Abstract
Inhaled and intravenously administered adenosine induces mast cell-mediated (histamine-dependent) bronchospasm in asthmatics without causing urticaria. A differential response to adenosine by human lung and skin mast cells is shown: low concentrations potentiate FcεRI-induced degranulation of human lung mast cells but not that of skin mast cells. Human lung mast cells were found to express ~3-fold more A3AR mRNA than skin mast cells, suggesting the involvement of the Gi-linked A3AR. Indeed, the adenosine-induced potentiation was sensitive to inhibition by pertussis toxin, and, furthermore, could be induced with an A3AR-specific agonist. This study reveals a previously unrecognized disparity in the response to adenosine by primary human mast cells from lung and skin that might explain why adenosine induces a pulmonary but not dermatologic allergy-like response in vivo. In addition, we identify the A3AR as a potentiating receptor of FcεRI-induced degranulation, thereby implicating it in the in vivo bronchoconstrictive response to adenosine in asthmatics.
Keywords: adenosine, human mast cells, G protein-coupled receptors, asthma, urticaria
INTRODUCTION
Inhaled and intravenously administered adenosine induces bronchoconstriction in asthmatics.(1;2) This response to inhaled adenosine is thought to be the result of a potentiating effect of the purine nucleoside on mast cell degranulation in the airways, because it is accompanied by an increase in histamine and tryptase levels in bronchoalveolar lavage fluid and can be largely antagonized by histamine receptor H1R antagonists (3–7). In addition, adenosine receptor antagonists protect against adenosine-provoked bronchoconstriction (8). Physiologic concentrations of adenosine to which mast cells might be exposed in tissues are uncertain, but adenosine concentrations in airway fluid have been estimated to average about 6 × 10−5 M for healthy subjects, and about 19 × 10−5 M for asthmatics (9). Whether the higher endogenous adenosine levels in asthmatic airway fluid also provokes mast cell activation and bronchospasm remains to be determined. Therefore, understanding the effects of adenosine and adenosine receptor activation on mast cell function is of considerable interest.
Although studies have shown that adenosine potentiates IgE-dependent degranulation of rodent mast cells (10), the effect on human mast cells is less clear. In some studies, adenosine increased IgE-mediated degranulation (11), while other studies have provided contradictory results. For example, adenosine inhibited, rather than potentiated, FcεRI-mediated degranulation of purified lung mast cells (12) and umbilical cord blood-derived mast cells (13). In addition, adenosine inhibited antigen-induced histamine release from human lung fragments (14), but was shown to augment IgE-mediated degranulation at 10 µM and inhibit degranulation at 1 mM by 4% pure preparations of lung mast cells (15). Thus, despite the evidence from rodent studies that adenosine enhances degranulation from immunologically-activated mast cells the effect of extracellular adenosine on human mature mast cells is incompletely understood.
Extracellular adenosine exerts its influence through surface-expressed G protein-coupled receptors (A1AR, A2aAR, A2bAR and A3AR) (16). The functional outcome of adenosine receptor stimulation generally depends on which G proteins are engaged. A2aAR and A2bAR are linked to adenylate cyclase activation and increased levels of intracellular cAMP via their predominant coupling with Gs proteins, while A1AR and A3AR utilize Gi proteins that are associated with the inositol triphosphate pathway and calcium mobilization (16). A2bAR also binds Gq proteins (17;18) and, thus, is linked to both adenylate cyclase activation through Gs and the inositol triphosphate production through Gq. A subject of intense research has been the identification of the adenosine receptor(s) responsible for the adenosine-induced hyper-reactivity in asthmatics and its effects on mast cells. The clinical observation that enprofylline, a phosphodiesterase inhibitor with antagonistic properties against A2bAR (but not A2aAR), provided protection against bronchoconstriction provoked by AMP (8) raised the notion that A2bAR was responsible for mediating the adenosine-induced bronchoconstriction in asthmatics (4). In rodents, in vivo and in vitro studies with genetically-deficient mice have shown clearly that activation of A3AR on mast cells induces airway hyper-responsiveness to inhaled adenosine (19) and potentiates IgE-induced degranulation of bone marrow-derived mast cells (20;21). In humans, biochemical studies with the mast cell leukemia cell line, HMC-1, have provided intriguing evidence of a possible role for both A2bAR and A3AR in cytokine production (22–24); however, these studies could not directly address the role of these receptors on IgE-induced mast cell degranulation because the HMC-1 cell line does not express FcεRI (25). Therefore, the adenosine receptor(s) involved in adenosine-mediated augmentation of FcεRI-induced degranulation of human lung mast cells has not been identified.
Here, we use genetically non-modified primary mast cells dispersed and purified from human lung and skin to show that extracellular adenosine affects FcεRI-mediated degranulation from lung mast cells in a bi-modal fashion – it potentiates at low (10−7–10−5 M) and inhibits at high (10−4–10−3 M) concentrations. In contrast, degranulation of skin mast cell is not potentiated by adenosine, but like lung mast cells is inhibited at high adenosine concentrations. Further, the potentiating effect of adenosine on degranulation of lung mast cells is mediated via a Gi protein-dependent pathway that can be initiated by direct stimulation of A3AR. These data clarify the longstanding issue regarding the effect of adenosine on IgE-induced degranulation of mature human lung mast cells and identify A3AR as the potentiating adenosine receptor. Together, these data help to explain why adenosine evokes clinical signs of mast cell degranulation in lung rather than skin.
METHODS
Mast cell isolation and purification
Fresh samples of human skin and lung were obtained from the Department of Pathology at Virginia Commonwealth University, Cooperative Human Tissue Network of the National Cancer Institute or the National Disease Research Interchange, as approved by the Human Studies Internal Review Board at Virginia Commonwealth University. The tissues were digested at 37°C with collagenase and hyaluronidase (Sigma, St. Louis, MO) for 1 h (lung) or 3 × 1 h (skin) in Minimal Essential Medium Eagle (2% FCS) (lung) or HBSS buffer (1X HBSS 0.04% NaHCO3, 1% fetal bovine serum, 1% HEPES, 0.1% CaCl2) (skin) containing amphotericin B and Antibiotic/Antimycotic solution. After digestion, the samples were filtered through 70 µm and 40 µm nylon cell strainers, washed and separated on a Percoll gradient. Cells at the buffer/Percoll interface were collected and washed. CD117+ lung mast cells were positively selected using the MACS human CD117 MicroBead Kit (Miltenyi Biotec, Auburn, CA) according to manufacturer’s instructions. The cells were re-suspended at 5×105 cells/ml in X-VIVO media (Cambrex, Walkersville, MD) containing stem cell factor (100 ng/ml; gift of Swedish Orphan Biovitrum, Stockholm, Sweden) and cultured in 24-well plates with weekly medium changes. To test for purity, the cells were assessed cytochemically after cytocentrifugation by metachromatic staining with acidic toluidine blue and by flow cytometry with 22E7 mAb. Typically, mature skin mast cells were of 95 – 100% purity by 6 weeks of culture and used experimentally between 8 – 12 weeks. Purified lung mast cells were used after 1 – 2 weeks of culture.
