Abstract
Various neuropeptides have emerged recently as potent immunomodulatory factors with potential for their therapeutic use on immune disorders. Here we highlight the most recent data relevant in the field and we offer our opinion how neuropeptide therapy might impact clinical immune diseases, and the challenges in this field that must be overcome before achieving medical progress. We also review recent reports describing the antimicrobial effects showed by some neuropeptides and the therapeutic, physiological and evolutionary consequences of this new finding. Finally, we discuss how a physiologically functional neuropeptide system contributes to general health and how neuropeptides educate our immune system to be tolerant.
Keywords: Neuropeptide, Autoimmunity, Inflammation, Antimicrobial, Tolerance, Parasite, Neuroimmunology
Introduction
The fact that the immune and neuroendocrine systems interact with each other was suspected and postulated more than a century ago. More recently it has been clearly established that the immune system communicates in a bidirectional way with the nervous system. This dialogue implies that the immune system acts as a sixth sense informing the nervous system that a systemic immune/inflammatory response to infection or tissue injury is occurring. The nervous system responds to this call by modifying behavioral responses (fever, reduction of locomotor activity, appetite and reproduction,…) and by triggering molecular pathways, in general anti-inflammatory and immunosuppressive in nature, which restrain and limit the immune response (secretion of glucocorticoids through the activation of the hypothalamus-pituitary-adrenal axis is the best example) (1). In addition, in conditions of physical and psychological stress, the brain controls the immune system (1). It is obvious that this communication can only be maintained through a mutual biochemical language, with cytokines/interleukins produced by immune cells being recognized by receptors expressed on cells of the neuroendocrine system, and vice versa, with cells of the immune system recognizing neurotransmitters, neuropeptides and hormones produced by the nervous and endocrine system (1). One of the most interesting issues in this scenario is that immune cells (including lymphocytes, macrophages, dendritic cells, mast cells and polymorphonuclear cells) can also produce neuropeptides in response to antigenic and inflammatory signals, and that these neuropeptides act in an autocrine/paracrine manner through specific receptors expressed on immune cells (2). In the last two decades, the list of neuropeptides acting as cytokine-like factors in the regulation of the immune response has been growing. Most of these neuropeptides show a clear anti-inflammatory profile, acting with apparent redundancy mainly on macrophages, microglia and neutrophils, in an attempt to keep under control the innate inflammatory response (Table 1, Figure 1). Fewer neuropeptides modulate the acquired immune response. Even more limited is the number of neuropeptides with a prominent role in the regulation of immune tolerance, and therefore, exhibiting the potential for therapy in autoimmune disorders (Table 1). In the last few years, several publications have extensively reviewed the role of neuropeptides and hormones in the regulation of the immune system, discussed the mechanisms involved, and analyzed the potential beneficial effects of neuropeptide-based therapies in inflammatory diseases (3-6). The aim of the present work is to highlight the most recent data relevant to the following outstanding questions related to the plethora of neuropeptide regulation of the immune system: 1) how far are we from the clinical application of neuropeptides in disorders with an immunological base; 2) does a neuropeptide-based treatment increase susceptibility to opportunistic infections; and finally, 3) how does a physiologically functional neuropeptide system contribute to general health. Moreover, we will offer our opinion on how neuropeptide therapy might impact clinical immune diseases, and the challenges in this field that must be overcome before achieving medical progress.
Table 1. Neuropeptides with relevant roles in inflammation or autoimmunity.
| Neuropeptide | Beneficial effect on experimental models |
|---|---|
| With Pro-inflammatory effects: | |
| Substance P/Neurokinins | Use of antagonists in inflammatory bowel |
| Bradykinin | disease and lung inflammation |
| CRH | |
| With Anti-inflammatory effects: | |
| Somatostatin | |
| CGRP | |
| Galanin | Sepsis, Endotoxemia |
| VIP/PACAP | Pancreatitis |
| α-melanocyte stimulating hormone | Inflammatory bowel disease, colitis |
| ACTH | Nephritis |
| Opoid peptides | Liver inflammation |
| Urocortin | Airway/lung inflammation |
| Atrial natriuretic peptide | Neurodegenerative diseases |
| Nociceptin | Brain injury |
