Abstract
Proliferating cell nuclear antigen (PCNA) is a critical player in cell proliferation. It interacts with a myriad of cellular proteins in genomic DNA replication and cell cycle control. This makes PCNA an attractive target for developing antiproliferative therapeutics. Indeed the binding of a human tumor suppressor protein, p21, to PCNA contributes to its antiproliferative effect in cells. In this work we reported a fluorescence polarization-based binding assay for determining the affinity between the p21 peptide and human PCNA. In order to improve the potency of the p21-based PCNA antagonist, we exploited the homotrimeric structure of PCNA and developed multivalent peptide-based PCNA antagonists. The di- and trivalent p21-based antagonists bind to PCNA with low nanomolar dissociation constant. Moreover, we showed that the multivalent PCNA antagonists inhibited PCNA-dependent DNA synthesis in a human cell extract with improved avidity when compared to the monovalent p21 peptide. The fluorescence polarization assay holds promise for the discovery of potent small-molecule PCNA inhibitors given its ready adaptability to a high-throughput screening format.
Keywords: PCNA, multivalent binding, fluorescence assay, DNA synthesis, antagonist
Controlled cell proliferation is crucial for the normal growth of human tissue and organs. Abnormality in cell proliferation usually leads to neoplastic diseases and cancer. Normal cell proliferation requires the orchestrated actions of proteins from various essential cellular pathways including DNA replication, DNA repair, and cell cycle regulation [1; 2]. Numerous studies pointed to the proliferating cell nuclear antigen (PCNA) as a master regulator of cell growth in humans [3]. PCNA possesses the striking ability of interacting with a myriad of proteins functioning in DNA replication/repair, translesion DNA synthesis, chromatin remodeling, and cell cycle checkpoint regulation [4; 5]. The unique properties of PCNA make it an attractive target for controlling eukaryotic cell proliferation. Indeed the human tumor suppressor protein, p21, exerts inhibitory effects on cell growth by targeting PCNA [6; 7]. Several studies have shown that depression of PCNA expression can effectively inhibit cell growth [8; 9]. In the past decade there have been impressive advances in understanding the structural and functional properties of PCNA [3]. The valuable information derived from these studies set the stage for developing potent PCNA antagonists for controlling cell proliferation. The development of effective PCNA antagonists will also contribute to the treatment of cancer and other common diseases such as rheumatoid arthritis [10]. Furthermore the development of PCNA antagonists may find valuable application in tissue engineering given that regulating and preventing the neoplastic outgrowth of implanted tissue is important for the success of tissue regeneration [11; 12; 13].
Proliferating cell nuclear antigen, as suggested by its name, was initially identified as an abundant protein species in proliferating cells. Later X-ray crystallography revealed that PCNA belongs to a universal protein family called ‘clamp’ protein, which functions as a processivity factor for the replicative DNA polymerase [5; 14]. One important function of PCNA is to greatly increase the processivity of DNA polymerase by tethering it to the DNA template. PCNA also orchestrates a number of other essential cellular pathways by recruiting numerous proteins to the replication fork [3]. Many of these proteins bind to PCNA through a PIP (PCNA-interacting protein) box located either internally or at the terminus of the protein sequence [4]. The PIP box sequence can be generalized to Qxx(M/L/I)xxF(Y/F) (x stands for variable amino acid). A previous study suggested that the affinity of PCNA binding protein is influenced by the variable residues of the PIP box as well as the sequence that flanks the PIP box [15].
An earlier study demonstrated that suppression of PCNA expression by antisense oligonucleotides selectively inhibits gastric cancer cell proliferation [8]. Similar observation was made for smooth muscle cells in rat carotid artery injury models by suppressing PCNA expression [9]. Therefore PCNA represents an attractive target for controlling cell proliferation in different cell types. It is known that the human tumor suppressor protein, p21, exerts an inhibitory effect upon cell growth by binding to PCNA [6]. p21 is a member of the CDK-inhibitor protein family [16]. The N-terminal region of p21 binds to cyclins and inhibits the cyclin-dependent kinases that function at the major transition points of the cell cycle [17; 18]. The C-terminal region of p21 binds directly to the interdomain connector (IDC) loop binding site on PCNA. The binding of p21 peptide to PCNA blocks the association of DNA polymerases with PCNA, thus preventing DNA synthesis. Notably, a 20 amino-acid peptide spanning the C-terminus of p21 was found to be sufficient for interaction with PCNA. Overexpression of this peptide in mammalian cells was able to arrest cell division [19; 20]. Subsequent biochemical studies revealed that p21 peptide binds to PCNA tightly, with a dissociation constant of 88 nM [21].
Given the potential of p21 peptide in controlling cell proliferation, several labs have developed peptides that bind to PCNA with an improved affinity [15; 21; 22]. Interestingly, it was found that shortening of the p21 peptide resulted in a severe loss of binding affinity to PCNA. To circumvent this problem, peptides with shorter length were rationally designed based on the consensus sequence of the PIP box. However, only one of the designed peptides demonstrated a slightly higher binding affinity [21]. Another study employed peptides derived from various PCNA binding proteins including the p66 subunits of human DNA polymerase δ and the human FEN1 endonuclease [15]. However, the affinity constant of the peptides were found to be at least 200-fold lower compared to the p21 peptide [15]. Random peptide libraries were also investigated to select for PCNA-binding peptides [23; 24]. Although novel peptides were identified, no significant improvement in binding affinity was achieved. The limited success in developing highly potent PCNA binding peptides stresses the need for new design strategies that are different from the strategies previously employed.
Numerous biological processes in nature involve multivalent binding interactions. In many cases the binding affinity between individual molecules is often weak; however multivalent interactions function to increase the binding affinity and stabilize complex formation [25; 26]. Exploiting multivalency researchers have developed a number of polyvalent inhibitors targeting various proteins and receptors [27; 28; 29; 30]. In most studies multivalent interactions were of significantly higher affinity compared to their respective monovalent counterpart.
In this work, we have developed a method to monitor PCNA binding by fluorescence polarization. With this new assay method, we quantified the binding affinity between a fluorescently labeled p21 peptide and human PCNA. Moreover a fluorescence-based competition assay was developed to quantify the binding affinity between the PCNA antagonists and PCNA. To improve the affinity of the p21-based PCNA antagonist, we exploited the multiple binding sites on the homotrimeric PCNA and generated multivalent antagonists consisting of two or three repeats of the native p21 peptide. Both divalent and trivalent p21 peptides bound to PCNA with improved affinity. We also demonstrated that the multivalent p21-based peptides effectively inhibit DNA synthesis in a human cell-free extract. Our results support the notion that PCNA antagonists with improved efficacy can be attained by exploiting the multivalency of the PCNA binding sites.
