin; however, it remains unclear whether this effect translates to all television viewing (including non–food-related television shows).
Repeated consumption of a rewarding food results in the formation of new linked memories that condition the individual to anticipate reward not only in response to the food but also in response to any environmental stimulus that is often paired with the reward. With regard to the lifestyle factors analyzed, all three, when experienced habitually, should strengthen memory traces that trigger reward expectancy to food cues: that is, when presented with rewarding food or food cues, people who often suffer from sleep deprivation or who often watch television or drink alcohol while eating are more likely to experience a greater reward response as a result. In addition, both alcohol and television likely become their own conditioned cues for those who consume food in conjunction with these factors. This is particularly concerning given that obese individuals have been shown to be more responsive to environmental food cues (51), and converging evidence indicates a strong role for cues in addictive-like overeating (52).
Is our inhibitory control out of control?
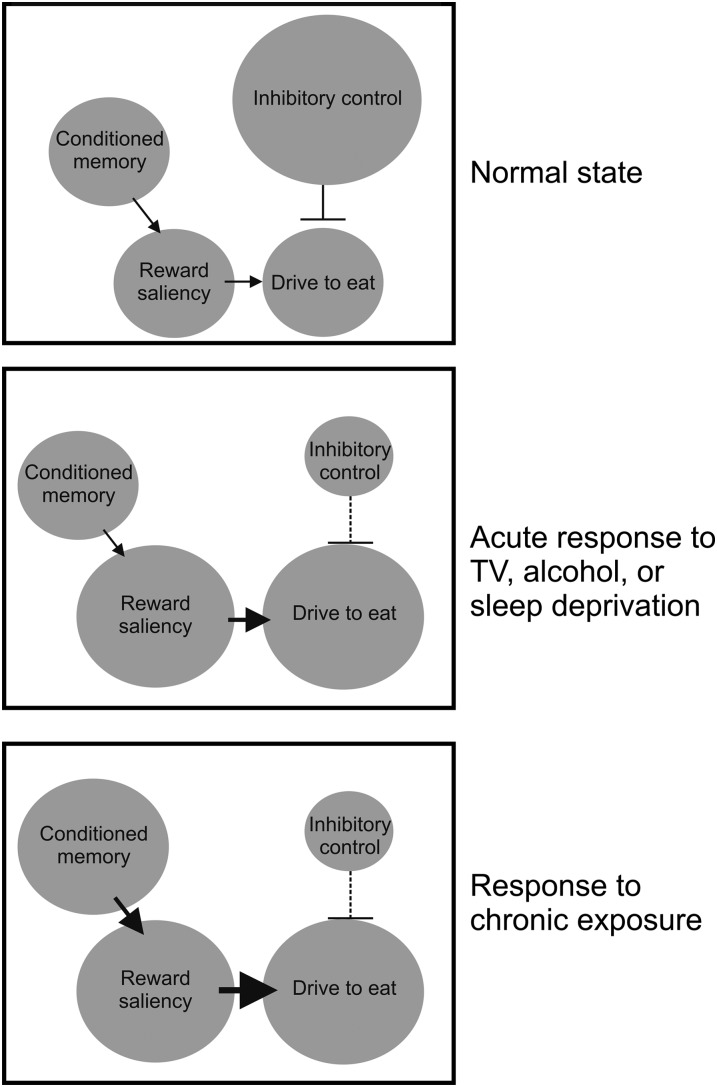
Whereas these alterations in reward-response likely contribute to the increased eating that results from these behaviors, they may be only part of the story. These behavioral patterns may, in addition to increasing the saliency of food reward, decrease one's capacity or tendency to exhibit inhibitory control. Compulsive behaviors, including excessive eating, are motivated by a combination of enhanced activation of reward saliency and disrupted activity in brain regions involved in inhibitory control (53). The orbitofrontal cortex, cingulate gyrus, and dorsolateral prefrontal cortex are all involved in inhibitory control (54). Neuroimaging data have implicated the prefrontal cortex as a brain region that is particularly vulnerable to sleep deprivation (55). Similarly, both alcohol and fast-paced television have been reported to impair prefrontal and executive function, respectively (56, 57). Thus, it may be that these behaviors increase food intake in the short term not only by increasing reward saliency but also by decreasing inhibitory control (Figure 4).
