Abstract
Objective
To explore possible benefits of a nicotinic acetylcholine receptor (nAChR) agent for autistic symptoms based on postmortem observation of nAChR abnormalities (deficient α4β2 nAChRs, excess α7 nAChRs) in brains of patients with autism.
Method
Mecamylamine, because of its safety record in children with other disorders, was chosen for this first exploration. Twenty children with autism spectrum disorder age 4–12 years were randomly assigned for 14 weeks to placebo (n=8) or mecamylamine (n=12) in ascending fixed doses: 0.5 mg/day for 6 weeks, 2.5 mg for 2 weeks, then 5 mg/day for 6 weeks. Improvement was rated by a blinded independent evaluator. Because of small sample, data analysis was descriptive.
Results
Eighteen participants (10 mecamylamine, 8 placebo) completed the study. All doses were well tolerated; the only side effect of note was constipation (50% compared with 25% of placebo group). Three children had clinically nonsignificant electrocardiographic QT prolongation. Both groups showed modest to moderate improvement, but differences between groups were negligible. On the primary outcome measure, the Ohio Autism Clinical Impressions Scale, 90% of the active treatment group showed improvement at some point (but only 40% sustained it), compared with 62% on placebo. Of the four in active treatment that sustained improvement, three had a maximum dose of 0.13–0.15 mg/kg/day, while those who regressed had doses ≥0.18 mg/kg/day. Graphed means suggested better outcome with lower mg/kg and longer medication duration. Four parents spontaneously reported reduced hyperactivity and irritability and better verbalization and continued mecamylamine at their own expense.
Conclusion
Mecamylamine appeared to be safe, but not very effective in autism. The suggestion of better results at lower doses and longer exposure warrants consideration for future trials. The next step would be exploration of a more specific α4β2 nAChR agonist, such as varenicline.
Introduction
Neuropathological data from autopsied basal forebrain have indicated an abnormality of the cholinergic system in autism (Bauman and Kemper 1994). Compared to age-matched individuals without autism, the brains of deceased people with autism have an extensive (60%–70%) loss of high-affinity nicotinic acetylcholine receptors (nAChRs) in the mesocortex (Perry et al. 2001). Studies based on radioligand binding autoradiography, protein subunit immunochemistry (western blotting), and messenger RNA quantitation (reverse transcription–polymerase chain reaction) indicate that the principal nAChR subtype involved is that containing the α4 and β2 subunit combination. Neurochemical investigations of the cholinergic system in brain tissue have established an extensive loss of the α4β2 nAChR subtype in cortical and cerebellar regions of adults with autism (Perry et al. 2001; Lee et al. 2002; Martin-Ruiz et al. 2004). This subtype is upregulated following the administration of nicotinic agonists such as nicotine in both human (Breese et al. 1997; Court et al. 1998; Perry et al. 1999) and animal models (Mochizuki et al. 1998; Sparks and Pauly 1999; Kassiou et al. 2001). Parallel neurochemical studies have identified loss of the α4β2 nAChR in the cerebellum in autism that is similar to, but less extensive than that found in the cortex, and an increase in the α7 nAChR subtype (Lee et al. 2002).
It is not yet clear whether and how extensively this nicotinic cholinergic abnormality relates to the clinical phenotype; however, nAChRs have generally been implicated in attention (Wesnes et al. 1983; Wesnes and Warburton 1984; Wesnes and Parrott 1992; Levin et al. 1998), learning (Levin 2002), anxiety (Picciotto et al. 2002), and pain perception (Marubio and Changeux 2000), which may all be relevant in autism. Perhaps more important for long-term treatment, the α4β2 nAChR subtype has been implicated in neuroprotection and synaptic plasticity (McGehee 2002).
Two open label trials in children or adolescents with autism suggested that the cholinesterase inhibitor donepezil is of clinical benefit (Chez 2001; Hardan and Handen 2002). However, a controlled trial showed equivocal results (Handen et al. 2010, 2011). Cholinesterase inhibitors increase the level of acetylcholine and activate both muscarinic and nAChRs. However, nicotine, mecamylamine, and varenicline specifically target nAChRs. Nicotine has demonstrated efficacy and safety in placebo-controlled trials in children with Tourette's disorder (Silver et al. 2001a, 2001b), in placebo-controlled trials in adult attention-deficit/hyperactivity disorder (ADHD) (Conners et al., 1996) and in an open trial in adults age 20–30 years with Down syndrome (Bernert et al. 2001). The efficacy of nicotine in reducing inattention, hyperactivity, and irritability in other chronic disorders beginning in childhood, such as Down syndrome (Seidl et al. 2000; Bernert et al. 2001) and Tourette's disorder, supports the strategy of testing a nicotinic cholinergic agent in autism. Silver et al. (2001b) also reported an effect on mood; given the irritability and other associated mood problems often associated with autism spectrum disorders (ASDs), this provides further support for a hypothesized benefit in ASD.
