Abstract
Background: Vitamin D deficiency contributes to secondary hyperparathyroidism, which occurs early in chronic kidney disease (CKD).
Objectives: We aimed to determine whether high-dose cholecalciferol supplementation for 1 y in early CKD is sufficient to maintain optimal vitamin D status (serum 25-hydroxyvitamin D [25(OH)D] concentration ≥30 ng/mL) and decrease serum parathyroid hormone (PTH). A secondary aim was to determine the effect of cholecalciferol on blood pressure and serum fibroblast growth factor-23 (FGF23).
Design: This was a double-blind, randomized, placebo-controlled trial. Forty-six subjects with early CKD (stages 2–3) were supplemented with oral cholecalciferol (vitamin D group; 50,000 IU/wk for 12 wk followed by 50,000 IU every other week for 40 wk) or a matching placebo for 1 y.
Results: By 12 wk, serum 25(OH)D increased in the vitamin D group only [baseline (mean ± SD): 26.7 ± 6.8 to 42.8 ± 16.9 ng/mL; P < 0.05] and remained elevated at 1 y (group-by-time interaction: P < 0.001). PTH decreased from baseline only in the vitamin D group (baseline: 89.1 ± 49.3 to 70.1 ± 24.8 pg/mL; P = 0.01) at 12 wk, but values were not significantly different from baseline at 1 y (75.4 ± 29.5 pg/mL; P = 0.16; group-by-time interaction: P = 0.09). Group differences were more pronounced in participants with secondary hyperparathyroidism (group-by-time interaction: P = 0.004). Blood pressure and FGF23 did not change in either group.
Conclusions: After 1 y, this oral cholecalciferol regimen was safe and sufficient to maintain serum 25(OH)D concentrations and prevent vitamin D insufficiency in early CKD. Furthermore, serum PTH improved after cholecalciferol treatment, particularly in patients who had secondary hyperparathyroidism. This trial was registered at clinicaltrials.gov as NCT00427037.
INTRODUCTION
Chronic kidney disease (CKD)4 is an increasing health burden in the United States and internationally (1, 2). A key feature that occurs early in the progression of CKD is secondary hyperparathyroidism (3), which increases risk of bone and cardiometabolic comorbidities (4). Secondary hyperparathyroidism is classically attributed to vitamin D deficiency and the progressive decline in renal 1-α-hydroxylase, which is the enzyme responsible for the conversion of 25-hydroxyvitamin D [25(OH)D] to 1,25-dihydroxyvitamin D [1,25(OH)2D, the biologically active form of vitamin D], which acts in a negative-feedback loop to downregulate parathyroid hormone (PTH) (5). The National Kidney Foundation's 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend measurement of serum 25(OH)D in stages 3 and 4 CKD if serum PTH concentrations are elevated and supplementation with ergocalciferol (vitamin D2) if serum 25(OH)D is below the optimal vitamin D status (<30 ng/mL) (6). Several studies in adults that examined the ability of the KDOQI guidelines to both maintain optimal vitamin D status and reduce PTH concentrations in early-stage CKD have shown mixed results (7). Furthermore, the effectiveness of ergocalciferol compared with cholecalciferol (vitamin D3) in raising and maintaining 25(OH)D concentrations has been questioned (8–10). Clinical trials of cholecalciferol supplementation in early-stage CKD have also provided mixed results with regard to PTH suppression (7); however, to our knowledge, there have been no long-term, randomized, placebo-controlled clinical trials that used cholecalciferol in adults with CKD published to date.
Vitamin D deficiency may also increase risk of cardiovascular disease (CVD) (11–13), which is significantly greater in CKD (14). Blood pressure–lowering effects of vitamin D have been hypothesized as a mechanism (11), although meta-analyses in the general population have not provided convincing evidence (15, 16). Studies of vitamin D (ergocalciferol or cholecalciferol) supplementation on blood pressure in predialysis CKD are few and have not shown significant effects; however, the studies are limited by their relatively short-term design (17). To our knowledge, it has not been determined whether longer-term cholecalciferol supplementation improves blood pressure in early-stage CKD.
The primary aim of this study was to determine whether cholecalciferol supplementation for 1 y (50,000 IU/wk for 12 wk followed by 50,000 IU every other week for 40 wk) was sufficient to maintain optimal vitamin D status and concomitantly reduce circulating PTH concentrations in CKD patients by using a randomized, placebo-controlled design. Secondary outcomes were changes in blood pressure and fibroblast growth factor-23 (FGF23), which is involved in the vitamin D–PTH regulatory axis and is predictive of mortality in CKD (18, 19).
SUBJECTS AND METHODS
Subjects
Participants were screened and recruited from diabetes and nephrology clinics at the Atlanta Veterans Affairs (VA) Medical Center from August 2008 to December 2010. Inclusion criteria for this study were as follows: subjects were aged 18–90 y and had an estimated glomerular filtration rate (eGFR) of <90 mL · min−1 · 1.73 m−2 calculated by using the Modification of Diet in Renal Disease Study equation (20). Exclusion criteria were as follows: current use of active vitamin D analogs, calcimimetics, or supplemental intake >1000 IU vitamin D; history of liver failure, intestinal malabsorption, or chronic diarrhea and serum calcium (corrected for albumin) >10.5 mg/dL; calcium × phosphorus product >70; or current use of any medication that could influence vitamin D metabolism, such as phenytoin, phenobarbital, or rifampin.
