Abstract
Depression predicts fall risk among older adults, and this relationship may be partially explained by depression-associated executive dysfunction, relevant to navigating demanding environments. This pilot study examined timed stepping accuracy under simple and complex dual-task conditions, using an instrumented walkway based on the Trail Making Test. Participants were balance-impaired older adults, either with (n = 8; major depressive disorder [MDD]) or without (n = 8; nondepressed [ND]) MDD. After accounting for comfortable gait speed and age, the MDD group was significantly slower than the ND group on the walkway with the highest cognitive demand and demonstrated greater dual-task cost, both of which were correlated with performance on traditional measures of executive functioning. No group differences were observed on the walkway with the least cognitive demand. Balance-impaired older adults with MDD demonstrate increased stepping accuracy time under cognitively demanding conditions, reflecting executive dysfunction and an additional contribution to increased fall risk.
Keywords: late life depression, dual task, gait
Introduction
Cognitive factors are increasingly recognized as contributors to fall risk in older adults. Executive dysfunction has been found to be the primary cognitive domain that predicts falls, including injurious falls, among healthy older adults,1–4 with other cognitive skills, such as memory, being less related to falling.2 Intact executive functions allow an individual to process continuously changing data, to appropriately allocate attentional resources to the task at hand, and to evaluate the success of a response and compensate for shifting internal and external demands.5
In the context of fall risk, poor executive functioning translates into reduced ability to adjust gait in accord with changing stimuli, such as barriers, changes in gradient, and so on. The ability to dual task, or walk while performing a cognitive task, is thought to reflect executive functioning skills and correlates with primary measures of executive functioning.1,6,7,9 Among 201 community-dwelling older adults, Herman and colleagues1 recently found that those who performed in the lowest quartile of executive functioning at baseline were 3 times more likely to fall during 2-year follow-up. Falls have been associated with greater dual-task cost, defined as the difference in performance on walking measures (such as speed or swing time variability, ie, variability in time taken by the swing leg to move in between contacts with the floor), with or without simultaneous performance of a cognitive task, such as counting backward or serial subtractions (eg, refs 1,10). Moreover, among the 213 older adults living independently in senior housing facilities followed for 1 year, only dual-task walking speed predicted recurrent falls, even though key variables such as age, sex, body mass index, Geriatric Depression scale (GDS) score, Mini-Mental State Examination (MMSE) score, distance vision, and prescription for psychoactive drugs were included in a fully adjusted model.10 It is important to keep in mind that this study included only individuals with GDS score <10 on the GDS-15, and that the mean number of depressive symptoms on the GDS-15 was less than 4. Thus, it is unlikely that many patients included in this study would have had major depressive disorder (MDD). While there are a number of factors beyond dual-task performance that consistently predict falls, the increase in fall risk with dual-task performance is not altogether surprising, given the attention diverted from focusing solely on walking (see Bock and colleagues11 for discussion).
To date, studies of dual-task performance have excluded individuals with depression, despite that late life depression has been associated with poor executive functioning skills, relative to normal controls.12–14 Executive dysfunction during late life depression has been coined “the depression executive dysfunction syndrome of late life” and is thought to reflect disruption to frontostriatal brain pathways.15,16 In addition depressive symptomatology has been associated with increased prospective17–19 and retrospective20 falls. Executive dysfunction, as reflected in measures of dual-task cost, may be one possible explanation for why individuals with depressive symptoms are at greater risk of falling, relative to normal controls.
The current pilot study sought to investigate whether depression significantly impacts dual-task performance among older adults with MDD, relative to nondepressed controls (ND). We recruited balance-impaired individuals at risk of falling in order to isolate depression status as the independent variable, by decreasing potential differences in functional status between groups. The dual-task paradigm employed in the current study required participants to step accurately across a series of walk-ways with various cognitive demands (ie, sequences of letters and/or numbers), similar to the paper version of the Trail Making Test.28 While not a traditional dual-task measure, it requires that participants engage in 2 tasks with competing cognitive demands (ie, stepping while following a sequence of stimuli), meeting the formal definition for what entails a dual-task measure (cf,ref 22). Considering the literature to date on executive dysfunction among older adults with MDD and with studies of fall risk linking executive functioning to performance on dual-task measures, we hypothesized that patients with MDD would perform more poorly on a measure of dual tasking (ie, Walking Trail Making Test-B [W-TMT-B]) but would perform equivalently to controls on measures that require less cognitive demand (eg, W-TMT-Baseline, W-TMT-A,). We also hypothesized that among the entire group, performance on W-TMT-B would be significantly correlated with performance on traditional paper-and-pencil measures of executive functioning skills.