Mast cell activation
Mast cells (106 cells/ml) were pre-treated (10 min) and activated (30 min) at 37°C in complete Tyrode’s Buffer (135 mM NaCl, 1 mM MgCl2, 20 mM Hepes, 5 mM KCl, 1.8 mM CaCl2, 5.6 mM glucose; pH 7.4) containing 0.05% bovine serum albumin at 37°C. The 10 min pre-incubation period with adenosine allowed time for adenosine to fully exert its effect before activating with anti-FcεRI mAb and was based on the methods used in several published studies including (13;14;26). The anti-FcεRIα mAb 22E7 (100 ng/ml) (generously provided by J. P. Kochan (Hoffman-LaRoche, Nutley, NJ) (27) was used to cross-link FcεRI and thereby activate these cells. Adenosine (Sigma-Aldrich, St. Louis, MO).was re-suspended in Tyrode’s Buffer. For experiments with Pertussis Toxin (PTX) (Sigma-Aldrich, St. Louis, MO), the cells were cultured overnight in X-VIVO 15™ media containing stem cell factor (100 ng/ml) without and with PTX (100 ng/ml). NECA and HEMADO (Tocris-Cookson, Ellisville, MO) were re-suspended in DMSO, and in these experiments the final concentration of DMSO was 0.1% and 0.025%, respectively.
After the activation period, cells and medium were separated by centrifugation. The cells were lysed with 1% Triton X-100 and degranulation was assessed by measuring the secretion of β-hexosaminidase activity using a colorimetric assay. Briefly, the release of p-nitrophenol from the substrate p-nitrophenyl N-acetyl-β-D-glucosaminide was measured as described.(28) Absorbance values were read at 405 nm with a SpectraMax 384 Plus UV-VIS plate reader (Molecular Devices Corporation, Chicago, IL). % release values from stimulated cells were calculated using the following formula: ((stimulated cell releasate/(stimulated cell releasate + stimulated cell lysate)) × 100. Net % release values were calculated by subtracting spontaneous from stimulated percent release values.
mRNA analysis
RNA from resting mast cells was isolated and DNase-treated, respectively, with the RNeasy Miniprep kit and RNase-Free DNase Set (Qiagen, GmbH, Germany). Cerebellum brain RNA (Ambion, Austin, TX) was used as a positive control. cDNA was synthesized (5 min at 65°C, 50 min at 50°C, 5 min at 85°C, 20 min at 37°C) from 250 ng total RNA using oligo(dT)15–20 and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) with a Perkin Elmer Master Gradient amplifier. The adenosine receptor primers were designed using the NIH primer designer tool Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). To prevent false positive amplification of genomic DNA, primer pairs that bind to unique sequences within the different exons and whose product spans across the large intron characteristic of A2aAR, A2bAR and A3AR and that separates exons 5 and 6 of A1AR were chosen. Using primers that bind to exon sequences ensured that the appropriately-sized PCR products were not the result of genomic DNA amplification since non-specific products could be easily accessed by size determination. Primers used (sense; antisense; product size; accession number): A1AR (5’- cattgggccacagacctact-3’; 5’-cagattgttccagccaaaca-3’; 238 bp; NM_000674.2 variant 1 and NM_001048230.1 variant 2); A2aAR (5’-cattgcctgcttcgtcct-3’; 5’-gatgcccttagccctcgt-3’; 136 bp; NM_000675.4); A2bAR (5’-ctccatcttcagccttctgg-3’; 5’-acaaggcagcagctttcatt-3’; 236 bp; NM_000676.2); A3AR: 5’-gggcatcacaatccacttct-3’; 5’-agggccagccatattcttct-3’; 171 bp; NM_000677.3 variant 2); GAPDH (5'-caatgaccccttcattgacc-3’; 5’-ttgattttggagggatctcg-3’; 159 bp; NM_002046.3); β-actin: 5’-gctcgtcgtcgacaacggctc-3’; 5’-caaacatgatctgggtcatcttctc-3’ (353 bp; Invitrogen, Carlsbad, CA). For RT-PCR, 1 µl of cDNA was added to the reaction mix (10 µM sense primer (1 µl), 10 µM antisense primer (1 µl), Taq DNA polymerase (2 units), 10X PCR buffer minus Mg2+ (5 µl), 10 mM dNTP mix (1 µl), 50 mM MgCl2 (1.5 µl)) in a 50 µl final volume with DEPC-H2O. RT-PCR protocol: (94°C for 4 min, (94°C for 1 min, 55°C for 1 min, 72°C for 1 min) × 35 cycles, 72°C for 10 min) products were electrophoresed in a 1.5% agarose gel with Tris-Borate-EDTA running buffer and stained with ethidium bromide. The stained gels were visualized on a BioRad Molecular Imager FX. For quantitative real time PCR analysis, 2 µl of cDNA were combined with 1 µl of sense and antisense primers (10 µM each for A2aAR, A2bAR and A3AR) and 12.5 µl of SYBR Green Supermix from the iScript™ SYBR Green RTPCR kit (Bio-Rad, Hercules, CA) in a final volume of 25 µl. A hot-start protocol (95°C for 5 min, (95°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec) × 30 cycles, 95°C for 1 min, 55°C for 1 min) was run on a Bio-Rad CFX96 Real Time System. A melting curve was generated at the end of each experiment to assess primer-dimer formation.
Statistical analyses
Statistical analysis was performed using GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego, CA, USA; www.graphpad.com.