FIGURE 4.
Diagram representing mechanisms that might explain the connection between the lifestyle factors of alcohol consumption, sleep deprivation, and television watching and food intake. By increasing the saliency of food reward and decreasing inhibitory control, these lifestyle factors encourage the acute drive to food consumption, particularly for rewarding food. Chronic exposure to these lifestyle factors also leads to conditioned memories, which in turn enhance reward response to food and the subsequent drive to eat. Thus, these lifestyle factors, experienced chronically, could lead to susceptibility to food addiction and obesity. TV, television.
Limitations
There are some limitations to this analysis. There were only 5 studies available for analysis in the case of sleep deprivation, and thus our findings that sleep deprivation increases food intake the following day are tentative and warrant further investigation. In addition, food items included for consumption varied from study to study; it could be that this had an impact on the cumulative effect sizes, because it has already been noted here that the effects of these manipulations are more prominent on certain types of food (eg, calorie-dense items). Demographic variables such as sex and age were also variable; however, all of the studies were conducted in healthy individuals, and the majority were conducted in young adults. This is a strength in that the data are more comparable; however, it highlights the need for further evaluation of the impact of these stimuli in other populations, including youth and the elderly. Young people are known to be relatively flexible in their habits, whereas the elderly tend to rely on more set routines. Thus, it may be that these lifestyle factors are more dangerous in younger populations. Finally, the effect sizes of these different factors are not perfectly comparable, because, in the case of sleep deprivation, the effect was significant for cumulative food intake on the day after deprivation, whereas for television and alcohol the effect was relative to a single meal intake.
Conclusions
Taken together, the results of this meta-analysis show that prominent Western lifestyle factors, such as sleep deprivation, alcohol consumption, and television watching, promote increases in acute caloric consumption. These increases are likely related to a series of neurologic and endocrine adaptations, which result in an increased value for food reinforcers, predisposing an individual to food addiction, adiposity, and ultimately obesity. Fortunately, emerging evidence suggests that curtailing these lifestyles can reverse this trend. A recent study showed that shifting sleep from a short to a healthier length, over a 6-y time span, was associated with the attenuation of fat mass gain (58). Similarly, research that investigated the effects of reduced television viewing on children found that children who watched <1 h of television/d had reduced body weights, BMIs, skin-fold thicknesses, and fat mass and were less likely to be categorically overweight (59). Finally, reduced alcohol consumption in early adulthood can help to attenuate weight gain and abdominal obesity in adulthood (60). These results thus highlight the importance of increasing awareness of the prominent role that these lifestyles play in inducing food intake and subsequent weight gain.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CDC and SJB: designed the study; CDC: analyzed data; and CDC, CB, SJB, and HBS: critically revised the manuscript for important intellectual content and contributed to writing the manuscript. All authors had full access to all of the data and take responsibility for the integrity and accuracy of the data analysis. The funding sources had no input in the design or conduct of this meta-analysis; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev 2011;12:e95–106 [DOI] [PubMed] [Google Scholar]
- 3.Booth KM, Pinkston MM, Poston WS. Obesity and the built environment. J Am Diet Assoc 2005;105:110–3 [DOI] [PubMed] [Google Scholar]
- 4.Hinkle A, Wu E. Communities of color issue briefing paper: addressing the obesity epidemic—public policies for healthy eating and physical activity environments, California Adolescent Nutrition and Fitness Program & California Pan Ethnic Health Network. Available from: http://www.canfit.org/pdf/CANFit-CPEHNbrief.pdf (cited 14 April 2005)