Experimental
Plasmid construction and gene cloning
The human PCNA gene was amplified by PCR using human cDNA as a template. The amplified PCR product was cloned into the E. coli expression vectors pET22b or pET28a using the restriction sites NdeI and XhoI. The PCNA/pET22b plasmid encodes human PCNA without tag, while the PCNA/pET28a plasmid allows the expression of human PCNA with an N-terminal 6xHis tag. To generate the expression plasmids for the p21 divalent peptide (KRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFS) and the p21 trivalent peptide (KRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFS), a synthetic gene was constructed by Genscript where repeats of the p21 peptide were linked together by flexible glycine/serine linkers. The amplified gene products were cloned into the E. coli expression vector pET28a using the restriction sites NdeI and XhoI. The di-and trivalent p21/pET28a plasmid allowed for the expression of peptides with an N-terminal 6xHis tag.
Protein expression and purification
Human PCNA was expressed in Rosetta(DE3) cells (Novagen). Cells were cultured at 37 °C until the OD600 reached 0.8. The cell culture was induced with 0.4 mM IPTG and cultured for 15 hours at 17 °C following the induction. For PCNA with no tag, the cells were harvested and sonicated in lysis buffer [50 mM Tris (pH 7.5), 50 mM NaCl, 5% glycerol, 2.5 mM β-mercaptoethanol, and 1 mM PMSF]. The cell free extract was loaded onto a DEAE sepharose column equilibrated with wash buffer [50 mM Tris (pH 7.5), 5% glycerol, and 2.5 mM β-mercaptoethanol] at a flow rate of 2.0 ml/min. The human PCNA was eluted off the column using a salt gradient between 170 and 250 mM NaCl. Protein purity was analyzed using SDS-PAGE and Coomassie Blue staining. The concentrated fractions were diluted in the wash buffer to a final NaCl concentration of 50 mM and then loaded onto a HiTrap Q FF anion exchange column (GE Healthcare) at a flow rate of 2.0 ml/min. PCNA was eluted off the column at a NaCl concentration of 340 mM. The PCNA fractions were pooled together and dialyzed against a buffer containing 10 mM Na2HPO4 (pH 7.5), 1.8 mM KH2PO4, 140 mM NaCl, 2.7 mM KCl, 5% glycerol, and 0.5 mM DTT. Protein purity was analyzed using SDS-PAGE and Coomassie Blue staining.
For purification of the 6xHis-tagged PCNA, the cells were resuspended and sonicated in lysis buffer [50 mM NaH2PO4 (pH 8.0), 500 mM NaCl, 5% glycerol, 1 mM β-mercaptoethanol, 1 mM PMSF, and 10 mM imidazole]. The cell free extract was incubated with Ni-NTA resin (Invitrogen) and washed extensively with lysis buffer. Bound PCNA was eluted with lysis buffer containing 100 mM imidazole. Eluted PCNA fractions were combined and diluted to a final NaCl concentration of 100 mM using a buffer containing 50 mM NaH2PO4 (pH 8.0), 5% glycerol, and 1 mM β-mercaptoethanol. Diluted protein solution was loaded onto a HiTrap Q FF anion exchange column (GE Healthcare) at a flow rate of 2.0 ml/min. Bound PCNA was eluted off the column at a NaCl concentration of 350 mM. Pure fractions were combined and buffer exchanged into a buffer containing 10 mM Na2HPO4 (pH 7.5), 1.8 mM KH2PO4, 140 mM NaCl, 2.7 mM KCl, 5% glycerol, and 0.5 mM DTT. Protein purity was analyzed using SDS-PAGE and Coomassie Blue staining.
The divalent and trivalent p21 peptides were expressed in the Rosetta(DE3) cells (Novagen). Cells were cultured at 37 °C until the OD600 reached 0.8. The cells were then induced with 0.4 mM IPTG and were cultured for an additional 8 hours at 37 °C. The cells were harvested and sonicated in lysis buffer [50 mM NaH2PO4 (pH 8.0), 8 M urea, 500 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol, and 10 mM imidazole]. Cell free extract was bound to Ni-NTA resin (Invitrogen) and washed extensively with lysis buffer. Urea was then removed by washing the column stepwise with the lysis buffer that contained 6 M, 3 M, 1 M, and 0 M urea. After the urea was completely removed, the divalent and trivalent p21 peptide was eluted with lysis buffer free of urea that contained 100 mM imidazole. The eluted peptides were dialyzed against a buffer containing 50 mM NaH2PO4 (pH 8.0), 150 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol. Purity of the divalent and trivalent peptides was analyzed using SDS-PAGE and Coomassie Blue staining.
Electrospray ionization mass spectrometry
For all ESI-MS analysis, the divalent and trivalent p21 peptide samples were desalted by HPLC chromatography using a Phenomenex Jupiter C18 column (300 Å, 10 μm, 250 mm × 10 mm) with an acetonitrile gradient at a flow rate of 4 ml/min. Mobile phase A (89.9% H2O, 10% acetonitrile, and 0.1% TFA) and B (9.9% H2O, 90% acetonitrile, and 0.1% TFA) were used to elute the peptides at approximately 30% acetonitrile. HPLC fractions containing the purified peptide were analyzed using a Q-TOF Ultima API-US quadrupole time-of-flight mass spectrometer (Micromass, Manchester, U.K.). The samples were injected into an API-ESI source. Mass spectra were acquired from 400 to 1800 m/z using a scan time of 0.5 s and an interscan time of 0.01 s. All data was processed using MaxEnt1.
Circular dichroism spectroscopy
Circular dichroism (CD) spectra were recorded using a Jasco J-810 spectropolarimeter for the peptides. CD data were collected for each sample in a 1 mm cell with 70 μM p21 peptide, 20 μM divalent p21 peptide, and 12 μM trivalent p21 peptide in a buffer containing 10 mM KH2PO4 (pH 8.0). The final spectra were obtained by averaging three independent data sets collected every 0.25 nm with an averaging time of 1 s and a bandwidth of 2 nm.