RESULTS
Extracellular adenosine at low concentrations augments FcεRI-induced degranulation of human mast cells from lung but not skin
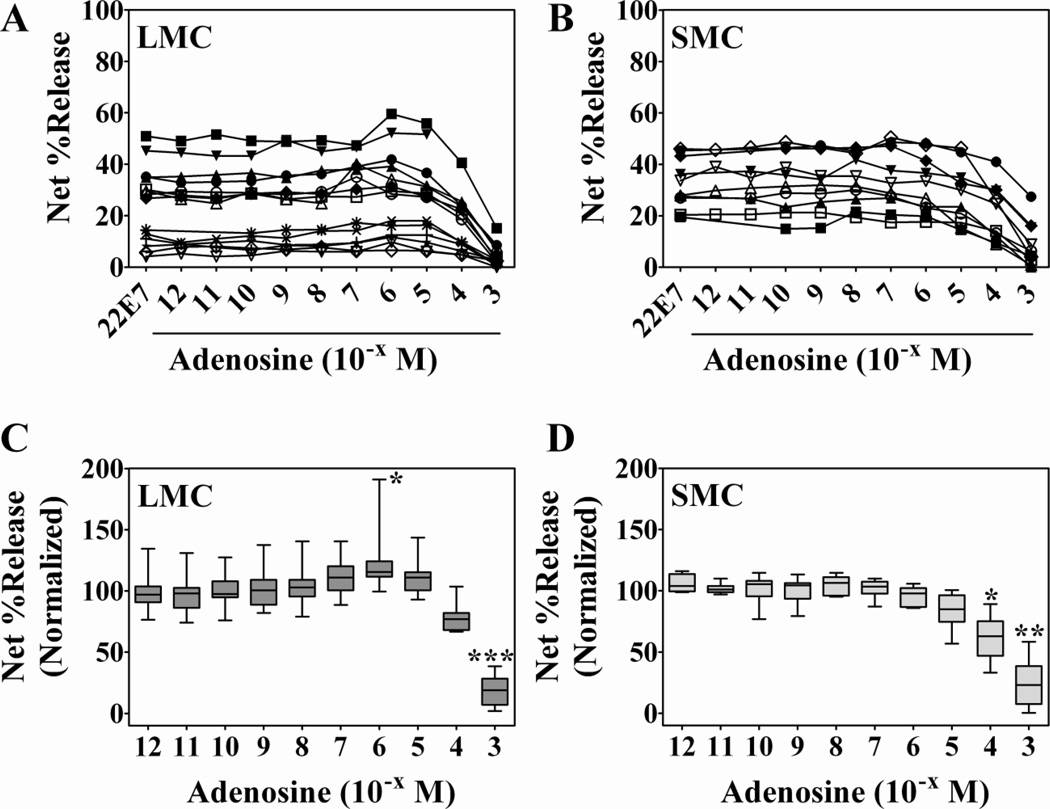
To examine the effect of adenosine on human mast cell degranulation, mast cells dispersed from lung (n = 14 preparations) and skin (n = 10 preparations) tissues, were pre-incubated with increasing doses of adenosine (10−12 – 10−3 M) and activated by cross-linking FcεRI with the IgG anti-FcεRIα mAb 22E7 (27). Degranulation was determined by measuring the release of β-hexosaminidase, a granule component, as described (28). Spontaneous release from lung and skin mast cells, respectively, was 11.5 ± 1.7 and 11 ± 1.3%. Net degranulation (22E7-induced minus spontaneous release of β-hexosaminidase) of mast cells activated with 22E7 alone was variable for different preparations (Figure 1A and B), particularly for lung mast cells. Therefore, to compare the different mast cell preparations, % net release values were normalized to the release values obtained with 22E7 alone (Figure 1C and D). An increase in the degranulation of FcεRI-mediated degranulation of lung mast cells was apparent at 10−7, 10−6 and 10−5 M concentrations (11.9 ± 4.4%, 25.4 ± 9.3% and 11.6 ± 4.2%, respectively) with the increase induced with 10−6 M adenosine being significant (p<0.05) (Figure 1C). In contrast, FcεRI-induced degranulation of skin mast cells was not enhanced by adenosine relative to degranulation induced by 22E7 alone (Figure 1B and 1D). The inability of adenosine to enhance degranulation of skin mast cells was not due to a defect in degranulation capacity since skin and lung mast cells responded equally well to calcium ionophore (65.5 ± 2.4% and 61.9 ± 4.1% release, respectively). Also, adenosine alone failed to induce degranulation of mast cells from either tissue (data not shown). Higher concentrations of adenosine strongly inhibited the degranulation response to FcεRI cross-linking from both tissues. At 100 µM and 1 mM adenosine, the percentage of maximal degranulation of lung mast cells was 78 ± 13 and 18 ± 13, respectively, with the inhibition at 1 mM being highly significant (Figure 1C). For skin mast cells, significant inhibition was observed at 100 µM (61 ± 18% of maximal) and 1 mM (24 ± 19% of maximal) adenosine. These results demonstrate a differential response by FcεRI-activated human lung and skin mast cells to low concentrations of extracellular adenosine: it potentiates the degranulation of mast cells from lung but not skin tissue. On the other hand, higher concentrations of adenosine inhibit FcεRI-induced degranulation of mast cells from both tissues. Thus, while the inhibitory effect of adenosine at high concentrations is common to mast cells from lung and skin, the potentiating response to low concentrations of adenosine is specific to lung mast cells.
Figure 1. Low concentrations of adenosine potentiate FcεRI-induced degranulation of human mast cells from lung but not skin.
Mast cells from human lung (A and C, n=14 preparations) and skin (B and D, n=10 preparations) were pre-incubated with adenosine for 10 min and then activated with anti-FcεRI mAb 22E7 (0.1 µg/ml) for 30 min at 37°C. β- Hexosaminidase % release was quantified and net % release was determined (A and B). Spontaneous release from lung and skin mast cells was 11.5 ± 1.7% and 11 ± 1.3%, respectively. For comparison, net % release values were normalized to those from cells activated in the absence of adenosine (C and D). Adenosine at 10−6 M induced a significant overall increase (25 ± 9%) in degranulation of lung mast cells, but not skin mast cells. Higher concentrations of adenosine inhibited degranulation of both subsets. Adenosine alone had no effect (not shown). *, p<0.05, by Kruskal-Wallis non-parametric analysis followed by Dunn’s multiple comparison post-test.
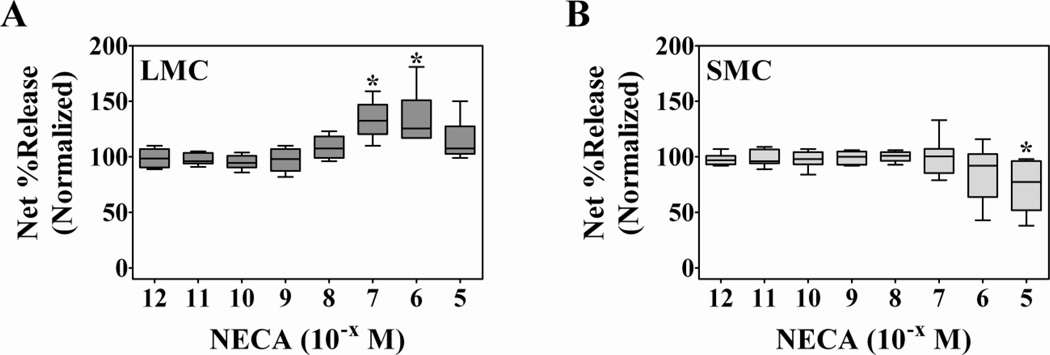
To assure that the potentiating effect of adenosine on degranulation of lung mast cells was due to adenosine receptor signaling and not a metabolic or by-product effect from adenosine degradation, identical experiments were carried out with the stable adenosine analogue 5’-N-ethylcarboxamideadenosine (NECA). As Figure 2 shows, NECA provoked responses for lung (n = 6 preparations) and skin (n = 8 preparations) mast cells that were comparable to those of adenosine. NECA significantly augmented the release of β-hexosaminidase from 22E7-activated lung mast cells (Figure 2A) at 10−7 M (33.5 ± 7% increase) and 10−6 M (34.5 ± 10% increase), but had no potentiating effect on the degranulation response of skin mast cells (Figure 2B). Spontaneous release for lung and skin mast cells, respectively, was 7 ± 2% and 5 ± 1%. Noteworthy, the insolubility of NECA at concentrations greater than 10−5 M prevented the examination of the effects of this analogue at higher concentrations. Nevertheless, significant inhibition of skin mast cell degranulation was observed at 10−5 M NECA. These results with NECA confirm the observations made with adenosine (Figure 1). Therefore, we conclude that the enhancing effect of adenosine on degranulation of lung mast cells was the result of adenosine receptor signaling and not an effect of by-products of adenosine metabolism.
