Abstract
Hypoxia has been shown to have a role in the pathogenesis of several forms of liver disease. The Hypoxia Inducible Factors (HIFs) are a family of evolutionarily conserved transcriptional regulators that affect a homeostatic response to low oxygen tension and have been identified as key mediators of angiogenesis, inflammation, and metabolism. In this review, we summarize the evidence for a role of HIFs across a range of hepatic pathophysiology. We describe regulation of the hypoxia inducible factors and review investigations that demonstrate a role for HIFs in the development of liver fibrosis, activation of innate immune pathways, hepatocellular carcinoma, as well as other liver diseases in both human disease as well as murine models.
Keywords: Hypoxia-Inducible Factor, Steatosis, Liver injury, Fibrosis, Inflammation
Introduction
The liver has a unique anatomical and functional niche within the body that profoundly affects its physiology and pathophysiology including its oxygen homeostasis. Afferent blood flow to the liver derives from both highly oxygenated blood in the hepatic artery as well as oxygen-depleted blood in the hepatic portal vein. Furthermore, the directional flow of mixed oxygenated and deoxygenated blood towards the central vein of the hepatic lobule creates a physiological oxygen gradient.(1) This gradient has been reported to result in oxygen tensions from about 60–65mmHg in periportal blood, falling to about 30–35 mmHg in perivenous portions of the liver parenchyma; by comparison, physiological arterial oxygen concentration in most other body tissues is about 74–104 mmHg, and venous oxygen concentration is 34–46 mmHg.(1) Hypoxia has profound consequences for tissues of an aerobic organism. In recent decades, our knowledge of the homeostatic response to hypoxia has increased to molecular genetic mechanisms.
The Hypoxia Inducible Factors are a family of heterodimeric transcription factors that act as master regulators of a homeostatic transcriptional response to hypoxia in virtually all cells and tissues. Active Hypoxia-Inducible Factor (HIF) consists of an alpha subunit and a beta subunit. Three alpha subunits, named Hypoxia Inducible Factor 1-α [HIF1α], Hypoxia Inducible Factor 2-α [HIF2α], or Hypoxia Inducible Factor 3α [HIF3α], have been described in humans, mice, and rats; all bind to a common β subunit named, alternatively, HIF1β, or the Aryl-Hyrdocarbon-Nuclear Receptor Translocator [ARNT].(2) Active HIF is named by its alp(1)ha subunit, hence, HIF1 is the active transcription factor consisting of HIF1α and ARNT, HIF2 is the dimer of HIF2α and ARNT, etc. HIF1 and HIF2 are the major hypoxia-inducible factors in humans, mice, and rats. Far less is known about the function of HIF3.(2)
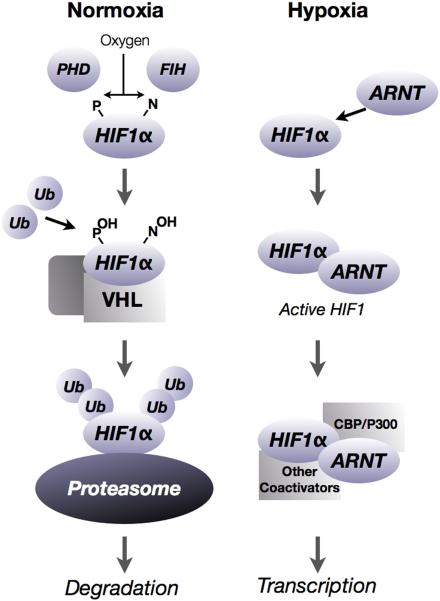
Post-translational degradation by the proteasome is a major pathway of HIF regulation. Under normoxia, and in the absence of other metabolic perturbations, the alpha subunits of HIF are rapidly hydroxylated by prolyl-hydroxylases (PHD1, PHD2, or PHD3) and scaffolded on a multimeric protein complex that includes the product of the Von Hippel Lindau tumor suppressor gene. Prolyl hydroxylation and presentation of HIF on the VHL scaffold leads to rapid ubiquitination and proteasomal degradation.(3) Under conditions of hypoxia, or perturbations in cellular redox state, HIFs escape hydroxylation and are free to form dimers with ARNT. Active HIF then translocates to the nucleus, where it binds to hypoxia-responsive elements (HREs) in the promoter region of target genes. HIF1 and HIF2 activate transcription of a broad range of target genes, (e.g., vascular endothelial growth factor, VEGF) with some overlap between the two factors.(4) Numerous other pathways have been implicated in posttranslational HIF regulation and have been reviewed elsewhere.(5) A simplified version of post-translational regulation of HIF is illustrated in Figure 1.
Figure 1.
Under conditions of normoxia, HIF alpha subunits are rapidly hydroxylated at proline and asparagine residues by hydroxylase enzymes. Hydroxylation of HIF1α and assembly on a protein scaffold consisting of the VHL tumor suppressor, along with other co-factors, results in the rapid ubiquitination of the alpha subunit and subsequent degradation by the proteasome. Conversely, in conditions of hypoxia, HIF alpha subunits escape degradation and are free to dimerize with the binding partner, the Aryl Hydrocarbon Nuclear Translocator (ARNT). The HIF heterodimer is named by the alpha subunit (hence, HIF1α-ARNT heterodimer is the active HIF1 transcription factor) which translocates to the nucleus and affects transcription of target genes, typically by binding to a hypoxia response element in the upstream promoter region of the target gene. CBP/P300 and other cofactors may be implicated in HIF transcription. Other pathways of HIF regulation have been described in detail elsewhere.
Hypoxic gene targets and their regulation by HIF
Dozens, even hundreds, of genes have been reported to be regulated by hypoxia and the hypoxia inducible factors.(4, 6) Notably, pivotal recent work in the biology of HIF has identified that a large number of hypoxia response genes many of which have been identified as HIF targets, lack a HRE in their promoter sequences, but that genes that contain an HRE in their promoter region are more likely to respond to hypoxic stimuli across a range of cell types.(6) Conversely, genes that are reported to be HIF targets may only be responsive to HIF within a narrow range of tissue types, depending upon cooperative interactions with other molecules including, for example, CBP-P300, sirtuin, beta catenin, and many others.(7, 8)
While a comprehensive list of HIF targets would be well beyond the scope of this article, several HIF targets that have been described in liver disease as summarized in Table 1. Notably, the gene families represented include proinflammatory and profibrotic mediators, as well as genes involved in tumor progression.(9–17)
Table 1.
Several gene targets of hypoxia inducible factors described in liver are listed, along with the broad category or categories of physiologic or pathologic activity to which major functions of the gene are attributed. Further information appears in the accompanying text.
