Abstract
Background: An impaired ability to compensate for calories and increased eating in the absence of hunger (EAH) has been associated with increased energy intake and weight gain in unrelated children.
Objective: The aims of this study were to compare caloric compensation [the percentage compensation index (%COMPX)] and EAH in weight-discordant siblings aged 5–12 y.
Design: In a crossover, behavioral genetics design, 47 same-sex sibling pairs (53% female, 55% full siblings) were served dinner once a week for 3 wk. Across conditions, siblings were served the same dinner, but 25 min before dinner, they either consumed in full or did not consume 1 of 2 preloads that varied in energy density (ED; 0.57 or 0.97 kcal/g). On the day when no preload was consumed, EAH was assessed after dinner and defined as the number of calories consumed from snacks.
Results: Overweight/obese siblings undercompensated [%COMPX: −48.8 ± 56.3 (mean ± SEM)] and therefore overate after the high-ED preload, whereas normal-weight siblings showed accurate compensation (%COMPX: 101.3 ± 51.9; P = 0.03). Furthermore, overweight/obese siblings consumed 34% more calories (93 kcal) in the absence of hunger than did normal-weight siblings (P = 0.01). Within-pair resemblances for %COMPX and EAH were stronger for full siblings (P < 0.049) than for half siblings (P > 0.23).
Conclusions: An impaired ability to regulate short-term energy intake, which includes incomplete adjustment for calorie differences in a preload and eating when satiated, may represent a behavioral phenotype for obesity in children. Future studies should test whether teaching children to focus on internal satiety cues may prevent at-risk children from overeating. This trial was registered at clinicaltrials.gov as NCT01598389.
INTRODUCTION
Obesity runs in families (1), which suggests that both genetic and environmental influences affect energy balance. At the same time, there is also evidence for significant differences in behavioral traits and phenotypic outcomes (eg, body weight and adiposity) among family members pointing to environmental influences not shared by family members (2, 3). Behavioral genetics designs are uniquely positioned to study the relation between genetic and environmental factors in accounting for individual differences in behavior. The discordant sibling design, in particular, controls for a known proportion of genetic variability among siblings (ie, 50% for full siblings, 25% for half siblings) and for the shared environment (4). Shared environments refer to environments that are correlated perfectly for family members and exert the same effect on the phenotypes of all family members (eg, household income) (5). Nonshared environments, on the other hand, refer to environments that are experienced differently by family members (eg, child feeding practices) (5) and, in the context of a discordant sibling design, are believed to make siblings within the same family different from one another (6, 7).
Eating in the absence of hunger (EAH)4 refers to children's susceptibility to eating when satiated in response to the presence of palatable snack foods (8). EAH is a behavioral eating trait that has been shown to increase short-term energy intake (9, 10) and to be associated with an increased risk of overweight (11, 12) in children. The trait further appears to be stable over time during childhood (11, 13), appears to be heritable (12), and has been shown to be increased in children whose caregivers use restrictive feeding practices and increased monitoring of food consumption (13, 14). Associations between EAH and adiposity (15) or EAH and child obesity risk status (16, 17) have been shown to be sex-specific.
Caloric compensation refers to adjustments in intake in response to changes in the caloric content of a preload and can provide a measure of individual differences in satiety (ie, effects of food after eating has ended) (18). It is assessed by administering a fixed amount of a food or nutrient (preload) and, after a predetermined time delay, measures its effects on subsequent intake (test meal) (18). Caloric compensation hence differs from EAH in that it assesses children's responsiveness to differences in the energy density (ED; kcal/g) of a preload based on feedback from internal cues of hunger and fullness. Studies with children have shown that the accuracy of their caloric compensation ability tends to decrease with age (19–21), be weaker in heavier children (22), and be influenced by parental feeding practices (19).
The aim of this study was to compare siblings who are discordant in their weight status in caloric compensation ability and EAH to elucidate potential differences in siblings’ ability to regulate short-term energy intake. We hypothesized that, within families and controlling for age differences, overweight and obese siblings would show poorer caloric compensation ability and greater EAH compared with normal-weight siblings, thereby pointing to an overall impaired ability to regulate short-term energy intake.
SUBJECTS AND METHODS
Experimental design
This study used a behavioral genetics, crossover design with repeated measures within participants. Siblings ate dinner at the Center for Weight and Eating Disorders at the University of Pennsylvania once a week for 3 wk. Across conditions, children were served the same dinner (pasta with tomato sauce, broccoli, applesauce, and milk). Twenty-five minutes before dinner, they either consumed in full (visits 2 and 3) or did not consume (visit 1) 1 of 2 preloads, which varied in ED (0.57 and 0.97 kcal/g). Dinner during the first visit served as a no-preload control condition, and EAH was assessed afterward. A graphic illustration of the sequence of experimental intake assessments is provided in Figure 1.
FIGURE 1.
Sequence of experimental intake assessments for eating in the absence of hunger and %COMPX. ED, energy density; %COMPX, percentage compensation index.
Participants and recruitment
Participants in this study were 47 same-sex sibling pairs (53% females) between 5 and 12 y of age and their mothers living in the greater metropolitan area of Philadelphia. Fifty-five percent of sibling pairs were full siblings; 45% of sibling pairs were half-siblings. Within families, all siblings had the same biological mother. Families were recruited through newspaper and online advertisements and flyers distributed in local pediatrician offices and grocery stores. Families from all racial and ethnic backgrounds were eligible to participate. To be included, sibling pairs had to be of the same sex, to be weight discordant (normal-weight versus overweight/obese; see Assessment of height and weight), to meet the age criteria (younger child: between 5 and 8 y of age; older child: between 9 and 12 y of age), and to like most foods that were served in the study (see Taste preference assessment). Only same-sex sibling pairs were included to reduce within-pair variability in energy intake because of sex differences. The age criteria were chosen to facilitate the counterbalancing of child weight status and age. Our goal was that for half of the sample (50%) the overweight/obese child would be the older sibling and the normal-weight child would be the younger sibling, whereas for the other half of the sample (50%), the overweight/obese child would be the younger sibling and the normal-weight sibling would be the older sibling. Children were excluded from participation if they had serious medical conditions known to affect food intake and body weight, had developmental or psychiatric conditions that might affect study compliance, had food allergies or nutrient intolerances (including lactose intolerance), or were taking medications known to affect appetite, food intake, or body weight.
