Abstract
Background
H5N1 avian influenza represents an episodic zoonotic disease with potential to cause a pandemic, and resistance is of considerable concern. We sought to generate high titer H5N1 antibodies in healthy volunteers for the purpose of developing hyperimmune IVIG.
Methods
We conducted a dose escalating unblinded clinical trial involving 75 subjects between the ages of 18 and 59 years. Three cohorts of twenty-five subjects were enrolled sequentially receiving 90, 120, or 180 mcg of H5N1 A/Vietnam/1203/04 vaccine for four doses 28 days apart.
Results
No significant dose related increases in the geometric mean titers (GMTs) of serum HAI were observed when comparing 90, 120, and 180 mcg. When analyzed together to determine the effect of additional vaccinations, HAI GMT after first, second, third and fourth vaccinations was 1:15.7, 1:22.2, 1:36.0, and 1:32.0, respectively (first vs baseline, P<0.0001; second vs first, P=0.02; third vs second, P<0.0001). The MN GMT after first, second, third, and fourth vaccinations was 1:17.5, 1:33.1, 1:55.7, and 1:68.4 respectively (all P<0.001).
Conclusion
Our study suggests that a third dose and fourth dose of the H5N1 A/Vietnam/1203/04 vaccine may result in a higher HA and MN GMT. There was no benefit to increasing the dose of the vaccine.
Keywords: avian influenza, vaccination, passive immunotherapy, IVIG
Introduction
H5N1 avian influenza currently represents an episodic zoonotic disease affecting at least 385 people in 14 countries. If this virus acquires the ability for sustained human-to-human transmission it could trigger the next influenza pandemic. It has previously been noted that there is a limited repertoire of antiviral drugs, and increasing resistance has been noted. [1] Recent studies reported that some H5N1 viruses isolated in Southern China and Southeast Asia were resistant to amantadine. [2],[3] Currently the circulating H5N1 viruses are susceptible to oseltamivir and zanamivir, though there have been descriptions of resistance to oseltamivir occurring on therapy, [4] and in circulating avian strains. [5]
Treatment with anti-influenza antibodies could potentially be of benefit in the treatment of avian influenza. Luke et al assessed passive immunotherapy during the 1918 pandemic, when severely ill patients were sometimes treated with plasma from convalescing survivors. In the eight published reports, the overall case fatality rate was 16% among 336 patients who received convalescent blood products, compared to 37% of 1219 untreated patients. [6] Zhou et al reported the prompt defervescence and cessation of viral shedding in a patient infected with H5N1 treated with convalescent plasma. [7]
A murine monoclonal antibody (mab) targeting a conserved site on the HA protected mice against lethal H1N1 and H2N1 influenza challenges. [9] Mabs to H1, given either intact or as Fab fragments, prevented lethal H1N1 infection in SCID mice. [10, 11] Humanized murine mabs prevented death when given to mice up to three days after an otherwise lethal H5N1 virus challenge. [12] Similarly, human mabs developed from Vietnamese H5N1 survivors protected mice when administered up to 72 hours after H5N1 virus challenge. [13]
Although monoclonal antibodies may have therapeutic potential, pooled human immunoglobulin from convalescent patients or vaccinees may be more readily available, more expeditiously approved for human use, and may be more likely to prevent the emergence of escape mutations due to the polyclonal nature. In view of this, we sought to determine a vaccination strategy to generate high titers of anti-influenza H5N1 antibodies in healthy volunteers for the purpose of developing a hyperimmune IVIG.
In previous studies using the influenza H5N1 vaccine (rgA/ Vietnam/1203/04 X A/PR/8/34), 451 healthy adults ages 18 to 64 years received two doses of 7.5, 15, 45, or 90 micrograms (mcg) or placebo by intramuscular (IM) injection. [14] A dose response relationship was demonstrated between vaccine and immunogenicity without a demonstrated plateau. Additional studies using purified influenza hemagglutinin vaccine (influenza A/Taiwan/1/86 (H1N1)) with HA doses up to 405 micrograms showed that increasing doses of HA resulted in increasingly higher levels of serum hemagglutination inhibiting and neutralizing antibody. [15] Therefore we sought to determine if additional and/or higher doses of the H5N1 A/Vietnam/1203/04 vaccine led to improved immunogenicity and higher antibody titers. We also sought to evaluate the Luciferase Immunoprecipitation System (LIPS) as a new platform for the assessment of immune response to the vaccine.
