Abstract
Introduction: Organisms of the Burkholderia cepacia complex (BCC) are important pathogens in cystic fibrosis (CF). The majority of those who acquire BCC develop chronic infection but it can also result in rapid decline in a significant minority. In addition, chronic infection with Burkholderia cenocepacia in particular is regarded as a contraindication to lung transplantation in many units. Whilst aggressive antibiotic therapy is employed in CF to eradicate Pseudomonas aeruginosa before infection becomes irreversibly established, no formal assessment of such strategies has been previously reported for BCC, despite the apparent widespread adoption of this practice. Methods: UK adult CF centers were surveyed about their current approach to new BCC infection. Outcomes of eradication therapy were assessed in patients attending the Manchester Adult CF Center with new BCC isolates between 1st January 2002 and 1st May 2011. Patients with previous infection with the same strain of BCC were excluded. BCC were identified at the national reference laboratories and confirmed by species-specific PCR and RecA sequencing. Results: Routine use of therapies to attempt eradication of new BCC is commonly used in the UK (12/17 centers who responded). This typically involves a combination of intravenous and nebulised antibiotics. Of 19 eligible cases of new BCC infection, the organism has been eradicated in 7 (37%). Three of these did not receive specific eradication therapy. Of 14 patients who have received eradication therapy and completed follow up, BCC were cleared in only 4 (29%). Conclusions: Attempted eradication of new BCC is a common practice in UK adult CF centers. A minority of patients clear the infection spontaneously and the effects of eradication therapies are at best modest. Early treatment may be associated with better outcomes, though there are insufficient data to support the use of any specific treatment regimen. A prospective, systematic evaluation of treatments and outcomes is required.
Keywords: cystic fibrosis, Burkholderia cepacia, Burkholderia cenocepacia, Burkholderia multivorans, Eradication
Introduction
Chronic respiratory infection is one of the hallmarks of cystic fibrosis (CF) lung disease and is the major cause of morbidity and mortality (Gibson et al., 2003). Infection starts early in life and tends to be persistent (Sly et al., 2009), though the nature of the most prevalent infecting micro-organisms typically changes with time (CF Foundation, 2009). In early childhood, chronic infection tends to be characterized by Staphylococcus aureus but in older subjects is dominated by gram negative organisms, particularly Pseudomonas aeruginosa which infects around 60% of adult CF patients in the UK (CF Trust, 2009). Other gram negative genera including Stenotrophomonas, Ralstonia, Achromobacter, and Pandorea are also increasingly cultured from CF sputum (Lipuma, 2010), and there is also growing recognition of the high prevalence of anaerobes (Tunney et al., 2008; Worlitzsch et al., 2009). However, one of the most important groups of organisms causing infection in the CF lung are the those of the Burkholderia cepacia complex (BCC). These currently infect around 3% of UK patients overall, and are predominantly found in adult patients, where the prevalence is four times greater than in those under 16 years (CF Trust, 2009).
Burkholderia cepacia complex were first identified as important pathogens in CF in the mid-1980s, when infection was recognized to be associated with increased mortality and morbidity (Tablan et al., 1985). In a minority of patients there may be a rapid and uncontrolled deterioration with septicemia and necrotizing pneumonia (termed “cepacia syndrome”) that usually results in early death (Isles et al., 1984). Patient to patient spread of organisms occurred through social contact (LiPuma et al., 1990), particularly for the epidemic (ET12) strain of Burkholderia cenocepacia which was responsible for the rapid spread of the organism through some CF units in the early 1990s (Govan et al., 1993). This caused considerable fear in the CF community and resulted in the application of a policy of strict segregation in clinical units. The prevalence of B. cenocepacia has fallen over the years due to the success of infection control measures preventing further spread and due to the death of chronically infected patients. As a result there has been considerable change in BCC epidemiology (Govan et al., 2007; France et al., 2008; Lipuma, 2010). Currently, new BCC infections are more likely to be environmentally acquired than through contact with other patients. The most commonly acquired BCC infection is now Burkholderia multivorans (Govan et al., 2007). New infections are usually with environmentally acquired strains (Mahenthiralingam et al., 2005), although rarely patient to patient transmission has also been reported (Biddick et al., 2003). In comparison with B. cenocepacia, the clinical significance of infection with the other BCC species is much less clear. With important exceptions involving epidemic spread (Govan et al., 2007), B. multivorans has not been associated with increased mortality or accelerated decline in key outcome measures, above that associated with chronic P. aeruginosa infection (Courtney et al., 2004; Jones et al., 2004; Kalish et al., 2006). There are also differences between the different BCC species post lung transplant. Patients with B. cenocepacia, but not those infected with other Burkholderia species, have poorer post lung transplant outcomes (Chaparro et al., 2001; De Soyza et al., 2001), and most transplant units will not consider these patients as candidates for lung transplantation. Concern about B. cenocepacia however means that patients with any BCC infection are often treated similarly (CF Trust, 2004). Strict segregation policies, designed to protect patients from acquiring and transmitting BCC to other patients, contribute to the anxieties felt by these patients and may cause additional psychological harm by excluding them from the full range of CF unit facilities (Duff, 2002).
