SUMMARY
D1 dopamine receptors are primary mediators of dopaminergic signaling in the CNS. These receptors internalize rapidly following agonist-induced activation but the functional significance of this process is unknown. We investigated D1 receptor endocytosis and signaling in HEK 293 cells and cultured striatal neurons using real-time fluorescence imaging and cAMP biosensor technology. Agonist-induced activation of D1 receptors promoted endocytosis of receptors with a time course overlapping that of acute cAMP accumulation. Inhibiting receptor endocytosis blunted acute D1 receptor-mediated signaling in both dissociated cells and striatal slice preparations. While endocytic inhibition markedly attenuated acute cAMP accumulation, inhibiting the subsequent recycling of receptors had no effect. Further, D1 receptors localized in close proximity to endomembrane-associated trimeric G protein and adenylyl cyclase immediately after endocytosis. Together, these results suggest a previously unanticipated role of endocytosis, and the early endocytic pathway, in supporting rapid dopaminergic neurotransmission.
INTRODUCTION
Dopamine (DA) is a major catecholamine neurotransmitter that controls a diverse range of physiological processes (Missale et al., 1998; Sibley, 1999). Disturbances of dopaminergic signaling have been implicated in many pathological conditions including Parkinson’s disease, schizophrenia, attention-deficit/hyperactivity disorder and addiction. Not surprisingly, dopaminergic signaling in the CNS is highly regulated and subject to precise temporal control. All of the known cellular actions of DA are mediated by G protein coupled receptors (GPCRs). D1 DA receptors are highly expressed within the brain. Their pharmacological properties suggest they mediate signaling in response to transient bursts of high extracellular DA concentration characteristic of phasic release (Heien and Wightman, 2006; Richfield et al., 1989) Upon binding DA, D1 receptors activate adenylyl cyclase (AC) through coupling to specific heterotrimeric G-proteins (Gs or Golf) and produce a dynamic increase in the concentration of cytoplasmic 3′-5′-cyclic adenosine monophosphate (cAMP) which transduces many D1 receptor-mediated signaling effects (Greengard, 2001; Neve et al., 2005).
In order for neurons to respond to physiologically relevant fluctuations in extracellular DA, D1 receptors must be able to reliably transduce and support changes in intracellular cAMP concentration over appropriate time intervals. After agonist-induced activation, D1 receptors are subject to a linked series of regulatory events which culminate in endocytic removal of receptors from the plasma membrane in numerous cell lines, as well as the intact brain (Ariano et al., 1997; Bloch et al., 2003; Dumartin et al., 1998; Martin-Negrier et al., 2006; Martin-Negrier et al., 2000; Mason et al., 2002; Ng et al., 1994; Tiberi et al., 1996; Vickery and von Zastrow, 1999). Previous studies of GPCRs indicate that endocytic removal of receptors from the cell surface can attenuate cellular signaling, and/or contribute to later functional recovery of cellular responsiveness by returning surface receptors by recycling. For some GPCRs, endocytosis promotes receptor dephosphorylation, thus promoting biochemical recovery (or resensitization) of receptors from the desensitized state after a refractory period (Lefkowitz, 1998; Pippig et al., 1995). However, none of these processes is thought to affect the signaling response to acute agonist activation. Further, D1 dopamine receptors can undergo dephosphorylation in the absence of endocytosis (Gardner et al., 2001). Thus the functional significance of D1 receptor endocytosis remains unknown.
Previous studies examining the relationship between signaling and endocytosis of D1 receptors have been carried out on a time scale of tens of minutes to hours, but fluctuations of extracellular DA in the CNS occur much faster-typically on the order of seconds to less than one minute (Heien and Wightman, 2006). Thus we considered the possibility that the functional significance of D1 receptor endocytosis involves more rapid events, and may have remained elusive due to the limited temporal resolution of previous work. In the present study, we applied recent advances in live imaging and fluorescent biosensor technologies to analyze both D1 receptor trafficking and receptor-mediated cAMP accumulation with greatly improved temporal resolution, beginning to approach that of physiological dopamine fluctuations. Our results show that D1 receptors endocytose more rapidly than previously recognized, and reveal an unanticipated role of regulated endocytosis of D1 receptors in promoting the acute response. Our findings thus identify a specific consequence of the endocytic machinery on D1 receptor-mediated signaling, and its function in a physiologically relevant model of dopaminergic neurotransmission.
RESULTS
Real-time analysis of D1 receptor endocytosis by live cell imaging
Flow cytometric analysis of surface accessibility of FLAG epitope-tagged D1 DA receptors (FD1R) in HEK 293 cells verified robust internalization in response to DA. Internalization was dose-dependent and rapid, approaching the steady state value with an estimated t1/2 of 3.9 min (Figure 1A). For greater temporal resolution, we employed live imaging by total internal reflection fluorescence (TIRF) microscopy and the pH-sensitive GFP variant superecliptic pHluorin (SpH, or SEP) fused to the N-terminal extracellular region of the D1 receptor (SpH-D1R). SpH is highly fluorescent at neutral pH, facilitating detection when in contact with the extracellular media. This fluorescence is rapidly quenched in the acidic environment of the endocytic pathway (Miesenbock et al., 1998; Sankaranarayanan et al., 2000). We used these properties to observe individual endocytic events in SpH-D1R expressing HEK 293 cells. In the absence of DA, SpH-D1R fluorescence was visible on the plasma membrane (Figure 1B, left). Bath application of DA caused rapid clustering of SpH-D1Rs into puncta that subsequently endocytosed (Figure 1B, right and Movie S1). Strikingly, an initial wave of SpH-D1R clustering and endocytosis occurred as soon as 30 seconds after agonist addition (Movie S1). Analysis of individual puncta by fluorescence intensity tracing verified their disappearance within 30 seconds to 1 minute after formation (Figure 1C). These properties are consistent with previous data indicating that D1 receptors endocytose primarily via clathrin-coated pits in HEK 293 cells (Vickery and von Zastrow, 1999), and with descriptions of clathrin-mediated endocytosis of signaling receptors imaged by TIRF microscopy (Puthenveedu and von Zastrow, 2006; Yudowski et al., 2006). Rapid endocytosis was further verified by the rate of DA-dependent reduction in integrated SpH-D1R surface fluorescence intensity (Figure 1D).
Figure 1. Rapid agonist-stimulated D1 receptor endocytosis and real-time examination of receptor-mediated regulation of cellular cAMP.
(A) Agonist-induced effects on surface receptor number as analyzed by fluorescence flow cytometry. Surface fluorescence of labeled FD1Rs measured at time 0 was defined as 100%. Data = mean fluorescence values +/− SEM, n = 3, 10,000 cells/condition, each time point in duplicate. (B) Representative images of SpH-D1R surface fluorescence visualized by TIRF microscopy prior to (left, inset) and 120 sec. after DA addition (right, inset). (C) Maximum intensity trace of representative SpH-D1R endocytic cluster vs. time. Trace represents area outlined in blue in (B), measurements taken every 4 sec. (D) Normalized integrated surface fluorescence of SpH-D1R measured every 3 sec. in the absence of agonist (Bleaching Control, n = 5 cells) or in response to DA (10μM DA, n = 20 cells). Integrated fluorescence value at time 0 was defined as 100%. Data = mean +/− SEM. (E) Representative pseudo-color image of Epac1-cAMP prior to (left) or 60 sec. after 10μM DA addition (right). Lookup table is scaled linearly to YFP/CFP emission ratio. (F) Change in normalized Epac1-cAMPs FRET signal in response to stimulation over a range of DA concentrations. Data = mean +/− SEM normalized FRET emission ratio at each time point, n = 8-16 cells per dose. Scale bars = 5μm. See also Figure S1.