A power analysis with α set at 0.05 and power and β set at 0.80 and 0.20, respectively, was performed to determine the number of sibling pairs needed in the study to detect a significant difference in percent compensation index (%COMPX) and EAH between weight-discordant siblings. On the basis of calculated SDs for EAH (8, 11, 13, 17, 23) and %COMPX (20, 21) from previous research, a sample size of 40 sibling pairs would detect mean differences in %COMPX as small as 24% and in EAH as small as 27 kcal between normal-weight and overweight/obese siblings, assuming a sibling correlation of 0.3. Because some participants did not meet the minimum intake requirements during some visits (see Statistical analysis), a total of 47 sibling pairs were enrolled in this study to make up for the missing intake observations.
Assessment of height, weight, and adiposity
At the screening visit, siblings’ height and weight were measured by a trained staff member. All measures were made while the siblings were wearing light clothing and after their shoes had been removed. Weight was measured on a digital scale (BWB-800; Tanita, Arlington Heights, IL; accurate to 0.1 kg), and standing height was measured with a wall-mounted stadiometer (Veder-Root, Elizabethtown, NC; accurate to 0.1 cm). Measurements were recorded in duplicate; the intraperson mean was used for analyses. Child age- and sex-specific BMI percentiles and z scores were calculated (24). Siblings were classified as normal-weight (BMI-for-age 5–84th percentile), overweight (BMI-for-age 85–94th percentile), or obese (BMI-for-age ≥95th percentile) (24). Weight discordance was defined as one sibling being of normal weight and the other being overweight or obese. To facilitate recruitment, 5 sibling pairs were included, for whom the BMI of the heavier sibling fell within 2% of the 85th BMI-for-age percentile (ie, corresponding to the 83rd percentile). Percentage body fat and fat-free mass (FFM; kg) were determined by dual-energy X-ray absorptiometry (Wi QDR Series Bone Densitometer; Hologic Discovery, Bedford, MA) during a separate visit at the end of the study.
Taste preference assessment
Once siblings met the criterion for weight discordance, preference for all foods and snacks served during the study was assessed by using a validated taste preference assessment (25). Each child first tasted a small amount of each of the dinner foods (pasta with tomato sauce, broccoli, and applesauce) and the preload (pudding). Children rated each food by using 3 cartoons depicting a smiling face (“yummy”), a neutral face (“just okay”), and a frowning face (“yucky”). In an effort to streamline the screening process and because the sensory properties of milk are known to children who regularly drink milk, we asked children to rate their preference for milk without tasting it. Only children who rated most of the foods with a neutral or smiling face were invited to participate in the study. Once children finished rating all foods, a rank-order preference assessment was performed during which children were asked to indicate the most “yummy” food, the next most “yummy” food, etc. Once children completed the preference assessment for the dinner foods and the preload, they repeated the assessment for the snack foods (potato chips, baked snack crackers, wafer biscuits, sponge cake with cream filling, chocolate chip cookies, and milk chocolate), which were to be offered during the EAH procedure. Preference assessments were conducted separately for each child in the absence of the other sibling.
Test meal
On all 3 study days, participants were served pasta with tomato sauce, broccoli, unsweetened applesauce, and 2% fat milk. The broccoli was served plain without any seasoning or added fat. All meal items were generally acceptable to children of this age group (26, 27). The ED (kcal/g), amounts (weight and volume), and energy contents (kcal) of foods and the milk served to siblings are shown in Table 1. Older siblings were served 40% larger portions of all foods and the milk to account for higher energy requirements. For both siblings, the portion size of the pasta entrée exceeded the 95th percentile of intake for children ages 6–11 y, based on data for spaghetti and tomato sauce from the Continuing Survey of Food Intakes by Individuals (28). The portion sizes of the broccoli, applesauce, and milk fell between the 75th and 90th percentiles of intake (28). The pasta was served on a 9-inch-diameter plate for the younger sibling and on a 10.25-inch-diameter plate for the older sibling. The milk was served in a 10-fl-oz transparent cup for the younger sibling and in a 14-fl-oz transparent cup for the older sibling with a lid and straw. The sizes of the bowls (12 oz) for the broccoli and applesauce were the same for younger and older siblings.
TABLE 1.
Amounts of foods and milk served at dinner and during the snack period
| Foods served | Energy density | Weight | Energy |
| kcal/g | g | kcal | |
| Dinner | |||
| Younger children (5–8 y) | |||
| Pasta1 with tomato sauce2 | 1.3 | 600 | 780 |
| Broccoli3 | 0.3 | 100 | 30 |
| Applesauce4 | 0.4 | 170 | 68 |
| Milk (2% fat) | 0.5 | 244 | 122 |
| Total energy provided | 1000 | ||
| Older children (9–12 y) | |||
| Pasta with tomato sauce | 1.3 | 840 | 1092 |
| Broccoli | 0.3 | 140 | 41 |
| Applesauce | 0.4 | 240 | 96 |
| Milk (2% fat) | 0.5 | 342 | 171 |
| Total energy provided | 1400 | ||
| Snacks | |||
| Younger (5–8 y) and older (9–12 y) children | |||
| Potato chips5 | 5.4 | 30 | 162 |
| Baked snack crackers6 | 4.7 | 70 | 329 |
| Wafer biscuits7 | 4.7 | 95 | 447 |
| Sponge cake with cream filling8 | 3.5 | 86 | 301 |
| Chocolate chip cookies9 | 4.8 | 132 | 634 |
| Milk chocolate10 | 5.0 | 200 | 1000 |
| Total energy provided | 2873 |
San Giorgio, Rotelle; New World Pasta Company.
