Abstract
Background: Dietary n−3 PUFAs are inversely associated with risk of sudden cardiac death (SCD); however, little is known about other fats and SCD. Furthermore, concerns have been raised that high n−6 PUFA intake may attenuate the benefits of n−3 PUFAs.
Objective: We examined associations and selected interactions between dietary fatty acids, expressed as a proportion of total fat and SCD.
Design: We conducted a prospective cohort study among 91,981 women aged 34–59 y from the Nurses’ Health Study in 1980. Over 30 y, we documented 385 SCDs.
Results: In multivariable models, women in the highest compared with the lowest quintile of SFA intake had an RR of SCD of 1.44 (95% CI: 1.04, 1.98). Conversely, women in the highest compared with the lowest quintile of PUFA intake had an RR of SCD of 0.57 (95% CI: 0.41, 0.78). Intakes of n−6 and n−3 PUFAs were both significantly associated with a lower risk of SCD, and n−6 PUFAs did not modify the association between n−3 PUFAs and SCD. MUFAs and trans fats were not associated with SCD risk. After further adjustment for coronary heart disease (CHD) and CHD risk factors potentially in the causal pathway, the association between PUFAs and SCD remained significant, whereas the association for SFAs was no longer significant.
Conclusions: Intake of PUFAs as a proportion of fat was inversely associated with SCD risk, independent of traditional CHD risk factors. These results support dietary guidelines to improve dietary fat quality by replacing intake of SFAs with n−6 and n−3 PUFAs.
INTRODUCTION
Sudden cardiac death (SCD)4 claims up to 300,000 lives in the United States annually (1) and accounts for >50% of cardiovascular and 15–20% of total mortality (2). Fatty acids, which are incorporated into the cell membrane, may modify myocyte electrical stability (3, 4) and alter vulnerability to ventricular arrhythmias, which are a primary cause of SCD (5, 6). Individual fatty acids may have varying effects on the propensity for arrhythmias. In experimental models, n−3 and some n−6 PUFAs have antiarrhythmic properties, whereas SFAs have pro-arrhythmic properties (3, 4, 7). Beyond antiarrhythmic effects, fatty acids may also influence risk of SCD through effects on lipids, blood pressure, atherosclerosis, thrombosis, and myocardial oxygen utilization (8–12).
Dietary fat quality may be an important modifiable factor for SCD. Previous epidemiologic studies support an inverse association between intake of n−3 PUFAs and SCD (13–16); however, little is known about the relation between intake of other fats and SCD risk. Furthermore, although dietary guidelines recommend increasing n−6 PUFA intake (17, 18), concerns have been raised that n−6 PUFAs may attenuate the health benefits of n−3 PUFAs (19, 20). To address these uncertainties, we examined the associations between intakes of the major classes of fatty acids and risk of SCD among women in the Nurses’ Health Study. We also evaluated the potential interaction between n−3 and n−6 PUFAs and SCD risk.
SUBJECTS AND METHODS
Study population
In 1976, 121,700 female registered nurses aged 30–55 y enrolled in the Nurses’ Health Study and provided information on lifestyle and medical history, which is updated biennially (21). Beginning in 1980, we ascertained dietary information through a food-frequency questionnaire (FFQ). In this analysis, we excluded women who had invalid dietary data at baseline in 1980 [ie, left ≥10 food items blank or had implausible energy intake (<600 or >3500 kcal/d)], which left 91,981 women for analysis. Informed consent was obtained from all women, and the research protocol was approved by the institutional review board at Brigham and Women's Hospital.
Dietary assessment
In 1980, we collected information on usual diet by using a 61-item FFQ. For each food item, participants were asked how often, on average, she had consumed a specified portion size over the past year. In 1984, diet was assessed by using an expanded 116-item FFQ, and similar questionnaires were used to update dietary information every 4 y from 1986 to 2006. On all questionnaires, we collected detailed information on type of fat or oil used in food preparation and brand or type of margarines. We calculated average fat intake by multiplying the frequency of consumption of each item by its fat content and summing across all foods. Fatty acid and other nutrient values were obtained from the Harvard University Food Composition Database, which is updated regularly by using direct analysis of fatty acids in commonly used margarines and processed foods. We considered intakes of SFAs, MUFAs, trans fatty acids, and PUFAs. For PUFAs, we examined separately n−6 [linoleic acid (18:2n−6) and arachidonic acid (20:4n−6)] and n−3 [intermediate-chain α-linolenic acid (ALA; 18:3n−3) and long-chain EPA (20:5n−3) + DHA (22:6n−3) from marine sources] PUFAs.
The questionnaire provides a reasonable measure of total and specific types of fat when compared with four 1-wk dietary records collected over 1 y; correlation coefficients were 0.57 for total fat, 0.68 for SFAs, 0.48 for PUFAs, and 0.58 for MUFAs (22). The correlation between long-term intake estimated from multiple FFQs and concentration in red blood cells was 0.27 for linoleic acid (18:2n−6), 0.41 for EPA (20:5n−3), 0.54 for DHA (22:6:n−3), and 0.48 for trans fats (23).
Endpoint ascertainment and definitions
Deaths were reported by next of kin, coworkers, or postal authorities or through a search of the National Death Index. Death certificates were obtained to confirm deaths, and we sought permission to obtain further information from medical records or family members. The next of kin was interviewed about the circumstances surrounding the death if not adequately documented in the medical record. Specific details for the classification of SCD have been described in detail elsewhere (6). Briefly, cardiac deaths were considered sudden if the death or cardiac arrest occurred within 1 h of symptom onset. To increase the specificity for an “arrhythmic death,” we excluded women with evidence of circulatory or neurologic impairment before death (5). We included unwitnessed deaths that could have occurred within 1 h of symptom onset and that had autopsy findings consistent with SCD in the analysis (ie, acute coronary thrombosis or severe coronary artery disease without myocardial necrosis or other pathologic findings to explain death; n = 41).
Modeling of dietary fat intake
Traditionally, in epidemiologic studies focusing on coronary heart disease (CHD) risk and lipids, the effect of dietary fat is assessed as an isocaloric substitution with carbohydrates. In the present analysis on SCD risk, the hypothesis was centered on the potential membrane-stabilizing effect of fatty acids. To our knowledge, carbohydrates do not have any direct pro- or antiarrhythmic properties and do not compete with fatty acids for incorporation into cell membranes. Therefore, in our primary analysis, fatty acid intake was quantified by a fat quality index, for which intake of a specific fatty acid was expressed as a percentage of total fat. In these models, we included total fat and total energy intake; thus, the β-coefficient for a specific fatty acid can be interpreted as the effect of that fatty acid substituting for all other fats. To best represent long-term diet and to reduce measurement error (24), we used a cumulative average measure of fat intake, placing greater weight on the most recent FFQ, as we hypothesized that recent dietary intake may be relatively more important with respect to SCD risk. For SCD incidence during 1984–1986, average fat intake was estimated as 1980 diet/2 + 1984 diet/2; for SCD incidence during 1986–1990, average fat intake was calculated as 1980 diet/4 + 1984 diet/4 + 1986 diet/2; for SCD incidence occurring between 1990–1994, average fat intake was calculated as 1980 diet/8 + 1984 diet /8 + 1986 diet/4 +1990 diet/2, and so forth.