Materials and Methods
Vaccine
The vaccine used in this study is a monovalent inactivated subvirion H5N1 vaccine (rgA/ Vietnam/1203/04 X A/PR/8/34) containing 90 mcg/mL A/H5N1 HA, as determined by single radial immunodiffusion, and manufactured by Sanofi Pasteur Inc, Swiftwater, PA.
Study Design and Subjects
We conducted a single center, dose escalating unblinded clinical trial. Written informed consent was obtained from potential subjects prior to screening. Healthy non-pregnant adults between the ages of 18 and 59 years who had no known allergy to vaccine components (including eggs), and met standard FDA criteria for plasma donation were eligible for this study. The study was conducted in accordance with an Institutional Review Board approved protocol.
Study Procedures
Three cohorts of twenty-five subjects each were enrolled sequentially. Cohorts 1-3 were assigned to receive 90 mcg, 120 mcg, or 180 mcg of H5N1 vaccine respectively. Each subject was to receive four doses of vaccine approximately 28 days apart in the deltoid or gluteal muscle. Subjects were allowed to choose the site of vaccination and were observed for 30 minutes after each immunization.
For the seven days after each vaccination subjects recorded the presence and severity of local and systemic symptoms on a diary card. Blood samples for antibody assays were collected before each vaccination and 4 weeks after the final dose.
After the vaccination phase of this protocol was completed, those subjects with a hemagglutinin inhibition titer above a given threshold (initially 1:640, and decreased to 1:160 by the end of the study) were asked to begin plasmapheresis.
All reported adverse events (AE) and serious adverse events (SAE) that occurred through Day 112 (4 weeks after the fourth vaccination) or anytime on plasmapheresis were recorded and reviewed by an independent safety monitoring committee. Stopping rules for safety were evaluated after each cohort was vaccinated at a given study point, prior to additional vaccinations, and prior to beginning enrollment at higher vaccine doses. Adverse events judged not related or unlikely related to the study interventions were excluded from this analysis.
Laboratory assays
Hemagglutination inhibition assays
Hemagglutination inhibition assays were performed at Southern Research Institute with the use of the influenza rgA/Vietnam/1203/2004 x A/PR/8/34 influenza (H5N1) vaccine strain. Hemagglutination-inhibition assays were performed according to established procedures with the use of horse erythrocytes. Serum samples were tested at an initial dilution of 1:10, and those that were negative were assigned a titer of 1:5. Previous studies with this vaccine reported the initial dilution as 1:20, and those negative at this dilution were assigned a titer of 1:10. [14]
Serum samples were tested in biweekly batches to determine eligibility for plasmapheresis (results not shown). At the end of the study, samples were tested under GLP in duplicate (different operators on different days). As 83 of 352 (23.6%) samples run in duplicate were found to differ from one another by more than two-fold, a third run was added for all samples. The geometric mean titer (GMT) was calculated for each serum sample. Replicate values not within two-fold of the other values were excluded from inclusion in the GMT calculation by procedures established prior to commencement of this study.
Microneutralization assays
Microneutralization assays were performed at Southern Research Institute according to established procedures using influenza rgA/Vietnam/1203/2004 x A/PR/8/34 influenza (H5N1). Serum samples were tested at an initial dilution of 1:20, and those that were negative were assigned a titer of 10. Serum samples were tested separately and in triplicate. The GMT was calculated for each serum sample. Replicate values not within two-fold of the other values were excluded from inclusion in the GMT calculation by procedures established prior to commencement of this study.
Luciferase Immunoprecipitation System (LIPS). Generation of Ruc-antigen fusion constructs
pREN2, a mammalian Renilla luciferase (Ruc) expression vector[16] was used to generate Heamagglutinin (HA) fusion protein constructs. A plasmid template for the HA of H5N1 Vietnam 1203 (from the CDC) was amplified by PCR and used to generate two non-overlapping DNA fragments of the HA. One of the fragments, HA-1, lacked the signal peptide and encoded amino acids 19-321 of the HA, while the HA-2 fragment encoded amino acids 332-550. Following subcloning into pREN2, the resulting constructs generated C-terminal fusions to Ruc. The plasmid DNA corresponding to each of these different pREN2 expression vectors was prepared using a Qiagen Midi preparation kit (Valencia, CA) DNA sequencing confirmed the integrity of these two DNA constructs.
