Abstract
A zinc(II)-dipicolylamine coordination complex selectively associates with anionic liposomes, including sterically protected PEGylated liposomes, and causes rapid leakage of encapsulated contents.
Encapsulating drugs inside liposomes is a well-known method of reducing drug toxicity and improving therapeutic efficacy.1 The current generation of government approved liposome/drug formulations use passive diffusion processes to release the drug, which makes it hard to regulate the delivery.2 One of the goals of nanomedicine is to improve liposome-based formulations by developing strategies to trigger drug release with spatiotemporal control3. The literature on triggered release liposomes includes systems that leak their encapsulated aqueous contents upon changes in local environmental factors such as temperature,4 pH,5 light,6 and ultrasound.7 There are also liposome systems that become leaky after selective chemical bond cleavage.8 Although many chemicals are known to disrupt bilayer membranes, the concept of selective non-covalent triggered release from liposomes is surprisingly underdeveloped.9 A likely reason for this situation is the challenge to find a liposome composition that can be selectively lysed by an exogenously added chemical that otherwise has little affinity for the host cell membranes. Previously, we have reported that zinc(II)-dipicolylamine (ZnDPA) coordination complexes have a remarkable ability to selectively associate with anionic bilayer membranes and not associate with zwitterionic membranes that are characteristic of healthy mammalian cells.10 We have utilized this membrane selectivity to develop in vivo imaging probes that target localized populations of anionic cells, such as apoptotic mammalian cells or bacteria, within living animal models.11 Here, we propose a new application using ZnDPA complexes; that is, a novel method of non-covalent triggered drug delivery (Scheme 1). We envision a two-step dosing strategy. The mammalian subject is treated intravenously first with appropriately functionalized, anionic liposomes that are filled with drugs. After time to allow liposome accumulation at the site of disease, a subsequent dose of ZnDPA complex targets the liposomes and induces leakage of the encapsulated drugs. A strategic advantage with this method of triggered liposome release is that it does not require any knowledge of the anatomical location of the site of disease. Herein, we describe in vitro liposome studies that demonstrate proof of the general concept.
Scheme 1.
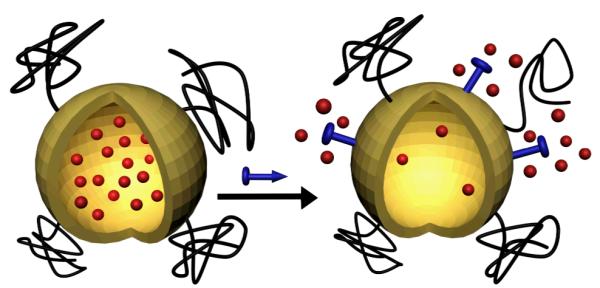
Triggered release from liposomes
The liposomes used in this study incorporated different ratios of three polar lipids, phosphatidylcholine (PC, zwitterionic head group), cholesterol (uncharged head group), and phosphatidylserine (PS, anionic head group). PS was chosen as the anionic target phospholipid for several reasons. It is typically the most common anionic phospholipid in the plasma membrane of healthy cells (5-15% of total phospholipid),12 but it is sequestered almost exclusively in the inner leaflet of the plasma membrane and not available for targeting by a hypothetical intravenous dose of ZnDPA complex 1. In comparison, a perfused disease-site containing a population of exogenous, PS-rich liposomes is expected to be a distinctive and biocompatible binding target for 1. Furthermore, it is known that association of metal cations or cationic molecules with PS-containing liposomes can induce membrane phase separation,13 a process that can enhance liposome leakage.14 Thus, the initial experimental goal was to ascertain if ZnDPA complex 1 could act as a chemical trigger and induce selective leakage of water-soluble contents from PS-rich liposomes.