Protocol
This was a 52-wk, double-blind, placebo-controlled clinical trial. Eligible participants were randomly assigned to receive 50,000 IU cholecalciferol (vitamin D3; Tischon)/wk for 12 wk followed by 50,000 IU cholecalciferol every other week for 40 wk (vitamin D group) or a matching placebo (Tischon). Participants were randomly assigned by using a 6-block randomization scheme. A member of the study team who was not involved in the recruitment of subjects generated a random allocation sequence, which was provided to the research pharmacist who assigned participants to the intervention. The primary investigator, all study personnel, and all participants were blinded to the intervention.
Participants were seen as outpatients at the Atlanta VA Clinical Research Unit at baseline and 12 and 52 wk. Seated blood pressure was measured at each visit by using an automated cuff after the subject sat quietly for ≥5 min; the average of 3 measurements was used. Whole blood was collected, processed for serum, and stored at −80°C at each visit for subsequent analysis of 25(OH)D, PTH, and FGF23. Twenty-four-hour urine was collected at baseline for all participants to determine the presence of microalbuminuria or proteinuria (>30 mg 24-h urine albumin/d) and was available at 52 wk in a subset of participants (n = 15). Three-day food records were available at baseline in 22 participants and in 13 participants at 52 wk. Serum calcium, phosphorus, albumin, creatinine, and alkaline phosphatase were abstracted from the electronic medical record or measured in collected serum if not available. This study was approved by the Emory Institutional Review Board and the VA Research and Development Committee, and all participants provided informed consent on enrollment.
Analytic methods
Serum 25(OH)D was assayed by using a chemiluminescent technique with an automated machine (Immunodiagnostic Systems iSYS automated machine). Our laboratory participates in the Vitamin D External Quality Assessment Scheme and the National Institute of Standards and Technology/NIH Vitamin D Metabolites Quality Assurance Program to ensure the accuracy of serum 25(OH)D measurements. Vitamin D insufficiency was defined as a serum 25(OH)D concentration <30 ng/mL, and vitamin D deficiency was defined as a serum 25(OH)D concentration <20 ng/mL (21). The intraassay and interassay were 1.8–4.0% and 10.1–13.0%, respectively. Intact PTH was assayed by using an ELISA technique (Immutopics International); the intraassay CV was 3.2%. FGF23 (C terminal) was assayed by using an ELISA (Immutopics International); the intraassay CV was 3.5%. Serum calcium, phosphorus, albumin, creatinine, and alkaline phosphatase were measured by using standard Atlanta VA Medical Center laboratory methods, as were urine calcium, albumin, phosphorus, and creatinine.
Food records
Patients were instructed to record dietary intake for 3 d before the baseline study visit and again before the final follow-up visit (52 wk). Food records are a reliable method of dietary assessment in CKD (22). All food records were collected and analyzed for dietary and supplemental vitamin D with Nutritionist Pro software (version 4.1.0; Axxya Systems LLC) by a single registered dietitian.
Statistical analyses
Descriptive statistics are reported as means ± SDs. Chi-square tests, t tests, and ANCOVA were used to determine differences between groups. Paired t tests or Wilcoxon's signed rank tests were used to determine changes in outcomes from baseline to 12 wk and from baseline to 52 wk for each treatment group. Mixed-model repeated-measures ANOVA and ANCOVA were also used to investigate the effects of group-by-time interactions. FGF23 was log10 transformed for analyses, and means are reported as back-transformed values. Post hoc analyses were performed according to secondary hyperparathyroidism status (PTH concentration >70 pg/mL) and according to CKD stage (2 compared with 3). All analyses were based on intention to treat. Statistical analyses were performed with JMP software (version 9.0.0; SAS Institute Inc); all tests were 2-sided and assumed a 5% significance level. A sample size of 20 participants per group was estimated to provide 80% power to detect a 30% difference in PTH for vitamin D treatment compared with the placebo with assumption of an SD of 15 pg/mL with α = 0.05. With the assumption of a 20% dropout rate, we aimed to randomly assign ∼50 subjects.
RESULTS
Patient demographics
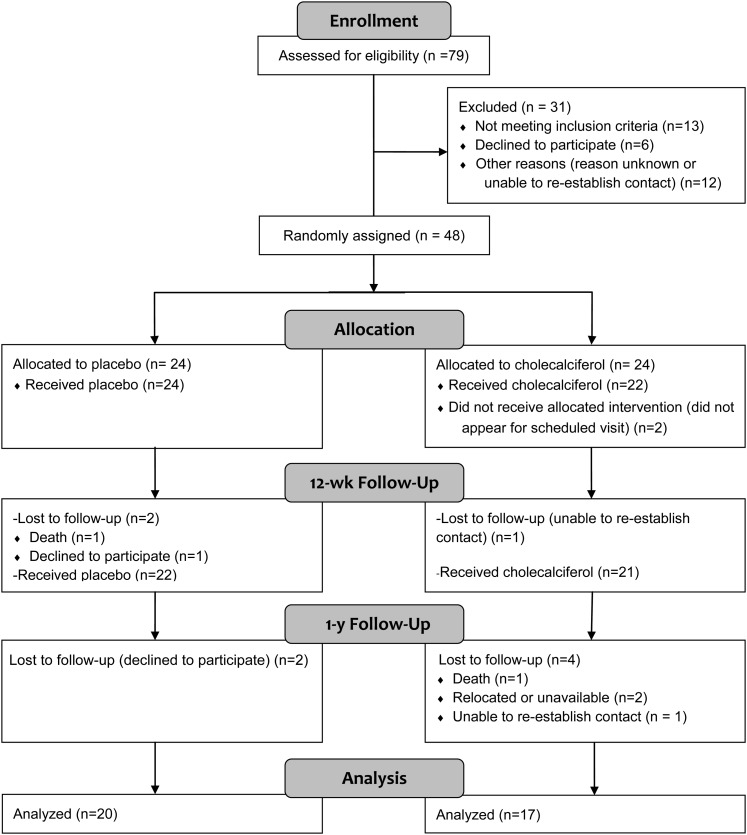
As indicated in Figure 1, 48 patients were randomly assigned to receive a placebo or vitamin D; however, 2 participants did not receive their allocated intervention. Data are presented in only participants that received treatment (n = 24 in the placebo group; n = 22 in the vitamin D group). One participant from each group died because of causes unrelated to the study. By 1 y, 7 participants in the vitamin D group and 4 participants in the placebo group were lost to follow-up; the group difference in loss to follow-up was not significant (P = 0.30; chi-square test). There were no reported adverse events related to the study.
