Abstract
l-Arginine is shown to protect hematopoietic progenitor (32D cl 3) cells from death due to exposure to γ radiation (137Cs). Some of the other intermediates in the urea cycle, namely ornithine and citrulline, plus urea itself, were not found to have any significant impact on cell survival after irradiation. Intriguingly, supplementation of irradiated cells with l-arginine results in decreased production of peroxynitrite, suggesting that suppression of superoxide generation by nitric oxide synthase in one or more microenvironments is an important factor in the observed radioprotection. The absence of any radioprotective effect of l-arginine in cells at 3% oxygen also confirms the involvement of one or more oxygen-derived species. Knockdown experiments with nitric oxide synthase (NOS) siRNAs in cells and NOS knockout animals confirm that the observed radioprotection is associated with nNOS (NOS-1). l-Arginine also ameliorates the transient inhibition of the electron-transport chain complex I that occurs within 30 min of completing the dose (10 Gy) and that appears to be a functional marker for postirradiation mitochondrial oxidant production.
INTRODUCTION
Due to the presence of endogenous antioxidant species, the measurable generation of secondary oxidants/reductants (such as superoxide and hydrogen peroxide) during low doses of ionizing radiation to cells/tissues can be troublesome to detect (1). However, there seems to be an amplification process resulting in larger amounts of reactive oxygen (ROS) and/or nitrogen species (RNS) that occurs soon afterward and persists for several minutes (2). This increase in ROS/RNS production has been linked to Ca2+-dependent processes in the mitochondrion and a reversible mitochondrial permeability transition (3, 4). Analogous findings have been reported (5–7) for unirradiated cells exposed to the culture medium transferred from irradiated cells (bystander response). Mitochondrial superoxide generation has been demonstrated to be critically important in the mechanism(s) of postirradiation damage because manganese superoxide dismutase (MnSOD, SOD-2) overexpression can significantly ameliorate the effects of ionizing radiation both in cultured cells (8, 9) and in vivo (10–13). Alternatively, overexpression of MnSOD may be radiosensitizing in some cell lines (14). However, there is presently no evidence for MnSOD being other than radioprotective in hematopoietic progenitor cells, where the additional expression of mitochondrially targeted catalase appears to afford further radioprotection (15). The mechanistic consequences of changing nitric oxide (NO) levels after irradiation are potentially complicated and may include protective cellular signaling pathways (2). Nevertheless, the majority of relevant studies continue to report detrimental effects associated with elevated postirradiation NO production (3, 13, 16–18). Taken together, these observations are consistent with a key role for the powerful oxidant peroxynitrite, formed during the extremely rapid reaction between nitric oxide and superoxide (19–21), in one or more mechanisms of radiation toxicity.
The primary function of nitric oxide synthase (NOS) is to catalyze the NADPH-dependent conversion of l-arginine and oxygen to NO and citrulline. However, all NOS isoforms also generate superoxide (O2−) (22), and the constitutive neuronal (nNOS, NOS-1) and the inducible (iNOS, NOS-2) isoforms continue to yield O2− as the main product when depleted of substrate l-arginine (23, 24). Consequently, it is noteworthy that the nNOS isoform has been shown to be transiently activated (i.e., with respect to NO production) by ionizing radiation in Chinese hamster ovary cells (3); also, the mitochondrially localized NOS [mtNOS, an nNOS variant (25)] has been implicated in postirradiation damage to rat bladder (18). Based upon these observations, we propose that, after irradiation, NOS is stimulated to synthesize NO, resulting in l-arginine becoming transiently depleted in some microenvironments, thus leading to increased O2− production in amounts that cannot be efficiently removed by endogenous SOD. If there is any residual NO temporarily pooled or still being synthesized in other microenvironments, then by diffusion this could react rapidly with the excess O2−, resulting in the generation of damaging peroxynitrite. If this argument is correct, it follows that the addition of l-arginine should be radioprotective. Accordingly, we set out to test this hypothesis in hematopoietic progenitor cells.
MATERIALS AND METHODS
Cell, Cell Culture and Enzyme Isolation
Hematopoietic progenitor cells (32D cl 3) established from the nonadherent cell population removed from a continuous mouse bone marrow line (26) were cultured as described previously (27). Unless stated to the contrary, culture media were purchased from Invitrogen, and all reagents, ACS grade or better, were obtained from Sigma-Aldrich. Additions of l-arginine (5 mM, final concentration) to both sample and control cells were made 1 h prior to performing the irradiations unless otherwise stated; this treatment had no discernable effect on viability. 32D cl 3 cells were irradiated with doses ranging from 0 to 10 Gy and plated in 4% methylcellulose to obtain clonogenic survival curves. Colonies of more than 50 cells were counted 7 days later, and all experiments were performed at least in triplicate. Mitochondria were isolated from cells or bovine heart by differential centrifugation. Complex I was purified from bovine mitochondria according to the method of Sazanov et al. (28) employing dispersion/solvation in n-dodecyl-β-d-maltoside (Anatrace) and standard chromatographic methods.
Knockdown (siRNA) Procedures
32D cl 3 cells were grown in medium containing IL-3 and washed twice with PBS buffer. The cells (2 × 107) were suspended in 200 μl PBS buffer, mixed with 5 μl (50 μM) siRNA (Ambion Silencer Select), and transferred to 0.4-cm gap cuvettes (Bio-Rad Gene Pulser). After 20 min incubation on ice, electroporation was carried out at a voltage of 250 V and capacitance of 400 μF (Bio-Rad Gene Pulser II). The cells were incubated for 10 min on ice and then transferred to T-75 flasks containing 32D normal medium (RPMI 1640 medium + 10% FBS, 15% WEHI, 1% l-glutamine and 1% PS). Assays of NOS activity in the presence of the inhibitors L-NPA (Nω-propyl-l-arginine, Cayman Chemical) with a 150-fold increased specificity for nNOS over eNOS (29) and the relatively nonspecific l-NAME (Nω-nitro-l-arginine methyl ester) confirmed the decrease in each NOS selectively “knocked down” (see Supplementary Material S1; http://dx.doi.org/10.1667/RR1281.1.S1). Irradiation of cells prior to plating for survival curves (described above) were performed 48 h later.