Real-time measurement of cAMP dynamics using a FRET-based biosensor
Given that significant endocytosis of D1 receptors occurred within ~1 minute of DA addition, we examined D1 receptor-mediated signaling over a similar time scale. We employed the FRET-based cAMP biosensor, Epac1-cAMPs, to measure DA stimulated cAMP production in real time, in individual cells, without phosphodiesterase inhibitors (Nikolaev et al., 2004). Cells expressing FD1R and Epac1-cAMPs showed a robust decrease in the normalized FRET emission ratio after DA addition, indicating elevated cytoplasmic cAMP concentration (Figure 1E). DA application produced both a rapid decrease of YFP emission and a corresponding increase in CFP emission, verifying that the observed changes were indeed due to decreased FRET (Figure S1A). Incubation of FD1R/Epac1-cAMPs expressing cells with a range of DA concentrations revealed a significant and dose-dependent increase in cytoplasmic cAMP concentration beginning within 20 seconds (Figure 1F). We did not observe significant changes in Epac1-cAMPs FRET in the absence of agonist (Figure S1B). Dual-channel fluorescence imaging indicated that co-expression of Epac1-cAMPs did not prevent agonist-induced endocytosis of D1 receptors (Figure S1C).
Endocytosis promotes acute D1 receptor-mediated signaling
In light of the temporal overlap between agonist-induced D1 receptor trafficking and signaling, we asked if there is a causal relationship between these processes. We employed a number of experimental manipulations to inhibit receptor endocytosis, and examined effects on acute cAMP accumulation using the Epac1-cAMPs FRET biosensor. Hypertonic sucrose (HS) inhibits clathrin-mediated endocytosis of a number of membrane proteins, including D1 receptors, by disrupting the normal clathrin lattice structure (Gardner et al., 2001; Heuser and Anderson, 1989; Vickery and von Zastrow, 1999). HS indeed inhibited FD1R endocytosis, as verified by fluorescence microscopy (Figure S2A) and quantified by fluorescence flow cytometry (Figure S2B). Further, this manipulation partially attenuated acute D1 receptor-mediated cAMP accumulation measured using the Epac1-cAMPs biosensor (Figure S2C).
Dynasore inhibits clathrin/dynamin-dependent endocytosis by interfering with the GTPase activity of dynamin (Kirchhausen et al., 2008). Dynasore visually reduced regulated endocytosis of FD1Rs (Figure 2A) and caused a near complete blockade of this process as quantified by fluorescence flow cytometry (Figure 2B). DA-stimulated cAMP accumulation was significantly inhibited by dynasore (Figure 2C). Dynasore caused a small shift in baseline fluorescence signal due to its low level of intrinsic fluorescence (Figure S2D) but this was easily corrected by subtraction (see Supplemental Experimental Procedures). Importantly, dynasore did not affect the cAMP accumulation elicited by receptor-independent activation of adenylyl cyclase with forskolin (Figure S2E). Thus chemical inhibition of endocytosis produces significant inhibition of cellular cAMP accumulation mediated specifically by D1 receptor activation.
Figure 2. Endocytic inhibitors reduce acute, D1 receptor-mediated, cAMP accumulation in HEK 293 cells.
(A) Subcellular distribution of surface-labeled FD1Rs in response to 10μM DA in the absence (left) or presence (right) of 80μM dynasore. (B) Agonist-induced effects on surface receptor number in the presence of 80μM dynasore (white) or 0.2%DMSO (black) as analyzed by fluorescence flow cytometry. Fluorescence measured at time 0 was defined as 100%. Data = mean surface fluorescence +/− SEM, n = 3, 10,000 cells/condition, each time point in triplicate. (C) Change in normalized Epac1-cAMPs FRET in response to 10μM DA in the presence of 80μM dynasore (open squares) or 0.2% DMSO (Vehicle; filled black circles). Data = mean +/− SEM normalized FRET ratio at each time point, n = 21-22 cells per group. (D) Representative (n = 3) immunoblot analysis of siRNA mediated knockdown of clathrin in FD1R-expressing cells with GAPDH loading control. (E) Agonist-induced effects on surface receptor number for cells transfected with clathrin siRNA (crosshatch) or non-silencing control siRNA (black) as analyzed by fluorescence flow cytometry. Fluorescence measured at time 0 was defined as 100%. Data = mean surface fluorescence +/− SEM, n = 3, 10,000 cells/condition, each time point in triplicate. (F) Change in normalized Epac1-cAMPs FRET in response to 10μM DA in cells transfected with clathrin siRNA (open triangles) or non-silencing control siRNA (filled circles). Data = mean +/− SEM normalized FRET ratio at each time point, n = 14-15 cells per group. (G) Agonist-induced effects on surface receptor number for cells expressing wild-type (black) or 360-382 deletion mutant D1 receptors (grey), as analyzed by fluorescence flow cytometry. Fluorescence measured at t = 0 was defined as 100%. Data = mean surface fluorescence +/− SEM, n = 3, 10,000 cells/condition, each time point in triplicate. (H) Change in normalized Epac1-cAMPs FRET in response to 10μM DA in cells expressing wild-type (closed circles) or 360-382 deletion mutant D1Rs (grey squares). Data = mean +/− SEM normalized FRET emission ratio at each time point, n = 30-31 cells per group. (I) Mean difference in cAMP response between each endocytic manipulation and corresponding control at 20 sec. (black) or 120 sec. (white) after DA addition. Data = difference in mean normalized FRET ratio +/− variance at each time point. Scale bar = 5 μm. (*p<0.05, **p<0.01 by 2-tailed unpaired t-test) See also Figure S2.
We next used an independent genetic approach, based on depleting clathrin heavy chain with a validated small interfering RNA (siRNA). Knockdown was confirmed biochemically by immunoblot (Figure 2D). Clathrin knockdown blocked FD1R internalization measured by flow cytometry (Figure 2E), and significantly inhibited acute DA-stimulated cAMP accumulation (Figure 2F).
Given that clathrin-coated pits mediate endocytosis of a wide range of membrane cargo, it is possible that the inhibited signaling produced by all of our endocytic manipulations could reflect an indirect consequence not specific to endocytosis of the D1 receptor itself. To address this, we used a receptor-specific mutation to inhibit endocytosis of D1 receptors. A middle portion of the D1 receptor cytoplasmic tail (residues 360-382) is not essential for ligand binding or receptor function but contains several residues subject to agonist-induced phosphorylation that affect endocytic trafficking of D1 receptors (Jackson et al., 2002; Kim et al., 2004; Lamey et al., 2002; Mason et al., 2002). We constructed a mutant version of the D1 receptor with these residues deleted and verified significant inhibition of DA-induced endocytosis compared to the wild-type receptors expressed at the same level (Figure 2G). The endocytosis-defective mutant D1 receptor produced a significantly blunted cellular cAMP accumulation response relative to its wild type counterpart (Figure 2H). Nevertheless, dose-response analysis revealed unchanged potency (indistinguishable EC50, Figure S2F). This verifies that deletion of residues 360-382 did not simply prevent activated receptors from coupling to the transduction pathway, as would be indicated by a rightward shift. Interestingly, the earliest phase of D1 receptor-mediated cAMP accumulation did not seem to be affected by dynasore pretreatment or expression of the endocytosis-deficient mutant. The mean effects of each of the endocytic manipulations at 20 sec. and 120 sec. after agonist treatment are summarized in Figure 2I.