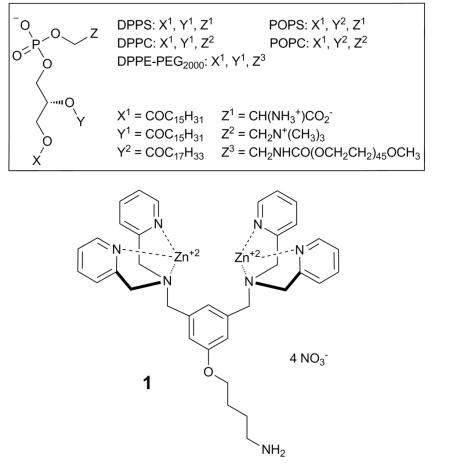
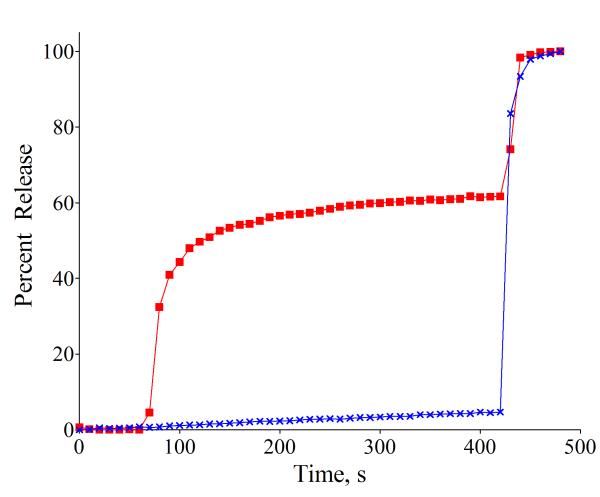
A standard carboxyfluorerscein (CF) leakage assay was employed to measure liposome release.15 Using film hydration and nanopore extrusion methods, the CF is trapped in high concentration (50 mM in 5 mM TES, 145 mM NaCl, pH 7.4) inside unilamellar liposomes (~200 nm diameter), which produces self-quenching of the fluorescent dye. Leakage from the liposomes leads to dilution of the CF and a large increase in fluorescence intensity. The first leakageexpe riments encapsulated a solution of CF inside anionic liposomes composed of DPPC:cholesterol:POPS 67:28: 5 (10 μM total lipid).‡ Addition of Zn(NO3)2 or the apo-ligand of 1 (see ESI) to a sample of the liposomes did not induce leakage, but as shown in Fig. 1, addition of chemical trigger 1 (10 μM) produced rapid and substantial dye release into the external solution (Table 1, entry 1). Negligible leakage was induced when the experiment was repeated with zwitterionic liposomes composed of DPPC: cholesterol 67:28 (Fig. 1 and Table 1, entry 2). This outcome was expected because ZnDPA complexes, such as 1, hardly associate with liposomes having zwitterionic surfaces (mimic of healthy mammalian cells). In other words, the 5 mol % of anionic PS is an essential component for ZnDPA complexation and triggered leakage. A more subtle result is the observation that replacing the unsaturated POPS with saturated DPPS leads to a significant decrease in the amount of CF release (Table 1, entry 3). This is a remarkable effect considering the small fraction of the PS component (5 mol %) within the liposome membrane and the very close structural similarity of DPPS and POPS. Another important observation is that changing the saturated DPPC to unsaturated POPC (that is, using liposomes composed of POPC:cholesterol:POPS, 67:28: 5) also eliminated most of the triggered release effect (Table 1, entry 50 4).
Fig. 1.
Percent CF release induced by addition of 1 (10 μM) at 60 s to liposomes (10 μM total lipid containing 50 mM CF) in 5 mM TES, 145 mM NaCl, pH 7.4, followed by liposome lysis with 20 % (v/v) Triton X-100 (20 μL) at 420 s. (red squares) Anionic DPPC:Cholesterol:POPS, 67:28:5; (blue crosses) Zwitterionic DPPC:Cholesterol, 67:28.
Table 1.
Percent release of CF from liposomes upon exposure to chemical trigger 1.a
| Membrane composition | |||||||
|---|---|---|---|---|---|---|---|
| Entry | DPPC | POPC | DPPS | POPS | DPPE- PEG2000 |
Chol. | % Rel. b |
| 1 | 67 | 0 | 0 | 5 | 0 | 28 | 55 |
| 2 | 67 | 0 | 0 | 0 | 0 | 28 | 3 |
| 3 | 67 | 0 | 5 | 0 | 0 | 28 | 4 |
| 4 | 0 | 67 | 0 | 5 | 0 | 28 | 9 |
| 5 | 67 | 0 | 0 | 5 | 2 | 28 | 63 |
| 6 | 67 | 0 | 0 | 5 | 8 | 28 | 84 |
| 7 | 67 | 0 | 0 | 0 | 2 | 28 | 24 |
| 8 | 67 | 0 | 0 | 0 | 8 | 28 | 19 |
Percent release at 120 s after addition of chemical trigger 1 in buffer (5 mM TES, 145 mM NaCl, pH 7.4); [total lipid] = [1] = 10 μM; 37 °C.