Clinicians have attempted eradication of newly acquired P. aeruginosa infections for over 25 years (Littlewood et al., 1985), and there is now good evidence that this practice is effective at clearing initial infection and delaying the onset of chronic infection (Valerius et al., 1991; Conway and Lee, 2009). Despite the success of this approach with P. aeruginosa and with methicillin-resistant S. aureus (MRSA; CF Trust, 2008), eradication has not been extended in a formal, systematic way to any other of the major CF pathogens, including BCC. Notwithstanding, attempted eradication of newly acquired BCC infections is commonly attempted and has been standard practice in our large tertiary adult CF center for the last decade or so. The goals of this study were twofold: to describe the management of newly acquired BCC infections in adult CF centers in the UK and to examine the results of eradication strategies in our own center. The second goal involves reporting the new acquisitions of BCC organisms in our adult patients over the last 9 years, the impact of transient BCC infections, and the results of eradication strategies.
Materials and Methods
Current approaches to new BCC infection
In order to establish how newly isolated BCC infections are currently treated in the UK, CF center directors were surveyed at the annual UK CF Center Director meeting. Non-returns were followed up by email.
Outcome of eradication therapy
Patients attending the Manchester Adult CF Center, a large tertiary-referral adult CF center (370 patients) in the North West of England, were included if they had new respiratory isolates of any BCC species between 1st January 2002 and 1st May 2011. Patients with infection caused by other species of Burkholderia, or with their first isolate of the same BCC organism before this date, and those whose first episode of infection with the same BCC was prior to transferring their care to our center were not included. Date of acquisition was defined as the date the organism was first cultured on selective medium from sputum samples. BCC infection was classified as having been eradicated if at least three consecutive cultures failed to yield the organism over a minimum period of 12 months, as per current UK CF Trust recommendations (CF Trust, 2004).
Historical annual rates of BCC infection and acquisition were calculated from records of the numbers of infections that occurred in a calendar year, with the total numbers of patients taken as those attending the unit at the end of that year. Incidence was expressed as the number of cases per 100 patients. Data include those from a previously published series (France et al., 2008).
Throughout this period, BCC-selective media were routinely used in the microbiology laboratory for analysis of all CF sputum samples. Organisms detected on selective media were formally identified as BCC at the national reference laboratories and confirmed by species-specific PCR and RecA sequencing. Genotyping by pulsed field gel electrophoresis (PFGE) was performed on all isolates and repeated when the interval between repeat positive samples was greater than 12 months.
There was no formal eradication protocol during the study period, but patients with new BCC infections were typically offered admission for intravenous (IV) antibiotics, followed by nebulised tobramycin with or without additional oral antibiotics. The exact choice, in the absence of evidence based guidelines or a formal protocol, was determined by organism sensitivities, patient allergies and preference, and logistics.
Results
Current approaches to new BCC infection
Responses were obtained from 36 CF units (75% of all UK specialist CF centers), representing over 6300 patients and including 19 pediatric and 17 adult centers. Because of the rarity of new BCC infection in pediatric centers, many of these reported little or no recent experience of the issue. For this reason, only replies from the 17 adult centers (representing 3560 patients) have been analyzed further.
Twelve adult CF centers, representing 2860 patients, always attempt eradication of newly isolated BCC. Two additional centers attempt eradication only in the presence of additional indications. Only two units had a formal eradication policy. Intravenous antibiotics were used in all cases, for a median of 2 (range 2–6) weeks, typically comprising combined IV tobramycin and meropenem with additional therapy consisting of co-trimoxazole (n = 4), ceftazidime (n = 5), and chloramphenicol (n = 2).
Nebulised antibiotics (typically tobramycin or meropenem) were also used in 13 of these 14 centers, for a median of 12 weeks (range 8 weeks – indefinite). Five centers used additional oral antibiotics, for a median of 7 (2–12) weeks. This most commonly involved minocycline (n = 4) and/or co-trimoxazole (n = 5).
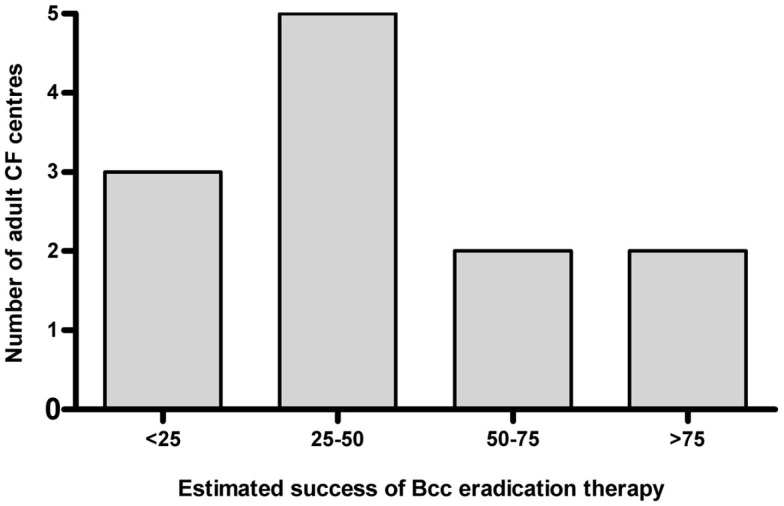
Of the 12 centers that always attempted eradication, 8 (67%) estimated success rate of eradication therapy to be less than 50% (Figure 1). In the five centers where eradication was not routine, factors that dissuaded clinicians were perceived poor success of treatment (n = 5), toxicity (n = 3), cost (n = 1), and lack of experience with this approach (n = 2).