Mecamylamine has clinical effects similar to nicotine in Tourette's disorder when used at low doses in combination with an antipsychotic drug (Silver et al. 2001b). Further, low doses of mecamylamine have been safely administered to a large number of children in a clinical trial in ADHD (Targacept, Inc., data on file). Extensive cardiac monitoring failed to show any change in cardiovascular functioning associated with daily exposures of mecamylamine up to 1.0 mg. The results of this trial failed to reach the level of significance on ratings of ADHD symptoms, but the safety of the drug in children at low doses was established. Further, Lipiello (2006) reported evidence supporting the hypothesis that normalization of cholinergic tone by selective antagonism of nAChRs may reduce the burden of core autism symptoms.
Many other neurotransmitter systems have been implicated one way or another in the etiology of autism (Lam et al. 2006). However, a nicotinic cholinergic abnormality may relate to other neurotransmitter anomalies in important ways. Reductions in hippocampal gamma-aminobutyric acidA (GABAA) receptors (Blatt et al. 2001), glutamate decarboxylase (Fatemi et al. 2002), glutamate AMPA receptor, and glutamate transporter in the cerebellum (Purcell et al. 2001) implicate both GABA and glutamate. However, it is likely that there is a significant pathological link between the α4β2 nAChR and GABA, so that nicotinic therapy may ameliorate dysfunctional GABA inhibition. Nicotinic receptors modulate GABA inhibition via both α4β2 and α7 nAChR subtypes (Alkondon and Albuquerque 2001).
Nicotine is traditionally considered to be an agonist and mecamylamine an antagonist. However, both drugs are associated with receptor desensitization. This indicates that in some respects, nicotine exerts a longer-term effect similar to an antagonist. Mecamylamine has a range of action across different doses, including cognitive enhancement (Mihailescu et al. 1998; Giniatullin et al. 2000; Buccafusco and Terry, 2002; Shytle et al. 2002).
An α4β2 nAChR agonist would be a more intuitive nicotine-class drug to try in a pilot study, given the deficit of α4β2 nAChRs documented in autism. For example, varenicline is an α4β2 nAChR agonist and might be considered a more direct treatment of the α4β2 nAChR deficiency, but its safety in children has not yet been established. The established safety of mecamylamine in studies of ADHD and Tourette's disorder made it a logical molecule to try first. Preliminary anecdotal clinical evidence suggested that in autistic patients, mecamylamine is associated with reduced compulsive behavior, improved social interaction and understanding, and more sophisticated conversation. Therefore we conservatively elected to try mecamylamine in this pilot trial.
Methods
Study design and treatment
This was a parallel-group, double-blind, placebo-controlled pilot trial of oral mecamylamine. Twenty children were randomly assigned in a ratio of 3:2 to receive mecamylamine (0.5 to 5.0 mg) or placebo. The acute phase of the trial lasted 14 weeks, followed by a 10-week open-label trial for placebo nonresponders. This pilot trial was funded by a grant through Autism Speaks. The Ohio State University Institutional Review Board approved the protocol and all parents/guardians provided written informed consent prior to participation.
Dosage started at 0.5 mg/day oral mecamylamine or matched placebo. Those who still had room for improvement and no limiting side effects at 6 weeks increased to 2.5 mg/day for 2 weeks and if still room for improvement and no limiting side effects at that dose, escalated to 5 mg/day for 6 weeks. Dose decreases to manage adverse effects were permitted at any time. Nonexclusionary background medications were continued as needed. Progress was monitored in clinical visits at the end of weeks 1, 2, 4, 6, 7, 8, 9, 10, 12, and 14.
Subjects
The subjects were 4 to 12 years of age, met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) criteria for Autistic Disorder or Pervasive Developmental Disorder Not Otherwise Specified based on clinical interview by a child psychiatrist and corroborated by the Autism Diagnostic Interview-Revised (ADI-R) by a research-certified administrator, and had an IQ of >36 or a mental age of >18 months. Exclusion criteria included the use of antipsychotic medications 3 months prior to baseline, psychoactive medications in the process of adjustment, systemic corticoids, or unstable seizure disorder. Subjects were also excluded if they began a major behavioral intervention within 2 months prior to baseline or planned to during the trial.
Assessments
Outcome measures included the Ohio Autism Clinical Impressions Scale (OACIS, Butter and Mulick 2006), the OSU Autism Rating Scale–DSM-IV (OARS-4; OSU RUPP 2005; http://psychmed.osu.edu/resources.htm#instruments), the Repetitive Behavior Scale (Bodfish et al. 1999), the Aberrant Behavior Checklist (Aman and Singh 1986, 1994), the Social Responsiveness Scale (Constantino et al. 2003), and target symptom assessment (Arnold et al. 2003). The OACIS was administered at every visit. The Repetitive Behavior Scale, Aberrant Behavior Checklist, and Social Responsiveness Scale were collected at baseline and every 2 weeks. The target symptom assessment was completed at baseline and weeks 2, 6, 8, 10, and 14. The OARS-4 was administered at baseline and weeks 6, 8, and 14.
In addition, cognition was assessed at baseline, weeks 6, 8, and end of treatment using a continuous performance task (Aman 1991), a cancellation task (Aman et al. 2008), and the Delayed Match-To-Sample Task (Aman and Turbott 1986; Aman 1991; Aman et al. 2003). Language was assessed at baseline and end of treatment using the Expressive Vocabulary Test-Second Edition (Williams 2007).
