Abstract
Central sleep apnea (CSA) describes a group of conditions in which cessations in air flow occur without respiratory effort. In contrast, obstructive sleep apnea patients have ongoing respiratory effort during respiratory events. However, considerable overlap exists in the pathogenesis and clinical presentation of obstructive sleep apnea and CSA. A good working knowledge of the mechanisms underlying CSA is important for optimal clinical care. In general, CSA can be classified into those with excessive drive (eg, Cheyne-Stokes breathing) versus those with inadequate drive (eg, sleep hypoventilation syndrome). One critical factor contributing to the cessation of air flow during sleep is the concept of the apnea threshold, such that a PaCO2 value below a certain level will lead to cessations in breathing. PaCO2 can fall below the chemical apnea threshold when drive is excessive (eg, robust chemosensitivity) or when hyperventilation is occurring (eg, following arousal). Another important factor is the loss of the so-called wakefulness drive to breathe, such that some rise in PaCO2 is likely to occur at the onset of sleep. A variety of factors contribute to this rise, including upper-airway collapse and diminished chemosensitivity (particularly during rapid-eye-movement sleep). In patients with low central drive, this further loss of drive at sleep onset can lead to marked hypercapnia in some cases. The treatment of CSA is also reviewed in some detail, including a role for positive airway pressure (eg, bi-level positive airway pressure in hypoventilation patients) and optimization of medical therapy (eg, in Cheyne-Stokes breathing). A paucity of research exists in this area, emphasizing the opportunities for young investigators who are interested in this field.
Keywords: central sleep apnea, CSA, obstructive sleep apnea, OSA, lung, Cheyne-Stokes breathing, sleep hypoventilation syndrome, sleep, continuous positive airway pressure, CPAP
Introduction
Central sleep apnea (CSA) is defined by the cessation of air flow without respiratory effort.1 This condition is in contrast to obstructive sleep apnea (OSA), in which ongoing respiratory effort is present during respiratory events.2–5 Although these definitions are quite distinct, in reality, considerable overlap is present between OSA and CSA from the standpoint of underlying mechanism and clinical presentation.6,7 For example, the majority of respiratory events are in fact hypopneas, in which obstructive versus central physiology is often difficult to distinguish clinically. Thus, this paper will focus on the clinical syndromes that fall under the umbrella of the term CSA, but we recognize that aspects of obstructive apnea are also important for this discussion and are covered thoroughly by other participants in this Journal Conference.
A number of forms of CSA exist, as will be detailed (Table 1). This classification is critically important, since it provides insight into underlying mechanism and dictates the type of therapy that the patient will require. Many patients are troubled by the diagnosis of CSA since it is widely assumed to be related to some form of brain dysfunction. Patients frequently present with a chief complaint that they had been told by their doctor, “There is something wrong with my brain.” Thus, reassurance can be an important component of the therapeutic approach to CSA. For example, cessation of respiratory effort is predictable when substantial hypocapnia occurs during sleep (ie, a PaCO2 value below the so-called apnea threshold will produce cessation of ventilation).8–10
Table 1.
Forms of Central Sleep Apnea
| Type of Central Apnea | Mechanism | Treatment |
|---|---|---|
| Sleep transition apnea | Ventilatory response to arousal drives PCO2 below apnea threshold | Reassurance Sleep hygiene Hypnotic therapy Oxygen |
| Narcotic-induced central apnea | Unclear, suppressed output from respiratory pattern generator | Reduce narcotic dose Consider advanced PAP device |
| Cheyne-Stokes breathing | High loop gain from extravascular lung water and robust chemoresponsiveness | Optimize medical therapy Consider PAP |
| Complex sleep apnea | CPAP reduces upper-airway resistance, improving the efficiency of CO2 excretion | Reassurance Expectant management |
| Idiopathic central apnea | Unknown | Acetazolamide Consider bi-level PAP |
PAP = positive airway pressure
CPAP = continuous positive airway pressure
Control of Breathing
In most cases the major determinant of minute ventilation is the PCO2. During wakefulness the PCO2 is tightly maintained near 40 mm Hg. However, during sleep the chemosensitivity to both carbon dioxide and oxygen falls. A PCO2 of 45 mm Hg occurs during stable sleep, in part due to upper-airway collapse, and minute ventilation is reduced (largely through a drop in tidal volume without an increase in respiratory rate). Chemosensitivity also varies with sleep stage. As will be discussed, transitions from wakefulness to sleep and vice versa may show some in-stability in breathing as the PCO2 set-point changes.
The Concept of Loop Gain
Another useful concept is that of loop gain, which is a measure of the stability or instability in the ventilatory control system.11–14 Loop gain refers to the propensity of an individual to develop periodic breathing. Those with high loop gain are prone to instability, whereas those with low loop gain are quite resistant to periodic breathing. Younes et al have developed the proportional-assist ventilation technique to assess ventilatory loop gain.12,15 Individuals with high loop gain develop periodic breathing with minimal proportional-assist ventilation support, whereas those with low loop gain do not develop periodic breathing, even with considerable proportional-assist ventilation support.
