Abstract
Background: Children from ethnic minority and low-income families in the United States have higher rates of poor health and higher mortality rates. Diet, an acknowledged correlate of health, may mediate the known race-ethnic and socioeconomic differentials in the health of US children.
Objective: The objective was to examine the independent association of race-ethnicity, family income, and education with nutritional and lipid biomarkers in US children.
Design: We used data from the NHANES 2003–2006 to examine serum concentrations of vitamins A, D, E, C, B-6, and B-12; serum concentrations of folate, carotenoids, and lipids; and dietary intakes of corresponding nutrients for 2–19-y-old children (n = ∼2700–7500). Multiple covariate–adjusted regression methods were used to examine the independent and joint associations of race-ethnicity, family income, and education with biomarker status.
Results: Non-Hispanic blacks had lower mean serum concentrations of vitamins A, B-6, and E and α-carotene than did non-Hispanic whites. Both non-Hispanic blacks and Mexican Americans had higher mean serum vitamin C, β-cryptoxanthin, and lutein + zeaxanthin but lower folate and vitamin D concentrations compared with non-Hispanic whites. In comparison with non-Hispanic whites, non-Hispanic blacks were less likely to have low serum HDL cholesterol or high triglycerides. Family income and education predicted few biomarker or dietary outcomes, and the observed associations were weak. Moreover, modification of race-ethnic differentials by income or education (or vice versa) was noted for very few biomarkers.
Conclusion: Race-ethnicity, but not family income or education, was a strong independent predictor of serum nutrient concentrations and dietary micronutrient intakes in US children and adolescents.
INTRODUCTION
Children from ethnic minority and low-income families in the United States have higher rates of poor health and higher mortality rates (1–4). The prevalence of acknowledged risk factors for disease (higher adiposity, total cholesterol, and glycated hemoglobin concentrations) also shows variation by ethnicity and socioeconomic position in US children (5–10). Ethnic and socioeconomic disparities in the health of US children and adolescents reflect a confluence of cultural norms, family resource constraints, risk environments, and gaps in knowledge of desirable health behaviors. Diet, an intervenable risk factor for a number of chronic diseases, may be one of the mediators of the ethnic and socioeconomic differences in health outcomes. Arguably, dietary intakes of key protective and risk-increasing nutrients among children may also be expected to vary with ethnicity and family socioeconomic position. Yet, few studies have systematically examined the independent association of ethnicity, income, and education with nutrient profiles in US children. An additional problem in examination of these associations is the acknowledged bias in assessment of dietary intakes of children and adolescents (11, 12). Nutritional biomarkers, however, are not subject to respondent errors and can reflect longer-term dietary exposures, micronutrient bioavailability, and in vivo utilization (13, 14); therefore, biomarkers may be particularly valuable in examining possible ethnic and socioeconomic disparities in dietary exposures in children.
A sizable body of evidence has contributed to our understanding of race-ethnic differences in micronutrient status in adults (15–27). For children, race-ethnic differences in prevalence of marginal vitamins A and D and iron nutritional status have received the greatest attention (28–36). However, there is a surprising paucity of information on whether other nutritional biomarkers in children also show ethnic differentials. Moreover, in most studies that examined these associations in children, few have examined the independent associations of race-ethnicity, family income, and education. Because race-ethnic group membership, family income, and education can potentially relate to health (and diet) by several interrelated and unique pathways, it is important to understand their independent contributions for design of interventions. To fill these gaps, we used nationally representative data to examine the independent associations of race-ethnicity, family income, and family education with available nutritional and lipid biomarkers in US children and to compare the associations of race-ethnicity, income, and education with biomarkers to those observed for dietary intakes.
SUBJECTS AND METHODS
We used public domain data from the continuous NHANES 2003–2004 and 2005–2006 for this study (37–39). The study was approved by the Queens College Institutional Review Board with an exempt review. The continuous NHANES is conducted annually by the National Center for Health Statistics (NCHS)5, CDC, and data are released for 2 y at a time (40). Each NHANES is a stratified, multistage, cluster-probability sample of the US population and includes an at-home interview and an examination in a mobile examination center (MEC). The MEC examination includes a complete medical examination, anthropometric measurements, an interview to collect dietary information, and collection of blood and urine samples with the use of standardized procedures. The 2003–2004 and 2005–2006 surveys oversampled low-income persons, adolescents, blacks, and Mexican Americans. In each survey, the unweighted response rates for the MEC-examined sample of 2–19-y-olds were >80% (40).
Biomarkers
We examined serum concentrations of water-soluble vitamins [vitamin C, vitamin B-6 (as pyridoxal phosphate), serum and red blood cell (RBC) folate, and vitamin B-12], fat-soluble vitamins and carotenoids [vitamin A (as retinol), vitamin D (as 25-hydroxycholecalciferol), vitamin E (as α-tocopherol), α-carotene, β-carotene, β-cryptoxanthin, lutein + zeaxanthin, and trans lycopene], and serum lipids (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) for this study. In both survey cycles (2003–2004 and 2005–2006), serum folate and vitamins D and B-12 were available for 2–19-y-olds; carotenoids, total or HDL cholesterol, and vitamins A, C, and E were available for 6–19-y-olds; LDL cholesterol and triglycerides were available for 12–19-y-olds. For vitamin B-6, we followed the NCHS advisory and limited our analysis to data from the 2005–2006 survey for 2–19-y-olds (the NCHS advisory cautions against combining the data for 2003–2004 with 2005–2006 because of methodologic differences) (39). Although serum lipids and serum and RBC folate were available for the NHANES 2007–2008, because of changes in laboratory method, site, or equipment from the earlier surveys, we limited our analysis to the 2003–2004 and 2005–2006 surveys.
All procedures for phlebotomy, subsequent handling of blood samples, analysis of samples, and reporting procedures followed a standardized protocol specified by the NHANES (38, 39). The assay methods used for measurement of serum analytes are detailed in the NHANES documentation and included the following: radioimmunoassay for vitamin D, radioassay for serum/RBC folate and serum vitamin B-12, direct immunoassay for HDL cholesterol, and enzymatic methods for serum total cholesterol and triglycerides, reversed-phase HPLC with fluorometric detection for vitamin B-6, HPLC with electrochemical detection for vitamin C, 2-step Diasorin procedure (Diasorin Corp) for vitamin D, and HPLC with photodiode array detection for vitamins A and E and the carotenoids. Total LDL cholesterol was calculated by using the Friedewald equation (38, 39).
Race-ethnicity and measures of socioeconomic position
In the US population, race-ethnicity tends to be related with socioeconomic position (SEP) (41–44). However, each SEP indicator (eg, income and education) also relates to health independent of race-ethnicity, and it has been suggested that investigation of race-ethnic health disparities should include ≥2 different measures of SEP (42). Thus, we included family income and education of head of household as indicators of family SEP. In the continuous NHANES, race-ethnicity is self-reported. The NCHS combines this information and provides adjudicated race-ethnicity categories as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and all others. For the 1999–2002 and 2003–2006 survey periods, the NHANES analytic guidelines do not recommend estimates for all Hispanics or non–Mexican American Hispanics because of inadequate sample size for non–Mexican American Hispanics and oversampling of Mexican Americans (45). For our analysis, race-ethnicity was categorized as non-Hispanic white, non-Hispanic black, and Mexican American. We considered family income assessed by family poverty-income ratio (PIR) and educational level of the head of household as indicators of family SEP. PIR is computed by the NCHS and is a ratio of family income to the poverty threshold established by the US Census Bureau. The poverty threshold varies with family size and is inflation adjusted; a PIR <1 is considered below poverty. We operationalized the PIR as <130%, 130–349%, and ≥350% for 2 reasons: 1) a PIR of <130% is used as the cutoff for several federal food assistance programs and 2) these cutoffs are often used by the NCHS for reporting of NHANES dietary and health data. The educational level of the family head was operationalized as <12 y, 12 y, some college, and college degree or higher.
Analytic sample
All MEC-examined respondents aged 2–19 y in the NHANES 2003–2004 and 2005–2006, with at least one measured biomarker of interest, were eligible for inclusion in the study (n = 7527). We excluded pregnant and lactating respondents (n = 115), those who were missing family PIR (n = 402) or family education information (n = 253), those with unknown supplement use status in the past month (n = 8) or who reported supplement use since fasting time (n = 12), those with other race-ethnicity (n = 658), and those who were missing information on chronic disease status (n = 16) or minutes of fasting before phlebotomy (n = 1) from the eligible sample for a final analytic sample of 6062. Some biomarkers were measured for ages ≥6 y; the final analytic sample for 6–19 y olds was 4908. Serum LDL cholesterol and triglycerides were available for 1502 respondents aged ≥12 y.
