Summary
Actin polymerization at the immune synapse is required for T cell activation and effector function; however, the relevant regulatory pathways remain poorly understood. We showed previously that binding to antigen presenting cells (APCs) induces localized activation of Cdc42 and Wiskott-Aldrich Syndrome protein (WASP) at the immune synapse [1]. Several lines of evidence suggest that Tec kinases could interact with WASP-dependent actin regulatory processes [2]. Since T cells from Rlk−/−, Itk−/−, and Rlk−/− × Itk−/− mice have defects in signaling and development [3], we asked whether Itk or Rlk function in actin polymerization at the immune synapse. We find that Itk−/− and Rlk−/− × Itk−/− T cells are defective in actin polymerization and conjugate formation in response to antigen-pulsed APCs. Itk functions downstream of the TCR, since similar defects were observed upon TCR engagement alone. Using conformation-specific probes, we show that although the recruitment of WASP and Arp2/3 complex to the immune synapse proceeds normally, the localized activation of Cdc42 and WASP is defective. Finally, we find that the defect in Cdc42 activation likely stems from a requirement for Itk in the recruitment of Vav to the immune synapse. Our results identify Itk as a key element of the pathway leading to localized actin polymerization at the immune synapse.
Results and Discussion
Itk Is Required for Actin Remodeling during T Cell-APC Interactions
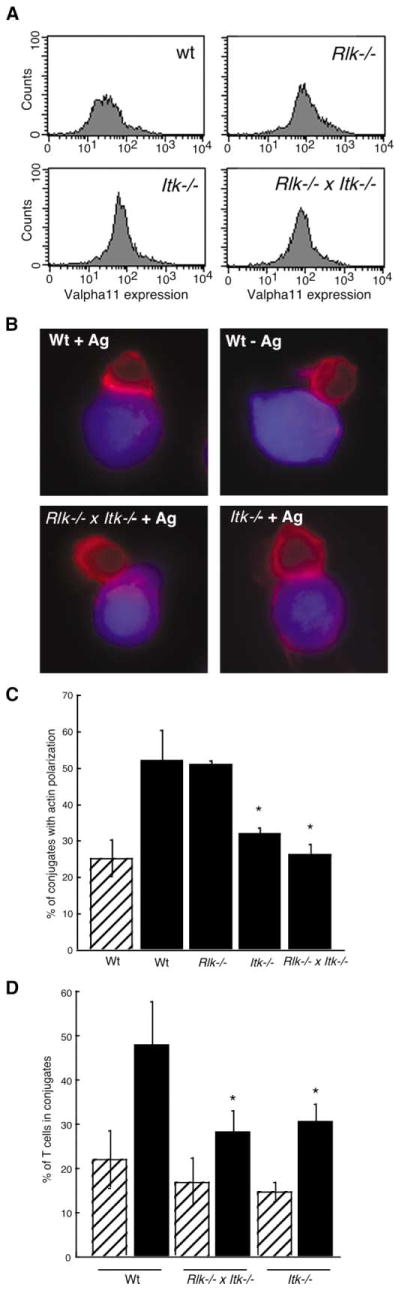
T cells from mice deficient in the Tec family kinases Itk and Rlk show graded defects in Ca2+ flux, proliferation, and cytokine production [3]. Since T cells deficient in the actin regulatory proteins WASP or Vav-1 have similar defects [4, 5], and Tec kinases interact with both proteins [6–8], we hypothesized that Tec kinases regulate actin remodeling in T cells. To test this, conjugates were formed between primary T cell blasts from wild-type (wt), Itk−/−, Rlk−/−, or Rlk−/− × Itk−/− TCR transgenic mice and antigen-pulsed B cells, and the distribution of F-actin was analyzed after labeling with rhodamine phalloidin. As expected, conjugates formed with wt T cells exhibited a sharp band of F-actin at the immune synapse in the presence of antigen (Figure 1B). In contrast, no antigen-dependent actin accumulation was observed in either Itk−/− or Rlk−/− × Itk−/− T cells. Quantitative analysis showed that for Itk−/− and Rlk−/− × Itk−/− T cells, the frequency of conjugates showing actin polymerization at the immune synapse was at background levels (defined by conjugates in the absence of antigen), while actin responses were normal for Rlk−/− T cells (Figure 1C). The actin defect in Itk-deficient T cells was accompanied by defects in the formation of stable conjugates with APCs (Figure 1D). These defects are not attributable to differences in surface expression levels of the AND Tg TCR; expression of Vα11 on all mutant T cell blasts was as high as on wt controls (Figure 1A).