FIGURE 1.
Flow diagram of participant enrollment. Subjects with stage 2–3 chronic kidney disease (n = 79) were screened for participation in the study. Forty-eight subjects were randomly assigned to receive a placebo or vitamin D. Two subjects did not receive their allocated intervention; data are presented in only the 46 participants who received treatment. Thirty-seven subjects completed the 1-y study and were included in the final analysis.
In all participants, 46.8% of subject had stage 1–2 CKD, and 53.2% of subjects had stage 3–4 CKD. The mean (±SD) eGFR was 62 ± 15 mL · min−1 · 1.73 m−2. In subjects with stage 1 or 2 CKD, all but one participant had microalbuminuria or proteinuria (>30 mg 24-h urine albumin/d), diabetes, or hypertension. Fifty-seven percent of participants were vitamin D insufficient [25(OH)D concentration <30 ng/mL]. Mean serum 25(OH)D and PTH concentrations of all subjects at baseline were 29.5 ± 8.2 ng/mL and 83.4 ± 37.8 pg/mL, respectively. Other baseline characteristics of the 2 groups are shown in Table 1. The groups were similar with regard to age, BMI, sex, race-ethnicity, kidney function, hypertension status, blood pressure, and serum PTH concentrations. More patients in the placebo group than in vitamin D group were enrolled during the summer and autumn seasons (91.7% compared with 68.2%, respectively; P = 0.04; chi-square test) and had documented vitamin D supplement use (45.8% compared with 13.6%, respectively; P = 0.02; chi-square test), and baseline serum 25(OH)D concentrations were higher in the placebo group than in the vitamin D group (32.1 ± 8.7 compared with 26.7 ± 6.8 ng/mL; P = 0.03; t test). Reported vitamin D dietary intake at the follow-up visit did not significantly differ from baseline (P = 0.59; paired t test); therefore, the average vitamin D intake from baseline and follow-up was calculated and reported. Vitamin D intake did not significantly differ between treatment groups (P = 0.12). There was a higher prevalence of type 2 diabetes in the vitamin D group than in the placebo group (86.4% compared with 54.2%; P = 0.02; chi-square test).
TABLE 1.
Baseline characteristics by treatment group1
| Variables | Vitamin D (n = 22) | Placebo (n = 24) | P |
| Age (y) | 62.3 ± 10.52 | 62.6 ± 8.9 | 0.90 |
| BMI (kg/m2) | 32.8 ± 5.1 | 31.5 ± 7.5 | 0.49 |
| Men [n (%)] | 20 (90.9) | 22 (91.7) | 0.93 |
| African American [n (%)] | 11 (50.0) | 11 (45.8) | 0.77 |
| eGFR (mL · min−1 · 1.73 m−2) | 62.5 ± 15.6 | 61.2 ± 15.7 | 0.78 |
| CKD stage 2/3 (n)3 | 11/11 | 10/14 | 0.47 |
| Hypertension [n (%)] | 20 (90.9) | 21 (87.5) | 0.71 |
| Total diabetes [n (%)]4 | 18 (81.8) | 17 (70.8) | 0.38 |
| Type 2 diabetes [n (%)] | 19 (86.4) | 13 (54.2) | 0.02 |
| Serum phosphorus (mg/dL) | 3.8 ± 0.6 | 3.7 ± 0.7 | 0.49 |
| Serum calcium (mg/dL) | 9.4 ± 0.4 | 9.5 ± 0.3 | 0.15 |
| Serum 25(OH)D (ng/mL) | 26.7 ± 6.8 | 32.1 ± 8.7 | 0.03 |
| Vitamin D insufficient [25(OH)D <30 ng/mL] [n (%)] | 15 (68.2) | 11 (45.8) | 0.13 |
| Serum PTH (pg/mL) | 89.1 ± 49.2 | 78.21 ± 22.8 | 0.61 |
| PTH >70 pg/mL [n (%)] | 11 (50) | 14 (58.3) | 0.57 |
| FGF23 (RU/mL) | 55.5 ± 34.8 | 42.5 ± 26.7 | 0.16 |
| SBP (mm Hg) | 127 ± 15 | 131 ± 17 | 0.44 |
| DBP (mm Hg) | 71 ± 10 | 73 ± 10 | 0.45 |
| Summer and autumn enrollment [n (%)] | 15 (68.2) | 22 (91.7) | 0.04 |
| Vitamin D supplement use [n (%)] | 3 (13.6) | 11 (45.8) | 0.02 |
| Dietary vitamin D (IU/d)5 | 108 ± 74 [11] | 180 ± 129 [11] | 0.12 |
P values were determined by using a 2-sample t or chi-square test. CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor-23; PTH, parathyroid hormone; RU, relative units; SBP, systolic blood pressure; 25(OH)D, 25-hydroxyvitamin D.
