Abstract
Conditionally replicating adenoviruses (CRAds) that replicate in tumor but less in normal cells are promising anticancer agents. A major determinant of their potency is their capacity for infecting target cells. The primary receptor for serotype 5 adenovirus (Ad5), the most widely used serotype in gene therapy, is the coxsackie-adenovirus receptor (CAR). CAR is expressed variably and often at low levels in various tumor types including advanced breast cancer. We generated a novel p16/retinoblastoma pathway-dependent CRAd, Ad5.pK7-Δ24, with a polylysine motif in the fiber C-terminus, enabling CAR-independent binding to heparan sulfate proteoglycans (HSPG). Ad5.pK7-Δ24 mediated effective oncolysis of all breast cancer cell lines tested. Further, we utilized noninvasive, fluorescent imaging for analysis of antitumor efficacy in an orthotopic model of advanced hormone refractory breast cancer. A therapeutic benefit was seen following both intratumoral and intravenous delivery. Murine biodistribution similar to Ad5, proven safe in trials, suggests feasibility of clinical safety testing. Interestingly, upregulation of CAR was seen in low-CAR M4A4-LM3 breast cancer cells in vivo, which resulted in better than expected efficacy also with an isogenic CRAd with an unmodified capsid. These results suggest utility of Ad5.pK7-Δ24 and the orthotopic model for further translational studies.
Keywords: adenovirus, breast cancer, oncolytic virus, heparan sulfate proteoglycans
Introduction
A total of 20–85% of patients diagnosed with breast cancer, depending on the initial stage, tumor biology and adjuvant treatment, will develop metastatic disease, which features median survival of approximately 2 years.1 Despite extensive research and advances in treatments, metastatic breast cancer remains essentially incurable and is the most common cause of cancer-related mortality in women. The disease is particularly difficult to treat when resistant to antiestrogen therapies, that is, becomes hormone refractory. Adenoviral cancer gene therapy has been proposed to possess potential as an effective treatment alternative for disseminated disease refractory to available modalities. In clinical cancer trials, nonreplicating and replicating adenoviruses have been remarkably safe, with hundreds of patients treated without treatment-related mortality.2 Further, recently completed randomized trials have validated the dramatic inherent potential of even early generation viruses.3–5 However, in most trials, when faced with bulky disease, antitumor efficacy has been low owing to the limited dispersion of the therapeutic agent in advanced tumor masses.
In an attempt to improve transduction of solid tumors, conditionally replicating adenoviruses (CRAds) have been constructed. Their therapeutic effect is potentiated owing to replication and subsequent oncolysis of tumor cells followed by further infection by the progeny virions.6 Type I CRAds utilize the similarity between the requirements of DNA viruses and carcinogenesis for inactivation of the major growth control pathways.7 Inactivation of such functions from the virus genome retains the ability for replication in tumor but not normal tissue. Specifically, a useful prototype approach has been deletion of 24 bp in the constant region 2 (CR2) of the E1A gene, which results in a mutated E1A protein unable to bind the retinoblastoma (Rb) protein.8,9 In quiescent normal cells, this binding is required for locking the cell cycle into S-phase for subsequent effective viral replication.10 The selectivity of this mutation has been demonstrated previously.8,9 The Rb/p16 pathway is mutated in a majority of human tumors including advanced breast cancers.11,12 In contrast to E1B region mutations,13 the CR2 deletion does not hinder replication in tumor cells.8,9
An issue concerning the use of adenoviruses for cancer therapy is the variable and often low expression of the primary receptor, coxsackie-adenovirus receptor (CAR), which has been reported for various tumor types,14 including breast cancer.15,16 Previously, it has been demonstrated that the oncolytic potency of CRAds is mostly related to their capability for entering target cells.17,18 Strategies have been proposed to circumvent dependence on CAR by using CAR-independent entry pathways, including retargeting complexes and genetic capsid modifications.19 With regard to genetic capsid modifications, an Arg-Gly-Asp (RGD) moiety has been introduced into the C-terminus20 or the HI-loop of the fiber21 to allow internalization via αvβ-integrins. Another useful approach has been utilization of non-CAR binding fibers for CAR-independent entry.22–24
Heparan sulfate proteoglycans (HSPGs) are common constituents of the extracellular matrix and have angiogenic and growth-promoting characteristics.25 Further, HSPGs have been found highly expressed in advanced breast cancers.26–29 Here, we incorporated an HSPG binding polylysine moiety into the C-terminus of a CR2 mutated (i.e. Rb/p16 pathway selective) CRAd. We hypothesized that this might lead to effective transduction and subsequent oncolysis of advanced breast cancer cells.