LIPS analysis
Extracts containing the Ruc-HA-1 and Ruc-HA-2 proteins fusions were prepared from transfected Cos1 with buffer A containing 50% glycerol as previously described. [17] A “master plate” was constructed by diluting patient sera 1:10 in assay buffer. For evaluating antibody titers by LIPS, 40 μl of buffer A, 10 μl of diluted human sera (1 μl equivalent), and 50 μl of of Ruc antigen from the Cos1 cell extract diluted in buffer A were added to each well of a second polypropylene plate in which the assay was conducted. Using these extracts, the immunoprecipitation assay was performed in a 96-well plate format at room temperature with the input for these immunoprecipitation assays for HA-1 and HA-2 proteins was 3.1 ×106 and 1.63 ×106, respectively. After the final wash, the plate was blotted and the LU measured in a Berthold Centro LB 960 plate reader luminometer using coelenterazine substrate mix. All LU data presented were obtained from the average of two independent experiments and corrected for background.
Statistical Analysis
Binary variables were compared between different dose cohort groups by means of Fisher’s exact test. The 95% exact confidence interval (CI) of a proportion was constructed by using the binomial distribution. Geometric mean and geometric standard deviation were calculated for antibody titers, and the comparison of antibody titer between dose groups after a vaccination was performed by applying analysis of variance (ANOVA) to log2 titers. In order to utilize all available antibody data and account for correlation among multiple antibody titers of the same study subject, generalized estimating equations (GEE) method [18] was employed to analyze log2 titers. For all GEE analyses, an exchangeable correlation structure was used as the working assumption.
All P values are two-sided, and P values of less than 0.05 were considered to be statistically significant. Data analyses were performed with the use of STATA, version 10.0 (Stata Corp LP).
Results
Seventy-five subjects were enrolled between December 2006 and March 2007. Seventy-one subjects completed all four vaccinations, and 69 of these subjects had sera available for all time points including Day 112 (4 weeks after the fourth vaccination). Three subjects withdrew prior to completing all vaccinations (two subjects received one vaccination, and one subject received two vaccinations). Two additional subjects completed all vaccinations but did not return for the final follow-up visit. The reasons for not completing the study included 3 subjects with scheduling conflicts/inability to dedicate the time, 1 subject with an ankle fracture, 1 subject with a small bowel obstruction from a previous appendectomy. The remaining subject was lost to follow-up after completing four vaccinations.
Baseline demographic characteristics of enrolled subjects are shown in Table 1. No significant differences in baseline age, gender, race or ethnicity were noted between the cohorts.
Table 1.
Demographics
| Vaccine Dose Group |
||||
|---|---|---|---|---|
| Baseline Characteristics | 90 mcg (n=25) |
120 mcg (n=25) |
180 mcg (n=25) |
P value |
| Age1 | ||||
| Mean | 40.4 | 39.6 | 34.8 | 0.18 |
| Std Dev | 11.1 | 12.4 | 10.9 | |
| Min-Max | 21-58 | 18-58 | 22-57 | |
|
| ||||
| Sex2 | ||||
| Female | 15 | 13 | 13 | 0.88 |
| Male | 10 | 12 | 12 | |
|
| ||||
| Race2 | ||||
| White | 17 | 19 | 14 | 0.26 |
| Black | 3 | 2 | 7 | |
| Asian | 3 | 2 | 4 | |
| Hispanic | 0 | 2 | 0 | |
| American Indian | 1 | 0 | 0 | |
| Other | 1 | 0 | 0 | |
|
| ||||
| Baseline HAI1 | ||||
| Geometric Mean | 5.7 | 5.1 | 5.6 | 0.53 |
| Geometric Std Dev | 1.5 | 1.1 | 1.7 | |
| Baseline MN1 | ||||
| Geometric Mean | 10.0 | 11.4 | 11.8 | 0.01 |
| Geometric Std Dev | 1.0 | 1.2 | 1.4 | |
Analysis of variance;
Fisher’s exact test.
Safety
Two SAEs were reported during the study period. One subject was hospitalized after the first vaccination for small bowel obstruction related to a previous appendectomy, and judged not related to the study. One subject who did not disclose a previous history of sickle cell anemia was hospitalized after the fourth vaccination with a vasoocclusive crisis. This was judged unlikely related to the study. No deaths occurred during the study period.
