The concept of ‘β-cell rest’, or suppression of insulin release from β-cells, was originally developed in the context of type 1 diabetes mellitus. Clinicians noted that the initiation of insulin therapy in newly diagnosed patients often led to partial remission, the so-called ‘honeymoon period’, which is characterized by increased endogenous insulin secretion and reduced exogenous insulin requirements. This was first formally documented by Jackson et al. in 1940, who reported ‘a regimen of control designed to approximate normal conditions of metabolism’, which markedly decreased insulin requirements in children with type 1 diabetes (1). The first controlled clinical trial of β-cell rest was performed in the 1970’s, when Mirouze et al. reported increased ‘remission’ rates in patients treated with intensive vs. conventional insulin therapy (2). These and other observations generated the hypothesis that decreased demand on β-cells can improve insulin secretion and β-cell viability. This concept was subsequently expanded into the field of type 2 diabetes (3). In the context of this review, we use the term β-cell rest ‘inclusively’, meaning that the agents used to induce β-cell rest may have many additional beneficial effects on glucose metabolism beyond their direct action on β-cells. Most obvious is the case of exogenous insulin, which ameliorates glucotoxicity and simultaneously reduces endogenous insulin secretion – effects that are difficult to tease apart, especially in the clinical setting. Another important distinction is difficult or impossible to make in the clinical arena: whether improved β-cell function is observed because of more work performed by existing β-cells or by a greater number including new β-cells.
Pathophysiology of beta-cell dysfunction in diabetes
Both type 1 and type 2 diabetes are characterized by progressive β-cell loss, resulting in an increased insulin secretory demand on the remaining β-cells. In type 1 diabetes, β-cell destruction is caused by an ongoing autoimmune process. In type 2 diabetes, β-cell mass is also decreased relative to non-diabetic controls matched for body mass index. While the underlying β-cell defect in type 2 diabetes remains unknown and is under intense investigation, it is clear that insulin resistance leading to increased insulin demand and β-cell overstimulation plays an important role. With the rising prevalence of overweight and obesity in the general population, insulin resistance may also accelerate the progression toward overt type 1 diabetes in individuals prone to autoimmune diabetes as increased workload of β-cells has been associated with augmented antigenicity (4). Recent studies provide good evidence for a link between nutrition, lipids, and subsequent inflammation and activation of the immune system (5, 6).
Oxidative stress is another important factor resulting in β-cell dysfunction, as β-cells are highly sensitive to the detrimental effects of reactive oxygen species (ROS). This may be, in part, because of a relative deficiency in antioxidant enzymes such as glutathione peroxidase, superoxide dismutase, and catalase, which normally help to reduce ROS. Particularly in type 2 diabetes, exposure to high levels of glucose and free fatty acids likely increases oxidative stress in the β-cells, contributing to impaired insulin secretion, reduced β-cell proliferation and to increased β-cell apoptosis (7, 8).
Mechanisms of beta-cell rest
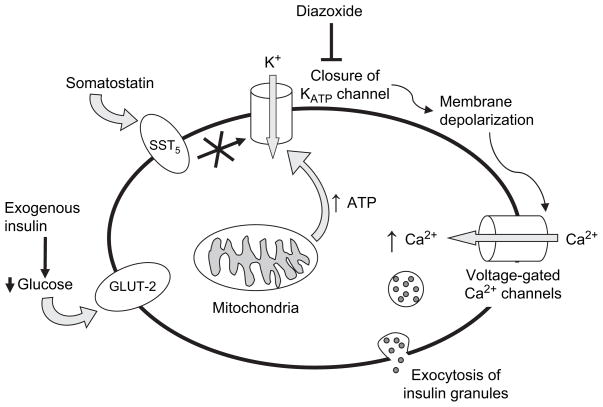
Exogenous insulin is the agent most frequently used to induce β-cell rest. Its effect on normalization of blood glucose greatly diminishes the insulin secretory demand, thus decreasing endogenous insulin production (Fig. 1). Other agents that directly inhibit insulin secretion from β-cells, such as adenosine triphosphate (ATP)-sensitive potassium channel (KATP channel) openers and somatostatin, typically require coadministration of insulin in order to avoid hyperglycemia.
Fig. 1.
Schematic presentation of glucose-mediated insulin secretion and medications that block insulin secretion. Glucose transporter 2 actively transports glucose into β-cells. Lower extracellular glucose concentrations induced by exogenous insulin lead to less glucose uptake. Glucose metabolism induces adenosine triphosphate (ATP) production, causing closure of the ATP-sensitive potassium (KATP) channels. The altered membrane potential leads to depolarization and influx of calcium via voltage-gated calcium channels. Closure of KATP channels can be directly blocked by KATP channel openers, such as diazoxide, or indirectly blocked by second-messenger systems triggered by interaction of somatostatin with the sst5 receptor. Somatostatin may also have direct effects on calcium channels.