In secondary analysis, we modeled fatty acid intake as a percentage of total energy by using multivariable nutrient-density models, which included total energy intake, percentage of energy from protein, and percentage of energy from all other individual fatty acids simultaneously (24). In these models, the β-coefficient for a fatty acid can be interpreted as the effect of substituting a specific percentage of energy from fat for the same percentage of energy from carbohydrates.
Statistical analysis
First, we assessed whether a diagnosis of an intermediate event, in the causal pathway between fatty acids and SCD [myocardial infarction (MI), stroke, coronary revascularization, angina, diabetes, hypertension, and hypercholesterolemia], was associated with a subsequent change in fatty acid intake (25). If intake of a specific fatty acid significantly changed after the diagnosis of the intermediate event, we stopped updating information for that fatty acid at the beginning of the interval during which the intermediate endpoint developed, to avoid time-dependent confounding by the intermediate event. If intake of a fatty acid did not change significantly, these diagnoses could not be important confounders. Thus, we continued to update dietary information to avoid misclassification of the participants’ long-term diet. This approach represents our best efforts to control for confounding by intermediate events and minimize misclassification of an individual's long-term dietary pattern. Importantly, the interpretation of each fatty acid—whether we stop updating at a particular diagnosis or continue updating—remains the same and represents the long-term intake of fat.
Each woman contributed person-months of follow-up from the date of return of the 1980 questionnaire to incident SCD, date of death, or end of follow-up (June 2010). Spearman correlation coefficients were used to evaluate associations among fatty acid subclasses, and Cox proportional hazards models estimated HRs as estimates of the RR. Multivariable models included various cardiovascular disease risk factors, lifestyle factors, and presence of chronic disease at baseline (see below for a full list of covariates). Fatty acid intake and other covariates, except for family history of MI, were updated during follow-up and were included as time-varying covariates in the models. In primary analysis, we adjusted for prevalent cardiovascular disease and other diseases we considered as intermediates at baseline. In secondary analyses, we included these intermediate endpoints in the model as time-varying covariates to address potential mechanistic pathways between fatty acids and SCD.
We grouped women into quintiles according to the distribution of fat intake in the entire population and conducted a test for linear trend by assigning the median value of fat intake to each quintile and modeling this variable as a continuous variable. Additionally, we examined the possibility of a nonlinear relation between fatty acids and SCD risk using restricted cubic spline transformations of the fatty acids. We tested for deviations from linearity using the likelihood ratio test, comparing the multivariate model with the linear term to the multivariate model with the linear and the cubic spline terms combined. If the deviation from linearity was significant (P < 0.05), we estimated the overall significance of the nonlinear relation of fatty acid to SCD risk, using a likelihood ratio test comparing the multivariate model with fatty acid as a linear term and spline variables to the model with covariates only. In secondary analysis, we grouped women into quintiles based on the fat intake among the cases only, to ensure an equal distribution of cases in each quintile. To test formally for interaction between n−3 and n−6 PUFAs, we included a cross-product term with both fats as continuous variables in multivariable models and used a likelihood ratio test, comparing the model with and without the interaction term. All statistical analysis was performed by using SAS software (version 9; SAS Institute Inc), and a P value <0.05 was considered statistically significant.
RESULTS
Population characteristics
Over 30 y of follow-up, we documented 385 SCDs. Women with a higher proportion of fat intake as PUFAs had a lower total fat intake and were less likely to smoke or use postmenopausal hormone therapy (Table 1). In contrast, women with a higher intake of SFAs and MUFAs had a higher intake of total fat, had a higher BMI, exercised less, and were more likely to smoke.
TABLE 1.
Characteristics of 91,981 women in the Nurses’ Health Study across quintiles of fatty acid intake at baseline in 19801
| SFA |
MUFA |
PUFA |
trans Unsaturated fat |
|||||||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Fatty acid intake, median (% of total fat) | 32.8 | 38.0 | 43.9 | 35.9 | 39.2 | 43.7 | 10.5 | 15.8 | 20.7 | 3.7 | 5.1 | 7.3 |
| Fatty acid intake, range (% of total fat) | <34.5 | 36.9–39.1 | >41.8 | <37.0 | 38.5–40.0 | >42.0 | <12.2 | 14.8–16.8 | >19.0 | <4.1 | 4.8–5.5 | >6.5 |
| Age (y) | 47 ± 72 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 47 ± 7 | 46 ± 7 | 47 ± 7 | 47 ± 7 | 47 ± 7 |
| BMI (kg/m2) | 24.1 ± 4.5 | 24.4 ± 4.4 | 24.5 ± 4.5 | 24.1 ± 4.5 | 24.3 ± 4.5 | 24.6 ± 4.5 | 24.5 ± 4.5 | 24.4 ± 4.5 | 24.3 ± 4.6 | 24.1 ± 4.4 | 24.4 ± 4.4 | 24.5 ± 4.6 |
| Exercise (h/wk) | 3.9 ± 2.9 | 3.9 ± 2.9 | 3.8 ± 2.9 | 4.1 ± 2.9 | 3.9 ± 2.9 | 3.7 ± 2.8 | 3.8 ± 2.9 | 3.9 ± 2.9 | 3.8 ± 2.9 | 4.2 ± 3.0 | 3.9 ± 2.9 | 3.7 ± 2.8 |
| Total fat intake (% of energy) | 36.9 ± 8.3 | 39.5 ± 7.6 | 38.5 ± 7.9 | 33.4 ± 8.0 | 37.9 ± 6.7 | 41.7 ± 7.8 | 41.5 ± 7.7 | 37.2 ± 7.1 | 34.1 ± 8.1 | 38.5 ± 9.0 | 39.9 ± 7.7 | 37.4 ± 7.4 |
| Current smoking (%) | 27 | 28 | 31 | 27 | 27 | 31 | 31 | 27 | 27 | 30 | 29 | 28 |
| Current hormone therapy (%) | 25 | 25 | 25 | 25 | 25 | 25 | 26 | 25 | 24 | 26 | 25 | 25 |
| Family history of MI, age <60 y (%) | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Regular aspirin use (%) | 14 | 14 | 16 | 14 | 15 | 15 | 15 | 15 | 15 | 14 | 15 | 15 |
| History of disease (%) | ||||||||||||
| Diabetes | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 2 | 2 |
| Hypertension | 17 | 16 | 17 | 16 | 16 | 18 | 17 | 16 | 16 | 17 | 16 | 17 |
| Hypercholesterolemia | 7 | 6 | 5 | 5 | 5 | 7 | 5 | 6 | 8 | 6 | 5 | 6 |
| CHD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
All variables except age were age-standardized. CHD, coronary heart disease; MI, myocardial infarction; Q, quintile.