Prego, Traditional Tomato Sauce; Campbell Soup Company.
Hanover, Petite Broccoli Florets (frozen); Hanover Foods Corporation.
Motts, Natural Applesauce (unsweetened); Mott's LLP.
Frito Lay.
Goldfish; Pepperidge Farm.
Nilla Wafers; Kraft Foods.
Twinkies; Hostess.
Chips Ahoy; Kraft Foods.
M&M's; Mars.
Preload
Preloads consisted of ready-to-eat vanilla or chocolate puddings, which differed in ED (0.57 or 0.97 kcal/g). Low-ED puddings were sugar-free and contained sugar substitutes. Younger children (5–8 y of age) were served 100 g (0.35 cups) pudding in both conditions, which provided 57 kcal in the lower-ED condition and 97 kcal in the higher-ED condition. Older children (9–12 y of age) were served 140 g (0.50 cups) pudding in both conditions, which provided 80 kcal in the lower-ED condition and 136 kcal in the higher-ED condition. The puddings were similar in macronutrient composition. The percentages of energy derived from fat, carbohydrate, and protein were 15%, 79%, and 7% for the low-ED vanilla pudding and 12%, 84%, and 4% for the high-ED vanilla pudding, respectively. The percentages of energy derived from fat, carbohydrate, and protein were 18%, 71%, and 11% for the low-ED chocolate pudding and 12%, 81%, and 7% for the high-ED chocolate pudding, respectively. Of the children who participated in the caloric compensation protocol, 79% chose to consume the vanilla-flavored preload and 21% chose to consume the chocolate-flavored preload. Pudding preloads were served in an 8-oz styrofoam bowl.
Snacks
Snacks were presented to participants in individual 12-oz bowls and included generous portions of potato chips, baked snack crackers, wafer biscuits, sponge cake with cream filling, chocolate chip cookies, and milk chocolate. One sibling pair was not served the sponge cake with cream filling for cultural/religious reasons. The type, amount, and energy content of each of the snack foods are shown in Table 1. Snack portions were similar in volume but not in weight. The amounts served exceeded the 95th percentile amounts consumed per eating occasion for children in this age group for most snacks. Because each of the 6 snack foods provided more energy than children were likely to consume, portions were not tailored to children's age.
The experimental dinner meals and snacks were prepared in the research kitchen of the Center for Weight and Eating Disorders according to a standardized protocol. All foods and the milk were preweighed before being served to participants and were reweighed after the participants had finished eating to determine the amount consumed by each child to the nearest 0.1 g.
Assessment of caloric compensation ability and eating in the absence of hunger
The siblings’ caloric compensation ability was assessed in 2 ways. First, a caloric compensation index (%COMPX) (20–22) was computed as follows:
 |
where EIlowED corresponds to calories consumed from the test meal after the low-ED preload, EIhighED corresponds to calories consumed from the test meal after the high-ED preload, preloadhighED corresponds to calories consumed from the compulsory high-ED preload, and preloadlowED corresponds to calories consumed from the compulsory low-ED preload. Given that the caloric contents of the preloads (ie, preloadhighED and preloadlowED) are constants, %COMPX represents a linear transformation of the difference in intake across the 2 preload conditions. A %COMPX of 100% equals perfect compensation. %COMPX scores <100% indicate undercompensation (or overeating), whereas %COMPX scores >100% indicate overcompensation (or undereating). Second, calories consumed from the test meal and total calories (test meal + preload) were compared across experimental conditions (ie, no-preload control, high-ED preload, and low-ED preload). EAH was assessed during the first test visit and referred to the number of calories consumed from the snacks while being fully satiated.
Procedures
On the day of each dinner test visit, mothers were instructed to have their children consume a typical lunch and an afternoon snack (if desired) and not to consume any foods or beverages (except water) after 1500. On arrival at the Center at 1700, mothers completed a brief report verifying that their children were well in the past 24 h and had complied with the fasting instructions.
At the beginning of each visit and again after consumption of dinner, subjective measures of hunger were obtained from each child participant by using 3 cartoon figures depicting a “hungry,” “half-full,” and “full” state (10). During the second and third test visits only, child participants were served 1 of 2 preloads 25 min before dinner. Participants were instructed to consume the preloads in full over a period of 10 min. After a 15-min delay, subjects were served dinner, which they consumed ad libitum over a period of 20 min. The first test visit, during which no preload was served, was considered a baseline visit. EAH was assessed after the completion of dinner during the first test visit by using the “free access procedure” (13). Specifically, after a 5-min delay after the completion of dinner, participants were given unrestricted access to a variety of snack foods, which they consumed ad libitum over a period of 15 min. During this time, children were also provided with age-appropriate magazines.
Children were instructed that they could eat as much or as little as they wanted during dinners and the EAH assessment. At the beginning of the second and third visits, siblings were told that they would not be receiving snacks after dinner, but that they instead will be served pudding before dinner. During all 3 test visits, siblings consumed their dinners and snacks in the same room, but a room divider prevented them from seeing each other in an effort to minimize the influence of social and visual cues on siblings’ food intake.
Statistical analysis
Data were analyzed by using the SAS System for Windows (version 9.2; SAS Institute) and SPSS (version 19; SPSS Inc). We confirmed the normality of distribution of continuous variables by using the Shapiro-Wilk test in conjunction with distribution plots and summary statistics (eg, skewness and kurtosis). Data points (ie, dinner intakes) for children who consumed <90% of the preload in at least one preload condition (n = 7) or for children whose total energy intake was <150 calories on at least one occasion (n = 2) were not included in the analysis. Children who were identified to not meet these minimum intake requirements indicated that they no longer liked or could not finish the pudding or that they were tired or not feeling well.