| Gene | Function | Reference |
|---|---|---|
| Plasminogen activator Inihibitor-1 (PAI-1) | Inflammation, thrombosis, metastasis | Kietzmann (1999), Anh (2010) |
| Prolyl-4 hydroxylase alpha-2 | Fibrosis, collagen stabilization | Copple (2011) |
| Heme Oxygenase 1 (HO1) | Oxidative stress, inflammation | Yeligar (2011) |
| Vascular endothelial growth factor | Angiogenesis, tumor vascularization, ischemia-reperfusion | Corpechot (2002), Maeno (2005), Nakamura (2005), Redaelli (2005) |
| Erythropoietin | Erythrocyte proliferation, liver regeneration | Rankin (2007) |
| Ceruloplasmin | Copper metabolism | Martin (2005) |
| Glukokinase | Metabolism | Roth (2004) |
| Bone Morphogenetic Protein | Tumorigenesis | Maegdefrau (2009) |
Oxygen and the Liver
The physiological gradient of oxygen tension across the hepatic lobule has profound effects on the function of hepatic parenchymal and nonparenchymal cells. Periportal hepatocytes and perivenous hepatocytes differ in their expression of many enzymes involved in glucose transport or metabolism, including insulin receptor, glucagon receptor, phosphoglycerate kinase (PGK1), L-type pyruvate kinase, and numerous others.(18) Consequently, periportal hepatocytes tend to sub-specialize in oxidative energy metabolism, glucose production, and synthesis of urea and bile, whereas perivenous hepatocytes are major sites of glucose uptake, glutamine formation and xenobiotic metabolism.(1)
Physiological exposure of hepatocytes to varying levels of oxygen tension also has consequences for the ability of hepatocytes to respond to hypoxic stress. Primary rat hepatocytes cultured in conditions approximating periportal oxygen tensions were able to survive transient anoxia with less cell death and cytokine release than hepatocytes cultured in conditions approximating perivenous oxygen tension. This suggests that in conditions of oxygen deprivation, such as increased hepatic metabolic demand, tissue ischemia, or other conditions, perivenous hepatocytes may be primed to increased injury when oxygen tension drops beneath a threshold level.(19)
Ischemia-Reperfusion Injury
Understanding and controlling ischemia reperfusion (IR) injury is a major goal of liver biology, particularly as IR injury often occurs in the context of reperfusion of the transplanted liver and in emergencies with low arterial pressure. Through a variety of mechanisms, including the production of reactive oxygen species and inflammatory mediators, IR injury can cause major morbidity including predisposing to graft failure. HIF1α induction has been described as an early event, preceding apoptosis, in ischemia-reperfusion injury.(20) Hepatic ischemiareperfusion has been described to upregulate the HIF target Vascular Endothelial Growth Factor (VEGF).(21) Unsurprisingly, HIF1α tends to accumulate during ischemia, but HIF1α DNA binding has been shown to decrease during reperfusion.(22) Some data suggests that HIF1α-dependent upregulation of the transferrin gene contributes to reactive species formation and liver injury in reperfusion, likely through iron-dependent reactive species accumulation.(23) A protective effect of HIF1α induction on ischemia-reperfusion injury has also been decribed in in vitro models.(24) Consistent with those results, knockout or silencing of the HIF-degrading PHD1 gene recently has been shown to attenuate IR injury.(25) Blocking the HIF1α target VEGF via a soluble VEGF antagonist reduced IR injury by inhibiting leukocyte migration and cytokine production.(26) More recent work describes a protective effect of HIF1α stabilization on hepatocyte apoptosis in ischemia reperfusion injury via an interaction between the Wnt signalling pathway and HIF, presenting data that suggests that a stabilizing interaction between beta-catenin and HIF1α promotes hepatocyte survival in ischemia/reperfusion injury.(8) Much of the work on HIFs in ischemia reperfusion injury relies upon HIF1α, and further work may clarify the role of other isoforms, such as HIF2α.
Hypoxia in Obstructive Sleep Apnea and Liver Disease
An association described between obstructive sleep apnea (OSA) and non-alcoholic fatty liver disease and/or NASH remains controversial.(27) Several studies have linked OSA, and in particular, the incidence of apneic-hypopneic episodes, to elevation of liver enzymes and the histologic appearance of NASH.(28, 29) A major confounding factor is the frequent comorbidity of obesity and/or the metabolic syndrome; however, one recent study suggested that even among obese patients, nocturnal oxygen desaturation contributed to insulin resistance and liver injury, including fibrosis, inflammation and ballooning necrosis, but not the appearance of steatosis.(30) A study of 83 patients with OSA and matched controls suggested that there was a relationship between OSA and progression of steatosis to steatohepatitis, based on serum levels of type III procollagen.(31) In a larger study of 218 patients with OSA, severe OSA (defined as greater than 50 Apneic/Hypopneic episodes/hour, AHI) was associated with an increased liver enzymes (OR 5.9, p<0.02). Patients with AHI greater than 50/hour were also much more likely to have steatosis, lobular necrosis, and fibrosis by liver biopsy.(32)
Several studies in mouse models have offered some data to corroborate these observations. In one study, chow-fed mice were exposed to either room air or 12 hours of room air and 12 hours of chronic, intermittent hypoxia (CIH, approximately 5% oxygen for periods of 30 seconds followed by 21% oxygen for periods of 30 seconds). After 12 weeks on the CIH regimen, mice developed increased serum ALT, serum triglycerides, and serum cholesterol, as well as increased NFκB DNA- binding activity in liver nuclear extracts.(33) In mice genetically predisposed to obesity, CIH increased liver triglycerides and phospholipids, as well genes of lipid biosynthesis, including SREBP1c, Acetyl-coenzyme A Carboxylase, and Steroyl-CoA Desaturases 1 and 2.(34) In a third study, WT mice were maintained on a high-fat diet and exposed to either room air (21% oxygen) or room air with period of intermittent hypoxia (as described above) for six months. At the conclusion of the study, CIH mice had a marked elevation in serum AST and ALT, some increase in inflammatory cytokines, and increased serum and liver malondialdehyde, myeloperoxidase, and alpha-1 collagen; however, liver triglycerides were unchanged, and only a mild enhancement of oil-red O staining was observed in CIH-treated mice.(35) The lack of an increase in steatosis compared to controls in perhaps not unexpected given that control group mice were also treated with 6 months of high-fat-diet feeding.
Acetaminophen Poisoning
The model that is emerging from these studies suggests that milder periods of hypoxia, such as moderate OSA, are insufficient by themselves to cause progression to hepatitis/steatohepatitis. However, when CIH is added to a primary insult, such as diet-induced steatosis, there is a predilection towards progression of liver injury. Corroborating evidence from other disease models includes the observation that sublethal acetaminophen poisoning resulted in fulminant liver failure when given in combination with CIH. (36) A recent study combined CIH with daily injections of low-dose acetaminophen (APAP, 200mg/kg) in mice for 4 weeks. At the end of the study period, CIH/APAP mice had markedly elevated liver enzymes, including serum AST, ALT, GGT, and total bilirubin whereas no elevation was observed in mice with APAP alone, and only AST increased in mice with CIH alone.(36)