Figure 1. Actin Polymerization at the Immune Synapse and Conjugate Formation Are Reduced in Rlk−/− × Itk−/− and Itk−/− T Cells.
(A) Primary T cell blasts from AND TCR Tg mice were analyzed by flow cytometry to verify comparable surface expression of the Tg TCR.
(B) Primary T cell blasts were allowed to conjugate to CMAC (blue)-dyed B cells ± Ag for 10 min, were fixed, and were stained with rhodamine phalloidin to detect F-actin (red). Note the lack of F-actin enrichment at the immune synapse in conjugates formed with the mutant T cells.
(C) Conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were selected at random and were scored for accumulation of F-actin at the immune synapse. Data are mean ± SD from at least 3 independent experiments, with 50 conjugates each (the asterisk indicates a significant difference from wt + Ag, p < 0.001).
(D) Conjugation efficiency in the absence (hatched bars) or presence (solid bars) of Ag was analyzed by flow cytometry as described in the Experimental Procedures. Data are mean ± SD from at least three independent experiments (the asterisk indicates a significant difference from wt + Ag, p < 0.05).
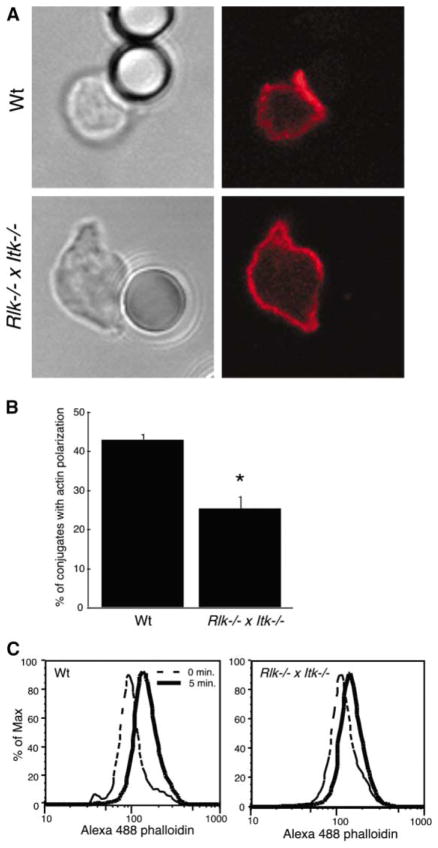
Since Tec kinases mediate signaling through both TCR and CD28 [9, 10], we used anti-TCR-coated beads to determine whether actin defects occur in the absence of CD28 engagement. Polymerization of actin at the bead interface was observed in wt T cells, but not in Rlk−/− × Itk−/− T cells (Figure 2). Moreover, costimulation with anti-CD28 under conditions that increase proliferation did not rescue the actin defect (data not shown). Thus, Itk is required for actin polymerization even in the absence of signaling through CD28. Since it has been reported that T cell activation by anti-TCR beads prepared in this way involves coligation of integrins by serum attachment factors [11], T cells were stimulated by crosslinking of soluble anti-CD3 in the absence of serum, and total F-actin content was assessed by flow cytometry. F-actin content in wt T cells increased by about 2-fold at 5 min of stimulation; this response was substantially blunted in the Rlk−/− × Itk−/− T cells (Figure 2C). Thus, while Itk may also function downstream of other costimulatory or integrin signaling pathways, we conclude that Itk functions downstream of TCR ligation in the pathway leading to net actin polymerization during conjugate formation. These results confirm and extend previous studies in Itk-deficient murine T cells [12] and Jurkat cells expressing kinase-inactive Itk [13].