Dietary nutrient intake
We also examined 24-h dietary intake of energy, fat, and the micronutrient corresponding to the respective biomarker. The NHANES collected a 24-h dietary recall by using the USDA's Automated Multiple-Pass Method, administered by a trained dietary interviewer, during the MEC interview (38, 39). Unlike the NHANES 2007–2008, estimates of 24-h total nutrient intake (diet + supplements) are not available for the 2003–2006 survey years. The supplement use information in the NHANES 2003–2004 and 2005–2006 was obtained in the household interview, and the NCHS does not provide estimates of 24-h supplemental nutrient intake for these survey years (38, 39) because of issues related to the format of supplement data and where and how they were collected.
Covariates
To determine whether race-ethnicity, family income, and education were independently associated with serum biomarker concentrations, we accounted for the effect of other potential confounders of these associations. These included sex, vitamin/mineral supplement use over the previous 30 d, self-reported disease condition (asthma, diabetes, or high blood pressure), nicotine exposure (assessed as serum cotinine concentration), month of MEC examination, BMI percentile for age and sex, and inflammatory condition (assessed as serum C-reactive protein concentration). Additional covariates were as follows: for vitamin D and serum lipids, hours of television and computer use; for vitamin E and carotenoids, serum total cholesterol. For dietary outcomes, the covariates included age, sex, month of MEC examination, day of recalled intake, self-reported history of chronic disease, and BMI percentile for age and sex.
Statistical methods
The independent associations of race-ethnicity, family income, and education with biomarker and dietary outcomes were examined by using multiple regression methods with biomarker concentration or dietary intake as a continuous outcome. Because the age interval of 2–19 y or even 6–19 y represents a range of developmental stages and differences in dietary autonomy, and possible interactions of race-ethnicity with family income and education, we began with models that included all covariates and 5 interaction terms (age by race, age by PIR, age by education, race by PIR, and race by education) to examine whether the associations of race-ethnicity, income, and education differed by age and SEP categories. In a backward stepwise multiple linear regression, we subsequently deleted the interaction term with the least significance and reexamined the significance of the remaining interaction term or terms. We used P < 0.01 as the criterion for determining the significance of the interaction terms to adjust for the number of steps in the elimination procedure (0.05/5 interaction terms for each biomarker = 0.01). If no significant interactions were observed, we present the main effects of race-ethnicity, PIR, and education; otherwise, we present findings that reflect the relevant interactions. All examined P values are based on a Satterthwaite adjusted F test statistic that takes account of the complex sample design and sample weighting and makes a correction for the df (46).
We also examined the independent association of race-ethnicity, income, and education with prevalence of serum concentration of selected micronutrients and lipids relative to a threshold indicating at-risk status by using multiple logistic regression models. The cutoffs used were based on previous NCHS publications (20, 47), and recommendations of expert committees (48–50) and were as follows: vitamin C (<0.2 mg/dL), vitamin B-6 (<20 nmol/L), serum folate (<2 ng/mL), RBC folate (<95 ng/mL), vitamin B-12 (<200 pg/mL), retinol (<20 μg/dL), vitamin D (<20 ng/mL), vitamin E (<500 μg/dL), total cholesterol (≥200 mg/dL), HDL cholesterol (≤40 mg/dL), LDL cholesterol (≥130 mg/dL), and triglycerides (≥130 mg/dL).
We log-transformed each reported biomarker concentration for multiple linear regression analyses. The estimates presented in the tables and figures were adjusted for multiple covariates and back-transformed to obtain geometric means and their 95% CIs. All analyses were performed by using SAS version 9.2 (SAS Institute) and SAS callable SUDAAN release 10.0.1 (Research Triangle Institute) software for analysis of data from multistage stratified cluster survey designs. The analyses included the appropriate sample weights to account for unequal probability of sampling and sample nonresponse (51). For serum LDL cholesterol and triglycerides, we followed the NCHS recommendations and limited our analysis to the morning-examined subsample with >8.5 h of fasting before phlebotomy (37, 38); separate weights are provided by the NCHS for this subsample. The tabular and graphic results are adjusted means (sometimes called predicted margins) derived from multiple linear regression and multiple logistic regression models (52).
RESULTS
Non-Hispanic whites had higher family PIR and education than did non-Hispanic blacks and Mexican Americans (Table 1). Supplement use over the month preceding the MEC examination was more frequent among non-Hispanic white children and adolescents. Self-report of asthma, diabetes, or high blood pressure was more frequent among non-Hispanic black children and adolescents. Mean serum cotinine was higher in non-Hispanic blacks and mean serum C-reactive protein was higher in Mexican Americans relative to other race-ethnic groups (Table 1).
TABLE 1.
Sociodemographic, lifestyle, and other characteristics of 2–19-y-old respondents with measured serum vitamin and lipid concentrations: NHANES 2003–20061
| n | Non-Hispanic white (n = 1792) | Non-Hispanic black (n = 2195) | Mexican American (n = 2075) | |
| Age group (%) | ||||
| 2–5 y | 1154 | 17.3 ± 1.12 | 18.3 ± 1.0 | 23.2 ± 0.9 |
| 6–11 y | 1529 | 31.8 ± 1.6 | 33.0 ± 1.4 | 36.1 ± 0.9 |
| 12–19 y | 3379 | 50.9 ± 1.9 | 48.7 ± 1.8 | 40.7 ± 1.1 |
| Sex (%) | ||||
| Male | 3078 | 52.6 ± 1.4 | 51.1 ± 1.4 | 51.7 ± 1.1 |
| Female | 2984 | 47.4 ± 1.4 | 48.9 ± 1.4 | 48.3 ± 1.1 |
| Poverty-income ratio (%) | ||||
| <130% | 2663 | 20.8 ± 2.3 | 51.7 ± 2.5 | 50.6 ± 2.1 |
| 130–349% | 2186 | 38.8 ± 2.1 | 34.9 ± 1.7 | 37.4 ± 1.6 |
| >349% | 1213 | 40.3 ± 2.5 | 13.5 ± 1.7 | 12.0 ± 1.4 |
| Household head educational level (%) | ||||
| <12 y | 2029 | 9.5 ± 1.2 | 31.1 ± 1.8 | 53.1 ± 2.6 |
| 12 y | 1498 | 27.3 ± 2.3 | 25.6 ± 2.1 | 21.6 ± 1,8 |
| Some college | 1711 | 38.0 ± 1.8 | 30.8 ± 1.5 | 18.7 ± 1.8 |
| College degree or higher | 824 | 25.2 ± 2.3 | 12.5 ± 1.6 | 6.6 ± 1.1 |
| Used dietary supplement in past 30 d (%) | 1468 | 37.5 ± 1.8 | 17.5 ± 1.3 | 24.1 ± 2.1 |
| Self-reported diabetes, asthma, or high blood pressure (%) | 1073 | 16.9 ± 0.9 | 21.5 ± 1.0 | 12.7 ± 0.7 |
| Month of MEC examination (%) | ||||
| November to April | 3166 | 35.5 ± 4.5 | 48.5 ± 8.3 | 76.5 ± 6.1 |
| May to October | 2896 | 64.5 ± 4.5 | 51.5 ± 8.3 | 23.5 ± 6.1 |
| BMI for sex-age percentile (%) | ||||
| <5th | 178 | 3.2 ± 0.5 | 3.1 ± 0.4 | 3.0 ± 0.4 |
| 5th to <85th | 3676 | 65.4 ± 1.9 | 60.5 ± 1.4 | 58.4 ± 1.3 |
| 85th to <95th | 982 | 16.5 ± 1.0 | 15.4 ± 1.0 | 17.8 ± 0.6 |
| ≥95th | 1145 | 14.9 ± 1.4 | 21.0 ± 1.1 | 20.8 ± 1.4 |
| Hours of fasting before phlebotomy3 | 6062 | 6.9 ± 0.2 | 7.8 ± 0.2 | 7.7 ± 0.2 |
| Serum cotinine (ng/mL) | 5471 | 0.18 (0.13, 0.25)4 | 0.30 (0.23, 0.37) | 0.06 (0.04, 0.07) |
| Serum CRP (mg/dL) | 5889 | 0.043 (0.040, 0.046) | 0.050 (0.046, 0.054) | 0.062 (0.057, 0.067) |
CRP, C-reactive protein; MEC, mobile examination center.