| Melanin concentrating hormone | Ischemia (various organs) |
| Adrenomedullin | Gingivitis/Oral inflammation |
| Chollecystokinin | Osteoarthritis |
| Neuropeptide Y (NPY) | |
| Cortistatin | |
| Ghrelin | |
| With effects on Autoimmunity: | |
| NPY | |
| VIP/PACAP | |
| Ghrelin | Experimental autoimmune encephalomyelitis |
| Renin-angiotensin | Rheumatoid arthritis |
| Cortistatin | Systemic lupus erytematous |
| α-melanocyte stimulating hormone | Crohn's disease |
| Urocortin | Experimental autoimmune uveoretinitis |
| Adrenomedullin | Type 1 diabetes |
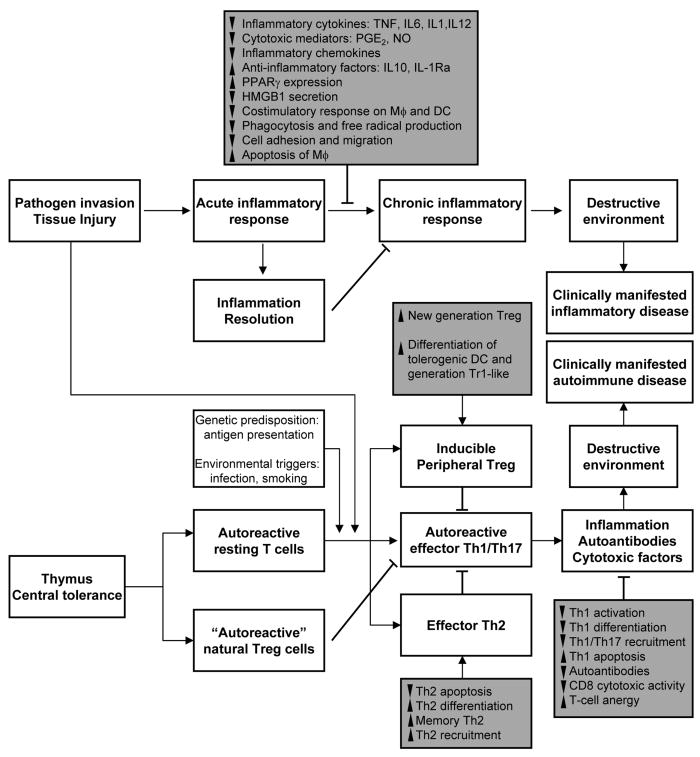
Figure 1. Regulation of the inflammatory and autoimmune responses by neuropeptides.
Grey boxes depict the mechanisms involved on the inhibition of inflammation and autoimmunity by neuropeptides. Black arrow, stimulation. Back-crossed line, inhibition. ▲, increase; ▼, decrease. Treg, regulatory T cells; Tr-1, type 1 Treg; DC, dendritic cells; MΦ, macrophages; NO, nitric oxide; PGE2, prostaglandin E2; PPARγ, peroxisome proliferator-activated receptor-γ; HMGB1, high-mobility group box-1; IL, interleukin; TNF, tumor necrosis factor; IL-1Ra, IL-1 receptor antagonist.
Are neuropeptides ready for the clinical practice in immune disorders?
Table 1 shows some of the experimental models where neuropeptides have been proven effective reducing clinical progression. A major question is how close we are from an effective clinical translation of neuropeptide-based treatments in inflammatory and autoimmune disorders. Early evidence demonstrated advantages of neuropeptide treatment over current therapies. The most relevant advantage is that instead controlling a unique factor, neuropeptides regulate a wide spectrum of inflammatory mediators, including cytokines, chemokines and cytotoxic factors (Figure 1). For example, VIP, PACAP and cortistatin decrease the production of the inflammatory mediator PGE2 by inhibiting the expression of cycloxygenase 2 (COX2) in macrophages, dendritic cells and microglia (7,8). Of special interest is the suppressive effect observed for some of these neuropeptides on activated microglia. Thus, VIP and ghrelin suppress the inflammatory response induced by beta-amyloid fibrils and by the neurotoxin MPTP on microglia (9-11). This raised the possibility of using these neuropeptides in Alzheimer's and Parkinson's disease, characterized by inflammation-induced neurodegeneration. Similar to VIP (12), ghrelin was recently shown to protect mice from neurodegeneration in a model Parkinson's disease by blocking microglial activation (11). Moreover, deactivation of microglia by ghrelin seems to play a major role in the protective effect of this neuropeptide in experimental autoimmune encephalomyelitis (EAE) (13).
Another critical factor that has been recently found to be downregulated by neuropeptides is the high-mobility group box-1 (HMGB1). HMGB1 is a DNA-binding protein that has been implicated as an essential and sufficient mediator of lethal endotoxemia and sepsis. Different studies demonstrated that VIP, urocortin, ghrelin and PACAP inhibit the secretion of HMGB1 by activated macrophages in vitro and in vivo, and that this effect is related to a protective role of these neuropeptides in established sepsis (14-17). Since HMGB1 is a late mediator of sepsis, neuropeptide treatments quite attractive for an immediate clinical application in human sepsis, since this would extend the therapeutic window to a clinically relevant time frames (18).
Most of the neuropeptide effects on inflammation are directly related to the downregulation of inflammatory mediators. With the exception of IL-10 and IL-1Ra, no other anti-inflammatory factors were found to be affected by neuropeptides until the publication of two recent papers reporting the induction by ghrelin and adrenomedullin of peroxisome proliferator-activating receptor-γ (PPARγ) in activated macrophages (19,20). Beside its involvement in the regulation of fatty acid and glucose metabolism, and in the proliferation and differentiation of adipocytes, PPARγ is a ligand-activated transcription factor recently identified in the anti-inflammatory pathways in endothelial cells and macrophages. Regulation of PPARγ by neuropeptides is not only relevant for switching-off the inflammatory response in conditions such as endotoxemia, but also in non-traditionally considered inflammatory disorders such as atherosclerosis, where PPARγ plays a major role in the regulation of cholesterol accumulation in macrophages and formation of “foam cells” in plaques. There is strong evidence that atherosclerosis presents the characteristics of an inflammatory disorder with a Th1-mediated autoimmune response directed against components of the arterial wall (21). Since neuropeptides such as VIP and PACAP downregulate Th1 type responses, anti-inflammatory neuropeptides emerge as attractive candidates to treat this vascular disease.