Figure 2. Defects in Actin Polymerization Occur Downstream of TCR Engagement.
(A) Splenic T cells were allowed to conjugate to anti-TCR-coated beads, were fixed, and were stained with Alexa 594-phalloidin to detect F-actin (red). Note the lack of F-actin enrichment at the bead interface with the Rlk−/− × Itk−/− T cell.
(B) Bead conjugates formed as in (A) were scored for accumulation of F-actin. Data are mean ± SD from six independent experiments (the asterisk indicates a significant difference from wt, p < 0.0001).
(C) Splenic T cells were stimulated for 5 min by soluble anti-CD3 crosslinking and were fixed, and F-actin content was assessed after labeling with Alexa 488-phalloidin.
Adhesive defects in Itk-deficient T cells were detectable only in the presence of antigen, indicating that “inside-out” signaling pathways required for high-avidity binding of the β2 integrin LFA-1 are Itk dependent. Indeed, we find that Rlk−/− × Itk−/− T cells exhibit defective TCR-activated adhesion to immobilized ICAM-1 (L.D.F. and P.L.S., unpublished data). Similarly, overexpression of dominant Itk mutants inhibits TCR-activated adhesion to fibronectin mediated by β1 integrins [13]. Since high-avidity integrin binding requires interaction with cortical actin, it seems likely that the defects in adhesion via β1 and β2 integrins stem from the failure to appropriately remodel F-actin.
Itk Is Required for Localized Cdc42 Activation and Downstream Activation of WASP
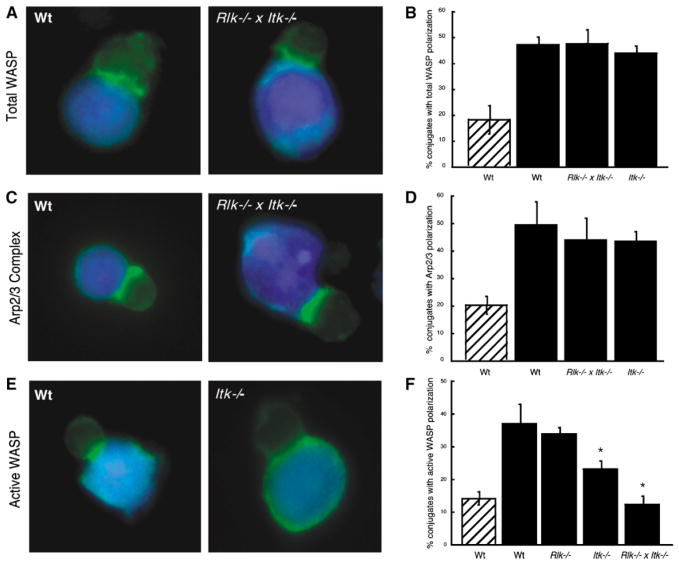
To investigate the molecular basis for the actin defects, we asked whether Itk functions in the cascade leading to WASP activation. This cascade involves localized activation of Cdc42, which binds to and induces large conformational changes in WASP, which then activates the formation of branched actin structures by Arp2/3 complex [14, 15]. We have shown that WASP recruitment and activation are separable, with recruitment occurring via interactions with SLP76 and Nck, and activation occurring via binding to Cdc42-GTP accumulated at the same site [1, 16]. As shown in Figure 3, WASP and Arp2/3 complex were recruited efficiently to the immune synapse in conjugates formed with both wt and Itk-deficient T cells (either Rlk−/− × Itk−/− or Itk−/−).
Figure 3. WASP and Arp2/3 Are Recruited to the Immune Synapse, but WASP Remains in an Inactive State.