Some evidence relates to the interaction between the HIF pathway and acetaminophen toxicity. HIF1α nuclear protein was observed to accumulate within 1 hour after a toxic dose (300mg/kg) of APAP; this increase in HIF1α was prevented by N-Acetylcysteine.(37) Pretreatment with Salidroside, an extract of an herbal compound used in Chinese medicine to ameliorate mountain sickness, was able to prevent ALT, AST, and serum TNFα in a mouse model of sublethal APAP toxicity as well as a decrease in HIF1α immunostaining compared to untreated controls.(38) It is unknown whether the role of HIF1α in APAP injury is protective or deleterious; for example, treatment of APAP-challenged mice with hyperbaric oxygen was able to improve survival, even though it increased HIF1 protein levels and the downstream target Glut1.(39) Recent work showed that deletion of HIF1α was protective against APAP toxicity at 6 but not 24 hours, suggesting that HIF1α may play more of a role in early, rather than late APAP toxicity. (40) In that study, a conditional knockout of HIF1α was generated through an inducible ubiquitin promoter, resulting in whole body deficiency of HIF1α. Mice were then exposed to acetaminophen, and mice with HIF deficiency appeared to have decreased liver damage at 6 hours, but not at 24 hours. The emerging picture suggests that HIF1α is a component of the cellular response to acetaminophen injury, but that the complexity of this metabolic insult involves other factors as it develops over time. It is possible that further research will identify therapies that can modify hypoxia inducible factor activity and thereby alter the progression of acetaminophen toxicity at particular points in the evolution of liver injury._
Alcohol Mediated Liver Injury
For several decades, it has been appreciated that alcohol creates alterations in hepatic portal blood flow and oxygen utilization within the liver.(41, 42) A seminal study in the field identified centrilobular injury as a consequence of hepatic oxygen demand in alcohol fed rats that were briefly exposed to low atmospheric oxygen tension, and were able to demonstrate protection against this effect with the co-administration of the thioamide anti hyperthyroid drug propylthiouracil.(41) The diverse effects of chronic ethanol on cellular signaling, cellular metabolism and organ physiology has been reviewed elsewhere. (43) The development of hypoxia in alcohol-exposed liver has also been reviewed elsewhere.(44) Exposure of thin liver slices to acute ethanol was reported to increase oxygen consumption over three decades ago.(45) Acute ethanol causes a rapid increase in liver metabolism, including rapid induction of alcohol detoxifying enzymes and hepatic oxygen consumption within 2–3 hours.(46) More recently, acute ethanol was found to result in increased areas of staining using the hypoxia-specific marker pimonidazole; this effect appeared to be at least partially dependent on functional hepatic Kupffer cells, and was also apparent in a model of chronic ethanol treatment.(47, 48)
Recent gene array data from ethanol-fed and pair-fed mice demonstrated upregulation of multiple genes in the glycolytic pathway, as well as genes in lipid metabolic pathways in the livers of chronically alcohol fed mice.(49) Although not explored in that publication, most, if not all of these genes may be regulated by HIF1α. An earlier report suggested an upregulation of HIF1α mRNA in the livers of chronic alcoholics.(50) One group offered some data to indicate that HIF1α mRNA is cyclically regulated with the urinary alcohol cycle in a model on continuous, intragastric ethanol feeding.(51) More recently, in the hypercholesterolemic ApoE(−/−) mouse, ethanol significantly increased HIF1α protein in liver, and a synergistic upregulation with tobacco smoke was observed.(52) Our own recent work has demonstrated that chronic alcohol administration increased HIF activation in the liver and that increased hepatiic steatosis in the setting of alcohol developed in a HIF-dependent manner.(53)
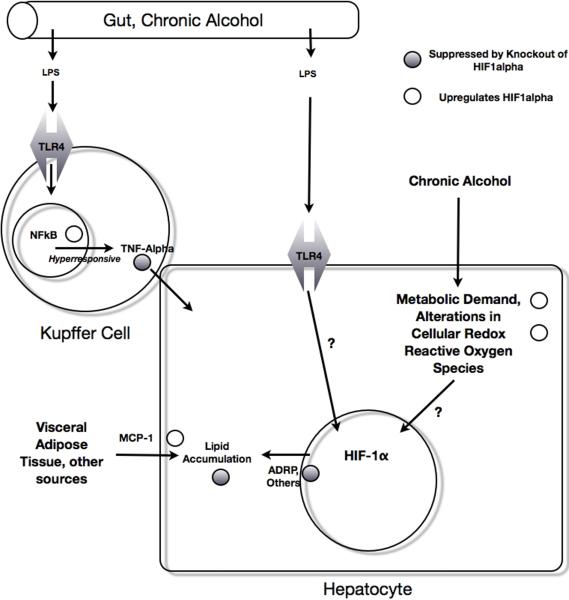
It has been well established that alcoholic liver disease proceeds in part through a combination of prolonged metabolic insult coupled with activation of signaling through innate immune mechanisms. Given the role of HIFs in innate immunity as described further below, it is quite possible that the contribution of HIFs to alcoholic liver disease proceeds both through pathways of innate immunity as well as through pathways in the hepatocyte, including hepatic lipid accumulation. A schematic illustrating the convergence of innate immune pathways with HIF1α in an experimental model of steatohepatitis is given in Figure 2.
Figure 2.
Pathways of HIF involvement in the Pathogenesis of Alcoholic Liver Disease Chronic alcohol causes increased gut permeability, which results in increased LPS entering the portal circulation. This stimulates TLR4 receptors in the hepatic macrophage, which in turn cause TNFα secretion via an NFκB, HIF1α dependent mechanism. LPS may also stimulate hepatic TLR4 to result in HIF1α activation (Nath, in submission.) Chronic alcohol increases results in an increase in HIF1α in whole liver, possibly mediated through the known effects of ethanol on increased metabolic demand, alteration in cellular redox state, and the creation of reactive oxygen species, all of which can increase HIF1α. HIF1α activation increases lipid accumulation. MCP-1 also increases lipid accumulation, and was shown to increase HIF1α in a hepatoma cell line in vitro.
Hypoxia inducible factors and immunity
In alcoholic liver disease (ALD) and non-alcoholic steatohepatitis (NASH), activation of Toll-like receptor 4 (TLR4) by gut-derived endotoxin has been demonstrated to contribute to disease pathogenesis. (54) Several reports indicate that activation of HIF1α plays a pivotal role downstream of LPS signaling through TLR4. LPS upregulated hepatic HIF1α in rats, as well as HIF1α target gene aldolase.(55) In macrophages, LPS stimulation upregulated HIF1α target genes, including VEGF, PAI-1, and iNOS, as well as HIF DNA binding and HIF1α mRNA and protein.(56) Using a cre-lox system of targeted HIF1α mutation to a transcriptionally inactive form, one group recently reported that knockdown of HIF1α transcriptional activity in cells of the myeloid lineage (LysMCre/HIFflox/flox mice) resulted in protection from LPS-induced sepsis. LysMCre/HIFflox/flox mice had lower levels of pro-inflammatory cytokines, including IL-6, IL-12, and TNFα, and maintained blood pressure and body temperature in the face of LPS challenge at levels that induced septic shock in wild-type mice.(57) Subsequent work indicated that LPS-induced HIF1α activity is dependent upon transcriptional regulation through the inflammatory master regulator group of proteins NFκB.(58) NFκB transcriptional activity is predominantly regulated through the inhibitory action of Inhibitor of κB proteins (IκB), which themselves are targeted for degradation by phosphorylation via the action of IκB Kinases (IKKα, IKKβ, the latter being the major isoform.) IKKβ deletion, then, renders cells unable to phosphorylate IκB and thereby inhibits NFκB signaling. Stimulation of bone-marrow-derived macrophages from mice in which IKKβ had been deleted by cre-lox mediated recombination (IKKβ-null mice) resulted in diminished expression of HIF1α target gene mRNAs. Additionally, HIF1α mRNA was suppressed in IKKβ-null mice prior to any stimulation, indicating that NFκB may regulate HIF1α at the transcriptional level.(59) Although a role for HIF1α activation in NASH has not been thoroughly investigated, pharmacological inhibition of IKK proteins, analogous to IKKβ-null strategies, was able to prevent steatosis and the development of NASH.(60)
These data suggest that the activation of the pro-inflammatory cascade downstream of LPS-TLR4 signaling may be at least partially dependent upon functional HIF1α signaling. In contrast, in other cell types, some data suggests that HIF1α may suppress T-cell mediated inflammation. HIF1α knockout in T-lymphocytes prevented sepsis and mortality after cecal ligation and puncture (CLP), and T-Cell specific HIF1α(−/−) mice had significantly lower levels of serum ALT 72 hours after CLP challenge than WT mice.(61) Knockout of HIF1α in T-and exvivo stimulation of T-cells from T-cell specific HIF1α (−/−) mice resulted in higher levels of IL2 and IFNg, suggesting that the survival benefit of T-Cell specific HIF1α knockout may be at least partially due to a derepression of HIF1α inhibition of pro-inflammatory cytokine release.(62)
Hypoxia Inducible Factors: A Common Mechanism of Lipid Accumulation?
