Abstract
Background
Ultrasound (US) exposed microbubble (MB) contrast agents have the capability to transiently enhance cell membrane permeability. Using this technique in cancer treatment to increase the efficiency of chemotherapy through passive, localized delivery has been an emerging area of research.
Purpose
Investigation of the influence of US parameters on MB mediated drug delivery in cancer.
Methods
2LMP breast cancer cells were used for in vitro experiments and 2LMP tumor-bearing mice were used during in vivo experiments. Changes in membrane permeability were investigated after the influence of MB-mediated US therapy parameters (i.e. frequency, mechanical index, pulse repetition period, US duration, and MB dosing and characteristics) on cancer cells. Calcein, a non-permeable fluorescent molecule, and Taxol, chemotherapeutic, were used to evaluate membrane permeability. Tumor response was also assessed histologically.
Results
Combination chemotherapy and MB-mediated US therapy with optimized parameters increased cancer cell death by 50% over chemotherapy alone.
Discussion
Increased cellular uptake of chemotherapeutic was dependent upon US system parameters.
Conclusion
Optimized MB-mediated US therapy has the potential to improve cancer patient response to therapy via increased localized drug uptake, which may lead to a lowering of chemotherapeutic drug dosages and systemic toxicity.
Keywords: ultrasound, microbubble contrast agents, cancer, drug delivery, chemotherapy
1. INTRODUCTION
Cancer is the second most common cause of death in the USA with a projected 570,000 deaths in 2010 (American Cancer Society, 2010). The efficiency of a drug, such as chemotherapy, to be delivered and taken-up by cancerous cells ultimately determines the effectiveness of any systemic treatment (Orive, 2003). The lack of tumor response to chemotherapy is well documented in many cancer types. Breast cancer studies have shown only 60% response to anthracycline-based chemotherapy with 14% of that being complete response (Carey, 2006). Literature has shown that adjuvant chemotherapy has no benefit to pancreatic cancer patients (Neoptolemos, 2001). Head and neck cancer shows between 12–50% 5-year survival rate using different chemotherapeutic options (Argiris, 2008). In general, the 5-year cancer survival rate in the United States is 68% (American Cancer Society, 2010). This insufficient response of cancer to a multitude of chemotherapeutic drugs highlights the need for improved delivery mechanisms to aid in increased localized drug uptake in the targeted cancer cells.
Diagnostic ultrasound (US) imaging has become a powerful clinical tool due to its real-time capability, portability, minimal exposure to radiation and inexpensive cost. The application of microbubble (MB) contrast agents to traditional US introduces contrast-enhanced US. MBs are flexible polymer, surfactant, or protein shelled gas-filled colloidal particles ranging in size from 1–8 μm (DeJong, 2000). When MBs are exposed to an US field, the mechanical force causes them to oscillate between states of rarefaction and compression. While this resonance produces nonlinear backscattered US signals useful for imaging purposes, under certain acoustical conditions MBs physical interaction with tissue has also been shown to temporarily enhance cellular permeability (Miller, 2000; Ward, 1999). This technique of using US exposed MBs to transiently enhance membrane permeability is an emerging area of research and could lead to improved tumor cell drug internalization (van Wamel, 2006). MB-mediated US therapy is impacted by both US exposure conditions and MB characteristics (Karshafian, 2009). Therefore, optimization of US-based therapy may improve the efficiency of systemic chemotherapeutic delivery and impact cancer treatment.
The concept of modulating membrane permeability has become increasingly popular with the intention of introducing active compounds, such as drugs and gene therapy vectors, into diseased cells (Miller, 2002). It has been shown that in the presence of MBs, US pressure (or mechanical index, MI) at low levels can increase the intracellular uptake of chemotherapeutic drugs and genetic materials such as polynucleotides and proteins (Hueber, 2000; Miller, 1999; Anwer, 2000; Hosseinkhani, 2003; Mukherjee, 2000; Zderic, 2002). During MB-mediated US therapy, cell membrane disruption appears similar to plasma wounds and are actively repaired within minutes after therapy ceases (Schlicher, 2006; McNeil, 2003). In cancer cells, US induced membrane permeability in combination with anti-cancer drugs such as Bleomycin and Adriamycin have been shown to increase drug uptake, demonstrating a promising technique for cancer therapy (Iwanaga, 2007; Wu, 2006). MB-mediated US therapy has also been shown to be beneficial in vivo to increase delivery of molecules. Localized cellular delivery of DNA using MB-mediated US therapy has been investigated in vivo in areas of enhanced cancer gene therapy, for cardiovascular applications and for bone formation (Miller, 2002). These applications have shown increased results using MBs to help penetrate the cellular membrane and/or endothelial barrier. Enhanced delivery of cytotoxic agents to tumors through this therapeutic method has also been used and proven to retard tumor growth in mice (Iwanaga, 2007). MB-mediated US immunogenic therapy for solid tumors has also been evaluated and shown to produce a 55% cure rate in a xenograft tumor model (Casey, 2010). MB-mediated US therapy in combination with chemotherapy has shown potential to enhance drug uptake at US targeted cancer sites, with future potential to decrease systemic toxicity.
In the United States, it is estimated that over 1.5 million new cases of cancer will be diagnosed in 2010 (American Cancer Society, 2010). As cancer has become a global problem, it has become increasingly important to detect, monitor and treat patients effectively. The impact of a therapeutic drug depends on the rate and ability to permeate into the desired tissue. The effectiveness of a therapeutic drug to resolve the cancer condition is directly dependent upon the amount delivered to the tumor over time. To improve therapy effectiveness, novel strategies in treatment are needed to overcome the current barriers of poor uptake resulting from tortuous vasculature, limited drug dosages and high tumor interstitial pressure (Jain, 2001). Specifically, there is an immediate need for promising strategies such as MB-mediated US therapy that can produce improved chemotherapeutic drug delivery and localized tumor uptake. The objective of this study was to investigate the influence of both US exposure and MB properties on MB-mediated ultrasound cancer drug delivery in vitro and in vivo and to optimize these conditions to enhance drug uptake.
2. MATERIALS AND METHODS
2.1 Cell lines and culture methods
In this study, 2LMP human breast cancer cells (MDA-MB-231, lung metastatic pooled) were used as a biological model for investigating MB-mediated US therapy as cancer therapy. The 2LMP cell line was maintained in DMEM, 10% FBS, and 1% L-glutamine. All cells were cultured 70% to 90% confluence before passaging. Cells were grown at 37° C and in 5% CO2 and 90% relative humidity. Appropriate cell numbers for all experiments were determined using a hemocytometer and trypan blue dye exclusion.
2.2 In vitro ultrasound treatment with fluorescent uptake
2LMP cells (1×106) were suspended in cell buffer (PBS with 5% FBS) in 75×12 mm Polystyrene tubes combined with 1 mL of calcein (MP Biomedicals, LLC, Solon, OH), 1×10−3 M concentration, and MBs. The brands of MBs studied were Definity (Lantheus Medical Imaging North Billerica, MA), SonoVue (Bracco International BV, Amsterdam, Netherlands), and Levovist (Schering AG, Berlin, Germany). Cells were exposed to US in a water bath of temperature 37° C. During experiment duration, tubes were continuously rotated using a mechanical stepper motor at a rate of 24 degrees per second allowing direct US exposure to the entirety of the cells. The transducer was immersed and stabilized at a far field distance of approximately 12 cm from the cells. Control samples underwent the same procedure, replacing exposure with sham US. The objective of these experiments was to quantify the cellular uptake of low molecular weight fluorescent molecules due to transient enhancements of membrane permeability. Membranes were disrupted using MB-mediated US therapy and a range of exposure conditions. The fluorescent signal from cellular-entrapped calcein was quantified using flow cytometry techniques immediately following therapy and reported as a percentage of signal recorded in control cell populations.