Results
Ad5.pK7-Δ24 displays efficient oncolysis of breast cancer cells in vitro
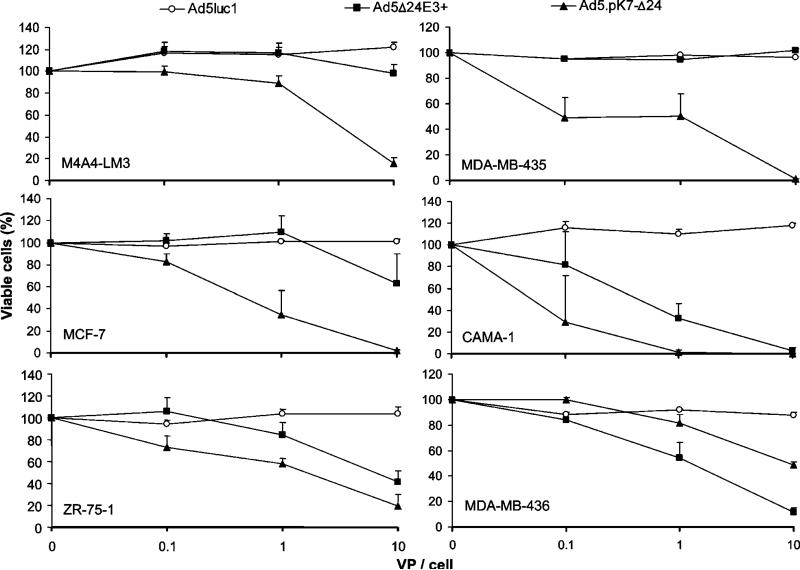
Seven lysines and a glycine–serine (GS)-linker sequence were incorporated in the C-terminus of the fiber of serotype 5 adenovirus (Ad5).pK7-Δ24 enabling HSPG binding. As a control virus, we utilized an isogenic virus, Ad5Δ24E3+, which has a wild-type fiber (Figure 1). Oncolytic potency of Ad5.pK7-Δ24 was analyzed by the mitochondrial activity-based 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Monolayers of MDA-MB-435, M4A4-LM3, MDA-MB-436, ZR-75-1, CAMA-1 and MCF-7 breast cancer cell lines were infected with Ad5.pK7-Δ24, Ad5Δ24E3+ and a nonreplicating control virus, Ad5luc1 (Figure 2). In all but one cell line (MDA-MB-436), significantly higher cell killing efficacy was observed for Ad5.pK7-Δ24 when compared to Ad5Δ24E3+. In MCF-7, Ad5.pK7-Δ24 was significantly more effective in all viral doses (P<0.05, P<0.05, P<0.01 for doses 0.1, 1 and 10 virus particles (VP)/cell, respectively) than Ad5Δ24E3+. In MDA-MB-435 and M4A4-LM3, significantly more effective cell killing was observed with the highest dose (P<0.0000001 and P<0.01, respectively). The difference was statistically significant on CAMA-1 cells with 1 VP/cell (P<0.01) and in ZR-75-1 with doses 0.1 and 1 VP/cell (P<0.05 and P<0.01, respectively). The E1-deleted control virus Ad5luc1 did not cause oncolysis in any of the cells tested (a significant difference versus Ad5.pK7-Δ24 in all cell lines).
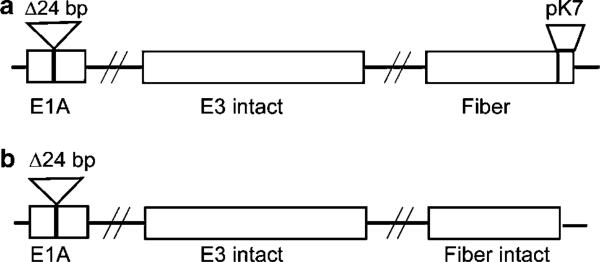
Figure 1.
Structure of the oncolytic viruses used in the study. Ad5.pK7-Δ24 (a) and Ad5Δ24E3+ (b) have a 24-bp deletion in the Rb-binding site of the adenoviral E1A, resulting in selective replication in cells deficient in the Rb/p16 pathway, such as breast cancer cells. Seven lysines and a GS linker sequence are incorporated into the C-terminus of the fiber of Ad5.pK7-Δ24, enabling binding to HSPGs.
Figure 2.
Ad5.pK7-Δ24 displays effective killing of breast cancer cell lines. Cells were infected with 0, 0.1, 1 or 10 VP/cell of Ad5.pK7-Δ24, Ad5Δ24E3+ (an isogenic control virus with a wild-type fiber) or Ad5luc1, a nonreplicating control virus, which was included to control for the effect of virus alone. Cell viability was measured with the MTS assay. The OD490 values of uninfected cells were set as 100%. Data are expressed as means±s.e. of quadruplicate experiments. In all but one cell line (MDA-MB-436), oncolysis was significantly enhanced with Ad5.pK7-Δ24 compared to Ad5Δ24E3+ or Ad5lucI (all P<0.05).
Efficacy of an intratumoral administration of Ad5.pK7-Δ24 in an orthotopic murine model of advanced hormone refractory breast cancer
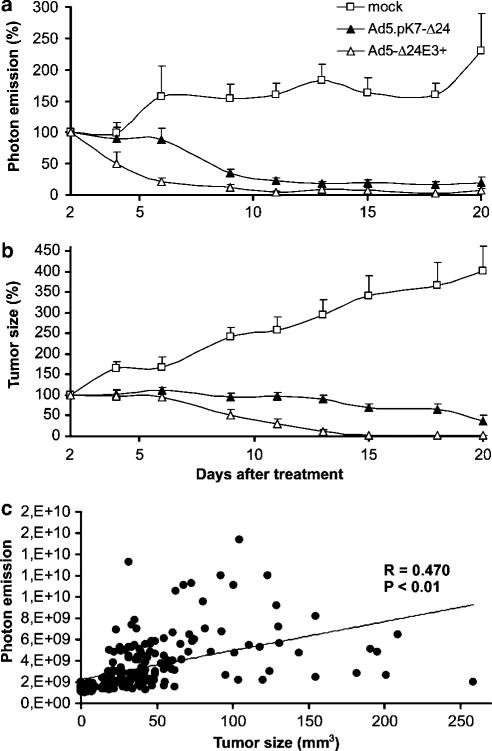
M4A4-LM3 cells expressing green fluorescent protein (GFP) were injected to both left and right uppermost mammary fat pads of nude mice. These cells metastasize rapidly to local and distant lymph nodes whereas lung metastases occur later.30 To establish advanced disease, the primary tumor was allowed to grow for 17 days before intratumoral injections of Ad5.pK7-Δ24, Ad5Δ24E3+ or growth medium on 3 successive days. Antitumor response was monitored by noninvasive imaging of fluorescence emission and by measuring the tumor with calipers in three dimensions (Figures 3a–c, and 4). All Ad5.pK7-Δ24-treated mice had significantly smaller tumors starting from day 9 after treatment when compared to the mock group (all P-values less than 0.01). One of the Ad5.pK7-Δ24-treated mice showed a complete tumor regression of one tumor on day 20 after the treatment but relapsed and had to be killed on day 46. Another mouse from the Ad5.pK7-Δ24-treated group also showed a complete tumor regression of one tumor and relapse did not occur.
Figure 3.