Figure 1.
Estimated success of BCC eradication therapy in UK adult CF centers. In all but one of these centers, the practice was routinely applied to all new BCC infections.
Epidemiology of new BCC infections
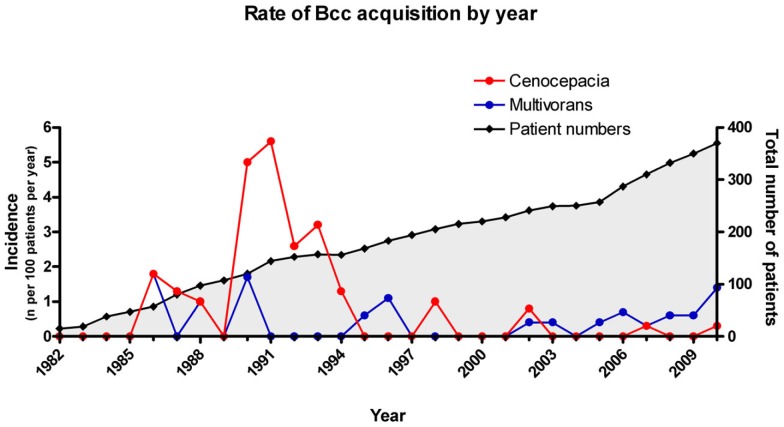
The changing epidemiology of BCC infections at the Manchester Adult Center over the last 30 years are presented in Figure 2. Rates of B. cenocepacia infection have dropped sharply since partial, and then full, segregation of patients was introduced in 1991 and 1994 respectively (France et al., 2008). Rates of B. multivorans acquisition on the other hand have remained static at an average of 1 new case per 200 patients over the last 10 years.
Figure 2.
Relative incidence of newly acquired B. multivorans and B. cenocepacia infections in CF patients attending the Manchester Adult CF Center. Incidence is expressed as number of cases in a calendar year per 100 patients. Total number of patients refers to that attending the clinic at the end of the year.
Between 1 January 2002 and 1st June 2011 there were 22 new BCC isolates: 16 (73%) B. multivorans, 4 B. cenocepacia (17%) and 1 each of B. latens, and B. vietnamiensis. A single new isolate of B. gladioli was excluded from further analysis. One of the B. cenocepacia infections was due to the epidemic ET12 strain, acquired through patient to patient contact after the patient chose to ignore explicit infection control advice. All of the remaining infections were due to individual sporadic strains, with the possible exception of the single infection with B. latens. This was one of a localized cluster of three patients with B. latens, in whom it is not clear whether cross-infection has occurred or whether the patients have been infected from a common environmental source (Horsley et al., 2011).
Mean age of acquisition was 29 years (range 18–51 years; see Table 1). Data on baseline clinical variables and eradication therapy are missing in one case. In the remaining 21 patients, mean forced expiratory volume in 1 s (FEV1) was 56% predicted (27–94%) and mean body mass index (BMI) was 21.1 (17.0–25.8) at time of first isolate. Seventeen patients (81%) were chronically infected with P. aeruginosa. 10 patients were also infected with S. aureus (48%), and 14 (67%) with one or more of Aspergillus sp., Candida sp., or other yeasts.
Table 1.
Demographics, treatment, and outcomes of patients with new BCC infections.
| Patient number | Burkholderia species | Other chronic respiratory infections | Age (years) | FEV1% predicted | BMI | Eradication regimen | Outcome number + ve isolates [time in days between 1st and last] |
|---|---|---|---|---|---|---|---|
| 1 | multivorans | SA, yeast | 20 | 54.9 | 23.7 | 14 day M, Co, T then 28 day S, T(N) | Chronic |
| 2 | multivorans | PA, Asp, C, Sten, Pan, MRSA | 27 | 72.4 | 18.8 | 14 day M, T, Ch, D | Chronic |
| 3 | cenocepacia | PA, C | 30 | 36.5 | 25.6 | 14 day M, T, Cz, Co | Chronic |
| 4 | multivorans | PA, Asp | 23 | 75.4 | 22.4 | 21 day Mi, Co then 14 day M, Te | Chronic |
| 5 | multivorans | PA, SA, Asp | 31 | 94 | 25.1 | 28 day Co, Cf | Chronic |
| 6 | multivorans | SA | 21 | 61.6 | 20.7 | 28 day Co, R, T(N) | Chronic |
| 7 | multivorans | PA, SA, Asp | 19 | 82.1 | 22.1 | 28 day Cz, T, Co, Cf, M(N) | Chronic |
| 8 | multivorans | PA, Asp | 35 | 27.1 | 17 | 28 day M and T(N) | Chronic |
| 9 | multivorans | PA, SA, Asp, AX | 20 | 73.3 | 20 | 28 day Mi, Co, T(N) | Chronic |
| 10 | multivorans | PA, C | 51 | 49.2 | 18.1 | 7 weeks Cf, Mi | Chronic |
| 11 | cenocepacia | ? | 24 | ? | ? | ? | Chronic |
| 12 | latens | PA, SA, Asp, C | 25 | 39.8 | 18.8 | None | Chronic |
| 13 | multivorans | PA, Asp, yeasts | 42 | 39.2 | 22.7 | 12 weeks Mi, Co, 14 day Az, Ta, M | Eradicated 5 [56] |
| 14 | cenocepacia | PA, SA, C, Trich | 24 | 55.1 | 21.8 | 14 day Te, Ta, Co, Mi | Eradicated 3 [19] |
| 15 | multivorans | PA, Asp | 18 | 32 | 17.7 | 28 day Mi, Co, Cf | Eradicated 1 |
| 16 | multivorans | PA | 38 | 45.3 | 19.8 | 28 day Mi, Co, T(N) | Eradicated 5 [44] |
| 17 | multivorans | PA, HI | 21 | 36.9 | 21.9 | None | Eradicated 1 |