Methods
Participants
Participants included 8 older adults with a Diagnostic and Statistical Manual of Mental Disorder (Fourth Edition [DSM-IV] diagnosis of MDD (3 males and 5 females) and 8 older controls with no history of MDD (2 males and 6 females). A 17th individual was excluded from the study due to technological problems with the equipment. The 2 groups of adults ranged in age from 63 to 91 and were recruited from University research participant registries and outpatient clinics at University and Veterans Affairs hospitals in Ann Arbor, Michigan. Exclusion criteria included a history or diagnosis of any traumatic brain injury, loss of consciousness of >3 minutes, dementia, diagnoses known to affect gait, such as parkinsonism, epilepsy, history of cerebrovascular accident (CVA), or amputation of a lower extremity, bipolar depression or psychosis, current substance abuse, untreated diabetes, medical instability (eg, acute, terminal, or worsening major medical condition), currently undergoing major medical treatment such as chemotherapy or radiation, inability to walk without an assistive device, severe weight-bearing pain such that it would interfere with gait-related tasks, and inability to speak English fluently. All participants were administered an institutional review board (IRB)-approved standardized screen over the telephone given by a research assistant to determine initial eligibility. Diagnosis of MDD and ruling out of psychiatric comorbid conditions were made by a licensed psychologist (S.L.W.) using the Structured Clinical Interview for DSM-IV.23 Both MDD and ND participants were included only if they scored ≥24 on the MMSE,21 to rule out generalized cognitive impairment, and performed of unipedal stance time (UST) in <5 seconds to indicate fall risk.24,25 The 2 groups also were equivalent with regard to medical comorbidities, as these variables are thought to negatively impact cognitive functioning (see Boyle et al26) and theoretically, performance on the W-TMT. Of the 8 MDD patients, 5 were treated with an antidepressant medication. Briefly, 2 participants were treated with citalopram only, 2 were treated with citalopram plus bupropion, and 1 was treated with duloxetine and clonazepam. There were no significant differences in demographic characteristics between groups (see Table 1). Hamilton Depression Rating scale (HDRS)36 scores were 16.0 ± 4.8 in the MDD group and 1.9 ± 1.1in the ND sample.
Table 1.
Demographic and Clinical Characteristics of Major Depressive Disorder (MDD) and Nondepressed Controls (ND)
| MDD, M ± SD | ND, M ± SD | |
|---|---|---|
| Age | 75.0 ± 9.6 | 78.5 ± 8.0 |
| Education | 15.9 ± 2.6 | 16.0 ± 2.4 |
| Sex | 5F,3M | 6F,2M |
| HDRS-17 | 16.0 ± 4.8 | 1.9 ± 1.1 |
| Time to complete 10 m walk (seconds) | 12.5 ± 3.2 | 18.1±13.2 |
| Charlson comorbidity index | 3.8 ± 1.5 | 4.3 ± 1.0 |
| Number of falls | 1.0 ± 1.8 | 0.5 ± 0.5 |
| Number of medications | 4.8 ± 3.2 | 5.1 ± 3.0 |
| MMSE | 27.9 ± 2.4 | 28.1 ± 2.1 |
Abbreviations: HDRS, Hamilton Depression Rating scale; MMSE, Mini-Mental State Examination; F, female; M, male.
All > .05, except HDRS (< .001).
Walking Trail Making Task
The W-TMT27 included four 10-m (5 m up, 5 m back) walk-ways containing 33 instrumented stepping targets of 48 mm diameter. Successful steps were those in which the forefoot met the target within 2 cm. Each participant was sized to wear a standardized flat-soled walking shoe with aluminum forefoot to activate the instrumented targets. Participants were shadowed by a research assistant who walked slightly to the side and behind the person. Participants were instructed to move on to the next target when the system read their steps and indicated a recorded response eliciting a beep. Participants were told not to use handrails unless they felt unsteady and in need of support beyond the shadowing of a research assistant.
For the current study, 4 types of pathways of increasing complexity were used (see Figures 1 and 2). The first walkway was used to measure the time to walk up and back a 5-m walkway (10 m total) at comfortable gait speed, with no targets. The W-TMT-Numbers Only (W-TMT-Baseline) consisted of stepping on numbers in consecutive order (ie, 1-2-3). The W-TMT-A pathway was a somewhat more complex version of W-TMT-Baseline and contained distracter numbers, requiring the participant to choose the correct set of consecutive numbers on which to step. The W-TMT-B walkway involves the greatest cognitive demand and requires participants to alternate steps between numbers and letters (ie, 1-A-2-B-3-C), and again maintain a set despite distracters. Participants completed 3 trials on each walkway. Their average completion time across each of the 3 trials was used as the basis for performance. Baseline walking time for each participant was measured first with comfortable walking speed on a walkway containing no targets. Following the 3 trials, participants were administered W-TMT-Baseline to ensure an understanding of the instructions. The remaining order of the walkways for each participant was then counterbalanced. For each walkway, the participants were told to imagine the walkway as an icy sidewalk and the numbered and lettered white dots as dry spots on which they can safely step without slipping. Consistent with the paper-and-pencil version of the TMT, accuracy was emphasized and errors were corrected by redirecting participants to go back and resume from the last correct response. In this way, the time measure. reflects errors, as with the standard paper-and-pencil version,28 and the number of errors is not considered in outcome measures. We also calculated cognitive demand cost, or the percentage of additional time required for participants to complete the more demanding W-TMT-B, relative to the less demanding W-TMT-A. This was calculated as, [(W–TMT–B)–WTMT– A)/W–TMT–A] × 100.
