Abstract
In this issue of Immunity, Chaussabel et al. (2008) apply an inductive approach to pathway discovery identifying modular units that govern human immune biology.
“It is impossible for the human intellect to grasp ideas of absolute continuity of motion. Laws of motion of any kind only become comprehensible to man when he can examine arbitrarily selected units of that motion. But, at the same time, it is this arbitrary division of continuous motion into discontinuous units which gives rise to a large proportion of human error.”
—Leo Tolstoy, War and Peace, III:3
It is increasingly appreciated that monothematic, deductive reasoning applied to the testing of individual genes or proteins does not efficiently embrace the complexity of human immune biology (Marincola, 2007; Benoist et al., 2006). Deductive reasoning aims at confirmation of hypotheses by minimizing experimental variables. However, this reasoning when applied to the clinics needs to confront the uncontrollable nature of human biology molded by the heterogeneous genetic background of patients, their phenotypic adaptations to environmental forces, and, in some instances, the rapid evolution of disease dictated by unstable viral or neoplastic genomes. Thus, nonlinear mathematics may better fit the purpose of comprehending the host reaction to a pathogenic insult in its globality (Dalgleish, 1999). Indeed, biology manifests several characteristics of chaotic systems in which repetitions, given a sufficient number of permutations, progressively exfoliate random associations leaving a bare stem of recurrent patterns linked by necessity to a particular phenomenon. Identification of these recurrent themes segregates relevant from irrelevant observations. Thus, as an alternative to deductive reasoning, inductive reasoning moves from observation to broader generalizations, allowing the formulation of evidence-based hypotheses. This reasoning is the impetus of the work by Chaussabel et al. (2008) in which they applied inductive reasoning to pathway discovery and identified operational units comprising sets of functionally related genes that are differentially expressed by peripheral blood mononuclear cells (PBMCs) in distinct immune pathologies. This discovery provides a framework for an evidence-based approach to system immunology.
Inductive reasoning applied to immunology has to confront the daunting number of permutations arising from thousand of genes interacting with one another. Current technology allows the accumulation of genome-wide information about coordinate expression patterns. However, the extraordinary volume of data generated is not matched by the capacity of the human brain that is “poorly equipped to handle the multidimensionality that results from these broad analyses” (Benoist et al., 2006). Thus, the promises offered by the “omic” revolution produced fewer results than originally anticipated because, in part, of an unprepared audience of biologists and clinicians whose familiarity with bioinformatics principles is not commensurate to the present needs (Bialek and Botstein, 2004).
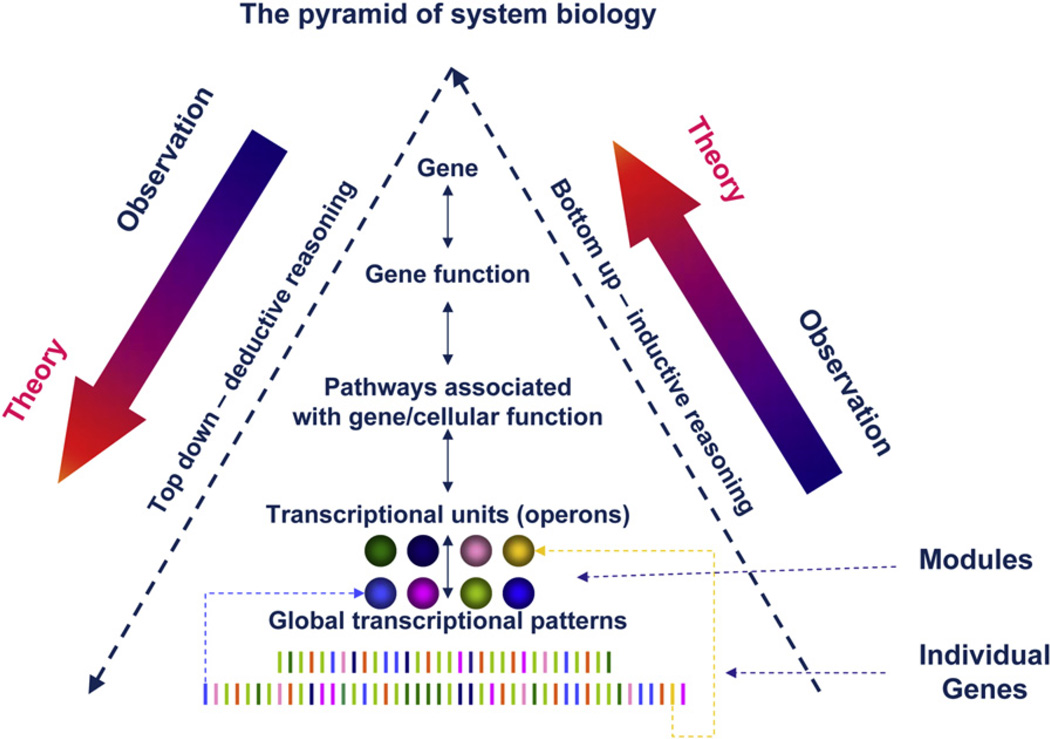
Deductive reasoning applied to biology traditionally follows a “top-down” approach: A gene or protein is responsible for a given phenotype through a direct cause-effect relationship. However, because genes and their products interact and modulate each other’s expression, this linearity is rarely unambiguous and clusters of genes need to be assembled into molecular pathways to explain biological functions. In turn, integration of experimentally defined pathways constructs virtual networks in which biological interactions are predicted according to theoretical algorithms (Avila-Campillo et al., 2007). These networks are used to interpret high-density data but because they are predominantly derived from experimental models, their clinical relevance remains questionable.