| 18 | multivorans | PA | 31 | 87.4 | 25.8 | None | Eradicated 1 |
| 19 | vietnamiensis | PA, SA, C, Serratia | 27 | 83.7 | 21.1 | None | Eradicated 1 |
| 20 | cenocepacia | Asp, Ewingella, yeasts | 36 | 25.2 | 18.8 | Isolated whilst on treatment | See notes1 |
| 21 | multivorans | PA, SA | 22 | 62.1 | 22.9 | 14 day M, Co, T then 6 weeks Mi, Co, Cf | Too early |
| 22 | multivorans | MRSA, Asp | 42 | 35.5 | 19.1 | 21 day Ta, M, T then 6 weeks Co, D | Too early |
BMI, body mass index; SA, Staphylococcus aureus; PA, Pseudomonas aeruginosa; Asp, Aspergillus fumigatus; C, Candida sp.; Sten, Stenotrophomonas maltophilia; Pan, Pandorea sp.; MRSA, methicillin-resistant Staphylococcus aureus; AX, Achromobacter xylosoxidans; Trich, Trichosporon beigelli; HI, Haemophilus influenzae; Serratia, Serratia marascens; Ewingella, Ewingella americana.
Az, aztreonam; Cf, ciprofloxacin; Ch, chloramphenicol; Co, co-trimoxazole; Cz, ceftazidime; D, doxycycline; M, meropenem; Mi, minocycline; R, rifampicin; T, tobramycin; Ta, tazocin; Te, temocillin; (N), nebulised; d, days; wks, weeks. ?, data missing.
Intravenous therapies highlighted in bold.
For those patients in whom BCC were eradicated, the number of BCC positive sputum cultures, and the time interval between the first and last of these, are given in the final column. All patients with chronic infection have had multiple positive cultures.
Cases 19–22 (shaded in gray) have incomplete data and have been excluded from subsequent analysis.
1Burkholderia cenocepacia identified only after patient death.
Eradication
In two cases it is still too early to report on eradication outcomes. In one further case, B. cenocepacia was identified on a single isolate only after the patient had died. These cases are highlighted in Table 1, and excluded from further analysis.
Of the remaining 19 cases, eradication has been achieved in 7 (37%); comprising 5 cases of B. multivorans and 1 each of B. vietnamiensis and B. cenocepacia (see Table 1). However, four of these seven were only single isolates (three of B. multivorans and one of B. vietnamiensis), three of which did not receive any specific therapy.
Although antibiotics for BCC eradication were prescribed in 14 completed cases, and included combinations of 2–5 antibiotics for a median of 28 days (maximum 84 days), only four of these patients (29%) successfully cleared the infection. There was no significant association between receiving eradication therapy and successful eradication of BCC infection (p = 0.25, Fisher’s exact test), see Table 2. In one case (#11), it is unclear whether eradication therapy was prescribed, and this has been excluded from the analysis in Table 2.
Table 2.
Summary of numbers of patients treated and treatment outcomes.
| BCC eradicated | Chronic infection | Total | |
|---|---|---|---|
| No treatment | 3 | 1 | 4 |
| Eradication therapy | 4 | 10 | 14 |
| Total | 7 | 11 | 18 |
The total of 18 patients does not include one case where the records are unclear as to what treatment the patient received (#11 in Table 1).
Overall, 4 of 14 treated patients and 3 of 4 untreated patients eradicated BCC. However, these three untreated cases were all single isolates, in whom subsequent negative cultures were already available.
There was no consistent eradication regimen applied during the period of study, see Table 1. Antibiotic regimens consequently reflect individual microbiological sensitivities, patient allergies, and preferences. It has therefore not been possible to identify any aspects of treatment regimens, either the antibiotics used, the length of treatment or route of delivery, that impact on outcomes. Overall, 10 patients received IV antibiotics as either part or whole of their eradication therapy. Intravenous regimens contained meropenem in 8/10 cases, tobramycin in 6 and co-trimoxazole in 4 cases. In eight cases, oral antibiotics were used to supplement or to extend IV therapy, and in six cases were used without prior IV antibiotics. Oral regimens contained co-trimoxazole in 10/14 cases, minocycline in 8, doxycycline in 2, and ciprofloxacin in 5.