Weighted percentage ± SE (all such values) unless otherwise indicated.
Values are means ± SEs.
Geometric mean; 95% CI in parentheses (all such values).
Serum biomarker concentration
Interactions of race-ethnicity, family income, and education for predicting mean biomarker concentration
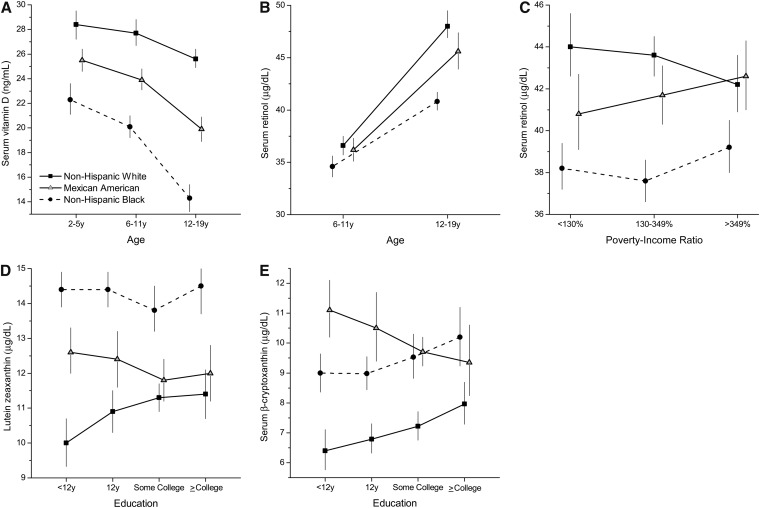
There was an interaction between age and race-ethnicity for vitamins D and A (P ≤ 0.0006), an interaction of race with PIR for vitamin A (P = 0.004), and an interaction of race with education for lutein/zeaxanthin and β-cryptoxanthin (P ≤ 0.005) (Figure 1). Mean serum vitamin D concentration was higher in non-Hispanic whites than in non-Hispanic blacks and Mexican Americans at every age (P < 0.0001). Serum vitamin D concentrations declined with age in all race-ethnic groups (P < 0.0001); however, the declines were steeper in the minority groups than in non-Hispanic whites.
FIGURE 1.
Interactions of race-ethnicity with age [serum vitamin D (A) and retinol (B)], poverty-income ratio (serum retinol; C), and education [serum lutein + zeaxanthin (D) and β-cryptoxanthin (E)] in US children and adolescents, P < 0.01 (Satterthwaite adjusted F test): NHANES 2003–2006. The estimates are adjusted means (and 95% CIs) from multiple linear regression models with each biomarker as a log-transformed dependent variable and included age (2–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), self-reported history of chronic disease (yes or no), hours of fasting (continuous), history of supplement use in past month (yes or no), BMI percentile for age and sex (continuous), serum cotinine, C-reactive protein, serum total cholesterol (for carotenoids), and hours of television and computer use (for vitamin D).
Mean serum retinol concentration was higher at age 12–19 y in all race-ethnic groups (P < 0.0001); however, the race-ethnic differences in serum retinol concentration were significant only in 12–19-y-olds (P = 0.0001). There were race-ethnic differences in serum retinol concentration in all PIR categories (P ≤ 0.0001).
Mean concentrations of lutein + zeaxanthin and β-cryptoxanthin differed by race-ethnicity at all categories of education (P < 0.0001); however, the education effect was positive and greater in non-Hispanic whites only (P ≤ 0.05). Among Mexican Americans, higher education predicted lower mean serum β-cryptoxanthin concentration (P = 0.02).
Race-ethnic differences in mean biomarker concentrations
With the exception of β-carotene and total and LDL cholesterol, race-ethnicity was a significant independent predictor of all examined biomarker outcomes (Table 2).
TABLE 2.
Multiple variable–adjusted geometric means and 95% CIs of biomarkers by race-ethnicity in US children and adolescents: NHANES 2003–20061
| Serum biomarker | n | Age | Non-Hispanic white | Non-Hispanic black | Mexican American | P (race-ethnicity)2 |
| y | ||||||
| Water-soluble vitamins | ||||||
| Vitamin C (mg/dL) | 4684 | 6–19 | 0.96a (0.91, 1.01) | 1.16c (1.12, 1.21) | 1.07b (1.01, 1.14) | <0.0001 |
| Pyridoxal phosphate (nmol/L)3 | 2672 | 2–19 | 56.1b (53.8, 58.6) | 49.3a (46.4, 52.4) | 56.3b (51.0, 62.1) | 0.01 |
| Serum folate (ng/mL) | 5388 | 2–19 | 13.8b (13.4, 14.3) | 12.6a (12.2, 13.0) | 13.0a (12.5, 13.5) | <0.0001 |
| Red blood cell folate (ng/mL) | 5389 | 2–19 | 257c (251, 263) | 214a (210, 218) | 242b (235, 250) | <0.0001 |
| Vitamin B-12 (pg/mL) | 5382 | 2–19 | 594a (579, 610) | 704b (686, 723) | 600a (580, 620) | <0.0001 |
| Fat-soluble vitamins and carotenoids (μg/dL) | ||||||
| α-Carotene | 4635 | 6–19 | 1.95b (1.80, 2.10) | 1.63a (1.47, 1.81) | 2.22c (2.02, 2.44) | 0.0001 |
| β-Carotene | 4427 | 6–19 | 10.7 (10.2, 11.3) | 10.6 (9.9, 11.3) | 10.9 (10.2, 11.5) | 0.8 |
| trans Lycopene | 4637 | 6–19 | 21.0b (20.4, 21.7) | 23.7c (22.8, 24.8) | 19.5a (18.8, 20.1) | <0.0001 |
| Vitamin E | 4637 | 6–19 | 803b (790, 815) | 770a (759, 781) | 797b (781, 814) | 0.0001 |
| Serum lipids (mg/dL) | ||||||
| Total cholesterol | 4654 | 6–19 | 160 (158, 162) | 160 (158, 162) | 158 (156, 161) | 0.6 |
| Total cholesterol (morning subsample)4 | 1441 | 12–19 | 156 (153, 160) | 156 (153, 160) | 157 (152, 162) | 1.0 |
| HDL cholesterol | 4654 | 6–19 | 51a (50, 52) | 57b (56, 58) | 51a (50, 53) | <0.0001 |
| HDL cholesterol (morning subsample)4 | 1441 | 12–19 | 50a (48, 51) | 56c (54, 57) | 52b (50, 54) | <0.0001 |
| LDL cholesterol (morning subsample)4 | 1437 | 12–19 | 85 (83, 88) | 85 (82, 89) | 84 (80, 88) | 0.8 |
| Triglycerides (morning subsample)4 | 1441 | 12–19 | 84a (79, 89) | 61b (58, 64) | 84a (80, 89) | <0.0001 |
The adjusted values are back-transformed predicted margins from multiple linear regression models with each biomarker as a log-transformed dependent variable and included age (2–5, 6–11, 12–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), self-reported history of chronic disease (yes or no), hours of fasting (continuous), history of supplement use in the past month (yes or no), BMI percentile for age and sex (continuous), serum cotinine, and C-reactive protein. Models for carotenoids and vitamin E also included serum total cholesterol, and models for vitamin D and serum lipids included hours of television and computer use. Adjusted values in each row not sharing a common superscript letter are significantly different, P < 0.05 (Satterthwaite adjusted F test).
Satterthwaite adjusted F test for differences between race-ethnic groups.
NHANES 2005–2006.
Limited to the subsample with morning examination and >8.5 h of fasting.
Water-soluble vitamins
Serum vitamins C and B-12 concentrations were higher in non-Hispanic black children than in non-Hispanic whites and Mexican Americans (P < 0.0001). However, serum and RBC folate concentrations were lower in non-Hispanic black and Mexican American children compared with non-Hispanic whites (P < 0.01). The mean serum vitamin B-6 concentration in non-Hispanic black children was also lower than that in non-Hispanic whites and Mexican Americans.
Fat-soluble vitamins and lipids
Serum concentrations of α-carotene were lower and those of trans lycopene were higher in non-Hispanic blacks than in non-Hispanic whites and Mexican Americans (P < 0.0001). Serum vitamin E concentrations in non-Hispanic blacks were lower than in non-Hispanic whites. Non-Hispanic blacks had higher mean HDL cholesterol and significantly lower mean triglyceride concentrations compared with the other 2 race-ethnic groups (P < 0.0001).