Mean ± SD (all such values).
The correlations between the various fatty acid classes and individual PUFAs are presented in Table 2. Linoleic acid, the most abundant n−6 PUFA, was strongly correlated with total n−6 PUFAs (r = 0.95, P < 0.001), whereas ALA was strongly correlated with total n−3 PUFAs (r = 0.73, P < 0.001).
TABLE 2.
Spearman correlations between dietary intake of fatty acid classes (% total fat) and individual PUFAs among women in the Nurses’ Health Study, 1980–20061
| SFAs | MUFAs | transFat | TotalPUFAs | Total n−6 | Linoleic acid(18:2n−6) | AA(20:4n−6) | Total n−3 fats | ALA(18:3n−3) | Marine n−3 fats (EPA + DHA) | |
| SFAs | 1.0 | 0.05 | −0.07 | −0.65 | −0.64 | −0.66 | −0.14 | −0.36 | −0.24 | −0.32 |
| MUFAs | 1.0 | 0.33 | −0.21 | −0.18 | −0.21 | −0.01 | −0.33 | −0.33 | −0.34 | |
| trans Fat | 1.0 | 0.16 | 0.16 | 0.17 | −0.25 | −0.06 | −0.01 | −0.30 | ||
| Total PUFAs | 1.0 | 0.94 | 0.97 | 0.14 | 0.63 | 0.55 | 0.39 | |||
| Total n−6 | 1.0 | 0.95 | 0.10 | 0.62 | 0.47 | 0.34 | ||||
| Linoleic acid (18:2n−6) | 1.0 | 0.07 | 0.56 | 0.49 | 0.32 | |||||
| AA (20:4n−6) | 1.0 | 0.32 | 0.09 | 0.56 | ||||||
| Total n−3 | 1.0 | 0.73 | 0.70 | |||||||
| ALA (18:3n−3) | 1.0 | 0.39 |
P < 0.001 for all correlations. AA, arachidonic acid; ALA, α-linolenic acid.
Total fat, fat types, and risk of SCD
The age- and multivariable-adjusted associations between dietary fatty acids and SCD are displayed in Table 3. Total fat intake was not associated with risk of SCD. SFA intake was positively associated with risk of SCD in multivariable models (P-linear trend = 0.01). When modeled as a continuous variable, the multivariable RR for a 5% increment of dietary fat as SFAs was 1.11 (95% CI: 1.01, 1.23). Conversely, intake of PUFAs was inversely associated with risk of SCD (P-linear trend < 0.001). The multivariable RR for a 5% increment of dietary fat as PUFAs was 0.79 (95% CI: 0.69, 0.90). For total PUFAs, the association appeared to plateau in the upper quintiles of intake (P-nonlinear relation < 0.001). Although MUFAs and trans fats were positively associated with risk of SCD in age-adjusted models, these associations were attenuated and not significant in multivariate-adjusted models. In secondary analyses, which further adjusted for intermediate diseases, the associations between fatty acids and SCD were attenuated (Table 3). SFA intake was no longer significantly associated with risk of SCD; however, the inverse association remained significant for PUFAs.
TABLE 3.
Multivariable RRs of SCD by intake of total fat (% of total energy) and fatty acid subclasses (% total fat) among 91,981 women in the Nurses’ Health Study1
| Quintile of fatty acid intake |
P-trend |
||||||
| 1 | 2 | 3 | 4 | 5 | Linear2 | Nonlinear3 | |
| Total fat | |||||||
| Median (% of total energy) | 26.3 | 30.7 | 33.9 | 37.2 | 42.8 | ||
| Cases (n) | 94 | 97 | 65 | 64 | 65 | ||
| RR (95% CI) of SCD | |||||||
| Age-adjusted model | 1.0 (ref) | 1.21 (0.91, 1.61) | 0.93 (0.67, 1.28) | 1.06 (0.76, 1.47) | 1.40 (1.00, 1.96) | 0.17 | NA |
| Multivariable model4 | 1.0 (ref) | 1.15 (0.86, 1.53) | 0.82 (0.59, 1.13) | 0.85 (0.61, 1.19) | 0.96 (0.68, 1.36) | 0.38 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 1.15 (0.86, 1.53) | 0.83 (0.60, 1.14) | 0.88 (0.63, 1.23) | 1.00 (0.71, 1.42) | 0.55 | NA |
| SFAs | |||||||
| Median (% of total fat) | 32.8 | 35.7 | 38.0 | 40.3 | 43.9 | ||
| Cases (n) | 61 | 58 | 66 | 91 | 109 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 1.03 (0.72, 1.48) | 1.21 (0.85, 1.72) | 1.69 (1.21, 2.34) | 2.04 (1.49, 2.81) | <0.001 | NA |
| Multivariable model4 | 1.0 (ref) | 0.97 (0.68, 1.39) | 1.03 (0.72, 1.46) | 1.34 (0.96, 1.86) | 1.44 (1.04, 1.98) | 0.01 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.92 (0.64, 1.32) | 0.93 (0.65, 1.32) | 1.11 (0.80, 1.55) | 1.15 (0.83, 1.59) | 0.18 | NA |
| PUFAs | |||||||
| Median (% of total fat) | 10.5 | 13.6 | 15.8 | 17.8 | 20.7 | ||
| Cases (n) | 128 | 90 | 56 | 54 | 57 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.71 (0.54, 0.93) | 0.43 (0.31, 0.59) | 0.42 (0.30, 0.57) | 0.42 (0.30, 0.57) | <0.001 | <0.001 |
| Multivariable model4 | 1.0 (ref) | 0.80 (0.61, 1.05) | 0.54 (0.39, 0.74) | 0.56 (0.40, 0.78) | 0.57 (0.41, 0.78) | <0.001 | <0.001 |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.87 (0.66, 1.15) | 0.66 (0.48, 0.92) | 0.71 (0.51, 0.99) | 0.73 (0.53, 1.02) | 0.02 | <0.001 |
| MUFAs | |||||||
| Median (% of total fat) | 35.9 | 37.8 | 39.2 | 40.8 | 43.7 | ||
| Cases (n) | 70 | 67 | 69 | 86 | 93 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 1.04 (0.74, 1.45) | 1.11 (0.79, 1.56) | 1.36 (0.98, 1.87) | 1.47 (1.07, 2.02) | 0.01 | NA |
| Multivariable model4 | 1.0 (ref) | 1.09 (0.77, 1.53) | 1.14 (0.81, 1.59) | 1.21 (0.87, 1.68) | 1.18 (0.85, 1.63) | 0.29 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 1.10 (0.78, 1.54) | 1.12 (0.80, 1.57) | 1.05 (0.76, 1.46) | 0.95 (0.69, 1.32) | 0.60 | NA |
| trans Fat | |||||||
| Median (% of total fat) | 3.7 | 4.5 | 5.1 | 5.9 | 7.3 | ||
| Cases (n) | 76 | 75 | 68 | 71 | 95 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 1.10 (0.80, 1.52) | 1.00 (0.72, 1.39) | 1.07 (0.77, 1.48) | 1.39 (1.02, 1.89) | 0.04 | NA |
| Multivariable model4 | 1.0 (ref) | 1.06 (0.77, 1.46) | 0.97 (0.69, 1.35) | 0.98 (0.71, 1.36) | 1.16 (0.85, 1.57) | 0.41 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 1.03 (0.75, 1.43) | 0.95 (0.68, 1.32) | 0.92 (0.66, 1.28) | 1.04 (0.76, 1.41) | 0.95 | NA |
NA, not applicable; ref, reference; SCD, sudden cardiac death.