Uncertainty < 10% of the number.
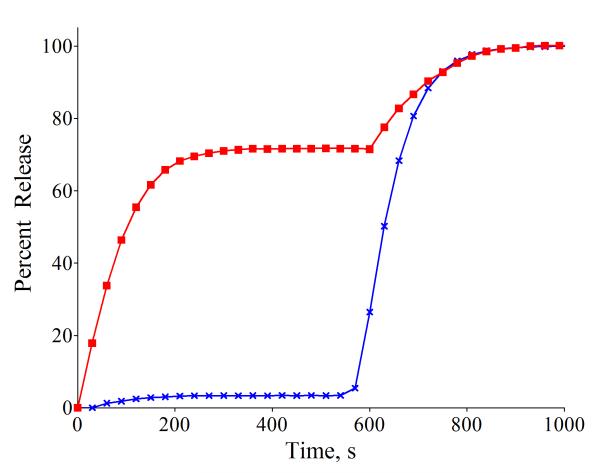
Additional leakage studies were conducted to shed light on the release mechanism. Previously, we have reported that a structurally different family of ZnDPA complexes can promote CF transport across liposome membranes using an anion counter-transport mechanism that involves direct coordination of the anionic CF with the lipophilic ZnDPA complex.15 In that case, CF transport was inhibited by the presence of anionic phospholipids. In contrast, the leakage process under study here is promoted by anionic phospholipid. Additional evidence that 1 does not employ the same anion counter-transport mechanism is the data in Fig. 2 showing that 1 triggers the leakage of uncharged glucose.16 The observation of glucose release is not only mechanistically important, but practically significant, as it suggests that 1 (or a next generation version) can trigger the leakage of any encapsulated water soluble analyte regardless of its charge. Another experimental result with important practical implications is the observation that 1 can trigger leakage from liposomes that are coated with long polyethyleneglycol (PEG) chains. It is well known in the drug delivery literature that circulation lifetime in the blood stream is greatly extended if the liposomes are PEGylated; that is, they incorporate a small fraction of amphiphilic DPPE-PEG2000 to sterically block self-aggregation of the liposomes and interaction with serum proteins.17 In the case of PS-rich liposomes, extensive steric protection is needed to prevent association with high-affinity blood coagulation proteins and liposome uptake by the mononuclear phagocytic system.18 But a potential strategic drawback with steric protection is diminished ability to release the liposome contents.8f However, entries 5-8 in Table 1 indicate that 1 can trigger CF leakage effectively from sterically protected PEGylated liposomes containing DPPE-PEG2000. Indeed the presence of DPPE-PEG2000 actually increases the amount of triggered release. This is not surprising since DPPE-PEG2000 contains an anionic phosphate diester head group that can promote membrane association of low molecular weight, chemical trigger 1. The fact that 1 can trigger leakage from sterically protected liposomes suggests that the leakage mechanism does not involve a liposome fusion process.19 Furthermore, dynamic light scattering measurements of liposomes treated with 1 show no significant change in the hydrodynamic diameter, which is not only evidence against liposome fusion but also against a lysis process that converts the liposomes into micelles.
Fig. 2.
(red squares) Percent glucose release induced by addition of 1 (10 μM) at 0 s to anionic liposomes (DPPC:Cholesterol:POPS, 67:28:5; 10 μM total lipid containing 0.3 M glucose) in 5 mM TES, 145 mM NaCl, pH 7.4, followed by liposome lysis with 20 % (v/v) Triton X-100 (20 μL) at 580 s. (blue crosses) Identical experiment but with no addition of 1.
The data indicates that the ternary mixture of cholesterol, saturated DPPC, and unsaturated POPS is a key factor for efficient triggered release by ZnDPA complex 1. The membrane phase behavior of this type of ternary mixture has been studied extensively and phase separation is a common phenomenon.20 It is known that binding of externally added Ca2+ to liposomes composed of cholesterol, saturated PC, and unsaturated PS induces phase separation and internal membrane budding.21 In the present case, DSC scans of liposomes composed of DPPC:cholesterol:POPS 67:28: 5 show that the presence of chemical trigger 1 induces a weak transition at about 44.5 °C (see ESI). We propose that association of cationic ZnDPA 1 with the anionic POPS head group induces domain formation and perhaps phase separation. This creates line tension and mismatched membrane thickness at the domain interfaces, which promotes liposome leakage.14 Consistent with this model is the observation that triggered CF leakage from liposomes composed of DPPC:Cholesterol:POPS 67:28:5, decreases substantially upon warming from 25 °C to 65 °C (ESI, Table S3). At increased temperature, the DPPC:Cholesterol:POPS 67:28:5 liposome membrane is more disordered (similar to entry 4, Table 1) and less susceptible to domain formation induced by association of 1 with the POPS head groups .