Enzyme Assays
Cells were pelleted by centrifugation, washed with phosphate buffered saline PBS, and initially resuspended in 50 mM potassium phosphate buffer, pH 7.4, prior to subsequent dilution in assay buffers. After sonication, all determinations were performed at least in triplicate. Protein concentration determinations were made using the bicinchoninic acid (BCA) assay kit supplied by Pierce Biotechnology (Rockford, IL). Ferrocytochrome c was prepared by the addition of sodium dithionite (EM Science) to beef heart ferricytochrome c, and excess reductant was removed by Sephadex-G25 column chromatography. Concentrations of ferrocytochrome c in stock solutions and assay media were determined spectrophotometrically (ε550 = 28 mM−1 cm−1; Δε550 = 18.5 mM−1 cm−1) (30). NADH dehydrogenase (complex I) activity was measured at 30°C in 50 mM potassium phosphate buffer, pH 7.4, by monitoring the NADH-dependent reduction of ferricyanide at 420 nm (Δε = 1.0 mM−1 cm−1) using 200 μM NADH and 0.5 mM K3Fe(CN)6 in the presence of 5 μM rotenone and 2 μg/ml antimycin A (31). Complex III (ubiquinone oxidase-cytochrome c reductase) activity was measured in 25 mM potassium phosphate, pH 7.2 (0.4 mM lauryl maltoside), by monitoring the reduction of 15 μM ferricytochrome c at 550 nm (Δε = 18.5 mM−1 cm−1) in the presence of 2 μg/ml rotenone and 2 mM KCN using reduced ubiquinone-2 at 30°C (32). Cytochrome c oxidase activity (complex IV) was quantified by monitoring the rate of cytochrome c (15 μM) oxidation at 550 nm (33) in 25 mM potassium phosphate buffer, 30°C, in the presence of 2 μg/ml antimycin A. Malate dehydrogenase activity was determined in 50 mM potassium phosphate, pH 7.4, 30°C, in the presence of 20 μM NADH and 5 mM oxaloacetate, monitoring the disappearance of reduced NADH spectrophotometrically at 340 nm. Activities of NOS isoforms were determined using the conversion of oxyhemoglobin to methemoglobin, observing absorbance changes at 400 nm, to indicate NO formation (34).
Western Blotting
Cell lysates were normalized to protein prior to Western blotting and/or probing for β-actin according to published methods (35). Mitochondria from cell lysates were isolated by differential centrifugation (25). Cell lysates or isolated mitochondria were mixed with Laemmli sample buffer (1:1) and boiled for 5 min. Native gel samples were not boiled and were run according to the manufacturer's instructions (Invitrogen). Ten micrograms of each sample was subjected to either 4–20% SDS-polyacrylamide or native blue gel electrophoresis, transferred onto polyvinylidene difluoride (PVDF) membranes, and blocked with 3% nonfat milk. Blots were incubated with primary antibodies (1:2,000) for at least 2 h prior to rinsing and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. Antibodies were purchased from Millipore and were detected using chemiluminescence according to the manufacturer's instructions (Western Lighting; Perkin Elmer).
Other Assays
Tyrosine nitration was quantified by the use of a competitive ELISA with chemiluminescence detection (Upstate, NY). Mitochondrial membrane depolarization was determined by measuring JC-1 fluorescence (excitation 485 nm; emission 535 and 590 nm) using CCCP (carbonyl cyanide m-chlorophenylhydrazone) to establish complete depolarization (Invitrogen). Cells (1 × 106) were incubated with 2 μM JC-1 at 37°C for 10 min prior to washing with PBS and resuspension in 2 ml PBS for fluorescence measurements. DADCF (2′,7′-dichlorofluorescein diacetate) was used for detection of ROS/RNS in cells. Fluorescence measurements (excitation 488 nm; emission 525 nm) were made after incubation of the dye with isolated cells at 37°C for 1 h, washing with PBS, and resuspension in 50 mM potassium phosphate buffer (prior to irradiation when required). Dihydrohodamine 123 was incubated with cells at a concentration of 10 μM for 1 h at 37°C prior to fluorescence detection (excitation 500 nm; emission 535 nm). MitoSoxe™ and DHE (dihydroethidium, or hydroethidine) were purchased from Invitrogen and used as described elsewhere (36). DAF-2 diacetate (4,5-diaminofluorescein diacetate) was added to cells 30 min prior to irradiation and cells were then immediately harvested for fluorescence measurements (excitation 485 nm; emission 538 nm).
Animal Procedures
Irradiations of mice were performed by placing five animals in a circular plexiglass container 20 cm in diameter and 2 cm in height in which they had free movement. The mice were then irradiated with a 137Cs source at a dose rate of 80 cGy per minute. The container was located on a rotating turntable during this procedure to ensure a uniform dose to all the mice. Sham control animals were handled in the same way except that they were never exposed to the radiation source. After irradiation, the mice (C57BL/6 NHSD “wild-type” and nNOS−/− knockouts) were euthanized immediately. The unirradiated control animals were euthanized at the same time. Hearts were rapidly excised and the pericardia were aerobically homogenized using calcium-free Ringer's solution. Dihydrorhodamine 123 was added and the samples incubated for 15 min (37°C under 20% O2). After sonication, the supernatants were obtained by centrifugation and the 530 nm fluorescence (490 nm excitation) was measured. Samples of the homogenized heart tissue intended for complex I, III and IV activity assays were cryogenically preserved by immersion in liquid nitrogen and stored for 1–2 days prior to analysis.
Instrumentation
Irradiation of cells was carried out on ice using a 137Cs irradiator. Doses of 10 Gy were administered at a dose rate of 1 Gy/min. Electronic absorption measurements were carried out with a Shimadzu UV-2501PC spectrophotometer. Fluorescence measurements were carried out using a Shimadzu RF-5301PC. Chemiluminescence measurements were performed using a BMG Labtechnology Fluostar Galaxy plate reader.
Statistics
Data are expressed as means ± SD. Differences in measured variables were assessed with Student's t test. P < 0.05 was considered to be statistically significant.
Animal Welfare
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh (IACUC protocol number 0503964A-1). Veterinary care was provided by the Department of Laboratory Animal Research of the University of Pittsburgh in accordance with the IACUC guidelines.
RESULTS
l-Arginine Protects Against Ionizing Radiation
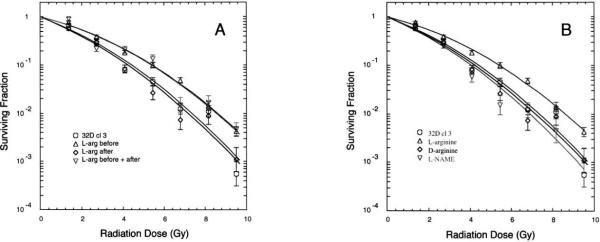
Clonogenic survival curves showed that the addition of l-arginine to the culture media (to 5 mM) 1 h prior to irradiation conferred significant protection of 32D cells from the damaging effects of ionizing radiation (Fig. 1A and Table 1), corresponding to a dose-modifying factor of 1.5 at ≤2 Gy (or 50% survival) and 1.2 at >5 Gy. This represents a modest but reproducible effect that translates into a fivefold increase in cell survival in the presence of l-arginine at doses of ~10 Gy. No improved survival was observed if the l-arginine was administered after the radiation. Therefore, at least in this particular system, l-arginine is clearly a protector (prophylactic) rather than a mitigator (therapeutic). The other enantiomer, D-arginine, did not produce a similar protective effect (Fig. 1B and Table 1), strongly suggesting that the mechanism by which l-arginine protects involves an enzymatic process rather than something like an electrostatic interaction of the amino acid with a membrane surface. Survival curves obtained in the presence of some other products of the enzymatic reactions of the urea cycle, namely urea, ornithine and citrulline, exhibited no significant differences from controls (not shown). Similarly, l-NAME, an inhibitor of NO synthesis but not O2− production by NOS (23), neither conferred protection from nor exacerbated the effects of ionizing radiation on 32D cells (Fig. 1B). These observations all strongly support the suggestion that added l-arginine is protective because it suppresses O2− production by NOS in response to irradiation.