2.3 Ultrasound Exposure Parameters
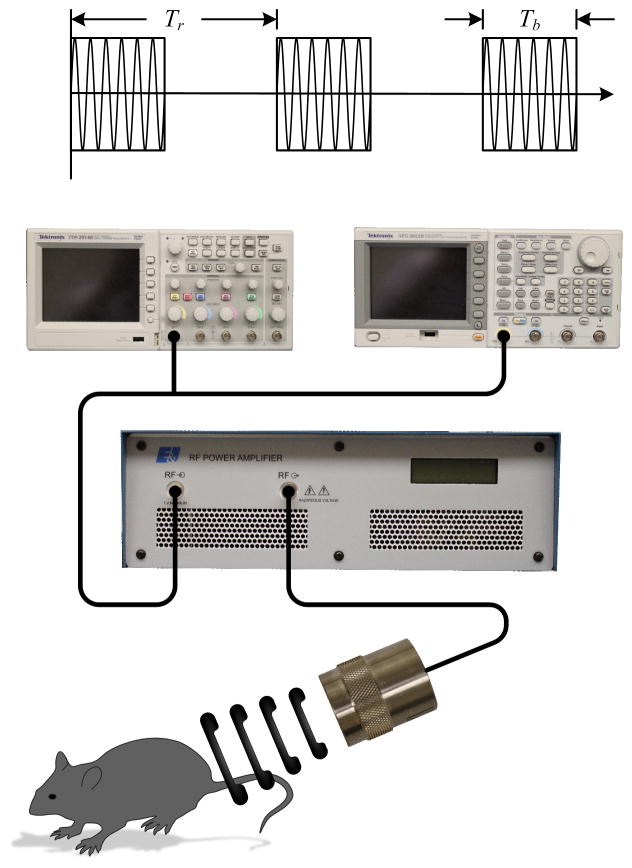
The custom experimental US setup involved single element (0.75 inch) immersion transducer (Olympus, Waltham, MA) in series with a signal generator (AFG3022B, Tektronix, Beaverton, OR) and power amplifier (A075, Electronics and Innovation, Rochester, NY) as illustrated in Figure 1. This study was completed in a series of experiments in order to investigate the influence of pertinent pulsed US parameters such as signal frequency (0.5, 1.0, or 2.25 MHz), duration (15, 60, 300, or 600 sec) and magnitude of exposure (MI of 0.1, 0.5, 1.0 or 2.0), pulse repetition period (PRP; 0.01, 0.1, 1.0 sec), MB dosage (10, 50, 250 μL) using a concentration of 14 million MBs/mL and brand (Definity, SonoVue, and Levovist). Concentrations of brands varied, therefore total MB count was held constant at 700,000 MBs (Definity (14 million MBs/mL, Levovist (7 million MBs/mL), SonoVue (7 million MBs/mL) ) for those experiments. MB concentration was determined using flow cytometry. Duty cycles for these experiments were fixed at 20%. Unless otherwise stated, default US parameters were 1.0 MHz frequency, an MI of 1.0, a PRP of 0.01 sec, duration of 300 sec and the default MB brand was Definity using a 50 μL dose.
Figure 1. Experimental Design Schematic.
(a) Experimental setup using a single element immersion transducer in series with a signal generator and power amplifier.
2.4 Intensity measurements
US intensity measurements were performed in a 37° C water bath using a hydrophone (Model HGL-0400, ONDA, Sunnyvale, CA) and preamplifier setup in series with a digital oscilloscope for voltage signal monitoring and recording. Individual immersed transducers were manipulated by a precision stepper motor (Velmex, Inc, Bloomfield, NY) in order to locate the spatial peak pressure maximum. The latter was determined by converting voltage to pressure measurements using hydrophone calibration data.
2.5 Flow cytometry
Fluorescent signals from internalized calcein molecules (600 Da) were quantified for each cell population using flow cytometry (Accuri C6, Accuri Cytometers Inc., Ann Arbor, MI). All experimental groups were analyzed in triplicate. Cells were normalized and average fluorescence per cells were calculated. For each experimental variant, data was normalized by control group fluorescence and reported as percent control. Cell viability tests were confirmed using membrane-impermeable propidium iodide (2 μL of 0.5 mg/mL, Fisher Scientific, Waltham, MA).
2.6 In vitro drug uptake
2LMP cells (1×106) were plated on acoustically transparent flasks (Opticell, Rochester, NY). After a 24 hr period to ensure proper seeding, Definity MBs (50 μL) were administered and cells underwent combination chemotherapy (Taxol, Parenta Pharmaceutics, Inc, Yardley, Pennsylvania) (100 nM, 0.84 μg) with MB-mediated US therapy. Taxol is a commonly known chemotherapy drug used in breast cancer, and was chosen as the model chemotherapeutic due to its ability to be evaluated in vitro and in vivo. Opticell flasks were placed in a custom built rack that was positioned attached at the bottom of a water bath of temperature 37° C. The transducer was immersed and stabilized at a far field distance of approximately 12 cm from the cells. The opticell flasks were inverted in order for the MBs to float to the top and act directly with the monolayer of cells. Control cells underwent chemotherapy only, MB-mediated US therapy only or no therapy. Following a 24 hr incubation period, plates were analyzed using either fluorescence microscopy (Olympus 1X70, Olympus American, Inc., Melville, NY) or flow cytometry. For flow cytometry, cells were trypsinized and stained with calcein AM (BD Biosciences, Franklin Lakes, NJ) and propidium iodide for measuring cell viability and death, respectively. Specifically, cells were stained with 1.0 μL of working calcein-AM stock (50 μL) and incubated for 15 min at 37° C. Subsequently, 2.0 μL of 0.5 mg/mL propidium iodide was added. Cells were then analyzed for fluorescence (1×103 event counts) using flow cytometry. All experimental groups were analyzed in triplicate. Light microscopy images were acquired and registered to fluorescence images to visibly validate cell viability and death tests.
2.7 In vivo ultrasound treatment
Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Forty-two 4-week-old nude athymic mice (The Jackson Laboratory, Bar Harbor, ME) were implanted subcutaneously in the flank with 2LMP cells (2×106). Approximately three wks post implantation, animals were sorted by average tumor size and grouped as follows (n = 6 per group): control (no drug or US), drug (Taxol) only, MB-mediated US only (MI of 0.5), or drug plus MB-mediated US therapy (MI = 0.1, 0.5, 1.0 or 2.0). Grouping was completed by taking caliper measurements of tumor size on Day 0 and sorting mice from smallest to largest tumor and then separating in order. Each group created had the same average size of tumor to ensure no biasing between groups. The US therapy groups were further stratified by the intensity of US exposure and used varying MI values of 0.1, 0.5, 1.0, or 2.0. All drug and MB-mediated US therapies were administered on days 0, 3, 7, 10, 14, and 17 of this study. Drug (37 μL, 6 mg/mL) and MBs (Definity, 30 μL) were administered via tail vein injections after dilution with saline to 100 μL. Drug and MB dosage were determined by the weight range of the animals and specification from respective companies. The remaining control groups received bolus injections of matched drug or MBs doses diluted to 100 μL with saline. Two min post injection, applicable groups underwent MB-mediated US therapy in a 37°C waterbath for 5 min using a transmit frequency of 1.0 MHz and PRP of 5 sec (20% duty cycle). Animals were weighed and tumors sizes were monitored using both caliper measurements and high-frequency (40 MHz) US imaging (Vevo 660, VisualSonics Inc, Toronto, CA) on days 0, 7, 12, 14, and 19. Using a standard normalized tumor size with an ellipse equation for tumor area and ellipsoidal formula for tumor volume, tumor measurements were tracked over time as percent change from day 0. On day 21, animals were humanely euthanized and tumors excised for histological analysis.