Paired t tests were used to compare normal-weight and overweight/obese siblings on %COMPX and EAH (primary outcomes). A mixed linear model (PROC MIXED) was used to further examine the influence of siblings’ age (younger or older) and sex on both outcome measures by adding these variables as a covariate to the model. To account for the dependence of siblings within the same family, a random effect of subject (sibling) nested within family was also added to the model. The interaction between sibling weight status and age or sex was tested for significance in all models. To assess nonshared environmental influences on child eating behaviors, within-pair difference scores were computed for %COMPX, EAH, age, BMI z score, percentage body fat, and FFM by subtracting, for each measure, the score of the normal-weight sibling from the score of the overweight/obese sibling. Positive scores indicate that the overweight/obese sibling scored higher on a given measure compared with his or her normal-weight sibling, whereas negative scores indicate that the obese sibling scored lower compared with his or her normal-weight sibling. A score of zero indicates no difference between the 2 siblings (29). We used general linear model regression analysis (PROC GLM) to assess the relation between difference scores in child eating behaviors (%COMPX and EAH) and difference scores in anthropometric measures (BMI z scores and percentage body fat). The influence of differences in siblings’ age on both outcome measures was also examined by adding this variable as a covariate to the regression model. Furthermore, intraclass correlations with 95% CIs (ρ 1-way random) (30) were performed to measure familial resemblances in %COMPX and EAH among full siblings and half siblings.
In addition, a mixed linear model (PROC MIXED) with repeated measures was used to further examine siblings’ compensation ability. The primary outcome was calories consumed at dinner repeated across 3 experimental conditions. The secondary outcome was total calories consumed at the meal (calories consumed at dinner + calories consumed from preload). The fixed-factor effects used in all models were preload condition (no preload, low-ED preload, or high-ED preload) and sibling weight status (normal-weight or overweight/obese). Again, to account for the dependence of siblings within the same family, a random effect of subject (sibling) nested within family was added to the model. The interaction between preload condition and sibling weight status was tested for significance in all models.
Descriptive statistics are reported as means (±SDs) for continuous variables or as percentages for categorical variables. Results from the mixed linear model analysis are presented as model-based means (±SEMs) unless otherwise indicated. Reported P values are 2-sided, and P < 0.05 was considered significant for all tests.
RESULTS
Child characteristics
The participants’ demographic and anthropometric characteristics are shown in Table 2. Approximately half of the siblings were male (47%). Most of them were African American (68%), and ∼20% of the sample was Hispanic. The average BMI-for-age percentile was 48.5 ± 19.5 for normal-weight siblings and 92.0 ± 6.0 for overweight/obese siblings. Of the heavier siblings, 57% were considered overweight, whereas 43% were considered obese.
TABLE 2.
Child demographic and anthropometric characteristics by sibling weight status (n = 47)
| Child characteristic | Normal-weight | Overweight/obese1 |
| Age (y) | 8.8 ± 2.32 | 9.0 ± 2.3 |
| Sex (n) | 22/25 | 22/25 |
| Male | ||
| Female | ||
| Race (%) | ||
| Black or African American | 68 | 68 |
| White | 9 | 9 |
| More than one race | 19 | 19 |
| Unknown | 4 | 4 |
| Ethnicity (%) | ||
| Hispanic | 19 | 21 |
| Not Hispanic | 55 | 53 |
| Unknown | 26 | 26 |
| Height (cm) | 131.6 ± 14.7 | 140.2 ± 16.4*** |
| Height-for-age z score | 0.02 ± 0.9 | 1.11 ± 0.9*** |
| Weight (kg) | 29.0 ± 8.7 | 46.2 ± 18.4*** |
| Weight-for-age z score | −0.08 ± 0.7 | 1.7 ± 0.7*** |
| BMI (kg/m2) | 16.4 ± 1.4 | 22.5 ± 4.9*** |
| BMI z score | −0.04 ± 0.5 | 1.60 ± 0.6*** |
| BMI-for-age percentile | 48.5 ± 19.5 | 92.0 ± 6.0*** |
| Body fat (%)3 | 19.4 ± 0.8 | 31.3 ± 1.3*** |
| Fat-free mass (kg)3 | 22.1 ± 7.0 | 28.9 ± 10.4** |
Paired t test for continuous variables: **P < 0.01, ***P < 0.001.
Mean ± SD (all such values).
Based on 44 sibling pairs who participated in the body-composition analysis.
Preference ratings
Foods served at dinner (including the preload) and during the snack period were generally well liked among children. With respect to the dinner foods, >90% of children gave a “yummy” or “just ok” rating for the main entrée (pasta with tomato sauce) and the fruit side dish (applesauce) and more than 80% of children gave those ratings for the vegetable side dish (broccoli) and the milk. The majority of children (>90%) rated each of the snack foods as “yummy” or “just ok,” whereas all children (100%) showed high liking ratings for the pudding preload. The distribution of preference rankings for each of the experimental foods from the taste preference assessment is shown in Table 3.
TABLE 3.
Distribution of child preference rankings of experimental foods (n = 47)
| Preference ranking for dinner and snack foods1 |
||||||
| Experimental foods | 1 | 2 | 3 | 4 | 5 | 6 |
| Dinner foods | ||||||
| Pasta with tomato sauce | 50 | 27 | 23 | — | — | — |
| Broccoli | 14 | 30 | 56 | — | — | — |
| Applesauce | 36 | 44 | 20 | — | — | — |
| Snacks | ||||||
| Potato chips | 21 | 18 | 14 | 19 | 17 | 11 |
| Baked snack crackers | 16 | 16 | 14 | 11 | 21 | 22 |
| Wafer biscuits | 10 | 10 | 20 | 25 | 18 | 17 |
| Sponge cake with cream filling2 | 21 | 13 | 10 | 13 | 19 | 22 |
| Chocolate chip cookies | 14 | 15 | 28 | 21 | 12 | 10 |
| Milk chocolate | 18 | 29 | 15 | 10 | 13 | 15 |
All values are percentages. Preference rankings ranged from 1 (most liked) to 3 (least liked) for dinner foods or from 1 (most liked) to 6 (least liked) for snack foods.