All the above results were obtained using 10 μM of chemical trigger 1, however, concentrations as low as 2 μM can induce decreased but significant amounts of liposome leakage (ESI, Table S2). A goal for future study is to optimize the structure of the chemical trigger and also the liposome membrane composition for efficient triggered release under pharmaceutically relevant conditions. A final point is the possibility of toxicity induced by the chemical trigger. Standard cell vitality assays showed that chemical trigger 1 is not toxic to human cells (see ESI) which agrees with the lack of toxicity seen in previously published cell and animal imaging studies using ZnDPA probes. This work was supported by the NIH (USA) and the University of Notre Dame.
Supplementary Material
Footnotes
Electronic Supplementary Information (ESI) available: [methods and spectraol data]. See DOI: 10.1039/b000000x/
Abbreviations: Chol (Cholesterol), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) , 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine [sodium salt] (DPPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine [sodium salt] (POPS), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] [ammonium salt] (DPPE-PEG2000).
Notes and references
- 1(a).Milla P, Dosio F, Cattel L. Curr. Drug. Metab. 2012;13:105. doi: 10.2174/138920012798356934. [DOI] [PubMed] [Google Scholar]; (b) Szebeni J, Muggia F, Gabizon A, Barenholz Y. Adv. Drug Delivery Rev. 2011;63:1020. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Malam Y, Loizidou M, Seifalian AM. Trends Pharmacol. Sci. 2009;30:592. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3(a).Alaouie AM, Sofou S. J. Biomed. Nanotechnol. 2008;4:234. [Google Scholar]; (b) Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. J. Pharm. Sci. 2008;97:4696. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]; (c) Lindner LH, Hossman M. Curr. Opin. Drug Discovery Dev. 2010;13:113. [PubMed] [Google Scholar]
- 4.Li L, ten Hagen TLM, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AMM, Lindner LH, Koning GA. J. Controlled Release. 2010;143:274. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Torres E, Mainini F, Napolitano R, Fedeli F, Cavalli R, Aime S, Terreno E. J. Controlled Release. 2011;154:196. doi: 10.1016/j.jconrel.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 6(a).Zasadzinski JA, Wong B, Forbes N, Braun G, Wu G. Curr. Opin. Colloid Interface Sci. 2011;16:203. doi: 10.1016/j.cocis.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qin G, Li Z, Xia R, Li F, O’Neill BE, Goodwin JT, Khant HA, Chiu W, Li KC. Nanotechnology. 2011;22:155605. doi: 10.1088/0957-4484/22/15/155605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7(a).Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. J. Controlled Release. 2010;148:13. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. J. Controlled Release. 2011;152:249. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 8(a).Andresen TL, Thompson DH, Kaasgaard T. Mol. Membr. Biol. 2010;27:353. doi: 10.3109/09687688.2010.515950. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li X, Zhao Y. Langmuir. 2012;28:4152. doi: 10.1021/la2050702. [DOI] [PubMed] [Google Scholar]; (c) Goldenbogen B, Brodersen N, Gramatica A, Loew M, Liebscher J, Herrmann A, Egger H, Budde B, Arbuzova A. Langmuir. 2011;27:10820. doi: 10.1021/la201160y. [DOI] [PubMed] [Google Scholar]; (d) Banerjee J, Hanson AJ, Gadam B, Elegbede AI, Tobwala S, Ganguly B, Wagh AV, Muhonen WW, Law B, Shabb JB, Srivastava DK, Mallik S. Bioconj. Chem. 2009;20:1332. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ong W, Yang Y, Cruciano AC, McCarley RL. J. Am. Chem. Soc. 2008;130:14739. doi: 10.1021/ja8050469. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Romberg B, Hennink WE, Storm G. Pharm. Res. 2007;25:55. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Guo X, Szoka FC., Jr. Acc. Chem. Res. 2003;36:335. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 9(a).Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang L. J. Am. Chem. Soc. 2011;133:4132. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheong I, Huang X, Thornton K, Diaz LA, Jr., Zhou S. Cancer Res. 2007;67:9605. doi: 10.1158/0008-5472.CAN-07-1565. [DOI] [PubMed] [Google Scholar]; (c) Volodkin D, Mohwald H, Voegel JC, Ball V. J. Controlled Release. 2007;117:111. doi: 10.1016/j.jconrel.2006.10.021. [DOI] [PubMed] [Google Scholar]; (d) McNally BA, Leevy WM, Smith BD. Suprmol. Chem. 2007;19:29. doi: 10.1080/10610270600902332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10(a).Lakshmi C, Hanshaw RG, Smith BD. Tetrahedron. 2004;60:11307. [Google Scholar]; (b) Hanshaw RG, O’Neil EJ, Foley M, Carpenter RT, Smith BD. J. Mater. Chem. 2005;15:2707. [Google Scholar]; (c) O’Neil EJ, Smith BD. Coord. Chem. Rev. 2006;250:3068. [Google Scholar]
- 11(a).Smith BA, Xiao S, Wolter W, Wheeler J, Suckow MA, Smith BD. Apoptosis. 2011;16:722. doi: 10.1007/s10495-011-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W, Suckow MA, Achilefu S, Smith BD. J. Am. Chem. Soc. 2010;132:67. doi: 10.1021/ja908467y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) White AG, Fu N, Leevy WM, Lee JJ, Blasco MA, Smith BD. Bioconjugate Chem. 2010;21:1297. doi: 10.1021/bc1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Leevy WM, Johnson JR, Lakshmi C, Morris J, Marquez M, Smith BD. Chem. Commun. 2006:1595. doi: 10.1039/b517519d. [DOI] [PubMed] [Google Scholar]; (e) Divittorio KM, Johnson JR, Johansson E, Reynolds AJ, Jolliffe KA, Smith BD. Org. Biomol. Chem. 2006;4:1966. doi: 10.1039/b514748d. [DOI] [PubMed] [Google Scholar]
- 12.Boon JM, Smith BD. Med. Res. Rev. 2002;22:251. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- 13(a).Ohnishi S, Ito T. Biochemistry. 1974;13:881. doi: 10.1021/bi00702a008. [DOI] [PubMed] [Google Scholar]; (b) Denisov G, Wanaski S, Luan P, Glaser M, McLaughlin S. Biophys. J. 1998;74:731. doi: 10.1016/S0006-3495(98)73998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bradley AJ, Maurer-Spurej E, Brooks DE, Devine DV. Biochemistry. 1999;38:8112. doi: 10.1021/bi990480a. [DOI] [PubMed] [Google Scholar]
- 14.Clerc SG, Thompson TE. Biophys. J. 1995;68:2333. doi: 10.1016/S0006-3495(95)80415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiVittorio KM, Leevy WM, O’Neil EJ, Johnson JR, Vakulenko S, Morris JD, Rosek KD, Serazin N, Hilkert S, Hurley S, Marquez M, Smith BD. ChemBioChem. 2008;9:286. doi: 10.1002/cbic.200700489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The glucose leakage experiment employed a standard coupled enzyme assay that consumes NADH and produces a change in absorbance. For further details, see the ESI and also: Westmark PR, Smith BD. J. Am. Chem. Soc. 1994;116:9343.
- 17.Allen C, Dos Santos N, Gallagher R, Chiu GNC, Shu Y, Li WM, Johnstone SA, Janoff AS, Mayer LD, Webb MS, Bally MB. Biosci. Rep. 2002;22:225. doi: 10.1023/a:1020186505848. [DOI] [PubMed] [Google Scholar]
- 18.Chiu GNC, Bally MB, Mayer LD. Biochim. Biophys. Acta. 2002;1560:37. doi: 10.1016/s0005-2736(01)00455-2. [DOI] [PubMed] [Google Scholar]
- 19.Holland JW, Hui C, Cullis PR, Madden TD. Biochemistry. 1996;35:2618. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 20(a).Veatch SL, Keller SL. Biophys. J. 2003;85:3074. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Baumgart T, Hess ST, Webb WW. Nature. 2003;425:821. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]; (c) Sasaki DY. Cell Biochem. Biophys. 2003;39:145. doi: 10.1385/CBB:39:2:145. [DOI] [PubMed] [Google Scholar]
- 21.Shimokawa N, Hishida M, Seto H, Yoshikawa K. Chem. Phys. Lett. 2010;496:59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.