Abstract
Enterococci, are used nationwide as a water quality indicator of marine recreational beaches. Prior research has demonstrated that enterococci inputs to the study beach site (located in Miami, FL) are dominated by non-point sources (including humans and animals). We have estimated their respective source functions by developing a counting methodology for individuals to better understand their non-point source load impacts. The method utilizes camera images of the beach taken at regular time intervals to determine the number of people and animal visitors. The developed method translates raw image counts for weekdays and weekend days into daily and monthly visitation rates. Enterococci source functions were computed from the observed number of unique individuals for average days of each month of the year, and from average load contributions for humans and for animals. Results indicate that dogs represent the larger source of enterococci relative to humans and birds.
Keywords: Indicator microbe organism, Enterococcus, recreational water quality, bather shedding, animal fecal load
1. Introduction
Measurements of fecal indicator bacteria in bathing waters are used to assess the microbiological safety of water contact at recreational beach sites. The fecal indicator bacteria currently recommended by the U.S. Environmental Protection Agency (EPA) for monitoring recreational marine beaches is enterococci. Beach advisories or closures are generally issued by local regulators when the measured enterococci levels exceed a specific threshold level (i.e. 104 CFU/100ml for single sample analyses, or a geometric mean of 35 CFU/100ml for multiple samples; U.S. EPA, 1986). These guideline levels for issuing beach advisories are based upon studies (Cabelli 1983) conducted at beaches impacted by point sources of sewage in temperate climates (e.g. sewer outfall). However, many beaches exist that are not impacted by point sources of pollution. Non-point sources at beaches include enterococci from human shedding during bathing activities (Elmir et al. 2007; Elmir et al. 2009), and from the feces deposited by animals (such as birds or dogs) which frequent the beach (Oshiro and Fujioka 1995; Jones and Obiri-Danso 1999; Haack et al. 2003; Kühn et al. 2003; Cox et al. 2005; DeGraef et al. 2005; Wither et al. 2005; Edge and Hill 2007; Wright et al. 2009). Moreover, loading from animals (e.g. birds and dogs) potentially results in different pathogen contributions in comparison to human sewage; therefore, the relationship between enterococci levels and human health may be different at beaches impacted by non-point sources compared to point source beaches (WERF 2009). Thus, when assessing enterococci data from a beach site, in particular a beach site characterized by non-point sources of pollution, the relative contribution of enterococci from different sources would be helpful when interpreting enterococci measurements with respect to the need for issuing beach advisories.
One approach for assessing different enterococci contributions is to compute the loads from the various non-point sources. Enterococci loads can be computed as the product of the “counts” of individual events and the enterococci load (CFU) per event. Very few studies have utilized “counts” at beaches to estimate enterococci loads. Grant et al. (2001) evaluated bird counts to estimate enterococci loads from a coastal wetland within southern California. Brinks (2009) compiled human visitation counts from lifeguard records to estimate risks associated with bathing at beaches. The focus of the current study was to illustrate the use of camera image data to provide counts of human bathing events and dog and bird visitations. This count information was combined with enterococci load information (Wright et al. 2009) to establish the relative contribution of different non-point sources to the study beach.
2. Study site
The study beach site is approximately 1600 m long and faces southwest towards Biscayne Bay in Miami-Dade County (Fig. 1). It is a low wave-energy beach as prevailing winds are in an offshore direction. Tides are semi-diurnal with an average range of 0.6 m. The beach is open to the public all year round, easily accessible by car, and the only county beach that allows pets. There are no point sources located in the vicinity of the beach (Fleming et al. 2004; Shibata et al. 2004), leaving human bathers and animals among the major non-point sources responsible for enterococci inputs to the beach. Human bathers have been found to shed enterococci when entering the water (Elmir et al. 2007; Elmir et al. 2009). At the study beach, dogs are frequent visitors in the company of humans, and birds are particularly numerous during winter months; thus, animal fecal events are also a potential source for enterococci contamination (Wright et al. 2009).
Figure 1.
Beach site. The arrow on the Florida map indicates the location of the beach. The x at the southeast end of the beach shows where the camera was set up.
3. Camera setup
A digital camera (Olympus Z8080) was installed in an environmentally protected housing with pan and tilt capability overlooking the southeastern 1/3 (550 m) of the beach. Under computer (PC) control, the camera took three adjacent 8 Mb (3264 × 2442) pixel images as a panorama of the beach every 15 min during daylight hours. During the night, the camera was shut off because the light would have been insufficient, and thus information was not available about whether humans or animals were present at night. However, the County routinely closed beach access at night, and therefore human and dog presence would be unlikely. The images were saved onto a computer magnetic storage disk for later retrieval. The camera control software (Erdman Video Systems, http://www.video-monitoring.com/) allowed complete control of camera functions, pan and tilt settings and scheduling of image taking.
To maintain image focus, care was taken to include the shoreline near the center portion of the images in order for the camera's automatic focusing mechanism to work properly. The evaluative metering mode of the camera was used for exposure settings. Since the processing and storage of an image required approximately 30 sec, the three images making up one panorama spanned 1 min. An example panorama for February 1, 2008 at 14:47 EST is shown in Fig. 2a. A full size cutout from the left image, indicated by the square, is shown in Fig. 2b to exhibit the resolution achieved. Due to the camera location at the southeastern end, a panorama covered approximately the southern 550 m of the beach.
Figure 2.


Sample panorama (a) with a full-size blow-up of a section (b) from February 1, 2008, 14:47EST
Images were collected from 2005 through 2008, although there were several gaps due to service or power failures. Nevertheless, during the course of the 3 years of camera operation, images for each day of the year were collected and available for analysis.