Figure 1.
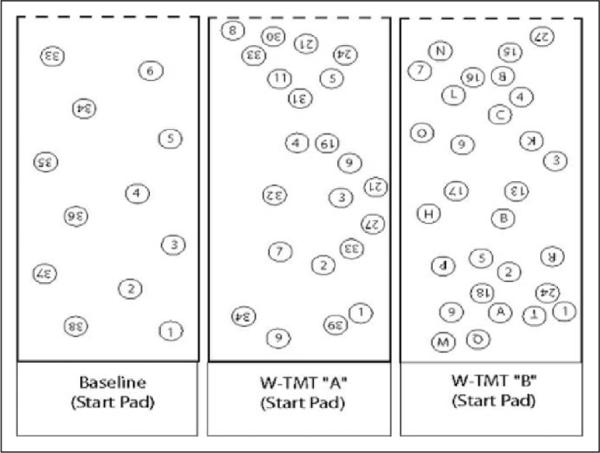
Walking trail making test, depicting W-TMT-Baseline, W-TMT-A, and W-TMT-B (partial walkway depiction). Figure depicts both up and back portions of the entire walkway. W-TMT indicates Walking Trail Making Test.
Figure 2.

Walking trail making test instrumented walkway.
Neuropsychological Measures
All eligible participants were administered a comprehensive neuropsychological battery, including standard measures of executive functioning, expected to play a role in gait performance.29 The Wisconsin Card Sorting Test (WCST30) is a set-shifting task in which participants are asked to categorize 2 decks of cards by the given characteristics of 4 key cards. The correct characteristic to which to match the cards changes throughout the task, and the examinees must decipher the categorical rule following examiner feedback (ie, correct/incorrect). The TMT28 is a 2-part timed paper-and-pencil measure with a numeric and numeric–letter task. On the first task (TMT-A), the participants are instructed to draw a line connecting numbers in numerical sequence as quickly and accurately as possible (ie, 1-2-3). The second task (TMT-B) requires the participants to draw a line in sequence alternating between numbers and letters (ie, 1-A-2-B-3-C). Two measures of verbal fluency were used, Controlled Oral Word Association Test (COWAT31) and Animal Naming.32 For the COWAT, a measure of phonemic fluency, participants are given a total of 3 letters, 1 at a time and asked to name as many words as possible, beginning with the letter indicated within 1 minute. Animal Naming was used as a measure of semantic fluency in which the participants were instructed to list as many animals as possible within 1 minute. The California Verbal Learning Test-233 (CVLT-2) is a verbal memory measure that includes 5 learning trials of a 16-item list of words, immediate recall following a distracter trial, and 30-minute delayed recall. The Brief Visual Memory Test–Revised34 (BVMT-R) is a visual memory measure that includes 3 learning trials of simple geometric designs and a 30-minute delayed recall trial. The American National Adult Reading Test (AMNART) is a word list reading task used to estimate premorbid verbal intellect.35
Procedure
All participants signed written informed consent prior to participation in the study, as approved by the VA Ann Arbor Healthcare System and University of Michigan IRBs. The participants then completed a clinical interview with a licensed psychologist (S.L.W.), including administration of the SCID-IV and HDRS.36 The participants were also administered the MMSE and UST during this time. Following the interview, the participants who met inclusion criteria were administered the comprehensive battery of neuropsychological measures and participated in the W-TMT.
Statistical Analyses
Repeated measures analysis of variance (rmANOVA) was performed for the walking trails measures, with W-TMT–Baseline, W-TMT–A, and W-TMT–B as within–participant variables, depression status (diagnosis yes/no) as the between–participant variable, and age and comfortable gait speed (ie, the time it takes to walk 10 m) as covariates, followed by post hoc ANOVAs. A separate ANOVA was performed for dual-task (cognitive demand) cost [(W–TMT–B) – WTMT–A)/W–TMT–A] × 100. To assess the degree to which performance on the W-TMT relates to performance on neuropsychological measures of executive functioning, we conducted partial Pearson product–moment correlation analyses, controlling for comfortable gait speed. Neuropsychological test scores for COWAT and WCST were age-corrected using normative data provided in the test manuals.32,37 Normative data for Animal Naming and TMT A & B were derived from the revised Heaton norms.38
Results
Walking Trail Making Test
The rmANOVA demonstrated a significant main effect of depression status F(1, 12) = 5.79, p < .05, η2 = .33 and a significant interaction of walkway × MDD status, MDD status, F(1, 12) = 4.89, p < .05, partial η2 = .29 (see Figure 3). Post hoc ANOVAs demonstrated that individuals with MDD were significantly slower than ND individuals on W-TMT-B, F(1, 12) = 6.52, p < .05, partial η2 = .35 and marginally slower than ND on W-TMT-A, F(1, 12) = 2.95, p = .11, partial η2 = .20 but not on W-TMT-Baseline, F(1, 12) = .70, p = .42, partial η2 = .06. Individuals with MDD demonstrated significantly greater cognitive demand cost (ie, approximately 45% greater) relative to the ND group, F(1, 12) = 4.83, p < .05, partial η2 = .29 (see Figure 4).