In our opinion, the most important finding in the last years has been the realization that some neuropeptides, including MSH, VIP, PACAP, urocortin, ghrelin, cortistatin and adrenomedullin, actively participate in the restoration of immune tolerance, especially under autoimmune conditions, by inducing the generation of regulatory T cells (Treg) (22-25). Treg are considered the “blue helmets” of the immune system playing a key role in the maintenance of self-tolerance (26). In fact, almost all autoimmune disorders are characterized by a decrease in Treg number or in functional impairment. Two major populations of Treg have been described. Naturally-occurring Treg are constitutively generated in thymus, and characterized by high expression of CD25 and the transcription factor FoxP3. Peripherally induced Treg consist of IL-10-secreting Tr1 cells, transforming growth factor-secreting Treg, and inducible FoxP3 Treg. Regardless of their origin, these Treg suppress autoreactive effector T cells in an antigen-dependent manner (26). Previous evidence, obtained mostly in vivo, suggested that neuropeptides induce the de novo generation of peripheral CD4+CD25+ IL-10-secreting T cells from the CD4+CD25- repertoire (22-25). The induction of tolerogenic dendritic cells (tolDCs) by MSH, VIP and PACAP appeared to play a major role in the generation of some of these Treg, at least in vitro (27). These previous findings clearly showed that the induction of Treg by neuropeptides was important for their potent antigen-specific effect in experimental autoimmunity, and raised the possibility of a cell-based strategy to treat autoimmunity and allogeneic transplantation by providing long-term tolerance to self-antigens and alloantigens (25,27). This is tremendously attractive from a therapeutic point of view, because the translation of important biological findings about Treg to a clinic setting has been limited by the inability to define their antigenic specificity and by the scarcity of circulating Treg. It has been proposed that the solution to this problem might lie in expanding the Treg cell population in vitro and by using selected antigens and peptides to generate antigen-specific Treg (28). A previous study from our laboratory demonstrated that it is possible to induce alloantigen-specific human Treg through the differentiation of tolDCs with VIP (29). Moreover, in two recent reports we confirmed that activation (through the T cell receptor or with alloantigens) of human blood CD4+CD25- lymphocytes in the presence of VIP resulted in the generation of CD4+CD25+FoxP3+ IL-10-expressing Treg cells with potent suppressive capacity on effector or allogeneic T cells (30,31). Both studies showed that blocking cell-cycle progression and the induction of an anergic state, as well as the expression of the suppressive molecule CTLA-4, are prerequisites for the induction of VIP-converted Treg, and that different molecular players and pathways are finely orchestrated at multiple levels. The clinical translation of these recent findings depends on whether the considerable variability that exists among patients affects the capacity of the neuropeptides to generate ex vivo Treg or tolDCs in GMP conditions with the same efficacy, reliability, homing capacity, and survival in vivo as observed in animal models and healthy human subjects.
Independent of the progression of clinical Treg-based therapies, the exciting results reported in the last few years support the implementation of therapeutic strategies based on the use of neuropeptides as drugs. In addition to their potent and diverse actions on various components in immunological disorders, as physiological endogenous compounds, neuropeptides are intrinsically non-toxic, and are not associated with dramatic side effects, as compared to existing anti-inflammatory and immunosuppressive drugs. In fact, most of these neuropeptides have been previously administered to humans for the treatment of sepsis and other disorders without apparent complications (32). Systemic infusion of ghrelin in patients with cachexia and chronic pulmonary infections led to a significant decrease in airway inflammation (33). One of the most relevant studies has been reported in 2010 by Prasse and coworkers (34), describing the therapeutic effect of VIP in patients with active sarcoidosis, a systemic disease characterized by the formation of granulomas affecting primarily the lung. Although its etiology remains unknown, numerous evidences indicate that an alveolar macrophage/Th1-driven inflammatory response in the granuloma is a central element in the evolution of sarcoidosis. This open labeled phase II study, using inhalation of nebulized VIP, demonstrated that VIP was safe and well tolerated, and that it significantly reduced the production TNFα by bronchoalveolar cells, the expression of costimulatory molecules on alveolar macrophages, and increased the numbers of bronchoalveolar CD4+CD25+FoxP3+ Treg (34). This is the first study showing the immunoregulatory effect of exogenous VIP in patients and describing the induction of Treg in human lung.
Despite the effectiveness recently described for the systemic and local administration of neuropeptides in human diseases, including immune disorders, an obstacle that has to be solved in order to translate neuropeptide-based treatments into viable clinic therapies is related to their nature as peptides, due to their sensitivity to degradation by peptidases. Recently, important progress has been made in increasing the neuropeptide half-life, and improving their targeted tissue delivery. Aminoacid modifications or substitutions have been previously proposed as a general strategy to increase neuropeptide stability (35). Onoue and coworkers have recently generated two VIP derivatives with considerable stability (more than 60 days at 40°C), which showed efficacy as an inhalable powder formulation in a model of asthma/COPD by reducing various inflammatory parameters (36,37). The efficacy of these VIP derivatives in other human disorders needs confirmation, but for now, they have tremendous promise for the clinic.