Multiple lines of evidence suggest that hypoxia and/or HIF may play a role in hepatic lipid accumulation. Numerous studies have implicated a role for hypoxia in altering lipid storage in various cell types. Rats exposed to chronic hypoxia accumulated foam cells in pulmonary alveoli.(63) Hypoxia was described to cause lipid-loading of macrophages, and this effect was prevented by HIF1α siRNA treatment.(63, 64) The differentiation of 3T3-L1 preadipocytes to an adipocytic phenotype was found to be partially dependent on HIF2α, which is transcriptionally regulated in adipocytic differentiation.(65) Forced expression of HIF1α in cardiomyocytes resulted in increased lipid accumulation, and was correlated to a suppression of peroxisomeproliferator-alpha DNA binding.(66) A recent study in breast cancer cell lines demonstrated an increase in HIF1 expression downstream of Akt signaling resulting in an increase in fatty acid synthase (FAS) which is over-expressed in several types of solid tumors.(67)
In hepatocytes, germline deletion of HIF2α resulted in neonatal death and a phenotype of severe steatosis.(68) Although this study suggests that the absence of HIF2α predisposes to steatosis, numerous other studies in vitro and in vivo have suggested that this observation does not apply to the role of HIFs in the adult liver. Hepatocyte specific deletion of the VHL gene is accompanied by a phenotype of hypervascularity and steatosis.(69) Simultaneous introduction of degradation-resistant transgenic constructs of HIF1α and HIF2α resulted in a similar phenotype of hepatic lipid accumulation; in that study, introduction of degradation-resistant HIF1α alone caused a mild phenotype of lipid accumulation, and introduction of degradation-resistant HIF2α alone caused a phenotype of hypervascularity, including the formation of cavernous hemangioma, without lipid accumulation.(4) More recently, a different group described lipid accumulation in a murine model of liver-specific HIF2 activation.(70) In that study, mouse models with cre-lox mediated deletion of VHLH, HIF1α, and/or HIF2α resulted in mice in which both HIF1 and HIF2 or only one or the other isoform was active. HIF2 appeared to play a major role in regulating hepatic lipid by various mechanisms, including the upregulation of lipid biosynthetic pathways, the suppression of fatty acid β-oxidation, or upregulation of the lipid droplet surface protein ADFP.(70) Newer studies have further extended and verified the dominant role of hepatic HIF2α on regulating hepatic lipid accumulation.(71) Our own group has shown that while hepatocyte-specific disruption of HIF1α is able to decrease the upregulation of hepatic lipid that occurs with chronic ethanol administration, constitutively active HIF1, using the HIF1dPA model of hepatocyte-specific HIF1α, results in steatosis that is further exacerbated by chronic ethanol exposure. Using in vitro approaches, we also described an upregulation of HIF1α in cultured hepatocytes that were exposed to the chemokine MCP-1, suggesting a pathway between cytokine stimulation of hepatocytes and hepatic lipid accumulation.(53) Further work is needed to clarify the relative contributions of HIF1α and HIF21α to hepatocyte lipid accumulation.
HIFs and Metal Accumulation in Liver Disease
Iron accumulation has a role in the pathogenesis of several hepatic diseases, including alcoholic liver disease, and hereditary hemochromatosis. Macrophage iron increased the severity of alcoholic liver disease in a rodent model.(72) In conditions of chronic iron deficiency, iron export is limited by production of hepcidin, which in turn degrades the iron efflux protein ferroportin. Using a model of hepatocyte specific HIF1α deletion, Peysonnaux and colleagues demonstrated that functional HIF1α is partially responsible for the downregulation of hepcidin in chronic iron deficiency.(73) In support of this, endothelial-cell ARNT-knockout mice, which are completely defective in HIF signaling, accumulated high levels of iron.(74) Hypoxia Inducible factors have been implicated in gut iron absorption, where some recent data showed that deletion of HIF2α, but not HIF1α, in intestinal cells resulted in downregulation of serum iron and intestinal expression of the divalent metal ion transporter-1 (DMT1). (75) A similar effect of HIF1α expression on DMT1 was observed in vitro in HEPG2 cells.(76)
Liver Fibrosis
Recent evidence indicates a profound effect of HIF1α on cholestatic liver injury. Moon et al. recently described the effect of HIF1α deletion in bile-duct ligated mice, a model of cholestatic liver injury.(77) Mice with a floxed HIF1α exon were mated to Mx-Cre mice enabling near total excision of floxed genetic elements in cells of the immune system and the liver and partial deletion in other body tissues following serial injections of poly-I:C. Deletion of HIF1α was followed with bile duct ligation (BDL) or sham ligation. In WT mice, an increase of pimonidazole stained areas and accumulation of HIF1α was observed as early as 3 days following BDL, indicating hypoxia. Both HIF1αflox/MxCre and WT mice displayed similar increases in ALT, AST, and serum bile acids, but HIF1αflox/MxCre mice were protected from increases in collagen synthesis and alpha-smooth muscle actin staining, both markers for tissue fibrosis, as well as profibrotic mediators including PAI-1 and Platelet-Derived Growth Factor (PDGF) A and PDGF-B.(77) In a series of in vitro experiments, the same group reported that production of profibrotic mediators was induced by culturing mouse hepatocytes in 1% oxygen. Using an siRNA approach, the authors demonstrated that the production of profibrotic mediators was completely prevented in ARNT-null cells, but only partially prevented in HIF1α-null cells, suggesting that other HIF isoforms (particularly, HIF2) play a role.(78)
These data in support of a role for HIF in liver fibrosis are rendered more compelling by evidence in other models of liver fibrosis. After five weeks of once-weekly diethylnitrosamine (DEN) injections (100mg/kg), pronounced collagen septa may be observed, and progression to cirrhosis is observed by 8 weeks. In DEN-treated mice, Vascular Endothelial Growth Factor (VEGF) isoforms were increased with increasing time of treatment, becoming strongly positive by northern blot and immunohistochemical staining by 8 weeks of DEN treatment, and were correlated with increase in tissue hypoxia as observed by pimonidazole staining.(11)
In vitro models have been in concordance with the previous findings. Stellate cell activation has been described as an initiating factor in liver fibrosis, and stellate cells cultured under hypoxia had increased collagen mRNA transcripts. (11) Exposure of the hepatic stellate cell line LX-2 to hypoxia stimulated HIF1α and VEGF mRNA accumulation by 8 hours, and was associated with evidence of increased signaling through the TGFb-SMAD dependent pathway. Comparison of a gene array using LX-2 cells in normoxia and hypoxia revealed several targets, including fibroblast growth factor-4, that have been implicated in fibrogenesis or inflammation.(79, 80) Another study also reported activation of HSC by hypoxia, and demonstrated that this activation was accompanied by secretion of proangiogenic cytokines, such as VEGF and ANG-1, which were able to stimulate HSC chemotaxis in an autocrine or paracrine fashion. Isolated stellate cells from HIF1α (−/−) mice also demonstrated that genes involved in fibrosis, including angiogenic and collagen-deposition factors, were at least partially dependent upon functional HIF1α.(81)
More recent work has argued for a dominant role for HIF2α in regulating hepatic fibrogenesis in the setting of steatohepatitis. Simultaneous, hepatocyte-specific VHL and HIF1α or HIF2α mouse mutants were generated and assessed for a number of fibrotic markers. The authors described that when mice with disruption of VHL (e.g., with increased expression of HIF1α and HIF2α) were treated for two weeks with an ethanol containing diet, they developed increased fibrosis and increased expression of the fibrosis marker smooth muscle actin. However, when simultaneous deletion of HIF2α, but not HIF1α, was carried out, this increase was prevented.(71)
Viral Hepatitis