Loop gain has a number of components, including the central respiratory controller (ie, controller gain), the efficiency of CO2 excretion (so-called plant gain), and the delays imposed by hemoglobin binding and the circulation (so-called mixing gain). Any of these various gains can serve to elevate the overall loop gain and create a propensity for breathing instability. The loop gain concept can be a challenging one, but can be more easily understood by using the thermostat analogy. The analogy of the respiratory system to regulate CO2 (ie, at 40 mm Hg) can be thought of by considering the temperature control system in a room trying to regulate room temperature (eg, at 20°C). The situations that lead to substantial instability in air temperature by analogy could lead to considerable fluctuations in PaCO2 in the ventilatory control system. A thermostat that is excessively sensitive (ie, turns on air conditioning when temperature rises to 20.001°C) would lead to temperature instability more than a thermostat that was less sensitive.
A similar phenomenon can occur in the ventilatory system in individuals with robust chemosensitivity (ie, those who breathe a lot with minimal CO2 elevation). Such individuals are prone to breathing instability or Cheyne-Stokes breathing (CSB) (Fig. 1). Similarly, a furnace that was overly powerful would tend to lead to marked temperature fluctuations. For example, if a spontaneous fall in room temperature to 19.5°C were to occur, a furnace that blows heat up to a room temperature of 50°C would tend to lead to more instability than a less robust furnace.16 By analogy, those individuals with high plant gain (very efficient CO2 excretion) would be more likely to develop marked CO2 fluctuations (and therefore periodic breathing) than an individual with less efficient CO2 excretion.17
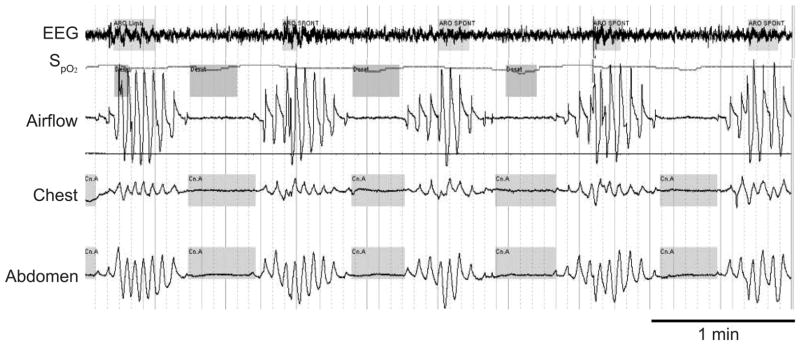
Fig. 1.
Cheyne-Stokes breathing. A crescendo-decrescendo pattern of breathing with cessations in air flow in the absence of respiratory effort. This breathing pattern is typically associated with desaturations and re-oxygenation (which may contribute to oxidative stress), arousals from sleep (often characterized by paroxysmal nocturnal dyspnea), and bursts of tachycardia (probably from catecholamine surges). (From Reference 1 with permission.)
Other situations that will lead to more unstable room temperature would be that the temperature regulation is in a small room (as opposed to a large indoor stadium) or a situation where the furnace and the thermostat are far removed from one another. By analogy, the ventilatory control system would not be prone to instability if the chemoreceptors were present in the lungs as opposed to the carotid bodies and brainstem. That said, the role of pathological delays in the circulation as a cause of periodic breathing has been questioned.18 Although Guyton et al showed in some classic experiments that prolonged circulatory delays could lead to breathing instability, these delays in some animals were on the order of several minutes (ie, beyond what could occur in a human even with severe heart disease).19
When studies have carefully examined congestive heart failure (CHF) patients with CSB, their circulatory delay is comparable to matched control CHF patients without Cheyne-Stokes.18 This finding may lead some to believe that pathological delays in the circulation are not important in control of breathing. However, a number of studies have shown improvements in both central and obstructive apnea with cardiac resynchronization therapy, with some data showing that improvements in circulatory delay are predictive of improvements in apnea-hypopnea index.20,21 These data suggest that circulatory delay may be important, although further data are clearly required.
Types of Central Sleep Apnea
In general, the mechanisms underlying CSA can be categorized into one of 2 groups: those with high central drive (or loop gain), and those with low central drive.