Family PIR and education differences in mean biomarker concentrations
Serum vitamin D concentrations increased with increasing PIR (P = 0.01). For all other biomarkers, PIR was not a significant independent predictor (Table 3). RBC folate concentration differed among categories of family education; however, the 95% CIs overlapped in most cases (Table 4). Serum α-carotene concentration increased with increasing years of education (P = 0.009).
TABLE 3.
Multiple variable–adjusted geometric means and 95% CIs of biomarkers by categories of PIR in US children and adolescents: NHANES 2003–20061
| PIR |
||||||
| Serum biomarker | n | Age | <130% | 130–349% | >349% | P (PIR)2 |
| y | ||||||
| Water-soluble vitamins | ||||||
| Vitamin C (mg/dL) | 4684 | 6–19 | 1.02 (0.97, 1.08) | 0.99 (0.94, 1.04) | 1.01 (0.96, 1.07) | 0.6 |
| Pyridoxal phosphate (nmol/L) | 2672 | 2–19 | 52.5 (49.7, 55.6) | 56.5 (52.9, 60.3) | 55.2 (52.2, 58.7) | 0.2 |
| Serum folate (ng/mL) | 5388 | 2–19 | 13.9 (13.4, 14.5) | 13.7 (13.2, 14.2) | 13.0 (12.4, 13.6) | 0.1 |
| RBC folate (ng/mL) | 5389 | 2–19 | 245 (239, 252) | 250 (245, 256) | 244 (238, 251) | 0.1 |
| Vitamin B-12 (pg/mL) | 5382 | 2–19 | 622 (596, 649) | 614 (597, 633) | 602 (585, 619) | 0.4 |
| Fat-soluble vitamins and provitamins | ||||||
| α-Carotene (μg/dL) | 4635 | 6–19 | 1.94 (1.78, 2.11) | 1.92 (1.73, 2.13) | 1.91 (1.75, 2.09) | 0.9 |
| β-Carotene (μg/dL) | 4427 | 6–19 | 11.0 (10.5, 11.6) | 10.6 (10.0, 11.2) | 10.6 (9.9, 11.4) | 0.4 |
| β-Cryptoxanthin (μg/dL) | 4606 | 6–19 | 8.15 (7.67, 8.66) | 7.81 (7.30, 8.35) | 7.81 (7.39, 8.24) | 0.4 |
| Lutein/zeaxanthin (μg/dL) | 4639 | 6–19 | 12.0 (11.4, 12.5) | 11.5 (11.2, 11.09) | 11.7 (11.1, 12.3) | 0.4 |
| trans Lycopene (μg/dL) | 4637 | 6–19 | 20.9 (20.3, 21.6) | 21.7 (21.1, 22.4) | 21.0 (19.9, 22.2) | 0.2 |
| Vitamin D (ng/mL) | 5325 | 2–19 | 23.6a (22.7, 24.5) | 24.0a (23.1, 25.0) | 24.7b (23.7, 25.9) | 0.01 |
| Vitamin E (μg/dL) | 4637 | 6–19 | 797a,b (779, 814) | 787a (773, 801) | 807b (795, 820) | 0.05 |
| Serum lipids (mg/dL) | ||||||
| Total cholesterol | 4654 | 6–19 | 160 (158, 163) | 158 (156, 160) | 161 (158, 163) | 0.1 |
| Total cholesterol (morning subsample)3 | 1441 | 12–19 | 157 (152, 161) | 156 (152, 160) | 157 (152, 162) | 0.9 |
| HDL cholesterol | 4654 | 6–19 | 52 (51, 53) | 52 (51, 53) | 52 (51, 53) | 0.3 |
| HDL cholesterol (morning subsample)3 | 1441 | 12–19 | 50 (48, 52) | 50 (48, 52) | 52 (51, 53) | 0.2 |
| LDL cholesterol (morning subsample)3 | 1437 | 12–19 | 85 (81, 89) | 85 (82, 88) | 85 (81, 89) | 1.0 |
| Triglycerides (morning subsample)3 | 1441 | 12–19 | 83 (77, 90) | 81 (76, 86) | 76 (70, 83) | 0.2 |
The adjusted values are back-transformed predicted margins from multiple linear regression models with each biomarker as a log-transformed dependent variable and included age (2–5, 6–11, 12–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family PIR (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), self-reported history of chronic disease (yes or no), hours of fasting (continuous), history of supplement use in the past month (yes or no), BMI percentile for age and sex (continuous), serum cotinine, and C-reactive protein. Models for carotenoids and vitamin E also included serum total cholesterol, and models for vitamin D and serum lipids included hours of television and computer use. Adjusted values in each row not sharing a common superscript letter are significantly different, P < 0.05 (Satterthwaite adjusted F test). PIR, poverty-income ratio; RBC, red blood cell.
Satterthwaite adjusted F test for differences between PIR groups.
Limited to the subsample with morning examination and >8.5 h of fasting.
TABLE 4.
Multiple variable–adjusted geometric means and 95% CIs of biomarkers by categories of educational level of household head in US children and adolescents: NHANES 2003–20061
| Educational level |
|||||||
| Serum biomarker | n | Age | <12 y | 12 y | Some college | College degree or higher | P (education)2 |
| y | |||||||
| Water-soluble vitamins | |||||||
| Vitamin C (mg/dL) | 4684 | 6–19 | 0.99 (0.93, 1.05) | 0.99 (0.93, 1.06) | 1.02 (0.96, 1.08) | 1.03 (0.97, 1.09) | 0.7 |
| Pyridoxal phosphate (nmol/L) | 2672 | 2–19 | 57.1 (50.5, 64.4) | 54.1 (49.9, 58.7) | 54.7 (51.9, 57.7) | 54.7 (49.8, 60.1) | 0.8 |
| Serum folate (ng/mL) | 5388 | 2–19 | 13.4 (12.9, 13.9) | 13.4 (12.9, 14.0) | 13.4 (12.9, 14.0) | 13.9 (13.2, 14.6) | 0.6 |
| Red blood cell folate (ng/mL) | 5389 | 2–19 | 250a,b (243, 256) | 246a,b (239, 254) | 242a (235, 248) | 255b (246, 263) | 0.04 |
| Vitamin B-12 (pg/mL) | 5382 | 2–19 | 627 (606, 649) | 603 (580, 627) | 606 (589, 624) | 621 (594, 650) | 0.3 |
| Fat-soluble vitamins and provitamins | |||||||
| Retinol (μg/dL) | 4639 | 6–19 | 41.0 (39.1, 42.9) | 41.6 (40.4, 42.8) | 42.3 (41.3, 43.6) | 42.9 (41.4, 44.5) | 0.3 |
| α-Carotene (μg/dL) | 4635 | 6–19 | 1.86a (1.68, 2.06) | 1.76a (1.62, 1.93) | 1.88a (1.76, 2.01) | 2.32b (1.96, 2.75) | 0.009 |
| β-Carotene (μg/dL) | 4427 | 6–19 | 10.6 (9.8, 11.5) | 10.4 (9.9, 11.0) | 10.6 (10.2, 11.1) | 11.4 (10.3, 12.5) | 0.2 |
| trans Lycopene (μg/dL) | 4637 | 6–19 | 20.8 (19.9, 21.8) | 22.1 (21.3, 22.9) | 21.0 (20.1, 22.0) | 20.9 (20.0, 21.8) | 0.1 |
| Vitamin D (ng/mL) | 5325 | 2–19 | 24.9 (23.7, 26.1) | 24.1 (22.9, 25.4) | 23.9 (23.0, 24.8) | 23.9 (22.9, 24.8) | 0.2 |
| Vitamin E (μg/dL) | 4637 | 6–19 | 791 (775, 806) | 800 (783, 817) | 797 (783, 812) | 796 (777, 815) | 0.8 |
| Serum lipids (mg/dL) | |||||||
| Total cholesterol | 4654 | 6–19 | 158 (155, 161) | 160 (157, 164) | 161 (159, 164) | 158 (155, 161) | 0.2 |
| Total cholesterol (morning subsample)3 | 1441 | 12–19 | 155 (149, 161) | 159 (154, 165) | 156 (151, 160) | 155 (148, 163) | 0.6 |
| HDL cholesterol | 4654 | 6–19 | 51 (50, 52) | 52 (51, 53) | 52 (50, 53) | 52 (50, 54) | 0.8 |
| HDL cholesterol (morning subsample)3 | 1441 | 12–19 | 51 (49, 53) | 51 (49, 52) | 51 (49, 53) | 50 (47, 53) | 0.9 |
| LDL cholesterol (morning subsample)3 | 1437 | 12–19 | 85 (81, 90) | 87 (82, 92) | 84 (80, 88) | 84 (78, 89) | 0.7 |
| Triglycerides (morning subsample)3 | 1441 | 12–19 | 74 (69, 79) | 85 (78, 93) | 78 (73, 83) | 82 (74, 91) | 0.1 |
The adjusted values are back-transformed predicted margins from multiple linear regression models with each biomarker as a log-transformed dependent variable and included age (2–5, 6–11, 12–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), self-reported history of chronic disease (yes or no), hours of fasting (continuous), history of supplement use in the past month (yes or no), BMI percentile for age and sex (continuous), serum cotinine, and C-reactive protein. Models for carotenoids and vitamin E also included serum total cholesterol, and models for vitamin D and serum lipids included hours of television and computer use. Adjusted values in each row not sharing a common superscript letter are significantly different, P < 0.05 (Satterthwaite adjusted F test).