FIG. 1.
Clonogenic survival curves after 0–10 Gy irradiation of cultured hematopoietic progenitor (32D cl 3) cells. Cells were plated in methylcellulose and colonies of greater than 50 cells were counted 7 days later. The theoretical fits to the data were calculated from the single-hit multitarget model (where D0 is the 37% survival dose, n is the extrapolation number, and Dq is the quasithreshold dose—see Table 1). Cells incubated (37°C) with l-arginine for 1 h prior to irradiation were found to be significantly more radioresistant than controls (P < 0.02). Panel A: Cells without supplemental l-arginine (◯) cells incubated in 5 mM l-arginine for 1 h prior to irradiation (▵); cells to which l-arginine was added (to 5 mM) immediately after irradiation (◇); cells to which l-arginine was added both before and after irradiation (▿). Panel B: Cells without supplemental l-arginine (◯); cells incubated in 5 mM l-arginine for 1 h prior to irradiation (▵); cells incubated in 5 mM d-arginine for 1 h prior to irradiation (◇); cells incubated in 5 mM l-NAME (Nω-nitro-l-arginine methyl ester) for 1 h prior to irradiation (▿).
TABLE 1.
Theoretical (single-hit, multitarget) Fits to Survival Curves of Figure 1
| Figure 1A parameters | ||
| Time of l-arginine additiona | D0 (Gy) | n |
| Control | 1.17 ± 0.08 | 7.46 ± 1.78 |
| Before irradiation | 1.53 ± 0.05 (*P = 0.013) | 6.96 ± 0.51 |
| After irradiation | 1.18 ± 0.11 | 7.10 ± 1.68 |
| Before and after irradiation | 1.56 ± 0.05 (*P = 0.018) | 6.54 ± 0.90 |
| Figure 1B parameters | ||
| Compound added before irradiationa | D0 (Gy) | n |
| Control | 1.07 ± 0.09 | 6.79 ± 1.10 |
| l-Arginine | 1.50 ± 0.07 (*P = 0.017) | 7.74 ± 0.67 |
| d-arginine | 1.13 ± 0.03 | 6.88 ± 0.60 |
| l-NAME | 1.13 ± 0.14 | 7.26 ± 1.29 |
Notes. The uncertainties given are standard errors.
When compared with controls, significant differences were observed only when l-arginine was added before irradiation.
All additions made to 5.0 mM concentration in the culture medium.
If this idea is correct, it is anticipated that the protection afforded by l-arginine should be significantly reduced if the radiation dose is given under low oxygen conditions. In fact, maintaining the cultured cells in a 3% oxygen atmosphere for 1 h prior to and 1 h after irradiation resulted in the protective effect of l-arginine being virtually eliminated, as is evident by comparing the final slopes (D0) of the survival curves (Table 2). Thus suppression of the level of an oxygen-derived species in the l-arginine-dependent mechanism of protection is confirmed. The unsurprising observation (apparent from the extrapolation numbers, n, in Table 2) that removal of oxygen is actually more protective than any effect of l-arginine does not detract from the present findings.
TABLE 2.
Theoretical (single-hit, multitarget) Fits to Survival Curves of Hematopoietic Progenitor (32D cl 3) Cells Comparing Normoxic and Hypoxic Conditions
|
D0 (Gy) |
n
|
|||
|---|---|---|---|---|
| 20% O2 | 3% O2 | 20% O2 | 3% O2 | |
| 32D cl 3 | 1.18 ± 0.08 | 1.13 ± 0.05 | 3.1 ± 1.1 | 13.9 ± 4.7 |
| 32D cl 3 + 5 mM l-argininea | 1.56 ± 0.02 (*P = 0.040) | 1.20 ± 0.01 | 3.1 ± 0.2 | 18.0 ± 3.3 |
Notes. The uncertainties given are standard errors.
When compared with controls, significant differences were observed only when l-arginine was added at 20% O2.
Additions made to the culture medium 1 h prior to irradiation.
To further test the working hypothesis and identify the NOS isoform in question, we performed a series of NOS (nNOS, iNOS and eNOS) knockdown experiments with the three siRNAs in 32D cells. Only suppression of nNOS was found to be radioprotective; moreover, addition of l-arginine to the medium of nNOS knockdown cells did not lead to any further radioprotection (Table 3). While the majority of these studies were performed with cells cultured and irradiated under 20% oxygen, a subset of experiments carried out in 4% oxygen (data not shown) also indicated that only knockdown of nNOS was radioprotective. These results appear to unambiguously demonstrate nNOS to be the isoform involved in the radioprotective effect of l-arginine. Furthermore, the finding that suppression of nNOS activity (and not that of eNOS or iNOS) confers radioprotection is in agreement with the recently reported observations of Rajagopalan et al. regarding bone marrow stromal cells (37).
TABLE 3.
Theoretical (single-hit, multitarget) Fits to Survival Curves of NOS Knockdown Hematopoietic Progenitor (32D cl 3) Cells
| siRNA | D0 (Gy) | n |
|---|---|---|
| Control | 1.25 ± 0.05 | 3.9 ± 2.4 |
| NOS1 | 1.48 ± 0.08 (*P < 0.05) | 1.5 ± 0.5 |
| NOS1 + 5 mM l-argininea | 1.59 ± 0.10 (*P < 0.05) | 1.4 ± 0.3 |
| NOS2 | 1.27 ± 0.17 | 4.6 ± 3.3 |
| NOS3 | 1.23 ± 0.13 | 3.1 ± 2.0 |
Notes. The uncertainties given are standard errors.
When compared with controls, significant differences were observed only with NOS1 knocked down, irrespective of the presence of l-arginine. Fits to survival curves are provided as Supplementary Material (http://dx.doi.org/10.1667/RR1281.1.S1).
Additions made to the culture medium 1 h prior to irradiation.