Cheyne-Stokes Breathing
CSB is common in patients with CHF.22 Studies suggest that 30–50% of patients with CHF with left-ventricular systolic dysfunction have CSB.23 Estimates of CSB prevalence have been variable in the literature, in part due to the increasing prevalence of obesity (which may be increasing the prevalence of OSA over CSB), and the studies that have been based in the Veterans Affairs hospitals involved exclusively men (since CSB is much more common in men than women). With these caveats, CSB remains the most well studied example of CSA to date. Despite the high prevalence figures and emerging literature, many cardiologists remain skeptical about the utility of sleep studies in patients with CHF.24 Several papers have examined the prognostic value of sleep abnormalities in patients with CHF.25
Lanfranchi et al examined polygraph studies in a cohort of patients with CHF in addition to demographic variables, echocardiographic, autonomic measures, and Holter monitoring.26 The multivariate analyses suggest that the best predictors of mortality in this cohort were apnea-hypopnea index (based on the polygraph) and the size of the left atrium. These data are consistent with the notion that CSB per se may be problematic in CHF, and not simply a marker of a “sicker” patient. However, other studies have been quite variable from the standpoint of the prognostic value of CSB in patients with CHF.27 Thus, therapeutic studies will probably be required to resolve this controversy as to whether CSB per se is problematic.
A number of interventional studies have been performed in the CSB arena, with somewhat conflicting messages. Nasal continuous positive airway pressure (CPAP) has been shown to improve oxygen tension as well as breathing pattern in CHF patients with CSB.28,29 Some data further suggest important improvements in left-ventricular ejection fraction with CPAP therapy, as compared to controls. The mechanisms underlying the improvement in hemodynamics are complex but probably include reductions in cardiac pre-load and after-load (Table 2).30–32 Given the small volume of blood in the pulmonary veins, steady-state cardiac output is critically dependent on right-ventricular function.33,34 That is, in steady state, left-ventricular output can also increase only if right-ventricular output increases, as the two must be equal. With CPAP in CSA, right-ventricular output is probably improved due to reductions in pulmonary artery pressure, which occur with alleviation of hypoxemia from CPAP application. However, further research is clearly required regarding the mechanisms underlying hemodynamic benefit from nasal CPAP therapy in CHF.35
Table 2.
Hemodynamic Effects of CPAP
| Increase right atrial pressure |
| Reduce cardiac pre-load |
| Improve oxygenation |
| Suppressed catecholamines |
| Reduced ventricular wall tension or after-load |
| Improved mitral regurgitation |
| Reduce pleural pressure required for inspiration by improving lung compliance |
| Improved right-ventricular after-load through reduced pulmonary vasoconstriction from hypoxemia and catecholamines |
CPAP = continuous positive airway pressure
An initial pilot study in patients with CHF, by Sin et al, showed a potential improvement in transplant-free survival in those treated with CPAP, as compared to controls.28 That study, however, had a number of limitations, including a small sample size, a failure to use the intention-to-treat analytical technique, and lack of β-blocker therapy in the majority of treated patients. These data led to considerable initial enthusiasm, but the subsequent Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) study published in The New England Journal of Medicine failed to corroborate these results.29
The CANPAP study showed no important improvement in outcome among CSB patients given CPAP, as compared to matched controls. Of concern, the data suggested a deleterious outcome among CPAP-treated patients in the short term. In CANPAP there was some late improvement in outcome in CPAP-treated patients, such that the overall survival was equivalent among CPAP-treated patients, as compared to controls. Some minor improvements in morbidity were observed in the CANPAP study include left-ventricular ejection fraction, although most acknowledge the study as negative. The mechanism underlying the worsening of short-term outcome is unclear but may relate to hypovolemia among CPAP-treated patients. In theory, hypovolemic patients (who are pre-load dependent) may experience a fall in cardiac output with nasal CPAP therapy, whereas hypervolemic patients (who are more after-load dependent) may experience improved cardiac output with CPAP.30
A subsequent follow-up study from CANPAP examined various subgroups, not pre-specified in the initial study. Arzt et al reported on CPAP-treated patients in CANPAP who experienced resolution of central apnea, as compared to CPAP-treated patients who had persistent apnea.36 The data showed improved outcome among those with resolution of apnea, as compared with the other groups. There are 2 potential explanations for this finding. On the one hand, some might argue that treatments that lead to resolution of apnea are likely to yield improved outcome in CHF patients with CSB. On the other hand, some could argue that the resolution of apnea was a good prognostic sign (ie, those patients who had resolution of apnea may be different from those with persistent apnea for reasons independent of CPAP therapy). For example, patients who are adherent with their medications may be the ones in whom CSB resolves and therefore the breathing improvement may be a marker, but not a cause, of a good prognosis. The answer to this dilemma will require further study, but remains a source of considerable discussion.
These data have led to the conception of the CANPAP II study, which will test the utility of newer devices (which use proprietary algorithms to maintain ventilation relatively constant during fluctuations in patient effort) in the treatment of breathing abnormalities during sleep in CHF patients. If these newer devices are effective at eliminating CSB, then one might anticipate improvement in outcome if CSB itself is indeed problematic.