Therapeutic effect of intratumorally injected Ad5.pK7-Δ24 in an orthotopic animal model of advanced hormone refractory breast cancer. GFP-expressing M4A4-LM3 cells were injected into the mammary fat pads of NMRI nude mice and primary tumors were allowed to grow for 17 days. Mice received intratumoral injections of 3 × 108 VP of Ad5.pK7-Δ24, Ad5Δ24E3+ or no virus on 3 successive days (days 17, 18 and 19 after injections of tumor cells). Tumor size was followed by assessment of the number of viable tumor cells by noninvasive imaging of fluorescence (a) or by physical measuring of the tumors with a caliper in three dimensions (b). Photon count and tumor size are depicted as a percentage of initial values. Ad5.pK7-Δ24-treated mice had significantly less viable tumor cells when compared to the mock group (P<0.01). (c) A significant correlation was seen between the GFP expression and tumor size (P<0.01).
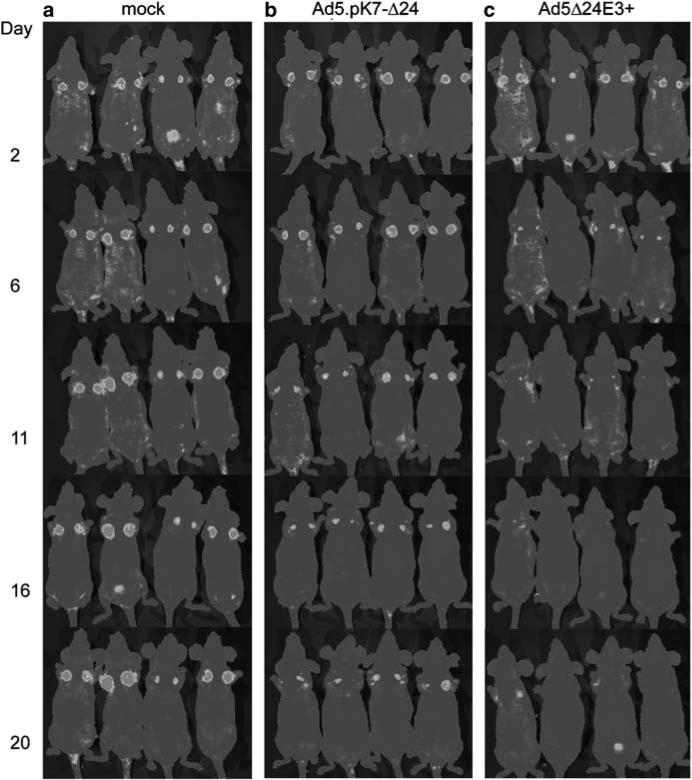
Figure 4.
Noninvasive imaging of viable tumor cells following treatment with Ad5.pK7-Δ24. Hormone refractory GFP-positive M4A4-LM3 breast cancer cells were injected into the mammary fat pads of NMRI nude mice and primary tumors were allowed to grow for 17 days. On days 17–19, mice were treated with intratumoral injections of either virus or growth medium. Fluorescence was imaged three times a week using a cooled CCD camera. A pseudocolor image was obtained (blue represents the lowest intensity and red the highest) and overlaid on a gray scale photographic image. (a) Mock-treated mice (b) Ad5.pK7-Δ24-treated mice and (c) Ad5Δ24E3+-treated mice. The left column indicates the imaging time point (days after last virus injection). (For color figure see online version.)
Ad5Δ24E3+-treated mice showed significant tumor regression compared to the mock group starting on day 4 until the last day of the experiment (all P-values less than 0.05). All of these mice showed a complete tumor regression of both tumors and relapse occurred in only one tumor. Ad5Δ24E3+ was significantly more effective than Ad5.pK7-Δ24 from day 4 to day 11 (all P-values <0.05).
The survival of the mice was also monitored (data not shown). Mice (n = 4) were killed when either tumor reached the size of 1 cm in any diameter. For Ad5.pK7-Δ24-treated mice and mock mice, median survival was 55.0 and 27.0, respectively (difference not significant). All of the mock mice had large primary tumors in both mammary fat pads as opposed to the Ad5.pK7-Δ24-treated mice, which frequently had a good response in one tumor whereas the other was distinctly smaller.
The difference between measurement of tumor size and photon emission is that only live tumor cells are expected to produce GFP, whereas stromal cells, cellular debris and extracellular matrix contribute to physical tumor size. Therefore, it was of interest to compare size and photon emission (Figure 3c). Good correlation was seen (P<0.01), although there seemed to be a trend for more rapid response in the photon count, as indicated by a more rapidly declining curve (Figure 3a).