Injection Site Reactogenicity
Pain and tenderness at the injection site were the most common AEs. Eighty-one percent of subjects (61/75) complained of pain and/or tenderness at the injection site after one or more vaccinations. The frequency of pain and tenderness decreased with subsequent injections (65%, 62%, 57%, 48% of subjects after the first, second, third, and fourth vaccination respectively). In six subjects, erythema was reported at the injection site for a total of nine episodes. In five subjects, injection site pruritus was reported a total of nine times. Mild edema at the injection site occurred in two subjects.
Systemic Reactogenicity
Excluding the local reactogenicity, 98 adverse events were reported from 36 subjects. The most common events were malaise, headache, and myalgia. The frequency of systemic adverse events decreased with subsequent injections (35%, 22%, 15%, 13% of subjects after the first, second, third, and fourth vaccination respectively). See Table 2 for a full list of adverse events. Self-limited lymphadenopathy was seen in 4 subjects for a total of 11 episodes, all occurring in the ipsilateral inguinal chain after vaccine injection into the gluteal muscle.
Table 2.
Adverse Events by Grade and Vaccine Dose
| Grade | Dose | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | 1 | 2 | 3 | 4 | 90 meg | 120 meg | 180 meg |
| Abdominal pain | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Acute sore throat | 2 | 0 | 0 | 0 | 2 | 0 | 0 |
| Allergic rhinitis | 3 | 0 | 0 | 0 | 0 | 3 | 0 |
| Anemia | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cough | 4 | 0 | 0 | 0 | 1 | 3 | 0 |
| Diarrhea | 2 | 1 | 0 | 0 | 1 | 2 | 0 |
| Disturbance in Skin Sensation | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Fasciculations | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Fatigue | 2 | 2 | 0 | 0 | 3 | 1 | 0 |
| Fever | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Headache | 18 | 0 | 0 | 0 | 8 | 8 | 2 |
| Light-headedness | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Lymphadenitis | 10 | 0 | 0 | 0 | 0 | 4 | 6 |
| Malaise | 14 | 0 | 0 | 0 | 6 | 5 | 3 |
| Myalgia | 18 | 1 | 0 | 0 | 8 | 7 | 4 |
| Nausea | 3 | 0 | 0 | 0 | 2 | 1 | 0 |
| Nonspecific rhinitis | 4 | 0 | 0 | 0 | 2 | 2 | 0 |
| Oral aphthous ulcer(s) | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pain - sciatic | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| Pain - radicular (arm) | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Sleep Disturbance | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Taste changes | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Vomiting | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total | 93 | 4 | 1 | 0 | 42 | 39 | 17 |
No dose related increase in systemic reactogenicity was observed. See Table 2.
Immunogenicity
Hemagglutination
Baseline HAI titers did not differ significantly between the cohorts (Table 1). The primary endpoint was the proportion of subjects in each cohort achieving a serum neutralizing antibody titer against influenza A/H5N1 of 1:320. The proportion of subjects reaching titers of 1:40 was also of study interest, as a titer of 1:40 is traditionally considered as protective. [14] A larger proportion of subjects in the 90 mcg cohort reached a HAI titer of 1:40 after one vaccination (50%, 12%, 29% for 90 mcg, 120 mcg, and 180 mcg cohorts respectively; Fisher’s exact test, P = 0.02). Otherwise no significant differences between cohorts after the same number of vaccinations were detected in the proportion reaching HAI titers of 1:40, 1:80, 1:160, and 1:320).
The HAI geometric mean titers (GMTs) for 90 mcg, 120 mcg, and 180 mcg dose groups for each study day are shown in Table 3. No significant dose related increases in the GMTs of serum HAI were observed when comparing 90 mcg, 120 mcg, and 180 mcg after each vaccination. Accounting for within-subject correlation, the GEE analysis applied to log2 titers measured after day 0, with both study day and cohort group included as categorical variables, showed that log2 titer significantly increased by 0.48 (95% CI 0.12-0.85), 1.22 (95% CI, 0.85-1.59), and 1.12 (95% CI, 0.73-1.50) from first vaccination to second, third, and fourth vaccination respectively. However, the GEE analysis suggested that the log2 titer was not significantly different between the dose groups (P= 0.07 for 120 mcg vs 90 mcg; P = 0.37 for 180 mcg vs 90 mcg).
Table 3.