Figure 3.
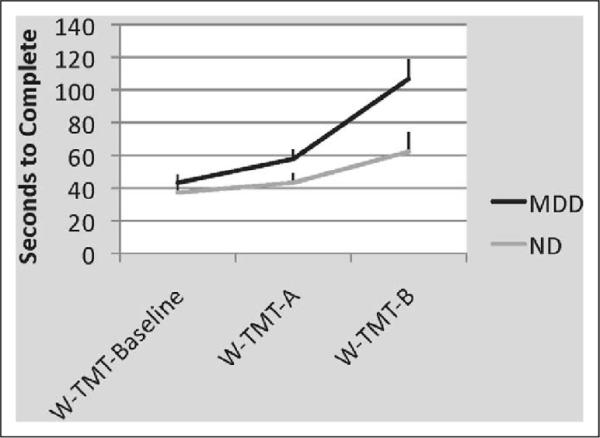
Average (± SE) speed to complete W-TMT (seconds.) There was a significant interaction of walkway × MDD status, F(1, 12) = 4.89, p < .05, partial η2 = .29 and a significant main effect of depression status, F(1, 12) = 5.79, p < .05, η2 = .33. Individuals with MDD were significantly slower than ND individuals on W-TMT-B, F(1, 12) = 6.52, p < .05, partial η2 = .35, and marginally slower than ND on W-TMT-A, F(1, 12) = 2.95, p = .11, partial η2 = .20 but not on W-TMT-Baseline, F(1, 12) = .70, p = .42, partial η2 = .06. W-TMT indicates Walking Trail Making Test; MDD, major depressive disorder; ND, nondepressed; SE, standard error.
Figure 4.
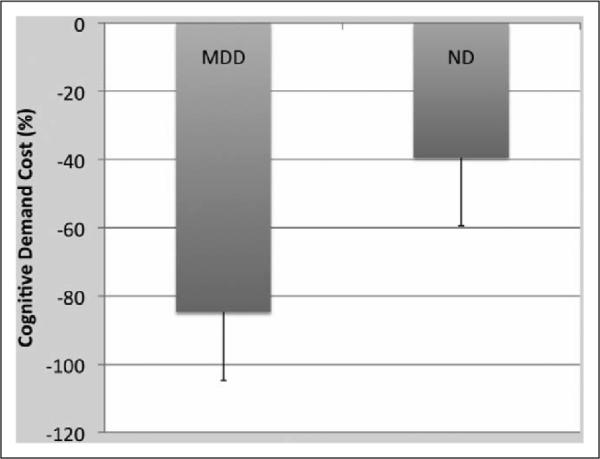
Mean cognitive demand cost (%) ± SE. Individuals with MDD demonstrated significantly greater cognitive demand cost relative to the ND group, F(1, 12) = 4.83, p < .05, partial η2 = .29. MDD indicates major depressive disorder; ND, nondepressed; SE, standard error.
In order to address the question of whether the presence of psychotropic medications in the MDD group was associated with performance on the W-TMT measures, we performed a set of post hoc analyses among the MDD group only. While there were no significant differences between medicated and unmedicated groups (albeit the sample sizes were likely too small to detect significant differences), there were some notable performance differences. After controlling for comfortable gait speed, patients in the MDD group medicated with antidepressant medications (n = 5) performed nominally more poorly than the unmedicated group (n = 3) on W-TMT-B (M = 109.25, SD = 38.71 and M = 84.27, SD = 39.99, respectively). The medicated group also performed nominally more poorly than the unmedicated group on the measure of dual-task cost (M = 99.07, SD = 41.17 and M = 53.21, SD = 23.92, respectively). The 2 groups performed roughly equivalently on W-TMT-Baseline (medicated: M = 38.54, SD = 15.99 and unmedicated: M = 43.22, SD = 16.52) and on W-TMT-A (medicated: M = 51.99, SD = 22.45 and unmedicated: M = 56.71, SD = 23.17).