2.8 Immunohistologic Analysis
Serial sections of 5 μm thickness were cut from formalin fixed, paraffin embedded tissue blocks and floated onto charged glass slides (Super-Frost Plus, Fisher Scientific) and dried overnight at 60° C. An H&E stained section was obtained from each tissue block. All sections subject to immunohistochemistry were de-paraffinized and hydrated with deionized water. The tissue sections were heat treated with 0.01M Tris-1 mM EDTA buffer (pH 9) using a pressure cooker (CEPC 800, Cook’s Essentials, China) for 5 min at maximum pressure (15 lb/in2). Following antigen retrieval, all sections were gently washed in deionized water and then transferred to TBST (0.05M Tris-based solution in 0.15M NaCl with 0.1% v/v Triton-X-100, pH 7.6). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min. To further reduce non-specific background staining, slides were incubated with 3% normal goat or horse serum for 20 min (Sigma, St. Louis, MO) according to the host where primary antibodies were produced. All slides were then incubated at 4° C overnight with either Ki67 or CD31 antibody. Negative controls were achieved by eliminating the primary antibodies from the diluents. Following washing with TBST, peroxidase-conjugated goat anti rabbit IgG (for CD31 and Ki67) (1:200, Jackson ImmunoResearch, West Grove, PA) was applied to the sections for 30 min at room temperature. Diaminobenzidine (DAB, Scy Tek Laboratories, Logan, UT) was utilized as the chromagen and hematoxylin (7211, Richard-Allen Scientific, Kalamazoo, MI) as the counterstain.
H&E sections were examined for cellular necrosis and reported as percent of the entire tumor cross-section (original magnification x5). Each CD31 section was examined (original magnification x40) to identify five separate areas containing the greatest microvessel density (MVD). Individual vessels from these five areas were counted (original magnification x200), averaged, and recorded as MVD. Ki67 sections were reviewed to determine level of cell proliferation within the tumors (original magnification x200).
2.9 Statistical Analysis
Data was summarized as mean ± SE. Statistical analyses were performed using the software package SAS 9.2 (SAS, Cary, NC). Assessment of in vitro cell death following drug plus MB-mediated US was performed using an analysis of variance (ANOVA) test for cell death. After ANOVA, statistical comparisons between groups were made with Tukey-Kramer multiple comparison procedure. Assessment of in vivo tumor size (both area and volume) was conducted with ANOVA test using day 21 data (percent change). After ANOVA, statistical comparisons between groups were made with Tukey-Kramer multiple comparison procedure. Evaluation of percent necrosis from histological analysis was performed on MB-mediated US therapy groups with ANOVA statistical testing to determine differences in influence of MI. MVD was assessed using ANOVA testing. A p-value less than 0.05 was considered statistically significant.
3. RESULTS
3.1 In vitro fluorescence tracer uptake
3.1.1 Frequency
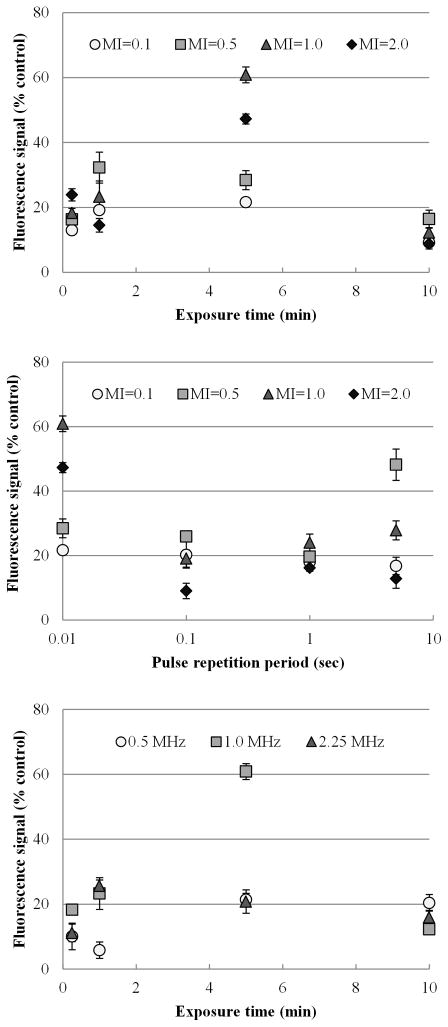
Within the three US transmission frequencies explored (0.5 MHz, 1.0 MHz and 2.25 MHz), a 1.0 MHz frequency showed the maximum level of extracellular fluorescent tracer uptake as shown in Figure 2a. At 5 min of exposure, 0.5, 1.0 and 2.25 MHz frequency US resulted in increased tracer uptake levels of 21.36 ± 1.96, 60.84 ± 2.42 and 20.80 ± 3.60 % respectively. The group with 1.0 MHz frequency presented the greatest increase in uptake level compared to the other frequencies at 5 min exposure (p < 0.0001). MB-mediated US therapy using a transmission frequency of 0.5 MHz only significantly differed from results found using 2.25 MHz at an exposure duration of 1 min (p = 0.001). At exposure times of 15 sec, 5 min and 10 min, there were no significant differences in extracellular tracer uptake levels between 0.5 MHz and 2.25 MHz (p > 0.05).
Figure 2. In vitro characterization of US parameters.
(a) Changes in frequency altering fluorescent uptake over a range of MB-mediated US exposure times. (b) Shown are the changes in fluorescent uptake after varying the MI over a range of exposure times. (c) As the MI and the PRF changes, the percent increase of fluorescence signal changes.
3.1.2 Mechanical Index
Increasing the magnitude of US exposure does not always translate to greater cellular permeability and extracellular tracer uptake during MB-mediated US therapy as shown in Figure 2b. For the exposure times studied, therapy using very low magnitude US (MI of 0.1) produced a very small increase in extracellular fluorescent tracer uptake that ranged over time from 9.24 ± 1.44 to 21.62 ± 1.38 %. This data set showed a near constant linear trend when compared to other US conditions. Specifically, using an MI of 1.0 produced significantly higher tracer uptake levels ranging from 12.24 ± 1.39 to 60.84 ± 2.42 % (p < 0.0001), peaking at an exposure time of 5 min. MB-mediated US therapy exposure at an MI of 0.5 produced a shifted left curve that peaked at an exposure time of 1 min with an uptake of 32.28 ± 4.79%, while cellular exposure at an MI of 2.0 peaked at 5 min with a corresponding increase in extracellular tracer uptake of 47.24 ± 1.54 %. Importantly, there were no significant differences in the quantity of dead cells found between each control and test group (p = 0.57).
3.1.3 Pulse repetition period
At lower PRPs (e.g., 0.01 sec), MB-mediated US therapy using higher MI values were more effective at modulating cellular permeability and increasing extracellular tracer uptake as shown in Figure 2c. In total, a PRP of 0.01 sec did not show a difference compared to results using a PRP of 0.1 and 1 sec (p > 0.05), but trended toward significance. US exposure using an MI of 1.0 produced a significant increase in extracellular tracer uptake at a PRP of 0.01 compared to 0.1 and 1.0 (p < 0.001).
