Abstract
Dorsal root injury results in substantial and often irreversible loss of sensory functions as a result of the limited regenerative capacity of sensory axons and the inhibitory barriers that prevent both axonal entry into and regeneration in the spinal cord. Here, we describe previously unknown effects of the growth factor artemin after crush injury of the dorsal spinal nerve roots in rats. Artemin not only promoted re-entry of multiple classes of sensory fibers into the spinal cord and re-establishment of synaptic function and simple behavior, but it also, surprisingly, promoted the recovery of complex behavior. These effects occurred after a 2-week schedule of intermittent, systemic administration of artemin and persisted for at least 6 months following treatment, suggesting a substantial translational advantage. Systemic artemin administration produced essentially complete and persistent restoration of nociceptive and sensorimotor functions, and could represent a promising therapy that may effectively promote sensory neuronal regeneration and functional recovery after injury.
Traumatic injury to the spinal dorsal roots often results in permanent sensory deficits1,2. Injured peripheral axons fail to enter the spinal cord at the dorsal root entry zone (DREZ) because of inhibitory barriers and an apparently limited regenerative capacity2–6. Oligodendrocytes, astrocytes, microglia and macrophages of the CNS produce growth inhibitory proteins, including Nogo, myelin-associated glycoprotein, and chondroitin sulfate proteoglycans3,4,7,8, that can alter the cytoarchitecture of regenerating peripheral axons and can cause growth cone collapse and cessation of growth3,7,9. Strategies aimed at altering the hostile central environment to permit axonal regrowth have shown some success. Increasing the levels of neurotrophic factors (for example, neurotrophin-3, nerve growth factor or glial cell line–derived neurotrophic factor, GDNF) by endogenous or exogenous means results in penetration of the DREZ by peripheral axons regenerating locally into the spinal cord3,10,11 and limited restoration of nociceptive and sensorimotor functions11. To date, however, the extent of restoration of sensory functions by growth factors has been incomplete, and growth factors have not promoted recovery of more complex behaviors (for example, touch-evoked grasping). To promote sensory axonal regrowth in the spinal cord, the growth factors have been administered by intrathecal infusion8,10–12 or via viral vectors13,14. These techniques present a considerably greater challenge for translation into clinical practice than that presented by systemic administration15. Moreover, clinical trials show that nerve growth factor and GDNF produce substantial side effects, including pain, weight loss, bowel urgency and paraesthesias15,16, reflecting the relatively broad distribution of their receptors.
Artemin signals through the GDNF family receptor GFRα3, which complexes with ret proto-oncogene (RET), a tyrosine kinase receptor. GFRα3 binds artemin selectively and its expression is highly restricted to sensory neurons17–23. Our previous studies demonstrated that artemin reversed multiple behavioral and neurochemical features of chronic pain in rats with peripheral nerve injury, but only while systemic administration continued24. Here, we found that intermittent artemin, given over a period of 2 weeks, produced an apparently complete functional restoration of nociceptive and sensorimotor functions that persisted for at least 6 months. Notably, the regenerative effects of artemin occurred after systemic administration and occurred even after a 2-d delay from the time of injury; together with the highly restricted anatomical distribution of GFRα3, this route of administration offers substantial advantages over the intrathecal route or cellular delivery for potential clinical translation.
RESULTS
Artemin promotes axonal regeneration into the spinal cord
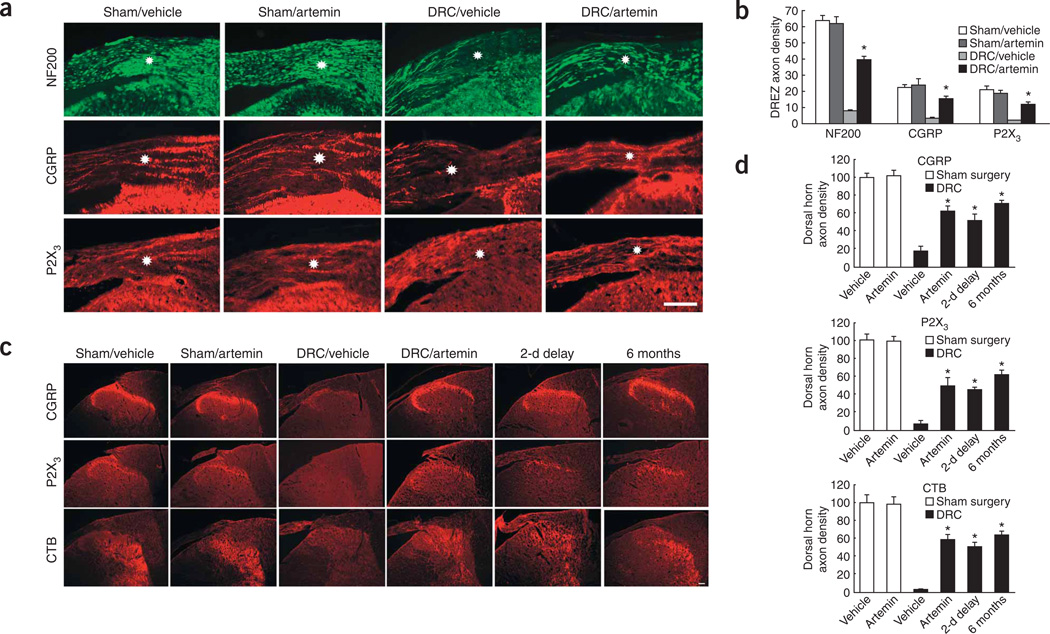
We used neurofilament 200 (NF200), calcitonin gene–related peptide (CGRP) and purinergic receptor P2X 3 (P2X3) immunolabeling to visualize myelinated, unmyelinated peptidergic and unmyelinated ‘peptide-poor’ fibers, respectively10. Together, these markers label nearly all dorsal root ganglion (DRG) neurons25–27. In addition, to trace the spinal-cord termination patterns of all regenerated fibers, we injected cholera toxin B (CTB) into the median nerve of the brachial plexus 5–7 d before the rats were killed. Artemin (1 mg per kg of body weight, subcutaneous injection) given on a Monday, Wednesday and Friday schedule for 2 consecutive weeks (the dosing schedule used for all experiments) starting either on the day of dorsal root crush (DRC) injury of the brachial plexus or beginning 2 d after the injury, promoted the regrowth of both myelinated and unmyelinated axons through the DREZ (Fig. 1). Sections from sham-operated animals showed uninterrupted immunofluorescence labeling for NF200, CGRP and P2X3 in axons from the periphery through the DREZ. Labeling for these markers terminated abruptly at the DREZ in the vehicle-treated DRC injury rats (Fig. 1c). In contrast, spinal cord sections from artemin-treated rats with DRC injury showed that the immunohistochemical markers for CGRP, P2X3, CTB and NF200 were found on the CNS side of the DREZ (Fig. 1a,c). Artemin normalized DRC-induced reductions in immunolabeled axon densities10 by approximately two-thirds (Fig. 1b,d). Moreover, in artemin DRC tissues, immunofluorescence for CGRP and P2X3 was found principally in the outer laminae of the dorsal horn, whereas that for CTB was distributed throughout the outer and intermediate laminae, corresponding to the normal termination patterns of these fibers28 (Fig. 1a,c). Artemin had no detectable effects in sham-operated animals (Fig. 1a–d).
Figure 1.
Systemic artemin administration promotes axonal growth across the DREZ into the spinal cord. (a) Immunofluorescence labeling of peripheral axons in the DREZ with NF200, CGRP and P2X3 2 weeks after sham injury or DRC injury and immediate treatment with six subcutaneous injections of artemin or vehicle. Scale bar represents 100 µm. (b) Quantification of axonal density in the DREZ in a constant area. * indicates a significantly (P < 0.001) greater axonal density in rats with DRC and artemin treatment compared with the same region from rats with DRC and vehicle treatment. (c) Immunofluorescence labeling for CGRP, P2X3 and CTB in the dorsal horn of the spinal cord after sham or DRC injury and treatment with six subcutaneous injections of artemin or vehicle, immediately or 2 d after injury. All images were taken 2 weeks after surgery, except those in the far right column, which were taken 6 months after surgery. Scale bar represents 200 µm. (d) Quantification of axonal density in the dorsal horn of the spinal cord in a constant area. * indicates a significantly (P < 0.001) greater axonal density in rats with DRC and artemin treatment compared with the same region from rats with DRC and vehicle treatment. Error bars, s.e.m.