- 5.WHO calls for action to restrict marketing of unhealthy foods and drinks to children. BMJ 2011;342:d503. [DOI] [PubMed] [Google Scholar]
- 6.Chaput JP, Klingenberg L, Astrup A, Sjödin AM. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obes Rev 2011;12:e12–20 [DOI] [PubMed] [Google Scholar]
- 7.Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, Brug J. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev 2010;11:695–708 [DOI] [PubMed] [Google Scholar]
- 8.Yeomans MR. Alcohol, appetite and energy balance: is alcohol intake a risk factor for obesity? Physiol Behav 2010;100:82–9 [DOI] [PubMed] [Google Scholar]
- 9.Bowman SA. Television-viewing characteristics of adults: correlations to eating practices and overweight and health status. Prev Chronic Dis 2006;3:A38. [PMC free article] [PubMed] [Google Scholar]
- 10.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, Born J, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr 2011;93:1229–36 [DOI] [PubMed] [Google Scholar]
- 11.Benedict C, Brooks SJ, O'Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab 2012;97:E443–7 [DOI] [PubMed] [Google Scholar]
- 12.Caton SJ, Bate L, Hetherington MM. Acute effects of an alcoholic drink on food intake: aperitif versus co-ingestion. Physiol Behav 2007;90:368–75 [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006;29:657–61 [DOI] [PubMed] [Google Scholar]
- 15.Lahti-Koski M, Pietinen P, Heliovaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK studies. Am J Clin Nutr 2002;75:809–17 [DOI] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr 2003;77:1312–7 [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476–82 [DOI] [PubMed] [Google Scholar]
- 19.Bellisle F, Dalix AM, Airinei G, Hercberg S, Péneau S. Influence of dietary restraint and environmental factors on meal size in normal-weight women: a laboratory study. Appetite 2009;53:309–13 [DOI] [PubMed] [Google Scholar]
- 20.Martin CK, Coulon SM, Markward N, Greenway FL, Anton SD. Association between energy intake and viewing television, distractibility, and memory for advertisements. Am J Clin Nutr 2009;89:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav 1998;64:279–85 [DOI] [PubMed] [Google Scholar]
- 22.Bellisle F, Dalix AM, Slama G. Non food-related environmental stimuli induce increased meal intake in healthy women: comparison of television viewing versus listening to a recorded story in laboratory settings. Appetite 2004;43:175–80 [DOI] [PubMed] [Google Scholar]
- 23.Hetherington MM, Anderson AS, Norton GN, Newson L. Situational effects on meal intake: a comparison of eating alone and eating with others. Physiol Behav 2006;88:498–505 [DOI] [PubMed] [Google Scholar]
- 24.Patel BP, Bellissimo N, Thomas SG, Hamilton JK, Anderson GH. Television viewing at mealtime reduces caloric compensation in peripubertal, but not postpubertal, girls. Pediatr Res 2011;70:513–7 [DOI] [PubMed] [Google Scholar]
- 25.Blass EM, Anderson DR, Kirkorian HL, Pempek TA, Price I, Koleini MF. On the road to obesity: television viewing increases intake of high-density foods. Physiol Behav 2006;88:597–604 [DOI] [PubMed] [Google Scholar]
- 26.Péneau S, Mekhmoukh A, Chapelot D, Dalix AM, Airinei G, Hercberg S, Bellisle F. Influence of environmental factors on food intake and choice of beverage during meals in teenagers: a laboratory study. Br J Nutr 2009;102:1854–9 [DOI] [PubMed] [Google Scholar]
- 27.Bellissimo N, Pencharz PB, Thomas SG, Anderson GH. Effect of television viewing at mealtime on food intake after a glucose preload in boys. Pediatr Res 2007;61:745–9 [DOI] [PubMed] [Google Scholar]
- 28.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9 [DOI] [PubMed] [Google Scholar]
- 30.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008;1:266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, Roy Choudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caton SJ, Marks JE, Hetherington MM. Pleasure and alcohol: manipulating pleasantness and the acute effects of alcohol on food intake. Physiol Behav 2005;84:371–7 [DOI] [PubMed] [Google Scholar]