A frequently asked question is regarding how CSB should be treated as of today. Although the answer to this question is unknown, optimization of medical therapy is probably the cornerstone of treatment, since CSB will often resolve with adequate medical therapy.37 The measurement of a serum brain natiuretic peptide level can sometimes be helpful, since patients who are apparently well treated medically often have room for improvement from the standpoint of medications and dosages.38 Similarly, studies have shown improvement in both OSA and CSB with cardiac resynchronization therapy.20,21 Other agents have been discussed, including oxygen,39 acetazolamide,17 and theophylline,40 but hard outcome data are lacking. The management of these patients is otherwise controversial.
Some would argue that, depending on the indication for the sleep study, treating patients for symptomatic benefit may be justified. For example, if a patient complains of non-restorative sleep or daytime sleepiness, one could argue to give a trial of CPAP therapy to improve symptoms. Because many CHF patients complain of fatigue rather than sleepiness,41 this clinical decision can be quite complex. Among CHF patients who also have a component of OSA one could argue that treatment with CPAP is justified, based on some improvements in hemodynamics that have been documented in prior studies.42,43 Although some short-term studies have shown some improvements in breathing pattern with the use of newer positive pressure devices, outcome data are again lacking.44,45 Because of the clinical overlap between OSA and CSB, and the potential improvement in morbidity with CPAP therapy in OSA plus CHF, we frequently will give these patients a trial of nasal CPAP with close clinical follow-up to document improvement in breathing pattern. However, we await further data before making more definitive recommendations.
Complex Apnea
Considerable controversy exists regarding complex sleep apnea.46 A number of entities can lead to poor outcome among CPAP-treated patients with OSA (Table 3); these entities should be ruled out before more esoteric diagnoses are sought. The definition of complex apnea is variable in the literature, but most reports suggest development of central apnea in OSA patients when initially exposed to CPAP.47 The term “treatment-emergent central apnea” was previously used to describe this phenomenon, which appears to occur in roughly 10% of CPAP titrations.1 Most of the published data suggest that this form of central apnea generally resolves with ongoing CPAP therapy.48,49 Chronic CPAP therapy is not a recognized risk factor for CSA, suggesting that these 10% of patients usually experience resolution of respiratory events with ongoing therapy. The mechanism underlying these events is unclear but probably related to the relief of upper-airway obstruction with the application of CPAP.50 By effectively removing a large resistance from the respiratory system, the efficiency of CO2 excretion is improved, and thus important hypocapnia can result. If the resulting PaCO2 falls below the CO2 apnea threshold,8 then cessation in respiratory effort will be expected.
Table 3.
Causes of CPAP-Refractory Sleep Apnea (Commonly Confused with Complex Apnea)
| Wrong CPAP level (over-titration, under-titration, weight change since titration) |
| CPAP non-adherence |
| Residual sleepiness on CPAP |
| Mask leak |
| Other sleep disorders (eg, Cheyne-Stokes breathing, chronic partial sleep deprivation) |
| Sleep transition apneas on CPAP initiation |
CPAP = continuous positive airway pressure
In roughly 1–2% of CPAP titrations there is persistence of these respiratory events despite ongoing CPAP therapy.49 This truly refractory group is uncommon clinically, and optimal therapy for these patients is not established. An emerging literature has been pushing the notion that newer devices are helpful to resolve this form of central apnea. However, randomized controlled trial data are lacking from the standpoint of hard clinical outcome. Thus, the management of treatment-emergent central apnea is unclear.51 For most cases, expectant management is adequate since these events are likely to resolve. For the remaining fraction of patients with truly refractory central apneas, a variety of options could be considered, including hypnotic therapy, oxygen therapy, acetazolamide, or potentially newer devices.52,53 However, further data will be required before any definitive recommendations can be provided. One issue that has received minimal attention is the concept that patients with treatment-emergent central apneas may have a poor initial experience with CPAP and thus may be non-adherent with therapy, even if the central events ultimately resolve. Randomized trials will ultimately be required on whether early intervention in these patients will lead to improved long-term adherence and outcomes.
The above discussion pertains primarily to patients with high drive or high loop gain. However, a number of patients who fall into the CSA category have low drive. For example, patients with hypercapnic COPD frequently experience worsening ventilation during sleep. The mechanism underlying this deterioration is complex but is thought to involve upper-airway collapse as well as the loss of the so-called wakefulness stimulus (or the wakefulness drive to breathe).50,54 In many cases these patients have hypopneas rather than apneas, and their etiology is often difficult to determine. As a result, many investigators refer to these individuals as having “sleep hypoventilation syndrome” rather than CSA per se. Regardless, these patients experience deterioration in their ventilation and often benefit from assisted ventilation.