Systemic artemin restores nociceptive functions
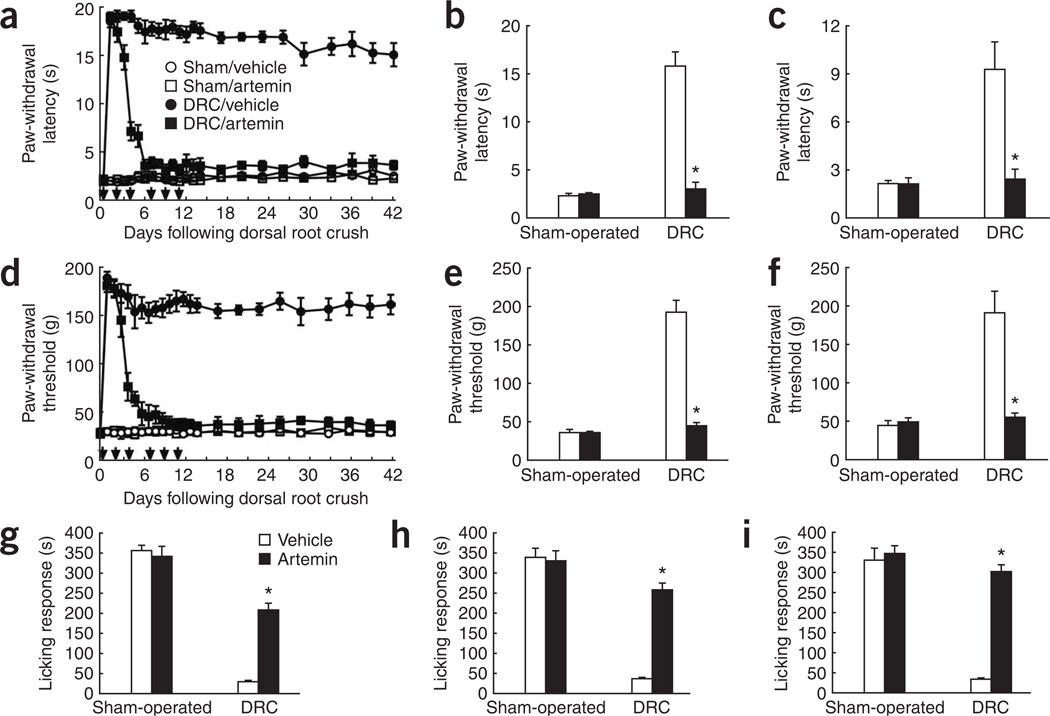
We recorded withdrawal responses of the ipsilateral forepaw from a 49 °C water bath or noxious pressure and licking of the forelimb in response to formalin injection after DRC injury or sham surgery. Animals with DRC injury that were treated with vehicle showed marked insensitivity to both noxious heat or pressure throughout the 6-week evaluation and at 6 months after DRC injury (Fig. 2). Artemin treatment caused a rapid, progressive recovery of thermal and mechanical thresholds in DRC injury rats. Nocifensive responses were present in 4 d and approached normal levels in 7 d of DRC (Fig. 2a,d). Termination of artemin treatment on day 11 did not affect the restoration of nociceptive responses, which remained virtually normal over the entire 42-d observation period (Fig. 2a,d) and 6 months later (Fig. 2c,f). Artemin did not alter the response thresholds in sham-operated groups at any time point (Fig. 2a–f). Artemin injection into the paw has been reported to produce thermal hyperalgesia resolving in 4 h29. Here, artemin did not alter the behavioral thresholds determined in sham-operated rats at either 24 h after subcutaneous injection or at any time studied. Formalin injection produced stereotypic licking behaviors that were abolished in animals with DRC; this behavior was restored by artemin (Fig. 2g–i) and improved responses were still present 6 months after DRC (Fig. 2i). Delaying initiation of artemin treatment by 2 d after DRC restored behavioral responses to noxious stimuli at 14 d (Fig. 2b,e,h) to an extent similar to that observed with immediate artemin treatment (Fig. 2a,d,g). In addition, DRC rats lost nociceptive responses to placing the forepaw in ice water (0 °C) (Supplementary Fig. 1 online), and these nocifensive responses were returned to normal baseline values after DRC by artemin treatment (Supplementary Fig. 1). The behavioral responses of the vehicle-treated and artemin-treated sham groups were not different from each other.
Figure 2.
Systemic artemin administration restores nociceptive responses. (a–i) Response to noxious thermal stimuli (a–c), noxious mechanical stimuli (d–f) or formalin injection into the forepaw (g–i) after sham or DRC injury and treatment with six subcutaneous injections of artemin or vehicle. Artemin or vehicle were administered immediately (a,c,d,f,g,i) or 2 d after injury (b,e,h). Responses are shown over a 6-week period following injury (a,d), at 14 days (b,e,g,h) or at 6 months (c,f,i) following injury. * indicates a significantly (P < 0.0001) restored response compared with DRC and vehicle treatment. Error bars, s.e.m.
Systemic artemin restores synaptic function
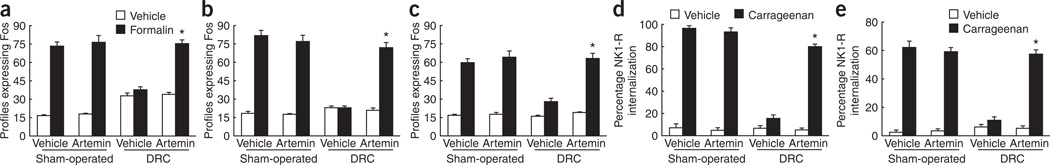
Noxious stimulus–induced expression of the proto-oncogene product Fos in the spinal dorsal horn is indicative of neuronal excitation of postsynaptic cells30–32. Forepaw formalin injection increased the numbers of Fos-positive spinal-cord cell profiles (Fig. 3a–c). DRC injury with vehicle treatment abolished the evoked spinal-cord expression of Fos 14 d after DRC (Fig. 3a,b and Supplementary Fig. 2 online). In contrast, artemin treatment either immediately or 2 d after DRC significantly improved formalin-induced Fos expression both 14 d and 6 months after root injury (P < 0.0001; Fig. 3a–c). Initiation of artemin treatment 2 d after DRC produced a similar improvement after 14 d (Fig. 3b).
Figure 3.
Quantification in the ipsilateral dorsal horn of formalin-induced Fos expression and evoked NK1-R internalization after carrageenan-induced inflammation. (a–c) Formalin-induced Fos expression. (d,e) NK1-R internalization evoked by noxious mechanical pinch (d) or light brush (e) after carrageenan-induced inflammation. Artemin or vehicle was administered immediately (a,c–e) or 2 d after injury (b). Responses are shown at 14 d (a,b,d,e) or 6 months (c) following injury. * indicates a significantly (P < 0.0001) restored response compared with DRC and vehicle treatment. Error bars, s.e.m.
Evoked internalization of the NK1 receptor (NK1-R) in the spinal dorsal horn by innocuous tactile or noxious mechanical stimuli in injured animals is indicative of postsynaptic responsiveness of dorsal horn neurons to substance P released from primary afferent fibers33. Noxious pinch elicited the internalization of NK1-R in NK1-R–positive dorsal horn profiles (Fig. 3d and Supplementary Figs. 3 and 4 online), and light brush following carrageenan injection caused internalization in most NK1-R–positive profiles (Fig. 3e) in the outer lamina of the sham-operated animals. Artemin treatment did not alter these responses in sham-operated animals (Fig. 3d,e). DRC injury reduced the pinch- and touch-evoked internalization, but the NK1-R–mediated response was restored following artemin treatment (Fig. 3d,e).