Safety assessments at screening/baseline and end point included physical exam, medical history, urinalysis, and for those able to cooperate, electrocardiogram, complete blood count (CBC), and blood chemistry. Vital signs, weight, and adverse events (AEs) were collected at each visit. A systematic side effects probe was also completed at every visit and concomitant medications were reviewed and recorded.
The OACIS-Improvement (OACIS-I, Butter and Mulick 2006), http://psychmed.osu.edu/resources.htm#instruments) is a 10-item clinician rated assessment designed to capture the clinician's global impression of the subject's improvement. The OACIS-I assesses improvement in social interaction, aberrant behavior, repetitive or ritualistic behavior, verbal communication, nonverbal communication skills, hyperactivity and inattention, anxiety and fearfulness, sensory sensitivities, restricted and narrow interests, and overall rating of autism. Each item is rated on a scale from 1 (Very much improved) through 4 (No change) to 7 (Very much worse). The OACIS-I was collected at every visit as the primary outcome measure. The main secondary measure was a z-score composite of all other scales.
The OARS-4 (OSU RUPP 2005), is a clinician-rated assessment based on a semi-structured interview with the subject's primary caregiver. Based on the DSM-IV symptoms of autistic disorder, it consists of 12 items rated from 0 (Never or Rarely-Not a Problem) to 3 (Very Often-A Severe Problem) comprising three subscales: (a) Impairment in social interaction, (b) Impairment in communication, and (c) Restricted, repetitive and stereotyped patterns of behavior, interests, and activities. It provides two summary scores: (a) A weighted score of severity of autism or autism spectrum symptoms (based on the 0–3 rating) and (b) A symptom count, based on how many symptoms received a rating >0. Each subscale symptom-count score can range from 0 to 4 with mean item scores ranging from 0.0 to 3.0.
Statistical methods
All subjects randomized were included in the analysis. The last-observation-carried-forward method was used to impute the missing data. The distribution of the subject baseline characteristics was compared across treatment groups. AEs were summarized by frequency counts and proportions. At each visit, pre-post change scores for all outcome measures were summarized by treatment groups using mean and standard deviation. Treatment effect size for all the outcome measures and the combined z-score was reported for visit week 14 only. Although we did not expect statistical significance at this sample size, Fisher's exact test was used to analyze the categorical variables. For continuous variables, p-values were calculated based on Wilcoxon ranked sum test. Simple linear regression was used to examine the relationship between the dose or weight and the mean OACIS item.
Results
Twenty children were randomized, 8 to placebo and 12 to active mecamylamine. Demographic and clinical characteristics of the 20 participants are presented in Table 1. At study entry, the placebo group had higher scores on: Aberrant Behavior Checklist (ABC) Stereotypy subscale (p=0.05), ADI-R Restricted, Repetitive, and Stereotyped Patterns of Behavior score (p=0.03), and Social Responsiveness Scale total (p=0.04). No significant differences were found for age, weight, sex, diagnosis, IQ, or entry scores for the OACIS, RBS, ABC Irritability, ABC Hyperactivity, ABC Lethargy/Social Withdrawal, ABC Inappropriate Speech, ADI-R Qualitative Abnormalities in Reciprocal Social Interaction or ADI-R Qualitative Abnormalities in Communication.
Table 1.
Sample Characteristics (n=20)
| Placebo (n=8) | Active (n=12) | Total (n=20) | |
|---|---|---|---|
| Age (year), mean, ±SD | 8.36, ±2.83 | 6.76, ±2.24 | 7.40, ±2.55 |
| Weight (kg), mean, ±SD | 40.26, ±20.69 | 26.68, ±7.83 | 32.40, ±15.75 |
| Height (cm), mean, ±SD | 131.31, ±19.61 | 122.14, ±13.61 | 126, ±16.56 |
| Gender | |||
| Male, n (%) | 6 (75) | 11 (91.67) | 17 (85) |
| Female, n (%) | 2 (25) | 1 (8.33) | 3 (15) |
| Race | |||
| White, n (%) | 8 (100) | 9 (75) | 17 (85) |
| Asian, n (%) | 0 (0) | 2 (16.67) | 2 (10) |
| Other, n (%) | 0 (0) | 1 (8.33) | 1 (5) |
| Diagnosis | |||
| Autistic disorder, n (%) | 7 (87.5) | 10 (83.33) | 17 (85) |
| PDD-NOS, n (%) | 1 (12.5) | 2 (16.67) | 3 (15) |
| IQa, mean, ±SD | 62.62, ±32.53 | 77.58, ±21.12 | 71.60, ±26.55 |
| IQ range (mean) | 17–124 (107) | 37–113 (76) | 17–124 (107) |