3.1.4 Duration of US exposure
Cells were exposed to MB-mediated US therapy for a range of duration (0.25, 1, 5, and 10 min) in addition to a variety of MI values, transmission frequencies, MB doses, and MB brands. Varying the duration of US exposure demonstrated that simply increasing the duration of US exposure does not always lead to increased cellular permeability and subsequent extracellular tracer uptake. As US parameters change, exposure time must be dictated for that specific constraint. Note that 5 min of US exposure resulted in the greatest increase in extracellular tracer uptake under the experimental conditions specified (figures 2a, 2b and 2c).
3.1.5 MB dose
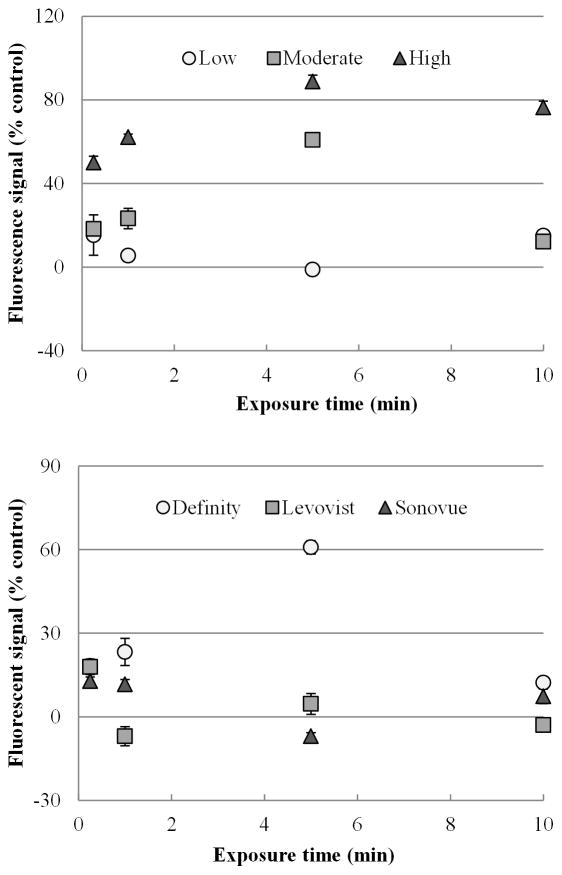
A trend emerged for MB-mediated US therapy, revealing as the quantity of MBs dose increased, the subsequent level of extracellular tracer increased as seen in Figure 3a. At an exposure time of 1 min, cells demonstrated a 5.47 ± 2.72 %, 23.28 ± 4.87 % and 62.21 ± 1.41 % increase in fluorescent tracer uptake as the MB dose went from low (10 μL), medium (50 μL), and high (250 μL), respectively. At an US exposure time of 5 min, increases in tracer uptake were found to be 1.14 ± 2.11 %, 60.84 ± 1.42 %, and 88.81 ± 3.02% for the low, medium and high MB doses, respectively. It is shown that MB dose is associated with increased uptake level significantly at both 1 min and 5 min (p < 0.0001, p < 0.0001). Within each time period, each of the doses were significant from each other (p < 0.05, p < 0.05).
Figure 3. In vitro characterization of MB parameters.
(a) Shown are changes in fluorescent uptake from varying MB dosing amounts over time. As the amount of MB administered increased, fluorescent uptake increased. (b) Shown are the changes in fluorescent signal uptake after varying MB brands over a range of exposure times. Definity resulted in the highest molecular uptake.
3.1.6 MB brand
Definity MBs were shown to have the greatest enhancement effect for modulating cellular membrane permeability and subsequent extracellular tracer uptake as seen in Figure 3b. At 15 sec, there were no significant differences (p = 0.09) between results obtained using the three MB brands, while at 1 and 5 min, extracellular tracer uptake using Definity was significantly increased compared results obtained using Sonovue and Levovist (p < 0.001).
3.2 In vitro drug uptake
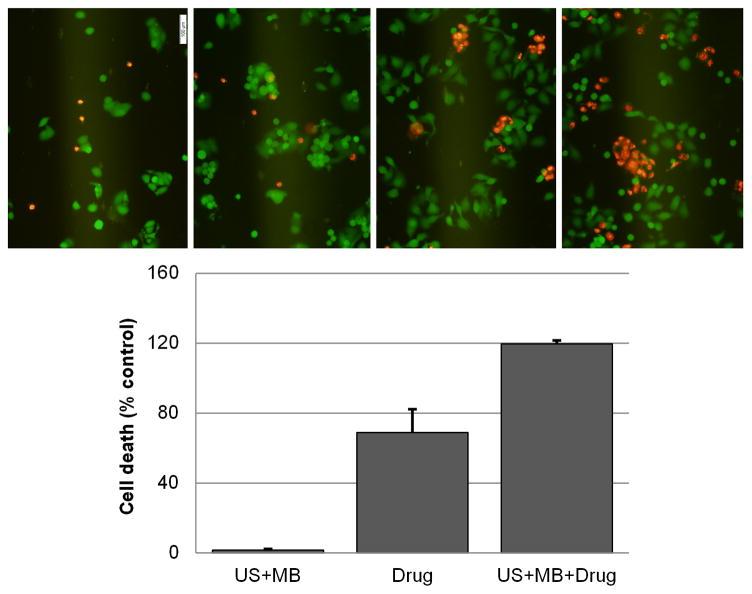
Combination drug and MB-mediated US therapy studies showed that the percentage of cell death increased by 120 % as compared to control data (p < 0.001) (Figure 4b). Combination drug and MB-mediated US therapy increased the percentage of cell death by approximately 50 % when compared to results obtained using drug alone (p < 0.05), as seen in Figure 4b. MB-mediated US therapy alone (no chemotherapeutic drug) showed no significant difference in the amount of cell death compared to control data (p > 0.05). Fluorescent images allowed qualitative analysis of viable and non-viable cells, showing an increased amount of cell death when comparing drug to MB-mediated US drug therapy. Representative pictures are shown in Figure 4a.
Figure 4. In vitro characterization of combination US MB mediated therapy and chemotherapy.
(a) A viability stain of dead cells (stained in red with propidium iodide) and viable cells (stained in green with calcein AM) were imaged using fluorescent microscopy viability stain 24 hrs after combination MB-mediated US therapy. (b) Shown are quantifications of cell death (% control) using flow cytometry 24 hrs following combination MB-mediated US therapy.