Artemin restores synaptic inputs from regenerated axons
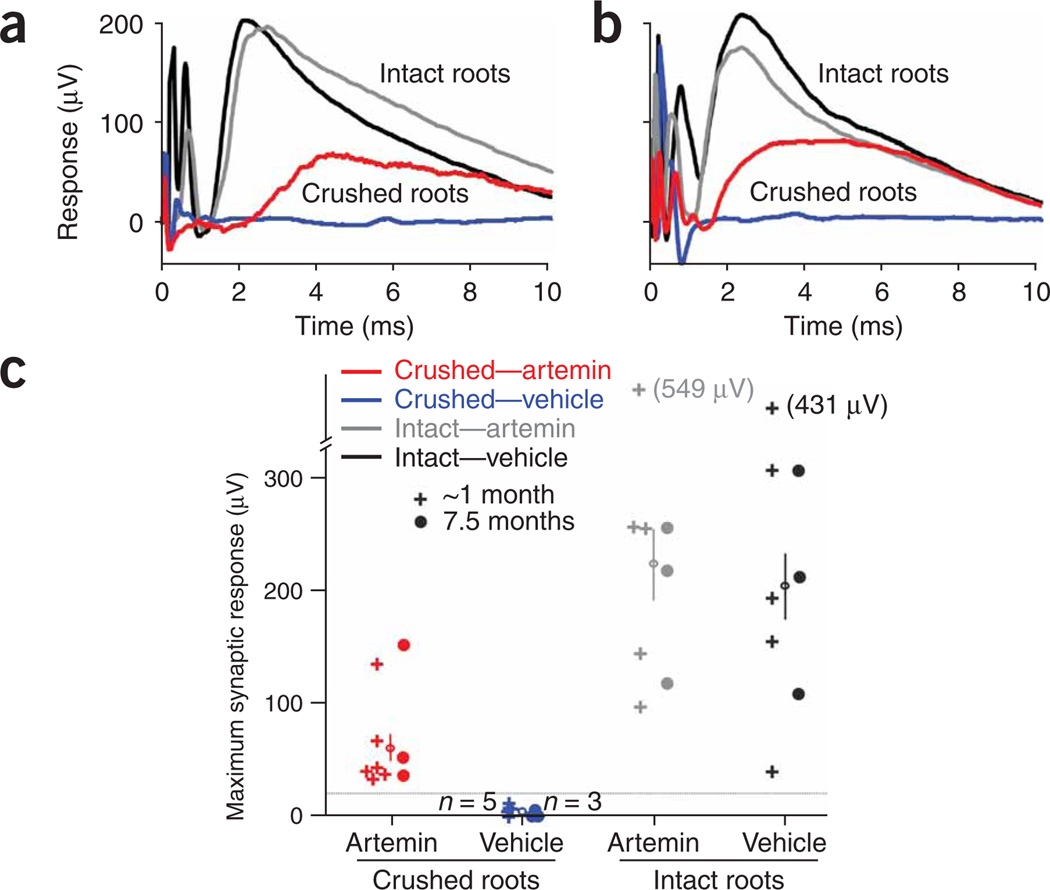
We assessed the recovery of synaptic function by regenerated sensory axons by taking extra-cellular recordings from the spinal cord in response to stimulation of the radial or median nerves. Typical responses mediated by unlesioned sensory axons had latencies and rise times of 1.0–1.5 ms and average peak amplitudes of 200–250 µV (Fig. 4a,b). These potentials represent the monosynaptic excitatory postsynaptic potentials (EPSPs) in spinal neurons that were evoked by activity in large-caliber myelinated sensory axons, and their amplitudes on the unlesioned side in artemin- and vehicle-treated animals were similar. The EPSPs were abolished by DRC and vehicle treatment in all of the animals tested (Fig. 4c). In contrast, all of the animals treated with systemic artemin showed clear recovery of synaptic function after DRC (Fig. 4c), with average EPSP amplitudes of ~60 µV, which is about one-third of the normal size. At 1 month post-lesion, the latencies and rise times of these EPSPs were longer (Fig. 4a) than those evoked by unlesioned sensory axons, consistent with the smaller and more variable diameter of regenerating axons, but these differences were largely eliminated by 7.5 months postlesion (Fig. 4b). Notably, we verified that all recorded responses were mediated via axons in the crushed dorsal roots. After recording responses with all roots (crushed and uncrushed) intact, we cut each of the crushed roots sequentially while recording the remaining responses. Cutting the previously crushed dorsal roots always abolished the response completely, therefore verifying that these responses were mediated only by axons in the crushed roots.
Figure 4.
Systemic artemin administration restores synaptic responses from sensory afferent fibers in spinal neurons. (a,b) Representative traces of field potentials recorded extracellularly in the ventral spinal cord in response to electrical stimulation of the median or radial nerves in the ipsilateral forelimb 1.4 (a) and 7.5 (b) months after DRC. On the unlesioned side of experimental animals (intact roots), the synaptic responses began 1.0–1.5 ms after the stimulus, with rise times of 1.0–1.5 ms in both vehicle-treated (black traces) and artemin-treated (gray traces) animals. In artemin-treated rats, there was substantial recovery of these synaptic inputs (red traces) both 1.4 (a) and 7.5 (b) months after DRC. In contrast, there was no recovery of synaptic function after DRC in vehicle-treated rats (blue traces), even at 7.5 months (b). (c) Scatter plot of the maximum synaptic response to stimulation of the medial or radial nerve recorded in all experimental animals. Each symbol represents the results from one animal, either after DRC or for unlesioned (intact) roots on the contralateral side of the same animal. The average maximum response for each group is shown with an open circle and vertical line (mean ± s.e.m.). The groups tested at ~1 month included postoperative times of 0.7–1.4 months. Colors for the different groups are the same as those used for the traces in panels a and b.
Systemic artemin restores sensorimotor functions
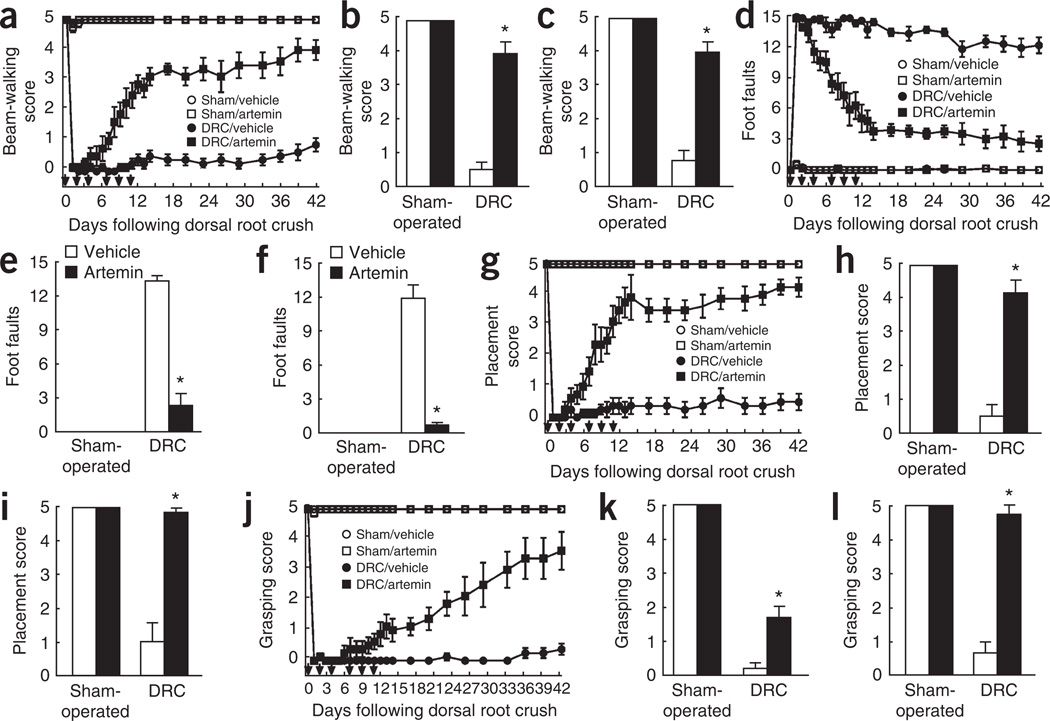
Sensory function, which we assessed using three behavioral tests11, was graded from 0 (no limb use) to 5 (normal) by an observer that was blinded to the treatments. Animals with DRC injury showed complete disruption of forelimb use and scores were consistently less than 1 over the entire 42-d testing period (Fig. 5). Artemin treatment produced a marked progressive improvement in beam-walking ability11 during the first 14 d, which continued beyond the termination of artemin injections, progressing at a slower rate over the remaining 42 d (Fig. 5a) and persisting at 6 months (Fig. 5c). Forelimb sensory deficit was further tested by allowing the rats to traverse a horizontal ladder and counting the incidence of slipping of a forepaw from the ladder11. Sham-operated animals rarely registered foot slips, whereas rats with DRC injury demonstrated an average of 14 incidents per trial (Fig. 5d–f). Artemin treatment resulted in a gradual, progressive improvement in the ability of the rats to walk across the ladder (Fig. 5d). Notably, improvement in sensorimotor function measured in these tests showed an apparent biphasic pattern, with very substantial improvement over the first 14-d period (Fig. 5d). Recovery was nearly complete 6 weeks after DRC and remained at almost normal levels at 6 months (Fig. 5d,f). Sensorimotor function was evaluated by a stabilization maneuver11, in which the rat is nudged forward and responds by placing its forelimbs in an outstretched position, palms flat and toes outspread. Vehicle-treated rats with DRC consistently failed to respond (Fig. 5g–i). In contrast, artemin-treated rats showed a marked recovery of the stabilization maneuver in 7 d, achieving nearly normal responses by day 14 (Fig. 5g). Recovery remained nearly complete at both 6 weeks and 6 months (Fig. 5g,i). Artemin treatment did not produce any changes in the behavior of sham-operated rats (Fig. 5g–i).