(A) Conjugates were labeled with an antibody that recognizes WASP in a conformation-independent manner. Both wt and Rlk−/− × Itk−/− T cells show recruitment of WASP to the immune synapse.
(B) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for WASP polarization.
(C) Conjugates were stained with an anti-Arp3 antibody. Both wt and Rlk−/− × Itk−/− T cells show accumulation of Arp2/3 complex at the interface.
(D) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for Arp2/3 polarization.
(E) Conjugates were stained with a monoclonal antibody that preferentially recognizes the open, activated form of WASP. Note that active WASP is not enriched at the immune synapse in the Itk−/− T cell.
(F) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of open WASP at the immune synapse.
Data in (B), (D), and (F) represent means from at least three independent experiments ± SD (the asterisk indicates a significant difference from wt + Ag, p < 0.01 for Itk−/−, and p < 0.001 for Rlk−/− × Itk−/− T cells).
The recruitment of Arp2/3 complex to the immune synapse is not necessarily a reliable indicator of WASP activation. Though several studies indicate that Arp2/3 complex binds only to active WASP [14, 17], there is also evidence for constitutive association [18]. Moreover, Arp2/3 complex could be recruited by other proteins. To directly address the activation state of WASP, we generated an antibody against an epitope that is masked by intramolecular interactions in the autoinhibited conformation and thus specifically recognizes active WASP (D.W.L., C.M. Labno, D.Y., J.K.B., and M.K.R., unpublished data). Using this antibody, we observed antigen-dependent WASP activation at the immune synapses in wt T cells, but only background levels of activation in Rlk−/− × Itk−/− T cells (Figures 3E and 3F). WASP activation in Itk−/− T cells was also significantly lower than in wt controls. Since no defect was evident in Rlk−/− T cells, the presence of Itk appears to be of primary importance for localized WASP activation.
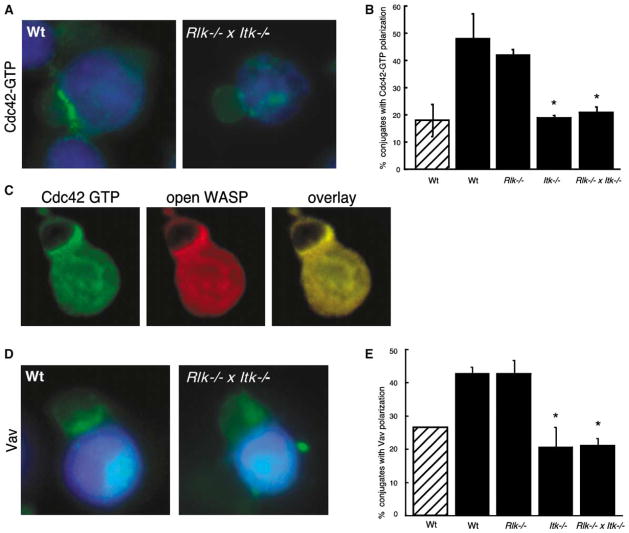
To determine if the failure to activate WASP results from a defect in Cdc42 activation, conjugates were labeled with recombinant GFP-WASP-GBD to detect Cdc42-GTP [1]. Wt and Rlk−/− T cells showed strong accumulation of Cdc42-GTP at the immune synapse. In contrast, both Itk−/− and Rlk−/− × Itk−/− T cells showed profound defects in Cdc42 activation (Figures 4A and 4B). Double-label analysis showed that Cdc42-GTP and active WASP colocalize at the immune synapse (Figure 4C). For both wt and Rlk−/− × Itk−/− T cells, every conjugate showing Cdc42-GTP accumulation also showed accumulation of active WASP (n ≥ 50 conjugates for each genotype). This was not the case when the conformation-unspecific WASP antibody was used to compare overall WASP recruitment with Cdc42 activation; 24% of Rlk−/− × Itk−/− T cells showed recruitment of WASP in the absence of Cdc42-GTP (n = 50 conjugates). Thus, in the absence of Itk, WASP can be recruited to the immune synapse without undergoing the conformational changes associated with Cdc42-induced activation.