4. Image Analysis
An input form was created in Microsoft Access to facilitate data entry, and lists were created in input boxes whenever possible to minimize data entry errors. The manual analysis of images consisted of visually recording the number of humans and animals, and the environmental conditions, as shown in Figure 3, in which all the categories are displayed. For consistency, one person counted all of the images used in this study. On the average 2 to 3 min were spent on an image, although the numbers of humans or animals in an image greatly affected the processing time. Even though environmental conditions were recorded at the same time, these data were not used in the current study.
Figure 3.
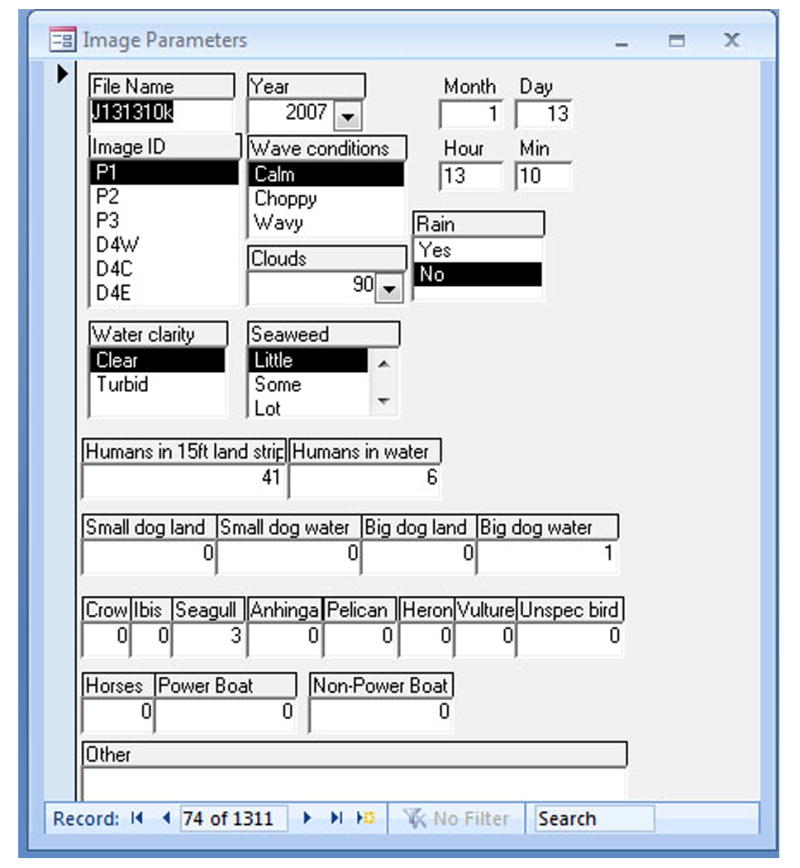
Database form (Microsoft Access) showing categories used in image analysis
The sheer number of images (i.e. 53,560 per year) precluded analyzing every one. Instead a method was devised to determine average intra-day distributions of humans and animals and average daily visitations for each month based on the analysis of a subset of the images. In this approach, first estimates of average intra-day distributions were determined by evaluating images hourly over a period of a week during each of four seasons. Next, once-a-day counts were determined for week days and weekend days of every month of the year. Finally, the intra-day distribution and the once-a-day counts were combined to determine average daily counts for each month.
Average intra-day distributions (i.e. the variation in counts throughout the course of a day) were established by separating a day into 4 time periods (i.e. 7:00 – 11:00, 11:01 – 13:00, 13:01 – 15:00, and 15:01 – 19:00). Hourly images for one week (a total of 252 images) during each season of the year (specifically, weeks in March, June, September and December were selected) were then analyzed for each time period to estimate the intra-day distribution for a given season. Average once-a-day counts for each month were estimated separately for week days and weekend days because the weekend days were expected to have substantially more visitors. For this purpose, counts were averaged from images closest to and between 13:00 and 14:00 for all Tuesdays and Thursdays in a month, representing a week day, and for all Saturdays and Sundays in a month, representing a weekend day. Approximately 9 Tuesdays and Thursdays were selected, each with 6 analyzed images (3 images in a panorama at 2 times), yielding a total of 54 images which were averaged to obtain the monthly once-a-day count corresponding to the 3rd time period (13:01 to 15:00) of an average week day. Saturdays and Sundays were averaged analogously to obtain the monthly once-a-day (period 3) count for weekend days. Therefore, the total number of images evaluated was reduced to 2304 (= 4∗252 + 12∗2∗54).
Since the once-a-day counts corresponded to period 3, these values were then scaled to obtain the counts for other periods of the day, i, in a given month, m, during an average week day as follows:
| (1) |
where Ni was the intra-day count for time period i = 1, .. , 4; Nm was the average once-a-day week day count for month m, N3 was the period 3 (13:01 – 15:00) count, and Nmi was the monthly average count for time period i. An analogous procedure was followed to obtain average weekend day counts for a given month. The described method assumed that the weekday or weekend day count in time period 3 (13:01 – 15:00) could be extrapolated to values in other time periods using the intra-day distribution. This assumption would fail if some animals only appeared on the beach at other time periods than period 3. For the most numerous animals on this beach, the images were checked, and it was found that the assumption was reasonably satisfied for humans, big dogs and seagulls, which were therefore the only objects included for further analysis.