Various recent reports described new strategies to improve neuropeptide delivery. Previously, formulations based on micelles or liposomes were shown to continuously release neuropeptides in vitro; recently, this strategy has been used in vivo in a model of autoimmune uveoretinitis (38). However, the size of neuropeptide-liposome formulations could compromise their access to the target organ. To avoid this potential problem, a recent study described a formulation of silver-protected VIP nanoparticles which functioned similar to naïve VIP deactivating microglia (39). This study provided for the first time proof of principle for the use of metal nanoparticles for neuropeptide targeting and drug delivery. The efficacy of nanoparticle-based therapy has to be demonstrated in vivo in inflammatory conditions.
In parallel, some of the tools used for gene therapy in other diseases emerged as an attractive alternative for neuropeptides delivery to target tissues. Adenoviral vectors and adeno-associated viral vectors expressing neuropeptides have been used previously to treat inflammatory and autoimmune disorders in animal models (32). To avoid immune responses generated by these vectors, we have recently evaluated the efficacy of lentiviral vectors expressing VIP in a model of autoimmune arthritis (40). The most remarkable effect of the lenti-VIP vectors was their potent therapeutic effect in established severe arthritis, probably due to long-term secretion of the neuropeptide. However, since these vectors integrate in almost all cells when administered systemically, further research should be focused on integration selectivity and on the incorporation of regulatory elements to switch-on/off their expression, such as Tet-on systems or tissue/cell promoter-selective elements. A potential alternative could be the use of a combined gene-cell therapy, in which the neuropeptide-containing vector is integrated in a certain cell ex vivo before inoculation. Following this strategy, we demonstrated recently that dendritic cells transduced with lenti-VIP vectors during differentiation have a therapeutic effect in EAE and sepsis models (41). A similar effect in EAE was reported for antigen-specific T cells transduced with adeno-associated vectors encoding α-MSH (42). These effects are based in the facts that the lenti-VIP-DCs and AAV-MSH-T-cells exhibit a tolerogenic/suppressive profile, migrating to the site of inflammation and peripheral lymphoid organs where they act as “Trojan horses”, continuously secreting the neuropeptide locally. This strategy not only increases the efficacy of the treatment, but also addresses the selectivity/safety issue. Finally, other groups have suggested that the administration of neuropeptide-binding proteins that have been isolated from the serum together with the corresponding neuropeptide would protect them from peptidases and increase their delivery in the proximity of the target cells. Recent advances have been made in this sense, especially with adrenomedullin (19), although further research is needed for most of the neuropeptides.
The development of metabolically stable neuropeptide analogs might represent a desirable approach especially for oral administration. However, since many of the immunoregulatory neuropeptides have pleiotropic effects, the systemic administration of stable analogs poses a safety issue. However, a surprising recent work describing the protective effect in EAE of orally administered unaltered α-MSH (43) opens up the interesting possibility of using natural neuropeptides in an oral regimen for the treatment of certain immune disorders.
Why do neuropeptide treatments not cause general immunosuppression?
Based on the wide spectrum of anti-inflammatory activities of most neuropeptides, a potential general immunosuppressive side-effect has been raised as an important concern related to neuropeptide-based therapies. However, although more studies are needed, the occurrence of opportunistic infections in animals, and more recently in humans treated with neuropeptides turned out to be quite scarce. One possible explanation is the immunomodulatory rather than immunosuppressive effect of most neuropeptides, particularly in terms of adaptive immune responses. The neuropeptide-induced generation of tolDC and the induction of Treg occur mostly in an antigen-dependent manner in models of autoimmune diseases, with minimal effects on the response to unrelated antigens.