| Entry OACIS-S score, mean, ±SD | 5.38, ±0.92 | 5.25, ±0.75 | 5.3, ±0.80 |
| Entry SRS total score, mean, ±SD | 120.63, ±30.55 | 95.08, ±19.72 | 105.30, ±27.09 |
| Entry RBS total score, mean, ±SD | 38.38, ±20.16 | 29.42, ±15.81 | 33, ±17.74 |
| Entry ABC scores: | |||
| Irritability, mean, ±SD | 12.88, ±9.60 | 12.75, ±9.42 | 12.80, ±9.24 |
| Lethargy, mean, ±SD | 17.00, ±9.37 | 10.33, ±6.46 | 13.00, ±8.23 |
| Stereotypy, mean, ±SD | 9.75, ±6.25 | 4.25, ±3.60 | 6.45, ±5.43 |
| Hyperactivity, mean, ±SD | 19.13, ±13.02 | 21.17, ±11.71 | 20.35, ±11.95 |
| Inappropriate Speech, mean, ±SD | 4.63, ±3.20 | 4.50, ±3.73 | 4.55, ±3.44 |
| Entry ADI-R Subscale A-Reciprocal Social Interaction, mean, ±SD | 26.63, ±5.80 | 23.00, ±5.56 | 24.4, ±5.80 |
| Entry ADI-R Subscale B-Communication, mean, ±SD | 16.25, ±3.77 | 13.17, ±3.93 | 14.40, ±4.07 |
| Entry ADI-R Subscale C-Restricted, Repetitive, and Stereotyped Patterns of Behavior, mean, ±SD | 7.00, ±2.07 | 4.92, ±1.73 | 5.75, ±2.10 |
This study utilized the Stanford Binet 5 (5 participants), the Leiter-R (13 participants), and the Mullen Early Scales of Learning (2 participants).
SD=standard deviation; PDD-NOS=Pervasive Developmental Disorder-Not Otherwise Specified; OACIS-S=Ohio Autism Clinical Global Impressions Scale-Severity; SRS=Social Responsiveness Scale; RBS=Repetitive Behavior Scale; ABC=Aberrant Behavior Checklist; ADI-R=Autism Diagnostic Interview-Revised.
Two of those in active treatment dropped out after 4 weeks with lack of improvement; in one case the child had stopped psychoactive medication to enter the study and had deteriorated to an intolerable extent. In the other case the family moved to an inconvenient distance. Their termination assessments were carried forward in the data analysis. The remaining 18 (10 mecamylamine, 8 placebo) finished the full 14 weeks of double-blind treatment, using all doses. No dose reduction for AEs was needed, but in one case after study completion the parents and physician decided that the child had done better with the middle dose than the high dose.
No statistically or clinically significant difference in the amount of improvement was seen between placebo and mecamylamine on any measure (Table 2a, b), except one subscale of the Social Responsiveness Scale on which placebo did better than active mecamylamine. In the active treatment group, nine (90% of the completers) showed some improvement on the OACIS-I overall score (rating < 4) at some point (but only four sustained that improvement after escalating to the highest dose), compared with five (62%) in the placebo group showing improvement at some point.
Table 2A.
Global Outcome Measures by Treatment Group and Time
| |
Placebo |
Active mecamylamine |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | Change week 6 | Change week 8 | Change week 14 | BL | Change week 6 | Change week 8 | Change week 14 | ES (p) of group differencea | |
| OACIS-S | |||||||||
| Overall autism | 5.63±0.74 | −0.25±0.46 | −0.38±0.52 | −0.63±0.74 | 5.25±0.75 | −0.17±0.41 | −0.33±0.49 | −0.58±0.79 | −0.05 (0.91) |
| Noncore items | 4.00±1.13 | −0.13±0.35 | −0.42±0.68 | −0.38±0.58 | 3.53+1.17 | −0.11±0.33 | −0.22±0.48 | −0.28±0.72 | −0.15 (0.75) |
| Autism symptoms | 5.20±0.93 | −0.14±0.28 | −0.41±0.41 | −0.52±0.52 | 4.70±0.60 | −0.21±0.30 | −0.37±0.45 | −0.49±0.50 | −0.06 (0.90) |
| OACIS-I | |||||||||
| Overall autism | 4.00b | −0.38±0.52 | −0.63±0.74 | −0.88±0.64 | 4.00b | −0.50±0.52 | −0.58±0.51 | −0.92±0.79 | 0.06 (0.90) |
| Noncore items | 4.00b | −0.13±0.56 | −0.46±0.73 | −0.38±0.72 | 4.00b | −0.17±0.58 | −0.31±0.63 | −0.25±0.75 | −0.17 (0.72) |
| Autism symptoms | 4.00b | −0.27±0.37 | −0.50±0.53 | −0.71±0.55 | 4.00b | −0.37±0.29 | −0.49±0.41 | −0.68±0.59 | −0.06 (0.90) |
| OARS | |||||||||
| Total impairment | 2.06±0.45 | −0.09±0.21 | −0.13±0.21 | −0.32±0.34 | 1.83±0.42 | −0.16±0.26 | −0.25±0.31 | −0.30±0.33 | −0.07 (0.89) |
| No. of symptoms | 10.5±1.07 | 0.13±0.35 | 0.13±0.35 | 0±0.53 | 10.08±1.16 | 0.08±0.29 | 0.08±0.29 | −0.25±0.45 | 0.51 (0.27) |
| Target symptoms | 5.00b | −0.73±0.96 | −1.33±0.87 | −1.56±1.05 | 5.00b | −0.69±0.78 | −1.10±1.08 | −1.46±1.38 | −0.08 (0.86) |
| Z Scorec composite | 0.38±0.99 | −0.32±0.56 | −0.61±0.73 | −0.73±0.90 | −0.25±0.70 | −0.44±0.60 | −0.59±0.64 | −0.62±0.71 | −0.14 (0.76) |
Negative ES (Cohen's d) reflects placebo showing nominally more improvement than active mecamylamine.