Several studies have illuminated the role of HIFs in the pathogenesis of viral hepatitis, including hepatitis B, hepatitis C, and hepatitis E. In a series of HCC cases secondary to primary HBV infection, Hepatitis B Virus X protein [HBx] was found to correlate with HIF1α expression, and transfection of HBx in HepG2 cells was found to increase HIF1α protein accumulation.(82) An earlier study similarly reported the stabilization of HIF1α protein in the presence of the HBx protein, and that this stability correlated with promoter activity of HIF1 at the Multi Drug Resistance-1 protein, an efflux drug transporter thought to be primarily responsible for chemoresistance in HCC. HIF1α siRNA treatment was able to abolish the activation of an MDR-1-luciferase construct induced by HBx transfection.(83) These findings were confirmed and extended in another study that reported that HBx protein increased levels of Metastasis Associated Protein 1 (MTA1) and Histone Deacetylase 1 (HDAC1). These two proteins in turn physically associated with HIF1α, and contributed to HIF1α stability.(84)
The hepatitis E virus (HEV) open reading frame protein 3 (ORF3) is a viral protein thought to be required for infection. In an in vitro system of hepatocyte cell lines expressing HEV ORF3, upregulation of several glycolytic pathway enzymes was reported, and correlated with increased expression and DNA-binding activity of HIF1α. This expression was correlated with increased Akt phosphorylation as well as increased phosphorylation of the CBP/p300 transcriptional co-activator via an ERK-dependent mechanism.(85)
Hepatitis C infection may interact with the HIF1α pathway via multiple mechanisms. Huh7 cells expressing the HCV core protein were reported to have increased VEGF expression and increased HIF1α DNA binding by EMSA; this binding was partially abrogated in the presence of PD98059, an ERK inhibitor.(86) Transient HCV infection in Huh7 cells was associated with HIF1α stabilization by 3 days; furthermore, in Huh7 cells expressing subgenomic HCV replicons, HIF1α was also stabilized. This stabilization again appeared to be dependent on multiple kinase and transcriptional pathways, as functional ERK and PI3K inhibition was able to prevent HIF1α protein accumulation, as was Stat3 inhibition and NFκB inhibition. HIF1α stability was accompanied by production of functional VEGF. (86) HIF stabilization by HCV was demonstrated to be insensitive to antioxidant treatment, and dependent upon derangement of mitochondrial respiration in HCV-infected cells.(87)
HIF in Liver Regeneration
HIF1α is rapidly induced in liver after partial hepatectomy, and remains upregulated for up to 24 hours.(12) Prolactin treatment was able to increase the proliferative response after partial hepatectomy, and was also able to upregulate HIF1α protein and VEGF.(88) However, in another study, hyperbaric oxygen pretreatment, which upregulates HIF1α protein, was unable to accelerate liver regeneration after partial hepatectomy; however, BRDU uptake, and indicator of cellular proliferation, was upregulated in hepatic sinusoidal endothelial cells.(89) More recent work has demonstrated that HIF1α deletion resulted in delayed recovery after partial hepatectomy, an effect that was attributed to decreased hepatic gluconeogenesis.(90)
Oncostatin M (OSM) is an IL-6-type cytokine secreted by leukocytes that has been described to have a role in liver regeneration, liver development, and angiogenesis.(91) A recent report offered data to demonstrate that OSM is able to up regulate HIF1α protein levels and HIF1α target genes, including PAI-1 and VEGF, in a Stat3 dependent mechanism. Further, the upregulation of HIF1 appeared to be at the transcriptional level.(91)
Hepatocellular Carcinoma
Significant evidence indicates that the HIFs play an important role in the pathogenesis and pathophysiology of hepatocellular carcinoma (79–90). HIF1α and VEGF were found to be expressed at higher levels in dysplastic nodules and implicated in malignant transformation.(92) This finding was confirmed in humans and extended by the description of HIF1 expression in chemically-induced preneoplastic lesions in mice.(93) Notably, this expression was independent of tissue hypoxia, as HIF1 positive areas did not differ from other regions of the liver in terms of needle-electrode measured oxygen tension and pimonidazole staining; however, HIF1 levels were effectively reduced by treatment with the PI3K inhibitor LY294002, raising the possibility of a PI3K-Akt dependent mechanism.(94) Recent data also suggest that inhibition of HIF may have a role in cancer therapy. Nonresectable hepatocellular carcinomas may be treated by transarterial catheter embolization (TAE) in which tumor vessels are occluded via catheter-guided placement of a coil or other occluding agent. Drawbacks of this approach include an uncertain survival benefit, as well as a possible induction of tumor neovascularization following TAE. Following the observation that neovascularization of embolized tumors proceeds with upregulation of VEGF, delivery of antisense oligonucleotides against HIF1α in combination with TAE was able to improve efficacy of TAE in promoting tumor necrosis and preventing neovascularization.(94) Furthermore, in that study, the ability of tumor cells to survive on glycolytic metabolism alone (the so-called Warburg effect) was inhibited through suppression of HIF1α glycolytic target genes, including the glucose transporter GLUT1 and Lactate Dehydrogenase A.(94)
The data from clinical studies paint a similar picture. In one series of cases, up to 50% of HBV-associated hepatocellular carcinomas expressed high levels of HIF1α, and HIF1α expression correlated with metastases and decreased survival.(82) Poor prognosis was also associated with expression of Metastasis Associated Protein-1 (MTA-1), which is a stabilizer of HIF1α.(95) Patients with MTA-1 positive cancer had larger tumors with increased incidence of microvessel invasion and nodal extension. The incidence of extrahepatic metastases was almost twofold higher (23% versus 12%, p<0.001) in patients with MTA positive lesions than in patients with MTA negative lesions. The prevalence of MTA-1 positive staining was higher in patients with HCC secondary to primary HBV infection than from other causes, including HCV infection or nonviral etiologies.(95)
Both HIF1α and HIF2α isoforms may be overexpressed in hepatocellular cancer. In one series, HIF2α expression was found to be present in 52% of HCC, and correlated with tumor size, capsule infiltration, portal vein invasion, and necrosis.(96) A subsequent larger study found HIF2α expression in 69.5% of HCC cases, as well as 55% of adjacent tissue, but no expression in noncancerous tissue, suggesting that HIF2α expression may be a preneoplastic feature of tumors or a feature of tumor-associated stroma. In that study, high HIF2α expression was also correlated with vascular endothelial growth factor expression, microvessel density, and decreased survival.(97)
Multiple studies have suggested that HIF1α is a prognostic factor for tumor recurrence in human and murine HCC.(98, 99) Some studies have shown that high expression of HIF1α in non-malignant liver tissue adjacent to resected HCC is a negative predictor of disease-free survival, and may correlate to upregulation of HIF1α dependent genes involved in cell migration and invasion.(100) Lastly, patients with HCC whose tumors had high levels of expression of p28(GANK) had a high risk of recurrence, metastasis, and high mortality; interestingly, high p28(GANK) expression correlated with higher levels of HIF1α.(101)
These observations imply that HIF1α inhibition may play a role in anticancer therapeutics. RNA-mediated inhibition of HIF1α was able to slow tumor growth.(102) The antitumor efficiency of doxorubicin was increased when combined with a HIF1α antisense oligonucleotide.(103) Rapamycin inhibits signaling by the Mammalian target of Rapamycin (mTOR) complex pathway, and has shown some efficacy against hepatocellular carcinoma. The prevention of HCC tumor growth by rapamycin in a rodent model was correlated to suppression of HIF1α by rapamycin.(104) Another compound, silibinin, was demonstrated to have some antitumor efficacy through phosphorylation of mTOR, and was also associated with suppression of HIF1α signaling.(105)
Summary
Hypoxia has been implicated in the pathogenesis of a broad range of hepatic disease. In most of these models, some data exists to implicate a role for hypoxia inducible factors. Consideration of the role of HIFs in liver diseases should include multiple cell types, as HIF1α activity has been implicated in hepatocytes as well as myeloid (Kupffer cells) and lymphoid (T-cells) lineage immune cells. Taken collectively, these findings strongly suggest that anti-HIF therapies promise useful interventions in the management of hepatic diseases of various etiologies.