Rapid endocytic trafficking of D1 receptors in cultured striatal neurons
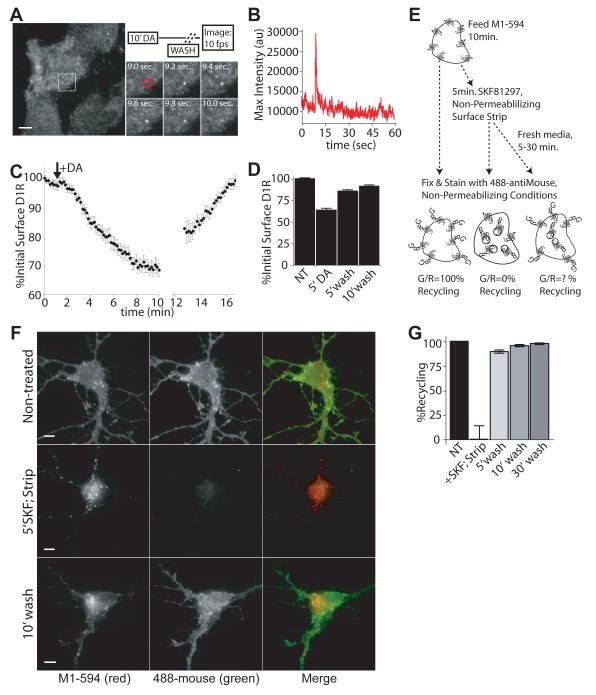
We next asked whether a causal relationship between D1 receptor endocytosis and acute cAMP signaling also exists in neurons. D1 receptors are expressed on a substantial fraction of GABAergic medium spiny neurons (MSNs), which represent the major cell type present within the striatum (Kreitzer, 2009). Because MSNs also express other DA receptor subtypes (notably D2 receptors that are oppositely coupled to AC), we used the D1-selective full agonist SKF81297 rather than dopamine in our neuronal studies. To quantitatively examine receptor internalization, FD1Rs were expressed in cultured striatal neurons and plasma membrane receptors labeled with monoclonal antibody conjugated to red Alexafluor (Figure 3A). Cells were incubated in the absence of agonist (top panel) or in the presence of the D1 receptor-specific agonist SKF81297 (bottom panel), and fixed under non-permeabilizing conditions. Labeled receptors still present in the plasma membrane were subsequently labeled with a green Alexafluor-conjugated secondary antibody. Accordingly red fluorescence represents the overall pool (internal and surface) of D1 receptors initially on the plasma membrane, and green fluorescence represents the fraction of D1 receptors that did not internalize. The ratio of these integrated fluorescence values was used to assess internalization across multiple neurons and experiments, establishing that D1 receptors internalize rapidly and robustly after activation in medium spiny neurons (Figure 3B).
Figure 3. Rapid, agonist-induced trafficking of D1 receptors in striatal neurons.
(A) Representative, epifluorescence images of FD1R-expressing striatal neurons after 10 min. incubation with (bottom) or without (top) of 1μM SKF 81297. (B) The total pool of FD1Rs initially present at the cell surface were labeled with Alexa594-conjugated primary antibody (red). After agonist treatment, neurons were fixed under non-permeabilizing conditions and incubated with Alexa488-conjugated secondary antibody (green) to label receptors that remained at the cell surface or recycled during the incubation period. (B) Quantification of FD1R internalization by change in normalized (surface/total) fluorescence ratio as described in (A). The average surface to total (green/red) fluorescence ratio measured in non-treated cells was defined as 1. Data = mean fluorescence ratios +/-SEM, n = 3, 31-36 cells per condition. (C) Representative images of SpH-D1R surface fluorescence in striatal neurons as visualized by TIRF microscopy prior to (left, inset), and 120 sec. after SKF 81297 addition (right, inset). Arrows indicate clusters of SpH-D1R fluorescence that formed after SKF addition and disappeared shortly thereafter, arrowhead indicates a cluster of that formed after SKF addition and persisted throughout imaging. (D) Kymograph of SpH-D1R fluorescence for endocytic events indicated by arrows in (C). Kymograph depicts surface fluorescence intensity (maximum intensity determination) as a function of time. Arrow indicates bath application of 1μM SKF 81297. (E) Average surface fluorescence of SpH-D1R expressing striatal neurons measured every 3 sec. in the absence of (Bleaching Control) or in response to 1μM SKF 81297. Initial fluorescence values normalized to 100%, data = background-corrected, mean surface fluorescence +/− SEM, n = 5-9 cells/condition. Scale bars = 5μm. See also Figure S3.
To examine receptor dynamics at the cell surface with greater temporal resolution, we imaged SpH-D1Rs by TIRF microscopy. SpH-D1R fluorescence was observed on the plasma membrane of both the cell body and dendrites (Figure 3C, left). Pronounced clustering of receptors occurred very rapidly after agonist addition (Figure 3C, right and Movie S2), characteristic of receptor concentration in clathrin-coated pits using this method (Yu et al., 2010; Yudowski et al., 2006). The vast majority disappeared within ~1 min of their formation, indicating the occurrence of rapid endocytic scission consistent with clathrin-dependent endocytosis. Figure 3D shows a representative kymograph of these events. Integrated SpH-D1R fluorescence intensity measurements established the overall kinetics of regulated D1 receptor endocytosis (Figure 3E, black circles). SpH-D1R surface fluorescence remained steady in the absence of agonist (Figure 3E, gray circles), confirming that D1 receptor endocytosis is agonist-dependent and that photobleaching was negligible. We also verified FD1R localization to clathrin-associated puncta within 2 minutes after agonist addition (Figure S3A), and to early endosomes marked by EEA1 within 5 minutes after agonist addition (Figure S3B) by dual labeling.
Endocytosis promotes acute D1 receptor-mediated signaling in striatal neurons
We adapted the FRET-based biosensor technology used to study HEK 293 cells to assess D1 receptor-mediated signaling in striatal neurons. Due to the typically lower expression of Epac1-cAMPs in neurons compared to HEK 293 cells, we used TIRF microscopy to achieve greater signal-to-noise ratio and facilitate FRET determination with high quantitative precision despite lower cytoplasmic biosensor concentration. The evanescent field produced by TIRF illumination field extends ~100 nm beyond the thickness of the plasma membrane and includes a significant volume of peripheral cytoplasm (Steyer and Almers, 2001). Acute D1 receptor activation induced by bath application of SKF81297 caused a pronounced decrease in the normalized (YFP/CFP) emission ratio of Epac1-cAMPs throughout the peripheral cytoplasm (Figure 4A), indicating increased cAMP concentration occurring rapidly after agonist application (Figure 4B). Dynasore caused a pronounced inhibition of D1 receptor endocytosis in MSNs (Figure S4) and, consistent with results from HEK 293 cells, inhibited acute agonist-induced cAMP accumulation (Figure 4C). Genetic inhibition of D1 receptor endocytosis, by 360-382 deletion, also blunted the rapid cAMP response (Figure 4D). These data provide two independent lines of evidence indicating that the endocytic machinery promotes acute D1 receptor-mediated cAMP accumulation in physiologically relevant neurons.