Sleep Transition Apnea
Sleep transition apneas refer to the fluctuations in ventilation that occur in otherwise normal individuals during the transition from wake to sleep.1 The pathogenesis of these apneas involves arousal from sleep, which is associated with an augmentation of ventilation,55,56 particularly immediately after waking up. In normal individuals with a PaCO2 = 40 mm Hg during wakefulness, PaCO2 = 45 mm Hg during stable non-rapid-eye-movement sleep. When these individuals wake up from sleep, they typically hyperventilate and drive down their PaCO2 to values below their normal awake eupnic PaCO2. If this rise in ventilation yields a PaCO2 below the so-called apnea threshold,8 then a cessation of breathing is predictable. Frequently, transient arousals from sleep occur and are followed by resumption of sleep, but the associated augmentation in ventilation leads to central apnea upon resumption of sleep.
The treatment of these individuals must be individualized as it depends on the underlying cause of the arousals. Depending on the etiology of the arousals (eg, obstructive apnea, periodic limb movement, sleep maintenance insomnia), the administration of appropriate treatment of underlying cause will help to alleviate these sleep transition apneas. In cases where transition apneas remain problematic, oxygen administration can be effective. Oxygen serves to raise PaCO2 through a variety of mechanisms (ie, Haldane effect, loss of hypoxic drive, loss of hypoxic pulmonary vasoconstriction yielding increased dead space, reduced minute ventilation raising effective FIO2, and sleep onset) (Table 4).57 If the oxygen serves to increase P aCO2 above the apnea threshold, then resolution of apnea can be anticipated. Hypnotic therapy may also be effective in patients with transition apneas, since these agents serve to raise PaCO2 and reduce recurrent arousals. These physiological effects would be predicted both to increase CO2 above the apnea threshold and to improve central apnea. Thus, a variety of approaches are useful for treating sleep transition apneas if they persist after addressing the underlying cause.
Table 4.
Mechanisms Underlying Oxygen-Induced Hypercapnia
| Loss of hypoxic pulmonary vasoconstriction, stripping perfusion from well-ventilated lung units, leading to increased pulmonary dead space |
| Haldane effect |
| Loss of central drive |
| Gradual increase in actual FIO2 from noninvasive source with falling minute ventilation |
| Sleep onset leading to loss of wakefulness drive to breathe |
Narcotic-Induced Central Apnea
Narcotic-induced central apneas are also being increasingly appreciated.1 These events occur in patients on chronic narcotic therapy, either due to chronic pain or due to drug abuse. Prevalence estimates vary, but the literature suggests that between 10% and 50% of chronic narcotic therapy patients have some form of central apnea. A number of features of these patients are noteworthy. These patients frequently have bradypnea (reduced respiratory rate), and thus a low respiratory frequency should alert the clinician to the possibility of narcotic therapy/use. In addition, at least in some cases, narcotic-induced central apneas are dose-dependent.1 As a result, these patients may experience resolution of their breathing abnormality upon reduction of their narcotic dosing (Fig. 2). Appropriate treatment of these patients is poorly studied. Efforts to reduce the narcotic dose may be facilitated by local analgesics or agents such as gabapentin (or non-narcotic analgesics), which may reduce narcotic requirements. Some data suggest that the use of newer devices may help resolve respiratory events, although outcome data are lacking.58
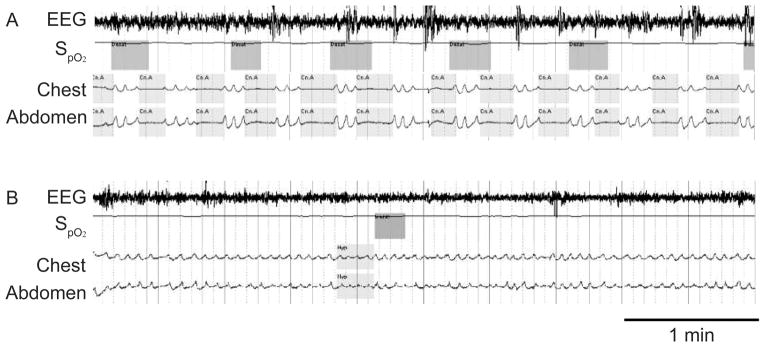
Fig. 2.
A: A patient with narcotic-induced central apnea. Note the cessations in air flow without respiratory effort. B: The breathing abnormality resolved on a lower dose of narcotic. (From Reference 1, with permission.)
Hypoventilation Syndromes
The approach to hypoventilation is most simply considered as patients who “can’t breathe” versus patients who “won’t breathe.” Can’t-breathe patients are ones with neuromuscular weakness or abnormalities in lung or chest wall mechanics that preclude adequate ventilation. Those who won’t breathe generally have abnormalities in central control with inadequate central drive to maintain a normal PaCO2. This distinction is often apparent based on the history and physical examination. The airway-occlusion pressure 0.1 s after the start of inspiratory flow (P0.1 or P100) can also be helpful, as it assesses the pressure generated at the mouth during the first 100 ms of a spontaneous inspiration, during which the airway is briefly occluded, unbeknownst to the patient. Those with low central drive have a low P0.1, whereas those with high central drive will generally have a normal or high P0.1. Clearly, severe neuromuscular disease would limit the ability to generate P0.1, so the test is far from perfect.59 However, it does provide a relatively robust metric of central drive, which is somewhat impervious to neuromuscular function and lung parenchymal disease. Measurement of hypercapnic chemosensitivity can also be helpful, since those with impaired central drive (eg, central congenital hypoventilation syndrome) will typically have blunted chemosensitivity.