List of abbreviations
- AHI
apneic/hypopneic episodes/hour
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- APAP
acetaminophen
- ARNT
aryl-hyrdocarbon-nuclear receptor translocator
- BDL
bile duct ligation
- CIH
chronic, intermittent hypoxia
- CLP
cecal ligation and puncture
- DEN
diethylnitrosamine
- DMT1
divalent metal ion transporter-1
- FAS
fatty acid synthase
- GGT
gamma-glutamyl transferase
- HBx
hepatitis B virus X protein
- HCC
hepatocellular carcinoma
- HDAC1
histone deacetylase 1
- HEV
hepatitis E virus
- HIF1
hypoxia inducible factor 1
- HIF1α
hypoxia inducible factor 1-α
- HIF2α
hypoxia inducible factor 2-α
- HIF3α
hypoxia inducible factor 3α
- HIFs
hypoxia inducible factors
- HREs
hypoxia-responsive elements
- HSC
hepatic stellate cells
- IKK
IκB Kinases
- iNOS
inducible nitric oxide synthase
- IR
ischemia reperfusion
- IκB
inhibitor of κB proteins
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MDR-1
multi-drug resistance 1
- MTA1
metastasis associated protein 1
- NASH
non-alcoholic steatohepatitis
- NFκB
nuclear factor kB
- ORF3
open reading frame protein 3
- OSA
obstructive sleep apnea
- OSM
oncostatin M
- PAI-1
plasminogen-activator-inhibitor-1
- PDGF
platelet-derived growth factor
- PGK1
phosphoglycerate kinase
- siRNA
small interference RNA
- SREBP-1c
sterol regulatory element binding protein 1-c
- TAE
transarterial catheter embolization
- TGFb-SMAD
transforming growth factor-beta
- TLR
toll-like receptor
- TNFα
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Bibliography
- 1.Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology (Baltimore, Md.) 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 2.Kietzmann T, Cornesse Y, Brechtel K, Modaressi S, Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. The Biochemical journal. 2001;354:531–537. doi: 10.1042/0264-6021:3540531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 4.Kim W, Safran M, Buckley M, Ebert B, Glickman J, Bosenberg M, Regan M, et al. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. The EMBO journal. 2006;25:4650. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimova EY, Kietzmann T. Hypoxia-inducible factors: post-translational crosstalk of signaling pathways. Methods Mol Biol. 647:215–236. doi: 10.1007/978-1-60761-738-9_13. [DOI] [PubMed] [Google Scholar]
- 6.Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37:4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 8.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology. 141:707–718. 718, e701–705. doi: 10.1053/j.gastro.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn YT, Chua MS, Whitlock JP, Jr., Shin YC, Song WH, Kim Y, Eom CY, et al. Rodent-specific hypoxia response elements enhance PAI-1 expression through HIF-1 or HIF-2 in mouse hepatoma cells. Int J Oncol. 37:1627–1638. doi: 10.3892/ijo_00000817. [DOI] [PubMed] [Google Scholar]
- 10.Yeligar SM, Machida K, Kalra VK. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem. 285:35359–35373. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology (Baltimore, Md.) 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 12.Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N. Expression of hypoxia inducible factor-1alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int. 2005;25:1002–1009. doi: 10.1111/j.1478-3231.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 13.Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, Dufour JF. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol. 2004;40:305–312. doi: 10.1016/j.jhep.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Peyssonnaux C, Zinkernagel A, Schuepbach R, Rankin E, Vaulont S, Haase V, Nizet V, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) The Journal of clinical investigation. 2007;117:1926. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105:4613–4619. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- 16.Roth U, Jungermann K, Kietzmann T. Modulation of glucokinase expression by hypoxia-inducible factor 1 and upstream stimulatory factor 2 in primary rat hepatocytes. Biol Chem. 2004;385:239–247. doi: 10.1515/BC.2004.018. [DOI] [PubMed] [Google Scholar]
- 17.Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, Schardt K, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- 18.Krones A, Jungermann K, Kietzmann T. Cross-talk between the signals hypoxia and glucose at the glucose response element of the L-type pyruvate kinase gene. Endocrinology. 2001;142:2707–2718. doi: 10.1210/endo.142.6.8200. [DOI] [PubMed] [Google Scholar]
- 19.Broughan TA, Naukam R, Tan C, Van De Wiele CJ, Refai H, Teague TK. Effects of hepatic zonal oxygen levels on hepatocyte stress responses. J Surg Res. 2008;145:150–160. doi: 10.1016/j.jss.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Cursio R, Miele C, Filippa N, Van Obberghen E, Gugenheim J. Liver HIF-1 Alpha Induction Precedes Apoptosis Following Normothermic Ischemia-Reperfusion in Rats. Transplantation proceedings. 2008;40:2042. doi: 10.1016/j.transproceed.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Tamagawa K, Horiuchi T, Uchinami M, Doi K, Yoshida M, Nakamura T, Sasaki H, et al. Hepatic ischemia-reperfusion increases vascular endothelial growth factor and cancer growth in rats. The Journal of surgical research. 2008;148:158–163. doi: 10.1016/j.jss.2007.12.787. [DOI] [PubMed] [Google Scholar]
- 22.Tacchini L, Radice L, Bernelli-Zazzera A. Differential activation of some transcription factors during rat liver ischemia, reperfusion, and heat shock. J Cell Physiol. 1999;180:255–262. doi: 10.1002/(SICI)1097-4652(199908)180:2<255::AID-JCP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Tacchini L, Fusar Poli D, Bernelli-Zazzera A, Cairo G. Transferrin receptor gene expression and transferrin-bound iron uptake are increased during postischemic rat liver reperfusion. Hepatology. 2002;36:103–111. doi: 10.1053/jhep.2002.33997. [DOI] [PubMed] [Google Scholar]
- 24.Alchera E, Tacchini L, Imarisio C, Dal Ponte C, De Ponti C, Gammella E, Cairo G, et al. Adenosine-dependent activation of hypoxia-inducible factor-1 induces late preconditioning in liver cells. Hepatology (Baltimore, Md.) 2008;48:230. doi: 10.1002/hep.22249. [DOI] [PubMed] [Google Scholar]
- 25.Schneider M, Van Geyte K, Fraisl P, Kiss J, Aragones J, Mazzone M, Mairbaurl H, et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology. 138:1143–1154. e1141–1142. doi: 10.1053/j.gastro.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006;168:695–705. doi: 10.2353/ajpath.2006.050759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoppin AG, Katz ES, Kaplan LM, Lauwers GY. Case records of the Massachusetts General Hospital. Case 31-2006. A 15-year-old girl with severe obesity. N Engl J Med. 2006;355:1593–1602. doi: 10.1056/NEJMcpc069023. [DOI] [PubMed] [Google Scholar]
- 28.Mishra P, Nugent C, Afendy A, Bai C, Bhatia P, Afendy M, Fang Y, et al. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28:1080–1086. doi: 10.1111/j.1478-3231.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 29.Jouet P, Sabate JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, et al. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17:478–485. doi: 10.1007/s11695-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 30.Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatsumi K, Saibara T. Effects of obstructive sleep apnea syndrome on hepatic steatosis and nonalcoholic steatohepatitis. Hepatol Res. 2005;33:100–104. doi: 10.1016/j.hepres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E, Lebeau B, et al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290–1296. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- 33.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, et al. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007–1013. doi: 10.1002/hep.21593. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, et al. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643–1648. doi: 10.1152/japplphysiol.00522.2005. [DOI] [PubMed] [Google Scholar]