Correlations With Measures of Neuropsychological Measures
Partial correlations, controlling for comfortable gait speed, were performed with measures of executive functioning, memory, visuospatial functioning, estimated premorbid verbal IQ (as measured by a word-list reading task) and the W-TMT measures. Results indicated that performance on W-TMT-B and cognitive demand cost was significantly related to performance on the WCST, Animal Naming, Trails A, Trails B, and CVLT-II learning trials 1–5 in the expected direction, with poorer performance on the cognitive measure associated with greater time to complete the W-TMT measure. Short- and long-delay free recall on the CVLT-II was related only to cognitive demand cost, again in the expected direction. Cognitive demand cost was also associated with performance on COWAT, a measure of phonemic fluency, but in the opposite direction than would be expected. Visual memory, estimated premorbid IQ, and visuospatial skills were not significantly correlated with any of the W-TMT measures. Performance on W-TMT-Baseline was not significantly correlated with any of the neuropsychological measures, while performance on W-TMT-A was significantly associated only with performance on Trails B (see Table 2). Figure 5 contains means and standard deviations for performance on cognitive measures. While the differences are not statistically significant between the 2 groups, all scores, with the exception of the COWAT, BVMT Long-Delay Free Recall, and BVMT Copy (the latter 2 on which the 2 groups performed roughly equivalently) were in the expected direction, with MDD performing more poorly than controls.
Table 2.
Pearson Product-Moment Correlation Coefficients between Walking Trail Making Test and Traditional Executive Functioning Measures
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | .44 | .27 | .09 | −.32 | −.06 | −.02 | .24 | <.01 | −.02 | −.01 | .22 | .03 | −.37 | −.32 | −.40 |
| 2 | – | .74** | .21 | −.23 | −.09 | −.10 | −.06 | −.03 | .12 | .02 | −.05 | −.25 | −.39 | −.58 | −.48 | |
| 3 | – | .68** | .02 | −.60* | −.51 | −.47 | −.37 | −.37 | −.19 | .06 | −.55* | − .67** | −.82*** | −.59* | ||
| 4 | – | .46 | −.81*** | −.73** | −.57* | −.25 | −.37 | −.24 | .51 | −.58* | −.75** | −.70** | −.62* | |||
| 5 | – | −.12 | −.07 | .01 | .29 | .12 | −.05 | .35 | .07 | −.18 | −.03 | −.12 | ||||
| 6 | – | .90*** | .77** | .40 | .64* | .44 | −.33 | .48 | .57* | .56* | .59* | |||||
| 7 | – | .90*** | .32 | .56* | .55* | −.39 | .58* | .59* | .54* | .52 | ||||||
| 8 | – | .34 | .52* | .52* | −.21 | .57* | .50 | .40 | .27 | |||||||
| 9 | – | .81*** | .22 | .32 | .28 | .36 | .49 | −.02 | ||||||||
| 10 | – | .58* | .32 | .13 | .43 | .40 | .22 | |||||||||
| 11 | – | .15 | −.01 | .48 | .30 | .35 | ||||||||||
| 12 | – | −.38 | −.21 | −.12 | −.16 | |||||||||||
| 13 | – | .44 | .66* | .17 | ||||||||||||
| 14 | – | .83*** | .56* | |||||||||||||
| 15 | .68** | .82*** | ||||||||||||||
| 16 | – | .51 |
Note. Scores for measures 5–10 and 12–16 are standardized, such that higher score reflects better performance. Score for measure 11 is raw, again with higher score reflecting better performance.
1. W-TMT-Baseline; 2. W-TMT-A; 3. W-TMT-B; 4. Cognitive Demand Cost; 5. American National Adult Reading Test; 6. California Verbal Learning Test-2, Trials 1–5; 7. California Verbal Learning Test-2, Short Delay Free Recall; 8. California Verbal Learning Test-2, Long Delay Free Recall; 9. Brief Visual Memory Test-R, Trials 1–3; 10. Brief Visual Memory Test-R, Long Delay Free Recall; 11. Brief Visual Memory Test-R, Copy; 12. Controlled Oral Word Association Test; 13. Animal Naming; 14. Trail Making Test A; 15. Trail Making Test B; 16. Wisconsin Card Sort Test, Perseverative Errors
p < .05;
p, .01;
p < .001
Figure 5.
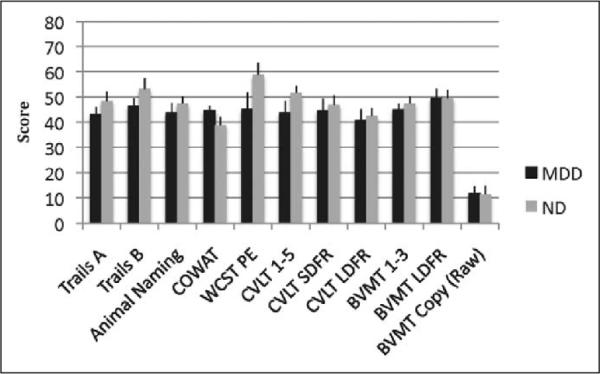
Mean scores on traditional neuropsychological measures by group ± SE. Differences between the MDD and ND groups were not significant at p < .05. All test scores are reported in T scores (M = 50, SD = 10) except BVMT-Copy (raw). MDD indicates major depressive disorder; ND, nondepressed; SE, standard error; BVMT, Brief Visual Memory Test.