Radiation Rapidly and Transiently Increases NO Production
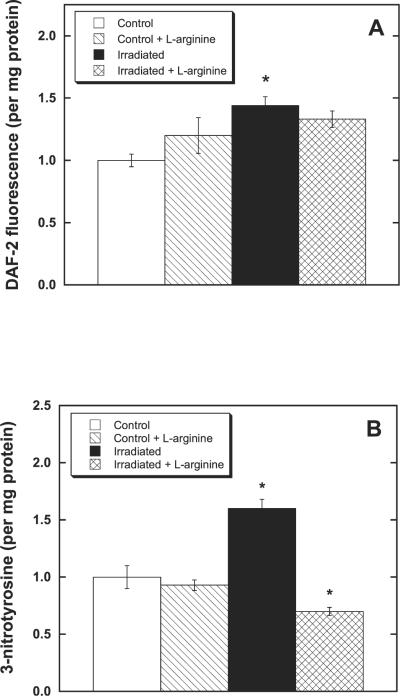
In the first 30 min after irradiation (10 Gy), elevated NO production was detected in 32D cells compared to the unirradiated controls (Fig. 2A). From 30 min to 6 h after exposure to ionizing radiation, there was no measurable increase in NO production observed in irradiated cells compared to controls (not shown). This observation is consistent with the elevated NOS activity previously reported postirradiation (2 Gy) in Chinese hamster ovary cells and also attributed to the nNOS isoform (3). An apparent small increase in NO production after the addition of l-arginine to unirradiated 32D cells was within experimental uncertainty (Fig. 2A). Therefore, with respect to stimulation of NO synthesis, the consequence of radiation exposure was certainly greater than any mass action effect stemming from l-arginine addition. It should also be noted that the elevated NO production measured in postirradiated 32D cells (± l-arginine) was only just detectable with the fluorometric indicator DAF-2. Consequently, the NO levels involved in the current experiments are significantly lower than those encountered in previous studies with NO donors (38) or after upregulation of iNOS (18), where the higher NO levels present clearly exacerbated the damaging postirradiation effects. In summary, within 30 min of irradiation, there had been a small “burst” of NO production in 32D cells that was not measurably affected by preincubation with l-arginine. This finding is fully in accord with the argument that the radioprotective effect of added l-arginine is not primarily due to any accompanying increase in NO synthesis.
FIG. 2.
Production of reactive nitrogen species after irradiation of hematopoietic progenitor cells (32D cl 3). Cells incubated (37°C) with l-arginine for 1 h prior to irradiation (10 Gy) were found to generate significantly less peroxynitrite (ONO2)− than controls (*P < 0.05). Panel A: NO detection after irradiation of 32D cells (± l-arginine). Cells were incubated with DAF-2 (4,5-diaminofluorescein diacetate) for 30 min prior to irradiation and then, within 30 min of irradiation, harvested for fluorescence measurements (excitation 485 nm; emission 538 nm). Panel B: Protein nitrotyrosine changes indicative of ONO2)− formation after irradiation of 32D cells (± l-arginine). Cells were irradiated and then analyzed 30 min later for the presence of 3-nitrotyrosine using an ELISA (see the Materials and Methods). Controls exhibited ~ mg nitrated tyrosine/mg protein.
Peroxynitrite (ONO2−) Formation is not Correlated with NO Production Alone
Protein nitrotyrosine formation is widely accepted to be indicative of ONO2− generation (21, 39, 40), and previous studies have demonstrated transient increases in protein nitrotyrosine formation after irradiation of various cell lines (3). Nitrotyrosine formation in 32D cells was monitored using a competitive ELISA (see Materials and Methods) for the quantification of protein 3-nitrotyrosines. At 30 min after irradiation (10 Gy), increased nitrotyrosine formation was observed (Fig. 2B) followed by a return to control values between 1 and 6 h postirradiation. However, cells treated with 5 mM l-arginine for 1 h prior to irradiation exhibited measurably less 3-nitrotyrosine formation at 30 min after irradiation, clearly demonstrating that there was less ONO2− generation compared to the irradiated cells not provided with supplemental l-arginine. Note that this result is contrary to the naïve expectation that any increase in NO production should lead to a consequent increase in ONO2− detected – the implication being that ONO2− formation is principally governed by the prevailing O2− level. The earlier studies with Chinese hamster ovary cells (3) indicated that maximal nitrotyrosine formation occurred at 5 min after irradiation (2 Gy), which is entirely in keeping with the present findings—that is, the present experiments do not distinguish effects at ~5 min from those within 30 min. In summary, considering the data in Fig. 2A and B together, it is apparent that while there is a small “burst” of NO production in 32D cells after irradiation, there must also be an accompanying and more significant elevation in the rate of O2− generation, resulting in increased ONO2− formation. These observations all strongly support the suggestion that added l-arginine is protective because it suppresses O2− generation by NOS after irradiation.
The Inner Mitochondrial Membrane is Transiently Depolarized Soon after Irradiation
The fluorescent dye JC-1 is frequently used as an indicator of mitochondrial membrane depolarization. The dye normally exists as a monomer exhibiting a green fluorescence at 535 nm (488 nm excitation). Upon dimerization, JC-1 emits red fluorescence at 595 nm (488 nm, excitation) proportional to ΔΨm (41, 42). Within 30 min postirradiation (10 Gy) the red-green fluorescence ratio observed in 32D cells showed clear evidence for depolarization of the inner mitochondrial membrane, followed by a return to control levels at 1 h, with little further change during the next 5–6 h (Table 4). Curiously, no significant difference was observed in the results of experiments performed in the presence and absence of l-arginine. Consequently, there is no indication in these data that the radioprotective effect of l-arginine might necessarily be linked to membrane polarization status. At 18 h postirradiation, corresponding to the time at which morphological features associated with apoptosis start to become apparent in 32D cells, there was further significant membrane depolarization, but once more the presence of added l-arginine had no discernable effect.
TABLE 4.
Changes in Mitochondrial Inner-Membrane Polarization in Hematopoietic Progenitor (32D cl 3) Cells after Irradiation (10 Gy)
| Control | + l-arginine | 10 Gy | 10 Gy + l-arginine | |
|---|---|---|---|---|
| Time = 30 min | ||||
| Green JC-1a | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Red JC-1a | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| Ratio (red/green) | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 |
| Time = 1 h | ||||
| Green JC-1a | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Red JC-1a | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
| Ratio (red/green) | 1.0 ± 0.2 | 1.2 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| Time = ± h | ||||
| Green JC-1a | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 |
| Red JC-1a | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Ratio (red/green) | 1.0 ± 0.2 | 1.2 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 |
| Time = 18 h | ||||
| Green JC-1a | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 2.1 ± 0.1 |
| Red JC-1a | 1.0 ± 0.1 | 1.2 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 |
| Ratio (red/green) | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
Note. The uncertainties given are standard errors.
Data were expressed per mg protein and then normalized to the control values.