Figure 5.
Systemic artemin administration promotes recovery of sensorimotor function. (a–l) We examined the ability to walk along an elevated beam over an open area (a–c), the number of foot slips when walking across a horizontal ladder (d–f), placement stabilization (g–i) and contact-evoked grasping (j–l) after sham or DRC injury and treatment with six subcutaneous injections of artemin or vehicle. Artemin or vehicle were administered immediately (a,c,d,f,g,i,j,l) or 2 d after injury (b,e,h,k). Responses are shown over a 6-week period following injury (a,d,g,j), or at 14 d (b,e,h,k) or 6 months (c,f,i,l) following injury. * indicates a significantly (P < 0.0001) restored response compared with DRC and vehicle treatment. Error bars, s.e.m.
Contact-evoked grasping was used as a measure of a highly complex sensorimotor response11. Normal and sham-operated rats lowered toward a cage consistently grasp the lid. This response was completely abolished by DRC injury (Fig. 5j–l); rats made forward-directed, waving-like movements of the forelimb, but grasping was never accomplished11. Treatment with systemic artemin produced a gradual, but progressive, restoration of contact-evoked grasping (Fig. 5j) that was almost monophasic over the entire 42-d observation period. Contact-evoked grasping reached normal levels by 6 months (Fig. 5l).
For all four behaviors, initiation of artemin treatment after a 2-d delay following DRC produced a level of restoration at 14 d (Fig. 5b,e,h,k) that was similar to that with immediate artemin treatment (Fig. 5a,d,g,j).
Functional recovery from dorsal root injury is long-lasting
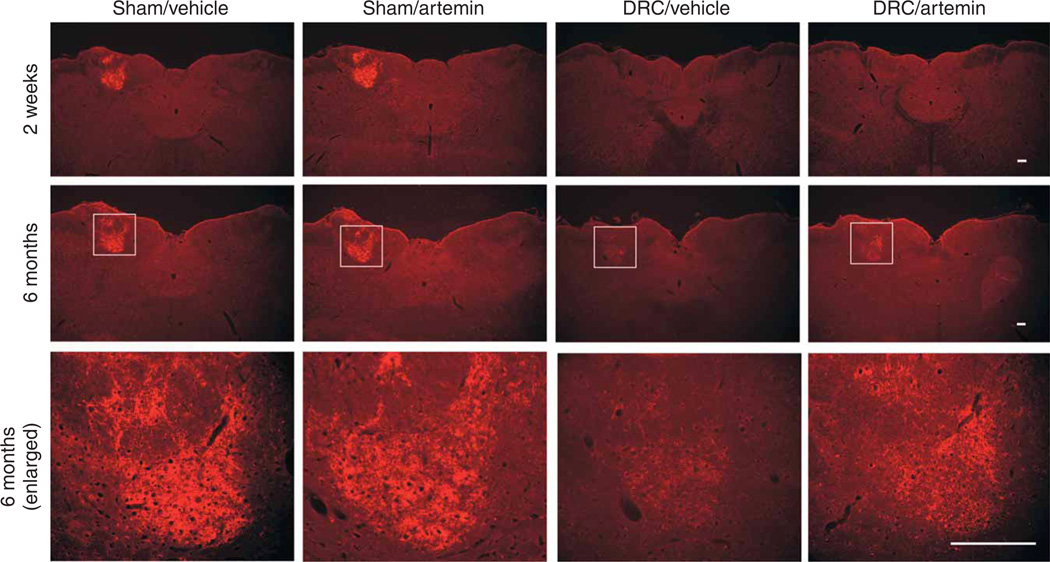
The neurochemical indices of regeneration of axons through the DREZ were consistent with the persistent improvements in behavior that we observed 6 months after the injury. The restoration of immunofluorescence labeling of CGRP, P2X3 and CTB into the spinal dorsal horn was still evident after 6 months in the artemin-treated animals (Fig. 1a,b), but was completely absent in vehicle-treated rats (data not shown). Most notable was the appearance of labeling for CTB in the cuneate nucleus 6 months after DRC in artemin-treated, but not vehicle-treated, rats (Fig. 6). The CTB axonal densities10 of the cuneate nucleus of sham-operated rats treated with artemin was 99.4 ± 7.43% of that of the comparable region of sham-operated, vehicle-treated rats determined at 6 months. Following DRC, the CTB axonal densities of vehicle-treated and artemin-treated groups were 1.32 ± 0.33% and 23.9 ± 4.63%, respectively, indicating a significantly (P < 0.0001) greater density with artemin treatment. Labeling for CTB was not present in the cuneate nucleus 14 d after DRC, as indicated by CTB axonal densities of 0.57 ± 0.18% and 0.49 ± 0.12% for vehicle-treated and artemin-treated groups, respectively. These observations raise the possibility that either regeneration of injured, or sprouting of uninjured, myelinated afferent fibers to this supraspinal nucleus could occur over a prolonged time course with artemin treatment. Although this observation is consistent with the slow rate of restoration of complex sensorimotor behavior, as indicated by contact-evoked grasping (Fig. 5j), mechanistic conclusions require additional experimental data.
Figure 6.
Systemic artemin administration increases CTB labeling in cuneate nucleus at 6 months. Immunofluorescence in cuneate nucleus of rats with DRC, but without artemin treatment (DRC/Vehicle), showed little CTB labeling either 2 weeks (top row) or 6 months (middle row) after DRC. After artemin administration, there was a clear increase in CTB labeling in the cuneate nucleus (DRC/artemin) at 6 months, but not at 2 weeks, after DRC. The bottom row shows high-magnification views of fluorescence for CTB in the cuneate nucleus. Scale bar represents 100 µm.
An alternative explanation of these results is that the intact C3 nerves provided a level of innervation that is sufficient to restore sensory function with artemin treatment. To address this possibility, dorsal roots from C4 to T2 were crushed and animals were treated with artemin or vehicle as described above. We carried out a second surgery 14 d after the initial surgery and verification of functional recovery with artemin treatment, in which we cut the C4–T2 dorsal roots, leaving the C3 root intact. This procedure resulted in a complete loss of function, indicating that contributions from the intact C3 root were not responsible for the functional recovery (Supplementary Fig. 5 online).