Finally to obtain average-daily counts for week days or weekend days for each month, the respective Nmi were averaged over the four periods of the day taking into account the duration of each time period as expressed in eq. (2):
| (2) |
Nma was the average-daily count for a given month with different values for week days and weekend days, and the numerical factors (4 and 2) in equation (2) correspond to the number of hours associated with each period and the denominator (12) corresponds to the total number of hours evaluated per day. This method assumed that averaging over a week for intra-day distributions and averaging over a month for daily counts sufficiently and uniformly sub-sampled the environmental conditions (for example, due to clouds, rain and tides), which might impact the number of individuals who visited the beach.
5. Visitation and Loads from Individuals, Methods
The daily average counts represented average numbers as seen by the camera. However, in order to determine the bather enterococci shedding loads, the number of unique individuals that actually visited the beach (i.e. individual visitation) had to be determined because the camera images taken every 15 minutes could show repeated instances of the same individuals.
5.1 Human Bathers
As the raw count of bathers in a picture only recorded how many people were in the water in that instant, two subsequent pictures of the same stretch of the beach might show some repeat and some new bathers. The estimation of the bather shedding load of enterococci depended on the number of new bathers, and therefore it was important to determine the actual bather visitation rate. Although the careful identification of individuals in the images could allow discrimination between repeat and new bathers, such analysis would be difficult and require much additional effort and time. Instead, the visitation rates were determined using additional data consisting of: the length of time a person spent at the beach, the duration spent in the water, and the number of bathing cycles (water entries). Since the main objective of this study was to illustrate the method, an informal survey of 23 study participants was taken of their bather behavior and used for this purpose. This bather behavior information was likely specific to the local area, and could vary with climatic region.
With the obtained average counts and bather behavior data, the visitation rates for human bathers were determined as follows. The average raw count of human bathers in the water was multiplied by the duration of the corresponding time period (recall that the day was separated into 4 time periods), and divided by the total time a person spent in the water. The resulting number represented the total number of unique bathers in that time period. For example, if the average instantaneous raw number of people in the water during the second time period (11:01 – 13:00) was 10, then during this time period, there were on average 10 people in the water at any instant of time. If the average time a bather spent in the water was 1 hour, there would be 20 unique bathers (10 raw count number ∗ 2 hr / 1 hr) in that time period. Since intra-day bather behavior information was not collected, the same behavior was assumed throughout the day. Thus, the same logic could be applied to evaluate the average number of new bathers per hour which was calculated by dividing the daily average bather count by the total time a person spent in the water.
The average number of enterococci shed by a bather was determined by Elmir et al. (2007; 2009). Their study exposed bathers to seawater in a controlled pool environment and measured the numbers of enterococci shed by sampling the pool water and applying the standard membrane filtration culture method. The researchers found that a bather shed enterococci on every re-entry into the water, which made it necessary to know the number of bathing cycles (from land to water) per bather which was information collected as part of the bather behavior survey. They also found that the number of enterococci shed depended on the bathing cycle for a person (i.e. the shedding during the first cycle was substantially greater than during the next cycle and so on), as well as whether there was body contact with sand or not prior to bathing.
Given the individual loading information and the number of bather individuals, the shedding load could be estimated.
5.2 Dogs
The approach used to determine the raw counts for dogs followed analogously the human approach using the intra-day distribution with the once-a-day counts to determine monthly average-daily numbers on a monthly basis. Since information analogous to the bather behavior was not obtained for animal behavior, their visitation rates had to be determined in a different manner. For dogs, visitation was obtained by summing average-daily counts for dogs in water and on land; then, assuming dogs would be subject to the behavior of their handlers, this instantaneous count was divided by the total beach stay duration of human visitors and finally multiplied by 12 (hours in a day).
Some dogs were observed by the researchers to have fecal events in the water, a type of event amenable to load quantification using image counts. From a parallel study (Wright et al. 2009), the average enterococci loading from a dog fecal event was 5.6 109 and 1.5 108 CFU for big (> 9 kg) and small dogs (< 9 kg), respectively. The camera images captured too few small dogs in the water to apply the described methodology, and therefore only big dogs were considered in the following. However, a rough estimate showed that the load from small dogs was at least an order of magnitude less than from big dogs due to the smaller load from a single event and the smaller number of small dogs.
It was assumed (since no statistics were available) that 50% of all dogs at the beach had a fecal event, and that the observed ratio of dogs in water to the total number of dogs at the beach (sum of dogs in water plus dogs on land) also applied to where their fecal events took place. Thus loads were computed as the product of the total dog visitation per day, 50% for the fraction of dogs with fecal events at the beach, the ratio of average daily dog count in water to total dogs in water and land, and 5.6 109 CFU per fecal event.
5.3 Seagulls
Amongst the birds, the only type of bird appearing consistently in large numbers was the seagull. During seasons outside of the summer, seagulls were often observed on the beach in substantial numbers at the water's edge or on emerging sandy banks during low tide. Seagulls generally flocked together, and the number of birds observed in a time period was simply used as representative of the number of individual birds. Because of a much higher rate of fecal events for birds, there was less need to determine the number of new birds. Additionally, seagull counts were assumed to be similar for weekend and week days. Thus, loads were computed by averaging both the weekend and week day average daily counts, which were then assumed to represent the hourly average daily seagull fecal event count (i.e. assuming one fecal event per hour). This quantity was then multiplied by 12 to obtain the total number of seagull fecal events per day.
Observations determined a wide range of enterococci per fecal event from 53 CFU (Wright et al. 2009) to several thousand. For this load calculation 1200 CFU per fecal event was used. The total load could be expected to enter the water with the rising tide at some time during the day.
6. Results
6.1 Image counts
Intra-day distributions for one season (e.g. for September as given in Table 1) showed the average counts of humans and/or animals at the beach at any one instant in time within the indicated time period of the day.
TABLE 1.