HAI and MN Geometric mean titer (95% confidence interval) by dose and by vaccination day
| Study Day | 90 mcg | 120 mcg | 180 mcg | Combined | P value1 | |
|---|---|---|---|---|---|---|
| HAI | Day 0 | 5.7 (4.9-6.7) |
5.1 (5.0-5.2) |
5.6 (4.4-7.0) |
5.5 (5.0-6.0) |
0.53 |
| Day 28 | 26.3 (12.6-55.0) |
9.9 (6.3, 15.5) |
15.3 (7.8-30.1) |
15.7 (11.0-22.5) |
0.08 | |
| Day 56 | 26.8 (13.4-53.3) |
18.2 (10.1-32.6) |
23.0 (12.0-44.1) |
22.2 (15.6-31.7) |
0.67 | |
| Day 84 | 52.5 (29.1-94.7) |
29.3 (16.9-50.9) |
30.6 (15.4-60.9) |
36.0 (25.7-50.5) |
0.30 | |
| Day 112 | 44.4 (24.2-81.3) |
21.2 (11.8-38.0) |
38.4 (19.7-74.7) |
32.0 (22.7-45.3) |
0.16 | |
|
| ||||||
| MN | Day 0 | 10 (10-10) |
11.5 (10.7, 12.4) |
11.8 (10.4, 13.4) |
11.0 (10.5, 11.6) |
0.01 |
| Day 28 | 14.5 (10.4, 20.2) |
17.3 (13.8, 21.5) |
21.3 (14.8, 30.7) |
17.5 (14.7, 20.8) |
0.20 | |
| Day 56 | 24.0 (16.0, 36.0) |
38.9 (27.2, 55.7) |
38.1 (25.1, 57.8) |
33.1 (26.5, 41.3) |
0.14 | |
| Day 84 | 47.4 (32.5, 69.2) |
54.0 (38.8, 75.2) |
68.3 (49.1, 95.1) |
55.7 (45.9, 67.6) |
0.32 | |
| Day 112 | 49.8 (34.2, 72.6) |
74.1 (55.6, 98.7) |
90.4 (64.4, 126.9) |
68.4 (56.5, 82.9) |
0.04 | |
P values were obtained using analysis of variance.
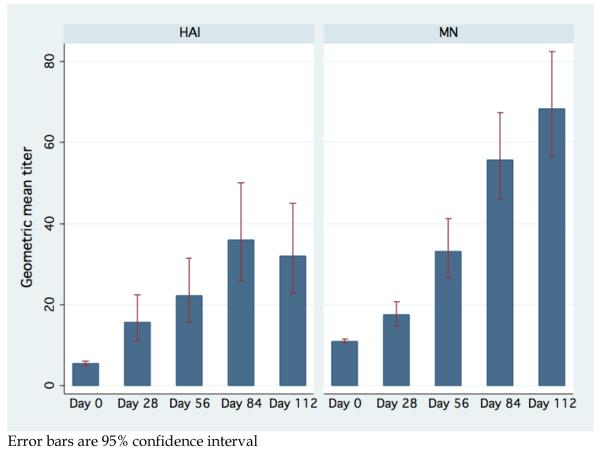
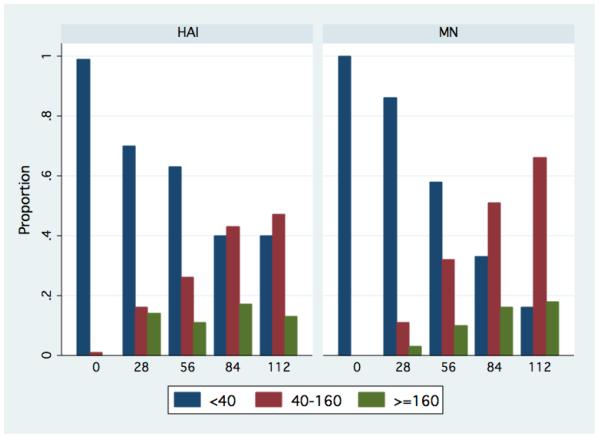
Given the limited sample size and the lack of difference in GMT between the cohorts, we analyzed all cohorts together to determine the effect of additional vaccinations beyond what was previously published. The HAI GMT for the combined cohort after first, second, third and fourth vaccinations was 1:15.7 (95% CI, 11.0-22.5), 1:22.2 (95% CI, 15.6-31.7), 1:36.0 (95% CI, 25.7-50.5), and 1:32.0 (95% CI, 22.7-45.3), respectively. See Figure 1. The paired t test showed that the mean HAI log2 titer increased significantly after each additional vaccination through the third vaccination (first vs baseline, P<0.0001; second vs first, P= 0.02; third vs second, P <0.0001). The difference between the third and the fourth vaccination was not significant (P=0.88). The proportion of subjects with a low (<1:40), medium (≥1:40 & <160), and high (≥160) antibody response (HAI and MN) at each study day are in Figure 2.