Mean ± SD (all such values).
One subject with stage 1 CKD was grouped with stage 2 subjects, and one subject with stage 4 CKD was grouped with stage 3 subjects.
Includes type 1 and 2 diabetes.
n in brackets.
Vitamin D status
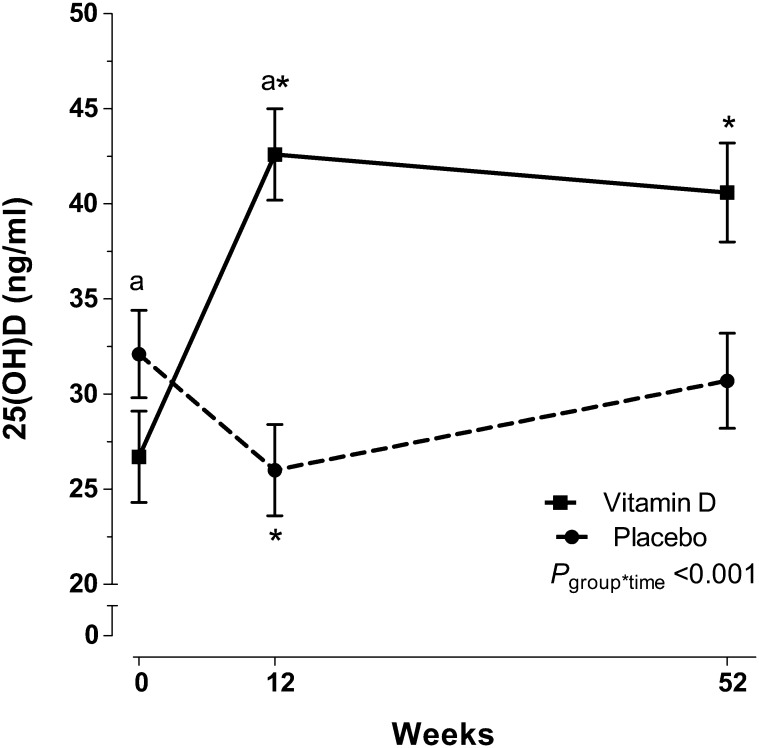
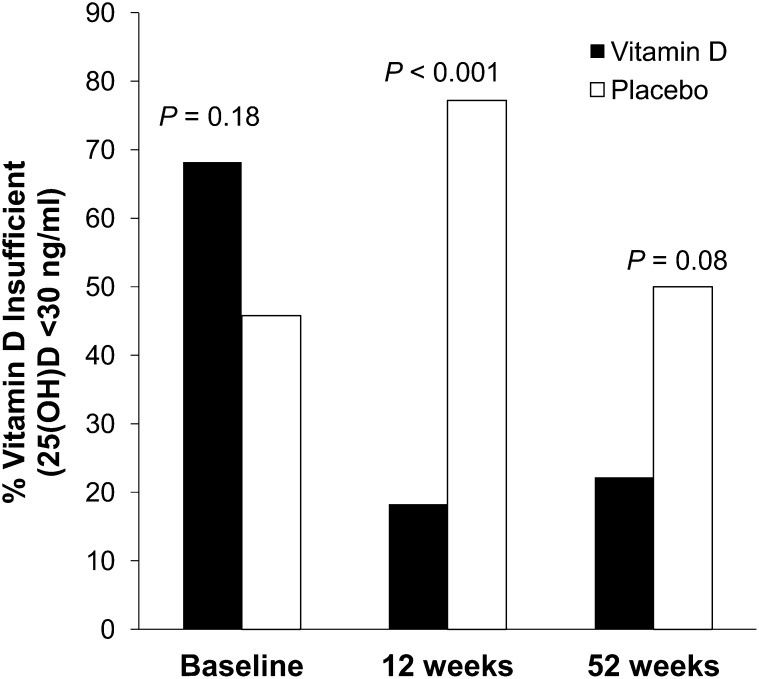
Serum 25(OH)D concentrations significantly increased by 12 wk after treatment in the vitamin D group [mean 25(OH)D concentration: 26.7 ± 6.8 to 42.5 ± 16.3 ng/mL; P < 0.001; paired t test; Figure 2] and remained elevated at 1 y compared with at baseline (40.3 ± 16.1 ng/mL; P = 0.003; t test). In the placebo group, serum 25(OH)D concentrations decreased significantly after 12 wk [mean 25(OH)D concentration: 32.1 ± 8.7 to 26.2 ± 6.8 ng/mL; P < 0.001; paired t test] and increased to baseline values by 1 y (31.2 ± 9.0 ng/mL; P = 0.16 for difference from baseline; t test). The group-by-time interaction for 25(OH)D was significant (P < 0.001; repeated-measures ANOVA). By 12 wk, 77.3% of participants in the placebo group were vitamin D insufficient, whereas 18.2% of participants in the vitamin D group were vitamin D insufficient (Figure 3; P < 0.001; chi-square test). By 1 y, 50% of participants in the placebo group were vitamin D insufficient, whereas 22.2% of participants in the vitamin D group were insufficient (P = 0.08; chi-square test).
FIGURE 2.