In this issue of Immunity, Chaussabel et al. (2008) apply “bottom-up” inductive reasoning to the understanding of human immune biology (Figure 1). Although the bioinformatics jargon may appear intimidating, the logic followed by the authors was straightforward. On the basis of the hypothesis that the transcription of PBMCs may be disease specific, the authors analyzed 239 PBMC samples from individuals with different immune conditions (juvenile idiopathic arthritis, systemic lupus erythematosus, acute infections, or liver-transplant recipients undergoing immune-suppressive therapy). In addition, two diseases that previously to this work had not been clearly associated with immune pathology were included: metastatic melanoma and type I diabetes. The authors observed that distinct clinical entities consistently displayed characteristic transcriptional patterns that consisted of clusters of coordinately expressed genes. Moreover, the investigators observed that genes belonging to each cluster shared similar functions, and therefore, each cluster represented a broad transcriptional unit that could in approximately half of the cases be subsequently assigned a functional interpretation: The authors called these transcriptional units “modules.” We will refer to them also as functional units when a broad function could be assigned to them or simply transcriptional units when no functional interpretation could be given on the basis of the current gene-annotation assignments. Good examples of functional units are the plasma cell and B cell signatures (M1.1 and M1.3, respectively) or interferon-inducible genes (M3.1), which have been shown to occur in various clinical conditions associated with chronic and/or acute inflammatory processes (Wang et al., 2008). Moreover, Chaussabel et al. (2008) observed that the pattern of expression of individual modules was disease specific and, at least in the case of systemic lupus erythematosus, correlated with disease activity. Thus, as in the cosmos, billion of stars form a galaxy (module), and each galaxy participates in a cluster (physiologic status), which, in turn, is part of a supercluster (patient’s condition); our genome can be potentially divided in functional modules, and the more or less harmonious interactions among them can be followed with the relative simplicity with which astronomers follow the movement of billion of stars congregated into cosmic units. This reduction of information from hundred of genes into a few, functionally defined modular units will facilitate the interpretation of immunological phenomena offering, at the same time, integrated biomarkers for clinical correlations.
Figure 1. The Pyramid of System Immunology.
Most investigators have adopted a “top-down” approach to build networks that could explain cellular functions in their globality; the function of a given gene is the premise from which its interactions with other genes are deducted to formulate pathways that lead to coordinated cellular functions (a concept similar to the experimental observation of “operons”). Because the process proceeds in the downstream direction, it becomes increasingly speculative. Here, the authors applied a “bottom-up” (inductive) approach and identified operational (transcriptional) units that they called modules through the identification of genes co-coordinately expressed in distinct pathophysiological conditions; this evidence-based reasoning offers two advantages: It provides a road map for biomarker discovery, and it identifies transcriptional networks that may lead the basic scientist toward the identification of upstream events that lead to pathogenic processes and that, therefore, are most probably relevant to human suffering.
The authors tested the validity of their discovery by predicting modular patterns in independent patient samples; in addition, “transcriptional vectors” were calculated that simply represent the arithmetic average of the expression values for subsets of genes representing each individual module. Plotting the transcriptional vector for each module on a spider graph, one could create individual patients’ expression profiles that were strikingly different from those obtained from healthy volunteers and, in the case of systemic lupus erythematosus, that were predictive of disease activity and could be used to monitor disease progression.