Labeling the p21 peptide with BODIPY
A peptide based on the C-terminal sequence of human p21 (CRQTSMTDFYHSKRRLIFS) was synthesized by Genscript. This peptide contains the same sequence as amino acids 143-160 of the native p21 protein, except that a cysteine residue was introduced at the N-terminus. The fluorophore, BODIPY, was conjugated to the N-terminal cysteine in the p21 peptide. In a typical labeling reaction, p21 peptide was resuspended in labeling buffer containing 20 mM Tris (pH 7.2) and 400 μM tris(2-carboxyethyl)phosphine (TCEP). BODIPY FL N-(2-aminoethyl) maleimide solubilized in DMF was added to the p21 peptide solution at a molar ratio of 5:1 (BODIPY : p21 peptide). The incubation was allowed to proceed at room temperature for 3 hours. The reaction was then quenched with -mercaptoethanol. The BODIPY-p21 peptide was purified by HPLC chromatography using a Phenomenex Jupiter C18 column (300 Å, 10 μm, 250 mm 10 mm) with an acetonitrile gradient. The labeled p21 peptide eluted at approximately 30% acetonitrile. A scrambled p21 peptide (CFKDSRTYRRKMISLRTSFQH) generated by using Mobyle [31] was synthesized and labeled with BODIPY following the above described protocol for the native p21 peptide.
Binding assay by fluorescence polarization
The binding affinity between p21 peptide and PCNA was determined through fluorescence polarization. For a typical reaction 300 nM BODIPY-p21 peptide was incubated with increasing concentrations of PCNA (monomer concentration of 0-6.4 μM) in PBS buffer [10 mM Na2HPO4 (pH 7.5), 1.8 mM KH2PO4, 140 mM NaCl, and 2.7 mM KCl] containing 1 mM DTT at 25 °C in a 96-well plate. Fluorescence polarization was recorded on a Perkin-Elmer Fusion plate reader with a 485 nm excitation filter and a 535 nm emission filter with polarizer. Polarization values were plotted against PCNA concentration. Dissociation constants were determined by fitting the curve to equation 1 using GraphPad Prism (GraphPad Software, La Jolla, CA).
| (eq. 1) |
Y is the measured fluorescence polarization value at various PCNA concentrations. rmax is the fluorescence polarization value at the maximal binding, X is the monomeric concentration of PCNA, and Kd is the dissociation constant.
Competition-based fluorescence polarization assay for IC50 determination
The affinity of monovalent, divalent, and trivalent p21 antagonists for PCNA was assessed by the ability of the antagonist to compete for the binding sites on PCNA with the fluorescently labeled BODIPY-p21 peptide. In a typical assay reaction, the fluorescently labeled BODIPY-p21 peptide (300 nM) was incubated with PCNA (monomeric concentration of 800 nM) in PBS buffer [10 mM Na2HPO4 (pH 7.5), 1.8 mM KH2PO4, 140 mM NaCl, 2.7 mM KCl] containing 1 mM DTT at 25 °C in a 96-well plate. Increasing concentrations of the monovalent, divalent, or trivalent p21 antagonist (0-43 μM) were added to the reaction mixture and the polarization value was recorded on a Perkin-Elmer Fusion plate reader with a 485 nm excitation filter and a 535 nm emission filter. Fluorescence polarization values were plotted against the logarithm value of the concentration of the p21 antagonist. IC50 values were determined by fitting the dose response curve to equation 2 using GraphPad Prism (GraphPad Software, La Jolla, CA).
| (eq. 2) |
X is the logarithm of the p21 antagonist concentration in molar; Y is the fluorescence polarization value.
Determination of the dissociation constant of p21 antagonist
We first determined the fraction of the bound BODIPY-p21 peptide using equation 3 [32].
| (eq. 3) |
fB is the fraction of the bound BODIPY-p21. r is the observed fluorescence polarization. rf is the fluorescence polarization for free peptide and rmax is the fluorescence polarization at maximum peptide binding. R is ratio of the quantum yield of the bound and free BODIPY-p21 peptide. The determined R value is equal to 1.
To determine the dissociation constants of the p21 antagonist binding to PCNA, the total concentration of p21-based antagonist (mono-, di- and trivalent peptide) (Y) was plotted against the molar ratio of bound and free BODIPY-p21 peptide (X). X = fB/(1- fB). The curve was fit to equation 4 to determine the dissociation constant (Ki) of the PCNA antagonist [33].
| (eq. 4) |
P is the total monomeric concentration of PCNA, L is the concentration of BODIPY-p21, and Kd is the dissociation constant for BODIPY-p21 binding to PCNA.
Gel Filtration Chromatography
PCNA alone and PCNA complexed with the divalent or trivalent p21 peptide were analyzed using a Superose 12 gel filtration column (GE Healthcare) connected to an ÄKTA chromatography system (GE Healthcare). The divalent and trivalent p21 peptides were incubated with His-tagged PCNA trimer at a molar ratio of 1:1. Protein sample was loaded on to a calibrated Superose 12 gel filtration column equilibrated in buffer containing 20 mM Tris (pH 7.2), 20 mM NaCl, 2.5% glycerol, and 1 mM DTT at a flow rate of 0.5 ml/min. The protein elution was monitored by recording the UV-vis absorbance at 280 nm. The eluted fractions were further analyzed using SDS-PAGE and Coomassie Blue staining. To calculate the molecular weight of the target protein, a calibration curve was generated by plotting the log Mr value of known protein standards against the calculated kav. kav = (Ve - Vo)/(Vc -Vo), where Vo is the column void volume, Ve is the elution volume, and Vc is geometric column volume.
Inhibition of the PCNA-dependent DNA synthesis by p21 antagonists
A typical 20 μl DNA synthesis assay solution contained 5 nM DNA substrate and 5 μl HeLa cell extract in a reaction buffer containing 40 mM Tris (pH 7.8), 8 mM MgCl2, 1 mM DTT, 4 mM ATP, 100 μM dNTP, 40 mM phosphocreatine, and 1 unit of creatine phosphokinase. The DNA substrate was generated by annealing a 5’-P32 end-labeled 20mer DNA primer (5'-GAGTCACAACGTCGATCTTC-3') to a 50mer DNA oligo (5'-CTAGCGATGAGCACTGCTGCTGTGATCACAGA AGATCGACGT TGTGACTC-3'). The cytoplasmic HeLa cell extract was prepared as previously described [34]. To assay the inhibition of DNA synthesis by the PCNA antagonists, cell extracts were preincubated with the monovalent, divalent, or trivalent p21 peptides on ice for 40 min. DNA synthesis was initiated by the addition of the DNA substrate together with ATP, dNTPs, phosphocreatine, and creatine phosphokinase. DNA synthesis was allowed to proceed for 2 minutes at 37 °C.