The four patients in whom eradication was successful received: (i) 28 days of oral co-trimoxazole, ciprofloxacin, and minocycline, (ii) 28 days of oral co-trimoxazole and minocycline combined with nebulised tobramycin, (iii) 14 days of intravenous temocillin, tazocin, and co-trimoxazole combined with oral minocycline, and (iv) 14 days of intravenous aztreonam and tazocin followed by 14 days of intravenous meropenem combined with 12 weeks of oral minocycline and co-trimoxazole. In all four cases where eradication was successful, the BCC appear to have cleared quickly. One case was a single isolate of B. multivorans, one was B. cenocepacia present on three occasions a maximum of 19 days apart, one was B. multivorans present on five occasions a maximum of 44 days apart, and the final case was B. multivorans present on four occasions a maximum of 56 days apart. In the first three of these cases, the organism was not isolated again after eradication therapy was started. In the final case, no further BCC were isolated once intravenous therapies were started, after an initial failure of oral co-trimoxazole and minocycline alone.
After discussions, a single patient elected not to receive specific eradication therapy, and developed chronic infection with B. latens.
Chronic infection
Of the 13 chronically infected patients, 3 subjects have had intervals of over 1 year between positive isolates, all confirmed by genotyping as being due to the same organism. The maximum time interval between isolates was 29 months, with 26 negative sputum cultures. Genotyping by PFGE analysis confirmed that these two were the same strain of B. multivorans, representing either re-infection from the same environmental reservoir or persistent undetected infection.
Conclusion
This study highlights a number of important features of current practice with regards to BCC infection in CF, and is the first to report data on attempted eradication of new infections. We have shown that whilst this practice is widespread in the UK, it is poorly standardized and may be of little value in the majority of cases. Data from our own unit have shown that spontaneous clearance of new BCC infection occurs in at least 17% of cases, though the true value may be higher. It is not possible to know whether the cases apparently responding to eradication therapy would have cleared the infection without additional assistance, nor whether there are more cases of transient infection in CF that are acquired and cleared between sputum sampling at clinic.
The success of patient segregation is borne out by the low rates of new B. cenocepacia infection. This has resulted in a shift in BCC epidemiology compared to an earlier published series from the same CF clinic (Jones et al., 2004). In the period covered by the earlier paper (1982–2003), 33/57 (58%) of newly acquired BCC infections were due to B. cenocepacia, the majority of these caused by the epidemic ET12 strain, and 16 (28%) were caused by B. multivorans. Of the 18 new BCC infections in the unit since this earlier study, 14 (78%) have been due to B. multivorans, and only two have been due to a sporadic strain of B. cenocepacia (see Figure 2). This changing epidemiology is already well described (Govan et al., 2007; France et al., 2008) and the falling rates of new B. cenocepacia infection reflect the effects of routine surveillance for BCC (using specific culture media) combined with strict segregation policies. By contrast, rates of new sporadic BCC infection have remained relatively stable over this period (France et al., 2008; see Figure 2). These are probably acquired from as yet unknown environmental reservoirs, and the prevalence is likely to increase as the CF population gets older.
Little data exist on outcomes except for the two most common BCC infections and whilst it appears that B. multivorans in the majority of cases is not as virulent a pathogen as B. cenocepacia, the infection is not without consequence. Three studies have reported no greater decline in lung function or BMI with chronic B. multivorans, compared to that seen with chronic P. aeruginosa infection (Courtney et al., 2004; Jones et al., 2004; Kalish et al., 2006). In the two studies where it was reported, there was no apparent impact on survival of acquiring chronic infection with B. multivorans (Jones et al., 2004; Kalish et al., 2006). However, the numbers involved in both studies were relatively small (covering a total of 41 patients with B. multivorans infection), and the follow up period of the larger study was far too short to make definitive observations about survival (Kalish et al., 2006). These comparisons have only been performed against patients with chronic infection with P. aeruginosa, an organism already known to be responsible for a more rapid deterioration in health (Emerson et al., 2002), and not against those with no chronic infection. Only with a longer study, on greater numbers of patients, would we be confident that B. multivorans infection had no additional impact on survival. It is unlikely to be entirely benign however. In vitro, B. multivorans has been shown to induce an inflammatory response similar to that of B. cenocepacia (Kaza et al., 2011). Patients with chronic B. multivorans infection can suffer accelerated decline and the classical features of cepacia syndrome have been described (Zahariadis et al., 2003; Jones et al., 2004). Occasional strains of B. multivorans have also been associated with epidemic spread, though unlike with B. cenocepacia the mode of transmission has not been identified (Baldwin et al., 2008). In addition to the open question of the clinical significance, infection with BCC has important consequences for the patient. Segregation may involve reduced access to specialist facilities and this, combined with the anxiety of being infected with a member of the feared “cepacia” family of organisms, can contribute to the psychological impact of infection with these pathogens (Duff, 2002).
If it is accepted that infection with even the sporadic strains of BCC is a significant event, then the issue of how this is managed becomes relevant. There are no national or international guidelines that the authors are aware of, and no reports of how new BCC is approached in other countries. Despite both the lack of any prior evidence of efficacy and the requirement for expensive and potentially toxic therapies, attempted eradication of new BCC is commonly attempted in adult CF centers in the UK. What is more, even in those centers where eradication is routinely attempted, there is a broad pessimism about the outcomes, with two-thirds of centers recognizing the below-50% success rate that we have reported here.