One sibling pair did not taste, rate, or rank-order the sponge cake for cultural/religious reasons.
Caloric compensation
Results from the mixed-model analysis showed a nonsignificant trend for a condition-by-weight status interaction for energy intake at dinner (P = 0.09) but not for total energy intake (P = 0.13). When served no preload, the low-ED preload, or the high-ED preload before dinner, normal-weight siblings consumed 541 ± 37, 489 ± 37, and 428 ± 38 kcal at the meal, respectively (Figure 2A). Total energy intake (meal + preload) for the normal-weight siblings was 567 ± 37 kcal in the low-ED and 563 ± 37 kcal in the high-ED preload condition, respectively (Figure 2B). Overweight/obese siblings, on the other hand, consumed 626 ± 37, 536 ± 38, 558 ± 37 kcal at the meal in the no preload, low-ED preload, and high-ED preload conditions, respectively. Total energy intake (meal + preload) for overweight/obese siblings was 613 ± 37 kcal in the low-ED preload and 684 ± 37 kcal in the high-ED preload conditions, respectively.
FIGURE 2.
Mean (±SEM) energy intake at the meal (A) and total energy intake (meal + preload; B) consumed by normal-weight (n = 47) and overweight/obese (n = 47) siblings across preload conditions. The analyses indicated a nonsignificant trend for a condition-by-weight status interaction for meal energy intake (P = 0.09). Values are model-based means with preload condition and child weight status included in the linear mixed model. ED, energy density.
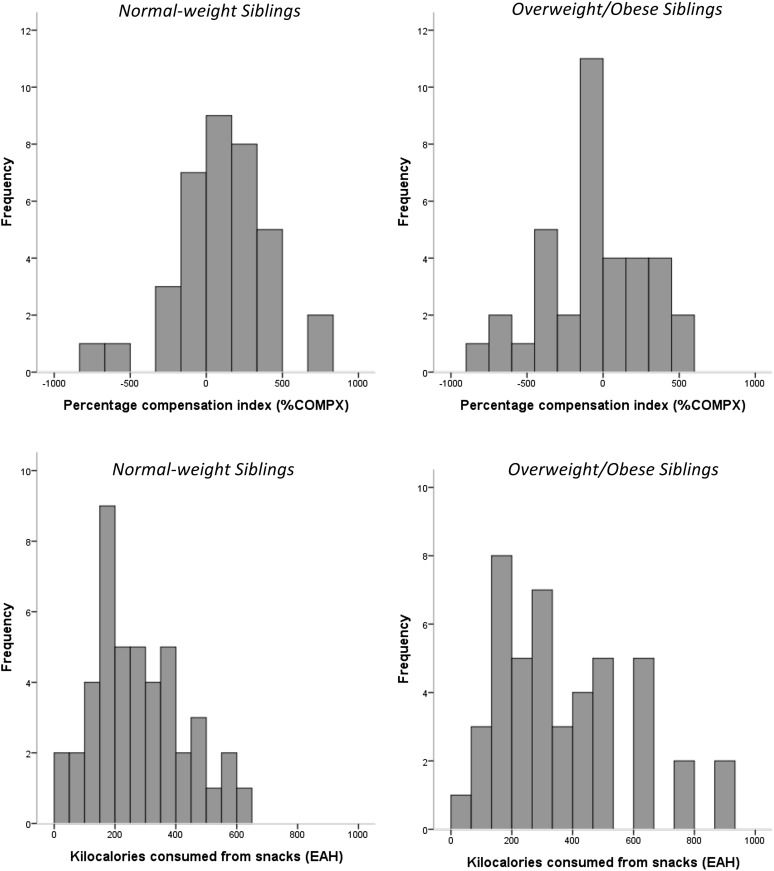
The individual responses for %COMPX by sibling weight status are shown in Figure 3. Data depicted in Table 4 show that %COMPX significantly differed between normal-weight and overweight/obese siblings (P = 0.03), indicating that overweight/obese siblings undercompensated (−47.8% ± 56.3) and therefore overate after the high-ED preload, whereas normal-weight siblings showed accurate compensation (101.3% ± 51.9). When adding covariates to the model, there was a significant weight status–by-age interaction (P = 0.02), which indicated that older normal-weight siblings overcompensated and therefore underate (177.5% ± 77.8), whereas younger overweight/obese siblings undercompensated and therefore overate (−125.7% ± 77.8; P = 0.002). Neither the interaction between siblings’ weight status and sex nor the main effect of sex on %COMPX was statistically significant (P > 0.13). Intraclass correlations indicated some sibling resemblance (familiality) in %COMPX among full siblings (ρ = 0.36; P = 0.049; 95% CI: −0.15, 0.81) but not among half siblings (ρ = 0.02; P = 0.47; 95% CI: −0.47, 0.51). Results from the general linear model analysis indicated that sibling differences in percentage body fat (P = 0.004), but not BMI in z score (P = 0.09) or FFM (P = 0.43), were significantly associated with sibling differences in %COMPX, even when adjusted for sibling differences in age.
FIGURE 3.
Histograms of individual responses for EAH and %COMPX by sibling weight status. EAH, eating in the absence of hunger; %COMPX, percentage compensation index.
TABLE 4.