Figure 2. The non-specific adenosine analogue NECA enhances FcεRI-induced degranulation of lung mast cells but not skin mast cells.
Human mast cells from lung (A, n = 6 preparations) and skin (B, n = 8 preparations) were pre-incubated with NECA for 10 min then activated with anti-FcεRI mAb 22E7 (0.1 µg/ml) for 30 min at 37°C. β-Hexosaminidase % release was quantified and net % release was determined. Spontaneous release for lung and skin mast cells, respectively, was 7 ± 2% and 5 ± 1%. For comparison, net % release values were normalized to those from cells activated in the absence of adenosine (LMC: 28.5 ± 2.2%; SMC: 41.9 ± 4.8%). NECA significantly enhanced degranulation of lung mast cells at 10−7 M (33.5 ± 7% increase) and 10−6 M (34.5 ± 10% increase), but had no potentiating effect on the degranulation of skin mast cells. NECA alone had no effect (not shown). *, p<0.05 by Kruskal-Wallis non-parametric test followed by Dunn’s multiple comparison post-test.
The A3AR is more highly expressed in human lung than skin mast cells
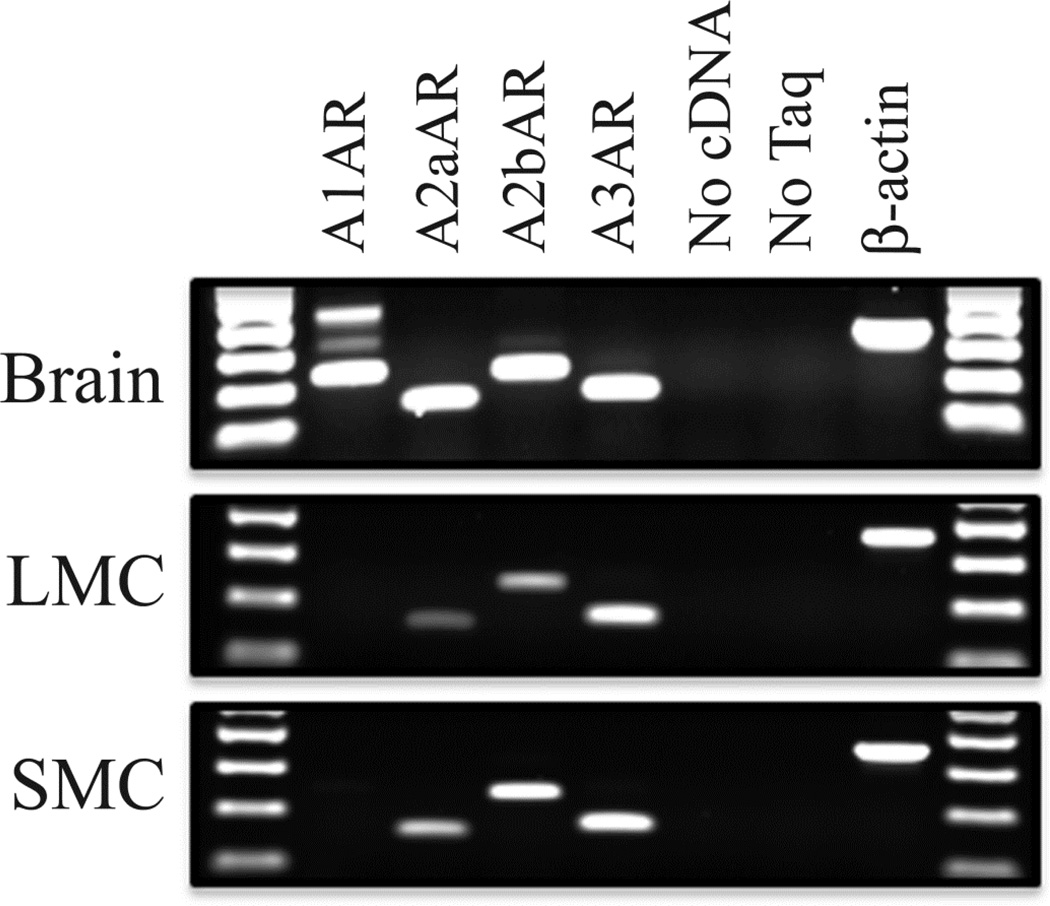
One possible explanation for the disparate response to adenosine by lung and skin mast cells would be a difference in adenosine receptor expression. Accordingly, the expression profiles of these receptors in resting mast cells from lung and skin tissues were examined. Standard RT-PCR reactions were performed with oligonucleotide primer pairs for each adenosine receptor and total RNA isolated from resting lung and skin mast cells (Figure 3). To minimize false positive amplification, 5' and 3' primers for each pair recognized sequences that arose in different exons and whose product therefore spanned across large introns of A1AR, A2aAR, A2bAR and A3AR. Total RNA from human cerebellum, where all four adenosine receptors are known to be expressed, was used as a positive control. This analysis demonstrated that human primary mast cells from skin and lung express mRNA transcripts for A2aAR, A2bAR and A3AR, but lack those for A1AR; thus, demonstrating no qualitative differences in the pattern of adenosine receptor expression. The absence of A1 and presence of A2a, A2b and A3 adenosine receptors also has been reported for HMC-1 cells (29).
Figure 3. Mast cells from human lung and skin express the same repertoire of adenosine receptors.