Test for linear trend calculated by assigning the median diet score in each quintile and modeling this as a continuous variable in regression models.
Test for nonlinear relation evaluated with a likelihood ratio test comparing the multivariate model with fatty acid as a linear term and cubic restricted spline variables to the model with covariates only. Nonlinear tests are presented when significant deviation from linearity was detected (P < 0.05), based on the likelihood ratio test, comparing the model with only the linear term with the model with the linear and the cubic spline terms.
The RRs are estimated from multivariate Cox proportional hazards models adjusted for age (in mo), total calories (quintiles), smoking (3 categories), BMI (3 categories), family history of myocardial infarction (none, before 60 y, after 60 y), menopausal status (yes or no), hormone therapy (current, past, never), exercise (4 categories), aspirin use (<1, 1–6, ≥7 times/wk), use of multivitamins (yes or no), use of vitamin E supplements (yes or no), alcohol use (4 categories), and history of diabetes, hypertension, hypercholesterolemia, coronary heart disease, and cancer at baseline (all yes or no). All models, except total fat, were also adjusted for the percentage of energy from total fat (quintiles).
Intermediate diseases include current diagnosis of hypertension, hypercholesterolemia, diabetes, coronary heart disease, and stroke as time-varying covariates.
Finally, when we created quintiles based on the fat intake among the cases only, we observed stronger associations and slightly increased power for SFAs and PUFAs and risk of SCD, although the overall interpretation of the results remains the same (see Supplemental Table under “Supplemental data” in the online issue). For example, the multivariate RR of SCD comparing quintile 5 with quintile 1 was 1.61 (95% CI: 1.16, 2.22) for SFA and 0.52 (95% CI: 0.37, 0.72) for PUFA. After further adjustment for potential intermediates, the associations of SFA and PUFA and risk of SCD were attenuated and no longer significant. In multivariate models, neither MUFAs nor trans fats were associated with risk of SCD.
n−6 and n−3 PUFAs and risk of SCD
Intakes of both n−6 and n−3 classes of PUFAs were associated with a lower risk of SCD, and the association appeared to plateau in the upper quintiles of intake for both classes (P-nonlinear relation ≤ 0.001; Table 4). Women in the highest compared with the lowest quintile of dietary fat from total n−6 PUFAs had a multivariable RR of SCD of 0.61 (95% CI: 0.44, 0.85), and this association was driven primarily by linoleic acid. The multivariable RR of SCD, comparing the highest with the lowest quintile of fat from n−3 PUFAs, was 0.49 (95% CI: 0.35, 0.69). Higher proportions of dietary fat from ALA and marine n−3 PUFAs were both associated with a lower risk of SCD. In secondary models that further adjusted for intermediary diseases, the inverse associations between linoleic acid, ALA, and marine n−3 PUFAs were attenuated but remained statistically significant.
TABLE 4.
Multivariable RRs of SCD by intake of n−6 and n−3 PUFAs (% of total fat) among 91,981 women in the Nurses’ Health Study1
| Quintile of fatty acid intake (% of total fat) |
P-trend |
||||||
| 1 | 2 | 3 | 4 | 5 | Linear2 | Nonlinear3 | |
| n−6 PUFAs | |||||||
| Total n−6 PUFAs | |||||||
| Median (% of total fat) | 8.2 | 11.4 | 13.6 | 15.6 | 18.6 | ||
| Cases | 124 | 91 | 63 | 50 | 57 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.73 (0.56, 0.96) | 0.50 (0.37, 0.68) | 0.41 (0.29, 0.57) | 0.45 (0.32, 0.62) | <0.001 | <0.001 |
| Multivariable model4 | 1.0 (ref) | 0.83 (0.63, 1.09) | 0.63 (0.46, 0.86) | 0.54 (0.39, 0.76) | 0.61 (0.44, 0.85) | <0.001 | <0.001 |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.92 (0.70, 1.21) | 0.76 (0.56, 1.04) | 0.70 (0.49, 0.98) | 0.79 (0.56, 1.09) | 0.04 | 0.001 |
| Linoleic acid | |||||||
| Median (% of total fat) | 7.8 | 11.0 | 13.2 | 15.2 | 17.9 | ||
| Cases | 121 | 86 | 69 | 57 | 52 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.69 (0.52, 0.91) | 0.54 (0.40, 0.72) | 0.45 (0.32, 0.62) | 0.39 (0.28, 0.55) | <0.001 | <0.001 |
| Multivariable model4 | 1.0 (ref) | 0.77 (0.58, 1.02) | 0.67 (0.49, 0.91) | 0.60 (0.43, 0.83) | 0.53 (0.38, 0.75) | <0.001 | <0.001 |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.85 (0.64, 1.13) | 0.82 (0.60, 1.11) | 0.77 (0.56, 1.08) | 0.68 (0.49, 0.96) | 0.02 | <0.001 |
