Abstract
Mitotic spindle bipolarity defines a unique division plane that promotes the successful transmission of genetic material during cytokinesis. The positioning and orientation of the spindle determines the symmetry of cell division and the relative location of daughter cells, which regulate cell fate decisions that contribute to embryonic development and tissue differentiation. Recent studies have identified integrins as regulators of spindle positioning and orientation, as well as spindle bipolarity and cytokinesis. This review summarizes and discusses the current effort focused on understanding how integrins regulate these mitotic events.
Introduction
The proper assembly and function of the mitotic spindle is essential for the segregation and transmission of genetic material [1]. Defects in chromosome segregation can lead to lethal errors that result in birth defects and contribute to tumor development. The assembly of the mitotic spindle is a complex process involving tightly regulated centrosome duplication, enhanced microtubule (Mt) nucleation, increased Mt dynamics, and the association of Mts with the cell cortex and kinetochores [2–4]. Spindle bipolarity is important for chromosome congression in metaphase and segregation in anaphase and contributes to cytokinesis by regulating the assembly of the contractile ring and the ingression of the cleavage furrow [5]. The positioning and orientation of the mitotic spindle are also important as they define the division plane, which contributes to embryonic development and tissue differentiation by determining the relative location of daughter cells and the differential inheritance of cytoplasmic determinants.
Increasing interest has focused on integrins as regulators of mitotic events. Integrins form a large family of α/β heterodimeric receptors that mediate cell adhesion to components of the extracellular matrix [6]. Integrin-mediated adhesion activates signals that regulate a number of cellular processes including cell migration, proliferation, and differentiation [6–8].
Integrin β subunit cytoplasmic domains (β tails) are central to integrin function. Integrin activity is conformationally regulated by proteins that bind to the β tail [9]. The adhesive function of integrins requires their ability to form transmembrane links with the actin cytoskeleton and β tails mediate this connection [10–13]. Additionally, integrins activate intracellular signaling proteins, such as the cytoplasmic tyrosine kinases FAK and Src, the Rho family of GTPases, including RhoA, Rac1, and Cdc42, and the lipid kinase, phosphatidylinositol-3-OH kinase (PI 3-kinase). β tails regulate signaling by many of these proteins [14–17], which are also known to control the actin and Mt cytoskeletons [18].
Here, we review recent studies linking integrins to the mitotic machinery, providing a new paradigm for integrin signaling. Our discussion aims to provide a framework to understand how integrins can influence the assembly and positioning of the mitotic spindle, as well as cytokinesis.
Spindle Positioning and Orientation
The identification of mechanisms that control the positioning and orientation of the mitotic spindle has interested biologists for decades. The ability of integrins to regulate cortical cues that position the mitotic spindle was first suggested by studies in NRK cells [19*]. In these cells, the spindle axis aligns parallel to the long axis of the cell. When cell shape (and therefore cell adhesion) was experimentally changed during mitosis, the spindle was repositioned in the direction of new long axis. These results indicated that NRK cells continually monitor the position of their spindles relative to changes in cortical cues and suggested a role for integrins, as they are known to regulate cell shape. Spindle repositioning was also shown to require astral Mts, as well as the motor protein dynein, which is thought to provide the pulling forces on astral Mts at the cortex [19*]. Astral Mts, dynein, and the cell cortex have also been implicated in the regulation of spindle positioning in Saccharomyces ceriviesae, Caenorhabditis elegans, and Drosophila melanogaster [20–22], suggesting that these mechanisms are evolutionarily conserved.
A correlation between cell shape and spindle orientation was also demonstrated using the integrin ligand fibronectin (Fn) to generate differently micropatterned substrata [23**]. When individual HeLa cells in interphase were adhered to a disc, spindles were randomly oriented in mitosis, but on a rectangular or triangular substratum, the spindle aligned with the long cell axis or the hypotenuse, respectively. Importantly, when cell shape was maintained, but areas of adhesion to Fn were restricted, the spatial distribution of adhesion sites dictated the position of the spindle. Regions of cell adhesion during interphase controlled the Src-dependent cortical localization of the actin-binding proteins cortactin and ezrin, which became associated with retraction fibers and remained localized marking the location of spindle poles as cells entered mitosis. Thus, the position of the spindle can be determined by adhesion-regulated cortical cues set during interphase [23**].