3.3 In vivo drug uptake
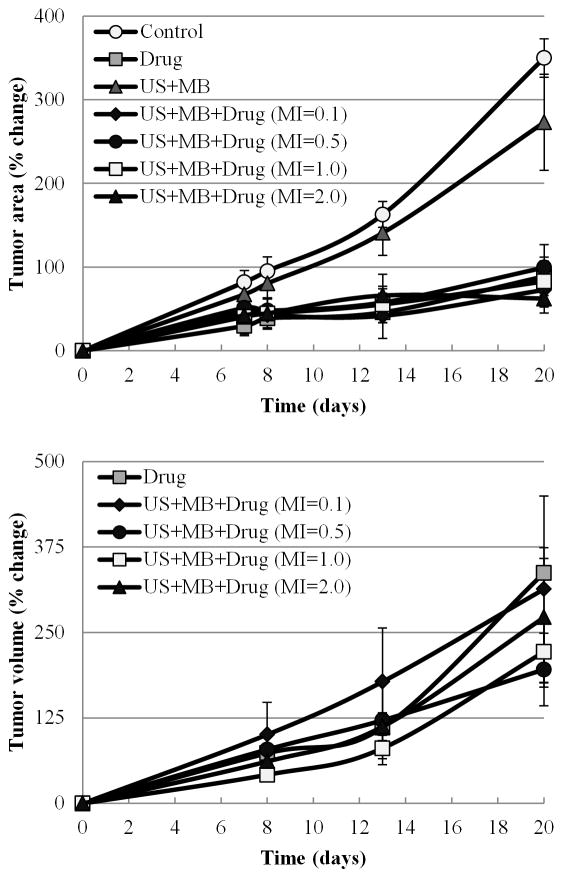
Evaluation of MB-mediated US therapy in a tumor-bearing mouse model using various magnitudes of US pressure (i.e., MI values of 0.1, 0.5, 1.0, and 2.0) consistently resulted in decreased tumor growth over groups receiving: chemotherapeutic drug alone, MB-mediated US therapy alone, or controls. MB-mediated US therapy using an MI value of 0.5 resulted in the highest impediment in tumor growth over the three week treatment period confirming that optimization of US parameters can further enhance antitumor drug effects (see figures 5, 6 and 7). As shown in figure 5a, terminal tumor area calculations via caliper measurements indicated no significant difference between any groups that were administered drug (p > 0.05) or between the control and US therapy alone groups (p > 0.05). However, there was a significant difference in terminal tumor sizes of non-drug groups (US alone and control groups) and those that received drug treatment (p < 0.001). Tumor assessment using high-frequency US imaging, shown in figure 5b, allowed for more precise tumor size measurements, particularly in the animal groups receiving drug therapy due to their smaller tumor sizes. Mean tumor volumes measured via high-frequency US for MB-mediated US drug therapy group animals (MI of 0.5) was 195.79 ± 53.01 mm3, which was significantly lower than that found in drug alone, 337.06 ± 21.36 mm3 (p = 0.037). MB-mediated US therapy using an MI of 1.0 also showed a significant enhancement of antitumor effects on tumor volume (221.61 ± 45.12 mm3) compared to the therapy with drug alone group (p = 0.04). There were no significant differences found between the MB-mediated US therapy groups with varied MI values as all groups demonstrated comparable antitumor effects. However, there was a discernible trend in therapeutic response and tumor growth retardation as MB-mediated US therapy and the magnitude of US exposure alternated from MI values of 0.1, 2.0, 1.0, to 0.5. The latter therapeutic condition demonstrated the smallest terminal group tumor size.
Figure 5. In vivo characterization of tumor size after combination MB-mediated US therapy.
(a) Control groups of saline treatment and US treatment only showed exponential growth of tumors (measurements of tumor area was performed using calipers). (b) Variations of tumor volume (measurements from high frequency US) are shown for chemotherapy and MB-mediated US treatment with chemotherapy groups. A MI of 0.5 depicted the best results with the smallest percent of growth of tumor.
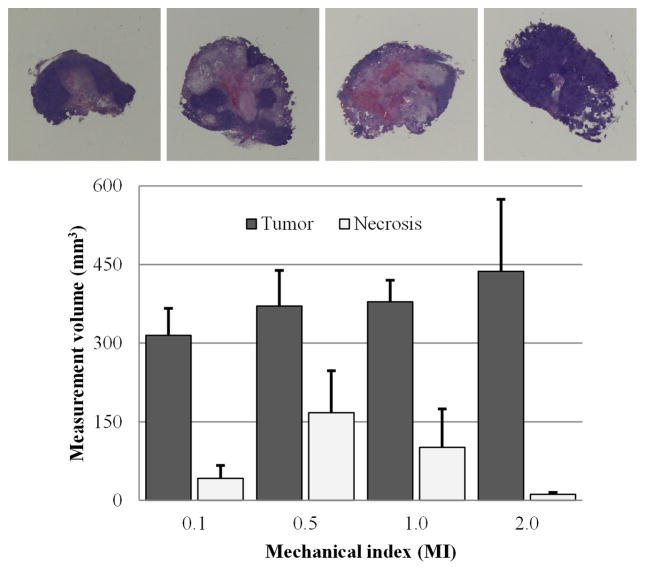
Figure 6. In vivo characterization of tumor necrosis per volume after combination MB-mediated US therapy.
(a) MB-mediated US treatment with various MIs was investigated in combination with chemotherapy. Shown are necrosis and tumor size from the varying treatments on the last day of experimentation. Representative histology slide are shown for each varying MI: (b) MI=0.1, (c) MI=0.5, (d) MI=1.0 and (e) MI=2.0. Control tumors are shown in (e) drug alone, (f) ultrasound alone, and (g) saline control. The control tumors were excluded from calculation due to ulcerations. MB-mediated US treatment with an MI of 0.5 resulted in the highest percentage of tumor necrosis.
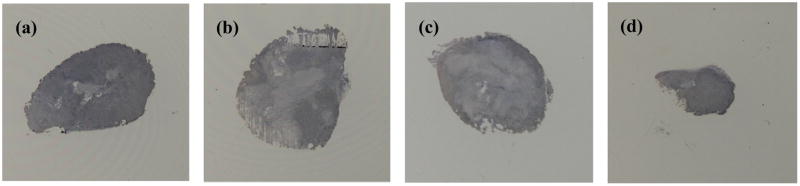
Figure 7. In vivo characterization of Ki67 staining.
MB-mediated combination therapy shows greatest effect of lowering levels of cellular proliferation localized to the tumor peripheral. Representative images are shown between varying MI values: (a) MI=0.1, (b) MI=0.5, (c) MI=1.0 and (d) MI=2.0.
As figure 6a details, MB-mediated US therapy using an MI value of 0.5 in combination with drug therapy also produced the highest degree of necrosis per tumor volume (40.72 ± 15.8 %), followed by therapy using an MI of 1.0 (24.92 ± 17.31 %), MI of 0.1 (15.31 ± 10.76 %) and MI of 2.0 (3.21 ± 1.10 %). MB-mediated US therapy using an MI value of 0.5 resulted in 17 and 3.5 times higher tumor necrosis levels than therapy with an MI values of 2.0 (p = 0.12) and 0.1 (p = 0.21), respectively. Due to tumor ulcerations in control group animals, they were excluded from necrosis percentages. These intratumoral necrosis levels suggest that MB-mediated US combination drug therapy using an MI value of 0.5 produces the greatest antitumor effect (p = 0.21), which coincides with tumor growth retardation. Representative pictures are shown in Figure 6b-e. The light pink area shown is necrotic tissue, where the deep purple area shown is viable cancerous tissue. There was no significant weight loss observed between day 0 to day 21 (p > 0.05) for each animal group. In addition, there were no observed differences in grooming and diet throughout the study duration indicating there was minimal adverse effects from the treatment. Immunohistologic cross-sectional slides of CD31 stained tumor tissue showed no significant differences in MVD counts between any of the four MB-mediated US combination drug therapy groups (p > 0.05). However, there was a noticeable decrease in MVD counts that trended towards significant in the MB-mediated US drug therapy group using an MI of 2.0. There were no differences in MVD counts between the control group animals (p = 0.18). Significant differences in MVD counts were found between MB-mediated US drug therapy groups using an MI of 2.0 (38 ± 2.57 counts) and both the drug only (55 ± 4.79 counts) and control (51 ± 3.26) animal groups (p < 0.05). Qualitative analysis of Ki67 staining confirmed results from the tumor necrosis analysis, exhibiting non-proliferative regions of tumor necrosis. Ki67 staining also revealed that throughout viable tumor regions, markedly lower levels of cellular proliferation were localized to the tumor peripheral and the greatest effect was found in the MB-mediated US combination drug therapy group animals exposed to US using an MI of 0.5 (Figure 7).