Diazoxide is the best known KATP channel opener. It inhibits insulin secretion by reversing the effect of glucose, which produces intracellular ATP via glycolysis and thus induces closure of KATP channels. This leads to subsequent opening of voltage-dependent calcium channels. Increased intracellular calcium concentrations induce translocation of insulin-rich vesicles to the cell membrane and exocytosis of insulin (Fig. 1). In addition, many other factors influence insulin secretion. Examples are pyruvate cycling (9), vagal stimulation (10), and incretin effects signaled via specific β-cell G-protein coupled receptors that activate the adenylyl cyclase pathway (11).
Diazoxide has the potential to decrease insulin secretion to fasting levels if given in adequate doses (12). Because of its lack of selectivity, diazoxide also acts on KATP channels in smooth muscle, causing a reduction in blood pressure. In fact, the earliest clinical use of diazoxide was as an antihypertensive agent in 1960 (13). Currently, diazoxide is administered most frequently for the treatment of hyperinsulinemic hypoglycemia of infancy (14). Numerous derivatives of diazoxide have been developed, which contain structural modifications that increase their potency and selectivity for β-cells. Few of these have been tested in humans, and none are Food and Drug Administration approved for clinical use. A review of these compounds has been published elsewhere (12).
Somatostatin and its analogs not only inhibit the release of insulin but also of growth hormone, glucagon, and gastrin (12, 15). Octreotide is the most frequently used somatostatin analog; it has clinical utility in a variety of hormone excess syndromes and gastrointestinal disorders. The inhibition of insulin release by octreotide has been exploited in the treatment of insulinoma and in hypothalamic obesity, in which insulin hypersecretion is thought to contribute to the pathophysiology (16). Octreotide inhibits insulin release via the sst5 receptor, while inhibition of glucagon, gastrin, and prolactin release are mediated via the sst2 receptor (17). Selective agonists for the sst5 receptor have been developed but have not been tested in humans (12). Two of these compounds (SS14 and SS28) have been shown to inhibit insulin release in vitro under conditions of both normoglycemia (6.1 mmol/L) and hyperglycemia (20 mmol/L) to a similar degree as native somatostatin (18). Another selective sst5 receptor agonist (DC-23-99) proved to be a more potent inhibitor of insulin release than somatostatin, while having less potent effects on glucagon release (19). These selective sst5 agonists may have future clinical utility for selective inhibition of insulin release.
Beneficial effects of beta-cell rest
At present, it is not entirely clear why β-cell rest has salutary effects, especially on a long-term basis. Several explanations have been suggested (Table 1): inhibition of endogenous insulin secretion might allow chronically overstimulated β-cells to replenish the immediately secretable insulin pool (20). It has also been speculated that glucokinase (GK) activity might recover during the period of decreased insulin secretion (21). Impaired function of GK and consequent impaired interaction with β-cell mitochondria have been linked to increased β-cell apoptosis (22).
Table 1.
Proposed mechanisms for beneficial effects of β-cell rest
| Restocking’ of cellular insulin stores (20) |
| Recovery of GK activity (21) |
| Reduction of gluconeogenic enzymes (e.g., GK, glucose-6-phosphatase, and phosphoenolpyruvate carboxykinase) (23) |
| Improved pancreatic islet blood flow (24) |
| Decreased islet fibrosis (25) |
| Decreased islet antigenicity (26–29) |
| Reduction in reactive oxygen species (8, 32, 33) |
In animal studies, improvement in insulin secretion and sensitivity from diazoxide appeared to be mediated by decreased activity of key enzymes regulating hepatic gluconeogenesis including hepatic GK, glucose-6-phosphatase, and phosphoenolpyruvate carboxykinase (23). Furthermore, blood flow to pancreatic islets is considerably increased after exposure to potassium channel openers such as diazoxide (24).
Beta-cell rest may also lead to decreased islet fibrosis, which is typically seen in long-standing type 1 and type 2 diabetes. A possible mechanism is reduced proliferation of pancreatic stellate cells (which produce extracellular matrix in pancreatic fibrosis), which are activated by high glucose and insulin levels (25). Beta-cell rest also may reduce the antigenicity of β-cells, which plays an important role in type 1 diabetes (26–29).
When β-cell rest is induced by insulin therapy, part of the benefit is clearly mediated by correction of glucotoxicity. Prolonged hyperglycemia leads to numerous harmful effects including decreased glucose utilization and insulin secretion in β-cells, downregulation of insulin transcription, upregulation of inflammatory cytokines, and β-cell apoptosis (30, 31). However, beneficial effects of β-cell rest may also result from decreased production of ROS independent of correction of hyperglycemia, with consequent improvement of β-cell function and reduction of apoptosis (8). This may be because of a direct effect of insulin as an anti-inflammatory hormone or may be secondary to disruption of proinflammatory processes triggered by glucose-dependent insulin secretion in the β-cell.