Comparison of transductional efficacy of the viruses and CAR/HSPG levels in vitro and in vivo
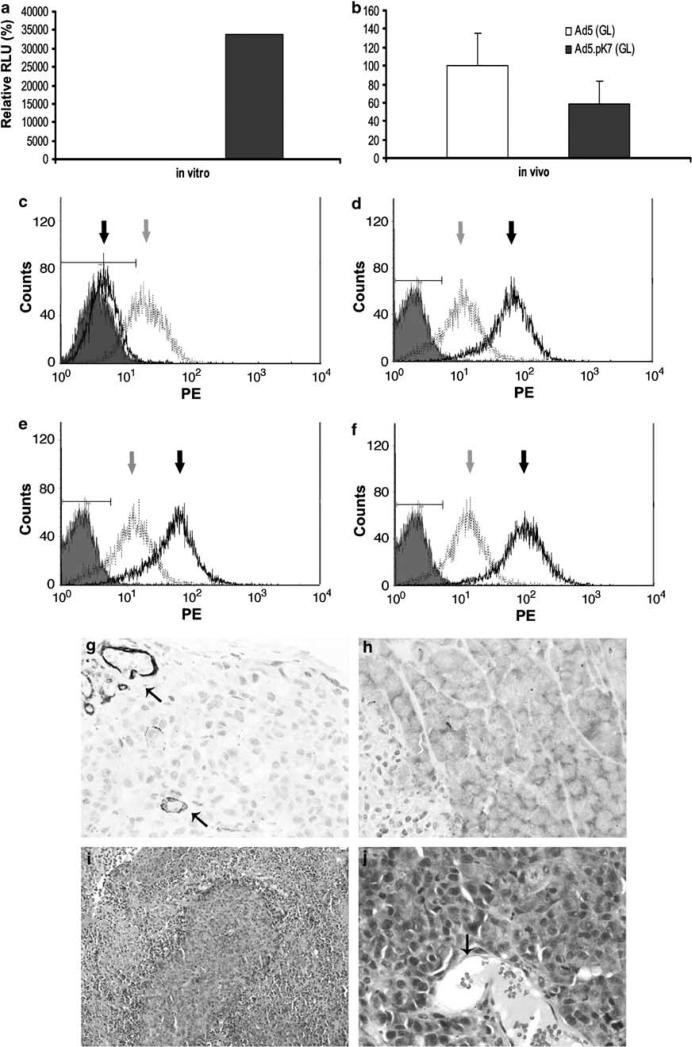
The discrepancy between in vitro and in vivo data on the efficacy of Ad5.pK7-Δ24 versus Ad5Δ24E3+ urged us to study whether there is a difference in CAR and HSPG levels in M4A4-LM3 cells in vitro and in M4A4-LM3 xenografts in vivo. To study transduction, we performed gene transfer assays with viruses containing the luciferase gene. In vitro, M4A4-LM3 cells were infected with Ad5.pK7 (GL), Ad5 (GL) or no virus and gene transfer efficacy was assessed. Ad5.pK7 (GL) was over 300 times more effective in gene transfer than Ad5 (GL) (P<0.00001, Figure 5a). In vivo, however, there was no significant difference between Ad5.pK7 (GL) and Ad5 (GL) (Figure 5b).
Figure 5.
Difference between relative gene transfer efficacy with a pK7-modified Ad versus an unmodified Ad5 capsid virus and between CAR and HSPG levels in vitro and in vivo. (a) M4A4-LM3 cells were infected in vitro with 40 VP/cell of either Ad5.pK7 (GL) or Ad5 (GL) and luciferase expression was assessed as a measure of gene transfer. The relative luciferase units (RLU) values of unmodified Ad5 (GL) were set as 100%. (b) In vivo M4A4-LM3 xenografts were allowed to grow for 28 days and 5 × 1010 VP of virus were injected intravenously and luciferase expression was assessed 48 h later from homogenized tumors. In vitro, Ad5.pK7 (GL) displays 300 times more efficient gene transfer when compared to Ad5 (GL) (a), but in vivo (b) there is no significant difference between the two viruses. (c–f) CAR expression was significantly upregulated in the M4A4-LM3 xenograft tumors grown in mice when compared to the cell line grown in vitro. M4A4-LM3 cells in vitro (c) or homogenized M4A4-LM3 tumors (d–f) were analyzed for their CAR (black line and arrow) and HSPG (gray line and arrow) expression by flow cytometric analysis. Grey shading is negative control. (g, h) Immunohistochemical staining was used to analyze whole-tumor sections for CAR and HSPG expression. Blood vessel endothelium (arrows) stained positive for HSPG, whereas the bulk of the tumor stained less intensively (g). In contrast, viable cancer cells stained positive for CAR and necrotic areas were negative (h). (i, j) Tumors were stained with hematoxylin and eosin to study their histopathology. Necrotic areas stained lighter and viable tumor tissue darker (i). Blood vessels (arrow) were surrounded by viable cancer cells with atypic morphology with enlarged/variable size nucleus (j). (For color figure see online version.)
To study CAR and HSPG expression in M4A4-LM3 cells and xenograft tumors, we performed flow cytometric analysis. CAR expression was significantly higher in tumor cells in vivo versus in vitro: over 98.3% (±0.8%) were CAR positive in vivo compared to just 3.2% (±2.5%) when the same cells were grown in vitro (P<0.01) (Figure 5c–f). No significant differences were seen in HSPG expression levels in vitro versus in vivo (83.2±15.2 and 78.7±7.1% positive cells, respectively). As an additional control, we compared gene delivery with Ad5 to delivery with Ad5-RGD in vitro versus in vivo, and the same upregulation of the Ad5 receptor CAR was seen, whereas the receptors involved with Ad5-RGD seemed unaffected (not shown).
Immunohistochemical staining and histology
Immunohistochemical staining of M4A4-LM3 xenograft tumors showed that HSPG staining was pronounced in blood vessel endothelial cells (see arrowheads in Figure 5g) but less pronounced in viable cancer tissue. In contrast, necrotic areas seemed to stain strongly (not shown). CAR was highly expressed in the cytoplasm of the healthy tumor tissue but not in the necrotic areas (Figure 5h), blood vessels nor the basement membrane. Tumors had large necrotic areas (shown as lighter color in Figure 5i). Blood vessels (see arrowhead in Figure 5j) rarely penetrated deep into the tumor and tumors were lined by a continuous basement membrane. Tumors were composed mostly of cancer cells and normal tissue was sparse (Figure 5i and j).