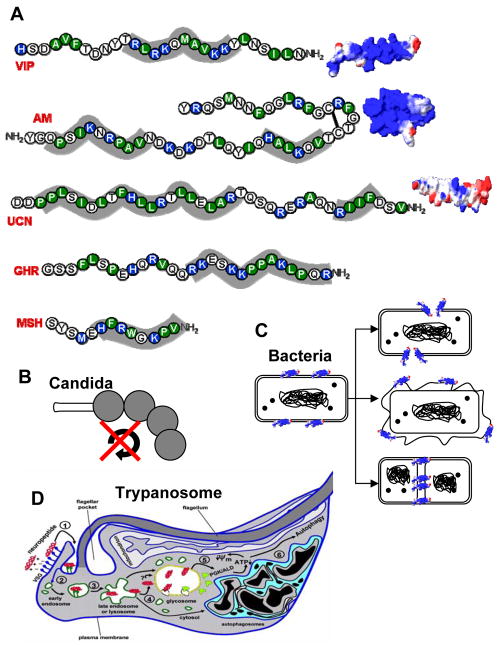
Another essential reason for the lack of general immunosuppression was revealed in experiments performed in sepsis models where neuropeptide treatment blunted the inflammatory response while reducing systemic bacteremia in the same time. These studies led to the discovery that the anti-inflammatory neuropeptides VIP, MSH, ghrelin and adrenomedullin have potent antimicrobial activities (14, 44-48). The antimicrobial effect of these neuropeptides has a very wide range, extending to a variety of gram-positive and gram-negative bacteria, yeast and parasites. Interestingly, based on structure and sequence, these neuropeptides have a number of similarities with the previously described natural antimicrobial peptides (i.e., defensins), including small size, aminoacid composition, amphipathic design and cationic charge (Figure 2A), and adopt conventional α-helical structures in membrane-like environments. Various mechanisms have been proposed to explain their antimicrobial activities based on direct interactions of the neuropeptide with pathogens (Figure 2B-D). The effect on Candida albicans is probably the most controversial. An initial report that an MSH-derived fragment of only three aminoacids blocked the cell cycle and the transition between budding and hyphal forms by signaling through a new melanocortin-like receptor (Figure 2B) has not been yet confirmed (47). What is more clearly established is that neuropeptides interact through their cationic residues with the negatively-charged cell surface of bacteria, followed by insertion of their hydrophobic domains which leads to cell wall/membrane destabilization and pore formation or cross the membrane and target various intracellular compartments (Figure 2C). It is estimated that a single transmembrane pore is enough to kill a bacteria. The mechanism involved in the microbicidal effect on African parasites such as Trypanosoma brucei, the causative agent of sleeping sickness, is different and unusual. In this case, neuropeptides are endocytosed by the parasite by being carried by negatively-charged surface glycoproteins constantly renewed to avoid recognition by immune cells. Once inside, neuropeptides integrate in and break the membrane of the lysosome. This should be enough to promote the death of the parasite, but at the same time, neuropeptides are released to the cytoplasm and reach the glycosome, a unique organelle that contains the glycosome enzymes. It appears that the neuropeptides disrupt the membrane of the glycosome and induce the translocation of the gycolytic enzymes to the cytoplasm, where they cause a collapse in the energetic metabolism of the parasite. Both lysosome disruption and ATP depletion cause the death of the parasite through an autophagy-like mechanism (48).
Figure 2. Antimicrobial activity of neuropeptides.
A. Neuropeptide sequences indicating hydrophobic (green) and positive-charged (blue) residues. Shadow areas indicate potential interacting motifs of neuropeptides with microbial membranes. Three-dimensional models for charge distribution in the peptides are shown (blue region: positive charge; red region, negative charge; white region, neutral charge). B. Neuropeptides inhibit cell cycle and budding-to-hyphal-form transition on yeast. C. After their binding to bacteria surface, neuropeptides can form membrane pores or destabilize membrane compromising membrane integrity/permeability or can inhibit the formation of septum which is essential for bacterial division. D. Cationic neuropeptides are endocytosed by parasites and alter cellular traffic and parasite metabolism, disrupt lysosomes and induce autophagy-like cell death. AM, adrenomedullin; VIP: vasoactive intestinal peptide; GHR, ghrelin; MSH, melanocyte-stimulating hormone; UCN, urocortin.
All these findings have important implications from both a therapeutic and physiological point of views. First, neuropeptides emerge as promising candidates for a new type of anti-pathogen agents based on their antibacterial and antiparasitic activities. Since neuropeptides affect unique processes/organelles of parasites not shared by the host, they might provide a more specific type of treatment. Second, neuropeptides could be considered as part of the innate defense because of high expression in most of the defense barrier-tissues (skin, oral cavity, and urogenital, gastrointestinal and respiratory tracts). In response to bacterial and parasite infections, local and systemic levels of neuropeptides increase. Third, although clearly involved in several physiological/developmental processes of pluricellular organisms, programmed cell death by autophagy is a new concept for unicellular organisms. However, from the point of view of the parasite as a colony and of its interaction with the host, the induction of trypanolytic neuropeptides during infection could mean an evolutionary advantage for the trypanosome, where neuropeptide released by cells of the hosts could lead to elimination of most of the parasite population and selection of the fittest parasites, favoring parasite transmission for a longer time by keeping the host alive (49). Fourth, from an evolutionary point of view, it is important to remember that most of these neuropeptides occur in the whole animal kingdom, appearing in very primitive organisms with very simple nervous and immune systems. One possibility is that the primeval role of neuropeptides was closely related to the natural immune defense as antimicrobial peptides, that they coevolved with the pathogens, and acquired additional functions during evolution. This could also explain the fact that fragments of neuropeptides (i.e. VIP6-28), that are produced by polymorphonuclear cells and that are not able to signal through the corresponding receptors on mammalian cells, maintain full parasiticidal activity (48), suggesting that, in fact, one of the major functions of neuropeptides is as antimicrobial peptides.
Does an altered neuropeptide system increase our susceptibility to immune disorders?