Satterthwaite adjusted F test for differences between education groups.
Limited to the subsample with morning examination and >8.5 h of fasting.
Race-ethnicity, family income, and education differentials in percentage of the population at risk by using biomarker cutoffs
The number of respondents with low serum concentrations of folate, vitamin B-12, and retinol was small [serum folate concentration <2 ng/mL (n = 1), RBC folate concentration <95 ng/mL (n = 27), vitamin B-12 concentration <200 pg/mL (n = 24), and serum retinol concentration of <20 μg/dL (n = 22)]. Therefore, further analyses were not performed for these biomarkers. For the remaining biomarkers, no examined interactions met the criteria for statistical significance; therefore, the main effects are presented in Table 5. Relative to non-Hispanic whites and Mexican Americans, a smaller percentage of non-Hispanic black children had vitamin C concentrations <0.2 mg/dL (P < 0.001). More than 50% of all non-Hispanic black children and nearly 30% of Mexican American children had serum vitamin D concentrations <20 ng/mL compared with ∼12% of non-Hispanic white children (P < 0.0001). Compared with non-Hispanic white children, Mexican Americans were less likely to have serum cholesterol concentrations ≥200 mg/dL (P = 0.01). The percentage of non-Hispanic black children with serum HDL cholesterol ≤40 mg/dL or triglyceride concentrations ≥130 mg/dL was smaller than the corresponding percentage of non-Hispanic white children (P < 0.0001). Family income and education were not independently associated with the risk of lower or higher concentrations of the examined biomarkers.
TABLE 5.
Multiple covariate–adjusted percentages and 95% CIs of the 2–19-y-old US children and adolescents below or above the at-risk cutoff: NHANES 2003–20061
| Serum biomarker2 | n | Age | Non-Hispanic white | Non-Hispanic black | Mexican American | P (race-ethnicity)3 | P (PIR)34 | P (education)3 |
| y | % | % | % | |||||
| Water-soluble vitamins | ||||||||
| Vitamin C | 4684 | 6–19 | 2.83b (1.66, 3.99) | 0.19a (0.01, 0.37) | 1.19b (0.30, 2.07) | 0.001 | 0.8 | 0.3 |
| <0.2 mg/dL | 64 | — | — | — | — | |||
| Pyridoxal phosphate | 2672 | 2–19 | 2.49 (1.12, 3.81) | 3.72 (2.57, 4.86) | 3.02 (1.04, 5.01) | 0.4 | 0.5 | 0.1 |
| <20 nmol/L | 99 | — | — | — | — | |||
| Fat-soluble vitamins | ||||||||
| Vitamin D | 5325 | 2–19 | 12.9a (10.8, 15.0) | 53.6c (48.0, 59.3) | 29.5b (25.3, 33.6) | <0.0001 | 0.4 | 0.4 |
| <20 ng/mL | 2112 | — | ||||||
| Vitamin E | 4637 | 6–19 | 1.69 (0.98, 2.40) | 1.89 (1.06, 2.71) | 1.63 (0.70, 2.55) | 0.9 | 0.2 | 0.9 |
| <500 μg/dL | 80 | — | — | — | — | |||
| Serum lipids | ||||||||
| Total cholesterol | 4654 | 6–19 | 11.8b (9.8, 13.9) | 9.49b (7.9, 11.1) | 6.75a (5.1, 8.3) | 0.01 | 0.3 | 0.6 |
| ≥200 mg/dL | 449 | — | — | — | — | |||
| Total cholesterol (morning subsample)5 | 1441 | 12–19 | 11.2b (7.7, 14.6) | 7.7a,b (5.6, 9.9) | 6.2a (3.1, 9.3) | 0.05 | 0.2 | 0.4 |
| ≥200 mg/dL | 123 | — | — | — | — | |||
| HDL cholesterol | 4654 | 6–19 | 13.3b (10.7, 15.9) | 5.81a (4.7, 6.9) | 12.0b (9.8, 14.3) | <0.0001 | 0.3 | 0.8 |
| <40 mg/dL | 513 | — | — | — | — | |||
| HDL cholesterol (morning subsample)5 | 1441 | 12–19 | 15.5b (11.2, 19.7) | 7.7a (5.8, 9.7) | 13.2b (9.6, 16.9) | 0.009 | 0.4 | 0.4 |
| <40 mg/dL | 174 | — | — | — | — | |||
| LDL cholesterol (morning subsample)5 | 1437 | 12–19 | 7.47 (5.14, 9.79) | 6.03 (4.02, 8.04) | 5.16 (2.01, 8.31) | 0.3 | 0.7 | 0.6 |
| ≥130 mg/dL | 95 | — | — | — | — | |||
| Triglycerides (morning subsample)5 | 1441 | 12–19 | 18.7b (13.3, 24.0) | 4.94a (3.02, 6.85) | 17.6b (12.6, 22.6) | <0.0001 | 0.1 | 0.2 |
| ≥130 mg/dL | 180 | — | — | — | — |
Multiple logistic regression model with each biomarker as a dichotomous outcome included age (2–5, 6–11, 12–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), self-reported history of chronic disease (yes or no), hours of fasting (continuous), history of supplement use in the past month (yes or no), BMI percentile for age and sex (continuous), serum cotinine, and C-reactive protein. Models for vitamin E also included serum total cholesterol, and models for vitamin D and serum lipids included hours of television and computer use. Value estimates in each row not sharing a common superscript letter are significantly different, P < 0.05 (Satterthwaite adjusted F test).
Numbers were too small for formal testing for serum folate <2 ng/mL (n = 1), red blood cell folate <95 ng/mL (n = 27), vitamin B-12 <200 pg/mL (n = 24), and retinol <20 μg/dL (n = 22).
Satterthwaite adjusted F test for differences between race-ethnic groups, PIR categories, and education categories.
PIR, poverty-income ratio.
Limited to the morning subsample and >8.5 h of fasting.
Dietary micronutrient intakes
Interactions of race-ethnicity, family income, and education for predicting mean dietary intake of selected micronutrients
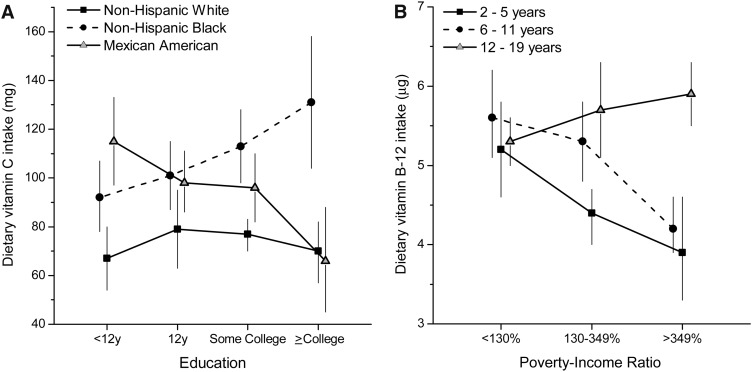
A race-by-education interaction was noted for vitamin C intake (P = 0.004), and an age-by-PIR interaction was noted for dietary vitamin B-12 (P < 0.0005) (Figure 2). Race differences in dietary vitamin C intake were noted in all categories of education, with higher intakes in non-Hispanic blacks and Mexican Americans. However, education was unrelated to dietary vitamin C intake in non-Hispanic whites. In non-Hispanic blacks, higher education predicted higher vitamin C intake, but in Mexican Americans it predicted lower mean intake (P = 0.01). There were no age differences in dietary vitamin B-12 intake in the lowest PIR category; however, age differences were noted in the other 2 PIR categories (P ≤ 0.006). In 2–11-y-olds, dietary vitamin B-12 intake declined with increasing PIR (≤0.01); this relation was not observed in 12–19-y-olds.