Ad5.pK7-Δ24 in a systemic treatment model of orthotopic hormone refractory advanced breast cancer
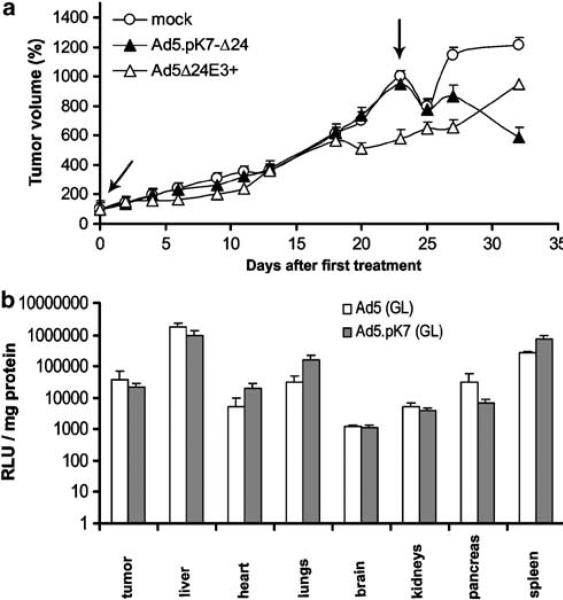
We allowed tumors to grow for 10 days for establishing advanced disease before intravenous injection of Ad5.pK7-Δ24, Ad5Δ24E3+ or growth medium. Twenty-three days after the first injection, mice received another injection (Figure 6a). In general, antitumor efficacy was less dramatic than with intratumoral injection. However, 32 days after the first virus injection, Ad5.pK7-Δ24-treated mice showed less GFP expression in the tumors when compared to both the mock-or Ad5Δ24E3+-treated mice (P<0.05). Despite a promising trend, there was no significant difference in survival between the groups. The median survivals were 27, 32 and 32 days in the mock, Ad5.pK7-Δ24 and Ad5Δ24E3+ groups, respectively. The capsid modification did not seem to have a big impact on the biodistribution of the agent (Figure 6b).
Figure 6.
(a) Therapeutic effect of intravenously injected Ad5.pK7-Δ24 in an orthotopic animal model of advanced hormone refractory breast cancer. GFP-expressing M4A4-LM3 cells were injected into the mammary fat pads of NMRI nude mice and primary tumors were allowed to grow for 10 days. Mice received two intravenous injections (3 × 1010 VP) of Ad5.pK7-Δ24, Ad5Δ24E3+ or no virus on days 10 and 33 (marked with an arrow) after the injection of cells (i.e. days 0 and 23 after treatment, respectively). Ad5.pK7-Δ24-treated mice had significantly fewer viable tumor cells on day 33 when compared to Ad5Δ24E3+ or mock-treated mice (P<0.05). Signal (photons) is depicted as a percentage of the initial photon count. (b) Biodistribution of Ad5.pK7 (GL) is similar to the isogenic Ad5 (GL) control. Bars denote s.e.
Discussion
Encouraging antitumoral efficacy and safety have been demonstrated in animal models with various CRAds. Nevertheless, this has not fully translated into similar clinical efficacy, which may be partly owing to poor transduction of a sufficient proportion of cells in solid tumor masses.31 A major determinant of oncolytic efficacy is infectivity, and therefore infectivity enhancement has emerged as an important goal.
In this study, we added seven lysine residues (pK7) into the fiber C-terminus of a CRAd to create Ad5.pK7-Δ24. HSPGs are expressed ubiquitously on cell surfaces and they may have a minor role in the binding of Ad2 and Ad5.32 However, advanced breast cancers have been reported to express significantly higher levels of HSPGs than normal breast cells.26–29 pK7 modification resulted in enhanced cell killing in vitro (Figure 2). Only in one cell line, MDA-MB-436, was the isogenic control CRAd Ad5Δ24E3+ superior to the pK7-modified counterpart, which may be a direct consequence of high CAR expression previously reported for this cell line.15 These data were well in accord with a previous paper where a polylysine modified CRAd was studied for therapy of glioma.33
To study the oncolytic efficacy of Ad5.pK7-Δ24 in a situation that models the clinical situation as closely as possible, we utilized a murine model of advanced breast cancer. M4A4-LM3 cells were labeled with GFP to allow noninvasive imaging. These cells are not hormone sensitive, which is another characteristic of the putative clinical target population, as anti-estrogen therapies would have priority over experimental approaches. Implantation of M4A4-LM3 xenografts into mammary fat pads results in early metastasis to regional lymph nodes, with later spread to distant lymph nodes and lungs.
Locally administered Ad5.pK7-Δ24 displayed significantly higher efficacy in comparison to the mock-treated group, but the isogenic control virus Ad5Δ24E3+ was even more effective. Oncolytic killing of tumor cells correlated closely with the reduction of fluorescent signal emitted from the tumors (Figure 3c). Importantly, photon emission was more sensitive and rapid in detecting antitumor response (Figure 3a and b). The tumors could be easily followed before and after treatment by noninvasive imaging. However, a slight drawback of GFP in noninvasive imaging is that organs, especially the liver, feature autofluorescence and that GFP is not surface-weighted. In other words, anything closer to the surface will appear brighter than structures deeper in the tissue.34
Interestingly, there seemed to be a discrepancy between the in vitro and in vivo efficacy of Ad5.pK7-Δ24 versus Ad5Δ24E3+. In vitro, the former was more oncolytic, whereas in vivo the latter seemed almost as effective after intravenous (Figure 6) or even superior following local delivery (Figures 3a, b and 4). Generally, it has been assumed that adenovirus receptors are expressed to a similar degree in vitro and in vivo. Further, this has been corroborated in many reports demonstrating similar infectivity data in vitro and in orthotopic models.21,22,35–37 Also, relatively good correlation has been seen between tumor cell lines grown two-dimensionally on plates or as three-dimensional spheroids.38–40 With regard to breast cancer, this has not been studied previously. However, a recent report suggested that the level and location of CAR were different when the same breast cancer cells were grown on plates versus in a three-dimensional model system.41 Furthermore, it was demonstrated with brain tumors that xenografts had higher CAR mRNA expression than the parental tumor cells.42 Moreover, with lung cancer cells, it was suggested that CAR may be required for effective xenograft formation.43
Our results suggest that orthotopic M4A4-LM3 tumors feature a higher CAR/HSPG ratio than the same cells grown in vitro (Figure 5c-f). Therefore, the infectivity of cells in vitro is not always the whole truth with regard to gene transfer and oncolysis in vivo. Also, given the nature of HSPGs as important components of the extracellular matrix of tumors, it is possible that binding to HSPGs may in fact hinder access of the virus to cancer cells. Nevertheless, binding to basement membrane HSPG might allow the virus to retain localization in the vicinity of the tumor for a longer time, resulting in a biological extended release system. It is also noteworthy that with intratumoral inoculation, high viral concentrations ensue, and part of the dose may be delivered to peripheral or even necrotic areas of the tumor. Both phenomena might negate differences in the transduction efficacy. Nevertheless, differences in CAR/HSPG-mediated transduction may help to shed some light on the biology of these moieties and their feasibility as utilization for viral antitumor approaches.