A direct role for integrins was demonstrated both in vivo and in cell culture [24*,25**]. Inhibiting β1 integrin expression or function dramatically affected the orientation of the spindle in HeLa cells [25**]. On Fn, spindle axes were parallel to the adhesion plane, but were randomly oriented on the non-integrin ligand poly-L lysine. A similar phenotype was observed when β1 integrin function was inhibited with RGD peptides, function-blocking antibodies, or siRNA [25**]. Integrins regulated spindle orientation by a mechanism that required astral Mts and actin polymerization.
The identification of integrin-regulated cortical cues is critical to understand how cell-matrix adhesion controls the orientation and positioning of the spindle. The localization of cortactin and ezrin correlated with the position of the spindle poles; however, it is not yet known whether these proteins directly contribute to these processes. Recently, phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) was shown to localize at the midcortex in metaphase when HeLa cells were adhered to Fn [26**]. Preventing the accumulation of PI(3,4,5)P3 by inhibiting PI 3-kinase induced spindle misorientation. Importantly, disrupting β1 integrin-mediated adhesion inhibited PI 3-kinase activity and the midcortical accumulation of PI(3,4,5)P3 during mitosis. Thus, integrins regulate the production and localization of PI(3,4,5)P3, which acts as a cortical cue to direct the orientation of the spindle. These results lead to the speculation that integrin signals from the basal cell surface to the midcortex are involved in a positive feedback loop similar to that establishing PI(3,4,5)P3 polarity during cell migration [27,28].
Local concentrations of PI(3,4,5)P3 can also be regulated by lipid phosphatases, such as PTEN (phosphatase and tensin homologue) [29]. Inhibiting the expression of PTEN in HeLa cells caused the dispersion of PI(3,4,5)P3 throughout the cortex and spindle misorientation [26**]. Thus, spatial inactivation of PI(3,4,5)P3 is also critical in directing the position of the spindle. It will be interesting to learn whether integrins regulate the localization of PTEN activity.
PI(3,4,5)P3 regulates signaling pathways by recruiting proteins containing pleckstrin homology domains (PH domains) to the plasma membrane [29]. At mitosis, dynein-dynactin motor complexes were found to concentrate at the midcortex by a PI(3,4,5)P3-dependent mechanism; however, PI(3,4,5)P3 was not required for their membrane association. These results suggest that an unidentified PH-domain containing protein provides a platform to concentrate these motor complexes at the midcortex [26**]. Learning the identity of this protein is an important future goal.
Many questions remain concerning how integrins regulate the orientation and positioning of the mitotic spindle. Current data suggest that integrin signaling is important at mitosis to direct the spindle parallel to the adhesion plane, whereas adhesive and contractile forces during interphase contribute to the positioning of the spindle with the long cell axis [19*,23**,26**,30]. Thus, in addition to identifying integrin signaling pathways and their cortical targets that control the cellular location of the spindle, it will be interesting to determine whether different sets of cues direct the orientation of the spindle parallel to the adhesion plane and its alignment with the long cell axis.
Integrin Signaling and Astral Microtubules
The interaction of astral Mts with specific regions of the cell cortex contributes to positioning the spindle at mitosis. The mechanisms involved may be similar to those used by integrins to regulate Mt dynamics during cell migration [31]. EB1 (end-binding protein1) and APC (adenomatous polyposis coli) bind to the plus end of Mts to promote their growth and association with the cell cortex during both processes [25**,32,33]. The interaction of APC with Mts is inhibited when it becomes phosphorylated by glycogen synthase-3β (GSK-3β [32]. Thus, the local inhibition of GSK-3β can regulate Mt dynamics at the cortex. In migrating cells, integrin-dependent regulation of Cdc42 inhibits GSK-3β through the activation of the Par6-PKCζ complex [34,35]. Integrins also inhibit GSK-3β by activating the PI 3-kinase-AKT pathway [36]. Thus, spindle positioning could be regulated by the integrin-dependent inhibition of GSK-3β at specific cortical sites by either mechanism.