Figure 1.
Aggregate HAI and Microneutralization geometric mean titer and 95% confidence interval by vaccination day
Figure 2.
Proportion of subject with a low (<1:40), medium (≥1:40 & <160), and high (≥160) antibody titer (HAI and MN) by study day
Microneutralization
The MN GMTs for 90 mcg, 120 mcg, and 180 mcg dose groups for each study day are shown in Table 3. No significant dose related increases in the GMTs of serum MN were observed when comparing 90 mcg, 120 mcg, and 180 mcg after the first three vaccinations. After the last vaccination, the GMT for 180 mcg dose group is significantly higher than the 90 mcg group (P = 0.02) but was not different than 120 mcg dose group (P=0.35). All cohorts were again combined together to determine the effect of additional vaccinations. The MN GMT for the combined cohort after first, second, third and fourth vaccinations was 1:17.5 (95% CI, 14.7-20.8), 1:33.1 (95% CI, 26.5-41.3), 1:55.7 (95% CI, 45.9-67.6), and 1:68.4 (95% CI, 56.5-82.9), respectively. The paired t test showed that the mean MN log2 titer increased significantly after each additional vaccination (all P<0.001).
Luciferase Immunoprecipitation System
To test whether we could use a surrogate for the hemaglutination inhibition assay, we studied the cohorts for changes in antibodies against HA as detected by Luciferase Immunoprecipitation System (LIPS). Two different HA fragments from the Vietnam 1203 strain were tested. One of the constructs, designated HA-1 corresponded to the N-terminal 300 amino acids of the HA, while a C-terminal protein HA-2 corresponded to the C-terminal 218 amino acids. Both the HA-1 and HA-2 constructs were highly expressed in Cos1 cells and used in a high throughput screening method to measure the antibodies in the immunized individuals. Analysis of anti-HA-2 antibodies showed that many individuals on Day 0 have a high level of HA-2 antibodies, increasing slightly over the duration of the study. As HA-2 is more conserved across influenza sub-types, this likely reflects pre-existing antibodies generated during seasonal influenza vaccination or infections.
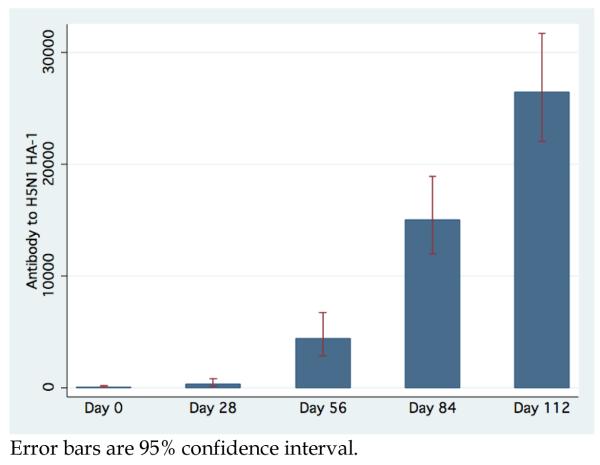
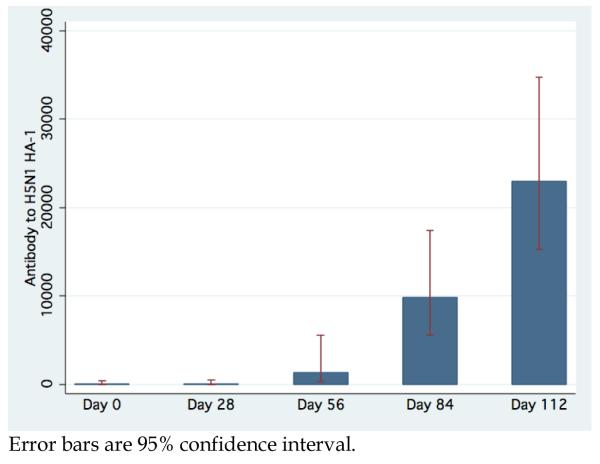
Antibodies to H5N1 HA-1 as measured by LIPS have a GMT for the combined cohort at baseline and after first, second, third and fourth vaccinations of 98.5 (95% CI, 47.8-203.0), 345.0 (95% CI, 147.4-807.6), 4,421.2 (95% CI, 2,870.8-6809.1), 15,038.6 (95% CI, 11,894.0-19,014.6), and 26,447.5 (95% CI, 21972.2-31834.4), respectively. (Figure 3) Anti-HA-1 measures seems to be positively correlated with HAI, with GMT for subjects with 1:5, 1:5-1:20, and >1:20 HAI titers given by 370.7 (95% CI, 218.0-630.3), 3,192.7 (95% CI, 1,238.5-8,223.7), and 11,520.5 (95% CI, 8,574.9-15,477.9). However, the difference in anti-HA-1 among subjects with HAI titer greater than 20 is not significant. Interestingly, 12 subjects that had no immune response to the vaccine as measured by HAI (HAI =5 through all vaccinations and follow-up) had demonstrable significant increase in anti-HA-1 antibodies after each vaccination (all P<0.003). (Figure 4) For this subset, the MN was 1:10.4 on Day 0 (95% CI 9.8-11.0) and increased after each vaccination to value of 1:63.5 on Day 112 (95% CI 39.7-101.43).