- 33.Poppitt SD, Eckhardt JW, McGonagle J, Murgatroyd PR, Prentice AM. Short-term effects of alcohol consumption on appetite and energy intake. Physiol Behav 1996;60:1063–70 [DOI] [PubMed] [Google Scholar]
- 34.Hetherington MM, Cameron F, Wallis DJ, Pirie LM. Stimulation of appetite by alcohol. Physiol Behav 2001;74:283–9 [DOI] [PubMed] [Google Scholar]
- 35.Tremblay A, St-Pierre S. The hyperphagic effect of a high-fat diet and alcohol intake persists after control for energy density. Am J Clin Nutr 1996;63:479–82 [DOI] [PubMed] [Google Scholar]
- 36.Westerterp-Plantenga MS, Verwegen CR. The appetizing effect of an apéritif in overweight and normal-weight humans. Am J Clin Nutr 1999;69:205–12 [DOI] [PubMed] [Google Scholar]
- 37.Caton SJ, Ball M, Ahern A, Hetherington MM. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav 2004;81:51–8 [DOI] [PubMed] [Google Scholar]
- 38.Yeomans MR. Short term effects of alcohol on appetite in humans: effects of context and restrained eating. Appetite 2010;55:565–73 [DOI] [PubMed] [Google Scholar]
- 39.Tremblay A, Wouters E, Wenker M, St-Pierre S, Bouchard C, Després JP. Alcohol and a high-fat diet: a combination favoring overfeeding. Am J Clin Nutr 1995;62:639–44 [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci; (Epub ahead of print 21 October 2011). [DOI] [PubMed] [Google Scholar]
- 41.Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 2012;17:452–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calissendorff J, Danielsson O, Brismar K, Röjdmark S. Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism 2006;55:1625–9 [DOI] [PubMed] [Google Scholar]
- 43.Ward BO, Somerville EM, Clifton PG. Intraaccumbens baclofen selectively enhances feeding behavior in the rat. Physiol Behav 2000;68:463–8 [DOI] [PubMed] [Google Scholar]
- 44.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev 2002;26:713–28 [DOI] [PubMed] [Google Scholar]
- 45.Yeomans MR, Hails NJ, Nesic JS. Alcohol and the appetiser effect. Behav Pharmacol 1999;10:151–61 [DOI] [PubMed] [Google Scholar]
- 46.Yeomans MR. Palatability and the microstructure of eating in humans: the appetiser effect. Appetite 1996;27:119–33 [DOI] [PubMed] [Google Scholar]
- 47.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J 2011;5:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr (Epub ahead of print 22 February 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson M, Spence JC, Raine K, Laing L. The association of television viewing with snacking behavior and body weight of young adults. Am J Health Promot 2008;22:329–35 [DOI] [PubMed] [Google Scholar]
- 50.Schuumlssler P, Kluge M, Yassouridis A, Dresler M, Uhr M, Steiger A. Ghrelin levels increase after pictures showing food. Obesity (Silver Spring) (Epub ahead of print 12 January 2012) [DOI] [PubMed]
- 51.Braet C, Claus L, Goossens L, Moens E, Van Vlierberghe L, Soetens B. Differences in eating style between overweight and normal-weight youngsters. J Health Psychol 2008;13:733–43 [DOI] [PubMed] [Google Scholar]
- 52.Alsiö J, Olszewski P, Levine AS, Schiöth HB. Feed-forward mechanisms: addiction-like behavioral and molecular adaptations in overeating. Front Neuroendocrinol (Epub ahead of print 28 January 2012) [DOI] [PubMed] [Google Scholar]
- 53.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 2008;363:3191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002;159:1642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res 2010;185:105–29 [DOI] [PubMed] [Google Scholar]
- 56.Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol 2010;91:289–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lillard AS, Peterson J. The immediate impact of different types of television on young children's executive function. Pediatrics 2011;128:644–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grund A, Krause H, Siewers M, Rieckert H, Müller MJ. Is TV viewing an index of physical activity and fitness in overweight and normal weight children? Public Health Nutr 2001;4:1245–51 [DOI] [PubMed] [Google Scholar]
- 60.Pajari M, Pietiläinen KH, Kaprio J, Rose RJ, Saarni SE. The effect of alcohol consumption on later obesity in early adulthood–a population-based longitudinal study. Alcohol 2011;45:173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.