RT-PCR analysis of all 4 adenosine receptors was carried out on RNA isolated from resting mast cells from lung (n=12 preparation) and skin (n=17 preparations). RNA from human brain, which expresses all 4 adenosine receptors, was used as a positive control. The RT-PCR products were electrophoresed on 1.5% agarose Tris-Borate-EDTA gels and stained with ethidium bromide. Mast cells from both tissues express A2aAR, A2bAR and A3AR, but not A1AR.
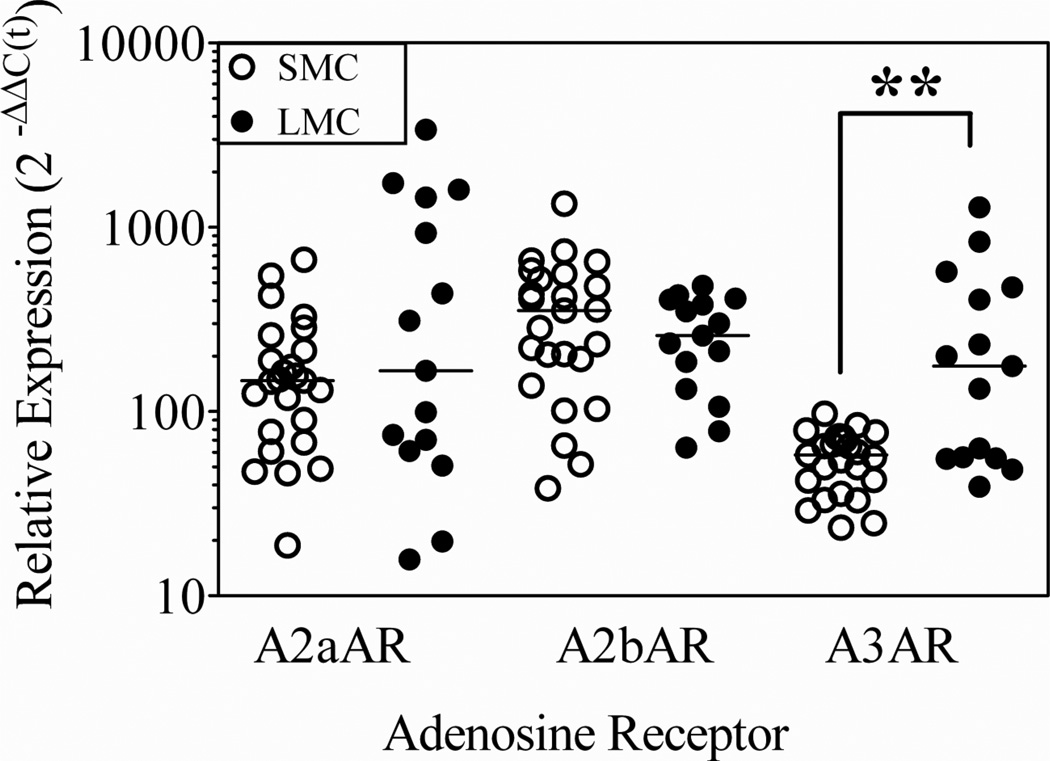
Quantitative real time PCR was used to determine if there were quantitative differences in receptor expression in resting human lung and skin mast cells. Using SYBR Green fluorescent dye for detection, C(t) values for each adenosine receptor and the housekeeping gene GAPDH were obtained, and comparative analyses were done using the 2−ΔΔC(t) method (Figure 4). The median [25%, 75%] values obtained with skin (n = 25 preparations) and lung (n = 15 preparations) mast cells, respectively, were the following: A2aAR: (147 [73, 236]; 166 [61, 1451]); A2bAR: (352 [165; 539]; 258 [132, 405]); A3AR: (58 [39, 73]; 176 [56, 471]). Skin and lung mast cells expressed statistically similar amounts of A2aAR and of A2bAR mRNA transcripts. In contrast, the median expression of A3AR was about 3 times greater in lung compared with skin mast cells (p<0.01). The significantly greater expression of A3AR in lung than skin mast cells suggested that this receptor might account, at least in part, for the differential response to low concentrations of adenosine. Therefore, functional assays were performed to determine if A3AR signaling was required and sufficient for the potentiating effect of adenosine on degranulation of lung mast cells.
Figure 4. Adenosine receptor A3AR is more highly expressed in mast cells from human lung compared with human skin mast cells.
Quantitative real time PCR was performed to determine if there were quantitative differences in adenosine receptor expression. The (median [25%, 75%]) values obtained with skin (n=25 preparations) and lung (n=15 preparations) mast cells, respectively, were determined. **, p=0.006 by non-parametric Mann-Whitney test.
Adenosine-evoked potentiation of FcεRI-stimulated degranulation of human lung mast cells is a Gi protein-dependent process mediated by the A3AR
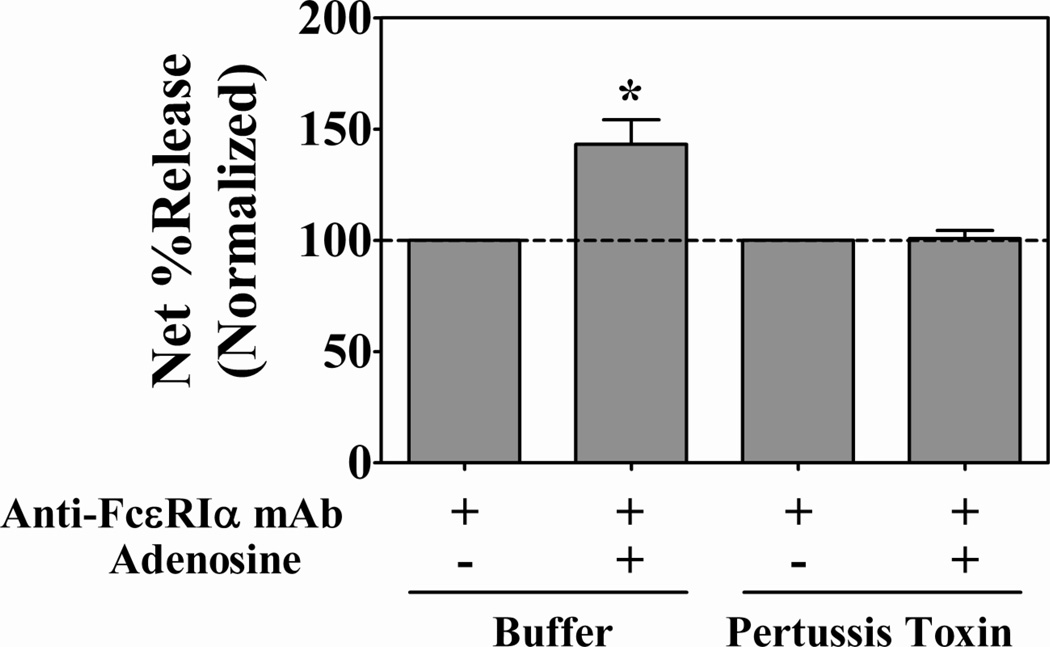
To test the possibility that the quantitative difference in expression of the A3AR might account for the differential response to low concentrations of extracellular adenosine, we took advantage of the fact that of the three possible adenosine receptors only A3AR signals through Gi proteins (22). Pertussis toxin (PTX), which inactivates Gi proteins by ADP ribosylation effectively inhibits Gi-mediated signal transduction (22;30;31), and, therefore, was used to determine if Gi protein activity was necessary for adenosine-augmented degranulation. Untreated and PTX-treated lung mast cells were activated with 22E7 in the presence or absence of adenosine and β-hexosaminidase release was determined. As expected, adenosine induced a significant increase (43 ± 11%) in FcεRI-mediated degranulation in the absence of PTX (Figure 5). In contrast, adenosine failed to augment the degranulation of lung mast cells whose Gi proteins had been inactivated with PTX. PTX did not inhibit lung mast cell degranulation as indicated by the similar response to calcium ionophore A23187 by untreated (71.9 ± 6.1% net percent release) and PTX-treated (75.7 ± 5.2% net percent release) mast cells. Thus, these data demonstrate that the potentiating effect of low concentrations of adenosine was entirely dependent on Gi protein-mediated signaling. This suggested that A3AR was the receptor responsible for the potentiating response to adenosine by lung mast cells.