Baseline for OACIS-I is 4 by definition, and baseline for Target Symptoms is 5 by definition; Therefore sd is 0 and meaningless.
Mean of Z scores from all scales (including Table 2B) based on baseline means and SD.
Lower scores are better on all measures.
BL=baseline; ES=effect size; OACIS-I=Ohio Autism Clinical Impressions Scale-Improvement; OARS=OSU Autism Rating Scale.
Table 2B.
Specific Outcome Measures by Treatment Group and Time
| |
Placebo |
Active |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BL | Change week 6 | Change week 8 | Change week 14 | BL | Change week 6 | Change week 8 | Change week 14 | ES (p) of group differencea | |
| Aberrant Behavior Checklist | |||||||||
| Irritability | 12.88±9.60 | −3.63±10.06 | −5.25±10.04 | −5.00±10.78 | 12.75±9.42 | −3.08±7.24 | −5.50±7.50 | −3.17±8.76 | −0.19 (0.68) |
| Lethargy | 17.00±9.37 | −4.13±7.53 | −6.38±8.72 | −7.50±9.56 | 10.42±6.61 | −4.5±6.68 | −5.00±7.86 | −4.25±6.97 | −0.40 (0.39) |
| Stereotype | 9.75±6.25 | −0.63±4.78 | −2.38±5.66 | −1.63±7.11 | 4.17±3.54 | −1.25±3.08 | −2.00±2.26 | −1.42±2.64 | −0.04 (0.93) |
| Hyperactivity | 19.13±13.02 | −4.63±10.91 | −4.88±12.47 | −5.50±12.06 | 21.08±11.74 | −6.67±10.87 | −6.75±10.55 | −6.92±11.25 | 0.12 (0.79) |
| Inappropriate speech | 4.38±3.16 | −0.5±2.00 | −1.13±2.42 | −1.75±2.38 | 4.50±3.73 | −1.58±2.78 | −1.92±2.02 | −1.50±3.90 | −0.07 (0.87) |
| Repetitive Behavior Scale | |||||||||
| Stereotypy | 7.38±3.16 | −0.88±4.64 | −2.13±4.22 | −0.63±6.21 | 4.58±2.87 | −1.67±2.93 | −1.83±2.62 | −1.92±2.94 | 0.29 (0.54) |
| Self-injury | 4.50±3.82 | −1.75±4.20 | −2.00±3.96 | −2.88±4.39 | 1.75±2.01 | −0.33±1.56 | −0.25±2.01 | −0.83±1.53 | −0.68 (0.15) |
| Compulsive | 7.25±4.53 | −2.63±2.56 | −3.75±2.49 | −3.50±3.38 | 5.50±3.90 | −2.83±3.83 | −3.25±3.25 | −2.42±3.55 | −0.31 (0.50) |
| Ritualistic | 5.88±4.91 | −0.38±2.26 | −2.13±3.40 | −2.00±3.02 | 6.00±4.63 | −1.75±4.25 | −2.42±3.99 | −1.75±4.58 | −0.06 (0.89) |
| Sameness | 9.38±6.14 | −1.25±3.77 | −2.75±4.53 | −2.50±3.21 | 8.08±5.62 | −4.00±5.94 | −3.92±6.26 | −4.67±6.05 | 0.42 (0.37) |
| Restricted | 4.13±2.75 | −1.00±2.93 | −1.25±2.82 | −1.13±2.64 | 3.92±3.23 | −2.00±2.22 | −1.92±2.27 | −1.58±2.50 | 0.18 (0.70) |
| Social Responsiveness Scale | |||||||||
| Receptive | 16.38±2.62 | −0.63±2.13 | −2.25±3.41 | −2.25±3.06 | 11.92±2.64 | 0.25±2.05 | 0±1.95 | 0.33±1.78 | −1.10 (0.03) |
| Cognitive | 22.75±6.04 | −0.88±2.23 | −2.63±6.67 | −2.13±5.74 | 19.50±2.88 | −1.67±4.85 | −1.67±4.68 | −2.50±5.39 | 0.07 (0.88) |
| Expressive | 39.88±11.68 | −3.00±6.68 | −6.63±10.77 | −7.25±8.55 | 34.33±8.14 | −2.00±7.83 | −3.75±7.74 | −4.17±7.31 | −0.39 (0.40) |
| Motivation | 19.13±4.26 | −1.38±2.45 | −4.25±5.57 | −5.75±4.89 | 13.00±4.69 | −2.92±4.78 | −3.50±4.56 | −3.83±4.55 | −0.41 (0.38) |
| Preoccupations | 22.50±7.67 | −2.00±4.81 | −4.50±5.45 | −4.88±6.92 | 16.33±6.88 | −1.75±7.82 | −2.92±7.42 | −3.33±7.43 | −0.21 (0.65) |
| EVT | 47.5±24.74 | — | — | 0±6.42 | 74.0±16.34 | — | — | −2.14±10.12 | 0.25 (0.66) |
Negative effect size (ES, Cohen's d) reflects more improvement in placebo group except for EVT, for which positive ES reflects more improvement in placebo.