Integrins can regulate Mt dynamics by additional pathways involving Rho GTPases. RhoA promotes the association and stabilization of Mts with the leading edge through an mDia-EB1-APC pathway [37,38]. Rac1 and Cdc42 can both promote Mt growth by activating the p21-activated serine-threonine kinase PAK1, which in turn phosphorylates and contributes to the inactivation of oncoprotein 18/stathmin [39,40], a protein that promotes MT depolymerization [41]. These pathways may similarly enhance the growth and cortical association of astral Mts at mitosis. Furthermore, integrins can promote the surface expression of lipid rafts [37,42], which may also contribute to the localization of cortical cues needed for spindle positioning.
Bipolar Spindle Assembly and Cytokinesis
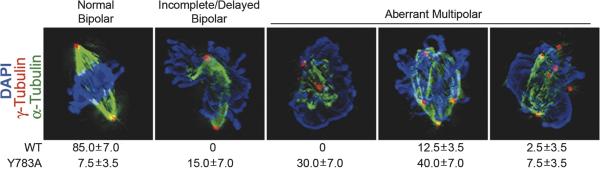
In a recent study, CHO cells expressing chimeric integrins that mediate adhesion to fibrinogen (Fg) [10,43] were used as a model system to demonstrate that the integrin –1 cytoplasmic tail regulates cell division [44**]. A tyrosine to alanine substitution in the membrane-proximal NPIY motif of the β1 tail (Y783A) known to perturb integrin function [45] dramatically inhibited the formation of the bipolar spindle and cytokinesis [44]. Importantly, these phenotypes were not cell type- or integrin-specific and were prevented when cells were adhered by endogenous integrins or by treatment of the mutant integrin with an activating antibody [44**].
The vast majority of cells adhered by the mutant integrin formed incomplete or multipolar spindles during the first mitosis on Fg (Fig. 1 and [44**]). In addition, Mt growth from the centrosome and spindle poles was inhibited by the Y783A mutation. Previous studies by others have suggested that the inhibition of centrosome function or the misregulation of centrosome duplication during interphase can cause mitotic defects, including mulitpolar spindles [46,47], suggesting that some of the mitotic defects caused by the Y783A mutation may be inherited from interphase. Furthermore, when cells adhered by their endogenous integrins were arrested at mitosis with nocodazole, washed and replated on Fg, the Y783A mutation also resulted in spindle and cytokinesis defects (CG Reverte unpublished data, [44**]). Taken together these results suggest that integrins affect spindle assembly by regulating events both during interphase and mitosis (Fig. 2).
Figure 1. Perturbing integrin function inhibits the bipolar spindle assembly.
CHO cells expressing recombinant Fg receptors containing either the wild type β1 tail (WT) or the mutant β tail containing a tyrosine to alanine substitution in the membrane-proximal NPIY motif (Y783A) cells were serum-starved and then replated on Fg-coated coverslips in the serum-free growth promoting medium CCM1. After 15–18 h, cells were immunostained as indicated. The percentage of cells with each major phenotype is indicated below a representative image. The results are the mean of three independent experiments ± SD and show that approximately 85% and 7.5% of cells adhered by the WT and Y783A mutant integrins, respectively, formed normal bipolar spindles. Images (100×) were collected as z-series for all three channels, similarly processed and deconvolved to generate 3D images. Bar: 5 μm. (Taken from Reverte et al (2006) Journal of Cell Biology 174: 491-497)
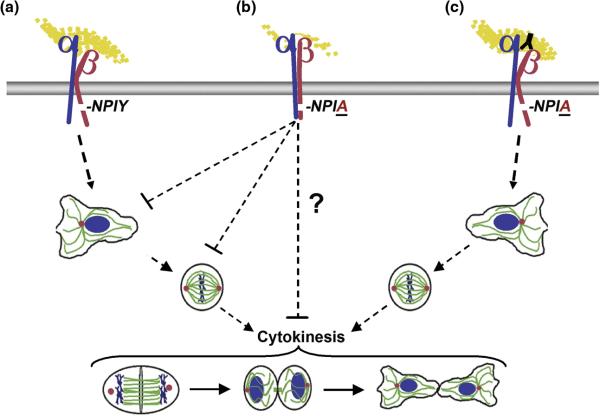
Figure. 2. Model for the Regulation of Spindle Assembly by Integrins.