Least-squares mean (±SEM) serum 25(OH)D concentrations in early chronic kidney disease subjects who were randomly assigned to receive vitamin D or a placebo for 1 y. Subjects with early-stage chronic kidney disease (mean eGFR: 62 ± 15 mL · min−1 · 1.73 m−2) were randomly assigned to receive 50,000 IU vitamin D/wk for 12 wk followed by 50,000 IU vitamin D every other week for 40 wk (n = 22) or an identically matched placebo (n = 24). Serum 25(OH)D concentrations are reported across time and by treatment group. The vitamin D group (solid line) had a significant increase in serum 25(OH)D by 12 wk, which remained elevated from baseline at 52 wk. The placebo group (dashed line) had a significant decrease in 25(OH)D by 12 wk. The group*time was determined with mixed-model repeated-measures ANOVA. *Significant change from baseline, P < 0.05 (paired t test); asignificant difference from placebo, P < 0.05 (t test). eGFR, estimated glomerular filtration rate; group*time, group-by-time interaction; 25(OH)D, 25-hydroxyvitamin D.
FIGURE 3.
Percentage of vitamin D–insufficient participants by treatment and week. The prevalence of vitamin D insufficiency in the vitamin D group (n = 22; black bars) was reduced by weeks 12 and 52. The vitamin D–insufficiency prevalence was higher in the placebo group (n = 24; white bars) than in the vitamin D group at weeks 12 (P < 0.001; chi-square test) and 52 (P = 0.08, chi-square test). 25(OH)D, 25-hydroxyvitamin D.
PTH concentrations
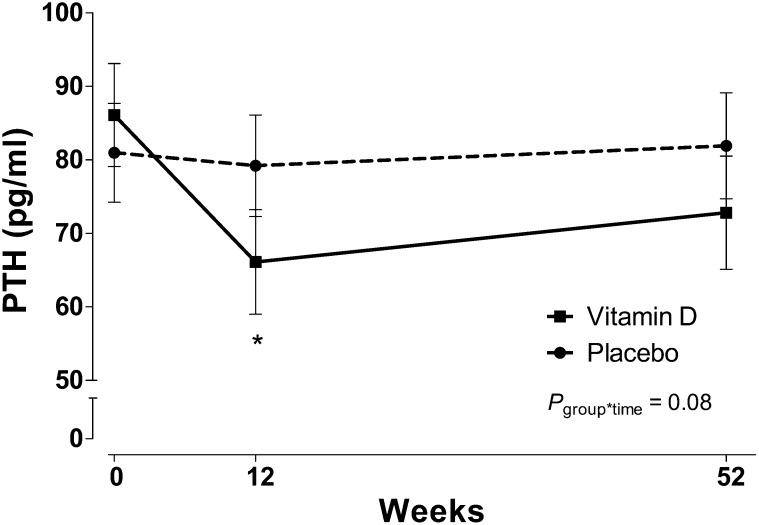
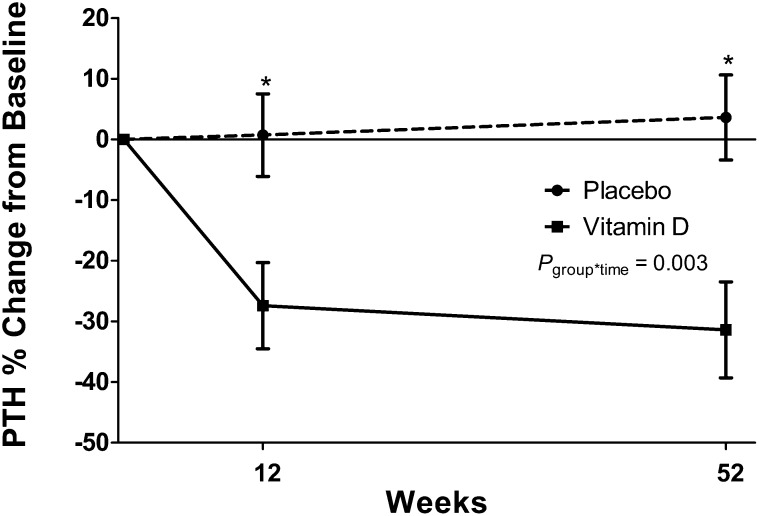
Serum PTH concentrations significantly decreased from baseline in the vitamin D group (mean PTH concentration: 89.1 ± 49.3 to 70.1 ± 24.8 pg/mL; P = 0.01; paired t test) at 12 wk, but values were not significantly different from baseline at 1 y (75.4 ± 29.5 pg/mL; P = 0.16; t test; Figure 4). PTH in the placebo group remained stable throughout the study. The group-by-time interaction for PTH approached significance in whole-group analysis before and after adjustment for baseline 25(OH)D values (P = 0.09 and P = 0.08 for repeated-measures ANOVA and ANCOVA, respectively). In a post hoc analysis in participants with an elevated PTH concentration (>70 ng/mL) at baseline, which was indicative of secondary hyperparathyroidism, the group-by-time interaction for PTH was significant (P = 0.004; repeated-measures ANOVA) and remained significant after adjustment for baseline 25(OH)D concentrations (P = 0.003; repeated-measures ANOVA). In participants with secondary hyperparathyroidism at entry, subjects randomly assigned to the vitamin D group, compared with subjects randomly assigned to the placebo group, had a greater percentage change in PTH concentrations from baseline to 12 wk (–27.4% compared with 0.7%, respectively; P = 0.01; ANCOVA) and to 52 wk (–31.4% compared with 3.6%, respectively; P = 0.005; ANCOVA) (Figure 5). In post hoc analyses by stage of CKD, the group-by-time interaction adjusted for baseline 25(OH)D in participants with stage 3 CKD was P = 0.08 (repeated-measures ANCOVA) with a decrease in PTH concentrations in the vitamin D group at 12 wk. The group-by-time interaction in participants with stage 2 CKD was P = 0.64.