To confirm that the inhibitory effect of the p21 antagonist on DNA synthesis was PCNA dependent, in a separate experiment increasing concentrations of PCNA were added to the cell extract treated with the p21-based peptide to rescue the DNA synthesis. All DNA replication reactions were quenched with a 5 μl 0.5 M EDTA solution. The assay solution was then treated with phenol/chloroform and precipitated with ethanol. Pellet was dissolved in formamide loading dye and separated on a 15% denaturing urea-polyacrylamide gel. The DNA product bands were imaged using PhosphorImager (Storm, GE Healthcare Bioscience). The full length DNA product was quantified using Imagequant 5.2 software (Molecular Dynamics). The amount of full-length DNA product in the presence of varying concentrations of the PCNA antagonist was normalized against the control reaction with no p21 antagonist added (treated as 100%). The IC50 value of inhibition was determined by fitting the dose response curve to equation 2 using GraphPad Prism (GraphPad Software, La Jolla, CA). X is the logarithm of the PCNA antagonist concentration in molar and Y is the percent of DNA synthesis relative to that in the control reaction.
Results
p21-PCNA binding assayed by fluorescence polarization
In order to quantify the binding affinity between PCNA and p21 peptide using fluorescence polarization, we utilized a p21-based peptide with a fluorophore BODIPY conjugated to its N-terminus. The peptide CRQTSMTDFYHSKRRLIFS contains the same sequence as the amino acids 143-160 of the human p21, except that a cysteine residue was introduced to the N-terminus of the peptide for fluorophore conjugation. The cysteine-containing p21 peptide was labeled using BODIPY FL N-(2-aminoethyl)maleimide following a standard thiol-labeling protocol. The labeled peptide was purified using HPLC and its identity was confirmed by electrospray ionization mass spectrometry (ESI-MS). The determined molecular weight of the labeled peptide (2791 Da) agreed well with the theoretical value of 2790 Da. This fluorescently labeled peptide was used for all later polarization assays.
Utilizing the fluorescently labeled peptide, we were able to quantify the binding affinity between p21 and human PCNA by measuring fluorescence polarization. Briefly, the BODIPY-p21 peptide (300 nM) was incubated with increasing concentrations of PCNA (monomer concentration of 0-6.4 μM). Changes in fluorescence polarization values associated with p21/PCNA binding were recorded for each PCNA concentration (Figure 1A). A Kd value of 342 ± 29 nM was determined for the p21 peptide binding to PCNA, which is higher than a dissociation constant (88 nM) determined by isothermal titration calorimetry (ITC) [21]. The lower affinity obtained for our p21 peptide is likely due to a shorter peptide sequence compared to the peptide used in the previous study (amino acids 141-160 of the native p21). We also observed a very similar binding affinity of the BODIPY-p21 peptide to the N-terminal His-tagged PCNA, with a Kd value of 251 ± 22 nM. This indicates that the N-terminal His-tag does not affect PCNA's interaction with the p21 peptide. Notably, a fluorescently labeled p21 peptide corresponding to amino acids 141-160 of the native p21 did not result in fluorescence polarization increase upon binding to PCNA. We attributed this to an increased N-terminal segmental rotation of the longer p21 peptide. Indeed, in the X-ray cocrystal structure of PCNA and a p21 peptide containing the amino acids 139-160 of the native p21, the amino acid residues 139-142 were found to be disordered [35]. Taken together it is conceivable that the N-terminal portion of the p21 peptide is highly flexible. As a control we generated a BODIPY-labeled scrambled p21 peptide (CFKDSRTYRRKMISLRTSFQH) and assessed its ability in binding PCNA using the same fluorescence polarization assay. No significant increase in fluorescence polarization was observed upon incubation of the peptide with PCNA (Figure 1B). This indicates that the scrambled p21 peptide lost its ability to bind PCNA. This observation supports the notion that the BODIPY-labeled p21 peptide binds to PCNA specifically.
Figure 1.
Fluorescence polarization binding assay for human PCNA and p21 peptide interaction. Fluorescence polarization values of BODIPY-p21 peptide (300 nM) (A) and BODIPY-labeled scrambled p21 peptide (300 nM) (B) were plotted against increasing concentrations of the human PCNA (monomeric concentration of 0-6.4 μM). The dissociation constant of BODIPY-p21 peptide for PCNA was determined through nonlinear curve fitting to equation 1 using GraphPad Prism (GraphPad Software, La Jolla, CA). No binding to PCNA was observed for the BODIPY-labeled scrambled p21 peptide.
Generation of divalent and trivalent p21 peptide-based antagonists
PCNA is a homotrimer with three identical p21 binding sites (Figure 2). Given the unique structure of PCNA, we designed novel PCNA antagonists that exploited the multiple p21-binding sites for high-affinity binding. Specifically, we designed multivalent PCNA antagonists in which two or three p21 peptides are linked by a flexible glycine/serine-rich linker (Figure 3A). The divalent p21 peptide (KRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFS) and trivalent p21 peptide (KRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFSGGSGGSGGSGGSGGSGGSKRRQTSMTDFYHSKRRLIFS) were expressed in E. coli cells and purified by Ni-affinity chromatography under denaturing condition. Following the purification, the denaturant was removed stepwise and the peptide was then eluted off the Ni-NTA column. The purified di- or trivalent p21 peptides were shown to be of high purity by SDS-PAGE and Coomassie Blue staining (Figure 3B). Electrospray ionization mass spectrometry (ESI-MS) was used to confirm the identity of the peptide (Figure 3C). We found that the N-terminal methionine of both peptides was post-translationally removed. The determined molecular weight of the di- and trivalent peptides (8470 and 12216 Da respectively) agreed well with the theoretical values of 8468 and 12215 Da respectively.
Figure 2.

The X-ray cocrystal structure of human PCNA complexed with the p21 peptide corresponding to amino acids 139-160 of the human p21. Each PCNA monomer (green, yellow, and blue) binds a p21 peptide (red). This image was generated using PyMol.
Figure 3.