With the development of genetic testing for central congenital hypoventilation syndrome (ie, PHOX-2b gene),60,61 an increasing number of patients are being diagnosed with this condition, even in adulthood.62 Central congenital hypoventilation syndrome represents the prototype for a “won’t breathe” disorder. Considerable progress has been made in our understanding of the genetics of this disorder as well as in terms of brainstem pathways. These patients frequently have autonomic and other systemic abnormalities, which are now being understood at a basic level. Important insights are now being gained regarding the links between respiratory and autonomic control, using sophisticated animal models of central congenital hypoventilation syndrome.63
Among patients who can’t breathe, a logical approach can be helpful to avoid missing potential causes. We recommend an anatomical approach whereby lesions anywhere along the following pathway could theoretically lead to hypoventilation: brainstem to bulbospinal tracts to C3–C5 spinal cord through upper motor neurons, anterior horn cell from cord to lower motor neurons to neuromuscular junction to muscle (diaphragm). Major abnormalities of the lung parenchyma and chest wall are usually obvious clinically or from pulmonary function tests. Neuromuscular processes, however, can be more subtle but can be suspected based on immediate orthopnea on history, low lung volumes on chest radiograph, lack of diaphragm movement on chest percussion, unexplained hypercapnia, or evidence of systemic weakness on examination. Thus, a careful clinical approach to the patient with hypoventilation can often be clinically important.64
Many patients with daytime hypercapnia will experience worsening of their blood gases at sleep onset. This worsening is probably multifactorial but related to loss of wakefulness drive to breathe as well as upper-airway collapse.54 Patients with hypercapnic COPD, obesity hypoventilation syndrome, neuromuscular disease, et cetera all have worsening elevation of PaCO2 during sleep.65 Definitive data regarding optimal management are lacking. Optimization of medical therapy for underlying conditions should clearly be emphasized (eg, weight loss, optimized bronchodilator therapy). However, bi-level positive airway pressure is frequently provided to hypercapnic patients in an effort to maintain if not improve PaCO2 during sleep. In some cases, nocturnal ventilation has been associated with improvement in daytime arterial blood gases, perhaps as a result of muscle rest or resetting of PaCO2 set point.
Randomized trial data for hypercapnic COPD are evolving, with the bulk of the existing data suggesting modest benefit to nocturnal bi-level positive airway pressure in these patients.66 While a number of negative studies exist, the positive studies have been primarily in COPD patients with marked hypercapnia.67 One recent multicenter randomized trial showed some improvement in adjusted mortality with nocturnal ventilation in COPD, as compared with the control group.66 However, quality of life deteriorated somewhat in the nocturnal-ventilation group, leading to caution in widespread advocacy for bi-level in optimal COPD management. A group in Germany, led by Windisch, has been investigating the use of so-called “high-intensity” nocturnal ventilation. They have considerable experience with the use of high levels of inspiratory pressure (up to 30 cm H2O) in COPD patients, without major complications.68,69 They have demonstrated excellent long-term outcome among consecutive series of patients with hypercapnic COPD who undergo high-intensity nocturnal ventilation. However, randomized trial data for high-intensity ventilation are currently sparse. As a result, the optimal management of hypercapnic COPD remains unclear until further data are available. At present, we do offer nocturnal ventilation to these patients in addition to oxygen supplementation (which has clear mortality benefit in hypoxemic COPD).70 For patients with hypercapnic respiratory failure without COPD, data are more sparse, but again bi-level positive airway pressure can sometimes be helpful in the management of these patients. Finally, we are aware of no data regarding optimal therapy for the overlap syndrome (OSA plus COPD) as reviewed elsewhere in this Journal.71
Summary
CSA is relatively uncommon, as compared with OSA. However, considerable overlap exists between CSA and OSA, from the standpoint of pathogenesis as well as disease manifestations. As a result, a good working knowledge about CSA is important. CSA can be divided into various subcategories, each with some unique features and management issues. In general, the topic is not well researched from the standpoint of mechanism or clinical trials, leaving considerable room for improvement for future investigators to target.
Footnotes
Dr Malhotra presented a version of this paper at the 45th Respiratory Care Journal Conference, “Sleep Disorders: Diagnosis and Treatment” held December 10-12, 2009, in San Antonio, Texas.