| Arachidonic acid | |||||||
| Median (% of total fat) | 0.15 | 0.19 | 0.22 | 0.26 | 0.33 | ||
| Cases | 76 | 82 | 63 | 78 | 86 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 1.12 (0.82, 1.53) | 0.84 (0.60, 1.18) | 0.99 (0.71, 1.36) | 0.99 (0.71, 1.37) | 0.78 | NA |
| Multivariable model4 | 1.0 (ref) | 1.13 (0.83, 1.55) | 0.84 (0.60, 1.18) | 0.97 (0.70, 1.34) | 0.89 (0.64, 1.24) | 0.32 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 1.14 (0.83, 1.56) | 0.86 (0.61, 1.20) | 0.98 (0.71, 1.36) | 0.88 (0.63, 1.23) | 0.30 | NA |
| n−3 PUFAs | |||||||
| Total n−3 PUFAs | |||||||
| Median (% of total fat) | 1.24 | 1.45 | 1.66 | 1.93 | 2.41 | ||
| Cases | 110 | 93 | 76 | 49 | 57 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.80 (0.60, 1.06) | 0.63 (0.47, 0.85) | 0.39 (0.27, 0.55) | 0.39 (0.28, 0.55) | <0.001 | <0.001 |
| Multivariable model4 | 1.0 (ref) | 0.80 (0.60, 1.05) | 0.67 (0.50, 0.91) | 0.45 (0.32, 0.64) | 0.49 (0.35, 0.69) | <0.001 | <0.001 |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.84 (0.63, 1.11) | 0.74 (0.55, 1.01) | 0.55 (0.39, 0.78) | 0.65 (0.46, 0.92) | 0.004 | <0.001 |
| α-Linolenic acid | |||||||
| Median (% of total fat) | 1.14 | 1.31 | 1.46 | 1.64 | 2.10 | ||
| Cases | 111 | 83 | 78 | 54 | 59 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.75 (0.56, 0.99) | 0.66 (0.49, 0.89) | 0.44 (0.31, 0.61) | 0.30 (0.20, 0.46) | <0.001 | NA |
| Multivariable model4 | 1.0 (ref) | 0.76 (0.57, 1.02) | 0.74 (0.55, 0.99) | 0.53 (0.38, 0.74) | 0.38 (0.25, 0.59) | <0.001 | NA |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.83 (0.62, 1.10) | 0.86 (0.64, 1.16) | 0.67 (0.48, 0.94) | 0.51 (0.33, 0.78) | <0.001 | NA |
| Marine n−3 fats (EPA + DHA) | |||||||
| Median (% of total fat) | 0.05 | 0.10 | 0.17 | 0.27 | 0.51 | ||
| Cases | 108 | 83 | 70 | 63 | 61 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.68 (0.51, 0.91) | 0.54 (0.40, 0.74) | 0.47 (0.34, 0.64) | 0.39 (0.28, 0.54) | <0.001 | <0.001 |
| Multivariable model4 | 1.0 (ref) | 0.72 (0.54, 0.96) | 0.63 (0.46, 0.86) | 0.56 (0.40, 0.77) | 0.50 (0.35, 0.70) | <0.001 | <0.001 |
| Multivariable model + intermediate diseases5 | 1.0 (ref) | 0.75 (0.56, 1.01) | 0.70 (0.51, 0.96) | 0.65 (0.47, 0.90) | 0.63 (0.44, 0.90) | 0.03 | 0.002 |
NA, not applicable; ref, reference; SCD, sudden cardiac death.
Test for linear trend calculated by assigning the median diet score in each quintile and modeling this as a continuous variable in regression models.
Test for nonlinear relation evaluated with a likelihood ratio test comparing the multivariate model with fatty acid as a linear term and cubic restricted spline variables to the model with covariates only. Nonlinear tests are presented when significant deviation from linearity was detected (P < 0.05), based on the likelihood ratio test, comparing the model with only the linear term with the model with the linear and the cubic spline terms.
The RRs are estimated from multivariate Cox proportional hazards models adjusted for age (in mo), percentage of energy from total fat (quintiles), total energy (quintiles), smoking (3 categories), BMI (3 categories), family history of myocardial infarction (none, before 60 y, after 60 y), menopausal status (yes or no), hormone therapy (current, past, never), exercise (4 categories), aspirin use (<1, 1–6, ≥7 times/wk), use of multivitamins (yes or no), use of vitamin E supplements (yes or no), alcohol use (4 categories), and history of diabetes, hypertension, hypercholesterolemia, coronary heart disease, and cancer at baseline (all yes or no).
Intermediate diseases include current diagnosis of hypertension, hypercholesterolemia, diabetes, coronary heart disease, and stroke as time-varying covariates.
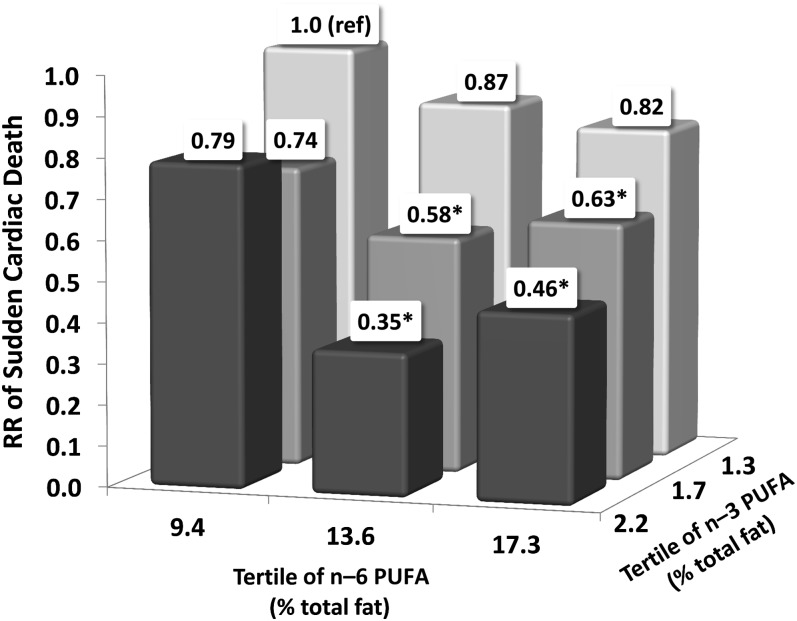
High intake of n−6 PUFAs did not modify the association between n−3 PUFAs and the risk of SCD (P-interaction = 0.82). Women with a higher intake of both n−6 and n−3 fats had a significantly lower risk of SCD than did women in the lowest tertile of both PUFAs (Figure 1). Furthermore, n−6 PUFAs did not attenuate the inverse association between ALA and risk of SCD (P-interaction = 0.40). Additionally, the ratio of n−6 to n−3 PUFAs was not associated with the risk of SCD. Women in the highest quintile of the n−6:n−3 ratio (median: 10.4) had an RR of 0.88 (95% CI: 0.64, 1.20) compared with women in the lowest quintile (median: 5.4).