This “bottom-up” approach is evidence based rather than based on theoretical assumptions. Therefore, the modules identified are likely to represent the downstream result of a clinically relevant determinism. Observations from other groups strongly support the validity of the approach: Critchley-Thorne et al. (2007) observed distinct transcriptional patterns in PBMCs from patients with metastatic melanoma compared to normal individuals; it would be interesting to reevaluate their analysis in the context of the modular network. He et al. (2006) observed that the transcriptional pattern of PBMCs from patients with chronic hepatitis C virus infection stimulated with interferon-α could predict treatment outcome after the systemic administration of the same agent. Thus, transcriptional patterns of PBMCs could serve as an easy-to-access lookout for a given disease status and could be exploited to identify biomarkers useful for diagnostic, prognostic, and therapeutic purposes (i.e., patient stratification during enrollment in clinical trials).
The results presented in this seminal paper are limited for a variety of understandable reasons: The diseases studied are few and limited to immune pathologies. We have observed that a panel of common cancer biomarkers could be identified by comparing a variety of primary and metastatic cancers of different histology to a broad array of normal tissues (Basil et al., 2006). It is possible the modular approach could discover better- defined functional units relevant to cancer and other diseases. As stated by the authors, future efforts should be aimed at broadening the database to other pathologies. The study addresses potential clinical implications of the modular approach by demonstrating a semi-quantitative relationship between transcriptional vectors and disease activity in the context of systemic lupus erythematosus. However, the predictive accuracy of the modular pattern at clinically relevant stages of disease and its relationship to treatment outcome in the context of lupus or other diseases will need to be tested for early diagnostic, prognostic, and predictive purposes. Furthermore, biomarkers are needed as surrogate endpoints; it will be important to test whether individual patient’s modular activity could serve as a useful monitor of response to therapy, early recurrence, and long-term survival compared to currently followed gene-specific biomarkers. A caveat of the present study is intrinsic to its design; by looking for recurrent themes within a particular disease, it is possible that subtle differences that are within each disease category and that may bear clinical significance in diagnostic, prognostic, or predictive terms may have been missed; although the predictive value of vector analysis in systemic lupus erythematosus suggest good quantitative correlation between transcriptional information and disease activity, it is possible that qualitative difference were excluded by eliminating less consistent expression patterns. This limitation could be over-come in future studies by subcategorizing individual diseases according to clinically relevant parameters.
In summary, Chaussabel et al. (2008) suggest an inductive approach to pathway discovery: Disease-specific gene-expression patterns are identified and condensed into few functional units; these are presumed to represent downstream effects of biological mechanisms determining the disease status. Some of them are representative of cell types, whereas others represent cellular functions such as immune-activation pathways, cell-cycle and metabolic functions, etc.; these observations have important scientific implications because an upstream look at the pathways controlling the activation of each functional unit may provide insight about disease pathogenesis. This evidence-based analysis represents a paradigm shift in which system biology (immunology) is approached from the bedside, yielding information most likely to be relevant to human suffering and confronting the basic immunologist and cell biologist with the challenge of aligning experimental observations with the reality of human disease approached in its uncontrollable complexity. Moreover, the modular approach offers practical applications as a global-biomarker- discovery tool that will need to be aggressively validated in the future.
REFERENCES
- Basil CF, Zhao Y, Zavaglia K, Jin P, Panelli MC, Voiculescu S, Mandruzzato S, Lee HM, Seliger B, Freedman RS, et al. Cancer Res. 2006;66:2953–2961. doi: 10.1158/0008-5472.CAN-05-3433. [DOI] [PubMed] [Google Scholar]
- Benoist C, Germain RN, Mathis D. Immunol. Rev. 2006;210:229–234. doi: 10.1111/j.0105-2896.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- Bialek W, Botstein D. Science. 2004;303:788–790. doi: 10.1126/science.1095480. [DOI] [PubMed] [Google Scholar]
- Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, et al. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. PLoS Med. 2007;4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. QJM. 1999;92:347–359. doi: 10.1093/qjmed/92.6.347. [DOI] [PubMed] [Google Scholar]
- He XS, Ji X, Hale MB, Cheung R, Ahmed A, Guo Y, Nolan GP, Pfeffer LM, Wright TL, Risch N, et al. Hepatology. 2006;44:352–359. doi: 10.1002/hep.21267. [DOI] [PubMed] [Google Scholar]
- Marincola FM. J. Transl. Med. 2007;5:21. doi: 10.1186/1479-5876-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Campillo I, Drew K, Lin J, Reiss DJ, Bonneau R. Bioinformatics. 2007;23:392–393. doi: 10.1093/bioinformatics/btl604. [DOI] [PubMed] [Google Scholar]
- Wang E, Worschech A, Marincola FM. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]