The multivalent p21 peptide-based PCNA antagonists. (A) p21 peptide repeats (red) linked through a flexible glycine/serine-rich linker (black). (B) SDS-PAGE analyses of purified di- and trivalent p21 peptides. N-terminally His-tagged divalent and trivalent p21 peptides were expressed in E. coli and affinity purified under denaturing conditions. Peptide purity was assessed using SDS-PAGE and Coomassie blue staining. (C) ESI-MS spectra of the purified divalent and trivalent p21 peptides.
Circular dichroism characterization of the multivalent PCNA antagonists
In order to gain structural information of the di- and trivalent p21 peptides, circular dichroism (CD) spectroscopy was used to characterize the secondary structure of the p21-based peptides. We found that the monovalent p21 peptide existed largely in a disordered structure (Figure 4). This is in accord with a previous study that characterized a similar p21 peptide using CD and NMR [36]. Although p21 peptide is largely disordered in solution, it can fold into α-helix or β-sheet depending on the chemical environment. The CD spectra of the trivalent and divalent peptides indicated disordered peptide structure, similar to the monomeric p21 peptide (Figure 4). It has been shown that despite existing in a largely disordered state in solution, p21 peptide can adopt a defined structure upon binding to PCNA [35; 36]. X-ray crystallography revealed that p21 peptide forms a β-sheet with the PCNA interdomain connector (IDC) loop [35]. It is likely that, except the flexible linker sequence, the multivalent p21 peptides become structured once they bind to the surface groove on PCNA.
Figure 4.
Circular dichroism (CD) spectra of the p21 peptide-based PCNA antagonists. The far UV-CD spectra of the native p21 peptide (blue), divalent p21 peptide (red), and trivalent p21 peptide (black) are overlaid.
Competition-based fluorescence polarization assay for PCNA binding
We devised a competition-based binding assay to determine the binding affinity of the p21 antagonists using the above-described BODIPY-p21. In this assay, increasing concentrations of the PCNA antagonists were added to a solution containing BODIPY-p21 and PCNA. Decrease in fluorescence polarization was observed as more p21 antagonist was titrated into the assay solution. The fluorescence polarization value was plotted against the logarithm value of p21 peptide concentration (Figure 5A). An IC50 value of 4.2 μM was determined for monovalent p21 peptide. To obtain the dissociation constant for the monovalent p21 peptide, the molar ratio of bound to free BODIPY-p21 peptide was calculated using equation 3 and plotted against the concentration of monovalent p21 peptide (Figure 5B). From the curve we determined the dissociation constant (Ki) of the monovalent p21 peptide to be 348 ± 14 nM. This value agrees well with the dissociation constant (342 ± 29 nM) determined for the labeled BODIPY-p21 peptide. These results suggest that the addition of the dye molecule to the N-terminus of the p21 peptide did not affect its binding affinity to PCNA.
Figure 5.
Competition-based fluorescence polarization assay revealed stronger PCNA binding of the multivalent p21 peptides. (A) Titration of the native p21 peptide (blue), divalent p21 peptide (black), and trivalent p21 peptide (red) (0-43 μM) into the assay solution containing BODIPY-p21 peptide (300 nM) and human PCNA (monomeric concentration of 800 nM). The polarization values are plotted against the logarithm values of the concentration of PCNA antagonist. The curve was fit to equation 2 using the Prism to obtain the IC50 value (4.2 μM for the native p21 peptide, 1.0 μM for the divalent p21 peptide, and 0.6 μM for the trivalent p21 peptide). (B-D) Plots of the total PCNA antagonist concentration against the molar ratio of bound/free BODIPY-p21 peptide in the presence of varied concentrations of p21 peptide (B), divalent p21 peptide (C) and trivalent p21 peptide (D). The dissociation constant of the p21-based peptides in binding to PCNA was determined through nonlinear regression fitting of the plot using equation 4.
Multivalent p21 peptides bind to PCNA with an improved affinity
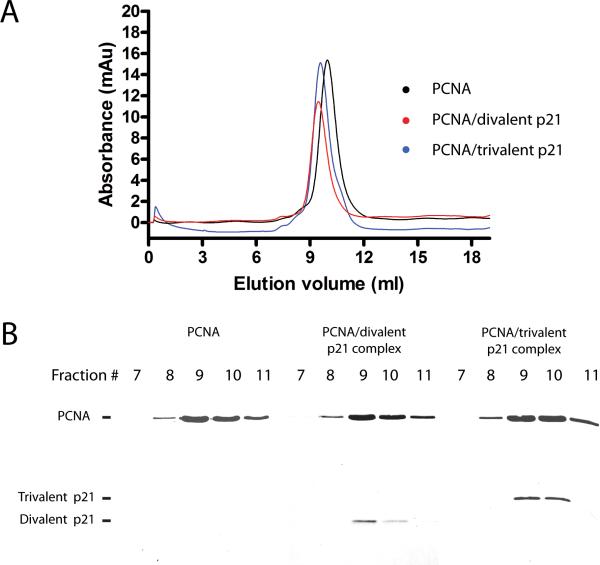
We next quantified the binding affinity of the di- and tri-valent p21 peptides for human PCNA. The affinity of divalent or trivalent p21 peptide for PCNA was determined following the method described for the monovalent p21 peptide. A Kd value of 30 ± 2 and 2.2 ± 0.2 nM was obtained for the divalent and trivalent p21 peptide, respectively (Figure 5C and D). The dissociation constants for the divalent and trivalent p21 peptides revealed a large increase in their affinity for PCNA compared to the monovalent p21 peptide. These represent a 12 and 158-fold increase in binding affinity for the di- and trivalent p21 antagonist respectively, which indicates a synergistic effect of the multivalent p21 binding to PCNA. We were interested in determining whether the di- and trivalent p21 peptides bind to a single PCNA trimer or more than one PCNA trimer. We analyzed PCNA complexes with either the divalent or trivalent p21 peptide using gel filtration chromatography in comparison to PCNA alone. PCNA trimer alone eluted from a Superose 12 gel filtration column with an apparent molecular weight of 122 kDa (Fig. 6A). This value is in accord with a previous report [37]. We next incubated PCNA with either divalent or trivalent p21 peptide at a molar ratio of 1:1 (PCNA trimer to peptide). If the di- or trivalent p21 peptides bind to multiple PCNA trimers, we would expect a large increase in molecular weight that doubles or triples that of the PCNA trimer. However, PCNA incubated with the di- or trivalent p21 peptide eluted as single peak with an elution volume slightly smaller than that of PCNA trimer alone (Figure 6A). SDS-PAGE analysis of the gel filtration elution fractions confirmed that the multivalent peptides coeluted with PCNA (Figure 6B). The calculated molecular weight of the PCNA/divalent p21 peptide and PCNA/trivalent p21 peptide complexes are both close to 149 kDa. The small difference in molecular weight of the divalent and trivalent p21 peptides (3.7 kDa) is small and thus was not resolved in the gel filtration chromatography. The determined molecular weight of the PCNA-peptide complexes was much smaller than that of two or three PCNA trimers, which rules out the binding of multiple PCNA trimers by the di- or trivalent p21 peptides.