Figure 5. Adenosine-induced potentiation of FcεRI-mediated degranulation of human lung mast cells is a Gi protein-dependent process.
Lung mast cells (n=4 preparations) were incubated overnight without and with pertussis toxin (100 ng/ml) to inactivate Gi proteins, washed, pre-incubated with adenosine (1 µM) for 10 min then activated with anti-FcεRI mAb 22E7 (0.1 µg/ml) for 30 min at 37°C. β-Hexosaminidase % release was quantified and net % release was determined. Spontaneous percent release values: (7.4 ± 1.2 (untreated); 7.7 ± 1.2 (PTX-treated)). For comparison, net % release values were normalized to those from cells activated with anti-FcεRI mAb alone ± pertussis toxin. Adenosine induced a 43 ± 11% increase in degranulation of control samples, but had no potentiating effect on the degranulation of PTX-treated lung mast cells. *, p<0.05 by two-tailed student’s t-test.
Targeted activation of A3AR on human lung mast cells is sufficient to enhance FcεRI-induced degranulation
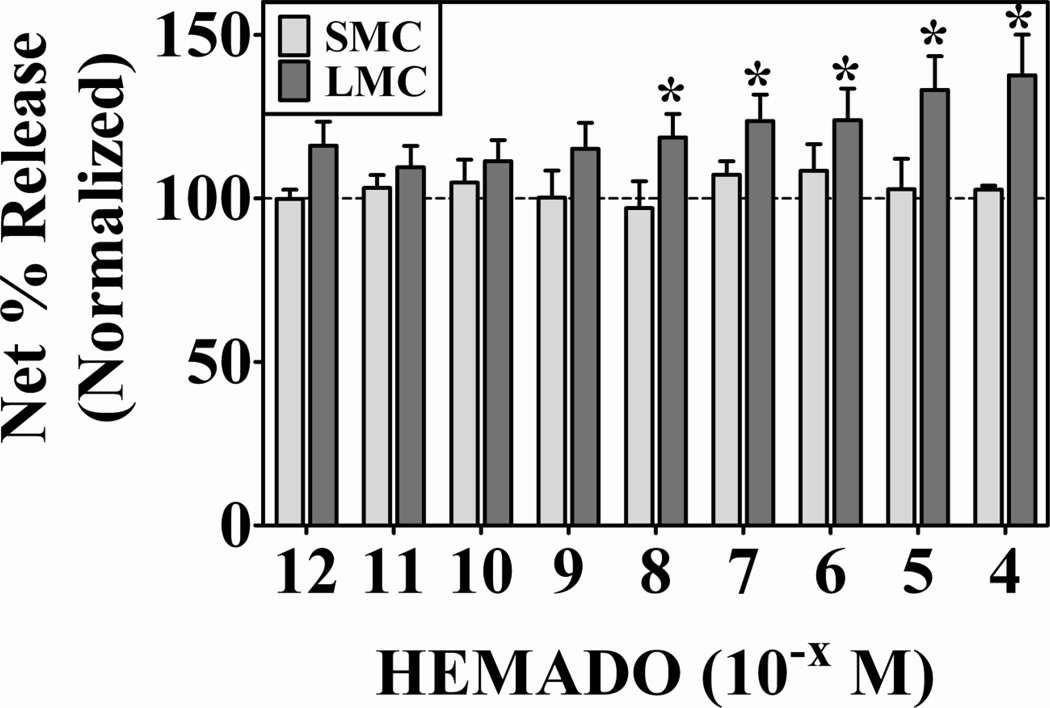
To directly test the idea that A3AR activation was sufficient to potentiate degranulation, this receptor was targeted with the specific agonist 2-(1-hexynyl)-N-methyladenosine (HEMADO). HEMADO was chosen because of its high affinity toward the human A3 adenosine receptor (Ki = 1.1 nM) and high selectivity over A2aAR (1,100-fold) and A2bAR (25,000-fold) (32;33). Lung (n = 8 preparations) and skin (n = 5 preparations) mast cells were activated with 22E7 in the presence or absence of HEMADO and β-hexosaminidase release was determined (Figure 6). Spontaneous release for lung and skin mast cells was 5.7 ± 0.6 % and 6.6 ± 1.3%. For comparison purposes, the net release values were normalized to those obtained from mast cells activated with 22E7 alone (28 ± 4% (skin) and 26 ± 5% (lung)). Figure 6 shows that HEMADO dose-dependently enhanced FcεRI-induced degranulation of lung mast cells relative to cells activated with 22E7 alone. Importantly, a significant increase in degranulation was observed with 10−8 M and 10−7 M HEMADO, concentrations at which A3AR, but not A2aAR or A2bAR, should be engaged according to published pharmacological data (32;33) demonstrating that A3AR activation is sufficient to potentiate FcεRI-induced degranulation of lung mast cells. HEMADO by itself did not induce degranulation of lung mast cells (data not shown). Figure 6 also shows that HEMADO had no enhancing effect on FcεRI-induced degranulation of skin mast cells. These data provide the first direct evidence that targeted activation of A3AR is sufficient to potentiate IgE-induced degranulation of human primary mast cells.
Figure 6. Targeted activation of A3AR augments FcεRI-induced degranulation of human lung mast cells, but not skin mast cells.