%COMPX and EAH and intraclass correlations (and 95% CIs) by sibling weight status1
| Intraclass correlation (ρ)2 |
|||||
| Normal-weight siblings | Overweight/obesesiblings1 | Sibling difference | Full siblings | Half siblings | |
| %COMPX | 101.3 ± 51.93 | −47.8 ± 56.3* | −149.1 ± 67.0 | 0.36* (−0.15, 0.81) | 0.02 (−0.47, 0.51) |
| EAH (kcal) | 276.5 ± 21.8 | 369.9 ± 31.8** | 93.4 ± 32.2 | 0.37* (−0.19, 0.66) | 0.16 (−0.27, 0.54) |
Between-sibling (normal-weight compared with overweight/obese) comparison with the use of a paired t test. *P < 0.05, **P < 0.01. EAH, eating in the absence of hunger; %COMPX, percentage compensation index.
Intraclass correlation (1-factor random, single measures).
Mean ± SEM (all such values).
Eating in the absence of hunger
Individual responses for EAH are shown in Figure 3 by sibling weight status. The calories consumed in the absence of hunger by normal-weight and overweight/obese siblings are provided in Table 4. Overweight/obese siblings, on average, consumed 34% more calories (93 kcal) during the EAH protocol than did normal-weight siblings (P = 0.006). When covariates were added to the model, a significant weight status–by-age interaction (P = 0.01) was observed, which indicated that older overweight/obese siblings consumed more calories in the absence of hunger (412 ± 38 kcal) than did older normal-weight (328 ± 38 kcal; P = 0.03) or younger normal-weight (258 ± 38 kcal; P = 0.001) siblings. In addition, a significant weight status–by-sex interaction (P = 0.009) was observed, which indicated that overweight/obese boys (411 ± 39 kcal) and overweight/obese girls (333 ± 37 kcal) consumed significantly more calories in the absence of hunger than did normal-weight girls (228 ± 37 kcal; P < 0.02). There was a trend for a main effect of siblings’ age on EAH (P = 0.06). Intraclass correlations indicated some sibling resemblance (familiality) in EAH among full siblings (ρ = 0.37; P = 0.03; 95% CI: −0.19, 0.66), but not among half siblings (ρ = 0.16; P = 0.23; 95% CI: −0.27, 0.54). Results from the general linear model analysis indicated that sibling differences in FFM (P = 0.03), but not in percentage body fat (P = 0.38) and BMI z score (P = 0.86), were significantly associated with sibling differences in EAH. When adjusted for sibling differences in age, sibling differences in FFM were no longer associated with sibling differences in EAH (P = 0.16). In an exploratory analysis, we examined whether the magnitude of the differences in BMI z score and percentage body fat affected the sibling difference in EAH by performing a median split of the BMI z score and percentage body fat difference. Results showed that siblings who were less discordant in BMI z score showed a mean difference in EAH of 84 ± 38 kcal, whereas those who were more discordant in BMI z score showed a mean difference in EAH of 121 ± 61 kcal; however, these differences were not statistically significant (P = 0.62). When this analysis was repeated with percentage body fat, the results showed that siblings who were less discordant in percentage body fat showed a mean difference in EAH of 61 ± 35 kcal, whereas those who were more discordant in percentage body fat showed a mean difference in EAH of 174 ± 74 kcal; however, these differences were not statistically significant (P = 0.18).
DISCUSSION
Through use of a behavioral genetics design, this study showed, for the first time, that overweight/obese siblings had a higher EAH and a lower %COMPX (ie, overeating in response to a high-ED preload) than did normal-weight siblings, thereby supporting our hypotheses that overweight/obese siblings would show poorer short-term compensation ability and a greater susceptibility to eating when no longer hungry in response to external food cues.
This study aimed to compare weight-discordant siblings on their compensation ability by using the %COMPX index. As hypothesized, overweight/obese siblings showed pronounced undercompensation (ie, overeating) after the high-ED preload, whereas normal-weight siblings, on average, exhibited a mean %COMPX score that was close to 100% (indicative of accurate caloric compensation). This finding confirmed our initial hypothesis and is in line with results from some (22, 31), but not all (32), previous studies conducted in unrelated children that showed that overweight and obese youth exhibit poorer caloric compensation ability compared with normal-weight youth. For example, a study with 3- to 7-y-old sibling pairs (32) showed that, when using a liquid preload, children's %COMPX was unrelated to child BMI z score and waist circumference. It is possible that differences in preload type (liquid or solid), preload timing (delay between preload and subsequent meal), and child ages between studies may account for these different findings. The findings from the current study suggest that strategies to improve caloric compensation among overweight/obese children may be needed to improve overall energy intake regulation. Additionally, there may be opportunities for parents and caregivers to guide children in their eating. For example, data by Birch et al (33) and Johnson (31) showed that responsiveness to the ED of a preload can be improved in children by teaching them to focus on internal cues of hunger and fullness. Hence, child feeding practices that focus on orienting children to their internal hunger and fullness cues while eating, rather than attempting to control children's food intake, may be useful strategies for parents and caregivers to improve their children's intake regulation (34).
Another aim of this study was to compare normal-weight and overweight/obese siblings in EAH. In this study, overweight/obese siblings consumed 34% more energy (93 calories) in the absence of hunger than did normal-weight siblings, thereby confirming our initial hypothesis. These data suggest that, when hunger is not present, overweight/obese children are prone to overeating in response to external food cues. Results from the paired t test analysis, but not from the difference score analysis, support findings from previous studies (11, 12, 15) that show a significant positive relation between child adiposity and EAH. Possible implications for these findings are that overweight/obese children may particularly benefit from a home food environment, which is structured in a way that minimizes exposure to food cues for palatable, energy-dense foods. Future studies are needed to determine whether the heightened reactivity to external food cues can be used to promote intake of healthy foods among overweight/obese siblings by creating a (home) food environment in which healthy foods, such as fruit and vegetables, are visible and easily accessible.