Intra-day counts by time period and source for September. Average daily count is a weighted average of the counts from all time periods weighted by the length of the time period.
| Time Period |
Human Land |
Human Water |
Big Dog Land |
Big Dog Water |
Seagull |
|---|---|---|---|---|---|
| 1 (7:00-11:00) | 4.30 | 1.39 | 0.10 | 0.07 | 15.20 |
| 2 (11:01-13:00) | 13.95 | 7.13 | 0.23 | 0.50 | 3.00 |
| 3 (13:01-15:00) | 17.82 | 11.42 | 0.34 | 0.40 | 3.60 |
| 4 (15:01-19:00) | 20.11 | 10.30 | 0.27 | 0.36 | 6.50 |
| Average Daily Count | 13.43 | 6.98 | 0.22 | 0.29 | 8.30 |
For September, humans visited the beach in greater numbers in the afternoon, while dogs were the most frequent in the water during the middle of the day (they were also most frequent on land during periods 2 and 3). The seagulls tended to be present at the beach predominately in the morning and late afternoon while during the middle of the day they were elsewhere, possibly foraging at sea. Intra-day distributions for humans in the water exhibited seasonal variations (Fig. 4). For easier comparison, the period counts were normalized by dividing by the corresponding average daily counts (listed in the figure legend) before plotting, The intra-day trends were similar for all seasons with bathers favoring time period 3 (13:00 to 15:00), especially during the colder seasons of the year.
Figure 4.
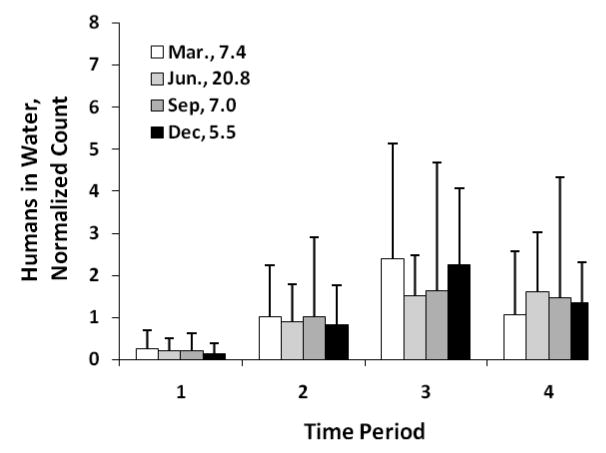
Intra-day distribution of humans in water by period of day and season. Error bars correspond to one standard deviation. The normalized numbers are the ratios of the period count divided by the average daily count (listed in the figure legend). Period 1 corresponds to 7:00 to 11:00, period 2 to 11:01 to 13:00, period 3 to 13:01 to 15:00, and period 4 to 15:01 to 19:00.
The intra-day distribution for dogs (Fig. 5) also showed higher counts during the noon and afternoon hours, although, generally there was less variation over the length of the day. A missing bar in the graphs was due to an exact count of zero. The seagulls (Fig. 6) on the other hand, showed distinctly higher counts during time periods 1 (7:00 to 11:00) and 4 (15:01 to 19:00), while very few birds used the beach during periods 2 and 3.
Figure 5.
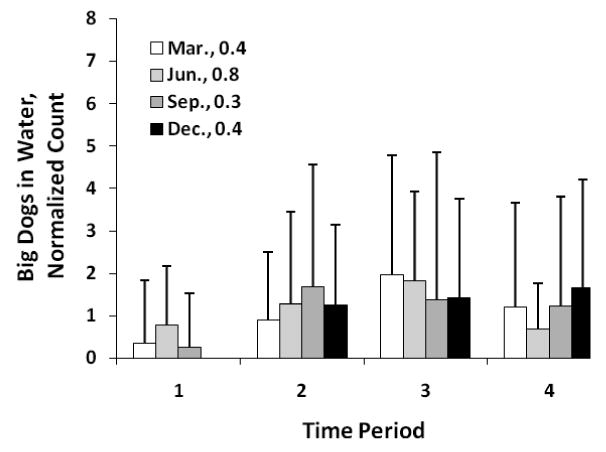
Intra-day distribution of big dogs (>9 kg) in water by period of day and season. Error bars and normalization as for Fig. 4.
Figure 6.
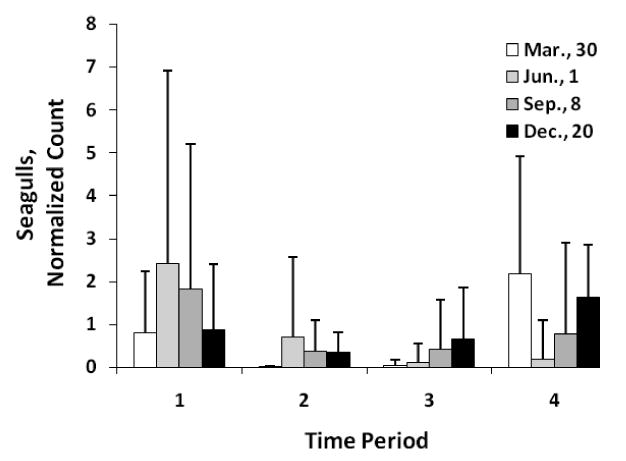
Intra-day distribution of total seagulls by period of day and season. Error bars and normalization as for Fig. 4
The raw human-bather count values in table 2 represented the averages of the once-a-day raw counts of humans in the water from images (Nm in eqs (1) and (2)). The daily average count in table 2 represented the average over the day of the number of people in the water (Nma in eq. (2)). If desired, the average raw counts for any of the 4 time periods of the day can be derived, using eqs. (1) and (2) and the numbers in tables 1 and 2. For example, for a September weekend day, the average once-a-day count in time period 3 would be Nm = 45.5, while the counts in each of the 4 time periods would be: N1=5.5, N2=28.4, N3=45.5, and N4=41.0. The average once-a-day raw human bather counts for each month along with the computed daily-average bather counts (table 2) showed significantly higher values (by a factor of 10) between summer and winter seasons. Moreover weekend days were observed to have more human counts (by about a factor of 2 to 10) than week days.