Mast cells from lung (n=8 preparations) and skin (n=5 preparations) were activated with anti-FcεRI mAb 22E7 (0.1 µg/ml) in the absence or presence of the A3AR-specific agonist HEMADO for 30 min at 37°C. β-Hexosaminidase % release was quantified and net % release was determined. Spontaneous release for lung and skin mast cells was 6 ± 4 % and 6.5 ± 1%. For comparison purposes, the net release values were normalized to those obtained from mast cells activated with 22E7 alone (28 ± 4% (skin) and 26 ± 5% (lung)). HEMADO dose-dependently enhanced the degranulation of lung mast cells, but not skin mast cells. HEMADO alone did not induce degranulation (not shown). *, p<0.05 by two-tailed student’s t-test.
DISCUSSION
In the current study, we demonstrate a previously unrecognized difference in response to adenosine by FcεRI-activated human mast cells dispersed from lung and skin tissue. Adenosine at low concentrations enhances the degranulation of lung mast cells, but has no potentiating effect on degranulation of skin mast cells. At higher concentrations, adenosine inhibits degranulation of mast cells from both tissues. The bi-modal response of lung mast cells to adenosine described here agrees with the finding of Peters et al. (15). However, the novel finding that adenosine fails to augment FcεRI-induced degranulation of human skin mast cells challenges the widely held notion that all mast cells respond to adenosine with enhanced IgE-induced degranulation. Although it remains to be determined whether the seemingly modest adenosine-induced increase in degranulation of lung mast cells observed here is physiologically significant, the fact that adenosine potentiates the degranulation of human mast cells from lung but not skin might explain why inhaled and intravenously administered adenosine induces a mast cell-mediated pulmonary response in asthmatics but not a dermatologic allergic-like response in vivo.
Because of the clinically-relevant mast cell-mediated bronchoconstrictive response to inhaled and intravenously administered adenosine by asthmatics, the identification of the adenosine receptor capable of potentiating the degranulation of human mast cells is of significant importance. The disparity in the FcεRI-mediated response of human lung and skin mast cells to adenosine presented here provided a unique genetically non-modified model with which to address this longstanding issue. Our finding that human lung mast cells express significantly more A3AR mRNA transcripts than skin mast cells suggested that the disparate augmenting response to adenosine could be due to differences in expression of this receptor. We attempted to determine if the difference in A3AR mRNA expression corresponded to receptor protein levels by Western blotting and flow cytometric analysis. Unfortunately, the commercially-available anti-adenosine receptor antibodies have proven ineffectual and unreliable in our hands and data on adenosine receptor protein expression in published literature is currently lacking. Therefore, a determination of receptor protein levels could not be made at this time. Nevertheless, the 3-fold higher expression level of A3AR mRNA in lung over skin mast cells corresponds to the functional outcome of adenosine-enhanced degranulation involving this receptor. The facts that the adenosine-induced increase in degranulation of lung mast cells was dependent on Gi protein signaling (since it was inhibited by PTX) and that direct stimulation of A3AR with the A3AR agonist HEMADO recapitulated the adenosine-induced enhancement provide solid evidence that A3AR is the receptor responsible for the potentiating response.
It is well-documented that the A3AR is the potentiating receptor of IgE-dependent degranulation of rodent mast cells: wild-type but not A3AR-deficient mice are susceptible to mast cell-mediated airway hyper-reactivity to inhaled adenosine (19), and A3AR is required for adenosine to enhance IgE-induced degranulation of bone marrow-derived mast cells (20;21). In humans, the precise identity of the adenosine receptor capable of potentiating FcεRI-induced degranulation has been unclear. Studies on canine and human transformed mast cell lines have suggested that A2bAR signals mediate this effect by acting through Gq, not the A3AR Gi pathway (17;18). However, if Gq proteins were primarily involved in this process, PTX-treatment should not have affected the degranulation response as it did in the present study since Gq proteins are insensitive to inhibition by PTX. Moreover, if A2bAR were responsible for the potentiating effect of adenosine, we would expect the same response in lung and skin mast cells since they express relatively similar amounts of A2bAR. Perhaps the most direct evidence in support of the A3AR as the potentiating adenosine receptor is that the highly discriminating A3AR agonist HEMADO enhances FcεRI-induced degranulation of lung mast cells. Unfortunately, similar experiments with A2bAR cannot be done at this time because A2bAR-specific agonists are not widely available, and, thus, a role for A2bAR signaling cannot be entirely ruled out. Nevertheless, the current study provides the first direct evidence in human primary lung mast cells to support a potentiating role for A3AR in FcεRI-induced degranulation.
At 100 µM and higher concentrations, adenosine significantly inhibited FcεRI-induced degranulation of both lung and skin mast cells. The identification of the adenosine receptor(s) behind this effect is currently being studied. Our preliminary experiments reveal that the inhibitory effect of adenosine at these concentrations is not prevented in skin mast cells treated with pertussis toxin (data not shown); thus, indicating that the Gi-coupled A3AR is not involved in this process. Given the difference in expression of A3AR and that mast cells from both tissues are affected equally by adenosine at these concentrations, these results agree with the present study. Since A3AR is not involved in the inhibitory phase, this implies that other adenosine receptors are responsible or that intracellular adenosine targets might be involved. The roles, if any, of A2aAR and A2bAR in the in the inhibition of FcεRI-induced degranulation by higher concentrations of adenosine are not presently known, but studies are currently underway to determine if these adenosine receptors are involved.
These present findings demonstrate a functional heterogeneity in response to adenosine by human mast cells from different tissues. Importantly, two types of human mast cells have been identified according to the protease composition of their secretory granules (34;35). MCT mast cells express only tryptase and predominate in the lung, whereas MCTC mast cells express tryptase as well as chymase, carboxypeptidase A3 and chathepsin G. MCTC mast cells are the only type found in normal skin, and account for a minor fraction of the mast cells in lung. Our finding that adenosine potentiates FcεRI-induced degranulation of mast cells from lung but not skin suggests that this could be due to a response of MCT rather than MCTC mast cells. Studies are currently being carried forward to determine if adenosine-induced potentiation of FcεRI-mediated degranulation and A3AR expression functionally and phenotypically segregate with the MCT phenotype. Interestingly, MCTC mast cells from skin and lung, but not MCT mast cells express the C5a receptor (CD88) and degranulate in response to C5a (36); thus, demonstrating the plausibility that A3AR expression and enhanced degranulation in the presence of adenosine might also functionally distinguish MCT mast cells from the MCTC type.