- 35.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293:G871–877. doi: 10.1152/ajpgi.00145.2007. [DOI] [PubMed] [Google Scholar]
- 36.Savransky V, Reinke C, Jun J, Bevans-Fonti S, Nanayakkara A, Li J, Myers AC, et al. Chronic intermittent hypoxia and acetaminophen induce synergistic liver injury in mice. Exp Physiol. 2009;94:228–239. doi: 10.1113/expphysiol.2008.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James LP, Donahower B, Burke AS, McCullough S, Hinson JA. Induction of the nuclear factor HIF-1alpha in acetaminophen toxicity: evidence for oxidative stress. Biochem Biophys Res Commun. 2006;343:171–176. doi: 10.1016/j.bbrc.2006.02.143. [DOI] [PubMed] [Google Scholar]
- 38.Wu YL, Piao DM, Han XH, Nan JX. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol Pharm Bull. 2008;31:1523–1529. doi: 10.1248/bpb.31.1523. [DOI] [PubMed] [Google Scholar]
- 39.Salhanick SD, Belikoff B, Orlow D, Holt D, Reenstra W, Buras JA. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF-1alpha expression. Acad Emerg Med. 2006;13:707–714. doi: 10.1197/j.aem.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Sparkenbaugh EM, Saini Y, Greenwood KK, Lapres JJ, Luyendyk JP, Copple BL, Maddox JF, et al. The Role of Hypoxia Inducible Factor-1 alpha (HIF-1{alpha}) in Acetaminophen Hepatotoxicity. J Pharmacol Exp Ther. doi: 10.1124/jpet.111.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Israel Y, Kalant H, Orrego H, Khanna JM, Videla L, Phillips JM. Experimental alcohol-induced hepatic necrosis: suppression by propylthiouracil. Proc Natl Acad Sci U S A. 1975;72:1137–1141. doi: 10.1073/pnas.72.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orrego H, Carmichael FJ, Saldivia V, Giles HG, Sandrin S, Israel Y. Ethanol-induced increase in portal blood flow: role of adenosine. Am J Physiol. 1988;254:G495–501. doi: 10.1152/ajpgi.1988.254.4.G495. [DOI] [PubMed] [Google Scholar]
- 43.Nath B, Szabo G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin Liver Dis. 2009;29:166–177. doi: 10.1055/s-0029-1214372. [DOI] [PubMed] [Google Scholar]
- 44.French SW. The role of hypoxia in the pathogenesis of alcoholic liver disease. Hepatol Res. 2004;29:69–74. doi: 10.1016/j.hepres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Videla L, Bernstein J, Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973;134:507–514. doi: 10.1042/bj1340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuki T, Thurman RG. The swift increase in alcohol metabolism. Time course for the increase in hepatic oxygen uptake and the involvement of glycolysis. Biochem J. 1980;186:119–126. doi: 10.1042/bj1860119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am J Physiol. 1996;271:G494–500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- 48.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–926. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 49.Yin HQ, Je YT, Kim M, Kim JH, Kong G, Kang KS, Kim HL, et al. Analysis of hepatic gene expression during fatty liver change due to chronic ethanol administration in mice. Toxicol Appl Pharmacol. 2009;235:312–320. doi: 10.1016/j.taap.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Chen SH, Zhang Y, Yu CH, Li SD, Li YM. Is the hypoxia-inducible factor-1 alpha mRNA expression activated by ethanol-induced injury, the mechanism underlying alcoholic liver disease? Hepatobiliary Pancreat Dis Int. 2006;5:560–563. [PubMed] [Google Scholar]
- 51.Li J, French B, Wu Y, Vanketesh R, Montgomery R, Bardag-Gorce F, Kitto J, et al. Liver hypoxia and lack of recovery after reperfusion at high blood alcohol levels in the intragastric feeding model of alcohol liver disease. Experimental and molecular pathology. 2004;77:184–192. doi: 10.1016/j.yexmp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Bailey SM, Mantena SK, Millender-Swain T, Cakir Y, Jhala NC, Chhieng D, Pinkerton KE, et al. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE−/−mouse: Implications for a “multi-hit” hypothesis of fatty liver disease. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 55.Scharte M, Han X, Uchiyama T, Tawadrous Z, Delude R, Fink M. LPS increases hepatic HIF-1alpha protein and expression of the HIF-1-dependent gene aldolase A in rats. The Journal of surgical research. 2006;135:262–267. doi: 10.1016/j.jss.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 56.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 57.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 58.van Uden P, Kenneth N, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. The Biochemical journal. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beraza N, Malato Y, Vander Borght S, Liedtke C, Wasmuth H, Dreano M, de Vos R, et al. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut. 2008;57:655. doi: 10.1136/gut.2007.134288. [DOI] [PubMed] [Google Scholar]
- 61.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, Ohta A, et al. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE. 2007;2:e853. doi: 10.1371/journal.pone.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolman M, Cervos-Navarro J, Sampaolo S, Cardesa A. Pathological changes in organs of rats chronically exposed to hypoxia. Development of pulmonary lipidosis. Histol Histopathol. 1993;8:247–255. [PubMed] [Google Scholar]
- 63.Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, Stahlman M, et al. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol. 2006;26:1871–1876. doi: 10.1161/01.ATV.0000229665.78997.0b. [DOI] [PubMed] [Google Scholar]
- 64.Jiang G, Li T, Qiu Y, Rui Y, Chen W, Lou Y. RNA interference for HIF-1alpha inhibits foam cells formation in vitro. Eur J Pharmacol. 2007;562:183–190. doi: 10.1016/j.ejphar.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 65.Wada T, Shimba S, Tezuka M. Transcriptional regulation of the hypoxia inducible factor-2alpha (HIF-2alpha) gene during adipose differentiation in 3T3-L1 cells. Biological & pharmaceutical bulletin. 2006;29:49–54. doi: 10.1248/bpb.29.49. [DOI] [PubMed] [Google Scholar]
- 66.Belanger A, Luo Z, Vincent K, Akita G, Cheng S, Gregory R, Jiang C. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochemical and biophysical research communications. 2007;364:567–572. doi: 10.1016/j.bbrc.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 67.Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 68.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 69.Park S, Haase V, Johnson R. von Hippel Lindau tumor suppressor regulates hepatic glucose metabolism by controlling expression of glucose transporter 2 and glucose 6-phosphatase. International journal of oncology. 2007;30:341–348. [PubMed] [Google Scholar]