Figure 4. Endocytic inhibition reduces acute D1 receptor-mediated cAMP accumulation in striatal neurons.
(A) Pseudo-color representation of Epac1-cAMP FRET in FD1R/Epac1-cAMPs expressing striatal neuron prior to (left) or 60 sec after addition of 1μM SKF 81297 (right). Lookup table is scaled linearly to YFP/CFP emission ratio. (B) Change in normalized Epac1-cAMPs FRET signal in response to stimulation with 1μM SKF 81297. Data = mean normalized FRET emission ratio +/− SEM at each time point, n = 7. (C) Change in normalized Epac1-cAMPs FRET in response to 1μM SKF 81297 in the presence of 80μM dynasore (open squares) or 0.2% DMSO (Vehicle; filled circles). Data represent mean normalized FRET emission ratio +/− SEM at each time point, n = 13-18 cells per group. (D) Change in normalized Epac1-cAMPs FRET in response to 1μM SKF 81297 in cells expressing wild-type (filled circles) or 360-382 deletion mutant D1Rs (open diamonds). Data = mean normalized FRET emission ratio +/− SEM at each time point, n = 12-15 cells per group. (*p<0.05, **p<0.01 by 2-tailed unpaired t-test) Scale bar = 5μm. See also Figure S4.
Endocytosis promotes D1 receptor-mediated regulation of action potential firing in dorsolateral striatum
Agonist-stimulation of D1 receptors in dorsolateral striatum increases neuronal excitability via PKA-dependent enhancement of L-type calcium currents (Abdallah et al., 2009; Hernandez-Lopez et al., 1997; Surmeier et al., 1995). Further, endogenous D1 receptors undergo agonist-induced internalization in this brain region (Dumartin et al., 1998; Muriel et al., 2002). To examine whether endocytosis affects integrated D1 receptor-mediated signaling, we performed whole-cell patch-clamp electrophysiology in intact brain slices containing the lateral dorsal striatum. Neurons were brought to a resting membrane potential of ~−90mV by passage of DC current via the patch amplifier and subsequently exposed to a series of 300msec current pulses to depolarize neurons and generate APs. In control slices pre-exposed to vehicle (0.2%DMSO), perfusion of SKF 81297 significantly enhanced AP generation as expected (Figure 5A top). Interestingly, pre-exposure of slices to dynasore strongly inhibited this response (Figure 5A bottom). This inhibitory effect of dynasore on D1 receptor-mediated AP firing was robust across experiments. Importantly, dynasore did not affect basal firing (Figure 5B). The time course of increased AP firing observed in vehicle-perfused slices is consistent with that of D1 receptor-mediated signaling in this preparation and, after accounting for perfusion lag time, closely paralleled that of acute cAMP signaling measured in dissociated MSNs. We further verified that dynasore did not alter the basic firing properties of MSNs in this preparation (Figure S5) using a previously established method (Hopf et al., 2010).
Figure 5. Dynasore prevents D1 receptor-mediated enhancement of action potential firing in lateral dorsal striatal neurons in brain slice.
(A) Example traces showing increased firing with the D1 receptor agonist SKF81297 (10μM) after pre-exposure to vehicle (0.2% DMSO), but not 80μM dynasore. Slices were incubated as indicated (bar in panel B) with either vehicle (0.2% DMSO, top pair of traces) or 80 μM dynasore (bottom pair of traces) prior to delivery of SKF81297. (B) Grouped data showing significant increase in firing with SKF81297 after pre-exposure to vehicle (34.7 +/− 12.8%, n=5) but not dynasore (−2.78 +/− 4.9%, n=5). Bars indicate the time course of drug perfusions relative to changes in AP firing. The percent change in number of action potentials (APs) generated relative to baseline was determined at the current step at baseline with 4 APs, or 5 APs. If no current steps at baseline had 4 APs, the baseline number of APs was determined by averaging each min for the 4 minutes before addition of DMSO or dynasore (*p<0.05 by 2-tailed unpaired t-test). See also Figure S5.
D1 receptors recycle rapidly after endocytosis, but recycling is not required for the acute cAMP response
We next investigated the mechanism by which endocytosis promotes acute D1 receptor-mediated signaling. One possibility is that endocytosis-dependent augmentation of cAMP accumulation might require subsequent receptor recycling. This would be predicted if endocytosis mediates a function in D1 receptor signaling akin to resensitization of other GPCRs. We imaged SpH-D1R insertion events with high temporal resolution using TIRF microscopy and rapid dequenching of fluorescence upon exposure to the neutral extracellular milieu. Vesicular insertion events delivering SpH-tagged receptors appear as ‘puffs’ of transiently increased surface fluorescence intensity, detectable by rapid (10 Hz) serial imaging (Yudowski et al., 2006). Such insertions were observed immediately after DA washout (Figure 6A and Movie S3), even after prolonged exposure of cells to the protein synthesis inhibitor cycloheximide (data not shown). This indicates that D1 receptors can indeed undergo rapid surface delivery. Insertion events were also observed in the continued presence of agonist, but this required distinguishing insertion events (Figure 6B) from the dimmer and longer-lasting receptor clusters representing clathin-coated pits (Yu et al., 2010; Yudowski et al., 2009; Yudowski et al., 2007; Yudowski et al., 2006). Integrated fluorescence intensity measurements (Figure 6C) and a conventional flow cytometric assay (Figure 6D) further verified recovery of the surface pool of receptors within several minutes after agonist washout.
Figure 6. D1 receptors rapidly recycle after endocytosis.
(A) Experimental schematic and representative images of SpH-D1R surface insertion events visualized by TIRF microscopy. Images acquired at 10 Hz after DA washout. (B) Maximum intensity trace of a representative SpH-D1R insertion event (red circle in A) vs. time. (C) Integrated surface fluorescence of plasma membrane SpH-D1R before and after DA addition and washout, as detected by TIRF imaging. Arrow indicates application of 10μM DA and gap indicates period of agonist washout. Data = mean integrated fluorescence values for each cell at a given time point +/− SEM, normalized to t = 0, n = 10. (D) Agonist-induced reduction and recovery of surface FD1R immunoreactivity determined by fluorescence flow cytometry. Surface FD1R immunoreactivity was determined in cells not exposed to agonist (NT), after 5 min incubation with 10μM DA (5′DA), or 5 min. DA followed by agonist washout and incubation in fresh media for 5 or 10 min. (5′wash, 10′wash). Data = mean surface FD1R fluorescence +/− SEM, normalized to the NT condition (defined as 100%) n = 3, 10,000 cells/condition, each condition in duplicate. (E) Experimental schematic to quantify recycling of internalized FD1Rs in striatal neurons by dual labeling and fluorescence ratio imaging. (F) Representative epifluorescence images obtained using the dual labeling procedure described in (E). (G) Quantification of FD1 receptor recycling across multiple neurons by fluorescence ratio imaging (see Experimental Procedures). Bars = mean percentage of internalized receptors that recycle 0 (+SKF;Strip), 5, 10 or 30 minutes after agonist washout, +/− SEM. 100% recycling = green to red fluorescence ratio measured in unstimulated neurons, 0% recycling = green to red fluorescence ratio in neurons treated with SKF for 5 min., stripped and immediately fixed. n = 3, 21-27 cells per group. Scale bars = 5μm.