Our own attempts to institute eradication therapy have been only partially successful. Not all patients have received treatment, and the approach has varied both with time and with microbiological and patient factors. The current practice is to offer an initial 2-week course of intravenous antibiotics containing three different agents, one of which is usually co-trimoxazole (which may be given orally if the patient is undergoing home therapy). This is then followed with 6 weeks of two to three oral antibiotics, again usually containing co-trimoxazole, combined with either nebulised tobramycin or meropenem. No conclusions can be drawn from the results presented here about the optimal nature of the eradication regimen, and the evidence for this treatment schedule is drawn from a combination of in vitro studies, case reports, and an attempt to minimize the potential adverse effects of treatment. Aaron et al. (2000) reported on the individual and combined antibiotic susceptibility of BCC to 10–15 different antibiotics. They did not differentiate by species, but since these were clinical isolates from the 1990s, they are likely to have been predominantly B. cenocepacia. They found a poor response to single agent treatments but much greater efficacy of combined therapy. The most effective combinations were those including meropenem, tobramycin and ceftazidime (effective against 93% isolates), septrin (88%), chloramphenicol (87%), or aztreonam (87%). A number of clinical reports have confirmed the efficacy of IV meropenem against BCC during exacerbation (Ciofu et al., 1996; Kuti et al., 2004; Weidmann et al., 2008), though eradication has not been an aim or a feature of these reports. In CF patients who had undergone lung transplantation, an aggressive approach to post transplant eradication of BCC was adopted by the Toronto lung transplant clinic. They treated patients with IV ceftazidime, chloramphenicol, tobramycin, septrin and nebulised tobramycin for 3 months post transplantation (Chaparro et al., 2001). In three out five cases of multi-resistant BCC, the regimen appears to have been successful in preventing re-infection post transplant. The other 20 patients who did not receive this regimen were all BCC positive after transplantation.
Nebulised tobramycin has been used with some success in 2 case reports of successful treatment of cepacia syndrome (Weidmann et al., 2008; Grimwood et al., 2009). Nebulised tobramycin in combination with nebulised amiloride has also been used as an eradication therapy in two case series. In the first, it was used to treat four adult CF patients with early growth of BCC, and appeared to be successful in three out of four cases (one B. cenocepacia; one B. ambifaria, one unclear; Middleton et al., 2005). However, a later report of outcomes in seven children reported successful eradication in only one patient (B. stabilis), when given twice daily in patients who had infection for up to 10 years (Ball et al., 2010). The numbers involved mean it is not possible to draw firm conclusions, but the difference in outcomes may relate to the chronicity of the infection. In those cases where eradication was successful, the infection had only been present for a short period of time. A similar observation can be made with the data presented here. In the four patients in whom eradication was successful, treatment was started within 2 months of the first isolate, and in three cases no further positive sputum cultures were found after the start of treatment. Early lack of response to eradication therapy may be an indication to change or abandon the approach.
The impact of eradication therapy in newly acquired BCC infection however has been disappointing. It is also possible that inadequate antibiotic treatment may cause more harm than good by inducing potentially harmful phenotypic change in the infecting organism (Zlosnik et al., 2010). In the current series, only 4 of 14 patients who received eradication therapy went on to clear the infection, though we cannot know whether these patients would have cleared the infection anyway. For even without specific therapy, infections may be cleared in a significant minority. Quite why this occurs is less clear. There is a highly diverse clinical course following acquisition of BCC infection, and spontaneous clearance may represent one end of a spectrum delineated by cepacia syndrome at the other. Phenotypic variation exists within individual BCC species, and exopolysaccharide production has been associated with clinical outcome (Zlosnik et al., 2010). Much remains unknown however about the interaction between BCC and the CF lung, and the influence of other host and bacterial virulence factors have yet to be defined. In addition, virulence may also change over time due to genetic recombination. There is already evidence of significant transfer of genetic information from epidemic B. cenocepacia to B. multivorans, a process that is believed to have occurred in the clinical environment (Baldwin et al., 2008).
A final important observation is that the interval between positive isolates can be prolonged. This emphasizes the need for continued surveillance and segregation. The current UK recommendation of at least 12 months of negative cultures before a patient is considered to have cleared the infection (CF Trust, 2004) may not be adequate for some patients.
It is important to recognize that there are significant limitations to the data presented here. The data are from a single center, involving a small number of subjects over a prolonged period during which the approach to new BCC infections has not been uniform. These results highlight the need for a thorough study of this question.