Given the systemic nature of the disease, it would be useful if efficacy could be achieved via intravenous delivery. Therefore, we utilized this route in mice with established advanced M4A4-LM3 tumors but saw only limited antitumor activity with Ad5.pK7-Δ24. Optimization of dose and schedule may yield improved results. Interestingly, Ad5.pK7-Δ24 seemed at least as effective as Ad5Δ24E3+ in this experiment. It is tantalizing to speculate that HSPG binding and subsequent extended localization near tumor cells might have played a role. High expression of HSPG in vasculature may contribute to the accumulation of systemically delivered Ad5.pK7-Δ24 into tumors (Figure 5i). Further, HSPGs have angiogenic characteristics,25 which might be a mechanism for HSPG expression in neo-vasculature and subsequent accumulation of pK7 modified virions to the tumor. Ad5.pK7-Δ24 may even have ‘antiangiogenic’ activity, if it results in the destruction of perivascular tissues. Further, as Ad5.pK7-Δ24 is not CAR-binding ablated, the virus may be able to utilize both CAR and HSPGs for spreading within the tumor.
In summary, Ad5.pK7-Δ24 is a novel CRAd that allows CAR-independent killing of tumor cells. Further studies are needed to investigate the full potential of this agent in the treatment of advanced cancers. One use of this virus might be re-treatment of patients previously treated with a CRAd with the Ad5 capsid, as utilization of a virus with a distinct capsid allows partial avoidance of pre-existing neutralizing antibodies, which are conformation specific.21,35,44 ‘Sero-switching’ the capsid might be a useful approach for retaining antitumor activity, as any CRAd will likely result in a neutralizing antibody response. Also, as many common and deadly tumor types such as colorectal, lung, pancreatic and ovarian cancers have been reported to frequently feature dysregulation of HSPG expression,45,46 Ad5.pK7-Δ24 might be useful for the treatment of a number of advanced cancer types. The capsid modification does not seem to affect the biodistribution much (Figure 6b), which may be useful with regard to safety, as Ad5-based CRAds have been safe in dozens of trials.
Materials and methods
Cell lines
GFP-expressing human breast cancer cell line M4A4-LM330 is an aggressive metastatic, hormone refractory subline derived from MDA-MB-435. Human transformed embryonic kidney cell line 293 and hormone refractory breast cancer cell lines MDA-MB-435, ZR-75-1, CAMA-1, MDA-MB-436 and MCF-7 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were maintained in the recommended conditions.
Construction of Ad5.pK7-Δ24
Ad5.pK7-Δ24 was constructed by co-transfecting (Effectene Transfection Reagent, Qiagen Corporation, Hilden, Germany) ethanol-precipitated Ad5.pK7 (GL) DNA47 with PacI- and PmeI-digested pShuttleΔ248 plasmid DNA in a 1:1 weight to weight ratio into 293 cells. Cells were incubated in a humidified incubator at +37°C with 5% CO2 for 8 h. Individual plaques were collected and purified for two rounds on A549 cells. Presence of the 24-bp deletion in E1A, absence of the wild-type E1A and the presence of an intact E3 region were confirmed with PCR as described.23 A PCR product from the fiber C-terminus (primers: F 5′-TCAGTCAAGTTTACTTAAACGGAG-3′ and R 5′-CTGTTACCCATGATATGATGCT -3′) was sequenced to verify the presence of the pK7 modification (sequencing primers: F 5′-GACACAACTCCAAGTGCATA-3′ and R 5′-GTTGAATACTAGGGTTCTGTGAG-3′). Ad5.pK7-Δ24 was amplified on A549 cells and purified using a standard double cesium chloride gradient method. Standard plaque assay and optical density (OD)260 were used for titering the virus. Production on A549 cells yielded titers of 1.6 × 1011 plaque-forming units (PFU)/ml and 2.2 × 1012 VP/ml.
Other recombinant adenoviruses
Conditionally replicating Ad5Δ24E3+48 and nonreplicating viruses Ad5lucI49 Ad5.pK7 (GL) and Ad5 (GL)47 have been described previously. Nonreplicating viruses were propagated on 293 cells, Ad5Δ24E3+ was propagated on A549 cells and all viruses were purified and titered as described previously. Production of Ad5Δ24E3+, Ad5 (GL), Ad5lucI and Ad5.pK7 (GL) yielded titers of 3.1 × 1012 VP/ml and 4 × 1011 PFU/ml, 2.9 × 1012 VP/ml and 2.7 × 1011 PFU/ml, 1.6 × 1012 VP/ml and 1.6 × 1011 PFU/ml and 3.2 × 1011 VP/ml and 2.7 × 1010 PFU/ml, respectively.
In vitro cytotoxicity assay
Cells in quadruplicate were infected with 0.1, 1 or 10 VP/cell for 1 h at 37°C in 50 μl of growth medium with 2% fetal calf serum (FCS), and incubated thereafter with growth medium with 5% FCS. Cell viability was measured using the CellTiter 96 AQUAEOUS One Solution Cell Proliferation Assay (MTS assay; Promega, Madison, WI, USA) on day 7 (MCF-7), day 11 (CAMA-1, M4A4-LM3) or 12 (MDA-MB-435).