Figure 3.
Aggregate GMT anti-HA-1 antibody titer and 95% confidence interval determined by LIPS by study day
Figure 4.
GMT anti-HA-1 titer antibody and 95% confidence interval determined by LIPS by study day in 12 Subjects with no immune response through all planned vaccinations as measured by HAI
Immunogenicity by vaccine site
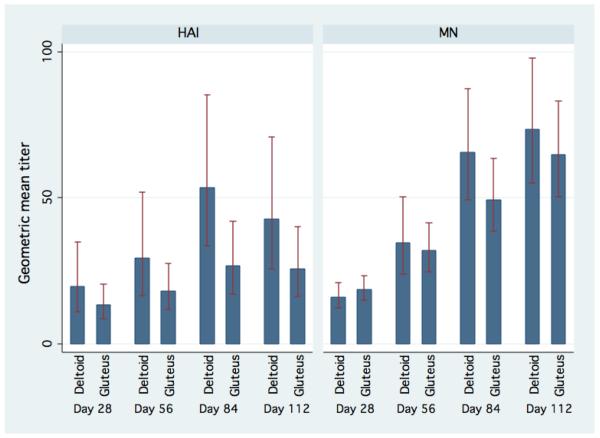
The vaccine is formulated at 90 mcg/ml, and standard practice would limit IM injection volumes to 1 ml in the deltoid and 2 ml in the gluteus. Therefore the 90 mcg cohort would receive one shot in the deltoid or gluteus, while the 120 and 180 mcg cohorts would receive two shots into the deltoid (one shot into each), or one shot into the gluteus. Unexpectedly, but likely in an effort by subjects to minimize anticipated discomfort, the majority of cohort 1 elected to receive 1 shot into the deltoid (81% of all vaccinations), while the majority of cohorts 2 & 3 received 1 shot in the gluteus (76% and 74% respectively). The GEE analysis showed that, adjusted for study day, receiving the vaccintion into the deltoid was associated with a 0.88 (95% CI 0.18-1.58; P=0.01) increase in mean log2 HAI titer and a 0.24 (95% CI -0.16-0.63; P=0.25) increase in MN compared to receiving the vaccination in the gluteus. (Figure 5)
Figure 5.
Aggregate HAI and Microneutralization geometric mean titer and 95% confidence interval by vaccination site and vaccination day
Discussion
The present study was conducted to determine the optimal immunization schedule to generate a high titer anti-influenza A/Vietnam/1203/2004 IVIG that could be used as a potential therapeutic for the treatment of avian influenza. Our results extend previous observations related to the immunogenicity of the H5N1 influenza virus sub-virion vaccines. Our study demonstrates similar GMT after two doses of 90 mcg vaccine as compared to previous studies with this vaccine when differences in methodology of HAI determination (accounting for a 1 dilution correction) is incorporated (1:26.8 vs 1:56.3). [14]
Our study suggests that some of the barriers to developing adequate antibody titers (generally accepted as 1:40) may be overcome by an additional dose of the vaccine increasing both the GMT and the percentage of the cohort above a titer 1:40. One previous study evaluated the efficacy of a third vaccination dose given 6 months after the primary series. [19] In that study the GMT HAI titer for the 90 mcg cohort decreased from 1:53.3 (95% CI, 38.1–74.7) 28 days after the primary series of two vaccinations to 1:25.6 (95% CI, 18.9–34.6) after 6 months. After a third vaccination at 6 months, the HAI titer increased to 1:69.8 (95% CI, 49.2–99.1). However, this was not maintained decreasing to 1:18.5 (95% CI, 14.5–23.6) by 6 months after the third vaccination.