FIGURE 2.
Interactions of race-ethnicity with education (dietary vitamin C intake; A) and age with poverty-income ratio (dietary vitamin B-12 intake; B) in US children and adolescents, P < 0.01 (Satterthwaite adjusted F test): NHANES 2003–2006. The estimates are adjusted means (and 95% CIs) from multiple linear regression models with each dietary variable as a dependent variable and included age (2–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), day of recalled intake (Monday–Thursday, Friday–Sunday), self-reported history of chronic disease (yes or no), and BMI percentile for age and sex (continuous).
Race-ethnic differentials in mean dietary nutrient intake
The reported 24-h mean intakes of all examined nutrients (except for folic acid, β-carotene, lycopene, and vitamin E) differed by race-ethnicity (Table 6). Compared with non-Hispanic whites, among non-Hispanic blacks the reported dietary intakes of total folate, vitamin B-12, retinol (μg), total vitamin A (retinol activity equivalent), α-carotene, and percentage of energy from saturated fat were lower and intakes of β-cryptoxanthin and lutein + zeaxanthin were higher. Among Mexican Americans, compared with non-Hispanic whites, the mean reported dietary intakes of most micronutrients were either not different [total folate, vitamin B-12, retinol (μg), vitamin A (retinol activity equivalent), α-carotene, and lutein + zeaxanthin], were lower (% of energy from total and saturated fat), or were higher (vitamin B-6 and β-cryptoxanthin).
TABLE 6.
Self-reported 24-h dietary energy and nutrient intake (multiple covariate–adjusted means and 95% CIs) of US children and adolescents by race-ethnicity: NHANES 2003–20061
| Dietary nutrient | n | Age | Non-Hispanic white | Non-Hispanic black | Mexican American | P (race-ethnicity)2 |
| y | ||||||
| Energy (kcal) | 6448 | 2–19 | 2127 (2078, 2176) | 2065 (1988, 2142) | 2093 (2011, 2174) | 0.3 |
| Water-soluble vitamins | ||||||
| Vitamin B-6 (mg)3 | 3286 | 2–19 | 1.74a,b (1.64, 1.84) | 1.69a (1.54, 1.84) | 1.87b (1.77, 1.97) | 0.02 |
| Total folate (μg) | 6448 | 2–19 | 394b (377, 412) | 365a (345, 385) | 403b (384, 423) | 0.05 |
| Folic acid (μg) | 6448 | 2–19 | 236 (221, 251) | 221 (206, 236) | 232 (215, 249) | 0.3 |
| Vitamin B-12 (μg) | 6448 | 2–19 | 5.33b (5.06, 5.61) | 4.72a (4.47, 4.96) | 5.41b (5.11, 5.71) | 0.0006 |
| Fat-soluble vitamins and carotenoids | ||||||
| Retinol (μg) | 5153 | 6–19 | 499b (469, 528) | 399a (372, 426) | 466b (435, 497) | <0.0001 |
| Vitamin A (μg RAE4) | 5153 | 6–19 | 604b (571, 637) | 495a (458, 532) | 580b (533, 626) | <0.0001 |
| α-Carotene (μg) | 5153 | 6–19 | 233b (191, 276) | 147a (109, 185) | 266b (191, 341) | 0.008 |
| β-Carotene (μg) | 5153 | 6–19 | 1095 (972, 1218) | 998 (808, 1188) | 1139 (901, 1376) | 0.5 |
| β-Cryptoxanthin (μg) | 5153 | 6–19 | 105a (88, 123) | 140b (117, 163) | 175b (141, 209) | 0.0009 |
| Lutein + zeaxanthin (μg) | 5153 | 6–19 | 758a (684, 832) | 955b (784, 1126) | 706a (615, 797) | 0.01 |
| Lycopene (μg) | 5153 | 6–19 | 5544 (5047, 6040) | 5242 (4605, 5879) | 6116 (4977, 7255) | 0.4 |
| Vitamin E (mg α-tocopherol) | 5153 | 6–19 | 6.3 (6.0, 6.6) | 6.1 (5.8, 6.4) | 6.2 (5.7, 6.6) | 0.6 |
| Dietary fats (% of energy) | ||||||
| Total fat | 5152 | 6–19 | 33.3b (32.8, 33.9) | 33.9b (33.3, 34.5) | 32.1a (31.5, 32.7) | 0.007 |
| Saturated fat | 5152 | 6–19 | 11.8b (11.6, 12.0) | 11.3a (11.1, 11.5) | 11.1a (10.8, 11.3) | 0.0001 |
The adjusted values are predicted margins from multiple linear regression models with each dietary variable as a continuous dependent and included age (2–5, 6–11, 12–19 y), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American), family poverty-income ratio (<130%, 130–349%, >349%), educational level of household head (<12 y, 12 y, some college, college degree or higher), month of mobile examination center examination (November–April, May–October), day of recalled intake (Monday–Thursday, Friday–Sunday), self-reported history of chronic disease (yes or no), and BMI percentile for age and sex (continuous). Adjusted values in each row not sharing a common superscript letter are significantly different, P < 0.05 (Satterthwaite adjusted F test).
Satterthwaite adjusted F test for differences between race-ethnic groups.
NHANES 2005–2006.
RAE, retinol activity equivalents.
PIR and education differentials in mean dietary nutrient intake
Dietary vitamin C intakes differed by categories of PIR (P = 0.03) (see Supplemental Table 1 under “Supplemental data” in the online issue) and intakes of β-carotene differed by categories of education (P = 0.01) (see Supplemental Table 2 under “Supplemental data” in the online issue). There was no independent association of family income or education for all other examined dietary outcomes.
DISCUSSION
The results of our study underscore the following: 1) race-ethnic differences in concentrations of micronutrient and lipid biomarkers remained after family income and education were accounted for, 2) race-ethnicity (but not family income and education) was a strong predictor of both biomarker and diet outcomes, and 3) the evidence of modification of the race-ethnic effect by family income and education was noted for very few outcomes. Overall, these findings indicate a possibly stronger influence of foodways associated with ethnicity relative to resource or knowledge constraints assessed as family income and education for the examined outcomes. The race-ethnic differentials in biomarkers may reflect the joint contribution of multiple interacting factors that include the following: cultural preferences for certain foods, their combinations, and their methods of preparation; biological influences on nutrient bioavailability and metabolism and supplement use; and social/neighborhood/environmental contexts that relate to food availability and demand, as effectors. The general lack of consistent family income associations with diet and biomarkers in children and adolescents may reflect the success of federal need-based food assistance and child nutrition programs in closing the income gap in micronutrient availability.
The observed race-ethnic gradients in serum concentrations of retinol and carotenoids in US children are consistent with previous reports (28–30, 53). Our results provide evidence for modification of the effect of race-ethnicity by family PIR or education for retinol, lutein + zeaxanthin, and β-cryptoxanthin. For these 3 biomarkers, the race-ethnic differences were present in every category of PIR (retinol) or education (lutein + zeaxanthin and β-cryptoxanthin). However, the magnitude of race-ethnic differences decreased with increasing PIR or education because the direction of the association of the biomarker with income or education differed among race-ethnic groups (Figure 1). For example, in non-Hispanic blacks and Mexican Americans, there appeared to be a positive (but nonsignificant) association of serum retinol and PIR; in non-Hispanic whites, this association was inverse. The expected positive association of education with serum concentrations of lutein + zeaxanthin and β-cryptoxanthin were significant only in non-Hispanic whites; in Mexican Americans, these associations were inverse. Although we did not observe race-by-PIR or -education interactions for self-reported dietary intake of vitamin A or lutein + zeaxanthin and β-cryptoxanthin (analogous to those observed for serum concentrations), we did find that higher education predicted lower dietary vitamin C intake in Mexican Americans (Figure 2). Collectively, these findings suggest possibly adverse effects of higher education on food selection of Mexican Americans. A possible explanation may be that higher education is related to greater dietary acculturation and less adherence to traditional foodways among Mexican Americans. In Mexican Americans, greater dietary acculturation has been reported to relate to adverse dietary outcomes (54, 55), and there is reported evidence of higher cardiovascular risk with higher education and SEP (56). Therefore, the nutritional advantages and healthful characteristics of traditional dietary patterns should be reinforced in all Mexican Americans irrespective of SEP.