Complex I Activity is Transiently Decreased soon after Irradiation
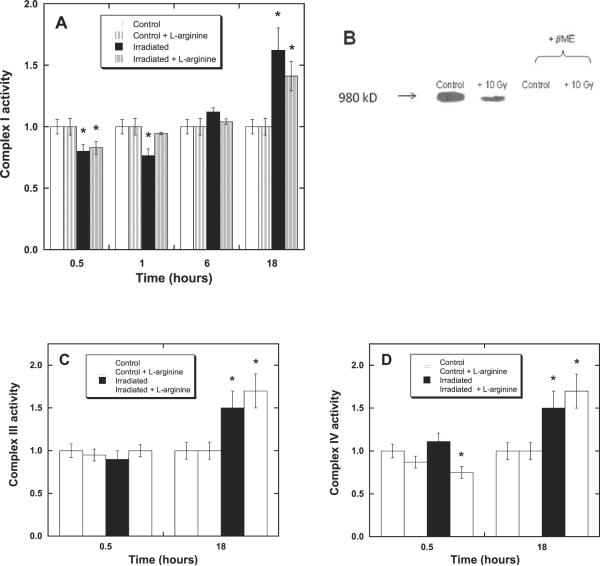
In an effort to ascertain the reason for the transient depolarization (ΔΨm), we examined the postirradiation electron-transfer activities of mitochondrial complexes I, III and IV. These are the electron-transport chain (ETC) complexes principally responsible for the proton gradient, and any change in their activity can be expected to result in a change in ΔΨm. We have shown previously (38) that irradiation of 32D cells at high doses (50 Gy) in the presence of NO donors causes irreversible damage, decreasing the activities of complexes I and III. In this current study, 32D cells were irradiated at 10 Gy in the presence and absence of l-arginine (added to 5 mM concentration 1 h prior to irradiation). Complex I was assayed for changes in activity at 30 min and 1, 6 and 18 h postirradiation (Fig. 3A). There was a reproducible decrease in complex I activity of irradiated samples (± l-arginine) within the first 30 min. More interestingly, 1 h after irradiation, the activities of l-arginine-treated samples had returned to control values, while those of irradiated samples containing no supplemental l-arginine remained low. At 6 h postirradiation, the complex I activity had returned to control levels in the absence of supplemental l-arginine. There was, however, an increase in complex I activity in both irradiated samples (10 Gy ± 5 mM l-arginine) at 18 h. This increase was found to be somewhat variable in magnitude (1.4- to 2-fold) and the time at which it was observed (occasionally as early as 6 h after irradiation). It is certainly tempting to associate the transient decrease in complex I activity observed at 30 min with the early changes in mitochondrial membrane potential (Table 4) even though the two sets of results at 1 h do not necessarily appear to be correlated, since there are at least two readily identifiable factors that may contribute to the apparent ambiguity. First, the electron-transfer activity measurements can be (and were) performed on snap-frozen samples, where the cessation of biological processes could be known almost to the minute. Therefore, an activity measurement corresponds to the status of an enzyme approximating to some instant in time, whereas measurement of ΔΨm, for example, requires viable mitochondria and actually represents an accumulated effect over a period of many minutes. Second, the complex I assay employed (ferricyanide reduction) is highly reproducible (.95% in our laboratory), whereas the depolarization measurement is much less so. Consequently, while a transient loss of complex I activity corresponding to about 15% of control values is readily detectable (Fig. 3A), any change in membrane depolarization of similar magnitude (in the data of Table 4) is really within experimental uncertainty.
FIG. 3.
Changes in activity of mitochondrial ETC complexes after irradiation. The electron-transfer activity of complex I, but not III or IV, was found to transiently decrease after irradiation (10 Gy) of 32D cells (± l-arginine) compared to controls (*P, 0.05), with recovery occurring faster in the presence of l-arginine. Panel A: Complex I (NADH dehydrogenase) activity at 0.5, 1, 6 and 18 h postirradiation, normalized to control rate 10 (±1) nmol K3Fe(CN)6/mg protein. Panel B: Changes in S-glutathiolation of isolated bovine complex I after irradiation. Native gel Western blots employing a monoclonal antibody (Millipore) to glutathione (see the Materials and Methods for details) show deceases in S-glutathiolation in irradiated (10 Gy) mitochondria demonstrating redox modification of reactive thiols. Addition ofβ-mercaptoethanol to both irradiated and control samples released the glutathione attached to complex I. Irradiated samples exhibited 64 ± 8% of the activity of unirradiated control samples (P < 0.05, data not shown). Panel C: Complex III (cytochrome c reductase) activity at 0.5 and 18 h postirradiation, normalized to control rate 42 ± 2 nmol cyt c/mg protein. Panel D: Complex IV (cytochrome c oxidase) activity at 0.5 and 18 h postirradiation, normalized to control rate 98 ± 10 nmol cyt c/mg protein.
Complex I is known to reversibly form disulfides with glutathione, leading to increased electron transport activity and decreased production of superoxide radical by the enzyme (43). Conversely, removal of glutathione from complex I (using β-mercaptoethanol, for example) increases superoxide production, and electron transfer activity is slowed. Irradiation of isolated bovine complex I diminished the S-glutathiolation of complex I relative to controls (Fig. 3B), and the rate of electron transfer was decreased to 64 ± 8% of the activity found for unirradiated controls. These observations are clearly in accord with the idea that the time-dependent variations in complex I activity detected in 32D cells after irradiation (Fig. 3A) may be due to changes in the S-glutathiolation level of the enzyme.
JC-1 Fluorescence and ETC Activity Changes Indicate Mitochondrial Proliferation
The green fluorescence of JC-1, when normalized to cell count or protein concentration, may be used as a measure of mitochondrial mass (44, 45). Intriguingly, at 18 h postirradiation, the green fluorescence intensity per mg protein was observed to have increased in 32D cells (Table 4), indicating a greater mitochondrial mass (or mitochondrial proliferation) in the irradiated cells (± l-arginine) compared with controls. Here again, as supplemental l-arginine had no effect, there is no indication that the mechanism of the observed radioprotective effect necessarily involves stimulation or suppression of mitochondrial biogenesis.
Examination of the postirradiation changes in electron-transfer function of complexes III and IV in 32D cells (Fig. 3C and D) revealed a very similar increase in these activities (1.5- to 2-fold over controls) compared to the results observed for complex I (Fig. 3A). Increases in malate dehydrogenase activity, an inner mitochondrial protein marker, were also observed (not shown). These results are fully in agreement with the observed changes in JC-1 green fluorescence (Table 4), confirming that substantial mitochondrial proliferation had occurred by about 18 h after irradiation (± l-arginine). In keeping with this finding, Nugent et al. recently reported (46) observing increased mitochondrial mass in both human keratinocytes and Chinese hamster ovary cells within 4 h of a 5-Gy dose (60Co).
Surprisingly, at 30 min postirradiation (10 Gy), there was no detectable change in complex III activity (Fig. 3C). This is contrary to our earlier studies at a higher dose (50 Gy) and in the presence of NO donors, where irreversible inhibition of complex III appeared to be the major effect (38). Similarly, there was no change in complex IV activity in 32D cells 30 min after irradiation, but there was a decrease in activity in both the l-arginine-treated samples (± radiation, Fig. 3D). This decrease in activity is almost certainly an experimental artifact due to residual NOS activity in l-arginine-containing samples, leading to detectable NO inhibition at the oxygen binding site of complex IV under the assay conditions.
Detection of Mitochondrially Localized Oxidants is Problematic in 32D Cells
Using the indicator dye 2′,7′-dichlorofluorescein (DCF), an increased production of oxidants at 30 min postirradiation followed initially by a return to control levels at 1 h and then by a second phase of oxidant production from 6 to 18 h was demonstrated in 32D cells. At each time, addition of supplemental l-arginine reduced the level of oxidants formed, but always within the limits of experimental uncertainty (Fig. 4). As rhodamine-based dyes are usually more readily taken up by mitochondria than fluorescein derivatives, we performed an analogous series of measurements employing rhodamine 123 (Rh123). However, while Rh123 fluoresecence is proportional to ROS/RNS production (47), it is also inversely proportional to inner-membrane depolarization (48) and, consequently, these two effects tend to cancel each other. Not surprisingly then, the results of these experiments (not shown) were also insignificantly small with regard to the postirradiation effects of added l-arginine.