Figure 6.
Gel filtration analysis of PCNA in complex with divalent and trivalent p21 peptide. PCNA trimer alone, PCNA trimer in complex with divalent p21 peptide (molar ratio 1:1), or PCNA trimer in complex with trivalent p21 peptide (molar ratio 1:1) were analyzed on a Superose 12 gel filtration column (GE Healthcare). Protein elution was monitored by UV-vis absorbance at 280 nm. (A) The chromatograms of PCNA alone, PCNA in complex with divalent p21 peptide and PCNA in complex with trivalent p21 peptide are plotted in black, red, and blue, respectively. (B) The SDS-PAGE analysis of the peak fractions of the gel filtration run of PCNA alone, PCNA in complex with divalent p21 peptide and PCNA in complex with trivalent p21 peptide
The p21-based antagonists inhibit PCNA-dependent DNA synthesis
PCNA plays an essential role in eukaryotic DNA replication. The binding of p21-based antagonists to PCNA can prevent the association of the replicative DNA polymerase to PCNA, thus preventing the PCNA-dependent DNA synthesis. Indeed, the monovalent p21 peptide (corresponding to amino acids 141-160 of the native p21) has been shown to inhibit DNA synthesis by polδ[20; 21]. We next determined whether the multivalent p21 peptides could inhibit PCNA-dependent DNA synthesis to a greater extent compared to the monovalent p21 peptide. Using the HeLa cell extract we examined the ability of the multivalent p21 peptides in inhibiting the PCNA-dependent synthesis on a primer-template DNA substrate. Agreeing with previous reports [20; 21], the native p21 peptide can effectively inhibit the DNA synthesis (compare lane 1 and 2 in Figure 7). To demonstrate that this inhibition was due to the binding of p21 peptide to PCNA, we titrated PCNA (0.1 - 2.5 μM) into the assay solution that contained 20 μM p21 peptide (lane 3 to 5 in Figure 7). We observed an increase in the amount of the full-length DNA product, confirming that p21 peptide inhibited DNA synthesis through its binding to PCNA. We found that to achieve a comparable level of inhibition of the DNA synthesis, lower concentrations of the di- and trivalent p21 peptide (0.6 and 0.5 μM respectively) were needed (compare lane 2, 7 and 13), which suggested a higher potency of the multivalent p21 peptides in inhibiting DNA synthesis. Titration of increasing amounts of PCNA into the assay solution reversed the inhibition by either the di- or trivalent peptide (see lane 7 to 11 and 13 to 17 in Figure 7). These observations support the notion that the multivalent p21 peptides also inhibit DNA synthesis by binding to PCNA.
Figure 7.
Inhibition of DNA synthesis by p21-based peptides in a HeLa cell-free extract is PCNA-dependent. The p21 peptide (20 μM), divalent p21 peptide (0.6 μM), or trivalent p21 peptide (0.5 μM) were incubated with HeLa call extract and 5 nM of DNA substrate containing a 5’ P32-end-labeled 20mer DNA annealed to a 50mer DNA template. Purified recombinant PCNA was added to the reaction mixture with increasing concentration (0.05-2.5 μM) as indicated. The DNA products were analyzed by gel electrophoresis and autoradiography on a 15% denaturing urea-polyacrylamide gel. DNA products were visualized using a PhosphorImager (Storm, GE Healthcare Bioscience).
To quantify the potency of the p21-based peptides as an antagonist of the PCNA-dependent DNA synthesis, increasing concentrations of the monovalent, divalent, or trivalent p21 peptide were titrated into the assay solution (Figure 8). For each of the three peptide antagonists, we observed a concentration-dependent decrease in the amount of the full-length DNA product. Fitting the dose response curve to equation 2 allowed us to determine the IC50 value for the inhibition of DNA synthesis by each p21-based PCNA antagonist. The monovalent p21 inhibited DNA synthesis with an IC50 value of 5.3 μM. Notably the di- and trivalent p21 peptide inhibited the DNA synthesis with a higher potency (IC50 value of 0.4 and 0.1 μM, respectively). The improved potency in inhibiting DNA synthesis agreed well with the higher binding affinity observed for the multivalent p21 peptides for PCNA compared to the monovalent p21 peptide.
Figure 8.
Increased potency of the multivalent p21 peptides in inhibiting DNA synthesis. (A) DNA synthesis in a HeLa cell extract in the presence of increasing concentrations of p21 peptide (0-50 μM), divalent p21 peptide (0 -1 μM) and trivalent p21 peptide (0 – 0.6 μM). A 5’ P32-end-labeled 20mer DNA annealed to a 50mer DNA template was added to the reaction mixture as a substrate. The DNA product was separated on a denaturing urea-polyacrylamide gel and visualized by autoradiography. The full length DNA product was quantified using the software QuantityOne (GE Healthcare Bioscience). (B) The percent of the full-length 50 bp DNA product relative to that in the absence of the PCNA antagonists was plotted against the logarithm value of the concentration of p21 peptide (blue), divalent p21 peptide (black), and trivalent p21 peptide (red). IC50 values were determined by nonlinear curve fitting to equation 2 using Prism (GraphPad Software, La Jolla, CA).
Discussion
PCNA is a key regulator of cell proliferation via its interactions with numerous essential protein factors, particularly the DNA polymerases. Remarkably, many PCNA-binding proteins, including p21, bind PCNA at a common site near the PCNA interdomain connector (IDC) loop. It has been shown that a p21 peptide binds to PCNA and prevents the genomic DNA replication [20; 21; 35]. This represents an important mechanism of regulating cell cycle progression in humans. Therefore, PCNA antagonists that mimic p21 in binding PCNA likely exert an anti-proliferative effect on eukaryotic cells. There have been early attempts in developing peptide-based PCNA antagonists. Several studies utilized the p21 peptide as an anti-cancer agent and showed that p21 peptides fused to cell-penetrating peptide tags displayed significant antiproliferative activity against human keratinocyte-derived HaCaT cells, DLD1 colon cancer cells, and CA46 lymphoma cells [38; 39; 40; 41]. Similarly, a GFP-fused p21 peptide inhibited cell proliferation of H1299 non-small cell lung carcinoma cells, U2OS osteosarcoma cells, and Saos2 osteocarcinoma cells [42].