FIGURE 4.
Least-squares mean (±SEM) PTH concentrations across time by treatment group in patients with chronic kidney disease who were randomly assigned to receive vitamin D or a placebo for 1 y. Subjects with early-stage chronic kidney disease (mean PTH concentration at baseline 83.4 ± 37.8 pg/mL) were randomly assigned to receive 50,000 IU vitamin D/wk for 12 wk followed by 50,000 IU vitamin D every other week for 40 wk (n = 22) or an identically matched placebo (n = 24). Serum PTH concentrations are reported across time and by treatment group. Serum PTH concentrations decreased from baseline at 12 wk in the vitamin D group (solid line) and did not change in the placebo group (dashed line). *Significant difference from baseline, P < 0.05 (paired t test). The group*time was determined with mixed-model repeated-measures ANOVA. group*time, group-by-time interaction; PTH, parathyroid hormone.
FIGURE 5.
Least-squares mean (±SEM) percentage changes in PTH concentrations from baseline by time and treatment group in patients with elevated baseline PTH concentrations (>70 pg/mL) adjusted for baseline 25(OH)D concentrations. Compared with placebo group (n = 24, dashed line), the vitamin D group (n = 22; solid line) experienced a significantly greater percentage decrease in PTH at weeks 12 and 52. *P-group difference < 0.05 (ANCOVA). group*time, group-by-time interaction. PTH, parathyroid hormone.
Blood pressure, FGF23, and other serum analytes
There were no significant changes in either group for blood pressure, serum creatinine, eGFR, or serum calcium, albumin, or phosphorus. There was no significant change in serum FGF23 concentrations in either group. Post hoc analyses revealed that, in patients with optimal vitamin D status [25(OH)D ≥30 ng/mL], FGF23 concentrations increased by 12 wk (mean FGF23 concentration: 31.55 ± 33.8 to 42.51 ± 34.7 relative units/mL; P = 0.03; paired t test) and returned to baseline concentrations by 52 wk (22.1 ± 31.5 relative units/mL; P = 0.72 for difference from baseline; t test) in the vitamin D group, although there was no change in FGF23 concentrations in the placebo group (group-by-time interaction: P = 0.04; repeated-measures ANOVA).
Urine analytes
Paired 24-h urine samples for baseline and 52-wk follow-up were available for 7 participants in the placebo group and 8 participants in the vitamin D group. There were no significant changes in urine excretion of calcium, creatinine, albumin, or phosphorus in either group. There was no significant change in the median (IQR) urine albumin in either group from baseline to 52 wk [vitamin D group: 19.6 mg/24 h (12.0–493.2 mg/24 h) to 18.6 mg/24 h (7.1–27.4 mg/24 h), P = 0.23; placebo group: 15.1 mg/24 h (7.9–53.3 mg/24 h) to 15.4 mg/24 h (12.3–62.4 mg/24 h), P = 1.00; Wilcoxon's signed rank tests). Hypercalcuria was not observed.
DISCUSSION
We performed a randomized, placebo-controlled trial of cholecalciferol supplementation for 1 y in patients with early CKD (stages 2 and 3). After 1 y, our cholecalciferol regimen was sufficient to maintain serum 25(OH)D concentrations and prevent vitamin D insufficiency. Furthermore, serum PTH concentrations improved after cholecalciferol treatment in patients who had secondary hyperparathyroidism, whereas PTH concentrations did not change in the placebo group. There were no changes in serum FGF23 concentrations or blood pressure over 1 y.
To our knowledge, this is the first long-term (≥1 y), randomized, placebo-controlled trial of cholecalciferol supplementation in adults with early CKD. Our data showed that a dose of 50,000 IU/wk for 12 wk followed by 50,000 IU every other week prevented vitamin D insufficiency [25(OH)D concentration <30 ng/mL] throughout the year, whereas patients who received the placebo remained vitamin D insufficient at rates reported in the literature (23, 24). In a separate cohort, we previously reported that 50,000 IU cholecalciferol/wk for 12 wk was sufficient to achieve optimal 25(OH)D concentrations in patients with CKD stages 3 and 4 (25). We extended these findings to 1 y in subjects with earlier-stage CKD. Several studies that followed protocols on the basis of KDOQI guidelines and by using ergocalciferol doses similar to ours have failed to achieve optimal serum 25(OH)D concentrations (26–28). In non-CKD populations, cholecalciferol has been shown to be more effective than ergocalciferol at raising and maintaining 25(OH)D concentrations (8–10). Although head-to-head trials of cholecalciferol compared with ergocalciferol in CKD are warranted, available data suggest that cholecalciferol may be more effective in the maintenance of an optimal vitamin D status.