To specifically examine recycling of the internalized receptor pool, we analyzed surface recovery of FD1R initially labeled in the plasma membrane of MSNs using a previously described method (Tanowitz and von Zastrow, 2003). Figure 6E depicts the experimental schematic. Representative images of the conditions used to quantify D1 receptor recycling are shown in Figure 6F. Recycling determinations averaged across multiple neurons and experiments are shown in Figure 6G. The majority (89.4+/-1.8%) of internalized FD1Rs return to the neuronal plasma membrane within 5 minutes after agonist washout. Together these results indicate that D1 receptors can indeed recycle very rapidly after endocytosis.
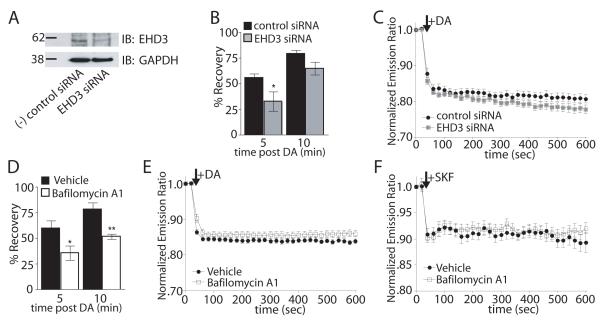
We next searched for inhibitors of D1 receptor recycling to examine whether, similar to endocytosis, recycling also plays a causal role in promoting the acute D1 receptor-mediated cAMP response. As D1 receptors return to the plasma membrane via similar membrane pathway as transferrin receptors (Vickery and von Zastrow, 1999), we investigated the effect of a validated siRNA targeting Eps15 homology domain containing protein 3 (EHD3). EHD3 localizes to recycling membrane structures (Galperin et al., 2002) and is required for efficient delivery of internalized transferrin receptors to the endocytic recycling compartment (Naslavsky et al., 2006). Knockdown of EHD3 was verified by immunoblot (Figure 7A). EHD3 siRNA significantly inhibited surface recovery of tagged FD1 receptors 5 minutes after agonist washout (Figure 7B) but did not affect acute D1 receptor-mediated cAMP accumulation (Figure 7C).
Figure 7. Inhibiting recycling does not affect acute D1 receptor-mediated cAMP accumulation.
(A) Representative (n = 3) immunoblot analysis of siRNA mediated knockdown of EHD3 in FD1R-expressing HEK293 cells with GAPDH loading control. (B) Recovery of surface FD1Rs measured by fluorescence flow cytometry. Recovery of surface FD1R fluorescence after 5 min DA incubation and 5 or 10 min after agonist washout was measured in cells transfected with siRNA targeting EHD3 (gray) or non-silencing control siRNA (black). %Recovery = [(Surface fluorescence after DA washout - average surface fluorescence after 5 min. DA incubation)/(average initial surface fluorescence - average surface fluorescence after 5 min. DA incubation)] × 100. Data = mean %Recovery +/− SEM, n = 3, 10,000 cells/well, each time point in triplicate. (C) Change in normalized Epac1-cAMPs FRET in response to 10μM DA in cells transfected with siRNA against EHD3 (grey squares) or non-silencing control siRNA (filled circles). Data = mean normalized FRET emission ratio at each time point +/− SEM, n = 20-28 cells per group. (D) Recovery of FD1Rs in the presence of 500nM Bafilomycin (white bars) or vehicle (black bars) as measured by fluorescence flow cytometry. Data = mean %Recovery +/− SEM, n = 3, 10,000 cells/well, each time point in triplicate. (E) Change in normalized Epac1-cAMPs FRET in response to 10μM DA in cells pretreated with Bafilomycin (open squares) or vehicle control (filled circles). Data = mean normalized FRET emission ratio at each time point +/− SEM, n = 29-30 cells per group. (F) Change in normalized Epac1-cAMPs FRET in response to 1μM SKF81927 in FD1 expressing striatal neurons pretreated with bafilomycin (open squares) or vehicle control (filled circles). Data = mean normalized FRET emission ratio at each time point +/− SEM, n = 11-14 neurons per group. (*p<0.05, **p<0.01, 2-tailed unpaired t-test).
Bafilomycin A1 is a specific inhibitor of the vacuolar H+-ATPase that inhibits recycling of a number of signaling receptors (Johnson et al., 1993; Presley et al., 1997). Pretreatment of FD1R-expressing HEK 293 cells with 500nM bafilomycin A1 significantly inhibited surface receptor recovery compared to cells pre-treated with vehicle (Figure 7D). Nevertheless, bafilomycin A1 also did not produce any detectable effect on acute D1 receptor-mediated cAMP accumulation in HEK 293 cells (Figure 8E) or striatal neurons (Figure 8F). These results indicate that the ability of D1 receptor endocytosis to augment the acute cAMP signal does not require subsequent receptor recycling to the plasma membrane. Instead, the results suggest that D1 receptors contribute to the acute signaling response upon entry to, or in transit through, an early endocytic intermediate.
Figure 8. Rapid endocytosis promotes colocalization of D1 receptors with ACV and Gs/olf.
(A) Representative confocal sections of FD1R (left, red) and ACV (center, green) immunoreactivity from striatal neurons incubated in the absence (top) or 2 min. after 1μM SKF81297 application (bottom). (B) Quantification of the fraction of D1R positive puncta that colocalize with ACV prior to (black) and after (white) 1μM SKF81297 addition. n = 4, 3-6 cells per condition. (C) Representative confocal optical sections of FD1R (left, red) and Gαs/olf (center, green) immunoreactivity from striatal neurons incubated in the absence of agonist (top) or 2 min. after 1μM SKF81297 application (bottom panel). (D) Quantification of the fraction of D1R positive puncta that colocalize with Gαs/olf prior to (black) and after (white) bath application of 1μM SKF81297 n = 4, 4-5 cells per condition. (*p<0.05, **p<0.01, unpaired t-test) (E) Proposed model of endocytosis-dependent augmentation of acute dopaminergic signaling. Activation of D1 areceptors stimulates ACV via receptor coupling to Gs/olf in the plasma membrane, creating an initial burst of cAMP production. Activated D1 receptors are rapidly concentrated in clathrin-coated pits, which undergo dynamin-dependent endocytosis and rapid uncoating. Additional cAMP is then generated from a nascent endocytic membrane compartment associated with Gs/olf and ACV. Endocytic membranes subsequently fuse with classical sorting endosomes that are not signaling-competent and receptors are inactivated by agonist dissociation in the increasingly acidic endosomal environment. Efficient trafficking of internalized D1 receptors back to the plasma membrane requires the vacuolar ATPase mediating continued endosomal acidification and EHD3 mediating membrane transfer from early to recycling endosomes. Manipulations that disrupt the function or trafficking through later sorting compartments (Bafilomycin, siRNA vs. EHD3) do not affect the magnitude of the acute cAMP response. Manipulations that interfere events initiating entry of activated D1 receptors into the endomembrane system (HS, dynasore, siRNA vs. clathrin, Δ360-382) inhibit acute cAMP signaling by preventing the second burst of cAMP production in this dynamic trafficking cycle.