Figure 4. Itk Is Required for Vav Recruitment and Cdc42 Activation at the Immune Synapse.
(A) Conjugates were labeled with recombinant GFP-WASP-GBD to detect Cdc42-GTP. Note the enrichment of activated Cdc42 at the immune synapse of the conjugate formed with the wt T cell, but not the Rlk−/− × Itk−/− T cell.
(B) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of Cdc42-GTP at the immune synapse.
(C) Double labeling was performed by using the GFP-WASP-GBD reagent to detect Cdc42-GTP (green) and mAb 26E6 to detect the open, active conformation of WASP (red). The overlaid image shows extensive colocalization of activated Cdc42 and WASP at the immune synapse (yellow).
(D) Conjugates were labeled with anti-Vav antibody. Note the enrichment of Vav at the immune synapse of the conjugate formed with the wt T cell, but not the Rlk−/− × Itk−/− T cell.
(E) Randomly chosen conjugates formed in the absence (hatched bar) or presence (solid bars) of Ag were scored for accumulation of Vav at the immune synapse. Data in (B) and (F) represent means from at least three independent experiments ± SD (the asterisk indicates a significant difference from wt + Ag, p < 0.001).
Itk Is Required for Vav Recruitment to the Immune Synapse
The results above suggest that Itk functions upstream of the relevant guanine exchange factor (GEF) for Cdc42. Although the role of Vav-1 as a direct GEF for Cdc42 is controversial [19, 20], we recently found that Vav-1-deficient Jurkat T cells are defective in Cdc42 activation at the immune synapse [16]. We therefore asked whether Itk is required for Vav recruitment. Although the background labeling of Vav was high in the absence of antigen, antigen-dependent recruitment of Vav was readily measurable in both wt and Rlk−/− T cells (Figures 4D and 4E). In contrast, antigen-dependent Vav recruitment was completely abolished in T cells lacking Itk. Similar defects were obtained when Jurkat T cells were cotransfected with an epitope-tagged Vav-1 construct and siRNA oligonucleotides to inhibit Itk expression. As in Itk−/− T cells, actin responses were defective and the efficiency of conjugate formation was diminished (D. Dombroski, C.M. Labno., J.K.B., and P.L.S., unpublished data). We conclude that Itk is required for Vav recruitment to the immune synapse. Since Itk-dependent phosphorylation of LAT can generate docking sites for Vav [21], LAT is a likely intermediary in this process. Itk-dependent phosphorylation of SLP-76 may also be required, since Vav recruitment requires tyrosine phosphorylation of SLP-76 [16].
Taken together, our findings show that Itk is an essential component of the actin regulatory cascade induced in T cells upon interaction with APCs. Unlike other aspects of T cell signaling in which loss of Rlk contributes to phenotypic severity [3], the actin defects are solely attributable to Itk deficiency. While this manuscript was in preparation, Grasis and coworkers reported that Itk−/− T cells have defects in antibody-induced actin remodeling [12]. Our work confirms and extends this finding, showing that these cells have defects in APC-induced actin responses and defining activation defects in key actin regulatory molecules. Importantly, the actin defects in Itk−/− T cells are not secondary to developmental defects; we find similar defects in Jurkat cells in which Itk expression has been depressed by using siRNA, and both Grasis et al. [12] and Woods et al. [13] have observed actin defects in Jurkat cells overexpressing Itk mutants.
At the molecular level, we conclude that Itk is required for recruitment and activation of Cdc42 and WASP at the immune synapse. To our knowledge, this represents the first study to demonstrate in situ the colocalization of an activated Rho GTPase and its activated effector protein and thereby provides experimental evidence supporting the broadly accepted paradigm that localized actin responses are controlled by localized activation of Rho GTPases. We find that in Itk-deficient T cells, WASP is recruited to the immune synapse but is not activated, consistent with our finding that WASP recruitment and activation are independent events [1]. We have recently described a minimal model for WASP activation at the immune synapse involving signaling through TCR, Lck, ZAP-70, SLP-76, Nck, Vav, and Cdc42 [16]. We now show that Itk is also a key component in this cascade.