Orthotopic breast cancer model in nude mice
Pathogen free 3-to 4-week-old female Naval Medical Research Institute (NMRI) nude mice were purchased from Taconic (Ejby, Denmark) and quarantined for 2 weeks. Mice were injected orthotopically into the left and right uppermost mammary fat pad with 2 × 106 M4A4-LM3 cells and a tumor was allowed to develop. In the local treatment model, when tumors reached the diameter of approximately 0.5 cm, mice were randomized into groups and injected with growth medium or 3 × 108 VP of Ad5.pK7-Δ24 or Ad5Δ24E3+ (all groups: n = 8 tumors in four mice). Viruses were diluted with mimimum essential growth medium without supplements and injected intratumorally in a total volume of 50 μl on 3 subsequent days (days 17, 18 and 19). Starting on day 20, mice were anesthetized using medetomidine (Domitor) and ketamine (Ketalar, Orion Pharma, Espoo, Finland) and imaged three times a week with the IVIS imaging system as recommended (Xenogen Corp., Alameda, CA, USA). Fixed size regions of interest were drawn around each tumor and chosen threshold values were set for minimum and maximum emission measured. Values were normalized to values obtained from a region of interest with no tumor. Tumors were measured in three dimensions using a caliber. The size was calculated by using formula ¾ π((0.5 × L)(0.5W)(0.5H)).
In the systemic treatment model, either growth medium or 3 × 1010 VP of Ad5.pK7-Δ24 (n = 14 tumors, seven mice) or Ad5Δ24E3+ (all groups: n = 14 tumors in seven mice) were injected in a total volume of 100 μl through the tail vein 10 days after the injection of cells, and again on day 33 (23 days after the first viral injection). On day 10, mice were imaged and tumor sizes were measured as above. When tumors reached 1 cm in any diameter, the mice were killed as required by animal regulations. Animal experiments were approved by the Provincial Government of Southern Finland.
Gene transfer assays
M4A4-LM3 cells in quadruplicates were infected for 30 min in room temperature with 40 VP/cell by adding Ad5.pK7 (GL) or Ad5 (GL) or no virus diluted in 200 μl of growth medium with 2% FCS. Cells were washed once and complete growth medium was added. Cells were incubated for 24 h at 37°C after which luciferase assay was performed (Luciferase Assay System, Promega). Background luciferase activities were subtracted from the data.
For in vivo studies, 2 × 106 cells of M4A4-LM3 were injected into the left uppermost mammary fat pad of NMRI nude mice and primary tumors were allowed to grow for 20 days. On day 20, 5 × 1010 VP of Ad5.pK7 (GL), Ad5 (GL) or no virus were injected through the tail vein. After 48 h, mice were killed and tumors were harvested. Tumors were homogenized in Cell Culture Lysis Buffer (Luciferase Assay System, Promega) and luciferase activity was analyzed as before. Mean background luciferase activity was subtracted from the data and the protein concentration was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) to allow normalization of the gene expression data for the number of cells.
Determination of receptor expression by flow cytometric analysis
M4A4-LM3 cells grown in cell culture flasks were washed with phosphate-buffered saline (PBS), harvested by incubating in 0.1% trypsin-ethylenediaminetetraacetic acid and suspended in PBS containing 2% FCS. Cells (2 × 105) were incubated with either anti-CAR primary antibody RmcB (1:100), anti-HSPG primary antibody 10E4 (SEIKAGAKU Co., Falmouth, MA, USA) (1:100) or buffer only for 30 min at 4°C. Cells were then rinsed with 2% FCS and incubated with 1:100 dilution of phycoeryhtrin (PE)-labeled goat anti-mouse immunoglobulin polyclonal antibody (BD Biosciences, Franklin Lakes, NJ, USA) for 30 min at 4°C. After cells were rinsed with 2% FCS, cells were immediately analyzed with flow cytometry.
To analyze receptor expression in M4A4-LM3 tumors grown in mammary fat pads, tumors were harvested on day 30, roughly homogenized with scalpels and further homogenized by exposing tissue to enzymatic dissociation for 2 h in 37°C using 0.2 Wünsch Units/ml of Liberase Blendzyme 1 (Roche Diagnostics, Indianapolis, IN, USA). Cells (2 × 105) were treated as described previously and analyzed by flow cytometry.
Immunohistochemical stainings
M4A4-LM3 xenograft tumors were harvested on day 30 and fixed in buffered formalin (10%), mounted on paraffin and deparafinized by treating with xylene and decreasing ethanol gradient. Deparafinized sections were treated with citrate buffer and methanol before blocking with 1.5% horse serum (Vectastain ABC kit, Vector Laboratories Inc., Burlingame, CA, USA). Slides were incubated overnight with 100 μl of antibody dilutions (10E4 for HSPG and RmcB for CAR, 1:200) and washed with PBS three times. Biotinylated secondary antibody (mouse IgG, 6 μl in 100 μl) was applied on the slides, and visualized by the Vectastain ABC system.
Histopathology
M4A4-LM3 xenograft tumors were harvested on day 30 and fixed in buffered formalin (10%) and cut in 5 μm sections. Deparafinized sections were stained with hematoxylin and eosin.
Biodistribution
Tumors were allowd to grow for 30 days. 3 × 1010 VP of Ad5 (GL) or Ad5.pK7 (GL) were injected through the tail vein. After 48 h, the mice (n = 7) were killed and organs were collected and analyzed for luciferase expression as described previously.35 Results were normalized to protein content of the organs by DC Protein Assay (Bio-Rad, Hercules, Manassas, CA, USA).
Statistical analysis
Cell viability and tumor size data were analyzed by two-tailed Student's t-test. P-value of <0.05 was considered statistically significant. Survival data were plotted into a Kaplan–Meir curve and groups were compared pairwise with log-rank test using SPSS 11.5.
Acknowledgements
This study was supported by HUCH Research Funds (EVO), Academy of Finland, Emil Aaltonen Foundation, Finnish Cancer Society, University of Helsinki, Sigrid Juselius Foundation, Helsinki Biomedical Graduate School, EU FP6 THERADPOX, Sohlberg Foundation, Biocentrum Helsinki, Instrumentarium Research Fund, Research and Science Foundation Farmos, the Finnish Breast Cancer Group and the Finnish Oncology Association.