A major target of vitamin D therapy in CKD is the management of secondary hyperparathyroidism. Studies based on KDOQI guidelines have yielded mixed results in their ability to reduce PTH concentrations (7, 26–28), although none of these studies were designed as randomized, placebo-controlled trials. In our study, cholecalciferol supplementation was effective in the reduction of PTH concentrations in patients with secondary hyperparathyroidism after 3 mo of treatment. Current guidelines may be limited because they recommend treatment of secondary hyperparathyroidism as opposed to prevention. A recent trial reported that ergocalciferol prevented secondary hyperparathyroidism in children with CKD stage ≥2 (29). Because serum PTH concentrations are relatively stable at eGFR values >60 mL · min−1 · 1.73 m−2 (30), it is likely that a longer follow-up period in our adult population would be necessary for vitamin D to have a preventative effect. Our data also indicated a more pronounced decrease in PTH in patients with stage 3 than stage 2 CKD after cholecalciferol treatment, which suggested that the effect of cholecalciferol on reducing PTH may be more readily apparent in CKD stage ≥3. This study established the efficacy of cholecalciferol for the reduction of PTH concentrations; however, longitudinal studies are needed to evaluate the preventative effects of vitamin D therapy in adults with all stages of CKD.
FGF23, which is a phosphaturic hormone that is elevated in CKD, is a major regulator in the vitamin D–PTH axis, and it predicts mortality in this population (18, 19). Vitamin D supplementation has been shown to both increase (31) and decrease (32) FGF23 concentrations in relatively healthy, vitamin D–deficient individuals. In our study of early CKD, cholecalciferol supplementation increased circulating FGF23 concentrations by 12 wk only in patients who had optimal vitamin D status at baseline [25(OH)D ≥30 ng/mL], although values returned to baseline by 52 wk. The complete actions and regulation of FGF23 are still being elucidated; however, FGF23 is known to directly inhibit renal 1-α-hydroxylase and promote 24-hydroxylase and, thereby, reduce circulating and tissue concentrations of 1,25(OH)2D (33, 34). Thus, the acute increase in FGF23 we observed may have been a compensatory mechanism to regulate renal 1,25(OH)2D production in early CKD patients with sufficient serum 25(OH)D concentrations, although we did not have serum 1,25(OH)2D to confirm. FGF23 concentrations in our early CKD population were not elevated to the extent seen in later-stage CKD (35) or with concentrations associated with mortality (19).
Epidemiologic data suggested that vitamin D deficiency is a risk factor for CVD in the general population (36). A recent retrospective study reported higher CVD-specific survival in CKD patients who successfully increased 25(OH)D concentrations with ergocalciferol supplementation (12). We did not find an effect of cholecalciferol treatment on blood pressure, which is consistent with the results of a 12-wk trial by Moe et al (17). Because blood pressure is highly sensitive to external factors (37), it is possible that other measures of cardiovascular risk, such as left ventricular mass index (38) or arterial stiffness (39–41), may be useful endpoints for vitamin D research on cardiovascular outcomes in CKD. The anticipated Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) Study did not find improvements in cardiac structure or function with paricalcitol treatment, although there was a reduction in CVD-related hospitalizations (42). Prospective clinical trials are required to identify the best measure of cardioprotective effects of vitamin D supplementation in early CKD and the optimal vitamin D formulation.
Albuminuria and proteinuria increase risk of disease progression and mortality in CKD (43). Cross-sectional studies have shown an inverse relation between circulating 25(OH)D and albuminuria (44, 45). Trials of active vitamin D analogs have resulted in decreased albuminuria (46, 47). Antiproteinuric effects of vitamin D may be mediated through several mechanisms including the downregulation of the renin-angiotensin system (48) and up-regulation of nephrin or renal megalin (48, 49). In a subset of patients with available 24-h urine samples, we did not find any changes in urine albumin in either group. However, our data can be considered only hypothesis-generating because of the limited sample size; larger, randomized, controlled trials will be needed to evaluate the potential effect of nutritional vitamin D on proteinuria.
Strengths of this pilot study were its long-term follow-up relative to previously published trials of cholecalciferol (7) and its prospective, double-blind, randomized, placebo-controlled design. Limitations included group differences at baseline including the greater report of dietary vitamin D intake and higher enrollment during the summer and autumn months in the placebo group, which led to higher baseline serum 25(OH)D concentrations. In addition, there was a greater prevalence of type 2 diabetes in the vitamin D group, which indicated a generally less healthy group. These occurrences were likely due to chance because of the randomized nature of the study; however, these occurrences may have influenced the study results. In addition, we recruited participants on the basis of the Modification of Diet in Renal Disease Study equation; an analysis of eGFR on the basis of the Chronic Kidney Disease Epidemiology Collaboration equation would have placed 6 participants in the normal range for eGFR at baseline. The exclusion of these participants did not alter the results. Other limitations were the small sample sizes that were available to analyze dietary records and 24-h urine and a lack of serum 1,25(OH)2D to further investigate FGF23 regulation and actions.
In conclusion, 1-y supplementation with high-dose oral cholecalciferol safely maintained serum 25(OH)D concentrations in the optimal range (≥30 ng/mL). This dose of cholecalciferol was also effective in the reduction of serum PTH concentrations in patients with secondary hyperparathyroidism. Additional study is needed to determine whether cholecalciferol can prevent development of secondary hyperparathyroidism in adults with early CKD as well as other outcomes relevant to CKD including cardiovascular risk and proteinuria.
Supplementary Material
Acknowledgments
We thank Ada Pabon and Albert Bonnet at the Atlanta VA Medical Center for their support with laboratory analyses; Breanna Wright, Shabnan Seydafkan, Meena Kumari, Lynn Schlanger, and Peggy Jenkins for their research recruitment and coordinating support; and Sarthak Khare for his assistance in sample preparation.