FIG. 4.
Changes in oxidant levels after irradiation of hematopoietic progenitor cells (32D cl 3). Levels of ONO2− and/or organic peroxides (products of H2O2 + peroxidase activity) are transiently increased compared to controls (*P < 0.05) after irradiation (± l-arginine). Oxidant detection by dichlorofluoroscein (DCF) after 10 Gy irradiation (± l-arginine) of 32D cells. Cells were incubated with DADCF (2′7″-dichlorofluorescein diacetate) for 1 h at postirradiation times of 0.5, 1, 6 and 18 h prior to measurement of oxidized DCF by fluorescence (excitation 488 nm; emission 525 nm). The data were evaluated per mg protein and then expressed normalized to the mean control value.
We could find no evidence (data not shown) for increased O2− production in irradiated 32D cells using dihydroethidium (DHE) or its mitochondrially targeted derivative MitoSoxe™. Again, this was not an entirely unexpected result as the reaction of O2− with NO to form ONO2− occurs at a diffusion-limited rate and almost certainly limits the sensitivity of DHE. Unlike the precursors of DCF and Rh123, DHE is insensitive to ONO2− (which we verified), and it follows that only if O2− is generated in huge excess (as might be observed in cells during the postcommitment stages of apoptosis or in tissue under inflammatory conditions) can DHE outcompete residual NO to yield a positive result.
Overall, interpretation of the experimental results with oxidant-sensitive dyes is ambiguous, but for reasons that are understood. Therefore, while these data do not offer much support for the hypothesis that l-arginine-depleted mtNOS is a postirradiation superoxide generator, they certainly do not refute the argument.
Oxidant Production in Cardiac Mitochondria
At whole-body doses of up to 10 Gy in mice, encompassing the LD50/30 for death stemming from bone marrow failure, there is no pronounced short-term radiation syndrome known to be associated with cardiac tissue. However, as cardiomyocytes are mitochondria-rich cells, myocardial tissue of mutant animals (in particular) does afford some unique opportunities for testing certain mechanistic ideas concerning the effects of radiation on mitochondria – provided, of course, that it is understood that the postirradiation adaptive response of cardiomyocytes can be expected to differ in some respects from those of more radiation-sensitive cell lines. We have previously demonstrated that murine heart mitochondria contain a NOS isoform that is absent from the myocardial cells of nNOS knockout animals; i.e., nNOS−/− is also mtNOS−/− (18, 25). Consequently, these mice provide a means of probing the hypothesis that mtNOS can be a significant source of O2− generation after irradiation.
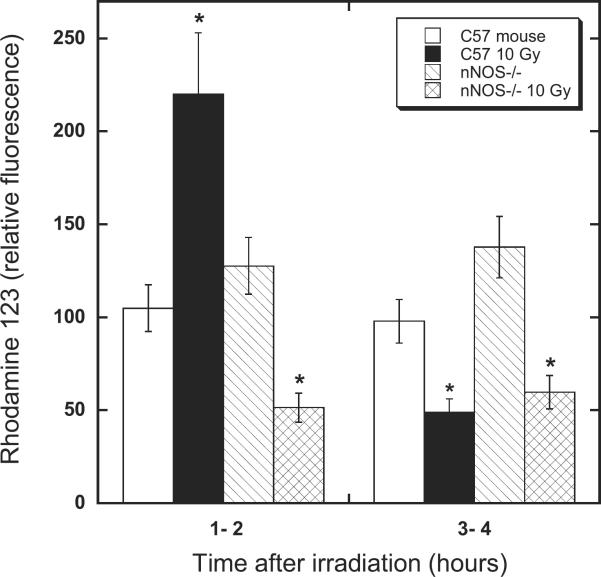
In practice, the isolation of cardiac mitochondria by differential centrifugation of freshly minced myocardium (subsequent to transport of animals from the irradiation facility to another laboratory where they were euthanized prior to excision of the hearts) took a little less than 1 h. Consequently, the earliest times at which data on such samples were collected were in the 1–2-h window after irradiation. Using increased Rh123 fluorescence as an indicator of oxidant generation (secondary to O2− production), it was evident that, as expected, the myocardial mitochondria of wild-type (C57) mice irradiated at 10 Gy exhibited about twice the oxidant production of unirradiated controls. Moreover, the oxidant production observed in the myocardial mitochondria from the mtNOS knockout (nNOS−/−) animals did not increase after irradiation (Fig. 5). Similar to the findings for the 32D cells (Fig. 4), the increased oxidant production evident in the case of the wild-type mouse mitochondria was a transient early event; at 3–4 h postirradiation, mitochondria from the wild-type and knockout mouse hearts both exhibited the same decreased Rh123 fluorescence compared with unirradiated controls (Fig. 5). A decrease in Rh123 fluorescence may be indicative of lower levels of oxidants (such as organic peroxides and peroxynitrite) and/or depolarization of the inner mitochondrial membrane (47, 48). Further work, beyond the scope of the present investigation, is necessary to distinguish between these possibilities. Fortunately, the important result relevant to the current work is unambiguous in demonstrating that, within the earliest practically accessible time window after irradiation (i.e. 1–2 h), mitochondria from the wild-type animals clearly did exhibit evidence for elevated levels of oxidant generation compared with unirradiated controls, while those from the knockout animals did not (Fig. 5). This observation provides further support for the notion that mtNOS is a very significant source of the transient O2− production during the early period after irradiation. Thus the results of these animal studies are fully in keeping with the results of the NOS knockdown experiments in 32D cells.
FIG. 5.
Postirradiation oxidant production in wild-type and nNOS−/− murine heart myocardium after 10 Gy total-body irradiation. Levels of ONO2− and/or organic peroxides (products of H2O2 + peroxidase activity) are transiently decreased in the knockouts (nNOS−/− ≡ mtNOS−/−) compared to wild-type (C57BL/6NHSD) animals (*P < 0.05) after irradiation. Animals were euthanized 1 h or 3 h postirradiation, hearts were rapidly excised, and the myocardia were homogenized using Ringer's solution. Dihydrorhodamine-123 was added and the samples incubated for 15 min immediately prior to obtaining the fluorescence data. Measurements of rhodamine-123 fluorescence (excitation 500 nm; emission 535 nm) were recorded within 2 h, or within 4 h, of completing the dose.
DISCUSSION
l-Arginine has previously been shown to mitigate radiation-induced immune dysfunction (49) and inflammation (50), but there is no conflict between these reported systemic effects and the present findings regarding a more acute response of 32 D cells. We interpret the lack of any mitigative activity of l-arginine or its breakdown products in the cultured cells to indicate that changes occurring after several hours are almost certainly less important in relation to the mechanism of protection than earlier events. That is, if administered after irradiation, the relevant cell-signaling cascade has already begun before enough of the l-arginine can be absorbed to have any significant effect. Previously, it has been established that mitochondrially localized MnSOD, but not the normal cytosolic CuSOD, is radioprotective in 32D cells (9). Furthermore, since O2− is a charged (anionic) species, it is slow to cross membranes, and, therefore, one or more mitochondrial sources of O2− must be involved in the acute radiation response. As all NOS isoforms generate O2− under some conditions, including l-arginine depletion in the case of nNOS (51), it follows that mtNOS [≡ nNOS (25)] may be one of the sources in question. The hypothesis that mtNOS is a significant source of the O2− involved in the acute radiation response is supported by several of the present findings, specifically, that (i) supplemental l-arginine is indeed protective (Fig. 1), (ii) there are indications of both increased NO synthesis and elevated peroxynitrite generation soon after irradiation (Fig. 2), (iii) nNOS knockdown 32D cells are protected (Table 3), and (iv) mitochondria-rich murine myocardium shows evidence of increased oxidant production in wild-type tissue compared with that from knockout animals devoid of mtNOS (Fig. 5).