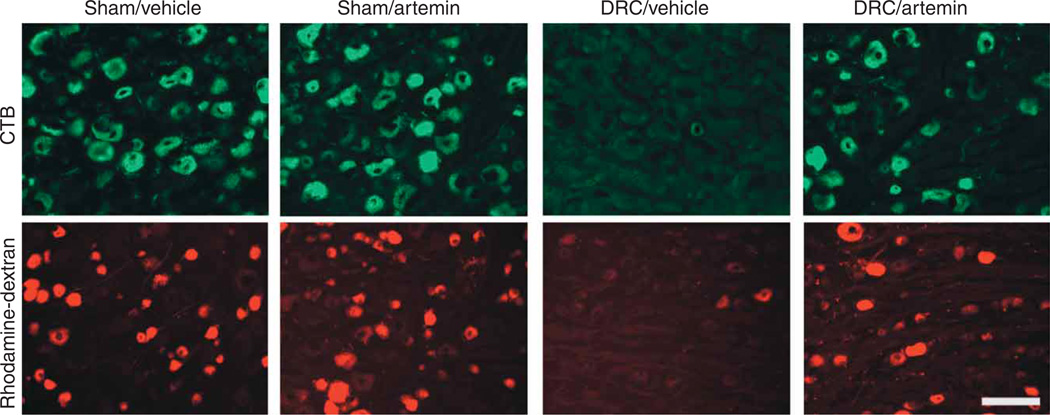
A second possibility is that a small number of axons remained intact and uninjured in the crushed dorsal root and that these spared axons might sprout, thus re-innervating the affected region and restoring function. To test this possibility, we crushed the C4–T2 dorsal roots unilaterally and immediately injected rhodamine-dextran or CTB into the ipsilateral dorsal horn. After 3 d, the ipsilateral DRG were examined for the presence of labeled cells. Virtually no CTB-labeled cells and fewer than 2% of rhodamine-dextran–labeled cells were observed (Fig. 7 and Supplementary Fig. 6 online). We also allowed possible regeneration to proceed for 2 weeks, injecting CTB or dextran on day 11. There were approximately 84 ± 13.7 CTB-positive profiles per section and 104 ± 10.1 rhodamine-dextran–positive profiles per section 3 d later in DRG tissue obtained from vehicle-treated, sham-operated animals, with a mean of 225 ± 18 total profiles counted per section. The DRG from vehicle-treated, DRC rats contained very few labeled neurons, as indicated by counts of only 4 ± 0.4 and 4.1 ± 0.7 profiles per section for CTB and rhodamine-dextran, respectively, out of 217 ± 20.7 total profiles counted per section. However, the incidence of labeling in DRG from artemin-treated, DRC rats (Fig. 7) was significantly increased to 52 ± 7.8 CTB-positive profiles per section (P < 0.0001) and 65.2 ± 8.1 rhodamine-dextran–positive profiles per section from a mean of 244.2 ± 32.3 total profiles counted per section (P < 0.0001). These data support the concept that regeneration is likely to contribute substantially to functional recovery. However, our data show that some axons do escape injury from the crush, so a possible contribution of sprouting of these uninjured fibers in the dorsal horn and perhaps cuneate nucleus to the observed functional recovery cannot be excluded.
Figure 7.
Systemic artemin administration increases retrodgrade labeling of DRG neurons from the dorsal horn. The tracers CTB or rhodamine-dextran were injected into the dorsal horn ipsilateral to surgery 11 d following DRC or sham injury and immediate treatment with six subcutaneous injections of artemin or vehicle. Fluorescence labeling is shown in DRG on day 14 after surgery. Scale bar represents 125 µm.
GFRα3 expression correlates with sensory recovery
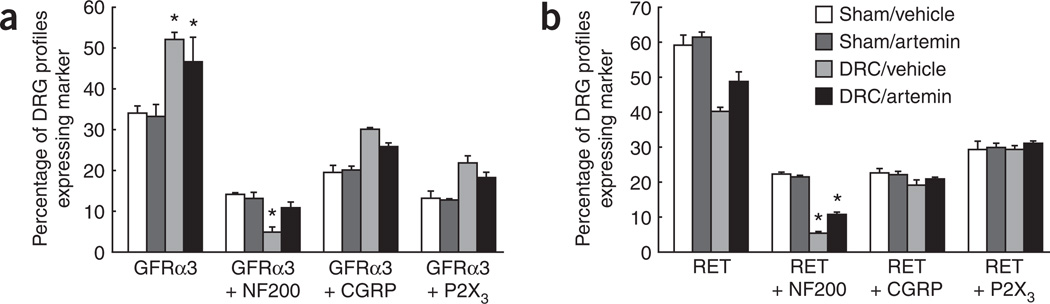
Artemin acts through the GFRα3 receptor coupled to the RET signaling protein34–37, and DRC-induced changes in the expression of GFRα3 or RET in peripheral nerves may influence their regeneration. Notably, artemin treatment in sham-operated rats did not alter GFRα3 or RET expression in sensory neurons. Approximately one-third of DRG neurons were GFRα3-immunoreactive in both the vehicle- and artemin-treated groups 14 d after sham surgery, whereas approximately two-thirds of DRG neurons were RET-immunoreactive (Fig. 8a,b and Supplementary Figs. 7 and 8 online). After DRC, however, the percentage of DRG neurons that were GFRα3-immunoreactive was significantly (P < 0.05) increased to 52 ± 1.8 (Fig. 8a and Supplementary Fig. 7). Artemin treatment partially normalized these changes.
Figure 8.
Systemic artemin normalizes expression of GFRα3 and RET. (a,b) Quantification of DRG neurons immunoreactive for NF200, CGRP or P2X3 and coexpressing either GFRα3 (a) or RET (b) 14 days after DRC or sham surgery and immediate treatment with artemin or vehicle. The data are expressed as the percentage of profiles (cells) in the DRG that expressed the indicated marker(s). DRC was associated with a significant (P = 0.011) increase in profiles expressing GFRα3, but not RET (P > 0.05), and a significant reduction in NF200-labeled profiles coexpressing either GFRα3 (P = 0.0003, ANOVA) or RET (P < 0.0001, ANOVA). The proportion of NF200-labeled profiles also expressing GFRα3 or RET was normalized (P = 0.0003) or partly normalized, respectively, by treatment with artemin. * indicates a significant (P ≤ 0.01, ANOVA) difference from the sham-operated, vehicle-treated groups. Error bars, s.e.m.
Labeling for either GFRα3 or RET and either NF200, CGRP or P2X3 was examined to identify changes in myelinated sensory axons (NF200) and unmyelinated peptidergic (CGRP) and nonpeptidergic (P2X3) nociceptors. Notably, 14 ± 0.5% of DRG neurons in sham-operated, vehicle-treated rats labeled for both GFRα3 and NF200, and 22 ± 0.85% of DRG neurons labeled for both RET and NF200, indicating that a significant (P < 0.05) proportion of myelinated sensory axons are subject to modulation by artemin. Notably, artemin treatment did not change these proportions in sham-operated rats (Fig. 8a,b and Supplementary Figs. 7 and 8 online). After DRC, however, the proportion of DRG neurons coexpressing GFRα3 and NF200 was reduced to 5 ± 1.2% and the proportion expressing NF200 and RET was reduced to 5 ± 0.9% (Fig. 8a,b and Supplementary Figs. 7 and 8). In contrast, there was a near doubling of DRG neurons that were immunoreactive for GFRα3 and either CGRP or P2X3, whereas those that were also immunoreactive for RET remained unchanged (Fig. 8a,b and Supplementary Figs. 7 and 8). Artemin treatment resulted in a partial normalization of the proportions of DRG neuronal populations expressing these markers (Fig. 8a,b and Supplementary Figs. 7 and 8). The percent of NF200-immunoreactive DRG profiles expressing GFRα3 was not significantly (P > 0.05) reduced in the DRC rats that were treated with artemin and the proportion expressing RET was significantly (P = 0.0074) greater in the artemin-treated DRC group compared with the vehicle-treated group. The observation that GFRα3, which is the only known receptor for artemin, is expressed selectively on DRG neurons, along with the apparent absence of expression of this receptor on reactive glia, myelin or macrophages38, provides a basis for suggesting that artemin may exert its neuroregenerative effects through an interaction with GFRα3 residing on peripheral neurons. It should be noted that non-GFRα3 mechanisms may also be possible, and these possibilities require further investigation.
Artemin treatment might also promote the growth of sensory axons through the DREZ by reducing the astrocytic scarring or axonal debris thatmay normally impede regeneration. In the current study, we found that DRC produced a marked increase in glial fibrillary acidic protein (GFAP) immunoreactivity in the DREZ 4 d after the nerve crush injury (Supplementary Fig. 9 online). Likewise, visualization of activated microglia with the macrophage marker ED1 progressively increased over a 2-week period after injury (Supplementary Fig. 10 online). Treatment with artemin did not produce any observable alterations in the intensity of immunoreactivity for either GFAP or ectodysplasin 1 14 d after the injury. Taken together with the apparent lack of expression of GFRα3 on non-neuronal cells38, these data indicate that artemin-induced functional recovery is likely to result from interactions with neurons.