Reduction of oxidative stress using insulin, independent of glycemia, has been shown in humans with type 2 diabetes. Bravi et al. measured markers of oxidative stress in patients with well-controlled type 2 diabetes [hemoglobin A1c (HbA1c) <7.0%] before and during euglycemic hyperinsulinemic clamp studies and in non-diabetic controls at baseline (32). They found that subjects with type 2 diabetes had a decreased glutathione (GSH) to GSH disulfide (GSSG) ratio compared with controls, indicating a decreased ability to protect β-cells from hydrogen peroxide-induced oxidative damage. The GSH/GSSG ratio increased in diabetic subjects during the hyperinsulinemic clamp to near that of normal controls, suggesting that exogenous insulin decreased oxidative stress independent of its effect on glycemia.
Langouche et al. have proposed that insulin may have an anti-inflammatory effect when used to maintain strict euglycemia in critically ill patients (33). They demonstrated that patients in the intensive care unit who were randomized to receive intensive insulin therapy had lower circulating levels of the intercellular adhesion molecule-1 and E-selectin. In addition, the group receiving intensive insulin therapy had lower levels of circulating nitric oxide (NO) and lower gene expression of inducible NO synthase. As high levels of NO are pro-inflammatory, it is possible that this may provide a link between intensive insulin treatment and preservation of β-cell function via anti-inflammatory effects.
Preclinical studies
Many rodent models of diabetes have been developed to further our understanding of pathophysiology and to develop treatment options. Examples are the non-obese diabetic (NOD) mouse, which serves as a model for type 1 diabetes, and the leptin-deficient ob/ob mouse, which is a common model of type 2 diabetes. Streptozotocin-induced diabetic animals have been used to study both main types of diabetes. Reviews of rodent models can be found elsewhere (34, 35). It is of note that results of rodent research should be extrapolated with great caution to clinical results in humans. Many important differences exist between rodent and human insulin genetics and physiology, and interventions that prevent or treat diabetes in models such as the NOD mouse frequently prove disappointing when tested in humans (34).
Insulin
While initiation of insulin therapy at the diagnosis of type 1 diabetes may result in temporary restoration of endogenous insulin production (the ‘honeymoon’ period), endogenous insulin secretion inevitably declines again to the point where exogenous insulin is necessary. Studies of insulin-induced β-cell rest directed at prevention of disease in diabetes-prone animals are therefore of special interest. Several investigators have found that treatment of diabetes-prone biobreeding (BB) rats with exogenous insulin and/or diazoxide resulted in a decreased incidence of diabetes (36, 37). Buschard et al. demonstrated that 8 d of insulin treatment in several rodent models of type 1 diabetes resulted in decreased expression of a monoclonal islet cell autoantibody (26). Other investigators have found that the expression of glutamic acid decarboxylase autoantigen is correlated with insulin secretion (4). Thus, β-cell rest may delay the onset of diabetes by decreasing the antigenicity of islets.
Other investigators have studied the effects of insulin-induced β-cell rest on animals with overt diabetes. Zhou et al. reported the effects of insulin at onset of diabetes in Sprague-Dawley rats fed high-energy chow and treated with streptozotocin to induce β-cell apoptosis (38). Two days after streptozotocin treatment, rats were given 7 d of subcutaneous insulin. Insulin mRNA levels were found to be 81.3% higher in insulin-treated diabetic rats compared with untreated animals. In addition, early insulin treatment improved the cytoplasmic insulin content of β-cells viewed on stained pancreas sections. More than 10 yr earlier, using a similar experimental approach, Kergoat et al. studied the effect of 1 vs. 5 d of insulin therapy in a streptozotocin-induced rat model of type 2 diabetes (39). In isolated perfused pancreas preparations, they found that the 1-d treatment regimen had little effect, but 5 d of insulin resulted in improved glucose-stimulated insulin secretion.
Other agents
Several studies have attempted to differentiate the direct, noxious effect of hyperglycemia from over-stimulation of β-cells. Yoshikawa et al. incubated islets isolated from Sprague-Dawley rats in a high-glucose environment (27 mmol/L glucose) for 48 h with either continuous or intermittent exposure to diazoxide (40). Despite hyperglycemia, both diazoxide exposure conditions resulted in improved β-cell function. Maedler et al. studied the effects of diazoxide and the β-cell-selective KATP channel opener NN414 on isolated human islets (41). They found that both KATP channel openers blocked the adverse effects of exposure to high glucose and the inflammatory cytokine interleukin-1β on β-cell secretory dysfunction and apoptosis.
Skak et al. investigated whether NN414 could be used to improve β-cell survival in diabetic BB rats (42). Animals were treated three times daily on days 1–19 with NN414, diazoxide, or vehicle. Almost half of the NN414-treated rats were found to have a β-cell mass (measured histologically) and function (measured by glucose-stimulated C-peptide) similar to that of non-diabetic controls vs. only 11% (4 of 36) of vehicle-treated rats. In addition, responsive NN414-treated rats were almost free of insulitis. Similar results were found in BB rats treated with insulin plus either diazoxide or NNC 55-0118 (43).