TABLE 2.
Average daily human-bather count by month as scaled from once-a-day raw human bather counts.
| WEEK DAY | WEEKEND DAY | |||
|---|---|---|---|---|
| Month | Raw Human Bather Count | Average Daily Count | Raw Human Bather Count | Average Daily Count |
| January | 1.6 | 0.72 | 6.9 | 3.02 |
| February | 1.4 | 0.60 | 4.5 | 1.92 |
| March | 3.8 | 1.58 | 40.6 | 16.97 |
| April | 9.9 | 4.71 | 60.7 | 28.94 |
| May | 4.5 | 2.47 | 52.8 | 29.30 |
| June | 11.2 | 7.41 | 42.2 | 28.00 |
| July | 22.4 | 14.48 | 47.9 | 30.91 |
| August | 10.3 | 6.46 | 48.9 | 30.71 |
| September | 5.3 | 3.25 | 45.5 | 27.83 |
| October | 4.0 | 2.18 | 33.9 | 18.47 |
| November | 1.6 | 0.79 | 4.1 | 2.01 |
| December | 4.5 | 2.00 | 3.4 | 1.52 |
Results of the bather behavior survey were listed in table 3. The average total time (and corresponding water time) spent by an individual at the beach varied from a low of 1.30 hours (0.13 hours) in January to a high of 2.90 hours (0.98 hours) in July. The average number of water entries or bathing cycles per individual increased from 0.41 in January to 2.42 in July.
TABLE 3.
Beach-goer average behavior by month. Total time represents the total time spent at the beach which is the sum of the time spent in the water plus the time spent on land. The length of the bathing cycle was calculated as the time in the water divided by the number of cycles.
| Month | Total Time (hours) | Time in Water (hours) | Number of Bathing Cycles |
|---|---|---|---|
| January | 1.30 | 0.13 | 0.41 |
| February | 1.64 | 0.27 | 0.46 |
| March | 1.99 | 0.41 | 0.50 |
| April | 2.33 | 0.55 | 0.55 |
| May | 2.52 | 0.69 | 1.17 |
| June | 2.71 | 0.84 | 1.80 |
| July | 2.90 | 0.98 | 2.42 |
| August | 2.71 | 0.84 | 1.80 |
| September | 2.52 | 0.69 | 1.17 |
| October | 2.33 | 0.55 | 0.55 |
| November | 1.99 | 0.41 | 0.50 |
| December | 1.64 | 0.27 | 0.46 |
Monthly human visitation (which takes into account the fact that the same individual may be observed in subsequent images) showed the effects of holidays, which appeared to explain the higher count values in December-January (Holiday Season) and March-April (School Spring Break) relative to adjacent months (table 4). The relatively low counts in June, compared to spring, may have been caused by the beginning of the rainy season, as June typically is the wettest month of the year in Miami, FL.
TABLE 4.
New bather individual visitation and total load
| Month | Bathing Cycles | Bather Individual Visitation in One Hour | Total Bather Individual Visitation in One Day | Total Load (CFU/day) | |||
|---|---|---|---|---|---|---|---|
| Week | Weekend | Week | Weekend | Week | Weekend | ||
| January | 0.41 | 5.6 | 23.7 | 67.4 | 284 | 1.58 107 | 6.65 107 |
| February | 0.46 | 2.2 | 7.2 | 27.0 | 86 | 7.03 106 | 2.25 107 |
| March | 0.50 | 3.9 | 41.5 | 46.4 | 498 | 1.33 107 | 1.43 108 |
| April | 0.55 | 8.6 | 52.7 | 102.9 | 632 | 3.23 107 | 1.99 108 |
| May | 1.17 | 3.6 | 42.2 | 42.7 | 507 | 2.65 107 | 3.14 108 |
| June | 1.80 | 8.8 | 33.4 | 106.1 | 401 | 8.46 107 | 3.20 108 |
| July | 2.42 | 14.7 | 31.5 | 176.9 | 378 | 1.63 108 | 3.49 108 |
| August | 1.80 | 7.7 | 36.6 | 92.4 | 440 | 7.37 107 | 3.51 108 |
| September | 1.17 | 4.7 | 40.1 | 56.2 | 481 | 3.49 107 | 2.99 108 |
| October | 0.55 | 4.0 | 33.6 | 47.6 | 404 | 1.50 107 | 1.27 108 |
| November | 0.50 | 1.9 | 4.9 | 23.3 | 59 | 6.68 106 | 1.70 107 |
| December | 0.46 | 7.5 | 5.7 | 89.6 | 68 | 2.34 107 | 1.78 107 |
In order to calculate the bather shedding load, the average numbers of enterococci shed as a function of bathing cycle were obtained in the study of Elmir et al. (2007) and are listed in table 5. The shedding from the first cycle at 571,000 CFU was twice as large as from the second cycle, and again a factor of approximately two larger than from the third cycle.
TABLE 5.