EVT=Expressive Vocabulary Test. The n for this measure is 13 (6 placebo and 7 active). This measure was only collected at baseline and end point.
BL=baseline; ES=effective size.
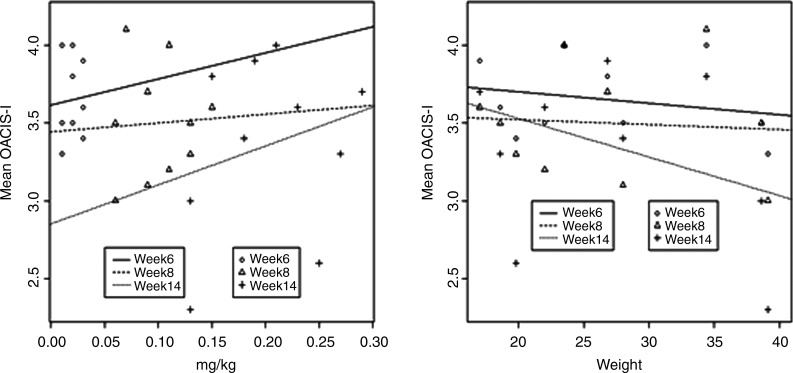
Of the four children in the active treatment group who sustained improvement through end point, three (75%) had a maximum dose between 0.13 and 0.15 mg/kg/day, while the remaining participants received doses of 0.18 mg/kg/day or more. The relationship among mg/kg/day dose, size of child, and duration of exposure is illustrated in Figure 1. Note that the OACIS-I score tends to be better at lower mg/kg/day doses, higher body weight, and longer duration of exposure. Unfortunately, duration is confounded with dose in the fixed escalation, but it appears that increased dose cannot explain the better response at 14 weeks because the response is inverse to the mg/kg/day dose.
FIG. 1.
Ohio Autism Clinical Impressions Scale-I (OACIS-I) Item Mean by Dose, Duration, and Weight. Lower score is better. Week 6=0.5 mg/day; week 8=2.5 mg/day; week 14=5 mg/day. Left panel: Lower mg/kg dose and longer duration are associated with better outcome. Better result at 14 weeks could be higher dose or longer duration of dosing because dose and duration are confounded, but duration seems the most likely association in view of the better outcome at lower mg/kg doses. Right panel: Association of better outcome with higher body weight (resulting in lower mg/kg doses in this fixed-dose titration). Results are not statistically significant at this sample size.
Safety measures
Table 3 shows AEs, including worsening of pre-existing conditions. Except for constipation, which occurred twice as often in the mecamylamine group as in placebo, the treatment groups were not different. Additionally, some AEs that were expected side effects, such as blurred vision and urinary retention, were not reported by any participants. No AE required dosage reduction.
Table 3.
Summary of Adverse Events by Treatment Groups on the Checklist of Expected Side Effects
| Adverse event checklist | Placebo (n=8) n (% of group) | Active treatment (n=12) n (% of group) |
|---|---|---|
| Constipation | 2 (25%) | 6 (50%) |
| Vomiting/nausea | 3 (37.5%) | 3 (25%) |
| Anorexia | 5 (62.5%) | 4 (33%) |
| Dry mouth | 1 (12.5%) | 1 (8%) |
| Mental symptoms | 4 (50%) | 6 (50%) |
| Sedation | 2 (25%) | 1 (8%) |
| Lung congestion | 6 (75%) | 9 (75%) |
| Urinary retention | 0 (0%) | 0 (0%) |
| Blurred vision | 0 (0%) | 0 (0%) |
| Dilated pupils | 1 (12.5%) | 1 (8%) |
| Weakness | 2 (25%) | 2 (16%) |
| Fatigue | 3 (37.5%) | 4 (33%) |
Physical exams at end point showed no new pathology except for one instance of pneumonia, which was judged not to be related to study treatment. One child had a “sticky heart valve” diagnosed in infancy and followed up by a cardiologist, with no worsening during the study. Vital signs, of particular interest given that mecamylamine was originally used as an antihypertensive medication, were unremarkable. The mean heart rate for the treatment group at baseline, 6, 8, and 12 weeks (respectively, in beats per minute) was 98, 96, 98, and 102, compared with 100, 103, 103, and 93 for placebo. The mean blood pressure (systolic/diastolic, in mm Hg) for baseline, 6, 8, and 12 weeks in the treatment group were 99/64, 96/64, 100/65, and 105/70, compared with 107/69, 105/65, 102/66, and 106/62 for placebo.