Dr Malhotra has disclosed relationships with Philips, Pfizer, Merck, Apnex, Itamar, Sepracor, Cephalon, Sleep Group Solutions, Sleep HealthCenters, Medtronic, and Ethicon. Dr Owens has disclosed no conflicts of interest.
References
- 1.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Badr MS. Pathophysiology of upper airway obstruction during sleep. Clin Chest Med. 1998;19(1):21–32. doi: 10.1016/s0272-5231(05)70429-9. [DOI] [PubMed] [Google Scholar]
- 4.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51(4):313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181(2):1891–93. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owens RL, Eckert DJ, Yeh SY, Malhotra A. Upper airway function in the pathogenesis of obstructive sleep apnea: a review of the current literature. Curr Opin Pulm Med. 2008;14(6):519–524. doi: 10.1097/MCP.0b013e3283130f66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90(1):13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 9.Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol. 2007;103(2):578–586. doi: 10.1152/japplphysiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bradley TD. Crossing the threshold: implications for central sleep apnea. Am J Respir Crit Care Med. 2002;165(9):1203–1204. doi: 10.1164/rccm.2203016. [DOI] [PubMed] [Google Scholar]
- 11.Khoo M. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122(2–3):167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 12.Younes M, Ostrowshi M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(5):1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 13.Cherniack NS. Respiratory dysrhythmias during sleep. New Engl J Med. 1981;305(6):325–330. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 14.Carley DW, Shannon DC. Relative stability of human respiration during progressive hypoxia. J Appl Physiol. 1988;65(3):1389–1399. doi: 10.1152/jappl.1988.65.3.1389. [DOI] [PubMed] [Google Scholar]
- 15.Meza S, Giannouli E, Younes M. Control of breathing during sleep assessed by proportional assist ventilation. J Appl Physiol. 1998;84(1):3–12. doi: 10.1152/jappl.1998.84.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement? Sleep. 2006;29(1):16–18. [PubMed] [Google Scholar]
- 17.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173(2):234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 18.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164(12):2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC, Cowley AW, Jr, Young DB, Coleman TG, Hall JE, DeClue JW. Integration and control of circulatory function. Int Rev Physiol. 1976;9:341–385. [PubMed] [Google Scholar]
- 20.Sinha AM, Skobel EC, Breithardt OA, Norra C, Markus KU, Breuer C, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44(1):68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, et al. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest. 2007;132(2):433–439. doi: 10.1378/chest.06-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javaheri S, Parker TJ, Wexler L, Michaels SE, Stanberry E, Nishyama H, Roselle GA. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122(7):487–492. doi: 10.7326/0003-4819-122-7-199504010-00002. Erratum in: Ann Intern Med 1995;123(1):77. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra A, Loscalzo J. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51(4):279–284. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure. Relationship to arterial PCO2. Chest. 1993;104(4):1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 26.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 27.Naughton MT, Lorenzi-Filho G. Sleep in heart failure. Prog Cardiovasc Dis. 2009;51(4):339–349. doi: 10.1016/j.pcad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–6. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 29.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, et al. Continuous positive airway pressure for central sleep apnea and heart failure. New Engl J Med. 2005;353(19):2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra A, Muse V, Mark E. Clinical pathological case. N Engl J Med. 2010 in press. [Google Scholar]
- 31.Fessler HE, Brower RG, Wise RA, Permutt S. Mechanism of reduced LV afterload by systolic and diastolic positive pleural pressure. J Appl Physiol. 1988;65(3):1244–1250. doi: 10.1152/jappl.1988.65.3.1244. [DOI] [PubMed] [Google Scholar]
- 32.Fessler HE. Heart-lung interactions: applications in the critically ill. Eur Respir J. 1997;10(1):226–237. doi: 10.1183/09031936.97.10010226. [DOI] [PubMed] [Google Scholar]
- 33.Magder S. More respect for the CVP. Intensive Care Med. 1998;24(7):651–653. doi: 10.1007/s001340050640. [DOI] [PubMed] [Google Scholar]
- 34.Magder S, Bafaqeeh F. The clinical role of central venous pressure measurements. J Intensive Care Med. 2007;22(1):44–51. doi: 10.1177/0885066606295303. [DOI] [PubMed] [Google Scholar]