In 90% pancreatectomized diabetic rats, a 5-d postoperative treatment with diazoxide was found to lower non-fasting plasma glucose values, improve glucose tolerance after oral glucose load, and induce a 50% increase in pancreatic insulin content compared with control (44). Kullin et al. used streptozotocin-induced β-cell damage in Sprague-Dawley rats as a model for insulitis and studied the effects of diazoxide and the selective KATP channel opener NNC 55-0118 (45). In isolated islets, both KATP channel openers protected rat islet morphology from streptozotocin-induced destruction and in addition preserved glucose oxidation rate and insulin content and secretion.
Studies of somatostatin and its analogs in animal models of diabetes have focused primarily on prevention or amelioration of diabetic complications (e.g., retinopathy and nephropathy). Data on the effects of somatostatin on β-cell function in animals are lacking.
Clinical studies
In 1940, Jackson et al. described 27 children with type 1 diabetes who were hospitalized and treated with regular insulin four times daily to achieve a mean blood glucose of 50 to 75 mg/dL for about 2 months (1). The authors noted that this treatment resulted in a decrease in insulin requirements by approximately 50%, presumably because of correction of glucotoxicity. In 1978, Mirouze et al. described the effects of a brief course of intensive intravenous insulin vs. conventional (three times daily subcutaneous insulin) therapy in recently diagnosed patients with type 1 diabetes (2). The primary end-point of the study was the rate of ‘remission’, defined as aglycosuria with fasting blood glucose <120 mg/dL and postprandial blood glucose <200 mg/dL without exogenous insulin. In the group that received intravenous insulin therapy, 75% (9 of 12) entered a remission period lasting a mean of 8 months vs. an 11% remission rate in controls. The authors concluded that ‘β-cells can be rested’ with ‘early adequate insulin therapy’, allowing preservation of residual β-cell function.
More recently, the Diabetes Control and Complication Trial (DCCT) in type 1 diabetes demonstrated that, among subjects who had C-peptide secretion at baseline, those who received intensive insulin therapy maintained higher C-peptide secretion over time as compared with those who received conventional insulin therapy (46). They concluded that intensive insulin therapy helped preserve endogenous insulin secretion and also resulted in improved metabolic control. In addition, subjects in the DCCT with higher C-peptide secretion had a lower risk of complications, including retinopathy, nephropathy, and severe hypoglycemia (47).
The Diabetes Prevention Trial (DPT-1) was designed to prevent type 1 diabetes by administration of subcutaneous or oral insulin (48, 49). This large-scale trial was based on the hypothesis that insulin may lead to immunomodulation and possible induction of tolerance; thus, insulin was not primarily used to induce β-cell rest. More than 103 000 first- and second-degree relatives of patients with type 1 diabetes were screened, and subjects were characterized according to their 5-yr risk of developing type 1 diabetes. The high-risk cohort was randomized to receive either close follow-up or 0.25 mg/kg/d of subcutaneous insulin. The intermediate risk group received placebo or oral insulin (7.5 mg/d). There proved to be no difference in the incidence of new-onset type 1 diabetes in the insulin-treated groups vs. controls (48). The negative results of the DPT-1, which countered the positive results seen in multiple animal trials, might be because of the low dose of insulin used to avoid hypoglycemia. Alternatively, animal data exist to suggest that β-cell rest with insulin must be initiated at an earlier stage in the development of diabetes in order to prevent new-onset disease (50). The Type 1 Diabetes TrialNet study group has initiated a follow-up trial to further explore the potential role of oral insulin to delay or prevent type 1 diabetes (51).
In 1988, Glaser et al. reported improved β-cell function after intensive insulin treatment in patients with poorly controlled type 2 diabetes (52). They studied 12 adult subjects with type 2 diabetes with HbA1c of 10.7–20.5% on maximal metformin and glibenclamide (glyburide) plus insulin (in 8 of 12 of these patients). Subjects were given intensive insulin therapy via continuous subcutaneous insulin infusion (CSII, or ‘insulin pump’) for approximately 17 d. Shortly (1–3 d) after cessation of CSII, subjects showed improved glycemia control and increased C-peptide responses to glucagon and intravenous glucose. This brief treatment with CSII also appeared to have long-term benefits: one patient was able to stop all antidiabetic agents for at least 1 yr after CSII, and an additional five subjects maintained good blood glucose control on oral hypoglycemic agents for 4 to over 24 months.
There has been a single uncontrolled clinical trial of short term (4–12 wk), twice daily insulin injections in 18 children with type 2 diabetes, who either had a history of diabetic ketoacidosis or had a HbA1c >9% (53). This study showed a marked improvement in HbA1c from 12.81% at baseline to 7.59% after treatment. The improvement persisted over a 12-month follow-up period.