Endocytosis drives D1 receptors into close proximity to both trimeric G protein and adenylyl cyclase
To assess whether it is possible for D1 receptors to promote acute cAMP accumulation from an endocytic intermediate, we investigated the physical organization of internalized D1 receptors relative to the relevant downstream cAMP transduction machinery. D1 receptors stimulate agonist-dependent cAMP production in striatal neurons by coupling to Golf, a trimeric G protein closely related to Gs, whose liberated α-subunit stimulates adenylyl cyclase V (ACV) (Neve et al., 2005). ACV is an integral membrane protein while Gs/olf α-subunits are membrane-tethered by palmitoylation and associate non-covalently with membrane-embedded βγ subunits. Gs/olf α-subunits dissociate from βγ and turn over their palmitoyl tether in response to receptor-mediated activation, allowing them to transiently redistribute to the cytoplasm (Marrari et al., 2007). Thus, for signaling to occur from activated receptors entering the endocytic pathway, both ACV and Gs/olf would need to exist in close proximity to D1 receptors.
We examined the distribution of D1 dopamine receptors relative to ACV, using dual label immunocytochemical staining of striatal neurons followed by laser-scanning confocal fluorescence microscopy. Prior to stimulation, the majority of surface-labeled FD1R immunoreactivity was concentrated at the cell periphery while endogenous ACV was detected both peripherally and associated with internal structures (Figure 8A, top). Surface-labeled D1 receptors moved to endocytic membrane structures within 2 minutes after agonist addition and a number of these colocalized with ACV (Figure 8A, bottom). The fraction of D1 receptor immunoreactive structures that also contained ACV is quantified in Figure 8B. We used the same approach to look at the subcellular localization D1 receptors in relationship to Gαs/olf proteins. Prior to stimulation, Gαs/olf immunoreactivity localized both peripherally and in association with internal structures, while D1 receptors showed a peripheral distribution consistent with plasma membrane localization (Figure 8C, top). Following acute receptor activation, D1 receptors redistributed to endocytic vesicles and Gαs/olf immunoreactivity colocalized with a significant fraction these structures (Figure 8C, bottom and Figure 8D). Examination of this distribution at higher magnification suggested that both downstream transduction proteins localize to sub-domains of D1 receptor-containing early endocytic membranes (insets in Figure 8A and 8C).
DISCUSSION
To our knowledge, the present results provide the first analysis of the relationship between D1 receptor trafficking and signaling in neurons, and on a time scale approaching that of physiological dopaminergic neurotransmission. Our results demonstrate that D1 receptors enter the endocytic pathway within ~1 min after activation by either DA or synthetic agonist and that receptor-mediated accumulation of cellular cAMP occurs with overlapping kinetics. They also establish a causal relationship whereby D1 receptor endocytosis augments acute dopaminergic signaling. We demonstrate that recycling is not required for this response and provide evidence that the endocytosis-dependent signal is generated from an early endosomal membrane, thus distinguishing the present results from endocytosis-dependent resensitization observed for several other GPCRs. Further, our results show that the endocytosis-dependent component of the D1 receptor-mediated signal is functionally relevant as it is required to increase AP firing in a native brain slice preparation.
Previous studies of D1 receptor-mediated signaling effects, measured over longer time intervals (> 30 minutes), have suggested that endocytosis either inhibits (Jackson et al., 2002; Zhang et al., 2007) or has no effect on dopaminergic signaling (Gardner et al., 2001). Further, endocytosis has been generally shown to attenuate cellular responsiveness, such as for AMPA receptor endocytosis and LTD (Malenka, 2003), or restore cellular responsiveness, such as for resensitization of β-adrenoreceptors after prolonged stimulation or stimulation and a refractory period (Pippig et al., 1995). We are not aware of previous evidence that endocytosis augments the acute cAMP response mediated by any signaling receptor. Our study focused on cells in which D1 receptors are primarily thought to endocytose via clathrin-coated pits. There is evidence that caveolae mediate a slower component of D1 receptor endocytosis in other cells, but this is not thought to affect the acute cAMP signal (Kong et al., 2007). As such, we believe that the presently identified role of endocytosis in supporting acute D1 receptor-mediated signaling is unique.
What is the mechanism by which rapid endocytosis contributes to dopaminergic signaling? We initially favored the hypothesis that this augmentation might occur by rapid cycling of receptors back to the plasma membrane. This was motivated by analogy with the resensitization paradigm established for several other GPCRs. As presently understood, however, the resensitization paradigm explains recovery of signal responsiveness after prolonged or repeated activation, and does not affect the acute signaling response (Lefkowitz, 1998; Pippig et al., 1995). Nevertheless, given the rapid kinetics with which D1 receptors were found to traverse the recycling pathway, we considered the hypothesis that D1 receptors might recycle so rapidly that their resensitization might have been missed by the previous paradigm. We rejected this hypothesis because genetic (EHD3 knockdown) and chemical (bafilomycin A1) inhibition of the recycling pathway did not affect D1 receptor-mediated cAMP accumulation, in contrast to the pronounced inhibition produced by various endocytic inhibitors.
Our results thus support the alternative hypothesis that endocytosis augments acute dopaminergic signaling by facilitating direct D1 receptor-mediated signaling from a membrane domain in the early endocytic pathway (Figure 9E). This is supported by immunocytochemical localization data showing close proximity between D1 receptors and downstream transduction machinery upon initial entry to the endocytic pathway. While there is no doubt that the plasma membrane is a major site of GPCR signaling, there is emerging evidence that signaling can also occur from the endocytic pathway, and there is presently no compelling reason to rule out endosomal signaling via trimeric G proteins (Calebiro et al., 2010; Sorkin and von Zastrow, 2009). Trimeric G proteins have previously been detected on endomembane compartments in mammalian cells and tissues (Marrari et al., 2007). Further, G protein α-subunits can mediate functionally significant signaling from endosomes in yeast (Slessareva and Dohlman, 2006). Recent evidence suggests that two other mammalian GPCRs (the TSH and PTH receptors) signal via G protein-linked activation of AC directly from the endosome membrane (Calebiro et al., 2009; Ferrandon et al., 2009). However, the endocytosis-dependent component of signaling described for these GPCRs is specific to a component of the cAMP response measured tens of minutes after initial receptor activation. Our studies establish a novel role of the endocytic machinery in augmenting rapid D1 receptor-mediated signaling, and show that D1 receptors achieve close proximity to essential downstream signaling components upon or shortly after endocytosis. It remains to be determined whether endomembrane signaling of D1 receptors is more effective than signaling from the plasma membrane, or if endocytosis restores signaling activity after termination at the plasma membrane.
The present discovery has intriguing implications for neuroscience. A variety of complex functions including learning and memory, locomotion and goal-directed behaviors such as food or drug seeking require precise regulation of dopaminergic signaling via D1 receptors (Kelley, 2004; Sibley, 1999). Further, recent studies in awake, behaving animals have shown transient spikes in DA concentrations that last on the order of seconds (Heien et al., 2005; Roitman et al., 2008; Tsai et al., 2009). Our data indicate that robust endocytosis of D1 receptors can occur, and is capable supporting cellular cAMP signaling on a time scale that approaches this physiology.