The final question is related to the physiological role played by endogenous neuropeptides in the maintenance of immune homeostasis. Previous evidence, obtained primarily in animals deficient in one of these neuropeptides or their receptors, indicated that in fact the absence of adrenomedullin, VIP, VIP/PACAP-receptors or urocortin resulted in higher susceptibility to endotoxemia, lung inflammation and Th1-mediated responses in vivo (reviewed in Ref. 32). A recent paper using PACAP-KO mice supported these findings in an autoimmune environment, showing higher EAE incidence and severity, which correlated with an increased inflammatory/Th1-driven response and a decrease in the number of antigen-specific Treg (50). Various reports suggested that similar conclusions in humans. Thus, monocytes and/or T cells isolated from patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis and multiple sclerosis express less receptors and respond less to VIP than cells from healthy subjects (51-54). This altered expression of VIP receptors seems to be related to an aberrant autoreactive Th1-driven inflammatory response in these patients, and even more important, it is genetically associated to a functional polymorphism in rheumatoid arthritis and spondylitis (52,54). Moreover, this polymorphism co-occurs with the HLA-B*2705 allele, which is strongly associated to ankylosing spondylitis (54), a particular setting that might be due to a founder effect or be the consequence of selective pressure.
Taken together, these results suggest that neuropeptides and their receptors are intrinsic regulators of immune tolerance, and that a defect in their signaling system affects the maintenance of immune homeostasis.
Conclusions
Helen Keller said in her masterpiece Optimist that “the highest result of education is tolerance”. The data reported in the last few years regarding the effect of neuropeptides on the immune system clearly indicate that certain neuropeptides play a critical role in the self-tolerance education of the immune system. It will probably take some time to have this knowledge translated into the clinical use of neuropeptides in immune disorders. Although many questions still have to be answered and new therapeutic approaches have to be developed, in our opinion, this day is getting closer.
Acknowledgments
This work has been partially supported by Spanish Ministry of Science and Innovation (E.G.R), National Institutes of Health (D.G.) and Spanish Ministry of Health and Junta de Andalucia (M.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elena Gonzalez-Rey, Email: elenag@ipb.csic.es.
Doina Ganea, Email: doina.ganea@temple.edu.
References
- 1.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Rey E, Delgado M. Anti-inflammatory neuropeptide receptors: new therapeutic targets for immune disorders? Trends Pharmacol Sci. 2007;28:482–491. doi: 10.1016/j.tips.2007.07.001. [DOI] [PubMed] [Google Scholar]
- •3.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review describing the effects of some anti-inflammatory neuropeptides, and the cellular and molecular mechanisms involved, on the induction of immune tolerance.
- 4.Lühder F, Lee DH, Gold R, Stegbauer J, Linker RA. Small but powerful: short peptide hormones and their role in autoimmune inflammation. J Neuroimmunol. 2009;217:1–7. doi: 10.1016/j.jneuroim.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Delgado M, Ganea D. Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–1151. doi: 10.1016/j.bbi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147:S202–S211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dello Russo C, Lisi L, Navarra P, Tringali G. Diverging effects of cortistatin and somatostatin on the production and release of prostanoids from rat cortical microglia and astrocytes. J Neuroimmunol. 2009;213:78–83. doi: 10.1016/j.jneuroim.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide inhibits cyclooxygenase-2 expression in activated macrophages, microglia, and dendritic cells. Brain Behav Immun. 2008;22:35–41. doi: 10.1016/j.bbi.2007.07.004. [DOI] [PubMed] [Google Scholar]
- •9.Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, Kim S, Lee D, Chung H, Oh MS, et al. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease by blocking microglial activation. Neurotox Res. 2009;15:332–347. doi: 10.1007/s12640-009-9037-x. [DOI] [PubMed] [Google Scholar]; This paper describes the therapeutic effect of ghrelin in a mouse model of Parkinson's disease It also confirms that deactivation of the inflammatory response mediated by microglia is an attractive strategy to treat some neurodegenerative diseases.
- 10.Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56:1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- 11.Bulgarelli I, Tamiazzo L, Bresciani E, Rapetti D, Caporali S, Lattuada D, Locatelli V, Torsello A. Desacyl-ghrelin and synthetic GH-secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by beta-amyloid fibrils. J Neurosci Res. 2009;87:2718–2727. doi: 10.1002/jnr.22088. [DOI] [PubMed] [Google Scholar]
- 12.Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 13.Theil MM, Miyake S, Mizuno M, Tomi C, Croxford JL, Hosoda H, Theil J, von Hörsten S, Yokote H, Chiba A, et al. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J Immunol. 2009;183:2859–2866. doi: 10.4049/jimmunol.0803362. [DOI] [PubMed] [Google Scholar]
- •14.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]; This works describes the antimicrobial effect of ghrelin and the inhibitory action on the secretion of HMGB1 in experimental sepsis Ghrelin emerges as an attractive factor to design new therapies for the treatment of sepsis showing a wide therapeutic window.