4. DISCUSSION
Improving cellular and vascular permeability to enhance chemotherapeutic drug uptake in cancer yields the potential to improve drug delivery and treatment efficacy. Development of more effective strategies for systemic delivery of these agents could permit lower drug dosing sessions throughout a therapeutic regimen, thereby, decreasing patient toxicity. MB-mediated US combination drug therapy is a promising method for addressing these concerns.
The in vitro component of our experimental study demonstrated that as the MB-mediated US therapeutic parameters were varied, the transient extent of cellular permeability and subsequent extracellular molecular uptake varied. Monitoring distributions of the membrane impermeable fluorescent tracer calcein allowed the effects of MB-mediated US therapy to be analyzed. US exposure parameters such as magnitude, duration, and pulse repetition period were shown to influence membrane permeability. Note that normalization by sham US (control) data was necessary since cell populations were not washed following incubation with the fluorescent dye. This leads to surface accumulation of fluorescent molecules and a weak fluorescent background signal. Viability studies showed there were no significant differences in the percentage of dead cells after receiving MB-mediated US therapy or sham US, suggesting that the incorporation of MBs or calcein had no net impact on cell viability. Increasing the exposure duration of MB-mediated US therapy was not shown to directly correlate with increased membrane permeability as determined by fluorescent tracer uptake. Interestingly, a previous study determined that cellular membranes can remain porous throughout a MB-mediated US therapeutic session (Pan, 2005), but decreasing MB concentrations toward the end of exposure can possibly decrease affects or allow molecules to flow back out of the cell. Extended periods of US exposure can also cause prolonged cell membrane damage (without cell death) leading to insufficient results considering the molecules are unconstrained and free to diffuse back outside the cells.
The in vitro studies revealed that maximal uptake of extracellular molecules occurred at an US transmission frequency of 1.0 MHz, which parallels findings by other groups (Hwang, 2005; Rahim, 2006). Stable cavitation of MBs is known to be dictated by, and proportional to, US wavelength (Krebs, 2004). For the transmission frequencies investigated and the size of the MBs, the optimal frequency is closest to 1.0 MHz. Since this US frequency was found optimal for our setup, it was used extensively throughout the duration of the experiments. A PRP of 0.01 seconds (PRF of 100 Hz) exhibited the greatest increase in membrane permeability and fluorescent uptake. Shorter pulse periods excite MBs more often, thus, leading to enhanced uptake. An MI of 1.0 showed the greatest increase in cellular permeability. At an MI of 0.1, it is inferred that the pressure amplitude is not sufficient to drive cavitation and induce molecular-level permeability effects in cell suspensions. At an MI of 0.5, stable cavitation occurs, which will increase cell membrane permeability, however it does not utilize the full MB potential. At an MI of 2.0, it is concluded that inertial cavitation dominates MB response, which minimizes mechanical interaction with cellular membranes due to MB destruction. The effects of increasing membrane permeability through MB-mediated US therapy have been shown to occur while MBs are still intact (Forbes, 2011). Therefore, these US parameters appear to lean towards stable MB cavitation. Stable cavitation has been shown to increase the effects of MB-mediated US therapy without inertial cavitation (Kamaev, 2004; Datta, 2008; Forbes, 2008). Given that an MI of 1.0 showed the greatest extracellular fluorescent tracer uptake, results suggest that stable MB cavitation was the dominant mechanism, thereby creating optimal US conditions for maximizing MB interaction with the cell suspensions. The duty cycle was purposely fixed throughout the entirety of the study so the time-average intensity of US exposure to the various cell groups was constant. MB resonance generated by the US parameters and interaction with cells ultimately leads to increased cellular permeability during MB-mediated US therapy. Limitations of the described in vitro studies could relate to the use of cell suspensions in comparison to cell monolayers. Cell monolayers exhibit more realistic conditions when comparing to in vivo work, yet cell suspensions were chosen for these experiments to allow for more parameters to be investigated. Having the cells suspended allows for immediate transition to quantitative analysis using flow cytometry. Another limitation of the study was that MB destruction analysis was not performed, which may have permitted complete differentiation between stable and inertial cavitation, yet literature sources allow us to point to stable cavitation as the positive affect of increased cellular membrane permeability.
MB properties influence the effectiveness of MB-mediated US therapy. Differences in MB brands have been previously studied and MB shell integrity and stability are important factors to consider before their use in MB-mediated US therapeutic applications (Dalecki, 2004). Definity was used for the majority of the study because of availability and approval for use in the United States for echocardiographic applications. Sonovue and Levovist are both lyophilized, dry powder MBs that are reconstituted in saline to enclose sulfur hexafluoride (SF6) gas and air, respectively, while Definity MBs are non-lyophilized encapsulating octafluoropropane (OFP) gas. OFP gas has a compressibility factor of 0.975, while air has a compressibility factor of 0.999 (Fowler, 1947; Vassernan, 1966). Definity and Sonovue both exhibit a lipid-shelled membrane, while Levovist contains a galactose-based shell (Bracco Diagnostics, 2001; RxMed, 1999; Lantheus Medical Imaging, 2008). Sonovue MBs has a mean diameter of 2.5 μm and 90% are smaller than 8 μm (Schneider, 1999). Definity MBs have a mean diameter between 1.1 to 3.3 μm with 98% less than 10 μm. Levovist MBs measure 2–8 μm in diameter, with 95% less than 10 μm. MB stability and ability to create stable cavitation may result from combinations of a shell, gas and lyophilized state. Definity proved to have the greatest enhancement effect of membrane permeability, but optimization for each MB type may need to occur. By adjusting ultrasound parameters such as frequency, concentration and attenuation, other types of MBs may also be able to improve membrane permeability. At 15 sec, there were no significant differences (p = 0.09) between the three MB brands, yet at 1 and 5 min, there was a significantly increased cell membrane permeability using Definity MBs, i.e. fluorescent uptake (p <0.001 and p < 0.001, respectively). This could be due to increased stability of this MB composition from the lipid shell and the OFP gas, allowing greater mechanical oscillations of the MBs and interactions with the cells before dissipating. Sonovue and Levovist were both lyophilized MBs, required reconstitution in the presence of liquid. However, Definity was non-lyophilized which may have contributed to the increased stability and performance during MB-mediated US therapy. Five min duration of US exposure had the greatest cell membrane permeability. At 5 min duration, a 5-fold increase in MB dose (from 10 μL to 50 μL) produced a 61.98 % increase in membrane permeability when combined with US exposure, yet an additional 5-fold increase in MB dosing (250 μL) showed only a 26.83% increase in membrane permeability. This trend indicates that there is a diminishing marginal response of cell membranes (permeability) exposed to increasing concentrations of MBs and US therapy. Because MBs are more stable at higher concentrations during stable cavitation (Calliada, 1998), increasing MB dosing may in fact hinder membrane permeability modulation and extracellular molecule uptake.