Despite efforts in improving the potency of the p21-based PCNA antagonist, few peptides showed better affinity to PCNA compared to the native p21 peptide[21]. In this work we exploited the multiple binding sites on the homotrimeric PCNA to develop multivalent p21 peptide-based antagonists for PCNA. We showed that the multivalent p21-PCNA interaction was markedly stronger compared to that of a monovalent p21 antagonist. The measured dissociation constants for the divalent and trivalent p21 peptides were 12 and 158-fold lower compared to that of the monovalent p21 peptide. Gel filtration chromatography revealed that the multivalent peptides bound to a single PCNA trimer. Moreover, we showed that the divalent and trivalent p21 peptides were more potent in inhibiting DNA synthesis in a HeLa cell extract with IC50 values 13 and 53-fold lower than that of the native p21 peptide. Our results support the notion that the multiple binding sites on the homotrimeric PCNA can be exploited to develop antagonists with improved potency.
The fluorescence-based assay that we developed can be readily adapted to a high throughput screening format. Indeed, fluorescence polarization-based high throughput screen has led to the discovery of several inhibitors that can disrupt crucial protein-protein interactions [43]. Examples include small molecule inhibitors of the c-Jun N-terminal kinase (JNK-1) and protein kinase C (PKC) [44; 45]. Additionally, fluorescence polarization assay was used in to identify compounds that prevented the dimerization of c-Myc and Max [46] and the interaction of thyroid hormone receptor with several coregulators [47]. To date, few small-molecule PCNA antagonist was reported. The lack of a suitable high throughput assay has hindered progress in this endeavor. Due to the better membrane permeability than peptides, small-molecule PCNA antagonists represent an attractive alternative to the peptide-based PCNA antagonist. We expect that the fluorescence polarization-based assay that we developed will help to identify potent and selective small molecules that bind to PCNA and exert anti-proliferative effect on cells.
Acknowledgement
The authors acknowledge support from the National Institutes of Health (P20RR017716) and the helpful suggestions from the reviewers.
Reference
- 1.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–74. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 2.Laiho M, Latonen L. Cell cycle control, DNA damage checkpoints and cancer. Ann Med. 2003;35:391–7. doi: 10.1080/07853890310014605. [DOI] [PubMed] [Google Scholar]
- 3.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–79. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–60. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang Z, Ai Y. Processivity factor of DNA polymerase and its expanding role in normal and translesion DNA synthesis. Biochim Biophys Acta. 2010;1804:1081–93. doi: 10.1016/j.bbapap.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waga S, Hannon GJ, Beach D, Stillman B. The P21 Inhibitor of Cyclin-Dependent Kinases Controls DNA-Replication by Interaction with Pcna. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 7.Kelman Z, Hurwitz J. Protein-PCNA interactions: a DNA-scanning mechanism? Trends in biochemical sciences. 1998;23:236–8. doi: 10.1016/s0968-0004(98)01223-7. [DOI] [PubMed] [Google Scholar]
- 8.Sakakura C, Hagiwara A, Tsujimoto H, Ozaki K, Sakakibara T, Oyama T, Ogaki M, Takahashi T. Inhibition of Gastric-Cancer Cell-Proliferation by Antisense Oligonucleotides Targeting the Messenger-Rna Encoding Proliferating Cell Nuclear Antigen. British Journal of Cancer. 1994;70:1060–1066. doi: 10.1038/bjc.1994.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons M, Edelman ER, Rosenberg RD. Antisense proliferating cell nuclear antigen oligonucleotides inhibit intimal hyperplasia in a rat carotid artery injury model. J Clin Invest. 1994;93:2351–6. doi: 10.1172/JCI117240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita Y, Kashihara N, Yamamura M, Okamoto H, Harada S, Maeshima Y, Okamoto K, Makino H. Inhibition of rheumatoid synovial fibroblast proliferation by antisense oligonucleotides targeting proliferating cell nuclear antigen messenger RNA. Arthritis Rheum. 1997;40:1292–7. doi: 10.1002/1529-0131(199707)40:7<1292::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Corsi KA, Schwarz EM, Mooney DJ, Huard J. Regenerative medicine in orthopaedic surgery. J Orthop Res. 2007;25:1261–8. doi: 10.1002/jor.20432. [DOI] [PubMed] [Google Scholar]
- 12.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Fux C, Mitta B, Kramer BP, Fussenegger M. Dual-regulated expression of C/EBP-alpha and BMP-2 enables differential differentiation of C2C12 cells into adipocytes and osteoblasts. Nucleic Acids Res. 2004;32:e1. doi: 10.1093/nar/gnh001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indiani C, O'Donnell M. The replication clamp-loading machine at work in the three domains of life. Nat Rev Mol Cell Biol. 2006;7:751–61. doi: 10.1038/nrm2022. [DOI] [PubMed] [Google Scholar]
- 15.Bruning JB, Shamoo Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure. 2004;12:2209–19. doi: 10.1016/j.str.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994;79:181–4. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 17.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 18.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–61. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 20.Warbrick E, Lane DP, Glover DM, Cox LS. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr Biol. 1995;5:275–82. doi: 10.1016/s0960-9822(95)00058-3. [DOI] [PubMed] [Google Scholar]
- 21.Zheleva DI, Zhelev NZ, Fischer PM, Duff SV, Warbrick E, Blake DG, Lane DP. A quantitative study of the in vitro binding of the C-terminal domain of p21 to PCNA: Affinity, stoichiometry, and thermodynamics. Biochemistry. 2000;39:7388–7397. doi: 10.1021/bi992498r. [DOI] [PubMed] [Google Scholar]