(A) The wild-type integrin containing an intact NPIY motif in the β1 tail is in the active conformation adhered to Fg, thereby activating signals that promote the nucleation of MT assembly from the centrosome and a radial MT network at interphase and the formation of the bipolar spindle at mitosis, ensuring the successful completion of cytokinesis. (B) The Y783A mutant (NPIA) suppresses cell adhesion on Fg and signaling to centrosomes, leading to the inhibition of MT assembly from the centrosome, bipolar spindle formation and cytokinesis. Current evidence suggests that integrin signaling both during interphase and mitosis impacts spindle assembly. It is not yet known whether perturbing integrin function can inhibit cytokinesis subsequent to spindle assembly. (C) Activating antibodies circumvent the requirement for a wild-type NPIY motif to regulate integrin conformation and restore integrin signaling pathways to promote centrosome function and spindle assembly in a NPIY-independent manner.
Studies to identify targets and mechanisms of integrin function inhibited by the Y783A mutation that contribute to the mitotic phenotypes are in progress. At present, it is known that the mutant integrin is defective in FAK/ Src signaling, the assembly of focal adhesions and actin stress fibers, and that activating the mutant integrin restores these processes (B Nieves, CW Jones, CG Reverte unpublished data, [44**]). It is not yet known whether restoring focal adhesion formation and/or FAK/Src signaling will be sufficient to restore centrosome function, bipolar spindle formation, and cytokinesis, or whether additional pathways are involved. In this regard, Mt growth can be restored in cells adhered by the mutant integrin with the addition of phorbol 12-myristate 13-acetate (PMA) by a mechanism involving the activity of PKCδ (CG Reverte, unpublished data). Furthermore, the focal adhesion proteins PAK1, HEF1, and ILK have recently been shown to regulate spindle assembly and other mitotic events [48–51]; it will be important to determine whether their mitosis-associated activities are inhibited by the mutant integrin.
Studies described above analyzing the role of cell adhesion in regulating spindle assembly and positioning/orientation suggest that the requirement for integrin function in specific mitotic events depends upon the activation of other surface receptors. In cells adhered to Fn in serum-containing medium, the inhibition of –1 integrins resulted in spindle misorientation without significant defects in spindle assembly [25**]. In contrast, spindle assembly and cytokinesis were both inhibited in cells adhered to Fg by the mutant integrin in a serum-free, growth-promoting medium [44**]. Similar results were observed when cells were plated in a serum-free growth medium in the absence of Fn [52]. Additional investigation is needed to define how β1 integrins cooperate with other cell surface receptors to regulate bipolar spindle assembly, positioning and cytokinesis.
In Vivo Models
Currently, there are two examples in which the integrin-regulation of the mitotic machinery in mammalian cells has physiological consequences. In mouse chondrocytes, the conditional deletion of the β1 gene resulted in the accumulation of binucleated chondrocytes with age, suggesting defects in cytokinesis; these mice also developed chondrodysplasia [53*]. Conditional deletion of the β1 gene in basal keratinocytes caused defects in spindle positioning. In these cells, the mitotic spindle normally assembles perpendicular to the basement membrane to promote an asymmetric cell division that generates a proliferative daughter cell that continues to adhere to the basement membrane and a committed suprabasal cell [24*]. Disrupting β1 integrin expression inhibited the correct orientation of the spindle and the proper differentiation of the epidermis [24*]. Interestingly, in Drosophila, the differentiation of the ovarian follicular simple epithelium requires integrins to promote the alignment of the spindle parallel to the basement membrane ensuring that each daughter remains adhered to the basement membrane to prevent inappropriate stratification [54]. Since the potential contribution of spindle defects to integrin-null phenotypes in mice has not been extensively analyzed, a re-examination of integrin-knockouts that lead to defects in differentiation events may prove interesting.