During in vitro experiments conducted with pre-determined optimized parameters, MB-mediated US therapy in combination with chemotherapy produced significant increases in cell death when compared to chemotherapy alone (p = 0.003). This difference in cell death is attributed to an increase in cellular permeability and intracellular drug loading. Noteworthy, there were no differences in cell viability levels when comparing cell groups exposed to sham US and MB-mediated US therapy alone (p = 0.30). This confirms again that MB-mediated US therapy alone is a mechanism that does not pose any additional biological effects over sham US. MB-mediated US therapy improves cell membrane permeability through generating small pores to allow for increased passive drug delivery. This is the source of enhanced cancer necrosis: MB-mediated US therapy produced a 50 % increase in cell death in vitro. Increasing cancer cell death without increasing drug dose could be an important attribution to overall patient care. One study limitations from the in vitro combination chemotherapeutic MB-mediated US therapy was that only a portion (estimated 25%) of the cell monolayer was directly exposed to the US beam. Given the analysis is conducted on cells from the entire monolayer, increasing the effective treatment area would increase the already positive response.
In vivo results showed that increasing drug delivery of Taxol to cancerous cells can improve tumor response through passively increasing drug uptake. These in vivo results showed a relationship between the terminal tumor size and terminal tumor necrosis from treatment. Tumor size decreases parallel tumor necrosis increases when optimal MB-mediated US parameters were used. Limitations of this study were the minimal number of parameters investigated in vivo. It was necessary to minimize changes in parameters to accurately determine which alterations were critical to improve therapy. Further investigations should include using the optimal MI found for drug uptake and varying the PRPs, followed by altering the drug concentration levels to determine the minimal dose possible to achieve desired affects while decreasing systemic toxicity. Another limitation to the study is there was not a biodistribution comparing drug accumulation in tumors. An MI of 0.5 had the greatest anti-tumor effect determined by both inhibition of tumor growth over the period of the study and end point tumor necrosis levels in vivo. An MI of 1.0 was favorable during the in vitro optimization studies. This slight variation between in vitro and in vivo is to be expected because temporarily opening cancer cells directly in vitro in suspension has less outside influences than within a murine model. There was no significant difference between the in vivo therapy with a MI of 0.5 and that using 1.0 and further studies will have to be done to conclude the differences between them in vivo. An MI of 2.0 exhibited very little tumor necrosis, 17x less than that with an MI of 0.5. Excessively high MI values have been shown to cause bursting of capillaries (Dalecki, 2004; Shi, 2006; Yeh, 2008; Miller, 2008), which in turn could decrease drug delivery to the tumor cells. Conversely, when a low MI is used, there is thought to be little MB cavitation, creating no additional increased drug delivery. An MI of 0.1 produced less tumor necrosis when compared to an MI of 0.5. It is hypothesized that the low pressure amplitude is not intense enough to sufficiently drive cavitation and induce cellular-level permeability effects, therefore showing no noticeable results. A PRP of 5 sec (PRF of 0.2 Hz) was chosen to allow sufficient time for MB recirculation. It is hypothesized that increasing drug delivery to endothelial cells is the chief mechanism to inhibit cancer growth.
Increases in endothelial cell death have been shown to reduce tumor size and increase necrotic activity within the tumor, yet they are extremely resistant cells (Cameron, 2005; Carmeleit, 2000; Karson, 1996; Haran, 1994). Overexpression of endothelial growth factor receptors have been found in many cancers such as head and neck, breast, pancreatic, colorectal, ovarian, and lung carcinomas. These growth factor receptors are an important factor in regulating cellular proliferation, differentiation and survival (Bo, 2008). A neoplastic tumor cannot grow beyond millimeters in size without recruitment of endothelial cells and new blood vessels to supply nutrition and oxygen for tumor cell survival (Folkman, 1975; Folkman, 1985). Increasing the delivery of Taxol, an anti-proliferation drug, to endothelial cells through MB-mediated US therapy will reduce the cell proliferation, resulting in starvation of the tumor and prohibiting increased tumor growth. Specifically, Taxol inhibits microtubule dynamics of cytoskeletal elements resulting in mitotic arrest (Subramanian, 2011). Additional positive antitumor effects of MB-mediated US therapy is the ability to increase vascular permeability. Significant results have shown the ability to increase tumor perfusion by degrading junctions between the endothelial cells causing increased flow of drug to the cancer cells (Shang, 2011). This in vivo phenomenon of enhancing angiogenesis has been shown on several tissue and cancer cells lines, such as colon cancer and skeletal muscle (Kodama, 2010; Zhao, 2010). The combination of increasing drug flow to induce endothelial cell death and increasing extravasation explains how MB-mediated US therapy can non-invasively and successfully improve cancer response to therapy. Future directions should include investigating the specific mechanisms of MB-mediated US therapy and conclusively determine whether endothelial cell uptake, extravasation, or a combination of the two is occurring under each chosen parameter.
5. CONCLUSION
Optimizing parameters of MB-mediated US therapy to improve cellular permeability is essential to enhance therapeutic delivery through increased uptake level in cancer cells. Combination chemotherapy and MB-mediated US therapy with optimized parameters increased cancer cell death by 50% over chemotherapy alone. Using a non-invasive approach to enhance effects of chemotherapy treatment in cancer patients is a novel mechanism for improving patient response to cancer treatment. Increasing the effectiveness of drug delivery is critical for further explorations in decreasing chemotherapy dosage amounts, leading to decreased systemic toxicity.
Acknowledgments
The authors are grateful for all the helpful suggestions and feedback from Drs. Eben Rosenthal and Kurt R. Zinn. This research was supported in part by NIH grant UL1RR025777, EP50CA089019-09 and NCI grant CA13148-38.
Footnotes
DECLARATIONS OF INTEREST
Authors state they have no declarations of interest.