- 15.Wu R, Dong W, Qiang X, Wang H, Blau SA, Ravikumar TS, Wang P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit Care Med. 2009;37:2421–2426. doi: 10.1097/CCM.0b013e3181a557a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Lv B, Wang H, Xiao X, Zuo X. PACAP inhibit the release and cytokine activity of HMGB1 and improve the survival during lethal endotoxemia. Int Immunopharmacol. 2008;8:1646–1651. doi: 10.1016/j.intimp.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink MP. Neuropeptide modulators of high mobility group box 1 secretion as potential therapeutic agents for severe sepsis. Am J Pathol. 2008;172:1171–1173. doi: 10.2353/ajpath.2008.080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miksa M, Wu R, Cui X, Dong W, Das P, Simms HH, Ravikumar TS, Wang P. Vasoactive hormone adrenomedullin and its binding protein: anti-inflammatory effects by up-regulating peroxisome proliferator-activated receptor-gamma. J Immunol. 2007;179:6263–6272. doi: 10.4049/jimmunol.179.9.6263. [DOI] [PubMed] [Google Scholar]
- 20.Demers A, Caron V, Rodrigue-Way A, Wahli W, Ong H, Tremblay A. A concerted kinase interplay identifies PPARgamma as a molecular target of ghrelin signaling in macrophages. PLoS One. 2009;4:e7728. doi: 10.1371/journal.pone.0007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George J. Mechanisms of disease: the evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:531–540. doi: 10.1038/ncpcardio1279. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Rey E, Chorny A, Varela N, O'Valle F, Delgado M. Therapeutic effect of urocortin on collagen-induced arthritis by downregulating inflammatory and Th1 response and inducing regulatory T cells. Arthritis Rheum. 2007;56:531–543. doi: 10.1002/art.22394. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AW, Kitaichi N. The diminishment of experimental autoimmune encephalomyelitis (EAE) by neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) therapy. Brain Behav Immun. 2008;22:639–646. doi: 10.1016/j.bbi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Rey E, Chorny A, O'Valle F, Delgado M. Adrenomedullin protects from experimental arthritis by downregulating inflammation and Th1 response and inducing regulatory T cells. Am J Pathol. 2007;170:263–271. doi: 10.2353/ajpath.2007.060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med. 2007;13:241–251. doi: 10.1016/j.molmed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Delgado M. Generating tolerogenic dendritic cells with neuropeptides. Hum Immunol. 2009;70:300–307. doi: 10.1016/j.humimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107:3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •30.Pozo D, Anderson P, Gonzalez-Rey E. Induction of alloantigen-specific human T regulatory cells by vasoactive intestinal peptide. J Immunol. 2009;183:4346–4359. doi: 10.4049/jimmunol.0900400. [DOI] [PubMed] [Google Scholar]; This is the first paper describing the ex vivo generation of alloantigen specific human Treg cells with a neuropeptide, and their potential application in allogeneic bone marrow transplantation It also points out some of the cellular events involved in such effect.
- 31.Anderson P, Gonzalez-Rey E. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human T cells at multiple levels. Mol Cell Biol. doi: 10.1128/MCB.01282-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson P, Delgado M. Endogenous anti-inflammatory neuropeptides and pro-resolving lipid mediators: a new therapeutic approach for immune disorders. J Cell Mol Med. 2008;2:1830–1847. doi: 10.1111/j.1582-4934.2008.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••33.Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–779. doi: 10.1016/j.pupt.2008.05.001. [DOI] [PubMed] [Google Scholar]; This paper describes that unmodified naïve ghrelin can be safely administered systemically and regulate airway inflammatory response in patients with lung infection.
- ••34.Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, et al. Inhaled Vasoactive Intestinal Peptide exerts immuno-regulatory effects in sarcoidosis. Am J Respir Crit Care Med. doi: 10.1164/rccm.200909-1451OC. in press. [DOI] [PubMed] [Google Scholar]; This is the first paper describing the immunomodulatory effect of any neuropeptide on humans, and the first reporting an agent that induces Treg cells in lungs of patients with autoimmune disorders It also proposes the inhalation of neuropeptides as a safe and effective way to administer them in humans.
- 35.Chapter MC, White CM, DeRidder A, Chadwick W, Martin B, Maudsley S. Chemical modification of class II G protein-coupled receptor ligands: frontiers in the development of peptide analogs as neuroendocrine pharmacological therapies. Pharmacol Ther. 2010;125:39–54. doi: 10.1016/j.pharmthera.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •36.Misaka S, Aoki Y, Karaki S, Kuwahara A, Mizumoto T, Onoue S, Yamada S. Inhalable powder formulation of a stabilized vasoactive intestinal peptide (VIP) derivative: anti-inflammatory effect in experimental asthmatic rats. Peptides. 2010;31:72–78. doi: 10.1016/j.peptides.2009.09.032. [DOI] [PubMed] [Google Scholar]; This is an important work describing that some selective changes in residues of neuropeptides greatly increases their stability, maintaining their function This opens attractive therapeutic possibilities for the clinical application of derivatives of neuropeptides in human diseases.