Interestingly, the phenotype of Itk-deficient T cells with respect to WASP recruitment and activation is identical to that of Vav-1-deficient Jurkat T cells [16]. Thus, it is attractive to speculate that the actin defects in Itk-deficient T cells stem from dysregulation of Vav function. Mislocalization of Vav could, in itself, lead to the observed defects; however, phosphorylation of Vav by Itk could also be required to regulate GEF activity. We previously reported that gross Vav phosphorylation proceeds normally in Rlk−/− × Itk−/− thymocytes [22], and we have obtained similar results in mature T lymphocytes from these mice. However, since Vav is phosphorylated at multiple sites [5], changes at specific sites associated with activation might not have been detected. There is conflicting evidence about the requirement for Itk kinase activity in actin regulatory pathways [12, 13, 23]. Thus, Vav activity may be regulated in a phosphorylation-independent manner, perhaps as part of the membrane recruitment mechanism. Finally, though we show here that Itk is required for Vav recruitment, there is also evidence that Vav influences Itk function [24]. Thus, it is possible that Itk functions as part of an interdependent group of signaling molecules, as proposed for Btk [25]. Additional studies will be required to resolve the mechanism(s) by which Itk and Vav interact.
In addition to regulating WASP via Cdc42, Itk could directly phosphorylate WASP at tyrosine 291 [7], a site that is revealed upon activation by Cdc42-GTP [26]. This raises the interesting possibility that Itk regulates WASP activity though a “feed-forward loop,” in which Itk first serves to activate Cdc42, revealing this phosphorylation site so that subsequent phosphorylation can modify WASP activity [26, 27]. Finally, we note that the defects in actin remodeling and conjugate formation in Itk-deficient T cells are much more profound than those in WASP-deficient T cells [28, 29]. This finding indicates that in addition to its role in WASP activation, Itk functions in other actin regulatory pathways. These could include Vav-dependent pathways and/or pathways dependent upon PLCγ and Ca2+ flux. The profound cytoskeletal defects in Itk-deficient T cells are likely to contribute to the defects in signaling, development, proliferation, and cytokine production in these cells.
Supplementary Material
Acknowledgments
We thank Anne Sperling and Matt Welch for gifts of antibodies and Frances Weis-Garcia and the Monoclonal Antibody Core Facility of Memorial Sloan-Kettering Cancer Center for their technical assistance in generating mAbs. We thank Shirley Bond for assistance with microscopy, Ana Venegas for technical assistance with mice, Jill Voss and Amy Dodson-Watts for administrative assistance, and members of the Burkhardt, Rosen, and Schwartzberg laboratories for many helpful discussions. Peptide synthesis, flow cytometry, antibody purification, and fluorescence microscopy were performed by using the University of Chicago Cancer Research Center Core Facilities. This research was supported by National Institutes of Health grants R01-AI44835 to J.K.B. and R01-GM56322 and Welch grant I-51544 to M.K.R. P.L.S. was supported in part by the Searle Scholars program/Chicago Community Trust, C.M. Lewis was supported by the Howard Hughes Medical Institute-National Institutes of Health Scholars Program, A.T. was supported by the Japan Society for the Promotion of Science, and L.D.F. was supported by the American Cancer Society.
Footnotes
Supplemental Data including the Experimental Procedures are available at http://www.current-biology.com/cgi/content/full/13/18/1619/DC1/.