Conclusions and Perspectives
The identification of integrins as regulators of mitotic events provides a new paradigm in integrin signaling. The mechanisms by which integrins regulate these events are just beginning to be unraveled. Most progress has been made in understanding how integrins regulate spindle positioning and orientation. Dissecting the mechanisms by which integrins promote spindle bipolarity may not be a trivial task as this process is more complex. Furthermore, integrins may initiate several signaling events during interphase that converge on a number of different pathways activated by other receptors to regulate various steps in bipolar spindle assembly.
Interestingly, both the activation and inactivation of integrin signaling may be required at mitosis. The observation that many cells in culture loosen their attachment to the matrix and round during mitosis suggests that integrin activity and signaling is downregulated [55]. Consistent with this idea, the tyrosine phosphorylation of FAK, p130Cas and paxillin, critical to the assembly of integrin signaling complexes, is inhibited at mitosis [56–58]. However, many mitotic cells maintain regions of adhesion on their basal surface in addition to retractions fibers, suggesting that a small subset of integrins remain activated. Further investigation is needed to determine how integrin signaling is regulated during mitosis. Perhaps, sophisticated imaging of the adhesion plane for the presence of organized adhesion structures containing activated integrin signaling proteins will be instructive.
Importantly, the finding that perturbing integrin function results in the generation of multipolar spindles has important implications for cancer biology, as multipolar spindles and the resulting aneuploidy are thought to contribute to tumorigenesis and metastasis [59–61]. Thus, understanding the mechanisms by which integrins promote spindle bipolarity may provide new therapeutic targets.
Acknowledgements
The authors thank Drs. Yu-Li Wang and Fumiko Toyoshima for helpful discussions and Ms. Debbie Moran for assistance in the preparation of the figures. Work from the LaFlamme laboratory was supported by grant GM51540 to SEL from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Compton DA. Spindle assembly in animal cells. Annu Rev Biochem. 2000;69:95–114. doi: 10.1146/annurev.biochem.69.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Nigg EA. Centrosome duplication: of rules and licenses. Trends Cell Biol. 2007;17:215–221. doi: 10.1016/j.tcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol. 2004;14:R797–805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 8.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 9.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 10.Ylanne J, Chen Y, O'Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otey CA, Vasquez GB, Burridge K, Erickson BW. Mapping of the alpha-actinin binding site within the beta 1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- 12.Pfaff M, Liu S, Erle DJ, Ginsberg MH. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 14.Berrier AL, Mastrangelo AM, Downward J, Ginsberg M, LaFlamme SE. Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains. J Cell Biol. 2000;151:1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrier AL, Martinez R, Bokoch GM, LaFlamme SE. The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J Cell Sci. 2002;115:4285–4291. doi: 10.1242/jcs.00109. [DOI] [PubMed] [Google Scholar]
- 16.Bodeau AL, Berrier AL, Mastrangelo AM, Martinez R, LaFlamme SE. A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tyrosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J Cell Sci. 2001;114:2795–2807. doi: 10.1242/jcs.114.15.2795. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 19*.O'Connell CB, Wang YL. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell. 2000;11:1765–1774. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used a novel micromanipulation approach to examine the relationship between cell shape and spindle positioning. These studies demonstrate that cells continually monitor position of the spindle relative to cortical cues and respond to changes in cell shape during mitosis by repositioning their spindles and do so by a mechanism requiring dynein and astral microtubules.
- 20.Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8:1110–1116. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- 23**.Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]; The authors used micro-contact printing to control the spatial distribution of desired extracellular matrix proteins on a substratum. These studies were the first demonstration that the extracellular matrix regulates the segregation of cortical components during interphase to regulate the spindle and division axis during mitosis.
- 24*.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors were the first to demonstrate that β1 integrins are essential for proper spindle orientation in vivo. They showed that β1 integrins are required in mouse epidermis to orient the spindle perpendicular to the basement membrane in basal keratinocytes, which is required for epidermal stratification and differentiation.