- 35.Atherton JJ, Moore TD, Lele SS, Thomson HL, Galbraith AJ, Belenkie I, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349(9067):1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 36.Arzt M, Floras JS, Logan AG, Kimoff RJ, Sériès F, Morrison D, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CAN-PAP) Circulation. 2007;115(25):3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 37.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99(12):1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 38.Karmpaliotis D, Kirtane AJ, Ruisi CP, Plonsky T, Malhotra A, Talmor D, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131(4):964–971. doi: 10.1378/chest.06-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javaheri S. Pembrey’s dream: the time has come for a long-term trial of nocturnal supplemental nasal oxygen to treat central sleep apnea in congestive heart failure. Chest. 2003;123(2):322–325. doi: 10.1378/chest.123.2.322. [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. New Engl J Med. 1996;335(8):562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 41.Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716–1722. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 42.Mansfield D, Gollogly N, Kaye D, Richardson M, Bergin P, Naughton M. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169(3):361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko Y, Floras J, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 44.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 45.Pittman S, Hill P, Malhotra A, et al. Stabilizing Cheyne-Stokes respiration associated with congestive heart failure using computer assisted positive airway pressure. Comp Cardiol. 2000;27:201–204. [Google Scholar]
- 46.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Current Opin Pulm Med. 2005;11(6):485–493. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 47.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468–475. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 48.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132(1):81–87. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 49.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 50.Skatrud J, Dempsey J, Badr S, Begle R. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol. 1988;65(4):1676–1685. doi: 10.1152/jappl.1988.65.4.1676. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra A, Bertisch S, Wellman A. Complex sleep apnea: it isn’t really a disease. J Clin Sleep Med. 2008;4(5):406–408. [PMC free article] [PubMed] [Google Scholar]
- 52.White DP. Central sleep apnea. Med Clin North Am. 1985;69(6):1205–1219. doi: 10.1016/s0025-7125(16)30983-x. [DOI] [PubMed] [Google Scholar]
- 53.White DP, Zwillich CW, Pickett CK, Douglas NJ, Findley LJ, Weil JV. Central sleep apnea. Improvement with acetazolamide therapy. Arch Intern Med. 1982;142(10):1816–1819. [PubMed] [Google Scholar]
- 54.Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. [PubMed] [Google Scholar]
- 55.Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 1997;778(1):127–134. doi: 10.1016/s0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- 56.Jordan A, McEvoy R, Edwards J, Schory K, Yang CK, Catcheside PG, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnea. J Physiol. 2004;558(Pt 3):993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malhotra A, Schwartz DR, Ayas N, Stanchina M, White DP. Treatment of oxygen-induced hypercapnia. Lancet. 2001;357(9259):884–885. doi: 10.1016/s0140-6736(05)71817-1. [DOI] [PubMed] [Google Scholar]
- 58.Javaheri S, Malik A, Smith J, Chung E. Adaptive pressure support servoventilation: a novel treatment for sleep apnea associated with use of opioids. J Clin Sleep Med. 2008;4(4):305–310. [PMC free article] [PubMed] [Google Scholar]
- 59.Whitelaw WA, Derenne JP. Airway occlusion pressure. J Appl Physiol. 1993;74(4):1475–1483. doi: 10.1152/jappl.1993.74.4.1475. [DOI] [PubMed] [Google Scholar]
- 60.Weese-Mayer DE, Silvestri JM, Marazita ML, Hoo JJ. Congenital central hypoventilation syndrome: inheritance and relation to sudden infant death syndrome. Am J Med Genet. 1993;47(3):360–367. doi: 10.1002/ajmg.1320470313. [DOI] [PubMed] [Google Scholar]
- 61.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164(1–2):38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Antic NA, Malow BA, Lange N, McEvoy RD, Olson AL, Turkington P, et al. PHOX2B mutation-confirmed congenital central hypoventilation syndrome: presentation in adulthood. Am J Respir Crit Care Med. 2006;174(8):923–927. doi: 10.1164/rccm.200605-607CR. [DOI] [PubMed] [Google Scholar]
- 63.Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105(2):404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapoport DM, Sorkin B, Garay SM, Goldring RM. Reversal of the “Pickwickian syndrome” by long-term use of nocturnal nasal-airway pressure. New Engl J Med. 1982;307(15):931–933. doi: 10.1056/NEJM198210073071507. [DOI] [PubMed] [Google Scholar]
- 65.Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 66.McEvoy RD, Pierce RJ, Hillman D, Esterman A, Ellis EE, Catcheside PG, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 67.Douglas NJ. Sleep in patients with chronic obstructive pulmonary disease. Clin Chest Med. 1998;19(1):115–125. doi: 10.1016/s0272-5231(05)70436-6. [DOI] [PubMed] [Google Scholar]
- 68.Windisch W. Impact of home mechanical ventilation on health-related quality of life. Eur Respir J. 2008;32(5):1328–1336. doi: 10.1183/09031936.00066407. [DOI] [PubMed] [Google Scholar]
- 69.Windisch W, Kostic S, Dreher M, Virchow JC, Jr, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of PaCO2. Chest. 2005;128(2):657–662. doi: 10.1378/chest.128.2.657. [DOI] [PubMed] [Google Scholar]
- 70.Barnes PJ. Chronic obstructive pulmonary disease. New Engl J Med. 2000;343(4):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 71.Owens R, Malhotra A. Sleep-disordered breathing and chronic obstructive pulmonary disease: the overlap syndrome. Respir Care. 2010;55(10) in press. [PMC free article] [PubMed] [Google Scholar]