Early insulin treatment in the course of type 2 diabetes has been suggested by many clinicians and clinical investigators but has not been widely accepted as a treatment modality. The availability of nasal or inhaled insulin may change clinical practice in the future as much of the hesitance stems from having to use injections. Two recent studies suggest that intensive insulin therapy at diagnosis of type 2 diabetes may lead to significant long-term improvement in β-cell function to the extent that subjects remain euglycemic off all diabetes therapy except appropriate diet. Ryan et al. gave 16 obese adult subjects with newly diagnosed type 2 diabetes a 2 to 3-wk course of intensive insulin therapy. At 1 yr follow-up, seven of the subjects remained near-euglycemic on diet therapy alone (54). Li et al. treated 138 consecutive adult patients with newly diagnosed type 2 diabetes with intensive insulin therapy using CSII for 2 wk. The remission rates (percent maintaining near-euglycemia on diet alone) were 72.6% at 3 months and 47.1% at 1 yr (55). Ilkova et al. showed similar results with a group of 13 subjects with newly diagnosed, diet-unresponsive type 2 diabetes. Subjects were treated with 2 wk of intensive insulin therapy using CSII. More than half maintained near-euglycemia on diet alone 12 months after CSII and 38.5% at 24 months. In four subjects, glycemia control deteriorated after 9–34 months but was restored by a second 2-wk course of CSII (56).
Prospective controlled trials of the effect of early β-cell rest in type 2 diabetes have not yet been conducted to confirm the above results. To address this issue, a trial is underway in adolescents and young adults with recently diagnosed type 2 diabetes, comparing the effect of conventional therapy (diet and metformin) with conventional therapy plus 2 wk of β-cell rest using insulin via CSII and diazoxide (57).
Clinical studies: other agents
Bjork et al. demonstrated in a small study of 20 young, adult patients with type 1 diabetes that the combination of insulin and diazoxide (4–6 mg/kg/d) produced greater β-cell rest (evidenced by lower C-peptide levels) than insulin alone (58). Importantly, subjects who received the 3-month course of diazoxide in this trial had higher residual endogenous insulin secretion at 1 yr compared with controls. Currently, Grill et al. are recruiting adult patients with recent onset type 1 diabetes to test whether low and intermittent (i.e., night time) dosing of diazoxide together with insulin administration can prevent β-cell loss during a 12-month observation period, and whether this approach is associated with fewer side effects of long-term diazoxide administration (59).
Thirty years ago, Greenwood et al. reported that treatment with diazoxide (5 mg/kg/d) led to improved insulin secretion in adults with type 2 diabetes (3). Diazoxide and insulin were administered to 10 diabetic subjects for 7 d. An additional four diabetic subjects received insulin plus placebo, serving as a control group. There was a substantial increase in the insulin response to stimulation with glucagon and tolbutamide after discontinuation of diazoxide compared with placebo. Guldstrand et al. replicated these results with an even shorter course of diazoxide (100 mg every 8 h × six doses) in eight obese individuals with type 2 diabetes (60). In this study, subjects were treated with insulin alone or insulin plus diazoxide in a randomly ordered treatment, with subjects serving as their own controls. Despite an identical degree of blood glucose control during the two treatment periods, insulin secretion was significantly better following treatment with diazoxide. The degree of C-peptide suppression achieved during these studies was not documented, however, and it is unclear if the benefit seen was actually because of resting of β-cells.
Laedtke et al. studied the effects of somatostatin in 11 adult subjects with type 2 diabetes. Subjects were given an overnight infusion of either somatostatin (60 ng/kg/min) or saline, while plasma glucose was maintained at 8 mmol/L (144 mg/dL) (61). To compensate for the lack of selectivity of somatostatin for the sst5 receptor, subjects received growth hormone and glucagon replacement during the somatostatin infusion. Immediately following cessation of the infusion, subjects exhibited improvement in mean insulin secretion rates to approximately that of non-diabetic controls, as well as improved insulin pulse mass and total insulin secretion. In an earlier study, five patients with type 2 diabetes had been studied after secondary failure of oral agents, with and without the addition of a long-acting somatostatin analog SMS 201-995 to an intermediate-acting insulin regimen (62). While β-cell function was not directly affected, postprandial glucose excursions were lower immediately after the administration of SMS 201-995, most likely because of the suppression of glucagon secretion and delayed intestinal absorption.
Few studies exist of somatostatin and its analogs in type 1 diabetes, including small numbers of subjects and reporting mixed results. Grunt et al. investigated the effect of octreotide on β-cell function in children with newly diagnosed type 1 diabetes (63). Ten children were given 50 μg/m2 of octreotide subcutaneously twice daily for 21 d after diagnosis. Although there were no differences in insulin requirement or HbA1c, subjects who had received octreotide had higher glucagon-stimulated C-peptide compared with controls at 6 and 12 months. Bjork et al. studied the effect of diazoxide compared with octreotide on C-peptide secretion and glycemia control in six subjects (64). After 1 wk, the glucagon-stimulated C-peptide response was markedly reduced in patients who had received diazoxide but unchanged after receiving octreotide, suggesting that this dose of octreotide (50 μg three times daily) is ineffective in inducing β-cell rest.