FIGURE 1.
Multivariable RRs of sudden cardiac death by joint classification of n−3 and n−6 PUFA intakes (% of total fat). The RRs were estimated from Cox proportional hazards models adjusted for age, total energy (quintiles), percentage of energy from protein and fat (in quintiles), smoking (3 categories), BMI (3 categories), family history of myocardial infarction (none, before 60 y, after 60 y), menopausal status (yes or no), hormone therapy (current, past, never), exercise (4 categories), aspirin use (3 categories), use of multivitamins (yes or no), use of vitamin E supplements (yes or no), alcohol use (4 categories), and history of diabetes, high cholesterol, high blood pressure, coronary heart disease, and cancer at baseline (all yes or no). *P < 0.05 compared with the referent category in Cox proportional hazards models. P-interaction = 0.82 (likelihood ratio test comparing the model with and without the interaction term).
Isocaloric models
In isocaloric models that compared the percentage of energy from specific fatty acids with the same energy intake from carbohydrate, the associations between total n−6 and n−3 PUFAs and SCD were attenuated but remained significantly inversely associated with the risk of SCD. However, the individual n−3 PUFAs ALA and marine n−3 fats were no longer significantly associated with SCD (Table 5). The RR of SCD, comparing the highest with the lowest quintiles of intake, was 0.75 (95% CI: 0.43, 1.32) for ALA and 0.75 (95% CI: 0.47, 1.20) for marine n−3 PUFAs. Additionally, a higher intake of SFAs, when energy from carbohydrates was replaced, was not associated with the risk of SCD.
TABLE 5.
Multivariable RRs of SCD by isocaloric substitution of fatty acids for carbohydrates in women1
| Quintile of fatty acid intake (% of energy) |
P-trend |
||||||
| 1 | 2 | 3 | 4 | 5 | Linear2 | Nonlinear3 | |
| Total PUFAs | |||||||
| Median (% of energy) | 3.8 | 4.8 | 5.5 | 6.2 | 7.4 | ||
| Cases | 121 | 86 | 60 | 59 | 59 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.74 (0.56, 0.98) | 0.55 (0.40, 0.75) | 0.56 (0.40, 0.76) | 0.57 (0.41, 0.77) | <0.001 | <0.001 |
| Multivariable model 14 | 1.0 (ref) | 0.78 (0.58, 1.04) | 0.58 (0.42, 0.81) | 0.56 (0.39, 0.79) | 0.49 (0.33, 0.71) | <0.001 | NA |
| Multivariable model 1 + intermediate diseases5 | 1.0 (ref) | 0.87 (0.65, 1.17) | 0.68 (0.49, 0.96) | 0.67 (0.47, 0.96) | 0.60 (0.42, 0.88) | 0.004 | NA |
| Total n−6 PUFAs | |||||||
| Median (% of energy) | 3.02 | 3.99 | 4.68 | 5.39 | 6.55 | ||
| Cases | 119 | 90 | 58 | 64 | 54 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.78 (0.59, 1.02) | 0.54 (0.39, 0.74) | 0.61 (0.45, 0.84) | 0.53 (0.38, 0.73) | <0.001 | <0.001 |
| Multivariable model 26 | 1.0 (ref) | 0.88 (0.66, 1.18) | 0.68 (0.48, 0.97) | 0.76 (0.53, 1.10) | 0.63 (0.41, 0.96) | 0.02 | 0.06 |
| Multivariable model 2 + intermediate diseases5 | 1.0 (ref) | 0.96 (0.72, 1.29) | 0.76 (0.54, 1.08) | 0.88 (0.61, 1.27) | 0.71 (0.47, 1.08) | 0.11 | <0.001 |
| Total n−3 PUFAs | |||||||
| Median (% of energy) | 0.46 | 0.54 | 0.60 | 0.67 | 0.79 | ||
| Cases | 109 | 76 | 75 | 72 | 53 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 0.73 (0.54, 0.98) | 0.74 (0.55, 1.00) | 0.69 (0.51, 0.94) | 0.49 (0.35, 0.68) | <0.001 | NA |
| Multivariable model 26 | 1.0 (ref) | 0.73 (0.54, 1.00) | 0.75 (0.54, 1.03) | 0.73 (0.52, 1.03) | 0.59 (0.40, 0.88) | 0.01 | NA |
| Multivariable model 2 + intermediate diseases5 | 1.0 (ref) | 0.77 (0.56, 1.05) | 0.79 (0.57, 1.10) | 0.79 (0.56, 1.12) | 0.69 (0.46, 1.02) | 0.09 | NA |
| SFAs | |||||||
| Median (% of energy) | 9.9 | 12.0 | 13.6 | 15.5 | 18.6 | ||
| Cases | 61 | 67 | 82 | 82 | 93 | ||
| RR (95% CI) of SCD | |||||||
| Age, energy-adjusted model | 1.0 (ref) | 1.25 (0.88, 1.77) | 1.62 (1.16, 2.26) | 1.68 (1.20, 2.34) | 2.07 (1.49, 2.88) | <0.001 | NA |
| Multivariable model 14 | 1.0 (ref) | 1.30 (0.88, 1.92) | 1.51 (0.99, 2.31) | 1.17 (0.73, 1.88) | 1.01 (0.59, 1.73) | 0.52 | 0.12 |
| Multivariable model 1 + intermediate diseases5 | 1.0 (ref) | 1.29 (0.88, 1.91) | 1.47 (0.96, 2.24) | 1.09 (0.68, 1.76) | 0.95 (0.55, 1.61) | 0.34 | NA |
NA, not applicable; ref, reference; SCD, sudden cardiac death.
Test for linear trend calculated by assigning the median diet score in each quintile and modeling this as a continuous variable in regression models.
Test for nonlinear relation evaluated with a likelihood ratio test comparing the multivariate model with fatty acid as a linear term and cubic restricted spline variables to the model with covariates only. Nonlinear tests are presented when significant deviation from linearity is detected (P < 0.05), based on the likelihood ratio test, comparing the model with only the linear term with the model with the linear and the cubic spline terms.