DISCUSSION
Systemic artemin treatment caused the regeneration of damaged axons, resulting in virtually complete and long-lasting restoration of nociceptive and sensorimotor functions. Artemin, given in six systemic injections over 11 d promoted the regeneration of multiple classes of nerve fibers through the DREZ, re-established the functional spinal synaptic connections, and restored nociceptive functions, sensorimotor functions and complex, directed sensorimotor behavior (contact grasping). The effects of artemin persisted for at least 6 months. Notably, artemin was administered systemically and promoted substantial functional recovery even after a 2-d delay in administration, suggesting that it has substantial and possibly permanent potential therapeutic application. Although several studies have reported varying degrees of success in promoting axonal regeneration and neuronal function with the use of neurotrophic factors, the translation of these findings into clinically useful procedures has been handicapped by the requirement for spinal injections, administration of genetically altered cells, the development of undesirable adverse effects and the rather limited scope of anatomical and functional recovery15. Our results, showing marked, long-term recovery of complex motor skills after the systemic administration of artemin, therefore represent a substantial advance with potential for translation into clinically applicable treatment of traumatic nerve injury.
The restoration of nociceptive sensory and synaptic function was demonstrated by the time-dependent return of behavioral responses to various noxious stimuli and of the evoked expression of Fos and internalization of NK1 receptors in postsynaptic spinal neurons. Artemin promoted growth ofNF200-, CGRP- or P2X3-labeled sensory fibers through the DREZ in a manner that was consistent with the level of expression of GFRα3 and RET found on these neurons after nerve injuries37. These findings suggest that limited regeneration (that is, approximately 50% of sensory fibers and synaptic potential amplitudes were approximately one-third of control values) is sufficient to restore behavioral responses to nociceptive stimuli, as well as to substantially improve sensorimotor function involving proprioception and touch. The time course and sensitivity to artemin-induced recovery of nociceptive and sensory functions were consistent with the relative proportion of myelinated and unmyelinated DRG cells expressing GFRα3. Most of the unmyelinated GFRα3 neurons are likely to be unmyelinated nociceptors11. GFRα3 expression was increased with DRC, suggesting that this population of artemin-sensitive nociceptors may account for the rapid and robust restoration of responses to noxious heat. Regeneration of central axons has been estimated to occur at approximately 2 mm per day39,40, which is consistent with the time frame for restoration of responses. The slightly slower recovery of responses to noxious mechanical stimuli may reflect a more restricted expression of GFRα3 on fibers mediating mechanical nociception.
Artemin-induced functional synaptic contact between large caliber sensory axons and spinal neurons was demonstrated electrophysiologically, a finding that is consistent with an almost complete restoration of proprioceptive and sensorimotor performance. The time course of recovery of placement stabilization, beam walking and horizontal ladder performance showed a two-phase pattern with a relatively rapid recovery rate up to approximately day 14 post-DRC, a slower rate of recovery at approximately 6 weeks and essentially full recovery by 6 months. Artemin may promote the regeneration of myelinated fibers into the spinal dorsal horn and permit the formation of synaptic connections with spinal neurons in the ventral horn, initially resulting in a relatively rapid, but incomplete, recovery of motor functions that is followed by a slower improvement over time. The population of artemin-sensitive myelinated sensory axons expressing GFRα3 is substantially smaller than that of unmyelinated fibers41,42, and this is reduced further by DRC. Nonetheless, this population still represents about 14% of DRG neurons and may be sufficient for the observed recovery of sensory behaviors. This conclusion is supported by the observation that artemin treatment protected against the DRC-induced loss of NF200-positive neurons that were labeled for GFRα3 or RET. Unlike other sensorimotor behaviors, artemin-induced contact grasping behavior recovered more slowly and was reached normal levels only 6 months after DRC. Contact-evoked grasping with force is thought to require increased levels of sensory-motor integration11, and the slower recovery of this behavior may reflect the requirement for reformation of more complex circuits.
The mechanism for artemin-mediated regeneration of injured axons is unknown. One possibility is that it occurs through interactions with the GFRα3 receptor. Dorsal roots were crushed10, rather than cut, and some small fibers escape injury. This conclusion was supported by immunohistochemical analysis, as well as by the observation that withdrawal thresholds from noxious heat and mechanical stimuli approached, but did not reach, the behavioral cutoff levels. Although sprouting of these uninjured fibers may contribute to the restoration of function, retrograde labeling of sensory neurons from the spinal dorsal horn shows that artemin strongly promotes regeneration of injured axons. The recovery of function did not appear to be associated with artemin-induced changes in the expression of inhibitory barriers, including the formation of the glial scar or changes in myelin debris. Notably, the effects of artemin occurred with a limited administration schedule, suggesting that once neurotrophic support is provided, continued improvement and functional connections may occur over long periods of time if the axons can be induced to pass the inhibitory barriers at the DREZ. This is supported by the fact that peripheral axons do possess the ability to regenerate without trophic support by exogenous factors and in the absence of inhibitory barriers (as found at the DREZ)43,44. Continued recovery may also be related to potential secondary effects that artemin-responsive DRG neurons may exert on artemin-nonresponsive DRG neurons. These possibilities and definitive demonstration of a role of GFRα3 in these effects will require confirmation through further experimentation.
Our results demonstrate that a limited dosing regimen of systemic artemin produces substantial and robust restoration of all sensorimotor functions in a model of dorsal root injury. Notably, artemin administration did not change sensory thresholds in uninjured animals. Especially noteworthy from a clinical perspective is the fact that persistent axonal regeneration and restoration of sensory function was produced by only six systemic injections of artemin, obviating the requirement for long-term treatment or for spinal infusions and their associated risks. Additionally, a delay of 2 d between DRC and initiation of artemin treatment did not impede regeneration of many primary afferent fibers across the DREZ or the restoration of nociceptive and sensorimotor function. This finding suggests that there is a window of opportunity for the treatment of traumatic dorsal root injuries. These results suggest that artemin offers substantial promise for the effective repair of damaged sensory neurons and treatment of debilitating nerve injuries.
METHODS
Animal surgery, artemin administration and tracing studies
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the institution in which they were carried out (University of Arizona or Tufts University), and were in accord with the US National Institutes of Health guidelines.
Unilateral C4–T2 dorsal root crush10 was performed on male Sprague-Dawley rats (Harlan), weighing 175–250 g. Rats were anesthetized with 2% halothane vaporized in a mixture of 95%O2/5% CO2 delivered at a rate of 2 liter min–1. Anesthesia was maintained with 0.5% halothane in O2. Under sterile conditions, a longitudinal incision (about 4 cm in length and 5 mm lateral from the midline) was made from the C2 to the T3 spinous processes and the paraspinal muscles from C3 to T3 were isolated and removed. Connective tissues and remaining muscles were removed and the left dorsal-lateral quarter of spinal arc was removed carefully from C3 to T2, exposing the C4–T2 dorsal roots. Each dorsal root was crushed three times, for 5–10 s per crush, midway between the DRG and the DREZ (that is, approximately 2 mm from the DREZ) with the sharply bent front tips of No. 7 forceps. On completion of the operation, hemostasis was confirmed, muscles were sutured in layers and the skin was closed with metal clips. Sham surgery was carried out under the same procedures, but without the root injury. Rat artemin24 or saline vehicle was given subcutaneously on a Monday, Wednesday and Friday schedule immediately after surgery, for a total of six injections over 2 weeks. Additional groups of rats were prepared with DRC 2 d before the initiation of the artemin treatments. For transganglionic tracing, the median nerve branch of the brachial plexus was exposed under sterile conditions and 5 µl of a 0.5% (wt/vol) solution of CTB10,27 (low salt, List Labs) was pressure-injected into the nerve at multiple injection sites 5–7 days before the rats were killed. To evaluate whether some nerve fibers may escape the crush, 0.2 µl of 5% CTB (List Labs) or 0.2 µl of 10% (wt/vol) tetramethylrhodamine-dextran (3,000 molecular weight, Molecular Probes) was microinjected into the ipsilateral dorsal horn of rats immediately after surgery or at day 11 after surgery, with each injection site being located adjacent to each dorsal root entry region from C4–T2. The injection period was 10 s. For CTB, the depth of the injection was 0.6 mm, and for rhodamine-dextran, the injection depth was 0.2 mm. We perfused the animals 3 d after microinjections, counted the labeled neurons in the DRG from C5–T1 in three randomly selected sections from each DRG and expressed each as a percent of counted DRG neurons; these percentages were averaged within groups.