Deleterious effects of beta-cell stimulation
If β-cell rest is good for β-cell function, then one might argue the converse: β-cell stimulation should worsen β-cell function. Sulphonylureas close KATP channels and thus insulin secretion. For the past 50 years, they have been widely used drugs for the treatment of type 2 diabetes; however, there is an ongoing debate about their potentially harmful effects on β-cells and the cardiovascular system. Several studies have shown that these agents induce apoptosis in β-cell lines, rodent islets, and islets isolated from human donors (65, 66). However, in the United Kingdom Prospective Diabetes Study, loss of β-cell function was not unique to sulfonylureas but occurred at a similar rate of decline in patients with type 2 diabetes on metformin or conventional (mainly diet) treatment, suggesting that factors other than β-cell stimulation had to be playing a role (67).
Therapeutic implications
Solid evidence based on basic and clinical studies has shown that β-cell rest has the potential to improve β-cell function both acutely and chronically. Although clinical trials of insulin for prevention of type 1 diabetes in humans have been disappointing, the protective effect of intensive insulin treatment in patients with type 1 diabetes has been clearly shown in the DCCT, and part of this effect may be because of induction of β-cell rest. However, the strategy of intensive β-cell rest using a combination of insulin and KATP channel openers has not yet been fully explored for prevention or delayed progression of new-onset type 1 diabetes. Diazoxide may be particularly attractive for this type of study as it is relatively safe and easy to administer.
The three studies demonstrating prolonged clinical remission of type 2 diabetes after a short period of β-cell rest using intensive insulin therapy are very intriguing (54–56). If confirmed by controlled clinical studies, this suggests a new paradigm for the treatment of type 2 diabetes, in which β-cell function may be preserved by the use of early insulin therapy for β-cell rest. Furthermore, as the ultimate goal is prevention of overt diabetes and its associated complications, β-cell rest may be part of a therapeutic approach for both types of diabetes in the preclinical state. Large, multicenter trials of β-cell rest may provide information on whether this strategy will ultimately prove beneficial in altering the natural history of both type 1 and type 2 diabetes.
References
- 1.Jackson RL, Boyd JD, Smith TE. Stabilization of the diabetic child. Am J Dis Child. 1940;59:332–341. [Google Scholar]
- 2.Mirouze J, Selam JL, Pham TC, Mendoza E, Orsetti A. Sustained insulin-induced remissions of juvenile diabetes by means of an external artificial pancreas. Diabetologia. 1978;14:223–227. doi: 10.1007/BF01219420. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1:444–447. doi: 10.1016/s0140-6736(76)91473-2. [DOI] [PubMed] [Google Scholar]
- 4.Palmer JP. Beta cell rest and recovery – does it bring patients with latent autoimmune diabetes in adults to euglycemia? Ann N Y Acad Sci. 2002;958:89–98. doi: 10.1111/j.1749-6632.2002.tb02950.x. [DOI] [PubMed] [Google Scholar]
- 5.Rother KI. Diabetes treatment – bridging the divide. N Engl J Med. 2007;356:1499–1501. doi: 10.1056/NEJMp078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 8.Fridlyand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes. 2004;53:1942–1948. doi: 10.2337/diabetes.53.8.1942. [DOI] [PubMed] [Google Scholar]
- 9.Newgard CB, Lu D, Jensen MV, et al. Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes. 2002;51 (Suppl 3):S389–S393. doi: 10.2337/diabetes.51.2007.s389. [DOI] [PubMed] [Google Scholar]
- 10.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JB, Arkhammar PO, Bodvarsdottir TB, Wahl P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr Med Chem. 2004;11:1595–1615. doi: 10.2174/0929867043365026. [DOI] [PubMed] [Google Scholar]
- 13.Black J. Diazoxide and the treatment of hypoglycemia: an historical review. Ann N Y Acad Sci. 1968;150:194–203. doi: 10.1111/j.1749-6632.1968.tb19045.x. [DOI] [PubMed] [Google Scholar]
- 14.Rother KI, Matsumoto JM, Rasmussen NH, Schwenk WF. Subtotal pancreatectomy for hypoglycemia due to congenital hyperinsulinism: long-term follow-up of neurodevelopmental and pancreatic function. Pediatr Diabetes. 2001;2:115–122. doi: 10.1034/j.1399-5448.2001.002003115.x. [DOI] [PubMed] [Google Scholar]
- 15.Gurrath MM. Peptide-binding G protein-coupled receptors: new opportunities for drug design. Curr Med Chem. 2001;8:1605–1648. doi: 10.2174/0929867013371798. [DOI] [PubMed] [Google Scholar]
- 16.Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 17.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 18.Zambre Y, Ling Z, Chen MC, et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol. 1999;57:1159–1164. doi: 10.1016/s0006-2952(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 19.Rossowski WJ, Coy DH. Specific inhibition of rat pancreatic insulin or glucagon release by receptor-selective somatostatin analogs. Biochem Biophys Res Commun. 1994;205:341–346. doi: 10.1006/bbrc.1994.2670. [DOI] [PubMed] [Google Scholar]