The HAI did not perform well in our study. HAI underestimated the MN titers, and did not reflect the overall immunogenicity of the vaccine (as demonstrated by antibodies measured by LIPS). Whether the antibodies generated in the absence of a HAI response would be protective is unclear, and deserves further study.
One surprising finding was that additional antigen dose did not appear to generate higher antibody titer. Interpretation of this data is confounded by the apparent 0.88-fold decrease in HAI antibody for those subjects that received the vaccine in the buttock. Given the local reactogenicity and the injectate volume required, using alternative vaccination locations such as the buttock may curtail local side effects but may also diminish immunogenicity.
No published studies have evaluated vaccination site on antibody response to the influenza vaccine. A randomized trial using the hepatitis B vaccine noted a significant less antibody titer in those subjects vaccinated in the arm versus buttock (Geometric mean 1454 vs 85 mIU/mL). [20] Some of this difference was attributable to needle length, and partially improved when a 2-inch needle was used instead of a 1-inch needle for buttock injections (GMT 387 vs 85 mIU/mL). Our study used a standard 1.5-inch needle for all injections. The etiology for these results in our study is unclear, and may represent difference in antigen processing at different vaccination sites, or simply related to the volume of injectate when vaccinations were given in the buttock. The influence injection site has on influenza vaccine immunogenicity deserves further study.
Through this study, we have collected 7.5 L of plasma from three subjects with a plasma GMT HAI of 1:256. This plasma will be processed into an IVIG product for further studies. While we did not achieve the objective of obtaining a large amount of anti-H5N1 influenza plasma for IVIG therapeutic studies, the findings did help elucidate vaccination hyper-immunization strategies for further studies.
Acknowledgments
We are grateful to the volunteers for their generous contributions to this study, our clinic for their hard work executing this study, and the Safety Monitoring Committee (John Treanor, Jim Campbell, and Ben Schwartz) for their time and oversight of the study.
This research was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and National Institute of Dental and Craniofacial Research, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Clinical Trials.gov identifier: NCT00383071
The authors report no conflicts relevant to this article.
References
- 1.Knobler S, Institute of Medicine (U.S.) Forum on Microbial Threats. Institute of Medicine (U.S.) Board on Global Health . The threat of pandemic influenza : are we ready? : workshop summary. National Academies Press; Washington, D.C.: 2005. [PubMed] [Google Scholar]
- 2.He G, Qiao J, Dong C, He C, Zhao L, Tian Y. Amantadine-resistance among H5N1 avian influenza viruses isolated in Northern China. Antiviral Res. 2008;77:72–6. doi: 10.1016/j.antiviral.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Cheung CL, Rayner JM, Smith GJ, et al. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J Infect Dis. 2006;193:1626–9. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 5.McKimm-Breschkin JL, Selleck PW, Usman TB, Johnson MA. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg Infect Dis. 2007;13:1354–7. doi: 10.3201/eid1309.07-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Guo Z, Pan X, et al. Passive immunotherapy for influenza A H5N1 virus infection with equine hyperimmune globulin F(ab’ )2 in mice. Respir Res. 2006;7:43. doi: 10.1186/1465-9921-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuno Y, Matsumoto K, Isegawa Y, Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol. 1994;68:517–20. doi: 10.1128/jvi.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–26. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–81. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson BJ, Boon AC, Lim AP, Webb A, Ooi EE, Webby RJ. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons CP, Bernasconi NL, Suguitan AL, et al. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 15.Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–73. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burbelo PD, Meoli E, Leahy HP, et al. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) Retrovirology. 2008;5:96. doi: 10.1186/1742-4690-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 19.Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008;197:580–3. doi: 10.1086/526537. [DOI] [PubMed] [Google Scholar]
- 20.Shaw FE, Jr., Guess HA, Roets JM, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine. 1989;7:425–30. doi: 10.1016/0264-410x(89)90157-6. [DOI] [PubMed] [Google Scholar]