In vivo measurements have shown that extracellular DA transients can vary in peak intensities from nano- to micromolar concentration. We have demonstrated that the D1 receptor undergoes rapid endocytosis in the upper range these concentrations (see Figure 1A). Interestingly, the peak concentration measured in each of these studies varied substantially depending on the experimental paradigm that elicited DA transients. Rewarding taste stimuli evoked DA transients in the nucleus accumbens with peak concentrations near 50nM (Roitman et al., 2008), whereas electrical stimulation of dopaminergic VTA neurons could elicit DA transients in the nucleus accumbens with peak amplitudes greater than 0.5 μM (Heien et al., 2005). In vivo microdialysis measurements of DA in the striatum of non-human primates showed dopamine concentrations greater than 1uM after self-administration of cocaine (Bradberry et al., 2000). Our data predict that low extracellular DA concentrations within the brain would stimulate little D1 receptor endocytosis, while higher extracellular DA concentrations would elicit robust D1 receptor endocytosis and promote acute ongoing dopaminergic signaling. Thus, endocytosis of D1 receptors may reflect a novel mechanism by which signal strength, signal duration and perhaps even the salience of a given stimulus could be effectively encoded at the cellular level.
It is important to note that although our studies examined D1 receptor mediated signaling with substantially improved temporal resolution relative to conventional biochemical approaches, we are not yet capable of resolving effects with the kinetics of physiological dopamine release thought to occur within the healthy brain. Thus our findings may be more applicable to drug-induced or pathological states. Future studies, delivering dopamine in a more transient manner and further improving the temporal resolution of analysis, will be important to investigate this issue.
In conclusion, the present study examined rapid D1 receptor-mediated signaling and endocytic trafficking and identified a role of the endocytic machinery in supporting a component of acute dopaminergic signaling. We believe that these findings establish a previously unanticipated relationship between the endocytic machinery and acute cAMP signaling, and do so in neurons that naturally respond to DA. We propose that endocytosis-supported signaling by D1 receptors likely represents a fundamental principle by which the nervous system shapes and maintains dopaminergic responsiveness at the level of the individual neuron.
EXPERIMENTAL PROCEDURES
cDNA constructs and reagents
The FLAG epitope-tagged human D1 dopamine receptor (FD1R), 360-382 deletion mutant and Epac1-cAMPs were previously described (Nikolaev et al., 2004; Vargas and von Zastrow, 2004; Vickery and von Zastrow, 1999). The superecliptic pHluorin-tagged D1 dopamine receptor (SpH-D1R) was constructed using an N-terminal cassette (Yudowski et al., 2006). For neuronal expression all constructs were cloned into pCAGGS (Niwa et al., 1991). The following synthetic RNA duplexes were obtained from the validated HP GenomeWide siRNA collection (Qiagen): Clathrin, HsCLC10; EHD3, HsEHD3_3; non-silencing control, AllStars Negative Control siRNA. Rhodamine-labeled duplexes were used in Epac1-cAMPs FRET experiments to verify delivery to the cells analyzed. Dynasore (Sigma) and bafilomycin A1 (Tocris Biosciences) were freshly prepared before use in DMSO. Additional details are included in Supplemental Experimental Procedures.
Cell culture and transfection
Human embryonic kidney 293 (HEK 293) cells were obtained from ATCC. Striatal neurons were prepared from embryonic day 17-18 Sprague Dawley rats, transfected upon plating and studied 10-14 DIV. Details are provided in Supplemental Experimental Procedures.
Live imaging of SpH-D1R localization and trafficking by TIRF microscopy
TIRF microscopy was performed at 37°C using a Nikon 2000E inverted microscope equipped with Perfect Focus, 100x/NA1.49 TIRF objective, Nikon 488 laser TIRF illuminator and standard 488/516 excitation cube, Lambda 10-3 emission filter wheel (520/50m filter) controlled via SmartShutter (Sutter Instruments) and interfaced to a PC running NIS-Elements Advanced Research software (Nikon). More details are included in Supplemental Experimental Procedures.
FRET imaging
Wide field FRET imaging was carried out at 37°C using a shuttered mercury arc lamp and standard CFP excitation (ET430/24x) and YFP emission (ET535/30m) bandpass filters (Chroma). TIRF FRET imaging was performed using 440nm and 514nm laser excitation and through-the-objective evanescent field illumination. YFP emission was collected using a 545/40m filter, and CFP emission was collected through a 485/30m filter. Corrected FRET ratios were obtained using the following equation: NFRET = [(IFRET-BGFRET)-(ICFP-BGCFP)BTDONOR-(IYFP-BGYFP)DEACCEPTOR)]/ICFP, where BTDONOR = donor bleed through, DEACCEPTOR = direct excitation of the acceptor, BGX = background fluorescence and IX = integrated fluorescence intensity measured in a given channel. Additional details are provided in Supplemental Experimental Procedures.
Immunocytochemical methods, fluorescence flow cytometry and biochemical methods
Immunocytochemical localization of receptors was carried out using M1 anti-FLAG monoclonal antibody (Sigma). Clathrin, EEA1, ACV and Gs/olf immunolocalization was carried out using mouse monoclonal anti-Clathrin (x-22) (abcam), mouse monoclonal anti-EEA1 (BD Biosciences), rabbit anti-ACV/VI (Santa Cruz) and mouse monoclonal GαS/olf (E-7) (Santa Cruz). Mean fluorescence intensity of Alexa647-labeled surface FD1Rs was collected using a flow cytometer (Becton Dickson). Samples were maintained on ice at the end of each experimental procedure. Ratiometric determination of agonist-induced changes in surface FD1R and surface recovery of internalized FD1R were performed using a modifications of previously described protocols (Haberstock-Debic et al., 2005; Tanowitz and von Zastrow, 2003). Immunoblot detection of clathrin heavy chain and EHD3 were carried out using mouse monoclonal anti-Clathrin HC (Santa Cruz) and rabbit polyclonal anti-EHD3 (abcam) and HRP conjugated secondary antibodies. Further details are included in Supplemental Experimental Procedures.