Electrocardiograms (ECGs) were successfully collected at screen and end point from 15 children (nine active and six placebo); the other five could not cooperate with ECG. The readings were generally normal with some exceptions; one child developed prolonged QT interval, another developed borderline QT interval, and a third went from borderline QT interval at screen to frank prolonged QT at end point. No physical signs or symptoms were associated with these ECG changes. Two children with previously diagnosed cardiac pathology (right atrial enlargement and valvular stenosis with left ventricular hypertrophy) were cleared for study participation by their cardiologists and experienced no exacerbation of these conditions during the study.
Urinalysis was obtained at screen and end point for 15 children (eight active and seven placebo) and blood chemistries and CBCs were obtained at screen and end point on 16 children (nine active and seven placebo); the remainder declined to cooperate. Lab values generally showed no change from screen to week 14. Six children (two active and four placebo) had abnormal lipids at screen; in three of these cases, abnormally high LDL cholesterol improved by 19 (placebo), 21 (placebo), and 21(active) points, respectively. No child showed new lipid abnormalities or worsening at 14 weeks. Other labs were unremarkable.
As one way of reducing the number of statistical tests while harnessing the multiple outcome measures incorporated, we constructed standard scores based on the principle clinical assessments of interest. The analysis for this z-score composite appears in the last line of Table 2a and is not significant.
Discussion
Anecdotally, there were four cases in which parents reported enough improvement that they chose to continue the mecamylamine after the study. In each of those cases, the parents stated that they noticed the greatest improvement in their child's irritability and hyperactivity, but also mentioned increased verbalization. The improved irritability would be compatible with the mood improvements reported by Silver et al. (2001b). However, these anecdotal improvements were not confirmed on a group basis by ABC irritability or hyperactivity scores or by Expressive Vocabulary Test (Table 2b). Nevertheless, the possibility of mood improvement may deserve further exploration.
Although mecamylamine appears safe for children with autism (despite three cases of clinically nonsignificant QT prolongation), the results of this pilot trial did not suggest clinically (or statistically) significant benefit on a group basis. Qualitative examination of the data suggested that improvement was greatest and was most likely to be sustained when the maximum daily dose was between 0.13 and 0.15 mg/kg. Possible reasons for failing to find benefit in the whole sample could be wrong dose or duration, restriction of benefit to a small minority, or simply that this medication is not useful in ASD. In view of the slightly better results at lower mg/kg/day dosage and at 14 weeks despite the absolute doses being higher at 14 weeks, the dosage and duration issues may deserve further exploration. Such exploration would need to monitor ECGs in view of the three cases with QT prolongation, which may be avoided by lower doses. However, those descriptive dose and duration impressions are limited by the small sample size.
Limitations
The most obvious limitation of this pilot trial is its small sample size spread over a large age range. Another is the confounding of dose with duration of treatment. Also, there was no attempt to sculpt doses to body size (other than clinical titration). In retrospect a dose-finding study prior to a placebo-controlled trial may have been useful.
Conclusions
Although it made sense to explore mecamylamine first because of its previous safety record in children, varenicline is more likely to address the specific α4β2 nAChR deficiency hypothesis, as it is a specific partial agonist of α4β2 nAChR. The response to mecamylamine was disappointing but not surprising, given that it does not particularly target the α4β2 nAChR. The next logical step may be to turn interest to a nicotinic agent with more α4β2 affinity. A cautious trial of varenicline or other α4β2 nAChR agonist may be the most rational extension of this trial (Deutsch et al. 2010; Anand et al. 2011).
Clinical Significance
These results are of most use to investigators. It appears that a second placebo-controlled trial of mecamylamine at a dose of 0.13–0.15 mg/kg/day for 3 months might be useful, although it would appear that resources might be better devoted to a trial of an α4β2 nAChR agonist. As it stands, the use of mecamylamine in children with autism would be off-label and without evidence of efficacy. If a clinician feels an individual trial is indicated for a child with hyperactivity and irritability unresponsive to the Food and Drug Administration-approved drugs, the dose most likely to help would be below 0.15 mg/kg/day maintained for several months. In view of the slight prolonging of QT interval, ECG monitoring would be desirable.
Disclosures
Dr. Arnold receives or has recently received research support or consulting honoraria from Lilly, Shire, Curemark, Neuropharm, Noven, Organon, Seaside Therapeutics, Targacept, Biomarin, and AstraZeneca. Dr. Aman has received consulting honoraria or research support from Bristol-Myers Squibb, Johnson and Johnson, Forrest, Novartis, and Supernus. Dr. Anand, Ms. Bates, Ms. Farmer, Ms. Hollway, Dr. Hurt, Dr. Li, Dr. Ramadan, Ms. Thompson, and Dr. Williams have no affiliations to report.
Acknowledgments
This pilot trial was funded by a grant from Cure Autism Now/Autism Speaks. It was further supported by award UL1RR025755 from the National Center for Research Resources (Ohio State University Center for Clinical and Translational Research). Participants were recruited with the assistance of the Interactive Autism Network (IAN) Research Database at the Kennedy Krieger Institute and Johns Hopkins Medicine–Baltimore, sponsored by the Autism Speaks Foundation.