Average bather enterococci shedding per bathing cycle
| 1st Cycle Load(CFU) | 2nd Cycle Load (CFU) | 3rd Cycle Load (CFU) |
|---|---|---|
| 5.71 105 | 2.84 105 | 1.65 105 |
The raw average daily dog counts and total individual dog counts (table 6) showed, as expected, higher values during the weekend days in comparison to the week days. The months of March and April showed the highest number of dogs at the beach and these numbers could be a result of spring break school vacations during this time of year, which resulted in more people and particularly dog use of the beach. There was a dramatic shift of dog counts from land to water during weekends in June and July, although the total numbers of dog visitations were approximately the same for week and weekend days. This was indicative of different usage patterns with more families and cookouts on weekends.
TABLE 6.
Big dog counts, visitation, and fecal loads.
| Month | Avg. Daily Count - :Land | Avg Daily Count - Water | Total Dog Visitation in One Day | Load to Water (CFU/day) | ||||
|---|---|---|---|---|---|---|---|---|
| Week | Weekend | Week | Weekend | Week | Weekend | Week | Weekend | |
| January | 0.33 | 0.41 | 0.12 | 0.44 | 4.15 | 7.77 | 3.22 109 | 1.13 1010 |
| February | 0.43 | 0.51 | 0.17 | 0.22 | 4.33 | 5.36 | 3.43 109 | 4.57 109 |
| March | 0.27 | 0.80 | 0.36 | 0.96 | 3.76 | 10.68 | 6.01 109 | 1.63 1010 |
| April | 0.50 | 0.83 | 0.31 | 0.78 | 4.17 | 8.29 | 4.51 109 | 1.13 1010 |
| May | 0.23 | 0.54 | 0.27 | 0.21 | 2.38 | 3.59 | 3.57 109 | 2.85 109 |
| June | 0.43 | 0.07 | 0.16 | 1.10 | 2.65 | 5.19 | 2.05 109 | 1.36 1010 |
| July | 0.49 | 0.28 | 0.48 | 0.84 | 4.02 | 4.64 | 5.57 109 | 9.74 109 |
| August | 0.41 | 0.48 | 0.73 | 0.66 | 5.03 | 5.04 | 9.02 109 | 8.20 109 |
| September | 0.40 | 0.60 | 0.29 | 0.66 | 3.29 | 5.99 | 3.92 109 | 8.83 109 |
| October | 0.14 | 0.35 | 0.22 | 0.43 | 1.84 | 4.03 | 3.13 109 | 6.26 109 |
| November | 0.15 | 0.29 | 0.00 | 0.14 | 0.89 | 2.64 | 0.00 | 2.41 109 |
| December | 0.16 | 0.47 | 0.07 | 0.07 | 1.65 | 3.92 | 1.43 109 | 1.43 109 |
Seagulls were most plentiful during the winter months when the people density was moderate (table 7). Summer months, characterized by the highest people and dog density, were nearly devoid of seagulls. As the seagulls did not appear to mind people very close by, this correlation was likely due to a seasonal pattern related to environmental conditions rather than a causal relationship. Thus, the seagull count was very similar for weekend and week days with a few exceptions. In March which had some of the highest usage rates of the year, the people and dog counts on weekends were much higher than on week days, and the seagull count responded inversely.
TABLE 7.
Seagull counts and fecal loads.
| Month | Week day Avg. Daily Count | Weekend day Avg. Daily Count | Total Daily Load (CFU/day) |
|---|---|---|---|
| January | 47 | 30 | 5.52 105 |
| February | 46 | 42 | 6.37 105 |
| March | 47 | 26 | 5.27 105 |
| April | 12 | 9 | 1.50 105 |
| May | 2 | 2 | 3.16 104 |
| June | 0 | 2 | 1.25 104 |
| July | 4 | 1 | 3.27 104 |
| August | 1 | 0 | 4.43 103 |
| September | 2 | 0 | 1.51 104 |
| October | 14 | 4 | 1.31 105 |
| November | 7 | 12 | 1.40 105 |
| December | 14 | 37 | 3.63 105 |
Since counts were available for humans both on land and in the water, a comparison of behavior as obtained from a survey and from camera image analysis was possible (table 8). The ratio of bathers to total beach visitors, R2, obtained from camera images should closely reflect the ratio, R1, of water time to total time derived from table 3. In general, the agreement was within 20 % (except February which may have been a particularly cold month as the South Florida area experiences weekly passages of cold fronts of various strengths during the winter months), suggesting that this visitor behavior statistic could be derived more reliably directly from image counts.
TABLE 8.
Beach visitor behavior. R1 is ratio of water time to total time. R2 is ratio of bathers to total beach visitors.
| Month | R1 | R2 |
|---|---|---|
| January | 0.10 | 0.12 |
| February | 0.16 | 0.11 |
| March | 0.21 | 0.20 |
| April | 0.24 | 0.26 |
| May | 0.27 | 0.26 |
| June | 0.31 | 0.29 |
| July | 0.34 | 0.40 |
| August | 0.31 | 0.40 |
| September | 0.27 | 0.35 |
| October | 0.24 | 0.31 |
| November | 0.21 | 0.13 |
| December | 0.16 | 0.17 |
6.2 Enterococcus Loads
For humans, the enterococci load (last two columns of table 4), not surprisingly, was substantially greater (up to 12 times in May) during weekend days than on week days, although the difference was smaller during the summer months. The human shedding load was computed at roughly 106 to 107 CFU/day during week days, and 107 to 108 CFU/day during weekend days.
The load for dogs on weekend days was up to 3 times higher than on weekdays (last two columns of table 6), although in summer months there was less difference between weekend and week days. Also for dogs, there seemed to be a “spring-break” effect with the highest load on weekend days in March. The fecal enterococci load for dogs was computed at 109 CFU/day on week days and 109 to 1010 CFU/day on weekend days.