The oxidant flux (Fig. 4) formed secondary to the O2− production, which is probably mostly peroxynitrite (Fig. 2B), almost certainly mediates the transient inhibition of complex I observed in 32D cells soon after irradiation (Fig. 3A). We have previously shown that complex I becomes irreversibly inactivated by higher doses of radiation (50 Gy) and/or concentrations of peroxynitrite in excess of physiological levels (38, 52). Now, however, working with the isolated bovine enzyme at reduced levels of insult, we have begun to find evidence for reversible inhibition of complex I due to the modification of redox-sensitive thiols by ionizing radiation (see Fig. 3B). Since both the inhibition of complex I activity and the concomitant loss of mitochondrial membrane potential have recovered within about 1 h after irradiation (Fig. 3A and Table 4), it is not clear that either of these events are necessary components of the radiobiological signaling sequence leading to cell death. The observed transient loss of complex I activity may simply be a functional marker of postirradiation oxidant production but an otherwise insignificant branch of the main radiobiological response.
At this juncture, it is not entirely clear what triggers the “burst” of NO synthesis leading to mtNOS becoming transiently depleted of l-arginine. In keeping with the suggestions of others (4, 5), we speculate that this could be due to a redox-regulated flux of Ca2+ ions entering the mitochondria, starting during irradiation and continuing for a few minutes after completion of irradiation. The possible involvement of Ca2+ fluxes is attractive because in addition to explaining the activation of mtNOS, inhibition of complex I activity (Fig. 3A) is known to result from increased Ca2+ levels (53, 54). This could in turn contribute to the depolarization of the mitochondrial membrane we observe at 30 min postirradiation (Table 4). It is additionally worth noting, however, that the effect of Ca2+ is probably not direct inhibition of complex I, since we find the measured activity of the enzyme isolated from other mitochondrial components purified from beef heart [i.e., purified from beef heart and assayed by the methods of Sharpley et al. (55)] to be insensitive to added Ca2+ in the concentration range 1 to 100 μM (data not shown).
An especially intriguing aspect of the current findings is that while the evidence points to a rapid postirradiation production of superoxide and secondary oxidants that can be ameliorated by l-arginine, from about 1 to 6 h after irradiation all the parameters we measured returned to control levels. Exactly what chain of events has been set in motion leading to cell death typically starting from around 18 h onward? We observed evidence for mitochondrial biogenesis (Table 4 and Fig. 3) correlated with inner membrane depolarization (Table 4) and elevated oxidant production (Fig. 4). The most straightforward interpretation of these observations is that irradiation of 32D cells initiated a redox-sensitive signaling cascade promoting biosynthesis of dysfunctional mitochondria. The dysfunctional mitochondria exhibit elevated oxidant generation, resulting in positive feedback to the redox-sensitive components of the deleterious signaling pathway. Of course, further details of this proposed pathway, including identification of its components, remain to be elucidated and will be the subject of future studies. There is no disagreement with the seemingly equivalent suggestion of Nugent et al. (46) that the increased mitochondrial mass is probably a stress response to mitochondrial dysfunction.
It is clear from the clonogenic survival curves (Fig. 1) that l-arginine is a protector, but not a mitigator, of the effects of ionizing radiation. Putative radioprotectors are of considerable interest in the practice of radiation oncology, where significant damage to surrounding healthy tissue is a problem when irradiating tumors. Also, of course, in the event of nuclear accidents, or more sinister scenarios leading to uncontrolled release of radioactivity, it would be desirable to have an effective protector available for use by first responders and any other individuals for whom the possibility of exposure may be predictable. Unfortunately, l-arginine is unlikely to be of any practical use in humans, being inefficiently absorbed by many cells and there is no active mitochondrial uptake mechanism. To elicit the protective effect we report here (Fig. 1) it was necessary to add l-arginine to millimolar concentrations in the cell culture medium 1 h prior to irradiation. In actuality, some means of metabolically boosting cellular l-arginine levels, or a precursor coupled to a mitochondrial-targeting system, are likely to be more efficacious than l-arginine itself.
ACKNOWLEDGMENT
This research was Supported by U19-AI068021 (JSG) Project 3 (JP and LLP).
Footnotes
The online version of this article (http://doi.org/10.1667/ RR1281.1) contains supplementary information that is available to all authorized users.