References
- American Cancer Society. Cancer facts and figures. Atlanta, Georgia: 2010. [Google Scholar]
- Anwer K, Kao G, Proctor B, Anscombe I, Florack V, Earls R, Wilson E, McCreey T, Unger E, Rolland A, Sullivan SM. Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration. Gene Ther. 2000;7:1833–1839. doi: 10.1038/sj.gt.3301302. [DOI] [PubMed] [Google Scholar]
- Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo A-H, Hou J-C, Lan Y-H, Tian Y-T, Zhang J-Y. Over-expression of EGFR on breast cancer. Chin J Cancer Res. 2008;20:69–72. [Google Scholar]
- Bracco Diagnostics. SonoVue package insert. 2001. [Google Scholar]
- Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: Basic principles. European Journal of Radiology. 1998;27:157–160. doi: 10.1016/s0720-048x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Cameron IL, Short N, Sun L, Hardman WE. Endothelial cell pseudopods and angiogenesis of breast cancer tumors. Cancer Cell International. 2005;5:17. doi: 10.1186/1475-2867-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheand MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Carmeleit P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Casey G, Cashman JP, Morrissey D, Whelan MC, Larkin JO, Soden DM, Tangney M, O’Sullivan GC. Sonoporation Mediated Immunogene Therapy of Solid Tumors. Ultrasound Med Biol. 2010;36:430–440. doi: 10.1016/j.ultrasmedbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Dalecki D. Mechanical Bioeffects of Ultrasound. Annu Rev Biomed Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–33. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong N, Frinking PJA, Bouakaz A, Ten Cate FJ. Detection of procedures of ultrasound contrast agents. Ultrasonics. 2000;38:87–92. doi: 10.1016/s0041-624x(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: A possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Forbes MM, Steinberg RL, O’Brien WD., Jr Frequency-dependent evaluation of the role of Definity in Producing Sonoporation of Chinese Hamster Ovary Cells. J Ultrasound Med. 2011;30:61–69. doi: 10.7863/jum.2011.30.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MM, Steinberg RL, O’Brien WD., Jr Examination of internal cavitation of Optison in producing sonoporation of Chinese hamster ovary cells. Ultrasound Med Biol. 2008;34:2009–2018. doi: 10.1016/j.ultrasmedbio.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler RD, Burford WB, Hamiton JM, Jr, Sweet R, Weber C, Kasper J, Litant I. Synthesis of Fluorocarbons. Industrial and Engineering Chemistry. 1947;39:292–298. [Google Scholar]
- Haran EF, Maretzek AF, Goldberg I, Horowitz A, Degani H. Tamoxifen Enhances Cell Death in Implanted MCF7 Breast Cancer by Inhibiting Endothelium Growth. Cancer Res. 1994;54:5511–5514. [PubMed] [Google Scholar]
- Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Ultrasound enhanced the transfection of plasmid DNA by non-viral vectors. Curr Pharmaceut Biotechnol. 2003;4:109–122. doi: 10.2174/1389201033489883. [DOI] [PubMed] [Google Scholar]
- Hueber PE, Pfisterer P. In-vitro and in-vivo transfection of plasmid DNA in the dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 2000;7:1516–1525. doi: 10.1038/sj.gt.3301242. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combined 1-MHz Ultrasound and microbubbles contrast agent treatment in vivo. Ultrasound Med Biol. 2005;31:553–564. doi: 10.1016/j.ultrasmedbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Iwanaga K, Tominaga K, Yamamoto K, Habu M, Maeda h, Akifusa S, Tsujisawa T, Okinaga T, Fukuda J, Nishihara T. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Therapy. 2007;14:354–363. doi: 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Kamaev PP, Hutcheson JD, Wilson ML, Prausnitz MR. Quantification of Optison bubble size and lifetime during sonication: dominant role of secondary cavitation bubbles causing acoustic bioeffects. J Acoust Soc Am. 2004;115:1818–1825. doi: 10.1121/1.1624073. [DOI] [PubMed] [Google Scholar]
- Karshafian R, Bevan PD, Williams R, Samac S, Burns PN. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol. 2009;35:847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Karson A, Yee E, Harlan JM. Endothelial Cell Death Induced by Tumor Necrosis Factor-α Is Inhibited by the Bcl-2 Family Member, A1. The Journal of Biological Chemistry. 1996;271:27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- Kodama T, Aoi A, Watanabe Y, Horie S, Kodama M, Li L, Chen R, Teramoto N, Morikawa H, Mori S, Fukumoto M. Evaluation of transfection efficiency in skeletal muscle using nano/microubbles and ultrasound. Ultrasound Med Biol. 2010;36:1196–205. doi: 10.1016/j.ultrasmedbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Krebs C, Odwin CS, Fleischer AC. Appleton and Lange Review for the Ultrasonography Examination. 3. New York, New York: McGraw-Hill Companies Inc; 2004. [Google Scholar]
- Lantheus Medical Imaging. Definity Packagage Insert. 2008. [Google Scholar]
- McNeil PL, Steinhardt RA. Plasma membrane disruption; repair, prevention adaptation. Annu Rev Cell Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- Miller DL, Averkiou MA, Bryman AA, Everbach EC, Holland CK, Wible JH, Wu J. Bioeffects Considerations for Diagnostic Ultrasound Contrast Agents. J Ultrasound Med. 2008;27:611–632. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- Miller DL, Bao S, Gies RA, Thrall BD. Ultrasonic enhancement of gene transfection in murine melanoma tumors. Ultrasound Med Biol. 1999;25:1425–1430. doi: 10.1016/s0301-5629(99)00105-2. [DOI] [PubMed] [Google Scholar]
- Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet. 2002;27:115–134. doi: 10.1023/a:1022983907223. [DOI] [PubMed] [Google Scholar]
- Miller DL, Quddus J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med Biol. 2000;26:661–667. doi: 10.1016/s0301-5629(99)00170-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, Thomas JD. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000;35:1678–1686. doi: 10.1016/s0735-1097(00)00575-1. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, Fernandez-Cruz L, Lacaine F, Pap A, Spooner D, Kerr DJ, Friess H, Büchler MW. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomized controlled trial. The Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- Orive G, Hernandez RM, Rodriguez Gascon A, Dominguez-Gil A, Pedraz JL. Drug Delivery in biotechnology: present and future. Curr Opin Biotechnol. 2003;14:659–664. doi: 10.1016/j.copbio.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Rahim A, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Physical parameters affecting ultrasound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med Biol. 2006;32:1269–79. doi: 10.1016/j.ultrasmedbio.2006.04.014. [DOI] [PubMed] [Google Scholar]
- RxMed. Levovist package insert. 1999. [Google Scholar]
- Schlicher RK, Radhakrishna H, Tolentino TP, Apkarian RP, Zarnitsyn V, Prausnitz MR. Mechanism of Intracellular Delivery by Acoustic Cavitation. Ultrasound in Med and Biol. 2006;32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- Schneider M. SonoVue, a new ultrasound contrast agent. European Radiology. 1999;9:S347–S348. doi: 10.1007/pl00014071. [DOI] [PubMed] [Google Scholar]
- Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364–9. doi: 10.1007/s12031-010-9451-9. [DOI] [PubMed] [Google Scholar]
- Shi WT, Forsberg F, Vaidyanathan P, Tornes A, Ostensen J, Goldberg BB. The influence of acoustic transmit parameters on the destruction of contrast microbubbles in vitro. Phys Med Biol. 2006;51:4031–4045. doi: 10.1088/0031-9155/51/16/010. [DOI] [PubMed] [Google Scholar]
- Subramanian IV, Devineni S, Ghebre R, Ghosh G, Joshi HP, Jing Y, Truskinovsky M, Ramakrishnan S. AAV-P125A-endostatin and paclitaxel treatment increases endoreduplication in endothelial cells and inhibits metastasis of breast cancer. Gene Therapy. 2011;18:145–154. doi: 10.1038/gt.2010.118. [DOI] [PubMed] [Google Scholar]
- Vassernan AA, Kazavchinskii YZ, Rabinovich VA. Thermophysical Properties of Air and Air Components. Moscow, Nauka: 1966. [Google Scholar]
- Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, de Jong N. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–55. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ward M, Wu J, Chiu JF. Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J Acoust Soc Am. 1999;105:2951–2957. doi: 10.1121/1.426908. [DOI] [PubMed] [Google Scholar]
- Wu J, Pepe J, Rincon M. Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics. 2006;44:e21–e25. doi: 10.1016/j.ultras.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Yeh CK, Su SY. Effects of acoustic insonation parameters on ultrasound contrast agent destruction. Ultrasound Med Biol. 2008;34:1281–1291. doi: 10.1016/j.ultrasmedbio.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Zderic V, Vaezy S, Martin RW, Clark JI. Ocular drug delivery using 20-kHz ultrasound. Ultrasound Med Biol. 2002;28:823–829. doi: 10.1016/s0301-5629(02)00515-x. [DOI] [PubMed] [Google Scholar]
- Zhao Y-Z, Gao H-S, Zhou Z-C, Tang Q-Q, Lu C-T, Jin Z, Tian J-L, Xu Y-Y, Tian X-Q, Wang L, Kong F-L, Li X-K, Huang P-T, He H-L, Wu Y. Experiment on the factors for enhancing the susceptibility of cancer cells to chemotherapeutic drug by ultrasound microbubbles. Journal of Drug Targeting. 2010;18:430–437. doi: 10.3109/10611860903434043. [DOI] [PubMed] [Google Scholar]