Preparation and purification of artemin
E. coli host BL21(DE3) plysS, expressing wild-type rat artemin containing an N-terminal 10 histidine tag with an enterokinase cleavage site, were lysed in phosphate-buffered saline (PBS, 5 mM NaPO4, 150 mM NaCl, pH 6.5) using a Gaulin press. After centrifugation (30 min at 4,700g) to isolate inclusion bodies, the pellets were washed twice with 20 volumes buffer (0.02 M Tris-HCl at pH 8.5 and 0.5 mM EDTA) and then washed twice with the same buffer containing Triton X-100 (2%, vol/vol) followed by two additional buffer washes without detergent. The final pellets were suspended in 50 ml of 6 M guanidine hydrochloride, 0.1 M Tris-HCl at pH 8.5, 0.1 M dithiothreitol, and 1 mM EDTA, and homogenized using a polytron homogenizer followed by overnight stirring at room temperature (18–21 °C). The solubilized proteins were clarified by centrifugation before denaturing chromatography through 5.5 liters of Superdex 200 preparative resin (GE Life Sciences) equilibrated with 0.05 M glycine/H3PO4 at pH 8.0 and eluted with 2 M guanidine hydrochloride at 20 ml min–1. Fractions containing artemin were pooled and concentrated approximately fivefold to 250 ml using an Amicon 2.5-liter stirred cell concentrator.
After filtration to remove any precipitate, the concentrated protein was subjected to renaturing sizing chromatography through Superdex 200 equilibrated with 0.1 M Tris-HCl at pH 7.8, 0.5 M guanidine hydrochloride, 8.8 mM reduced glutathione and 0.22 mM oxidized glutathione. The column was run using 0.5 M guanidine hydrochloride at 20 ml min–1. Fractions containing renatured artemin were identified by SDS-PAGE to determine the presence of the dimeric product under nonreducing conditions, pooled, and stored at 4 °C for further processing. The N-terminal histidine tag was removed enzymatically with lysyl-endopeptidase to produce 113 amino-acid artemin. The protein sample was made with 0.1 M NaCl, 25 mM HEPES at pH 8.0, and 0.15 M guanidine hydrochloride and lysyl-endopeptidase (WAKO) added at a 1:600 (wt/wt) ratio of protease to artemin. The samples were stirred at room temperature for 2 h, and the digest was subjected to Ni-NTA chromatography (Qiagen). The flow through from this chromatography step was subjected to further purification using SP-Sepharose (GE Life Sciences) eluted with 5 mM phosphate containing 150 mM NaCl. Eluted artemin was aliquoted and stored at −70 °C.
Behavioral observations
Restoration of nociceptive, sensorimotor and proprioceptive functions were assayed according to the behavioral protocols described previously, with some modifications10,11. Paw-withdrawal latency to noxious thermal stimulation was measured with a 49 °C water bath10; the forepaw ipsilateral to injury was immersed in a 49 °C water bath until the rat withdrew its paw or until the cutoff time of 20 s was reached. Ipsilateral forepaw withdrawal to noxious mechanical stimulation was tested with a Randall-Selitto noxious pinch device (Ugo-Basil)10 with the cutoff set at 250 g. Scoring of rat performance in contact-evoked grasping, beam walking, horizontal ladder and stabilization placement was carried out as previously described10,11. Contact-evoked grasping was measured by lowering rats head first toward a wire cage lid while holding the rat by the tail. The intact animals instinctively grasp the lid on contact with their forepaws. The number of successful grasps in five trials was counted. In the beam-walking test, the rats traversed a beam (1.5 m in length, 3 cm wide) and performance was scored according to the following scheme: 0 for no attempt at using the limb, 1 for walking motion/cycling, but no weight bearing, 2 for attempted weight bearing, but incorrect targeting (missing the beam) and placement (dorsal aspect down), 3 for weight bearing, but incorrect placement and frequent mistargeting, 4 for full weight bearing, but slight deficits in placement and infrequent mistargeting, 5 for normal walking with no deficits. The horizontal ladder test was carried out by allowing the rats to walk across a ladder with 8-cm-wide rungs that were 6 cm apart. Each foot slip of the ipsilateral forepaw was counted. In this test, rats walked toward a darkened ‘home box’ to which they had become accustomed before testing. In the stabilization-placement measurement, a rat was held on a bench-top facing away from the experimenter, with one forepaw drawn back against its side and then nudged forward. Rats normally brace themselves (extend the forepaws rostrally with palms flat against the bench-top, toes spread) against forward displacement. In all behavioral experiments, the experimenters were blinded with respect to the treatment group.
Immunohistochemistry
Rats were transcardially perfused with 10% (vol/vol) buffered formalin (Sigma), and cervical spinal cord, DRG and brainstems were removed, cryoprotected (in 20% (wt/vol) sucrose), frozen and sectioned (10 µm for DRG, 20 µm for spinal cord) on a cryostat. Sections were incubated with primary antibodies to CGRP (host rabbit/guinea pig, 1:10,000, Peninsula), P2X3 (host rabbit/guinea pig, 1:10,000, Neuromics), NF200 (host mouse, 1:5,000, Sigma), GFRα3 (host rabbit, 1:1,000, Biogen Idec, R11, 2 µg ml−1)29, Ret (host rabbit, 1:5,000, Biogen Idec, 2 µg ml−1)29, Fos (host rabbit, 1:5,000, Calbiochem), CTB (host goat, 1:5,000, List Labs) and to NK1-R (host rabbit, 1:5,000, a gift from P. Mantyh, University of Arizona)33 for 24–72 h. To delineate the DREZ, sections were stained with antibodies to glial fibrillary acidic protein (GFAP, host rabbit/mouse, 1:5,000, Sigma). We used Cy3-conjugated goat antibody to rabbit IgG (1:1,000, Jackson), Alex Fluor 488/594-conjugated goat antibodies to rabbit, mouse or guinea pig IgG (1:1,000, Molecular Probes), and Alex Fluor 594-conjugated donkey antibody to goat IgG (1:1,000, Molecular Probes) as secondary antibodies for 2 h. Analyses of colocalization of two neuromarkers in DRG or spinal cord were carried out using dual-labeling immunofluorescence.
Once the tissue was processed for one of the neuromarkers, it was then processed for the second label. The primary antibodies of both neuromarkers were raised from different species of host animals and the secondary antibodies were conjugated with either a red or green fluorescent compound. Quantitative analysis of axon density in the dorsal root along the central side of DREZ was carried out as previously described10,45. Briefly, one section per root from C4–T2 of each rat was randomly selected, and then the total area and the labeling-occupied area in defined DREZ by GFAP were measured with the MetaMorph imaging system 5.5 (Universal Imaging)10,42,45. All the values were expressed as the ratio of the density of the labeled area to the total area and then averaged. For image analysis of labeling in the dorsal horn, one section per segment of C4–T2 spinal cord region was randomly selected from each animal. The acquired images were set to a constant threshold level with MetaMorph45. The integrating density of the staining on each side of spinal dorsal horn for each section was recorded and expressed as the ratio of the density of the ipsilateral side to the contralateral side. Labeling with CTB was determined as the ratio of the density of the ipsilateral side to the average density in sham-vehicle group. Data for each treatment group was then expressed as mean and s.e.m.
For counts of the percentage of the labeled neurons in DRG sections, six to seven sections of DRG between C4 and T2 were randomly selected. In each section, a minimum of 150 cells were counted and the percentage of cells expressing each label were determined by counting the number of immunoreactive profiles and the total number of neuronal profiles, as determined by DAPI nuclear staining41. The mean percent and s.e.m. were thus determined on the basis of these samples (that is, n = 6–7). A total of three animals were studied by this procedure. The percent of positive cells was similar among animals in each treatment group.
Nociceptive reaction and Fos expression in formalin-induced inflammation
The experiments were carried out in awake, freely moving rats as described previously31. The plantar surface of the ipsilateral forepaw of the rat that received DRC and artemin or vehicle treatment was injected with 100 µl of 10% (vol/vol) formalin subcutaneously, and the time spent licking the injection site was recorded45,46. We perfused the rats with 10% buffered formalin 3 h after the injections and harvested C4–T2 spinal cord for immunofluorescence examination of postsynaptic Fos expression in the spinal dorsal horn. The control rats received the same amount of saline injections. We randomly selected three sections per segment between the C4–T2 spinal cord region from each animal and counted the number of cells expressing label for Fos in the ipsilateral dorsal horn (above the horizontal line crossing the central canal). The data are expressed as mean ± s.e.m. of Fos-positive profiles per section in each treatment group. Formalin did not elicit Fos expression in the contralateral dorsal horn.