An analysis of covariance showed significant weight status–by-age interactions for both %COMPX and EAH and a significant weight status–by-sex interaction for EAH, which indicated that the degree of under- and overcompensation and susceptibility to eating when no longer hungry was age- and (for EAH only) sex-dependent. These findings indicate that efforts to improve caloric compensation ability and reduce EAH in children need to take into account individual differences and tailor intervention components accordingly.
The analysis of the within-pair discrepancy (eg, difference score) is an estimate of the nonshared environment in behavioral genetics designs (29). In this study, we examined the relation between the difference scores in siblings’ eating traits (ie, %COMPX and EAH) and the difference scores in their percentage body fat, FFM, and BMI z score. Only the within-pair difference in percentage body fat, but not differences in sibling BMI z score or FFM, was significantly associated with the within-pair difference in %COMPX. For EAH, only the within-pair difference in FFM, when unadjusted for age, but not differences in percentage body fat or BMI z score, was associated with the within-pair difference in this eating trait. Siblings in this study showed a range in BMI and adiposity. By including overweight children (ie, those with a BMI-for-age between the 85th and 94th percentile), the weight discordance for some sibling pairs was fairly small [within-pair BMI-for-age percentile difference (minimum to maximum): 15–82]. It is possible that the variability in the magnitude of weight discordance may have accounted for the inconsistent findings in the difference score analysis.
This study further showed that within-pair resemblances in %COMPX and EAH were stronger in full siblings than in half siblings, pointing to potential genetic influences underlying these eating traits. Genetic influences have indeed been documented for both caloric compensation and EAH. Specifically, EAH has been shown to be heritable (h2 = 51%) (12) and associated with the BclI (rs41423247) polymorphism of the glucocorticoid receptor gene (35). Similarly, polymorphisms in the peroxisome proliferator–activated receptor γ- and β-adrenergic receptor genes have been associated with children's caloric compensation ability (36). There is a need for future studies to systematically test genetic influences on eating traits that are associated with hyperphagia in children by using behavioral genetics designs.
The strengths of this study include the unique cohort of children (same-sex and weight-discordant full and half siblings); the behavioral genetics design, which controlled for home environmental influences on eating traits; and the inclusion of a large number of minority children. To our knowledge, this also is the first study that concurrently assessed caloric compensation and EAH in related children to examine their ability to regulate short-term energy intake. The study also had limitations. One, although unique, the narrowly defined study sample may preclude a generalization of the study findings to a more heterogenous group of children. Also, despite comprehensive screening efforts, it is possible that children with undiagnosed developmental or psychiatric conditions may have entered the study. Second, future studies with weight-discordant siblings may benefit from excluding overweight children (BMI-for-age: 85–94th percentile), which would increase the within-pair weight discordance. Third, the inclusion of full and half siblings may have weakened the statistical power to detect within-pair differences in eating traits. Fourth, despite informing children about the timing of the snacks and puddings, it is possible that the randomization of the preload, but not EAH, conditions may have affected children's intake. Additionally, the large food portions served during dinner and the snacks may have led to overall greater intakes in children (37) and may have differentially affected overweight/obese children (38). Also, serving older children 40% more food during the preload/dinner may not have accurately accounted for individual differences in children's overall energy requirements. Last, it is possible that consumption of a chocolate-flavored preload, which slightly differed in macronutrient composition from the vanilla-flavored preload, may have affected intake among the subset of children (21%) who consumed the chocolate flavored preload.
In summary, overweight/obese siblings, when compared with normal-weight siblings, showed an impaired ability to adjust for calorie differences in a preload and consumed more snacks when satiated. These findings suggest that an impaired ability to regulate short-term energy intake, which includes adhering less to internal cues of hunger and fullness and eating when full, may represent a behavioral phenotype for obesity in children. Future studies should test whether teaching children to focus on internal satiety cues and structuring the home food environment in a healthy way may prevent at-risk children from overeating.
Acknowledgments
We acknowledge the contributions of the staff and students at the Center for Weight and Eating Disorders at the University of Pennsylvania who assisted with this study.
The authors’ responsibilities were as follows—TVEK: study design, data collection, statistical analysis, interpretation of the results, and writing of the manuscript; DBA: training and mentorship for TVEK, statistical analysis, and critical revision of the manuscript; LLB and VAS: training and mentorship for TVEK and critical revision of the manuscript; RHM: statistical analysis, interpretation of the results, critical revision of the manuscript; and MSF: training and mentorship for TVEK, study design, statistical analysis, interpretation of the results, and critical revision of the manuscript. None of the authors had a conflict of interest. DBA has, anticipates, or has had financial interests with the Frontiers Foundation; Vivus Inc; Kraft Foods; University of Wisconsin; University of Arizona; Paul, Weiss, Wharton & Garrison LLP; and Sage Publications.
Footnotes
Abbreviations used: EAH, eating in the absence of hunger; ED, energy density; FFM, fat-free mass; %COMPX, percentage compensation index.