Overall, this study addresses longstanding issues as to whether adenosine potentiates or inhibits the degranulation of human mast cells and which adenosine receptor(s) is(are) involved. We show that in primary human lung mast cells adenosine acts in a bi-modal fashion – enhancing FcεRI-induced degranulation at low concentrations and inhibiting it at higher concentrations. This unrecognized disparity in response to adenosine by primary human lung and skin mast cells might explain why bronchospasm but not urticaria is a side effect of adenosine administration. Furthermore, we provide the necessary evidence that identifies the A3AR as being responsible for adenosine-dependent potentiation of IgE-induced degranulation; thus, implicating A3AR as the culpable receptor in adenosine-induced bronchospasm of asthmatics. These findings provide an explanation as to why inhaled and intravenously administered adenosine induces a pulmonary but not allergy-like dermatologic response in asthmatics, and suggest that A3AR antagonists might be effective at preventing the adenosine-induced bronchospastic response.
ACKNOWLEDGMENTS
Supported by National Institutes of Health grants KO1HL092581 and Jeffress Memorial Trust grant to G.G. and R01AI20487 and U19AI077435 to L.B.S.
REFERENCES
- 1.Drake I, Routledge PA, Richards R. Bronchospasm induced by intravenous adenosine. Hum. Exp. Toxicol. 1994;13:263–265. doi: 10.1177/096032719401300407. [DOI] [PubMed] [Google Scholar]
- 2.Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br. J. Clin. Pharmacol. 1983;15:161–165. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polosa R, Ng WH, Crimi N, et al. Release of mast-cell-derived mediators after endobronchial adenosine challenge in asthma. Am. J. Respir. Crit Care Med. 1995;151:624–629. doi: 10.1164/ajrccm/151.3_Pt_1.624. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST. The Quintiles Prize Lecture 2004. The identification of the adenosine A2B receptor as a novel therapeutic target in asthma. Br. J. Pharmacol. 2005;145:1009–1015. doi: 10.1038/sj.bjp.0706272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips GD, Rafferty P, Beasley R, Holgate ST. Effect of oral terfenadine on the bronchoconstrictor response to inhaled histamine and adenosine 5'-monophosphate in non-atopic asthma. Thorax. 1987;42:939–945. doi: 10.1136/thx.42.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafferty P, Beasley R, Holgate ST. The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5' monophosphate in atopic asthma. Am. Rev. Respir. Dis. 1987;136:369–373. doi: 10.1164/ajrccm/136.2.369. [DOI] [PubMed] [Google Scholar]
- 7.Spicuzza L, Di MG, Polosa R. Adenosine in the airways: implications and applications. Eur. J. Pharmacol. 2006;533:77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Clarke H, Cushley MJ, Persson CG, Holgate ST. The protective effects of intravenous theophylline and enprofylline against histamine- and adenosine 5'-monophosphate-provoked bronchoconstriction: implications for the mechanisms of action of xanthine derivatives in asthma. Pulm. Pharmacol. 1989;2:147–154. doi: 10.1016/0952-0600(89)90039-2. [DOI] [PubMed] [Google Scholar]
- 9.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Marquardt DL, Parker CW, Sullivan TJ. Potentiation of mast cell mediator release by adenosine. J. Immunol. 1978;120:871–878. [PubMed] [Google Scholar]
- 11.Peachell PT, Columbo M, Kagey-Sobotka A, Lichtenstein LM, Marone G. Adenosine potentiates mediator release from human lung mast cells. Am. Rev. Respir. Dis. 1988;138:1143–1151. doi: 10.1164/ajrccm/138.5.1143. [DOI] [PubMed] [Google Scholar]
- 12.Duffy SM, Cruse G, Brightling CE, Bradding P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migration via the adenosine A2A receptor. Eur. J. Immunol. 2007;37:1653–1662. doi: 10.1002/eji.200637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Takei M, Nakahata T, Fukamachi H. Inhibitory effect of adenosine on degranulation of human cultured mast cells upon cross-linking of Fc epsilon RI. Biochem. Biophys. Res. Commun. 1998;242:697–702. doi: 10.1006/bbrc.1997.8040. [DOI] [PubMed] [Google Scholar]
- 14.Hillyard PA, Nials AT, Skidmore IF, Vardey CJ. Characterization of the adenosine receptor responsible for the inhibition of histamine and SRS-A release from human lung fragments. Br. J. Pharmacol. 1984;83:337–345. doi: 10.1111/j.1476-5381.1984.tb16493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters SP, Schulman ES, Schleimer RP, et al. Dispersed human lung mast cells. Pharmacologic aspects and comparison with human lung tissue fragments. Am. Rev. Respir. Dis. 1982;126:1034–1039. doi: 10.1164/arrd.1982.126.6.1034. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 17.Auchampach JA, Jin X, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptor and evidence that degranulation is mediated by the A2B receptor. Mol. Pharmacol. 1997;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- 18.Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol. Pharmacol. 1999;56:705–713. [PubMed] [Google Scholar]
- 19.Hua X, Chason KD, Fredholm BB, et al. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J. Allergy Clin. Immunol. 2008;122:107–113. 113. doi: 10.1016/j.jaci.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 21.Salvatore CA, Tilley SL, Latour AM, et al. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J. Biol. Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 22.Baram D, Dekel O, Mekori YA, Sagi-Eisenberg R. Activation of mast cells by trimeric G protein Gi3; coupling to the A3 adenosine receptor directly and upon T cell contact. J. Immunol. 2010;184:3677–3688. doi: 10.4049/jimmunol.0901333. [DOI] [PubMed] [Google Scholar]
- 23.Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J. Clin. Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryzhov S, Goldstein AE, Matafonov A, et al. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J. Immunol. 2004;172:7726–7733. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson G, Blom T, Kusche-Gullberg M, et al. Phenotypic characterization of the human mast-cell line HMC-1. Scand. J. Immunol. 1994;39:489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 26.Hughes PJ, Holgate ST, Church MK. Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem. Pharmacol. 1984;33:3847–3852. doi: 10.1016/0006-2952(84)90050-9. [DOI] [PubMed] [Google Scholar]
- 27.Riske F, Hakimi J, Mallamaci M, et al. High affinity human IgE receptor (Fc epsilon RI). Analysis of functional domains of the alpha-subunit with monoclonal antibodies. J. Biol. Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- 28.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J. Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- 29.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ. Res. 2003;92:485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 30.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- 31.Gilman AG. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 32.Klotz KN, Falgner N, Kachler S, et al. [3H]HEMADO--a novel tritiated agonist selective for the human adenosine A3 receptor. Eur. J. Pharmacol. 2007;556:14–18. doi: 10.1016/j.ejphar.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Volpini R, Costanzi S, Lambertucci C, et al. N(6)-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A(3) receptor and a starting point for searching A(2B) ligands. J. Med. Chem. 2002;45:3271–3279. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 34.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J. Histochem. Cytochem. 1989;37:1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 35.Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J. Immunol. 1991;147:247–253. [PubMed] [Google Scholar]
- 36.Oskeritzian CA, Zhao W, Min HK, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]