- 70.Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. HIF-2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009 doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, et al. Hypoxia-inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 54:472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiong S, She H, Zhang AS, Wang J, Mkrtchyan H, Dynnyk A, Gordeuk VR, et al. Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G512–521. doi: 10.1152/ajpgi.90327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yim SH, Shah Y, Tomita S, Morris HD, Gavrilova O, Lambert G, Ward JM, et al. Disruption of the Arnt gene in endothelial cells causes hepatic vascular defects and partial embryonic lethality in mice. Hepatology. 2006;44:550–560. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Lai Z, Ya K, Fang D, Ho YW, Lei Y, Ming QZ. Correlation between the expression of divalent metal transporter 1 and the content of hypoxia-inducible factor-1 in hypoxic HepG2 cells. J Cell Mol Med. 2008;12:569–579. doi: 10.1111/j.1582-4934.2007.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moon J, Welch T, Gonzalez F, Copple B. Reduced liver fibrosis in hypoxia-inducible factor-1{alpha}-deficient mice. American journal of physiology. Gastrointestinal and liver physiology. 2009;296:G582–592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Copple BL, Bai S, Moon JO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic Kupffer cells. Hepatol Res. 40:530–539. doi: 10.1111/j.1872-034X.2010.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novo E, Cannito S, Zamara E, ValfrË di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. The American journal of pathology. 2007;170:1942–1953. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi YF, Fong CC, Zhang Q, Cheung PY, Tzang CH, Wu RS, Yang M. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007;581:203–210. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 81.Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1alpha regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 31:230–244. doi: 10.1111/j.1478-3231.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie H, Song J, Liu K, Ji H, Shen H, Hu S, Yang G, et al. The expression of hypoxia-inducible factor-1alpha in hepatitis B virus-related hepatocellular carcinoma: correlation with patients' prognosis and hepatitis B virus x protein. Digestive diseases and sciences. 2008;53:3225–3233. doi: 10.1007/s10620-008-0296-9. [DOI] [PubMed] [Google Scholar]
- 83.Han HK, Han CY, Cheon EP, Lee J, Kang KW. Role of hypoxia-inducible factor-alpha in hepatitis-B-virus X protein-mediated MDR1 activation. Biochem Biophys Res Commun. 2007;357:567–573. doi: 10.1016/j.bbrc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Yoo YG, Na TY, Seo HW, Seong JK, Park CK, Shin YK, Lee MO. Hepatitis B virus X protein induces the expression of MTA1 and HDAC1, which enhances hypoxia signaling in hepatocellular carcinoma cells. Oncogene. 2008;27:3405–3413. doi: 10.1038/sj.onc.1211000. [DOI] [PubMed] [Google Scholar]
- 85.Moin SM, Chandra V, Arya R, Jameel S. The hepatitis E virus ORF3 protein stabilizes HIF-1alpha and enhances HIF-1-mediated transcriptional activity through p300/CBP. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01340.x. [DOI] [PubMed] [Google Scholar]
- 86.Nasimuzzaman M, Waris G, Mikolon D, Stupack D, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. Journal of virology. 2007;81:10249–10257. doi: 10.1128/JVI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Ripoli M, D'Aprile A, Quarato G, Sarasin-Filipowicz M, Gouttenoire J, Scrima R, Cela O, et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J Virol. 84:647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olazabal IM, Munoz JA, Rodriguez-Navas C, Alvarez L, Delgado-Baeza E, Garcia-Ruiz JP. Prolactin's role in the early stages of liver regeneration in rats. J Cell Physiol. 2009;219:626–633. doi: 10.1002/jcp.21707. [DOI] [PubMed] [Google Scholar]
- 89.Ren P, Kang Z, Gu G, Liu Y, Xu W, Tao H, Zhang JH, et al. Hyperbaric oxygen preconditioning promotes angiogenesis in rat liver after partial hepatectomy. Life Sci. 2008;83:236–241. doi: 10.1016/j.lfs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Tajima T, Goda N, Fujiki N, Hishiki T, Nishiyama Y, Senoo-Matsuda N, Shimazu M, et al. HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun. 2009;387:789–794. doi: 10.1016/j.bbrc.2009.07.115. [DOI] [PubMed] [Google Scholar]
- 91.Vollmer S, Kappler V, Kaczor J, Flugel D, Rolvering C, Kato N, Kietzmann T, et al. Hypoxia-inducible factor 1alpha is up-regulated by oncostatin M and participates in oncostatin M signaling. Hepatology. 2009;50:253–260. doi: 10.1002/hep.22928. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura K, Zen Y, Sato Y, Kozaka K, Matsui O, Harada K, Nakanuma Y. Vascular endothelial growth factor, its receptor Flk-1, and hypoxia inducible factor-1alpha are involved in malignant transformation in dysplastic nodules of the liver. Human pathology. 2007;38:1532–1546. doi: 10.1016/j.humpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka H, Yamamoto M, Hashimoto N, Miyakoshi M, Tamakawa S, Yoshie M, Tokusashi Y, et al. Hypoxia-independent overexpression of hypoxia-inducible factor 1alpha as an early change in mouse hepatocarcinogenesis. Cancer research. 2006;66:11263–11270. doi: 10.1158/0008-5472.CAN-06-1699. [DOI] [PubMed] [Google Scholar]
- 94.Sun X, Jiang H, Jiang X, Tan H, Meng Q, Sun B, Xu R, et al. Antisense hypoxiainducible factor-1alpha augments transcatheter arterial embolization in the treatment of hepatocellular carcinomas in rats. Human gene therapy. 2009;20:314–324. doi: 10.1089/hum.2008.164. [DOI] [PubMed] [Google Scholar]
- 95.Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, Seo DD, et al. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929–936. doi: 10.1002/hep.22124. [DOI] [PubMed] [Google Scholar]
- 96.Bangoura G, Yang L, Huang G, Wang W. Expression of HIF-2alpha/EPAS1 in hepatocellular carcinoma. World journal of gastroenterology : WJG. 2004;10:525–530. doi: 10.3748/wjg.v10.i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bangoura G, Liu Z, Qian Q, Jiang C, Yang G, Jing S. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World journal of gastroenterology : WJG. 2007;13:3176–3182. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dai CX, Gao Q, Qiu SJ, Ju MJ, Cai MY, Xu YF, Zhou J, et al. Hypoxia-inducible factor-1 alpha, in association with inflammation, angiogenesis and MYC, is a critical prognostic factor in patients with HCC after surgery. BMC Cancer. 2009;9:418. doi: 10.1186/1471-2407-9-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daskalow K, Rohwer N, Raskopf E, Dupuy E, Kuhl A, Loddenkemper C, Wiedenmann B, et al. Role of hypoxia-inducible transcription factor 1alpha for progression and chemosensitivity of murine hepatocellular carcinoma. J Mol Med. 88:817–827. doi: 10.1007/s00109-010-0623-4. [DOI] [PubMed] [Google Scholar]
- 100.Simon F, Bockhorn M, Praha C, Baba HA, Broelsch CE, Frilling A, Weber F. Deregulation of HIF1-alpha and hypoxia-regulated pathways in hepatocellular carcinoma and corresponding non-malignant liver tissue--influence of a modulated host stroma on the prognosis of HCC. Langenbecks Arch Surg. 395:395–405. doi: 10.1007/s00423-009-0590-9. [DOI] [PubMed] [Google Scholar]
- 101.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, Ren YB, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology. 53:181–192. doi: 10.1002/hep.24015. [DOI] [PubMed] [Google Scholar]
- 102.Greenberger L, Horak I, Filpula D, Sapra P, Westergaard M, Frydenlund H, AlbÊk C, et al. A RNA antagonist of hypoxia-inducible factor-1{alpha}, EZN-2968, inhibits tumor cell growth. Molecular cancer therapeutics. 2008 doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 103.Liu F, Wang P, Jiang X, Tan G, Qiao H, Jiang H, Krissansen G, et al. Antisense hypoxia-inducible factor 1alpha gene therapy enhances the therapeutic efficacy of doxorubicin to combat hepatocellular carcinoma. Cancer science. 2008;99:2055–2061. doi: 10.1111/j.1349-7006.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang W, Jia W, Xu G, Wang Z, Li J, Ma J, Ge Y, et al. Antitumoral Activity of Rapamycin Mediated Through Inhibition of HIF-1alpha and VEGF in Hepatocellular Carcinoma. Digestive diseases and sciences. 2008 doi: 10.1007/s10620-008-0605-3. [DOI] [PubMed] [Google Scholar]
- 105.GarcÌa-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2008 doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]