REFERENCES
- 1.Epperly MW, Osipov AN, Martin I, Kawai KK, Borisenko GG, Tyurina YY, et al. Ascorbate as a “redox sensor” and protector against irradiation-induced oxidative stress in 32D CL 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–61. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–54. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 3.Leach JK, Black SM, Schmidt-Ullrich R, Mikkelsen RB. Activation of constitutive nitric-oxide synthase activity is an early signaling event induced by ionizing radiation. J Biol Chem. 2003;277:15400–6. doi: 10.1074/jbc.M110309200. [DOI] [PubMed] [Google Scholar]
- 4.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 5.Lyng FM, Seymour CB, Mothersill C. Oxidative stress in cells exposed to low levels of ionizing radiation. Biochem Soc Trans. 2001;29:350–3. doi: 10.1042/0300-5127:0290350. [DOI] [PubMed] [Google Scholar]
- 6.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 2006;165:400–9. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Zhao Y, Han W, Zhao G, Zhu L, Wang J, et al. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br J Cancer. 2008;98:1839–44. doi: 10.1038/sj.bjc.6604358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong GH. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995;1271:205–9. doi: 10.1016/0925-4439(95)00029-4. [DOI] [PubMed] [Google Scholar]
- 9.Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, et al. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–78. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 10.Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 11.Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, et al. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys. 1999;43:169–81. doi: 10.1016/s0360-3016(98)00355-1. [DOI] [PubMed] [Google Scholar]
- 12.Epperly MW, Tyurina YY, Nie S, Niu YY, Zhang X, Kagan V, et al. MnSOD-plasmid liposome gene therapy decreases ionizing irradiation-induced lipid peroxidation of the esophagus. In Vivo. 2005;19:997–1004. [PubMed] [Google Scholar]
- 13.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greengerger EE, Nie S, et al. Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors. Radiat Res. 2007;167:289–97. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 14.Zhong W, Oberley LW, Oberley TD, Yan T, Domann FE, St Clair DK. Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Differ. 1996;7:1175–86. [PubMed] [Google Scholar]
- 15.Epperly MW, Melendez JA, Zhang X, Nie S, Pearce L, Peterson J, et al. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat Res. 2009;171:588–95. doi: 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anoopkumar-Dukie S, McMahon A, Allshire A, Conere TJ. Further evidence for biological effects resulting from ionizing radiation doses in the diagnostic X-ray range. Br J Radiol. 2005;78:335–7. doi: 10.1259/bjr/24426084. [DOI] [PubMed] [Google Scholar]
- 17.Chi C, Ozawa T, Anzai K. In vivo nitric oxide production and iNOS expression in X-ray irradiated mouse skin. Biol Pharm Bull. 2006;29:348–53. doi: 10.1248/bpb.29.348. [DOI] [PubMed] [Google Scholar]
- 18.Kanai A, Epperly M, Pearce L, Birder L, Zeidel M, Meyers S, et al. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H13–21. doi: 10.1152/ajpheart.00737.2003. [DOI] [PubMed] [Google Scholar]
- 19.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–42. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 20.Radi R, Denicola A, Alvarez B, Ferrer-Sueta G, Rubbo H. The biological chemistry of peroxynitrite. In: Ignarro LJ, editor. Nitric oxide biology and pathobiology. Academic Press; San Diego: 2006. pp. 57–82. [Google Scholar]
- 21.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 22.Rosen GM, Tsai P, Weaver J, Porasuphatana S, Roman LJ, Starkov AA. The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem. 2002;277:40275–80. doi: 10.1074/jbc.M200853200. [DOI] [PubMed] [Google Scholar]
- 23.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–80. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 24.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–9. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 25.Kanai A, Epperly M, Pearce L, Birder L, Zeidel, Meyers S, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberger JS, Sakakeeny MA, Humphries RK, Eaves CJ, Eckner RJ. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983;80:2931–5. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epperly MW, Bray JA, Esocobar P, Bigbee WL, Watkins S, Greenberger JS. Overexpression of the human manganese superoxide dismutase (MnSOD) transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat Oncol Investig. 1999;7:331–42. doi: 10.1002/(SICI)1520-6823(1999)7:6<331::AID-ROI3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Sazanov LA, Peak-Chew SY, Fearnley IM, Walker JE. Resolution of the membrane domain of bovine complex I into subcomplexes: implications for the structural organization of the enzyme. Biochemistry. 2000;39:7229–35. doi: 10.1021/bi000335t. [DOI] [PubMed] [Google Scholar]
- 29.Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by N omega-propyl-L-arginine. J Med Chem. 1997;40:3869–70. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- 30.Yonetani T. Cytochrome oxidase from beef heart muscle. In: Maehly AC, editor. Biochemical preparations. John Wiley & Sons; New York: 1966. pp. 14–20. [Google Scholar]
- 31.Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–16. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 32.Hatefi Y, Rieske JS. The preparation and properties of DPNH—cytochrome c reductase (complex I-III of the respiratory chain) Methods Enzymol. 1967;10:225–31. [Google Scholar]
- 33.Sinjorgo KM, Durak I, Dekker HL, Edel CM, Hakvoort TB, van Gelder BF, et al. Bovine cytochrome c oxidases, purified from heart, skeletal muscle, liver and kidney, differ in the small subunits but show the same reaction kinetics with cytochrome c. Biochim Biophys Acta. 1987;893:251–8. doi: 10.1016/0005-2728(87)90046-6. [DOI] [PubMed] [Google Scholar]
- 34.Hevel JM, Marletta MA. Nitric-oxide synthase assays. Methods Enzymol. 1994;233:250–8. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 35.Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopalan MS, Stone B, Rwigema JC, Salimi U, Epperly MW, Goff J, et al. Intraesophageal manganese superoxide dismutaseplasmid liposomes ameliorates novel total-body and thoracic radiation sensitivity of NOS1−/− mice. Radiat Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce LL, Epperly MW, Greengerger JS, Pitt BR, Peterson J. Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide. Nitric Oxide. 2001;5:128–36. doi: 10.1006/niox.2001.0338. [DOI] [PubMed] [Google Scholar]
- 39.Beckman JS. Protein tyrosine nitration and peroxynitrite. FASEB J. 1002;16:1144. doi: 10.1096/fj.02-0133lte. [DOI] [PubMed] [Google Scholar]
- 40.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–17. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 42.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–6. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 43.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, et al. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–65. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB, Hockenbery DM. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J Cell Biol. 1997;138:449–69. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kluza J, Marchetti P, Gallego MA, Lancel S, Fournier C, Loyens A, et al. Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene. 2004;23:7018–30. doi: 10.1038/sj.onc.1207936. [DOI] [PubMed] [Google Scholar]
- 46.Nugent SM, Mothersill CE, Seymour C, McClean B, Lyng FM, Murpphy JE. Increased mitochondrial mass in cells with functionally compromised mitochondria after exposure to both direct gamma radiation and bystander factors. Radiat Res. 2007;168:134–42. doi: 10.1667/RR0769.1. [DOI] [PubMed] [Google Scholar]
- 47.Ischiropoulos H, Gow A, Thom SR, Kooy NW, Royall JA, Crow JP. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 1999;301:367–73. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- 48.Sobreira C, Davidson M, King MP, Miranda AF. Dihydrorhodamine 123 identifies impaired mitochondrial respiratory chain function in cultured cells harboring mitochondrial DNA mutations. J Histochem Cytochem. 1996;44:571–9. doi: 10.1177/44.6.8666742. [DOI] [PubMed] [Google Scholar]
- 49.Shukla J, Chatterjee S, Thakur VS, Premachandran S, Checker R, Poduval TB. L-Arginine reverses radiation-induced immune dysfunction: the need for optimum treatment window. Radiat Res. 2009;171:180–7. doi: 10.1667/RR1241.1. [DOI] [PubMed] [Google Scholar]
- 50.Shukla J, Khan NM, Thakur VS, Poduval TB. L-Arginine mitigates radiation-induced early changes in cardiac dysfunction: the role of inflammatory pathways. Radiat Res. 2011;176:158–69. doi: 10.1667/rr2523.1. [DOI] [PubMed] [Google Scholar]
- 51.Xia Y. Superoxide generation from nitric oxide synthases. Antioxid Redox Signal. 2007;9:1773–8. doi: 10.1089/ars.2007.1733. [DOI] [PubMed] [Google Scholar]
- 52.Pearce LL, Kanai AJ, Epperly MW, Peterson J. Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide. 2005;13:254–63. doi: 10.1016/j.niox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Jekabsone A, Ivanoviene L, Brown GC, Borutaite Nitric oxide and calcium together inactivate mitochondrial complex I and induce cytochrome c release. J Mol Cell Cardiol. 2003;35:803–9. doi: 10.1016/s0022-2828(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 54.Matsuzaki S, Szweda LI. Inhibition of complex I by Ca2+ reduces electron transport activity and the rate of superoxide anion production in cardiac submitochondrial particles. Biochemistry. 2007;46:1350–7. doi: 10.1021/bi0617916. [DOI] [PubMed] [Google Scholar]
- 55.Sharpley MS, Shannon RJ, Draghi F, Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–8. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]