References
- 1.Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Wasp recruitment to the T cell: APC contact site occurs independently of cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 2.Takesono A, Finkelstein LD, Schwartzberg PL. Beyond calcium: new signaling pathways for Tec family kinases. J Cell Sci. 2002;115:3039–3048. doi: 10.1242/jcs.115.15.3039. [DOI] [PubMed] [Google Scholar]
- 3.Schaeffer EM, Schwartzberg PL. Tec family kinases in lymphocyte signaling and function. Curr Opin Immunol. 2000;12:282–288. doi: 10.1016/s0952-7915(00)00088-1. [DOI] [PubMed] [Google Scholar]
- 4.Snapper SB, Rosen FS. The Wiskott-Aldrich syndrome protein (WASP): roles in signaling and cytoskeletal organization. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 5.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ. Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem. 1996;271:25646–25656. doi: 10.1074/jbc.271.41.25646. [DOI] [PubMed] [Google Scholar]
- 7.Guinamard R, Aspenstrom P, Fougereau M, Chavrier P, Guillemot JC. Tyrosine phosphorylation of the Wiskott-Aldrich syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett. 1998;434:431–436. doi: 10.1016/s0014-5793(98)01016-3. [DOI] [PubMed] [Google Scholar]
- 8.Kline JB, Moore DJ, Clevenger CV. Activation and association of the Tec tyrosine kinase with the human prolactin receptor: mapping of a Tec/Vav1-receptor binding site. Mol Endocrinol. 2001;15:832–841. doi: 10.1210/mend.15.5.0631. [DOI] [PubMed] [Google Scholar]
- 9.Liao XC, Fournier S, Killeen N, Weiss A, Allison JP, Littman DR. Itk negatively regulates induction of T cell proliferation by CD28 costimulation. J Exp Med. 1997;186:221–228. doi: 10.1084/jem.186.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King PD, Sadra A, Teng JM, Xiao-Rong L, Han A, Selvakumar A, August A, Dupont B. Analysis of CD28 cytoplasmic tail tyrosine residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. J Immunol. 1997;158:580–590. [PubMed] [Google Scholar]
- 11.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–117. doi: 10.1034/j.1600-065x.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 12.Grasis JA, Browne CD, Tsoukas CD. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol. 2003;170:3971–3976. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 13.Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. EMBO J. 2001;20:1232–1244. doi: 10.1093/emboj/20.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 16.Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. SLP-76 coordinates Nck-dependent WASP recruitment with Vav-1/Cdc42-dependent WASP activation at the T cell-APC contact site. J Immunol. 2003;171:1360–1368. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 17.Higgs HN, Blanchoin L, Pollard TD. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry. 1999;38:15212–15222. doi: 10.1021/bi991843+. [DOI] [PubMed] [Google Scholar]
- 18.Prehoda KE, Scott JA, Dyche Mullins R, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Das B, Wei W, Van Aelst L, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17:1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movilla N, Dosil M, Zheng Y, Bustelo XR. How Vav proteins discriminate the GTPases Rac1 and RhoA from Cdc42. Oncogene. 2001;20:8057–8065. doi: 10.1038/sj.onc.1205000. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Villar JJ, Whitney GS, Sitnick MT, Dunn RJ, Venkatesan S, O’Day K, Schieven GL, Lin TA, Kanner SB. Phosphorylation of the linker for activation of T-cells by itk promotes recruitment of vav. Biochemistry. 2002;41:10732–10740. doi: 10.1021/bi025554o. [DOI] [PubMed] [Google Scholar]
- 22.Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnadieu E. Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol. 2000;165:3917–3922. doi: 10.4049/jimmunol.166.9.5540. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds LF, Smyth LA, Norton T, Freshney N, Downward J, Kioussis D, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and-independent pathways. J Exp Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides mechanisms of signal integration and molecular memory in WASP. Mol Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 27.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J Biol Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 28.Krawczyk C, Oliveira-dos-Santos A, Sasaki T, Griffiths E, Ohashi PS, Snapper S, Alt F, Penninger JM. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- 29.Cannon JL, Burkhardt JK. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol Rev. 2002;186:90–99. doi: 10.1034/j.1600-065x.2002.18609.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.