In conclusion, we are really no closer to answering the question of whether early BCC infection in CF can be eradicated with antibiotic therapy. We have shown that the practice of giving therapy to eradicate newly acquired BCC infections is widespread in adult CF units in the UK and have presented data, based on our own experience, that eradication is unsuccessful in the majority of cases. This is borne out by the estimates of success rate in other units. We have also shown that spontaneous clearance of infection occurs without treatment, which may be responsible for some of the apparent successes of eradication therapy. Looking to the future, and recognizing the potential clinical and psychological impact of untreated BCC infection, it is important that a rigorous and systematic evaluation be undertaken of the practice of eradication in order to avoid the inappropriate use of expensive and potentially harmful therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aaron S. D., Ferris W., Henry D. A., Speert D. P., Macdonald N. E. (2000). Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 161(Pt 1), 1206–1212 [DOI] [PubMed] [Google Scholar]
- Baldwin A., Mahenthiralingam E., Drevinek P., Pope C., Waine D. J., Henry D. A., Speert D. P., Carter P., Vandamme P., LiPuma J. J., Dowson C. G. (2008). Elucidating global epidemiology of Burkholderia multivorans in cases of cystic fibrosis by multilocus sequence typing. J. Clin. Microbiol. 46, 290–295 10.1128/JCM.01818-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R., Brownlee K. G., Duff A. J., Denton M., Conway S. P., Lee T. W. (2010). Can Burkholderia cepacia complex be eradicated with nebulised amiloride, and TOBI? J. Cyst. Fibros. 9, 73–74 10.1016/S1569-1993(10)60281-0 [DOI] [PubMed] [Google Scholar]
- Biddick R., Spilker T., Martin A., LiPuma J. J. (2003). Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans, and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol. Lett. 228, 57–62 10.1016/S0378-1097(03)00724-9 [DOI] [PubMed] [Google Scholar]
- CF Foundation (2009). Patient Registry 2009 Annual Report. Bethseda, MD: Cystic Fibrosis Foundation [Google Scholar]
- CF Trust (2004). The Burkholderia cepacia Complex: Suggestions for Prevention, and Infection Control, in Report of the UK Cystic Fibrosis Trust Infection Control Group. London: UK Cystic Fibrosis Trust [Google Scholar]
- CF Trust (2009). UK CF Registry Annual Data Report. London: UK Cystic Fibrosis Trust [Google Scholar]
- CF Trust. (2008). Methicillin Resistent Staphylococcus aureus, in Report of the UK Cystic Fibrosis Trust Infection Control Working Group. UK Cystic Fibrosis Trust: London [Google Scholar]
- Chaparro C., Maurer J., Gutierrez C., Krajden M., Chan C., Winton T., Keshavjee S., Scavuzzo M., Tullis E., Hutcheon M., Kesten S. (2001). Infection with Burkholderia cepacia in cystic fibrosis: outcome following lung transplantation. Am. J. Respir. Crit. Care Med. 163, 43–48 [DOI] [PubMed] [Google Scholar]
- Ciofu O., Jensen T., Pressler T., Johansen H. K., Koch C., Høiby N. (1996). Meropenem in cystic fibrosis patients infected with resistant Pseudomonas aeruginosa or Burkholderia cepacia, and with hypersensitivity to beta-lactam antibiotics. Clin. Microbiol. Infect. 2, 91–98 10.1111/j.1469-0691.1996.tb00212.x [DOI] [PubMed] [Google Scholar]
- Conway S. P., Lee T. W. (2009). Prevention of chronic Pseudomonas aeruginosa infection in people with cystic fibrosis. Expert Rev. Respir. Med. 3, 349–361 10.1586/ers.09.26 [DOI] [PubMed] [Google Scholar]
- Courtney J. M., Dunbar K. E., McDowell A., Moore J. E., Warke T. J., Stevenson M., Elborn J. S. (2004). Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J. Cyst. Fibros. 3, 93–98 10.1016/j.jcf.2004.06.006 [DOI] [PubMed] [Google Scholar]
- De Soyza A., McDowell A., Archer L., Dark J. H., Elborn S. J., Mahenthiralingam E., Gould K., Corris P. A. (2001). Burkholderia cepacia complex genomovars, and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet 358, 1780–1781 10.1016/S0140-6736(01)06808-8 [DOI] [PubMed] [Google Scholar]
- Duff A. J. (2002). Psychological consequences of segregation resulting from chronic Burkholderia cepacia infection in adults with CF. Thorax 57, 756–758 10.1136/thorax.57.9.756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R. L. (2002). Pseudomonas aeruginosa, and other predictors of mortality, and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 34, 91–100 10.1002/ppul.10127 [DOI] [PubMed] [Google Scholar]
- France M.W., Dodd M. E., Govan J. R., Doherty C. J., Webb A. K., Jones A. M. (2008). The changing epidemiology of Burkholderia species infection at an adult cystic fibrosis centre. J. Cyst. Fibros. 7, 368–372 10.1016/j.jcf.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Gibson R. L., Burns J. L., Ramsey B. W. (2003). Pathophysiology, and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168, 918–951 10.1164/rccm.200304-505SO [DOI] [PubMed] [Google Scholar]
- Govan J. R., Brown A. R., Jones A. M. (2007). Evolving epidemiology of Pseudomonas aeruginosa, and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2, 153–164 10.2217/17460913.2.2.153 [DOI] [PubMed] [Google Scholar]