Seagulls were found to have lower overall fecal loads, computed at 103 to 105 CFU per day (table 7). A strong seasonal effect was observed with respect to the seagull contribution, with higher contributions during the winter months and lower contributions during the summer.
7. Discussion
The analysis of camera images of human and animal events on a non point source recreational marine beach provided a detailed enumeration of activities at the beach. This was important since a growing number of recreational beaches have been determined to be without any known point sources; thus, the input from humans and animals can have increased impacts on the evaluation of the enterococci sources and consequently on beach bacterial water quality. The method introduced in this study to reduce the effort of image analysis to obtain distributions of quantitative counts relied on statistical homogeneity for week days or weekend days within a month, such that sub-sampling could be employed. To confirm these assumptions would require that many more images be analyzed, which was beyond the scope of this project. However, similar sub-sampling schemes have been applied successfully in related fields, such as traffic counting. The 25-fold reduction in effort realized by applying this method is substantial. Further reductions may be possible by re-using intra-day and monthly distributions if a later recount is desired or if counts for other beaches in the area are contemplated.
For this study beach, the number of enterococci released (in decreasing order based on magnitude) came from dogs, humans and birds. Due to uncertainty of night time bird use, the estimate of bird visitation numbers could possibly be under-estimated by a factor of two or more, however this would not substantially change the relative ranking.
In order to evaluate the impact on water quality, estimates of near-field water column concentrations from human shedding and dog fecal loading were calculated by using a conservative (small) near-field dilution volume of 1 m3. These estimates showed that the input from a human bather's shedding (table 5) would reduce to concentrations of an order of 10 CFU/100 ml in a matter of minutes, while a similar estimate for one dog fecal event would yield an order of 1000 CFU/100 ml. Thus, dogs were the only animals likely to produce enterococci concentrations high enough to trigger regulatory standards. Similarly, when based on the estimated total daily loads for the 550 m stretch of beach monitored by the camera, the impact from dogs was an order of magnitude greater on average than impact from bather shedding, even on a maximum usage day. The effect of bird feces and normal beach usage bather shedding were several orders of magnitude less than impact from dog feces. With respect to possible health effects, another study on this same beach found that report of human illness was not associated with measures of dog Bacteriodales (a source tracking marker), and as such it was not clear if dog feces in these waters as quantified in our study would have any definitive human health impacts (Sinigalliano et al in press)
7.1 Limitations
In the estimation of dog enterococci loading, only fecal events were considered. It was likely that dogs would shed similar to humans; however, due to their relatively low visitation numbers it seemed reasonable to ignore dog shedding relative to the much larger fecal loading.
Of note, aside from human and animal sources, the other potentially important sources of enterococci on non-point source marine recreational beaches not explored in this study were “run-off” and/or regrowth of the enterococci in the sand and water (U.S. EPA 2007).
Finally, the environmental data collected from the images were not directly used; however, it would seem feasible to use this information to define the effect of weather conditions on variations in counts and behavior in future analyses. Then, camera image analysis could be used to also assess shorter term contributions from the different sources, and with additional data, possibly even input resulting from rain or wind and waves.
8. Conclusions
Monitoring of a beach with an automated camera and subsequent analysis of images provided a robust way of establishing quantitative human and animal usage. Combined with estimates of individual loading from bathers and animals, the obtained counts could be translated into microbial loads. The averaging used in the described camera image analysis methodology was an efficient way to remove the short term variability caused by factors such as weather.
By automating the camera image analysis, there is further potential for near real-time assessment of the different sources by developing algorithms which estimate the enterococci loading given the information provided in a sequence of camera images. Such near real-time analysis could provide useful site information as a context for enterococci and pathogen monitoring data, and if combined with risk analysis, could help in the evaluation of potential health risks associated with the different sources contributing loads to the beach in all types of recreational waters. This, in turn, may assist regulators in making time-sensitive decisions regarding whether or not to limit beach access. Although applied in this study to estimate enterococci loads, the methodology and count results could be applied to other microbes or pathogens if individual load numbers are determined, providing additional information for health risk assessment.
Acknowledgments
The authors would like to dedicate this research to the memory of Ms Seana Campbell, a very talented, hardworking and creative young researcher who enriched all people whose lives she touched, and who died too young.
Contributions from Mary Wright and Michael Schoor – University of Miami, Chip Gaudio – Miami Seaquarium, Dr. Samir Elmir - Miami-Dade County Department of Health, and Chris Weaver and Rusty Erdman - Erdman Video. Special thanks go to Lincong and Jiangang Luo for their painstaking analyses of camera images.
This study was funded in part from the following sources: the National Center for Environmental Health (NCEH), Centers for Disease Control and Prevention (CDC); Florida Dept of Health (FL DOH) through monies from the Florida Dept of Environmental Protection (FL DEP); the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School (NSF 0CE0432368/0911373; NIEHS 1 P50 ES12736) and NSF REU in Oceans and Human Health, and the NSF SGER (NSF SGER 0743987) in Oceans and Human Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wang John D, Email: jwang@rsmas.miami.edu.
Solo-Gabriele Helena M, Email: hmsolo@miami.edu.
Abdelzaher Amir M, Email: a.zaher@umiami.edu.
Fleming Lora E, Email: lfleming@med.miami.edu.