Consistent with prior reports (32–36), we found race-ethnic differences in mean serum vitamin D concentration, which widened with increasing age. The widening of the race-ethnic differential with age may reflect greater age-associated decline in milk intake in non-Hispanic blacks relative to other ethnic groups (57) or a possible decrease in outdoor physical activity. The recent Institute of Medicine report (49) recognized the black-white differences in calcium economy and vitamin D nutritional status but did not provide race-specific values of vitamin D to indicate at-risk thresholds.
It has been suggested that race-ethnic differences in serum vitamin B-12 and folate concentrations may reflect race differentials in bioavailability and metabolism of these nutrients (58, 59). The results of our study provide some evidence in support of this argument. For example, non-Hispanic blacks had the lowest mean dietary intake but the highest mean serum vitamin B-12 concentrations (along with the lowest dietary, serum, and RBC folate). Similarly, although mean total dietary folate (and folic acid) intakes of Mexican Americans did not differ from those of non-Hispanic whites, the serum and RBC folate concentrations were significantly lower.
Our findings of significantly lower mean concentrations of vitamin C in non-Hispanic white children compared with non-Hispanic black and Mexican American children differ from those observed among adults in the NHANES III (19) and the NHANES 2003–2004 (20). In these reports, non-Hispanic white adults had higher serum ascorbate concentrations and family PIR was a positive predictor. However, non-Hispanic white children and adolescents in our study had the lowest mean ascorbate concentration, and a higher proportion was at risk for concentrations indicating marginal status. Moreover, neither PIR nor education was related with serum ascorbate after adjustment for race-ethnicity in multivariate-adjusted models. These results suggest that dietary vitamin C intake and sources may change during transition to adult years, and the assumption about relevance of ethnic foodways during adult years may not apply to all dietary components similarly.
Although summary estimates of micronutrient intake from self-reported diets are known to be measured with error (11, 12), our results show that non-Hispanic black and white differences in serum concentrations of several micronutrients (vitamin C, folate, vitamin A, α-carotene, β-cryptoxanthin, and lutein zeaxanthin) paralleled these differences in dietary intakes of corresponding micronutrients. This is all the more remarkable given the higher prevalence of supplement use among non-Hispanic white children. The correspondence between serum concentrations and dietary micronutrients in Mexican American and non-Hispanic white differentials was limited to only a few nutrients (vitamin C, vitamin E, and β-carotene). We can speculate that these ethnic differences in biomarker and dietary intake correspondence may possibly reflect ethnic differentials in reliability of the dietary assessment method used in the NHANES, completeness and accuracy of the database used to estimate micronutrient content of ethnic foods, differential bioavailability of micronutrients because of ethnic differences in food sources, and dietary supplement use. With the availability of total nutrient intakes from diet and supplements in recent surveys (60), future such analyses may provide a more nuanced interpretation of race-ethnic differentials in correspondence between biomarker and nutrient intakes.
Our study findings suggest that the serum lipid profile of non-Hispanic whites was more atherogenic than that of the comparison minority groups. Overall, non-Hispanic blacks had the most favorable lipid profile of all ethnic groups. The finding of higher mean serum HDL-cholesterol concentration and lower triglycerides in non-Hispanic black children and adolescents is in accord with prior such reports (6, 61–66). However, our result of null findings for association of serum lipids with SEP differs from an earlier report of effect modification by socioeconomic factors in black 9–11-y-olds (67) but concurs with the findings of Winkleby et al (8), who found that neither ethnicity nor family education was related to “non-HDL cholesterol concentration” in 4–24-y-olds in the NHANES III. We note that the race-ethnic differences in serum lipid profile observed in children and adolescents in our study have also been reported for US adults, which persisted after adjustment for SEP (61, 68).
Strengths of our study include a nationally representative sample of sufficient size with available information on family income and education to allow an assessment of their independent and joint associations with the outcomes examined in the study and the assessment of micronutrient status with the use of biomarkers, which avoids reliance on self-reported intakes.
In conclusion, there were distinct race-ethnic differentials, independent of family income and ethnicity, in serum concentrations and dietary intake of micronutrients and lipids in US children and adolescents. Although non-Hispanic blacks differed from non-Hispanic whites in several outcomes, significantly higher risk of marginal status was evident only for vitamin D. With the current safety net of food assistance and child-nutrition programs in place, overall results suggest little independent or interaction effect of SEP for the majority of the examined outcomes. Any interventions to affect a desired change will need to target ethnic groups.
Supplementary Material
Acknowledgments
We thank Lisa Licitra Kahle, Information Management Systems, Silver Spring, MD, for expert programming support and David Check, National Cancer Institute, Bethesda, MD, for graphic support.
The authors’ responsibilities were as follows—AKK: designed and conducted the research, wrote the manuscript, and had primary responsibility for the final content; and BIG: conducted the research (analytic strategy) and edited the initial manuscript drafts for important intellectual content. Both authors read and approved the final manuscript. Neither of the authors declared a conflict of interest.
Footnotes
Abbreviations used: MEC, mobile examination center; NCHS, National Center for Health Statistics; PIR, poverty-income ratio; RBC, red blood cell; SEP, socioeconomic position.
REFERENCES
- 1.National Research Council, Institute of Medicine; Committee on Evaluation of Children's Health Children's health, the nation's wealth: assessing and improving child health. Washington, DC: National Academies Press, 2004 [Google Scholar]
- 2.Starfield B, Robertson J, Riley AW. Social class gradients and health in childhood. Ambul Pediatr 2002;2:238–46 [DOI] [PubMed] [Google Scholar]
- 3.Starfield B, Riley AW, Witt WP, Robertson J. Social class gradients in health during adolescence. J Epidemiol Community Health 2002;56:354–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh GK, Kogan MD. Widening socioeconomic disparities in US childhood mortality, 1969-2000. Am J Public Health 2007;97:1658–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webber LS, Harsha DW, Phillips GT, Srinivasan SR, Simpson JW, Berenson GS. Cardiovascular risk factors in Hispanic, white, and black children: the Brooks County and Bogalusa Heart studies. Am J Epidemiol 1991;133:704–14 [DOI] [PubMed] [Google Scholar]
- 6.Belcher JD, Ellison RC, Shepard WE, Bigelow C, Webber LS, Wilmore JH, Parcel GS, Zucker DM, Luepker RV. Lipid and lipoprotein distributions in children by ethnic group, gender, and geographic location—preliminary findings of the Child and Adolescent Trial for Cardiovascular Health (CATCH). Prev Med 1993;22:143–53 [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 8.Winkleby MA, Robinson TN, Sundquist J, Kraemer HC. Ethnic variation in cardiovascular disease risk factors among children and young adults: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. JAMA 1999;281:1006–13 [DOI] [PubMed] [Google Scholar]
- 9.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distribution and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med 1998;27:879–90 [DOI] [PubMed] [Google Scholar]
- 10.Saaddine JB, Fagot-Campagna A, Rolka D, Narayan KM, Geiss L, Eberhardt M, Flegal KM. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care 2002;25:1326–30 [DOI] [PubMed] [Google Scholar]
- 11.Livingstone MB, Robson PJ, Wallace JM. Issues in dietary intake assessment of children and adolescents. Br J Nutr 2004;92(suppl 2):S213–22 [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute, Risk Factor Monitoring Branch. NCS dietary assessment literature review. Available from: http://riskfactor.cancer.gov/tools/children/review/ (cited October 2011)
- 13.Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr 2003;133(suppl 3):873S–4S [DOI] [PubMed] [Google Scholar]
- 14.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr 2003;133(suppl 3):875S–80S [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Sowell A. Serum alpha-tocopherol status in the United States population: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 1999;150:290–300 [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr 2006;84:375–83 [DOI] [PubMed] [Google Scholar]
- 17.Ford ES. Variations in serum carotenoid concentrations among United States adults by ethnicity and sex. Ethn Dis 2000;10:208–17 [PubMed] [Google Scholar]
- 18.Ganji V, Kafai MR. Population determinants of serum lycopene concentrations in the United States: data from the Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 2005;135:567–72 [DOI] [PubMed] [Google Scholar]
- 19.Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. J Nutr 2007;137:2456–63 [DOI] [PubMed] [Google Scholar]