The authors’ responsibilities were as follows—VT and JAA: designed the research, analyzed data, performed the statistical analyses, and had primary responsibility for the final content of the manuscript; JAA, VT, JL, KEC, SMZ, LH, and KSS: conducted the research; and all authors: wrote and edited the manuscript and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor-23; KDOQI, Kidney Disease Outcomes Quality Initiative; PTH, parathyroid hormone; VA, Veterans Affairs; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Castro AF, Coresh J. CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 2009;53(3 suppl 3):S46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–70 [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007;71:31–8 [DOI] [PubMed] [Google Scholar]
- 4.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011;6:913–21 [DOI] [PubMed] [Google Scholar]
- 5.Llach F, Velasquez Forero F. Secondary hyperparathyroidism in chronic renal failure: pathogenic and clinical aspects. Am J Kidney Dis 2001;38(5 suppl 5):S20–33 [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 suppl 3):S1–201 [PubMed] [Google Scholar]
- 7.Alvarez JA, Wasse H, Tangpricha V. Vitamin D supplementation in chronic kidney disease: a systematic review. Dermato-Endocrinology 2012;4 (in press). Available from: http://www.landesbioscience.com/journals/dermatoendocrinology/toc/volume/4/issue/2/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab 2011;96:E447–52 [DOI] [PubMed] [Google Scholar]
- 9.Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, Tangpricha V. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab 2009;94:2037–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854–8 [DOI] [PubMed] [Google Scholar]
- 11.Petchey WG, Johnson DW, Isbel NM. Shining D’ light on chronic kidney disease: mechanisms that may underpin the cardiovascular benefit of vitamin D. Nephrology (Carlton) 2011;16:351–67 [DOI] [PubMed] [Google Scholar]
- 12.Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Treatment of 25-OH vitamin D deficiency in older men with chronic kidney disease stages 3 and 4 is associated with reduction in cardiovascular events. Am J Ther (Epub ahead of print 17 August 2011) [DOI] [PubMed] [Google Scholar]
- 13.Judd SE, Tangpricha V. Vitamin d therapy and cardiovascular health. Curr Hypertens Rep 2011;13:187–91 [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305 [DOI] [PubMed] [Google Scholar]
- 15.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:1931–42 [DOI] [PubMed] [Google Scholar]
- 16.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 2010;5:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutiérrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off” hypothesis. Clin J Am Soc Nephrol 2010;5:1710–6 [DOI] [PubMed] [Google Scholar]
- 19.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011;305:2432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54 [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30 [DOI] [PubMed] [Google Scholar]
- 22.Bross R, Noori N, Kovesdy CP, Murali SB, Benner D, Block G, Kopple JD, Kalantar-Zadeh K. Dietary assessment of individuals with chronic kidney disease. Semin Dial 2010;23:359–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, Takasu J, Adler S, Norris K. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1144–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 2009;75:88–95 [DOI] [PubMed] [Google Scholar]
- 25.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract 2008;14:10–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 2007;50:59–68 [DOI] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S. Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: a randomized controlled trial. Am J Kidney Dis 2012;59:58–66 [DOI] [PubMed] [Google Scholar]
- 28.Qunibi WY, Abdellatif A, Sankar S, Hamdan Z, Lin FY, Ingle J, Cadena A, Gelfond J, Kasinath B. Treatment of vitamin D deficiency in CKD patients with ergocalciferol: are current K/DOQI treatment guidelines adequate? Clin Nephrol 2010;73:276–85 [DOI] [PubMed] [Google Scholar]
- 29.Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Wells D, Aitkenhead H, Manickavasagar B, Van't Hoff W, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clin J Am Soc Nephrol 2012;7:216–23 [DOI] [PubMed] [Google Scholar]
- 30.Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 2009;4:186–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol 2012;7:624–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uzum AK, Salman S, Telci A, Boztepe H, Tanakol R, Alagol F, Ozbey NC. Effects of vitamin D replacement therapy on serum FGF23 concentrations in vitamin D-deficient women in short term. Eur J Endocrinol 2010;163:825–31 [DOI] [PubMed] [Google Scholar]
- 33.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 2007;293:F1577–83 [DOI] [PubMed] [Google Scholar]
- 34.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004;113:561–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westerberg PA, Linde T, Wikstrom B, Ljunggren O, Stridsberg M, Larsson TE. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant 2007;22:3202–7 [DOI] [PubMed] [Google Scholar]
- 36.Leu M, Giovannucci E, Vitamin D. Epidemiology of cardiovascular risks and events. Best Pract Res Clin Endocrinol Metab 2011;25:633–46 [DOI] [PubMed] [Google Scholar]
- 37.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697–716 [DOI] [PubMed] [Google Scholar]
- 38.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 2010;5:905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrade J, Er L, Ignaszewski A, Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol 2008;3:1800–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raggi P, Bellasi A, Ferramosca E, Block GA, Muntner P. Pulse wave velocity is inversely related to vertebral bone density in hemodialysis patients. Hypertension 2007;49:1278–84 [DOI] [PubMed] [Google Scholar]
- 41.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 2011;58:186–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012;307:674–84 [DOI] [PubMed] [Google Scholar]
- 43.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, de Jong PE, El-Nahas M, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011;79:1331–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2007;50:69–77 [DOI] [PubMed] [Google Scholar]
- 45.Isakova T, Gutiérrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr 2011;21:295–302 [DOI] [PubMed] [Google Scholar]
- 46.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension 2008;52:249–55 [DOI] [PubMed] [Google Scholar]
- 47.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010;376:1543–51 [DOI] [PubMed] [Google Scholar]
- 48.Li YC. Vitamin D: roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens 2012;21:72–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int 2011;79:715–29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.