- Govan J.R., Brown P. H., Maddison J., Doherty C. J., Nelson J. W., Dodd M., Greening A. P., Webb A. K. (1993). Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 342, 15–19 10.1016/0140-6736(93)91881-L [DOI] [PubMed] [Google Scholar]
- Grimwood K., Kidd T. J., Tweed M. (2009). Successful treatment of cepacia syndrome. J. Cyst. Fibros. 8, 291–293 10.1016/j.jcf.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Horsley A., Perry C., Martin K., Webb K., Turton J., Kenna D., Jones A. (2011). Burkholderia latens infection in cystic fibrosis. J. Cyst. Fibros. 10, 291–292 10.1016/S1569-1993(11)60149-5 [DOI] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. (1984). Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104, 206–210 10.1016/S0022-3476(84)80993-2 [DOI] [PubMed] [Google Scholar]
- Jones A. M., Dodd M. E., Govan J. R., Barcus V., Doherty C. J., Morris J., Webb A. K. (2004). Burkholderia cenocepacia, and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax 59, 948–951 10.1136/thx.2003.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish L.A., Dunbar K. E., McDowell A., Moore J. E., Warke T. J., Stevenson M., Elborn J. S. (2006). Impact of Burkholderia dolosa on lung function, and survival in cystic fibrosis. Am. J. Respir. Crit. Care Med. 173, 421–425 10.1164/rccm.200503-344OC [DOI] [PubMed] [Google Scholar]
- Kaza S. K., McClean S., Callaghan M. (2011). IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth, and intracellular survival of bacteria. Int. J. Med. Microbiol. 301, 26–33 10.1016/j.ijmm.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Kuti J. L., Moss K. M., Nicolau D. P., Knauft R. F. (2004). Empiric treatment of multidrug-resistant Burkholderia cepacia lung exacerbation in a patient with cystic fibrosis: application of pharmacodynamic concepts to meropenem therapy. Pharmacotherapy 24, 1641–1645 10.1592/phco.24.16.1641.50960 [DOI] [PubMed] [Google Scholar]
- Lipuma J. J. (2010). The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 23, 299–323 10.1128/CMR.00068-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiPuma J. J., Dasen S. E., Nielson D. W., Stern R. C., Stull T. L. (1990). Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336, 1094–1096 10.1016/0140-6736(90)92571-X [DOI] [PubMed] [Google Scholar]
- Littlewood J. M., Miller M. G., Ghoneim A. T., Ramsden C. H. (1985). Nebulised colomycin for early Pseudomonas colonisation in cystic fibrosis. Lancet 1, 865. 10.1016/S0140-6736(85)92222-6 [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E., Urban T. A., Goldberg J. B. (2005). The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3, 144–156 10.1038/nrmicro1085 [DOI] [PubMed] [Google Scholar]
- Middleton P.G., Kidd T. J., Williams B. (2005). Combination aerosol therapy to treat Burkholderia cepacia complex. Eur. Respir. J. 26, 305–308 10.1183/09031936.05.00119504 [DOI] [PubMed] [Google Scholar]
- Sly P.D., Brennan S., Gangell C., de Klerk N., Murray C., Mott L., Stick S. M., Robinson P. J., Robertson C. F., Ranganathan S. C., Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) (2009). Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am. J. Respir. Crit. Care Med. 180, 146–152 10.1164/rccm.200901-0069OC [DOI] [PubMed] [Google Scholar]
- Tablan O. C., Chorba T. L., Schidlow D. V., White J. W., Hardy K. A., Gilligan P. H., Morgan W. M., Carson L. A., Martone W. J., Larson J. M. (1985). Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors, and clinical outcome. J. Pediatr. 107, 382–387 10.1016/S0022-3476(85)80511-4 [DOI] [PubMed] [Google Scholar]
- Tunney M. M., Field T. R., Moriarty T. F., Patrick S., Doering G., Muhlebach M. S., Wolfgang M. C., Boucher R., Gilpin D. F., McDowell A., Elborn J. S. (2008). Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 177, 995–1001 10.1164/rccm.200708-1151OC [DOI] [PubMed] [Google Scholar]
- Valerius N. H., Koch C., Hoiby N. (1991). Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 338, 725–726 10.1016/0140-6736(91)91446-2 [DOI] [PubMed] [Google Scholar]
- Weidmann A., Webb A. K., Dodd M. E., Jones A. M. (2008). Successful treatment of cepacia syndrome with combination nebulised, and intravenous antibiotic therapy. J. Cyst. Fibros. 7, 409–411 10.1016/j.jcf.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Worlitzsch D., Rintelen C., Böhm K., Wollschläger B., Merkel N., Borneff-Lipp M., Döring G. (2009). Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin. Microbiol. Infect. 15, 454–460 10.1111/j.1469-0691.2008.02659.x [DOI] [PubMed] [Google Scholar]
- Zahariadis G., Levy M. H., Burns J. L. (2003). Cepacia-like syndrome caused by Burkholderia multivorans. Can. J. Infect. Dis. 14, 123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlosnik J. E., Costa P. S., Brant R., Mori P. Y., Hird T. J., Fraenkel M. C., Wilcox P. G., Davidson A. G., Speert D. P. (2010). Mucoid, and Nonmucoid Burkholderia cepacia Complex Bacteria in Cystic Fibrosis Infections. Am. J. Respir. Crit. Care Med. 183, 67–72 10.1164/rccm.201002-0203OC [DOI] [PubMed] [Google Scholar]