- 37.Onoue S, Misaka S, Ohmori Y, Sato H, Mizumoto T, Hirose M, Iwasa S, Yajima T, Yamada S. Physicochemical and pharmacological characterization of novel vasoactive intestinal peptide derivatives with improved stability. Eur J Pharm Biopharm. 2009;73:95–101. doi: 10.1016/j.ejpb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Camelo S, Lajavardi L, Bochot A, Goldenberg B, Naud MC, Brunel N, Lescure B, Klein C, Fattal E, Behar-Cohen F, de Kozak Y. Protective effect of intravitreal injection of vasoactive intestinal peptide-loaded liposomes on experimental autoimmune uveoretinitis. J Ocul Pharmacol Ther. 2009;25:9–21. doi: 10.1089/jop.2008.0074. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Montesinos R, Castillo PM, Klippstein R, Gonzalez-Rey E, Mejias JA, Zaderenko AP, Pozo D. Chemical synthesis and characterization of silver-protected vasoactive intestinal peptide nanoparticles. Nanomedicine. 2009;4:919–930. doi: 10.2217/nnm.09.79. [DOI] [PubMed] [Google Scholar]
- 40.Delgado M, Toscano MG, Benabdellah K, Cobo M, O'Valle F, Gonzalez-Rey E, Martín F. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum. 2008;58:1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- ••41.Han D, Tian Y, Zhang M, Zhou Z, Lu J. Prevention and treatment of experimental autoimmune encephalomyelitis with recombinant adeno-associated virus-mediated alpha-melanocyte-stimulating hormone-transduced PLP139-151-specific T cells. Gene Ther. 2007;14:383–395. doi: 10.1038/sj.gt.3302862. [DOI] [PubMed] [Google Scholar]; This is an elegant work that propose a gene-cell therapy based in the use of encephalogenic-specific T cells as “Trojan horse” to deliver immunoregulatory neuropeptides at the sites of inflammation and antigen presentation.
- 42.Toscano MG, Delgado M, Kong W, Martin F, Skarica M, Ganea D. Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs. Mol Ther. 2010 Jan 12; doi: 10.1038/mt.2009.293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •43.Brod SA, Hood ZM. Ingested (oral) alpha-MSH inhibits acute EAE. J Neuroimmunol. 2008;193:106–112. doi: 10.1016/j.jneuroim.2007.10.026. [DOI] [PubMed] [Google Scholar]; An interesting work with surprising findings that opens the possibility to use neuropeptide-based therapies in an oral regime in autoimmune disorders.
- 44.El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Charnley M, Moir AJG, Douglas CWI, Haycock JW. Anti-microbial action of melanocortin peptides and identification of a novel X-Pro-D/L-Val sequence in gram-positive and gram-negative bacteria. Peptides. 2008;29:1004–1009. doi: 10.1016/j.peptides.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Madhuri, Shireen T, Venugopal SK, Ghosh D, Gadepalli R, Dhawan B, Mukhopadhyay K. In vitro antimicrobial activity of alpha-melanocyte stimulating hormone against major human pathogen Staphylococcus aureus. Peptides. 2009;30:1627–1635. doi: 10.1016/j.peptides.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Rauch I, Holzmeister S, Kofler B. Anti-Candida activity of alpha-melanocyte-stimulating hormone (alpha-MSH) peptides. J Leukoc Biol. 2009;85:371–372. doi: 10.1189/jlb.1008614. [DOI] [PubMed] [Google Scholar]
- ••48.Delgado M, Anderson P, Garcia-Salcedo JA, Caro M, Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2009;16:406–416. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]; This work describes an unusual mechanism of cell death for parasites using endogenous neuropeptides It discusses important and suggestive implications for the interactions of pathogens with hosts viewed from physiological and evolutionary points of views.
- 49.Campos-Salinas J, Gonzalez-Rey E. Autophagy and neuropeptides at the crossroad for parasites: to survive or to die? Autophagy. 2009;5:551–554. doi: 10.4161/auto.5.4.8365. [DOI] [PubMed] [Google Scholar]
- •50.Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work confirms the role played by endogenous neuropeptides on the maintenance of immune tolerance by using PACAP-deficient mice.
- 51.Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- •52.Delgado M, Robledo G, Rueda B, Varela N, O'Valle F, Hernandez-Cortes P, Caro M, Orozco G, Gonzalez-Rey E, Martin J. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis: altered expression and signal in immune cells. Arthritis Rheum. 2008;58:1010–1019. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]; The first paper that associates reduced expression of any neuropeptide/neuropeptide-receptor system with a genetic polymorphism in subjects with any autoimmune disease.
- 53.Juarranz Y, Gutierrez-Canas I, Santiago B, Carrion M, Pablos JL, Gomariz RP. Differential expression of vasoactive intestinal peptide and its functional receptors in human osteoarthritic and rheumatoid synovial fibroblasts. Arthritis Rheum. 2008;58:1086–1095. doi: 10.1002/art.23403. [DOI] [PubMed] [Google Scholar]
- 54.Paladini F, Cocco E, Cauli A, Cascino I, Vacca A, Belfiore F, Fiorillo MT, Mathieu A, Sorrentino R. A functional polymorphism of the vasoactive intestinal peptide receptor 1 gene correlates with the presence of HLA-B*2705 in Sardinia. Genes Immun. 2008;9:659–667. doi: 10.1038/gene.2008.60. [DOI] [PubMed] [Google Scholar]