- 20.Ritzel RA, Hansen JB, Veldhuis JD, Butler PC. Induction of beta-cell rest by a Kir6. 2/SUR1-selective K(ATP)-channel opener preserves beta-cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab. 2004;89:795–805. doi: 10.1210/jc.2003-031120. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo MA, Magnuson MA, Drain PF, Piston DW. A functional link between glucokinase binding to insulin granules and conformational alterations in response to glucose and insulin. J Biol Chem. 2002;277:34168–34175. doi: 10.1074/jbc.M112478200. [DOI] [PubMed] [Google Scholar]
- 22.Kim WH, Lee JW, Suh YH, et al. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54:2602–2611. doi: 10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- 23.Alemzadeh R, Holshouser S, Massey P, Koontz J. Chronic suppression of insulin by diazoxide alters the activities of key enzymes regulating hepatic gluconeogenesis in Zucker rats. Eu J Endocrinol. 2002;146:871–879. doi: 10.1530/eje.0.1460871. [DOI] [PubMed] [Google Scholar]
- 24.Jansson L, Kullin M, Karlsson FA, Bodin B, Hansen JB, Sandler S. K(ATP) channels and pancreatic islet blood flow in anesthetized rats: increased blood flow induced by potassium channel openers. Diabetes. 2003;52:2043–2048. doi: 10.2337/diabetes.52.8.2043. [DOI] [PubMed] [Google Scholar]
- 25.Hong OK, Lee SH, Rhee M, et al. Hyperglycemia and hyperinsulinemia have additive effects on activation and proliferation of pancreatic stellate cells: Possible explanation of islet-specific fibrosis in type 2 diabetes mellitus. J Cell Biochem. 2007;128:665–675. doi: 10.1002/jcb.21222. [DOI] [PubMed] [Google Scholar]
- 26.Buschard K, Brogren CH, Ropke C, Rygaard J. Antigen expression of the pancreatic beta-cells is dependent on their functional state, as shown by a specific, BB rat monoclonal autoantibody IC2. APMIS. 1988;96:342–346. doi: 10.1111/j.1699-0463.1988.tb05313.x. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson FA, Bjork EE. Beta-cell rest: a strategy for the prevention of autoimmune diabetes. Autoimmunity. 1997;26:117–122. doi: 10.3109/08916939709003855. [DOI] [PubMed] [Google Scholar]
- 28.Bjork E, Kampe O, Karlsson FA, et al. Glucose regulation of the autoantigen GAD65 in human pancreatic islets. J Clin Endocrinol Metab. 1992;75:1574–1576. doi: 10.1210/jcem.75.6.1464667. [DOI] [PubMed] [Google Scholar]
- 29.Hagopian WA, Karlsen AE, Petersen JS, et al. Regulation of glutamic acid decarboxylase diabetes autoantigen expression in highly purified isolated islets from Macaca nemestrina. Endocrinology. 1993;132:2674–2681. doi: 10.1210/endo.132.6.8504767. [DOI] [PubMed] [Google Scholar]
- 30.Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem. 2007;282:287–293. doi: 10.1074/jbc.M606118200. [DOI] [PubMed] [Google Scholar]
- 31.Poitout V, Robertson RPRP. Minireview: secondary beta-cell failure in type 2 diabetes – a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 32.Bravi MC, Armiento A, Laurenti O, et al. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2006;55:691–695. doi: 10.1016/j.metabol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Langouche L, Vanhorebeek I, Vlasselaers D, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115:2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 36.Gotfredsen CF, Buschard K, Frandsen EK. Reduction of diabetes incidence of BB Wistar rats by early prophylactic insulin treatment of diabetes-prone animals. Diabetologia. 1985;28:933–935. doi: 10.1007/BF00703140. [DOI] [PubMed] [Google Scholar]
- 37.Vlahos WD, Seemayer TA, Yale JF. Diabetes prevention in BB rats by inhibition of endogenous insulin secretion. Metabolism. 1991;40:825–829. doi: 10.1016/0026-0495(91)90010-t. [DOI] [PubMed] [Google Scholar]
- 38.Zhou YS, Gao Y, Guo XH, Li B, Wang S, Chi JM. Effects of timely insulin treatment on protection of beta cells in a rat model of type 2 diabetes mellitus. Chin Med J (Engl) 2004;117:1523–1529. [PubMed] [Google Scholar]
- 39.Kergoat M, Bailbe D, Portha B. Insulin treatment improves glucose-induced insulin release in rats with NIDDM induced by streptozocin. Diabetes. 1987;36:971–977. doi: 10.2337/diab.36.8.971. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa H, Ma Z, Bjorklund A, Grill V. Short-term intermittent exposure to diazoxide improves functional performance of beta-cells in a high-glucose environment. Am J Physiol Endocrinol Metab. 2004;287:E1202–E1208. doi: 10.1152/ajpendo.00255.2004. [DOI] [PubMed] [Google Scholar]