REFERENCES
- 1.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73 [DOI] [PubMed] [Google Scholar]
- 2.Dunn J, Plomin R. Why are siblings so different? The significance of differences in sibling experiences within the family. Fam Process 1991;30:271–83 [DOI] [PubMed] [Google Scholar]
- 3.Plomin R, Daniels D. Why are children in the same family so different from one another? Int J Epidemiol 2011;40(3):563–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison DB. The use of discordant sibling pairs for finding genetic loci linked to obesity: practical considerations. Int J Obes Relat Metab Disord 1996;20:553–60 [PubMed] [Google Scholar]
- 5.Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin North Am 2001;48:893–907 [DOI] [PubMed] [Google Scholar]
- 6.Plomin R. Nature and nurture: an introduction to human behavioral genetics. Pacific Grove, CA: Brooks/Cole, 1990 [Google Scholar]
- 7.Rodgers JL, Buster M, Rowe DC. Genetic and environmental influences on deliquency: DF analysis of NLSY kinship data. J Quant Criminol 2001;17:145–68 [Google Scholar]
- 8.Cutting TM, Fisher JO, Grimm-Thomas K, Birch LL. Like mother, like daughter: familial patterns of overweight are mediated by mothers’ dietary disinhibition. Am J Clin Nutr 1999;69:608–13 [DOI] [PubMed] [Google Scholar]
- 9.Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Am J Clin Nutr 1999;69:1264–72 [DOI] [PubMed] [Google Scholar]
- 10.Fisher JO, Birch LL. Parents’ restrictive feeding practices are associated with young girls’ negative self-evaluation of eating. J Am Diet Assoc 2000;100:1341–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr 2002;76:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher JO, Cai G, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity (Silver Spring) 2007;15:1484–95 [DOI] [PubMed] [Google Scholar]
- 13.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr 2003;78:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JO, Birch LL. Restricting access to foods and children's eating. Appetite 1999;32:405–19 [DOI] [PubMed] [Google Scholar]
- 15.Hill C, Llewellyn CH, Saxton J, Webber L, Semmler C, Carnell S, van Jaarsveld CH, Boniface D, Wardle J. Adiposity and ‘eating in the absence of hunger’ in children. Int J Obes (Lond) (Lond) 2008;32:1499–505 [DOI] [PubMed] [Google Scholar]
- 16.Kral TV, Moore RH, Stunkard AJ, Berkowitz RI, Stettler N, Stallings VA, Tanaka LM, Kabay AC, Faith MS. Adolescent eating in the absence of hunger and relation to discretionary calorie allowance. J Am Diet Assoc 2010;110:1896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obes Res (Silver Spring) 2006;14:131–8 [DOI] [PubMed] [Google Scholar]
- 18.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009;97:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birch LL, Fisher JO. Food intake regulation in children. Fat and sugar substitutes and intake. Ann N Y Acad Sci 1997;819:194–220 [DOI] [PubMed] [Google Scholar]
- 20.Johnson SL, Taylor-Holloway LA. Non-Hispanic white and Hispanic elementary school children's self-regulation of energy intake. Am J Clin Nutr 2006;83:1276–82 [DOI] [PubMed] [Google Scholar]
- 21.Cecil JE, Palmer CN, Wrieden W, Murrie I, Bolton-Smith C, Watt P, Wallis DJ, Hetherington MM. Energy intakes of children after preloads: adjustment, not compensation. Am J Clin Nutr 2005;82:302–8 [DOI] [PubMed] [Google Scholar]
- 22.Johnson SL, Birch LL. Parents’ and children's adiposity and eating style. Pediatrics 1994;94:653–61 [PubMed] [Google Scholar]
- 23.Francis LA, Birch LL. Maternal weight status modulates the effects of restriction on daughters’ eating and weight. Int J Obes (Lond) 2005;29:942–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 25.Birch LL, Sullivan SA. Measuring children's food preferences. J Sch Health 1991;61:212–4 [DOI] [PubMed] [Google Scholar]
- 26.Kral TV, Kabay AC, Roe LS, Rolls BJ. Effects of doubling the portion size of fruit and vegetable side dishes on children's intake at a meal. Obesity (Silver Spring) 2010;18:521–7 [DOI] [PubMed] [Google Scholar]
- 27.Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr 2011;93:284–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smiciklas-Wright H, Mitchell D, Mickle S, Cook A, Goldman J. Foods commonly eaten in the United States: quantities consumed per eating occasion and in a day, 1994-96. Houston, TX: US Department of Agriculture Research Service, 2002. [Google Scholar]
- 29.Rovine MJ. Estimating nonshared environment using sibling discrepancy scores : Hetherington EM, Reiss D, Plomin R, Separate social worlds of siblings. The impact of nonshared environment on development. Hillsdale, NJ: Lawrence Erlbaum Associates, 1994:33–61 [Google Scholar]
- 30.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30–46 [Google Scholar]
- 31.Johnson SL. Improving Preschoolers’ self-regulation of energy intake. Pediatrics 2000;106:1429–35 [DOI] [PubMed] [Google Scholar]
- 32.Faith MS, Keller KL, Johnson SL, Pietrobelli A, Matz PE, Must S, Jorge MA, Cooperberg J, Heymsfield SB, Allison DB. Familial aggregation of energy intake in children. Am J Clin Nutr 2004;79:844–50 [DOI] [PubMed] [Google Scholar]
- 33.Birch LL, Mcphee L, Shoba BC, Steinberg L, Krehbiel R. Clean up your plate—effects of child feeding practices on the conditioning of meal size. Learn Motiv 1987;18:301–17 [Google Scholar]
- 34.Anzman SL, Rollins BY, Birch LL. Parental influence on children's early eating environments and obesity risk: implications for prevention. Int J Obes (Lond) 2010;34:1116–24 [DOI] [PubMed] [Google Scholar]
- 35.Rutters F, Lemmens SG, Born JM, Bouwman F, Nieuwenhuizen AG, Mariman E, Westerterp-Plantenga MS. Genetic associations with acute stress-related changes in eating in the absence of hunger. Patient Educ Couns 2010;79:367–71 [DOI] [PubMed] [Google Scholar]
- 36.Cecil JE, Palmer CN, Fischer B, Watt P, Wallis DJ, Murrie I, Hetherington MM. Variants of the peroxisome proliferator-activated receptor gamma- and beta-adrenergic receptor genes are associated with measures of compensatory eating behaviors in young children. Am J Clin Nutr 2007;86:167–73 [DOI] [PubMed] [Google Scholar]
- 37.Fisher JO, Kral TV. Super-size me: Portion size effects on young children's eating. Physiol Behav 2008;94:39–47 [DOI] [PubMed] [Google Scholar]
- 38.Sharafi M, Fisher JO, Birch LL. Behavioral responses to portion size are moderated by children's weight status. Obesity (Silver Spring) 2009;17:S91 [Google Scholar]