Discussion
The current pilot study showed that among a small sample of individuals with MDD and balance impairment, increasing cognitive demand was associated with decreasing performance on a measure of timed stepping accuracy, relative to ND controls. This is the first study to assess performance on a task that includes cognitive and gait demands among older adults with MDD, and provide insights into why older adults with depressive symptoms may be at increased risk of falling, relative to their ND peers.17,19
Performance on traditional measures of executive functioning was associated with performance on the dual-task measure used in this study, and most strongly related to the measures reflecting the greatest cognitive demand (ie, W-TMT-B and dual-task cost). Executive dysfunction has been related to increased risk of falling in a number of studies1–3 and may help to account for the relationship between depressive symptoms and fall risk among older adults. While performance on measures of executive functioning was not significantly different between the MDD and ND groups in the present study, the MDD group performed nominally more poorly across most measures of executive functioning, despite equivalent levels of estimated premorbid verbal intellect. Larger sample sizes may achieve significant differences. Indeed, poorer performance on measures of executive functioning among older adults with MDD has been commonly observed.12,13,14,39 On the other hand, it is possible that dual-task performance detects more subtle cognitive decrements than those observed on paper-and-pencil measures. In the current study, a measure of phonemic fluency was the only test of executive functioning that did not follow this pattern, and it was weakly associated with the other measures of executive functioning used in this study (rs = −.12 to .38). Phonemic fluency has been shown to recruit fewer frontal regions among older adults as compared to semantic fluency tasks40 and thus may not be a sufficient stimulus to gauge increased fall risk in older adults.
In fact, in the current study, cognitive demand cost, measured as the relative difference between performance on W-TMT-A and W-TMT-B, was the most robust measure, with performance on the more complex Trails B declining from Trails A at nearly twice the rate of controls. It also correlated most highly with traditional measures of executive functioning. A dual-task cost difference of 45% in the MDD compared to the ND group in the current study is higher than between-group differences reported in other studies11,41,42 of dual-task cost also employing visual stimuli, with differences in cost between groups typically ranging from 10% to 20%. This lower cost may relate to samples studied, with most studies comparing young and older adults, or it may also be related to the specific dual-task paradigm employed in the current study. In fact, a study by Persad and colleagues29 who used the same paradigm to measure dual-task cost as was used in the current study, but with a larger sample size, also detected a cognitive demand cost difference of approximately 60% between patients with MCI in the executive functioning domain and age-matched healthy controls. The cognitive stimuli embedded in the walking task create competition for attentional resources for visual stimuli, which Bock and colleagues11 suggest is crucial to produce significant dual-task effects between groups. Thus, the paradigm employed in the current study may be more challenging for patient groups and have greater utility in clinical settings. It is likely that more executive functioning resources are elicited when required to shift mental set between 2 tasks with competing and similar attentional demands, as opposed to between 2 disparate pieces of information, such as making verbal responses while walking.
Performance on the W-TMT-B and dual-task cost was also associated with verbal memory skills, but not with visual memory, estimated premorbid verbal IQ, or visuospatial skills. On the one hand, this might suggest that W-TMT reflects both executive dysfunction and reduced verbal memory. On the other hand, among older patients with depression, verbal memory dysfunction and executive dysfunction commonly co-occur. In fact, executive dysfunction has been found to mediate verbal memory performance among older patients with depression, as successful encoding of verbal stimuli relies in part on intact executive functioning skills (eg, organizing information during the encoding phase).14
It is notable that all patients in the current study were balance impaired (UST < 5 seconds), which already places them at higher risk of experiencing an injurious fall.25 The presence of MDD was the only measurable difference between the 2 groups, suggesting that the presence of clinical depression, as opposed to other physical factors associated with fall risk, such as presence of medical comorbidities, contributes to performance on a measure of dual-task cost. It should also be noted that 5 of our 8 patients were medicated with antidepressants, with 4 of those 5 being prescribed an selective serotonin reuptake inhibitor (SSRI). Antidepressants may contribute to increased fall risk even when depressive symptoms have been taken into account; particularly among those prescribed SSRIs,43,44 an effect not observed in other studies.18 While our sample was too small to reliably assess for effects of medication, posthoc analyses suggested that our small sample of medicated patients performed nominally more poorly than the (even smaller) sample of unmedicated patients on the most complex measures (W-TMT-B and dual-task cost). There was a large amount of variability in the medicated and unmedicated groups, however. In order to tease apart the specific effects of antidepressant medications, a much larger sample of medicated and unmedicated patients would be required, matched for potentially confounding variables such as depression severity. In fact, in the current sample, the medicated patients reported nominally more severe depressive symptoms than the unmedicated group (HDRS score M = 17.00, SD = 3.8 vs M = 14.3, SD = 6.8, respectively).