The RRs in model 1 are estimated from multivariate Cox proportional hazards models adjusted for age (in mo), total energy (quintiles), percentage of energy from protein (quintiles), smoking (3 categories), BMI (3 categories), family history of myocardial infarction (none, before 60 y, after 60 y), menopausal status (yes or no), hormone therapy (current, past, never), exercise (4 categories), aspirin use (<1, 1–6, ≥7 times/wk), use of multivitamins (yes or no), use of vitamin E supplements (yes or no), alcohol use (4 categories), percentage of energy from remaining fatty acids (SFA, MUFA, PUFA, and trans fat, all in quintiles), and history of diabetes, hypertension, hypercholesterolemia, coronary heart disease, and cancer at baseline (all yes or no).
Intermediate diseases include current diagnosis of hypertension, hypercholesterolemia, diabetes, coronary heart disease, and stroke as time-varying covariates.
The RRs in model 2 are estimated from Cox proportional hazards models adjusted for covariates in model 1, except that n−3 and n−6 PUFAs (both in quintiles) replace total PUFAs.
DISCUSSION
In this prospective study, women consuming a higher proportion of dietary fat as SFAs had a greater risk of SCD, whereas women consuming a higher proportion of dietary fat as PUFAs had a lower risk. Higher intakes of n−6 and n−3 PUFAs were both associated with a lower risk of SCD, even after control for intermediate diseases within the causal pathway. Intakes of MUFAs and trans fats were not associated with risk of SCD in multivariable models.
The inverse association between n−3 PUFAs and SCD in this cohort is consistent with results from previous studies (14–16). Additionally, dietary n−6 PUFAs, particularly linoleic acid, was associated with lower SCD risk. These results are consistent with a retrospective case-control study, in which men in the lowest, compared with highest, quintile of linoleic acid in adipose tissue had a 5.7-fold greater risk of SCD (26). In previous studies examining CHD, linoleic acid has been more strongly associated with fatal (27, 28) compared with nonfatal endpoints (29). In combination with the current study, these data suggest that intake of linoleic acid may lower the propensity for fatal ventricular arrhythmias in the setting of CHD (3), which results in a lower incidence of fatal CHD, particularly SCD.
Diets high in n−6 PUFAs enhanced, rather than attenuated, the beneficial effects of n−3 PUFAs. Women with a high intake of both n−6 and n−3 PUFAs had the lowest risk of SCD, consistent with studies on CHD risk (30) and cardiovascular biomarkers (31). Furthermore, a high n−6:n−3 ratio was not associated with a greater risk of SCD, consistent with experimental evidence in which a high n−6:n−3 ratio did not inhibit n−3 PUFA incorporation into cell membranes (32). Thus, there is little evidence to support that a reduction in the n−6:n−3 ratio, particularly through a reduction in n−6 PUFAs, will lower the risk of SCD. Future research should explore whether the absence of an interaction between n−6 and n−3 PUFAs remains at higher intakes.
There are several potential mechanisms through which PUFAs may lower the risk of SCD. Atherosclerosis underlies most SCD events (33). PUFAs have effects on serum lipids, inflammation, blood pressure, endothelial function, thrombosis, and myocardial oxygen utilization (8–12) and thus may lower SCD risk through improvement in atherosclerotic pathways. When we adjusted for the interim development of CHD and CHD risk factors, the association between PUFA intake and the risk of SCD was attenuated but remained significant. This suggests that PUFAs likely also influence SCD risk through additional antiarrhythmic pathways not involved in atherosclerosis. PUFAs affect many cellular processes that may protect against ventricular arrhythmias, including influences on cardiac ion channels, particularly voltage-gated Na(+) and L-type Ca(2+) channels, β-adrenergic and other receptors, cell-signaling pathways, gap junction communication, and membrane fluidity (34, 35). The attenuation of risk estimates after adjustment for CHD and CHD risk factors was smaller for n−3 than for n−6 PUFAs; therefore, antiarrhythmic pathways may predominate for this class of fatty acids.
Whereas n−3 PUFAs have been consistently associated with a lower risk of SCD in observational studies of apparently healthy individuals such as ours, the effect of n−3 supplementation in clinical trials among post-MI patients is conflicting. In the GISSI-Prevensione trial, 850 mg long-chain n−3 PUFAs/d reduced SCD by 45% after 42 mo (36). However, in 2 recent post-MI trials, n−3 supplements did not significantly reduce SCD events beyond standard guideline-based clinical care (37, 38). The overall rate of SCD in these trials was low, and thus they were underpowered to detect moderate reductions in SCD. Additionally, in randomized trials, only individuals with low baseline intakes may benefit from supplementation, and this may be a small minority in some populations (39, 40). In the current study, the association between PUFAs and SCD appeared to reach a threshold at higher intakes.
In this analysis, we used a novel method to evaluate dietary fatty acids—a fat quality index for which each fatty acid is expressed as a percentage of total fat intake. Traditionally, the effect of fat intake on disease risk is assessed as a substitution for carbohydrates or all other sources of energy. However, in cell membranes, the increase in concentration of one fatty acid corresponds to a reduction of the same magnitude among all other fatty acids. Therefore, this fat quality index may be a better marker of the true biologic effect of dietary fatty acids, particularly for SCD, where the membrane effects of fatty acids may be crucial. For n−3 PUFAs, the relation with SCD was much stronger when expressed as a percentage of total fat, compared with a substitution for carbohydrates. Because n−3 PUFAs have the greatest antiarrhythmic actions relative to other fat types, the effect of n−3 PUFAs on SCD may be strongest when replacing other fats, rather than replacing carbohydrates or taking as a supplement to overall dietary fat intake, as done in clinical trials. Incorporation of dietary PUFAs into the cell membrane may also saturate at higher intakes as shown by the nonlinear relation between PUFAs measured in diet and in blood (23) and nonlinear relations between PUFA intake and SCD.
The association between SFA and SCD was significant only when expressed relative to intake of other fat and appeared to be mediated through the development of interim CHD and predisposing diseases. In earlier work, replacement of SFAs with PUFAs lowered the risk of CHD, whereas replacement with carbohydrates had little effect on risk (41). Current dietary guidelines focus on dietary fat quality, as opposed to quantity, and recommend replacing intake of less healthful fats (SFA and trans fat) with PUFAs (18). The fat quality index provides a novel method to quantify the overall fat quality of diets and individual foods to aid in the translation of the dietary guidelines.
Intake of MUFAs was not associated with risk of SCD, consistent with other findings for CHD (41). Although high blood concentrations of some SFAs and MUFAs have been associated with a higher risk of sudden cardiac arrest (42), biomarkers of SFAs and MUFAs do not reflect intake of endogenously synthesized fatty acids.