- 25**.Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 2007;26:1487–1498. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was the first to demonstrate that β1 integrins are required to ensure the mitotic spindle is oriented parallel to the adhesion plane in culture cells. They further showed that EB1, astral microtubules and actin polymerization were required for integrins to direct the orientation of the spindle.
- 26**.Toyoshima F, Matsumura S, Morimoto H, Mitsushima M, Nishida E. PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev Cell. 2007;13:796–811. doi: 10.1016/j.devcel.2007.10.014. [DOI] [PubMed] [Google Scholar]; The authors present an elegant study teasing out the underlying mechanisms for integrin-dependent control of spindle orientation. They demonstrated that integrins regulate the localized accumulation of PI(3,4,5)P3 to the midsection at the cortex, which contributes to the recruitment of dynein motor complexes that provide pulling forces on the spindle required for orienting it parallel to the adhesion plane.
- 27.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 30.Thery M, Bornens M. Cell shape and cell division. Curr Opin Cell Biol. 2006;18:648–657. doi: 10.1016/j.ceb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Manneville JB, Etienne-Manneville S. Positioning centrosomes and spindle poles: looking at the periphery to find the centre. Biol Cell. 2006;98:557–565. doi: 10.1042/BC20060017. [DOI] [PubMed] [Google Scholar]
- 32.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 33.Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol. 2007;178:1109–1120. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 35.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 36.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 38.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 39.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- 40.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of microtubule destabilizing activity of Op18/stathmin downstream of Rac1. J Biol Chem. 2004;279:6196–6203. doi: 10.1074/jbc.M307261200. [DOI] [PubMed] [Google Scholar]
- 41.Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 42.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 43.OToole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Reverte CG, Benware A, Jones CW, LaFlamme SE. Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J Cell Biol. 2006;174:491–497. doi: 10.1083/jcb.200603069. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors were the first to show that integrin signaling influences centrosome function and MT assembly required for the formation of the bipolar spindle at mitosis. Since defects in centrosome function and MT assembly were observed both during interphase and mitosis additional investigation is needed to dissect the specific requirements for integrin function at different points in the cell cycle.
- 45.O'Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- 46.Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Mandal M, Kumar R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002;3:767–773. doi: 10.1093/embo-reports/kvf157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol. 2008;180:681–689. doi: 10.1083/jcb.200710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orly J, Sato G. Fibronectin mediates cytokinesis and growth of rat follicular cells in serum-free medium. Cell. 1979;17:295–305. doi: 10.1016/0092-8674(79)90155-7. [DOI] [PubMed] [Google Scholar]
- 53*.Aszodi A, Hunziker EB, Brakebusch C, Fassler R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003;17:2465–2479. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors were the first to demonstrate that inhibiting β1 integrin function can block cytokinesis in vivo. Mice containing a chondrocyte-specific deletion of β1 integrins developed chondroplasia and showed defects in the G1/S transition and cytokinesis.
- 54.Fernandez-Minan A, Martin-Bermudo MD, Gonzalez-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicularepithelium monolayer. Curr Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 55.Glotzer M. Animal cell cytokinesis. Annu Rev Cell Dev Biol. 2001;17:351–386. doi: 10.1146/annurev.cellbio.17.1.351. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi R, Mazaki Y, Hirota K, Hashimoto S, Sabe H. Mitosis specific serine phosphorylation and downregulation of one of the focal adhesion protein, paxillin. Oncogene. 1997;15:1753–1761. doi: 10.1038/sj.onc.1201345. [DOI] [PubMed] [Google Scholar]
- 57.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma A, Richardson A, Schaefer EM, Parsons JT. Serine phosphorylation of focal adhesion kinase in interphase and mitosis: a possible role in modulating binding to p130(Cas) Mol Biol Cell. 2001;12:1–12. doi: 10.1091/mbc.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 60.D'Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 61.D'Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R, Whitehead C, Stivala F, Lingle WL, Salisbury JL. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat. 2002;75:25–34. doi: 10.1023/a:1016550619925. [DOI] [PubMed] [Google Scholar]