While purely speculative at this point, it will be important for future studies to address why depression is related to both executive dysfunction and risk of falling. It is possible, for example, that a common factor underlies all 3 symptoms. Pathology in frontostriatal cerebral circuitry would be a candidate explanation. White matter pathology has been associated with performance on fall risk indicaters,45–47 executive dysfunction,48 and depression.49,50 Nevertheless, the explanation is likely more complicated than this, as white matter abnormalities in frontostriatal circuitry has also been demonstrated among ND elders who perform poorly on fall risk indictors.45–47 Future research might consider how different combinations of physical, cognitive, and affective symptomatology place individuals at greater or lesser risk of falling. Such profiling could then be used in clinical settings to deter-mine individuals' risk profile and may suggest specific preventive and treatment regimens.
The current study does have a number of limitations that should be considered, in light of the findings. First, this is a pilot study with a small sample size, and thus findings should be confirmed with larger samples. Second, both the MDD and ND groups presented with a number of medical comorbidities and prescriptions for medications that may have contributed to their performance. It is not possible with the small sample to address specific effects of these variables, but larger studies should specifically test the extent to which specific medical diagnoses (e.g., diabetes, hypertension, etc.) contribute to performance on dual-task measures. Finally, the depressed sample in this study was diagnosed with MDD. To date, studies investigating the relationship between depression and fall risk have related scores on a measure of self-reported symptoms of depression to prospective17–19 or retrospective falls20 or to performance on indicators of fall risk,1 but have not compared differences between groups with clinical MDD with their ND counterparts. Because this pilot study recruited only elders with clinical significant depression, in line with most studies of geriatric depression in the psychiatry literature, we are unable to address how the presence of lesser levels of depressive symptoms might contribute to performance on dual-task measures. This is an important question for future study, as most older adults with depressive symptomatology do not meet full criteria for MDD (see ref 51). Finally, we did not specifically assess vision, and more specific deficits such as contrast sensitivity may have affected study results to some degree. At the same time, no participant reported difficulty viewing the stimuli and all participants were instructed to wear their corrective lenses prior to tasks. It will be important to more formally test vision in future studies.
Conclusions
A small sample of older adults with depression have prolonged stepping time performance under increasingly cognitively demanding conditions, suggesting the interference of executive dysfunction on gait in complex environments. A comprehensive assessment of fall risk should include measures of physical (eg, gait and balance), cognitive, and affective functioning.
Acknowledgments
We would like to thank Ciaran Considine, BS, Brennan Haase, BS, Hadia Leon, BS, and Kortni Meyers, BS, for their assistance with recruitment and data collection and scoring. We would like to thank Carol Persad, PhD, for assistance with Walking Trail Making Test measurements and James Ashton-Miller, PhD, for technical assistance. Thanks to Jon-Kar Zubieta, MD, PhD, for assistance with editing the manuscript. Thanks also to Turner Geriatric Clinic physicians for assistance with recruitment.
Funding The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Supported by a University of Michigan Depression Center Berman Award (SLW); E6388M (VA Career Development Award-Level 1; SLW), AG08808 (University of Michigan Claude D. Pepper Older Americans Independence Center, Biomechanics Core and Human Subjects Assessment Core); AG109675 (K24 Mid-Career Investigator Award in Patient-Oriented Research; NAB); Office of Research and Development, Medical Service and Rehabilitation Research and Development Service of the Department of Veterans Affairs; the Dorothy and Herman Miller Fund for Mobility Research in Older Adults; and the Michigan Alzheimer's Disease Research Center.
Footnotes
This manuscript was presented at the 3rd International Congress on Gait and Mental Function, February 2010, Washington D.C.
Declaration of Conflicting Interests The authors declared no conflicts of interest with respect to the author-ship and/or publication of this article.