- 41.Maedler K, Storling J, Sturis J, et al. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6. 2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- 42.Skak K, Gotfredsen CF, Lundsgaard D, Hansen JB, Sturis J, Markholst H. Improved beta-cell survival and reduced insulitis in a type 1 diabetic rat model after treatment with a beta-cell-selective K(ATP) channel opener. Diabetes. 2004;53:1089–1095. doi: 10.2337/diabetes.53.4.1089. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen SB, Sorensen TS, Hansen JB, Mandrup-Poulsen T, Hornum L, Markholst H. Functional rest through intensive treatment with insulin and potassium channel openers preserves residual beta-cell function and mass in acutely diabetic BB rats. Horm Metab Res. 2000;32:294–300. doi: 10.1055/s-2007-978639. [DOI] [PubMed] [Google Scholar]
- 44.Leahy JL, Bumbalo LM, Chen C. Diazoxide causes recovery of beta-cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes. 1994;43:173–179. doi: 10.2337/diab.43.2.173. [DOI] [PubMed] [Google Scholar]
- 45.Kullin M, Li Z, Hansen JB, Bjork E, Sandler S, Karlsson FA. K(ATP) channel openers protect rat islets against the toxic effect of streptozotocin. Diabetes. 2000;49:1131–1136. doi: 10.2337/diabetes.49.7.1131. [DOI] [PubMed] [Google Scholar]
- 46.The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 47.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 48.Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 49.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial – Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 50.Visser J, Klatter F, Vis L, Groen H, Strubbe J, Rozing J. Long-term prophylactic insulin treatment can prevent spontaneous diabetes and thyroiditis development in the diabetes-prone bio-breeding rat, while short-term treatment is ineffective. Eur J Endocrinol. 2003;149:223–229. doi: 10.1530/eje.0.1490223. [DOI] [PubMed] [Google Scholar]
- 51.National Institute of Diabetes and Digestive and Kidney Diseases et al. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2000. [Accessed 5 July 2007]. Oral insulin for prevention of diabetes in relatives at risk for type 1 diabetes mellitus. NLM identifier NCT00419562. (available from http://www.clinicaltrials.gov/ct/show/NCT00419562) [Google Scholar]
- 52.Glaser B, Leibovich G, Nesher R, Hartling S, Binder C, Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh) 1988;118:365–373. doi: 10.1530/acta.0.1180365. [DOI] [PubMed] [Google Scholar]
- 53.Sellers EA, Dean HJ. Short-term insulin therapy in adolescents with type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1561–1564. doi: 10.1515/jpem.2004.17.11.1561. [DOI] [PubMed] [Google Scholar]
- 54.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Xu W, Liao Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–2602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 56.Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 57.National Institute of Diabetes and Digestive and Kidney Diseases. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2000. [Accessed 5 July 2007]. Effect of short-term beta-cell rest in adolescents and young adults with type 2 diabetes mellitus. NLM identifier NCT00445627. (available from http://www.clinicaltrials.gov/ct/show/NCT00445627) [Google Scholar]
- 58.Bjork E, Berne C, Kampe O, Wibell L, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes. 1996;45:1427–1430. doi: 10.2337/diab.45.10.1427. [DOI] [PubMed] [Google Scholar]
- 59.Grill V. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2000. [Accessed 5 July 2007]. Efficacy of diazoxide in type 1 diabetes. NLM identifier NCT00131755. (available from http://www.clinicaltrials.gov/ct/show/NCT00131755) [Google Scholar]
- 60.Guldstrand M, Grill V, Bjorklund A, Lins PE, Adamson U. Improved beta cell function after short-term treatment with diazoxide in obese subjects with type 2 diabetes. Diabetes Metab. 2002;28:448–456. [PubMed] [Google Scholar]
- 61.Laedtke T, Kjems L, Porksen N, et al. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279:E520–E528. doi: 10.1152/ajpendo.2000.279.3.E520. [DOI] [PubMed] [Google Scholar]
- 62.Candrina R, Giustina G. Effect of a new long-acting somatostatin analogue (SMS 201-995) on glycemic and hormonal profiles in insulin-treated type II diabetic patients. J Endocrinol Invest. 1988;11:501–507. doi: 10.1007/BF03350169. [DOI] [PubMed] [Google Scholar]
- 63.Grunt JA, Al-Hakim H, Willoughby L, Howard CP. A randomized trial of a somatostatin analog for preserving beta cell function in children with insulin dependent diabetes mellitus. J Pediatr Endocrinol. 1994;7:331–334. doi: 10.1515/jpem.1994.7.4.331. [DOI] [PubMed] [Google Scholar]
- 64.Bjork E, Berne C, Karlsson FA. Induction of beta-cell rest in type 1 diabetes. Studies on the effects of octreotide and diazoxide. Diabetes Care. 1998;21:427–430. doi: 10.2337/diacare.21.3.427. [DOI] [PubMed] [Google Scholar]
- 65.Efanova IB, Zaitsev SV, Zhivotovsky B, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 66.Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501–506. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- 67.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]