Electrophysiology
Acute brain slices (250-300 μm) containing the dorsal striatum were prepared from P20-P28 male Sprague-Dawley rats. Electrophysiology was carried out in artificial CSF, using whole-cell recording of MSNs visualized by infrared-DIC, with 2.5 to 3.5 mm electrodes, as described in detail in Supplemental Experimental Procedures. All animal methods were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health and the Ernest Gallo Clinic and Research Center’s Institute for Animal Care and Use Committee.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Martin Lohse (University of Würzburg, Germany) for providing the Epac1cAMPs construct and Dr. Tomas Kirchhausen (Harvard Medical School) for providing dynasore used in initial experiments and instructions for its effective use. Data for this study were collected at the Nikon Imaging Center (NIC) at the University of California, San Francisco. We are grateful to Dr. Kurt Thorn, Director of the NIC, for valuable instruction and advice. We also thank Drs. Guillermo Yudowski and Kit Wong for advice and assistance, and Dr. Jin Tomshine for useful discussion. This work was supported by grants from the National Institutes of Health (DA-010711 and DA-010154 to M.Z., MH-24468 to S.J.K., AAA-015358 to F.W.H.) and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (AB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M, Carra S, Pierucci M, Di Giovanni G, Esposito E, et al. Impact of Serotonin 2C Receptor Null Mutation on Physiology and Behavior Associated with Nigrostriatal Dopamine Pathway Function. J Neurosci. 2009;29:8156–8165. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Sortwell CE, Ray M, Altemus KL, Sibley DR, Levine MS. Agonist-induced morphologic decrease in cellular d1A dopamine receptor staining. Synapse. 1997;27:313–321. doi: 10.1002/(SICI)1098-2396(199712)27:4<313::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Bloch B, Bernard V, Dumartin B. “In vivo” intraneuronal trafficking of G protein coupled receptors in the striatum: regulation by dopaminergic and cholinergic environment. Biology of the Cell. 2003;95:477–488. doi: 10.1016/s0248-4900(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of Self-Administered Cocaine and Cocaine Cues on Extracellular Dopamine in Mesolimbic and Sensorimotor Striatum in Rhesus Monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS biology. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends in Pharmacological Sciences. 2010;31:221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 Dopamine Receptor in Striatal Neurons In Vivo as Evidence of Activation by Dopamine Agonists. J Neurosci. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga J-P. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin E, Benjamin S, Rapaport D, Rotem-Yehudar R, Tolchinsky S, Horowitz M. EHD3: a protein that resides in recycling tubular and vesicular membrane structures and interacts with EHD1. Traffic. 2002;3:575–589. doi: 10.1034/j.1600-0854.2002.30807.x. [DOI] [PubMed] [Google Scholar]
- Gardner B, Liu ZF, Jiang D, Sibley DR. The Role of Phosphorylation/Dephosphorylation in Agonist-Induced Desensitization of D1 Dopamine Receptor Function: Evidence for a Novel Pathway for Receptor Dephosphorylation. Mol Pharmacol. 2001;59:310–321. doi: 10.1124/mol.59.2.310. [DOI] [PubMed] [Google Scholar]
- Greengard P. The Neurobiology of Slow Synaptic Transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim K-A, Yu YJ, von Zastrow M. Morphine Promotes Rapid, Arrestin-Dependent Endocytosis of {micro}-Opioid Receptors in Striatal Neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Wightman RM. Real-time measurements of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Wightman RM. Phasic Dopamine Signaling During Behavior, Reward, and Disease States. CNS & Neurological Disorders – Drug Targets (Formerly Current Drug Targets – CNS & Neurological Disorders) 2006;5:99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 Receptor Activation Enhances Evoked Discharge in Neostriatal Medium Spiny Neurons by Modulating an L-Type Ca2+ Conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. The Journal of cell biology. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang S-J, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced Nucleus Accumbens SK Channel Activity Enhances Alcohol Seeking during Abstinence. Neuron. 2010;65:682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Rafal MI, Ziad YC, Marie-France N, Mario T. Homologous regulation of the heptahelical D1A receptor responsiveness: specific cytoplasmic tail regions mediate dopamine-induced phosphorylation, desensitization and endocytosis. Journal of Neurochemistry. 2002;82:683–697. doi: 10.1046/j.1471-4159.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- Johnson LS, Dunn KW, Pytowski B, McGraw TE. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor’s internalization motif. Mol Biol Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kim O-J, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim K-M, Sibley DR. The Role of Phosphorylation in D1 Dopamine Receptor Desensitization: evidence for a novel mechanism of arrestin associtation. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Macia E, Pelish HE, William E, Balch C. J. D. a. A. H. Methods in Enzymology. Academic Press; 2008. Use of Dynasore, the Small Molecule Inhibitor of Dynamin, in the Regulation of Endocytosis; pp. 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong MMC, Hasbi A, Mattocks M, Fan T, O’Dowd B, George S. Regulation of D1 Dopamine Receptor Trafficking and Signaling by Caveolin-1. Mol Pharmacol. 2007;72:1157–1170. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and Pharmacology of Striatal Neurons. Annual Review of Neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Lamey M, Thompson M, Varghese G, Chi H, Sawzdargo M, George S, O’Dowd B. Distinct residues in the carboxy tail mediate agonist-induced desensitization and internalization of the human dopamine D1 receptor. J Biol Chem. 2002;277:9415–9421. doi: 10.1074/jbc.M111811200. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G Protein-coupled Receptors III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. The Journal of biological chemistry. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic Plasticity and AMPA Receptor Trafficking. Annals of the New York Academy of Sciences. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and Trafficking of Heterotrimeric G Proteins,Ć. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Negrier M-L, Giselle C, Bertrand B. Receptor recycling mediates plasma membrane recovery of dopamine D1 receptors in dendrites and axons after agonist-induced endocytosis in primary cultures of striatal neurons. Synapse. 2006;60:194–204. doi: 10.1002/syn.20296. [DOI] [PubMed] [Google Scholar]
- Martin-Negrier ML, Charron G, Bloch B. Agonist stimulation provokes dendritic and axonal dopamine D1 receptor redistribution in primary cultures of striatal neurons. Neuroscience. 2000;99:257–266. doi: 10.1016/s0306-4522(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Mason JN, Kozell LB, Neve KA. Regulation of Dopamine D1 Receptor Trafficking by Protein Kinase A-Dependent Phosphorylation. Mol Pharmacol. 2002;61:806–816. doi: 10.1124/mol.61.4.806. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Muriel M-P, Orieux G, Hirsch EC. Levodopa but not ropinirole induces an internalization of D1 dopamine receptors in parkinsonian rats. Movement Disorders. 2002;17:1174–1179. doi: 10.1002/mds.10256. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD Proteins and Rab11-FIP2: A Role for EHD3 in Early Endosomal Transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine Receptor Signaling. Journal of Receptors and Signal Transduction. 2005;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Ng G, Mouillac B, George S, Caron M, Dennis M, Bouvier M, O’Dowd B. Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. European Journal of Pharmacology. 1994;267:7–19. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. The Journal of biological chemistry. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Presley J, Mayor S, McGraw T, Dunn K, Maxfield F. Bafilomycin A1 Treatment Retards Transferrin Receptor Recycling More than Bulk Membrane Recycling. J Biol Chem. 1997;272:13929–13936. doi: 10.1074/jbc.272.21.13929. [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo Regulates Clathrin-Coated Pit Dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The Use of pHluorins for Optical Measurements of Presynaptic Activity. Biophysical Journal. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annual Review of Pharmacology and Toxicology. 1999;39:313–341. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- Slessareva JE, Dohlman HG. G Protein Signaling in Yeast: New Components, New Connections, New Compartments. Science. 2006;314:1412–1413. doi: 10.1126/science.1134041. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. The Journal of biological chemistry. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas GA, von Zastrow M. Identification of a Novel Endocytic Recycling Signal in the D1 Dopamine Receptor. J Biol Chem. 2004;279:37461–37469. doi: 10.1074/jbc.M401034200. [DOI] [PubMed] [Google Scholar]
- Vickery RG, von Zastrow M. Distinct dynamin-dependent and – independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. The Journal of cell biology. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Dhavan R, Chevalier MW, Yudowski GA, von Zastrow M. Rapid Delivery of Internalized Signaling Receptors to the Somatodendritic Surface by Sequence-Specific Local Insertion. J Neurosci. 2010;30:11703–11714. doi: 10.1523/JNEUROSCI.6282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Molecular biology of the cell. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. The Journal of neuroscience. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nature neuroscience. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SGN, Miller GM, Isacson O, Caron MG, Yao W-D. Inhibition of the Dopamine D1 Receptor Signaling by PSD-95. J Biol Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.