Reference
- 1.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65(10):1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21(5):540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46(5):M164–M170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 5.Giordani B, Persad C. Neuropsychological influences on gait in the elderly. In: Hausdorff JM, Alexander NB, editors. Gait Disorders: Evaluation and Management. Taylor & Francis Group; Boca Raton, FL: 2005. pp. 117–142. [Google Scholar]
- 6.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53(3):410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 7.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35(6):619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu-Ambrose T, Khan KM, Donaldson MG, Eng JJ, Lord SR, McKay HA. Falls-related self-efficacy is independently associated with balance and mobility in older women with low bone mass. J Gerontol A Biol Sci Med Sci. 2006;61(5):832–838. doi: 10.1093/gerona/61.8.832. [DOI] [PubMed] [Google Scholar]
- 9.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 10.Beauchet O, Annweiler C, Allali G, Berrut G, Herrmann FR, Dubost V. Recurrent falls and dual task-related decrease in walking speed: is there a relationship? J Am Geriatr Soc. 2008;56(7):1265–1269. doi: 10.1111/j.1532-5415.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 11.Bock O. Dual-task costs while walking increase in old age for some, but not for other tasks: an experimental study of healthy young and elderly persons. J Neuroeng Rehabil. 2008;5:27. doi: 10.1186/1743-0003-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. J Geriatr Psychiatry Neurol. 2004;17(4):195–201. doi: 10.1177/0891988704269823. [DOI] [PubMed] [Google Scholar]
- 13.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 14.Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2007;22(2):261–270. doi: 10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- 16.Alexopoulos G. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Andresen EM, Wolinsky FD, Miller JP, Wilson MM, Malmstrom TK, Miller DK. Cross-sectional and longitudinal risk factors for falls, fear of falling, and falls efficacy in a cohort of middle-aged African Americans. Gerontologist. 2006;46(2):249–257. doi: 10.1093/geront/46.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Byers AL, Sheeran T, Mlodzianowski AE, Meyers BS, Nassisi P, Bruce ML. Depression and risk for adverse falls in older home health care patients. Res Gerontol Nurs. 2008;1(4):245–251. doi: 10.3928/19404921-20081001-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheeran T, Brown EL, Nassisi P, Bruce ML. Does depression predict falls among home health patients? Using a clinical-research partnership to improve the quality of geriatric care. Home Healthc Nurse. 2004;22(6):384–389. doi: 10.1097/00004045-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter CR, Scheatzle MD, D'Antonio JA, Ricci PT, Coben JH. Identification of fall risk factors in older adult emergency department patients. Acad Emerg Med. 2009;16(3):211–219. doi: 10.1111/j.1553-2712.2009.00351.x. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Wright DL, Kemp TL. The dual-task methodology and assessing the attentional demands of ambulation with walking devices. Phys Ther. 1992;72(4):306–312. doi: 10.1093/ptj/72.4.306. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-IV (SCID-IV) American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- 24.Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil. 2000;81(5):587–591. doi: 10.1016/s0003-9993(00)90039-x. [DOI] [PubMed] [Google Scholar]
- 25.Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45(6):735–738. doi: 10.1111/j.1532-5415.1997.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander NB, Ashton-Miller JA, Giordani B, Guire K, Schultz AB. Age differences in timed accurate stepping with increasing cognitive and visual demand: a walking trail making test. J Gerontol A Biol Sci Med Sci. 2005;60(12):1558–1562. doi: 10.1093/gerona/60.12.1558. [DOI] [PubMed] [Google Scholar]
- 28.Reitan R, WD . The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tucson: 1985. [Google Scholar]
- 29.Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J Gerontol A Biol Sci Med Sci. 2008;63(12):1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 31.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd ed. AJA Associates; Iowa City: 1994. [Google Scholar]
- 32.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. 3rd Ed. Psychological Assessment Resources; Lutz, FL: 2005. [Google Scholar]
- 33.Delis DC, Kramer JH, Kaplan E, Ober BA. Calfornia Verbal Learning Test-Second Edition, Adult Version. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 34.Benedict RHB. Brief Visuospatial Memory Test-Revised: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1997. [Google Scholar]
- 35.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 37.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual- Revised and Expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- 38.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- 39.Elderkin-Thompson V, Kumar A, Mintz J. Executive dysfunction and visuospatial ability among depressed elders in a community setting. Arch Clin Neuropsychol. 2003;19(5):597–611. doi: 10.1016/j.acn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Meinzer M, Flaisch T, Wilser L, et al. Neural signatures of semantic and phonemic fluency in young and old adults. J Cogn Neurosci. 2009;21(10):2007–2018. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li KZ, Lindenberger U, Freund AM, Baltes PB. Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci. 2001;12(3):230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- 42.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15(3):417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- 43.Kerse N, Flicker L, Pfaff JJ, et al. Falls, depression and antidepressants in later life: a large primary care appraisal. PLoS One. 2008;3(6):e2423. doi: 10.1371/journal.pone.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vestergaard P. Skeletal effects of central nervous system active drugs: anxiolytics, sedatives, antidepressants, lithium and neuroleptics. Curr Drug Saf. 2008;3(3):185–189. doi: 10.2174/157488608785699432. [DOI] [PubMed] [Google Scholar]
- 45.Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010;31(7):1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74(1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 48.Elderkin-Thompson V, Hellemann G, Pham D, Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2009;24(5):459–468. doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- 49.Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60(12):1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 50.Gunning-Dixon FM, Hoptman MJ, Lim KO, et al. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16(4):255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- 51.Lyness JM, King DA, Cox C, Yoediono Z, Caine ED. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47(6):647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]