- 20.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–63 [DOI] [PubMed] [Google Scholar]
- 21.Dowd JB, Aiello AE. Did national folic acid fortification reduce socioeconomic and racial disparities in folate status in the US? Int J Epidemiol 2008;37:1059–66 [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988-2004. Am J Clin Nutr 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 23.Ganji V, Kafai MR. Trends in serum folate, RBC folate, and circulating total homocysteine concentrations in the United States: analysis of data from National Health and Nutrition Examination Surveys, 1988-1994, 1999-2000, and 2001-2002. J Nutr 2006;136:153–8 [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Bowman BA. Serum and red blood cell folate concentrations, race, and education: findings from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 1999;69:476–81 [DOI] [PubMed] [Google Scholar]
- 25.Dixon LB, Winkleby MA, Radimer KL. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 2001;131:1232–46 [DOI] [PubMed] [Google Scholar]
- 26.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008;87:1446–54 [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr 2005;82:442–50 [DOI] [PubMed] [Google Scholar]
- 28.Looker AC, Johnson CL, Woteki CE, Yetley EA, Underwood BA. Ethnic and racial differences in serum vitamin A levels of children aged 4-11 years. Am J Clin Nutr 1988;47:247–52 [DOI] [PubMed] [Google Scholar]
- 29.Ballew C, Bowman BA, Sowell AL, Gillespie C. Serum retinol distributions in residents of the United States: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2001;73:586–93 [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Gillespie C, Ballew C, Sowell A, Mannino DM. Serum carotenoid concentrations in US children and adolescents. Am J Clin Nutr 2002;76:818–27 [DOI] [PubMed] [Google Scholar]
- 31.Brotanek JM, Gosz J, Weitzman M, Flores G. Iron deficiency in early childhood in the United States: risk factors and racial/ethnic disparities. Pediatrics 2007;120:568–75 [DOI] [PubMed] [Google Scholar]
- 32.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004;158:531–7 [DOI] [PubMed] [Google Scholar]
- 33.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 2007;86:150–8 [DOI] [PubMed] [Google Scholar]
- 34.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics 2009;123:797–803 [DOI] [PubMed] [Google Scholar]
- 35.Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med 2008;162:513–9 [DOI] [PubMed] [Google Scholar]
- 36.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics 2009;124:e362–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey. Hyattsville, MD: US Department of Health and Human Services, CDC. Available from: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (cited October 2010)
- 38.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey 2003-2004. Hyattsville, MD: US Department of Health and Human Services, CDC. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/nhanes03_04.htm (cited May 2011)
- 39.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey 2005-2006. Hyattsville, MD: US Department of Health and Human Services, CDC. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm (cited May 2011)
- 40.Centers for Disease Control and Prevention; National Center for Health Statistics. NHANES response rates and CPS totals. Hyattsville, MD: US Department of Health and Human Services, CDC. Available from: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm (cited May 2011)
- 41.Adler NE. Overview of health disparities. In: Thomson GE, Mitchell F, Williams M, eds. Examining the health disparities research plan of the National Institutes of Health: unfinished business.Committee on the Review and Assessment of the NIH's Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities. Washington, DC: National Academies Press, 2006. [PubMed]
- 42.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA 2003;289:2709–16 [DOI] [PubMed] [Google Scholar]
- 43.LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health 2005;82:iii26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumanyika SK, Krebs-Smith SM. Preventive nutrition issues in ethnic and socioeconomic groups in the United States : Bendich A, Deckelbaum RJ, Primary and secondary preventive nutrition Totowa, NJ: Humana Press, 2001:325–55 [Google Scholar]
- 45.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey. Analytic guidelines. Hyattsville, MD: US Department of Health and Human Services, CDC, 2006. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm (cited May 2011)
- 46.Research Triangle Institute SUDAAN language manual, release 10.0. Research Triangle Park, NC: Research Triangle Institute, 2008 [Google Scholar]
- 47.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey. National report on biochemical indicators of Diet and Nutrition in the US population 1999-2002. Hyattsville, MD: US Department of Health and Human Services, CDC, 2008. Available from: http://www.cdc.gov/nutritionreport/99-02/pdf/nutrition_report.pdf (cited May 2011)
- 48.National Heart, Lung, and Blood Institute. The report of the expert panel: Integrated guidelines for cardiovascular health and risk reduction in children and adolescents. 2011. Available from: http://www.nhlbi.nih.gov/guidelines/cvd_ped/index.htm (cited November 2011)
- 49.Institute of Medicine, Food and Nutrition Board, Committee to Review Dietary Reference Intakes for Calcium and Vitamin D Dietary Reference Intakes for calcium and vitamin D. Washington, DC: National Academy Press, 2010 [Google Scholar]
- 50.Institute of Medicine, Food and Nutrition Board Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 51.Korn EL, Graubard BI. Analysis of health surveys. New York, NY: John Wiley and Sons, 1999 [Google Scholar]
- 52.Graubard BI, Korn EL. Predictive margins for survey data. Biometrics 1999;55:652–9 [DOI] [PubMed] [Google Scholar]
- 53.Neuhouser ML, Rock CL, Eldridge AL, Kristal AR, Patterson RE, Cooper DA, Neumark-Sztainer D, Cheskin LJ, Thornquist MD. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr 2001;131:2184–91 [DOI] [PubMed] [Google Scholar]
- 54.Ayala GX, Baquero B, Klinger S. A systematic review of the relationship between acculturation and diet among Latinos in the United States: implications for future research. J Am Diet Assoc 2008;108:1330–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuhouser ML, Thompson B, Coronado GD, Solomon CC. Higher fat intake and lower fruit and vegetables intakes are associated with greater acculturation among Mexicans living in Washington State. J Am Diet Assoc 2004;104:51–7 [DOI] [PubMed] [Google Scholar]
- 56.Diez Roux AV, Detrano R, Jackson S, Jacobs DR, Jr, Schreiner PJ, Shea S, Szklo M. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation 2005;112:1557–65 [DOI] [PubMed] [Google Scholar]
- 57.Fulgoni V, III, Nicholls J, Reed A, Buckley R, Kafer K, Huth P, DiRienzo D, Miller GD. Dairy consumption and related nutrient intake in African-American adults and children in the United States: continuing survey of food intakes by individuals 1994-1996, 1998, and the National Health and Nutrition Examination Survey 1999-2000. J Am Diet Assoc 2007;107:256–64 [DOI] [PubMed] [Google Scholar]
- 58.Caudill MA. Folate bioavailability: implications for establishing dietary recommendations and optimizing status. Am J Clin Nutr 2010;91:1455S–60S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmel R. Ethnic and racial factors in cobalamin metabolism and its disorders. Semin Hematol 1999;36:88–100 [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention; National Center for Health Statistics. National Health and Nutrition Examination Survey 2007-2008. Hyattsville, MD: US Department of Health and Human Services, CDC. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/diet07_08.htm (cited March 2012)
- 61.Tyroler HA, Glueck CJ, Christensen B, Kwiterovich PO., Jr Plasma high-density lipoprotein cholesterol comparisons in black and white populations. The Lipid Research Clinics Program Prevalence Study. Circulation 1980;62:IV99–107 [PubMed] [Google Scholar]
- 62.Webber LS, Osganian V, Luepker RV, Feldman HA, Stone EJ, Elder JP, Perry CL, Nader PR, Parcel GS, Broyles SL, et al. Cardiovascular risk factors among third grade children in four regions of the United States. The CATCH Study (Child and Adolescent Trial for Cardiovascular Health). Am J Epidemiol 1995;141:428–39 [DOI] [PubMed] [Google Scholar]
- 63.Lamb MM, Ogden CL, Carroll MD, Lacher DA, Flegal KM. Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999–2004. Am J Clin Nutr 2011;94:877–83 [DOI] [PubMed] [Google Scholar]
- 64.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr 2000;71:725–32 [DOI] [PubMed] [Google Scholar]
- 65.Herd SL, Gower BA, Dashti N, Goran MI. Body fat, fat distribution and serum lipids, lipoproteins and apolipoproteins in African-American and Caucasian-American prepubertal children. Int J Obes Relat Metab Disord 2001;25:198–204 [DOI] [PubMed] [Google Scholar]
- 66.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001-2006. Arch Pediatr Adolesc Med 2009;163:371–7 [DOI] [PubMed] [Google Scholar]
- 67.Zuckerman AE, Olevsky-Peleg E, Bush PJ, Horowitz C, Davidson FR, Brown DG, Walter HJ. Cardiovascular risk factors among black schoolchildren: comparisons among four Know Your Body studies. Prev Med 1989;18:113–32 [DOI] [PubMed] [Google Scholar]
- 68.Linn S, Fulwood R, Rifkind B, Carroll M, Muesing R, Williams OD, Johnson C. High density lipoprotein cholesterol levels among US adults by selected demographic and socioeconomic variables. The Second National Health and Nutrition Examination Survey 1976-1980. Am J Epidemiol 1989;129:281–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.