References
- Brinks M. The health risk of bathing in southern California coastal waters. Abstracts of the National Beaches Conference held in Huntington Beach, CA; Washington D.C.. 2009. p. 119. [Google Scholar]
- Cabelli V. U.S. EPA Report EPA-600/1-80-031. Cincinnati, OH: US Environmental Protection Agency; 1983. Health effects criteria for marine recreational waters. [Google Scholar]
- Cox P, Griffith M, Angles M, Deere D, Ferguson C. Concentrations of pathogens and indicators in animal feces in the Sydney watershed. Applied and Environmental Microbiology. 2005;71(10):5929–5934. doi: 10.1128/AEM.71.10.5929-5934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graef EM, Devriese LA, Baele M, Vancanneyt M, Swings J, Haesebrouck F, Decostere A. Identification of enterococcal, streptococcal and Weissella species in the faecal flora of individually owned dogs. Journal of Applied Microbiology. 2005;99(2):348–353. doi: 10.1111/j.1365-2672.2005.02588.x. [DOI] [PubMed] [Google Scholar]
- Edge TA, Hill S. Multiple lines of evidence to identify the sources of fecal pollution at a freshwater beach in Hamilton Harbour, Lake Ontario. Water Research. 2007;41(16):3585–3594. doi: 10.1016/j.watres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Elmir SM, Wright ME, Abdelzaher A, Solo-Gabriele HM, Fleming LE, Miller G, Rybolowik M, Shih P, Pillai SP, Cooper JA, Quaye EA. Quantitative evaluation of bacteria released by bathers in a marine water. Water Research. 2007;41(1):3–10. doi: 10.1016/j.watres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmir SM, Shibata T, Solo-Gabriele HM, Sinigalliano CD, Gidley ML, Miller G, Plano LRW, Kish J, Withum K, Fleming LE. Quantitative Evaluation of Enterococci and Bacteroidales Released by Adults and Toddlers in Marine Water. Water Research. 2009;43(18):4610–4616. doi: 10.1016/j.watres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Solo-Gabriele H, Elmir S, Shibata T, Squicciarini D, Jr, Quirino W, Arguello M, Van de Bogart G. A Pilot Study of Microbial Contamination of Subtropical Recreational Waters. Fl J Env Health. 2004 March;:29–33. [PMC free article] [PubMed] [Google Scholar]
- Grant SB, Sanders BF, Boehm AB, Redman JA, Kim JH, Mrše RD, Chu K, Gouldin M, McGee CM, Gardiner NA, Jones BH, Svejkovsky J, Leipzig GV, Brown A. Generation of enterococci bacteria in a coastal saltwater marsh and its impact on surf zone water quality. Environmental Science and Technology. 2001;35(12):2407–2416. doi: 10.1021/es0018163. [DOI] [PubMed] [Google Scholar]
- Haack SK, Fogarty LR, Wright C. Escherichia coli and enterococci at beaches in the Grand Traverse Bay, Lake Michigan: sources, characteristics, and environmental pathways. Environmental Science and Technology. 2003;37(15):3275–3282. doi: 10.1021/es021062n. [DOI] [PubMed] [Google Scholar]
- Jones K, Obiri-Danso K. Non-compliance of beaches with the EU directives of bathing water quality: evidence of non-point sources of pollution in Morecambe Bay. Journal of Applied Microbiology Symposium Supplement. 1999;85:101S–107S. doi: 10.1111/j.1365-2672.1998.tb05288.x. [DOI] [PubMed] [Google Scholar]
- Kühn I, Iversen A, Burman LG, Olsson-Liljequist B, Franklin A, Finn M, Aarestrup F, Seyfarth AM, Blanch AR, Vilanova X, Taylor H, Caplin J, Moreno MA, Dominguez L, Herrero IA, Möllby R. Comparison of enterococcal populations in animals, humans, and the environment – a European study. International Journal of Food Microbiology. 2003;88(2-3):133–145. doi: 10.1016/s0168-1605(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Oshiro R, Fujioka R. Sand, soil, and pigeon droppings: sources of indicator bacteria in the waters of Hanauma Bay, Oahu, Hawaii. Water Science Technology. 1995;31(5-6):251–254. [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming LE, Elmir S. Monitoring Marine Recreational Water Quality Using Multiple Microbial Indicators in an Urban Tropical Environment. Water Research. 2004;38(13):3119–3131. doi: 10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigalliano CD, Fleisher JM, Gidley ML, Solo-Gabriele HM, Shibata T, Plano LRW, Elmir SM, Wang JD, Wanless D, Bartowiak J, Boiteau R, Withum K, Abdelzaher A, He G, Ortega C, Zhu X, Wright M, Kish J, Hollenbeck J, Backer LCl, Fleming LE. Traditional and Molecular Analyses for Fecal Indicator Bacteria in Non-point Source Subtropical Recreational Marine Waters. Water Research. 200X doi: 10.1016/j.watres.2010.04.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Ambient Water Quality Criteria for Bacteria - 1986. Washington, DC: US EPA; 1986. EPA440/5-84-002. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Report of the Experts Scientific Workshop on Critical Research Needs for the Development of New or Revised Recreational Water Quality Criteria. Washington, DC: 2007. EPA 823-R-07-006, http://www.epa.gov/waterscience/criteria/recreation/. [Google Scholar]
- Water Environment Research Foundation (WERF) Report of the Experts Scientific Workshop on Critical Research and Science Needs for the Development of Recreational Water Quality Criteria for Inland Waters. Alexandria, VA: 2009. [Google Scholar]
- Wither A, Rehfisch M, Austin G. The impact of bird populations on the microbiological quality of bathing waters. Water Science and Technology. 2005;51(3/4):199–208. [PubMed] [Google Scholar]
- Wright ME, Solo-Gabriele HM, Elmir S, Fleming LE. Microbial Load from Animal Feces at a Recreational Beach. Marine Pollution Bulletin. 2009;58(11):1649–1656. doi: 10.1016/j.marpolbul.2009.07.003. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]