Mechanical stimulation and NK1-R internalization in carrageenan-induced inflammation
The mechanical stimulation experiment was carried out by the method described previously32. A subcutaneous injection of 100 µl of a suspension of 2% (wt/vol) λ-carrageenan (Sigma-Aldrich) in saline (pH 6.8) was administered into the plantar surface of the forepaw of the rat. After 3 h, the rats were subjected to non-noxious mechanical stimulation by light stroking of the dorsal forepaw at 1-s intervals over a period of 5 min with the wooden handle of a brush. Alternatively, rats were subjected to a noxious mechanical stimulation applied as a 30-s pinch with a hemostat applied to the distal part of the forepaw. For preparation for immunohistochemical visualization of internalization of NK1-R in the spinal dorsal horn, the rats were anesthetized and perfused for 15 min with PBS followed by 10% (vol/vol) buffered formalin. Frozen C4–T2 spinal cord sections (6 µm) were obtained and then incubated with NK1-R primary antibody (host rabbit, 1:5,000, a gift from P. Mantyh)32 overnight at 4 °C. After washing with PBS, the sections were transfer to secondary antibody (Cy3-conjugated goat antibody to rabbit IgG, 1:1,000, Jackson) for 2 h. To count profiles expressing label for NK1-R, three sections per segment between C4–T2 spinal cord region were randomly selected from each animal and the number of labeled NK1-R neurons and the number of neurons expressing label for internalized NK1-R in the ipsilateral dorsal horn were counted. Data are expressed as the percent of neurons demonstrating NK1-R internalization relative to the total profiles expressing label for NK1-R. Neurons were identified by use of DAPI mount medium42.
Electrophysiological methods
Animals were anesthetized using 2.5% (vol/vol) isoflurane for the duration of all terminal electrophysiology experiments. The cervical cord was exposed from C4 to T1 and stabilized with a spinal clamp on T2. Radial and/or median nerves were dissected and suspended on silver hooks for stimulation. Recordings were made with low-impedance metal microelectrodes (1-mm exposed tip) positioned 1 mm lateral of the midline and ~0.5 mm from the ventral surface of the cord (that is, in the ventral horn). The radial or median nerve was stimulated with square, 50-µs, 4-V pulses delivered at 2–5 Hz. Positive voltage changes are shown as upward deflections in Figure 4. Single responses were filtered (0.1–3 kHz), digitized (16 bits, 20-kHz sampling rate), averaged (50–100 individual traces) and stored for analysis off-line. The spinal preparation usually produced stable, replicable neuronal potentials for several hours. Recordings were made from the ventral horn at each segmental level (C5–T1) on both sides of the cord in response to ipsilateral stimulation of individual brachial nerves. Recordings were made first from the experimental (DRC) side and then from the control (intact roots) side to ensure that the preparation was in good physiological condition at the time regeneration was assessed. The peak magnitude of the ventral horn field potential at 2–6-ms latency was used as the physiological measure of the summed short-latency monosynaptic response in the cord at a given location. The maximum response observed among all recording sites was taken as the global estimate of synaptic function (the values plotted in Fig. 4c). To verify that the responses observed were elicited by the regenerated axons, we cut the dorsal roots at the end of each experiment; in every case, all of the responses in the spinal cord evoked by peripheral nerve stimulation disappeared.
Statistical analysis
Statistical comparisons among treatment groups were carried out with two-factor ANOVA. Significant differences in a treatment group over time were determined with ANOVA followed by the Fisher least significant difference or the Student-Neuman-Keuls test. Pairwise comparisons were made with the Student t-test. Significance was set at P = 0.05.
ACKNOWLEDGMENTS
This work was supported by grants from Biogen Idec.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Al-Qattan MM. Obstetric brachial plexus palsy associated with breech delivery. Ann. Plast. Surg. 2003;51:257–264. doi: 10.1097/01.SAP.0000063750.16982.E4. [DOI] [PubMed] [Google Scholar]
- 2.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraher JP. The transitional zone and CNS regeneration. J. Anat. 2000;196:137–158. [PubMed] [Google Scholar]
- 4.Neumann S, Skinner K, Basbaum AI. Sustaining intrinsic growth capacity of adult neurons promotes spinal cord regeneration. Proc. Natl. Acad. Sci. USA. 2005;102:16848–16852. doi: 10.1073/pnas.0508538102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starkey ML, et al. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp. Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Wong LF, et al. Retinoic acid receptor β2 promotes functional regeneration of sensory axons in the spinal cord. Nat. Neurosci. 2006;9:243–250. doi: 10.1038/nn1622. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Bloechlinger S. Neuroscience. It takes more than two to Nogo. Science. 2002;297:1132–1134. doi: 10.1126/science.1076247. [DOI] [PubMed] [Google Scholar]
- 8.Ramer LM, Borisoff JF, Ramer MS. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J. Neurosci. 2004;24:10796–10805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlstedt T. Regenerating axons form nerve terminals at astrocytes. Brain Res. 1985;347:188–191. doi: 10.1016/0006-8993(85)90911-4. [DOI] [PubMed] [Google Scholar]
- 10.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- 11.Ramer MS, et al. Neurotrophin-3–mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol. Cell Neurosci. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- 12.Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X3 expression in sensory neurons. J. Neurochem. 2001;77:864–875. doi: 10.1046/j.1471-4159.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J. Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J. Comp. Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- 15.Thoenen H, Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 16.Kordower JH, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Baloh RH, et al. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18:793–802. doi: 10.1016/s0896-6273(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 18.Baloh RH, et al. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi H, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarberg H, et al. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 21.Johnson EM, Jr, Taniuchi M, DiStefano PS. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988;11:299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]
- 22.Sanicola M, et al. Glial cell line-derived neurotrophic factor–dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc. Natl. Acad. Sci. USA. 1997;94:6238–6243. doi: 10.1073/pnas.94.12.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treanor JJ, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 24.Gardell LR, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat. Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- 25.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett DL, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol. Cell Neurosci. 1998;12:256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 28.Robertson B, Grant G. A comparison between wheat germ agglutinin- and choler-agenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurons in the rat with some observations in the cat. Neuroscience. 1985;14:895–905. doi: 10.1016/0306-4522(85)90152-6. [DOI] [PubMed] [Google Scholar]
- 29.Malin SA, et al. Glial cell line–derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J. Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris JA. Using c-fos as a neural marker of pain. Brain Res. Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 31.Hunt SP, Pini A, Evan G. Induction of c-fos–like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 32.Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein–like immunoreactivity in the rat spinal cord. J. Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honor P, et al. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J. Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sah DW, Ossipov MH, Porreca F. Neurotrophic factors as novel therapeutics for neuropathic pain. Nat. Rev. Drug Discov. 2003;2:460–472. doi: 10.1038/nrd1107. [DOI] [PubMed] [Google Scholar]
- 35.Sah DW, Ossipov MH, Rossomando A, Silvian L, Porreca F. New approaches for the treatment of pain: the GDNF family of neurotrophic growth factors. Curr. Top. Med. Chem. 2005;5:577–583. doi: 10.2174/1568026054367593. [DOI] [PubMed] [Google Scholar]
- 36.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors—implications for neural development. Curr. Opin. Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 37.Yan H, Newgreen DF, Young HM. Developmental changes in neurite outgrowth responses of dorsal root and sympathetic ganglia to GDNF, neurturin and artemin. Dev. Dyn. 2003;227:395–401. doi: 10.1002/dvdy.10294. [DOI] [PubMed] [Google Scholar]
- 38.Baloh RH, et al. GFRα3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc. Natl. Acad. Sci. USA. 1998;95:5801–5806. doi: 10.1073/pnas.95.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J. Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component B in two branches of a single axon. J. Neurosci. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett DL, et al. The glial cell line–derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J. Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRα3 is expressed predominantly in nociceptive sensory neurons. Eur. J. Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 43.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 44.Plunet W, Kwon BK, Tetzlaff W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J. Neurosci. Res. 2002;68:1–6. doi: 10.1002/jnr.10176. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, et al. Glial cell line–derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 46.Abbadie C, Lombard MC, Morain F, Besson JM. Fos-like immunoreactivity in the rat superficial dorsal horn induced by formalin injection in the forepaw: effects of dorsal rhizotomies. Brain Res. 1992;578:17–25. doi: 10.1016/0006-8993(92)90224-w. [DOI] [PubMed] [Google Scholar]