- 22.Kontopidis G, Wu SY, Zheleva DI, Taylor P, McInnes C, Lane DP, Fischer PM, Walkinshaw MD. Structural and biochemical studies of human proliferating cell nuclear antigen complexes provide a rationale for cyclin association and inhibitor design. Proc Natl Acad Sci U S A. 2005;102:1871–6. doi: 10.1073/pnas.0406540102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Zhang P, Liu L, Lee MY. A novel PCNA-binding motif identified by the panning of a random peptide display library. Biochemistry. 2001;40:4512–20. doi: 10.1021/bi010103+. [DOI] [PubMed] [Google Scholar]
- 24.Warbrick E. A functional analysis of PCNA-binding peptides derived from protein sequence, interaction screening and rational design. Oncogene. 2006;25:2850–9. doi: 10.1038/sj.onc.1209320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacchettini JC, Baum LG, Brewer CF. Multivalent protein-carbohydrate interactions. A new paradigm for supermolecular assembly and signal transduction. Biochemistry. 2001;40:3009–15. doi: 10.1021/bi002544j. [DOI] [PubMed] [Google Scholar]
- 26.Dam TK, Brewer CF. Multivalent lectin-carbohydrate interactions energetics and mechanisms of binding. Adv Carbohydr Chem Biochem. 2010;63:139–64. doi: 10.1016/S0065-2318(10)63005-3. [DOI] [PubMed] [Google Scholar]
- 27.Deniaud D, Julienne K, Gouin SG. Insights in the rational design of synthetic multivalent glycoconjugates as lectin ligands. Org Biomol Chem. 2011;9:966–79. doi: 10.1039/c0ob00389a. [DOI] [PubMed] [Google Scholar]
- 28.Fang F, Yu M. Viral receptor blockage by multivalent recombinant antibody fusion proteins: inhibiting human rhinovirus (HRV) infection with CFY196. J Antimicrob Chemother. 2004;53:23–5. doi: 10.1093/jac/dkh019. [DOI] [PubMed] [Google Scholar]
- 29.Dernedde J, Rausch A, Weinhart M, Enders S, Tauber R, Licha K, Schirner M, Zugel U, von Bonin A, Haag R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci U S A. 2010;107:19679–84. doi: 10.1073/pnas.1003103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee SR, Pullambhatla M, Shallal H, Lisok A, Mease RC, Pomper MG. A Modular Strategy to Prepare Multivalent Inhibitors of Prostate-Specific Membrane Antigen (PSMA). Oncotarget. 2011;2:1244–53. doi: 10.18632/oncotarget.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neron B, Menager H, Maufrais C, Joly N, Maupetit J, Letort S, Carrere S, Tuffery P, Letondal C. Mobyle: a new full web bioinformatics framework. Bioinformatics. 2009;25:3005–11. doi: 10.1093/bioinformatics/btp493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny CH, Ding W, Kelleher K, Benard S, Dushin EG, Sutherland AG, Mosyak L, Kriz R, Ellestad G. Development of a fluorescence polarization assay to screen for inhibitors of the FtsZ/ZipA interaction. Anal Biochem. 2003;323:224–33. doi: 10.1016/j.ab.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Dandliker WB, Hsu ML, Levin J, Rao BR. Equilibrium and kinetic inhibition assays based upon fluorescence polarization. Methods Enzymol. 1981;74(Pt C):3–28. doi: 10.1016/0076-6879(81)74003-5. [DOI] [PubMed] [Google Scholar]
- 34.Li JJ, Kelly TJ. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985;5:1238–46. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 36.Esteve V, Canela N, Rodriguez-Vilarrupla A, Aligue R, Agell N, Mingarro I, Bachs O, Perez-Paya E. The structural plasticity of the C terminus of p21Cip1 is a determinant for target protein recognition. Chembiochem. 2003;4:863–9. doi: 10.1002/cbic.200300649. [DOI] [PubMed] [Google Scholar]
- 37.Chen U, Chen S, Saha P, Dutta A. p21Cip1/Waf1 disrupts the recruitment of human Fen1 by proliferating-cell nuclear antigen into the DNA replication complex. Proc Natl Acad Sci U S A. 1996;93:11597–602. doi: 10.1073/pnas.93.21.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball KL, Lain S, Fahraeus R, Smythe C, Lane DP. Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr Biol. 1997;7:71–80. doi: 10.1016/s0960-9822(06)00029-7. [DOI] [PubMed] [Google Scholar]
- 39.Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–20. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 40.Bidwell GL, 3rd, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev. 2010;62:1486–96. doi: 10.1016/j.addr.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutoh M, Lung FD, Long YQ, Roller PP, Sikorski RS, O'Connor PM. A p21(Waf1/Cip1)carboxyl-terminal peptide exhibited cyclin-dependent kinase-inhibitory activity and cytotoxicity when introduced into human cells. Cancer Res. 1999;59:3480–8. [PubMed] [Google Scholar]
- 42.Mattock H, Lane DP, Warbrick E. Inhibition of cell proliferation by the PCNA-binding region of p21 expressed as a GFP miniprotein. Exp Cell Res. 2001;265:234–41. doi: 10.1006/excr.2001.5160. [DOI] [PubMed] [Google Scholar]
- 43.Heeres JT, Hergenrother PJ. High-throughput screening for modulators of protein-protein interactions: use of photonic crystal biosensors and complementary technologies. Chem Soc Rev. 2011;40:4398–410. doi: 10.1039/b923660k. [DOI] [PubMed] [Google Scholar]
- 44.Chen T, Kablaoui N, Little J, Timofeevski S, Tschantz WR, Chen P, Feng J, Charlton M, Stanton R, Bauer P. Identification of small-molecule inhibitors of the JIP-JNK interaction. Biochem J. 2009;420:283–94. doi: 10.1042/BJ20081899. [DOI] [PubMed] [Google Scholar]
- 45.Fowler A, Swift D, Longman E, Acornley A, Hemsley P, Murray D, Unitt J, Dale I, Sullivan E, Coldwell M. An evaluation of fluorescence polarization and lifetime discriminated polarization for high throughput screening of serine/threonine kinases. Anal Biochem. 2002;308:223–31. doi: 10.1016/s0003-2697(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 46.Kiessling A, Sperl B, Hollis A, Eick D, Berg T. Selective inhibition of c-Myc/Max dimerization and DNA binding by small molecules. Chem Biol. 2006;13:745–51. doi: 10.1016/j.chembiol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Arnold LA, Estebanez-Perpina E, Togashi M, Jouravel N, Shelat A, McReynolds AC, Mar E, Nguyen P, Baxter JD, Fletterick RJ, Webb P, Guy RK. Discovery of small molecule inhibitors of the interaction of the thyroid hormone receptor with transcriptional coregulators. J Biol Chem. 2005;280:43048–55. doi: 10.1074/jbc.M506693200. [DOI] [PubMed] [Google Scholar]