References
- 1.Bernard-Marty C, Cardoso F, Piccart MJ. Facts and controversies in systemic treatment of metastatic breast cancer. Oncologist. 2004;9:617–632. doi: 10.1634/theoncologist.9-6-617. [DOI] [PubMed] [Google Scholar]
- 2.Hemminki A, Alvarez RD. Adenoviruses in oncology: a viable option? Biodrugs. 2002;16:77–87. doi: 10.2165/00063030-200216020-00001. [DOI] [PubMed] [Google Scholar]
- 3.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Peng Z, Han D, Zhang S, Pan J, Tang P, Xiao S, et al. Clinical evaluation of safety and efficacy of intratumoral administration of a recombinant adenoviral-p53 anticancer agent (Genkaxin®). Mol Ther. 2003;7(Suppl. 2):422. Abstract 1096. [Google Scholar]
- 5.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Quan ZZ, Liu JW, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 6.Kanerva A, Hemminki A. Modified adenoviruses for cancer gene therapy. Int J Cancer. 2004;110:475–480. doi: 10.1002/ijc.20129. [DOI] [PubMed] [Google Scholar]
- 7.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 8.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 9.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 10.Helt AM, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JJ, Tiniakos DG, McIntosh GG, Autzen P, Henry JA, Thomas MD, et al. Retinoblastoma protein in human breast carcinoma: immunohistochemical study using a new monoclonal antibody effective on routinely processed tissues. J Pathol. 1996;180:65–70. doi: 10.1002/(SICI)1096-9896(199609)180:1<65::AID-PATH607>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 14.Bauerschmitz GJ, Barker SD, Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review). Int J Oncol. 2002;21:1161–1174. [PubMed] [Google Scholar]
- 15.Lucas A, Kremer EJ, Hemmi S, Luis J, Vignon F, Lazennec G, et al. Comparative transductions of breast cancer cells by three DNA viruses. Biochem Biophys Res Commun. 2003;309:1011–1016. doi: 10.1016/j.bbrc.2003.08.101. [DOI] [PubMed] [Google Scholar]
- 16.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- 17.Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- 18.Hemminki A, Dmitriev I, Liu B, Desmond RA, Alemany R, Curiel DT. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res. 2001;61:6377–6381. [PubMed] [Google Scholar]
- 19.Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- 20.Wickham TJ, Tzeng E, Shears LL, Roelwink PW, Li Y, Lee GM, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- 22.Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- 23.Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 24.Bernt KM, Ni S, Gaggar A, Li ZY, Shayakhmetov DM, Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol Ther. 2003;8:746–755. doi: 10.1016/j.ymthe.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Sharma B, Handler M, Eichstetter I, Whitelock JM, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102:1599–1608. doi: 10.1172/JCI3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda K, Maruyama H, Guo F, Kleeff J, Itakura J, Matsumoto Y, et al. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001;61:5562–5569. [PubMed] [Google Scholar]
- 27.Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 28.Burbach BJ, Friedl A, Mundhenke C, Rapraeger AC. Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells. Matrix Biol. 2003;22:163–177. doi: 10.1016/s0945-053x(03)00009-x. [DOI] [PubMed] [Google Scholar]
- 29.Burbach BJ, Ji Y, Rapraeger AC. Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells. Exp Cell Res. 2004;300:234–247. doi: 10.1016/j.yexcr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Goodison S, Viars C, Urquidi V. Molecular cytogenetic analysis of a human breast metastasis model: identification of phenotype-specific chromosomal rearrangements. Cancer Genet Cytogenet. 2005;156:37–48. doi: 10.1016/j.cancergencyto.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Kirn D. Clinical research results with dl1520 (onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Therapy. 2001;8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 32.Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–8780. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinoura N, Yoshida Y, Tsunoda R, Ohashi M, Zhang W, Asai A, et al. Highly augmented cytopathic effect of a fiber-mutant E1B-defective adenovirus for gene therapy of gliomas. Cancer Res. 1999;59:3411–3416. [PubMed] [Google Scholar]
- 34.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 35.Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 36.Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002;62:1266–1270. [PubMed] [Google Scholar]
- 37.Hemminki A, Zinn KR, Liu B, Chaudhuri TR, Desmond RA, Rogers BE, et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002;94:741–749. doi: 10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- 38.Lam JT, Bauerschmitz GJ, Kanerva A, Barker SD, Straughn JM, Wang JM, et al. Replication of an integrin targeted conditionally replicating adenovirus on primary ovarian cancer spheroids. Cancer Gene Ther. 2003;10:377–387. doi: 10.1038/sj.cgt.7700578. [DOI] [PubMed] [Google Scholar]
- 39.Lam JT, Kanerva A, Bauerschmitz GJ, Takayama K, Suzuki K, Yamamoto M, et al. Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy. J Gene Med. 2004;6:1333–1342. doi: 10.1002/jgm.635. [DOI] [PubMed] [Google Scholar]
- 40.Lamfers ML, Hemminki A. Multicellular tumor spheroids in gene therapy and oncolytic virus therapy. Curr Opin Mol Ther. 2004;6:403–411. [PubMed] [Google Scholar]
- 41.Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA. 2003;100:1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuxe J, Liu L, Malin S, Philipson L, Collins VP, Pettersson RF. Expression of the coxsackie and adenovirus receptor in human astrocytic tumors and xenografts. Int J Cancer. 2003;103:723–729. doi: 10.1002/ijc.10891. [DOI] [PubMed] [Google Scholar]
- 43.Qin M, Escuadro B, Dohadwala M, Sharma S, Batra RK. A novel role for the coxsackie adenovirus receptor in mediating tumor formation by lung cancer cells. Cancer Res. 2004;64:6377–6380. doi: 10.1158/0008-5472.CAN-04-1490. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Hemminki A, Siegal GP, Barnes MN, Dmitriev I, Krasnykh V, et al. Adenoviruses with an RGD-4C modification of the fiber knob elicit a neutralizing antibody response but continue to allow enhanced gene delivery. Gynecol Oncol. 2005;96:341–348. doi: 10.1016/j.ygyno.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 45.Blackhall FH, Merry CL, Davies EJ, Jayson GC. Heparan sulfate proteoglycans and cancer. Br J Cancer. 2001;85:1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, et al. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–1653. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki K, Alemany R, Yamamoto M, Curiel DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res. 2002;8:3348–3359. [PubMed] [Google Scholar]
- 49.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]