Important study limitations require discussion. The associations among this population of healthy women may not extend to secondary prevention, particularly among patients receiving state-of-the-art drug therapy. We had limited power to assess the role of effect modification by antecedent CHD in the setting of multiple comparisons. There may be sex differences in the association between fat and risk of SCD. ALA may be more effective at preventing SCD in women than in men (30), either directly (43) or through greater conversion of ALA to EPA and DHA (44). As with any observational study, residual confounding could explain, in part, the association between fatty acids and SCD. Importantly, the homogeneity among these women reduces potential confounding by factors, such as socioeconomic status, that associate with a healthy lifestyle; however, this homogeneity also limits the generalizability of our findings to other populations, such as those with higher intakes of PUFAs. Strengths of the current study include its long-term prospective design, repeated dietary assessments, and large number of rigorously confirmed SCDs.
In this prospective cohort of women, PUFAs, including both n−6 and n−3 PUFAs, were inversely associated with the risk of SCD. High intake of n−6 PUFAs enhanced, rather than attenuated, the inverse association between n−3 PUFA intake and risk of SCD. Furthermore, SFAs were positively associated with risk, although to a lesser extent. Overall, these results support dietary guidelines from the American Heart Association (17, 45) and USDA (18) to improve dietary fat quality by replacing SFA intake with n−6 and n−3 PUFAs for overall heart health.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SEC, EBR, and CMA: designed the research; SEC: performed the statistical analysis, drafted the manuscript, and had primary responsibility for the final content; and EBR, RKS, AMB, KMR, JEM, WCW, and CMA: contributed intellectual content to the manuscript. All authors read and approved the manuscript as written. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: ALA, α-linolenic acid; CHD, coronary heart disease; FFQ, food-frequency questionnaire; MI, myocardial infarction; SCD, sudden cardiac death.
REFERENCES
- 1.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2010;3:63–81 [DOI] [PubMed] [Google Scholar]
- 2.Myerburg RJ, Interian A, Simmons J, Castellanos A. Sudden cardiac death : Zipes DP, Cardiac electrophysiology: from cell to bedside. 4th ed. Philadelphia, PA: WB Saunders, 2004:720–31 [Google Scholar]
- 3.Kang JX, Leaf A. Prevention of fatal cardiac arrhythmias by polyunsaturated fatty acids. Am J Clin Nutr 2000;71:202S–7S [DOI] [PubMed] [Google Scholar]
- 4.McLennan PL. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr 1993;57:207–12 [DOI] [PubMed] [Google Scholar]
- 5.Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–64 [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation 2003;107:2096–101 [DOI] [PubMed] [Google Scholar]
- 7.Isensee H, Jacob R. Differential effects of various oil diets on the risk of cardiac arrhythmias in rats. J Cardiovasc Risk 1994;1:353–9 [PubMed] [Google Scholar]
- 8.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55 [DOI] [PubMed] [Google Scholar]
- 9.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev 2009;22:18–38 [DOI] [PubMed] [Google Scholar]
- 10.von Schacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res 2007;73:310–5 [DOI] [PubMed] [Google Scholar]
- 11.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation 2002;105:2303–8 [DOI] [PubMed] [Google Scholar]
- 12.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 2006;189:19–30 [DOI] [PubMed] [Google Scholar]
- 13.Albert CM, Manson JE, Hennekens CH, Ruskin JN. Fish consumption and the risk of myocardial infarction. N Engl J Med 1997;337:497–8 [DOI] [PubMed] [Google Scholar]
- 14.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n−3 fatty acids and the risk of sudden death. N Engl J Med 2002;346:1113–8 [DOI] [PubMed] [Google Scholar]
- 15.Siscovick DS, Raghunathan TE, King I, Weinman S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995;274:1363–7 [DOI] [PubMed] [Google Scholar]
- 16.Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n−3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J 2008;29:2024–30 [DOI] [PubMed] [Google Scholar]
- 17.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7 [DOI] [PubMed] [Google Scholar]
- 18.US Department of Agriculture, US Department of Health and Human Services Dietary guidelines for Americans. 7th ed. Washington, DC: US Government Printing Office, 2010 [Google Scholar]
- 19.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88 [DOI] [PubMed] [Google Scholar]
- 20.Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet 2003;92:109–32 [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, Monson R, Stason W, Hennekens CH. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 1987;317:1303–9 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998 [Google Scholar]
- 23.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74–81 [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40 [DOI] [PubMed] [Google Scholar]
- 25.Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol 2011;26:877–86 [DOI] [PubMed] [Google Scholar]
- 26.Roberts TL, Wood DA, Riemersma RA, Gallagher PJ, Lampe FC. Linoleic acid and risk of sudden cardiac death. Br Heart J 1993;70:524–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 2005;165:193–9 [DOI] [PubMed] [Google Scholar]
- 28.Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008;88:203–9 [DOI] [PubMed] [Google Scholar]
- 29.Kark JD, Kaufmann NA, Binka F, Goldberger N, Berry EM. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am J Clin Nutr 2003;77:796–802 [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111:157–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n−3 and n−6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003;108:155–60 [DOI] [PubMed] [Google Scholar]
- 32.Slee EL, McLennan PL, Owen AJ, Theiss ML. Low dietary fish-oil threshold for myocardial membrane n−3 PUFA enrichment independent of n−6 PUFA intake in rats. J Lipid Res 2010;51:1841–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008;51:213–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007;116:e320–35 [DOI] [PubMed] [Google Scholar]
- 35.McLennan PL, Abeywardena MY. Membrane basis for fish oil effects on the heart: linking natural hibernators to prevention of human sudden cardiac death. J Membr Biol 2005;206:85–102 [DOI] [PubMed] [Google Scholar]
- 36.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 37.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–9 [DOI] [PubMed] [Google Scholar]
- 38.Kromhout D, Giltay EJ, Geleijnse JM. n−3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26 [DOI] [PubMed] [Google Scholar]
- 39.Petrova S, Dimitrov P, Willett WC, Campos H. The global availability of n−3 fatty acids. Public Health Nutr 2011;14:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA 2011;305:1348–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu JH, Lemaitre RN, Imamura F, King IB, Song X, Spiegelman D, Siscovick DS, Mozaffarian D. Fatty acids in the de novo lipogenesis pathway and risk of coronary heart disease: the Cardiovascular Health Study. Am J Clin Nutr 2011;94:431–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen JH, Schmidt EB, Molenberg D, Toft E. Alpha-linolenic acid and heart rate variability in women examined for coronary artery disease. Nutr Metab Cardiovasc Dis 2005;15:345–51 [DOI] [PubMed] [Google Scholar]
- 44.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr 2006;83:1467S–76S [DOI] [PubMed] [Google Scholar]
- 45.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 2003;23:151–2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.