References
- Alkondon M. Albuquerque EX. Nicotinic acetylcholine receptor alpha 7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Aman MG. Applications of computerized cognitive-motor measures to the assessment of psychoactive drugs. In: Dodson W.E., editor; Kinsbourne M., editor; Hiltbrunner B., editor. Assessing Cognitive Function in Epilepsy. New York: Demos Publications; 1991. pp. 69–96. [Google Scholar]
- Aman MG. Buican B. Arnold LE. Methylphenidate treatment in children with low IQ and ADHD: Analysis of three aggregated studies. J Child Adolesc Psychopharmacol. 2003;13:27–38. doi: 10.1089/104454603321666171. [DOI] [PubMed] [Google Scholar]
- Aman MG. Hollway JA. McDougle CJ. Scahill L. Tierney E. McCracken J. Arnold LE. Vitiello B. Ritz L. Gavaletz A. Cronin P. Swiezy NB. Wheeler C. Koenig K. Ghuman J. Posey DJ. Cognitive effects of risperidone in children with autism and irritable behavior. J Child Adolesc Psychopharmacol. 2008;18:227–236. doi: 10.1089/cap.2007.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG. Singh NN. Aberrant Behavior Checklist Manual. East Aurora, NY: Slosson Educational Publications; 1986. [Google Scholar]
- Aman MG. Singh NN. Aberrant Behavior Checklist—Community. Supplementary Manual. East Aurora, NY: Slosson Educational Publications; 1994. [Google Scholar]
- Aman MG. Turbott SH. Incidental learning, distraction, and sustained attention in hyperactive and control subjects. J Abnorm Child Psychol. 1986;14:441–455. doi: 10.1007/BF00915437. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (DSM-IV) 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anand R. Amici SA. Ponath G. Robson JI. Nasir M. McKay SB. Nicotinic acetylcholine receptor alterations in autism spectrum disorders: Biomarkers, therapeutic targets. In: Eapen V., editor. Autism/Book 2. Tech Open Access Publisher; 2011. ISBN 978-953-307-493-1. [Google Scholar]
- Arnold LE. Vitiello B. McDougle CJ. Scahill L. Shah B. Gonzalez NM. Chuang S. Davies M. Hollway J. Aman MG. Cronin P. Koenig K. Kohn AE. McMahon DJ. Tierney E. Parent-defined target symptoms respond to risperidone in RUPP autism study: Customer approach to clinical trials. J Am Acad Child Adolesc Psychiatry. 2003;42:1443–1450. doi: 10.1097/00004583-200312000-00011. [DOI] [PubMed] [Google Scholar]
- Bauman ML. Kemper TL. Neuro-anatomic observations of the brain in autism. In: Bauman M.L., editor; Kemper T.L., editor. The Neurobiology of Autism. Baltimore: John Hopkins University Press; 1994. pp. 119–145. [Google Scholar]
- Bernert G. Sustrova M. Sovcikova E. Seidl R. Lubec G. Effects of a single transdermal nicotine dose on cognitive performance in adults with Down syndrome. J Neural Transmission: Supplementum. 2001;61:237–245. doi: 10.1007/978-3-7091-6262-0_19. [DOI] [PubMed] [Google Scholar]
- Blatt GJ. Fitzgerald CM. Guptill JT. Booker AB. Kemper TL. Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: An autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bodfish JW. Symons FJ. Lewis MH. The Repetitive Behavior Scale. Western Carolina Center Research Reports; 1999. [Google Scholar]
- Breese CR. Marks MJ. Logel J. Adams CE. Sullivan B. Collins AC. Leonard S. Effect of smoking history on (3H) nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Buccafusco J. Terry A. Nicotine, cognition in young, aged nonhuman primates. In: Levin E., editor. Nicotinic Receptors in the Nervous System. CRC Press; 2002. [Google Scholar]
- Butter E. Mulick J. The Ohio Autism Clinical Impressions Scale (OACIS) Columbus, OH: Children's Research Institute; 2006. [Google Scholar]
- Chez M. Donepezil treatment in autism; Amer Assoc Neurol Int Meeting 2001 (abstract). [Google Scholar]
- Conners K. Levin E. Sparrow E. Hinton S. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- Constantino JN. Davis SA. Todd RD. Schindler MK. Gross MM. Brophy SL. Metzger LM. Shoushtari CS. Splinter R. Reich W. Validation of a brief quantitative measure of autistic traits: Comparison of the Socialresponsiveness Scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Court JA. Lloyd S. Thomas N. Piggott MA. Marshall EF. Morris CM. Lamb H. Perry RH. Johnson M. Perry EK. Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87:63–78. doi: 10.1016/s0306-4522(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Deutsch SI. Urbano MR. Neumann SA. Burket JA. Katz E. Cholinergic abnormalities in autism: Is there a rationale for selective nicotinic agonist interventions? Clin Neuropharmacol. 2010;33:114–120. doi: 10.1097/WNF.0b013e3181d6f7ad. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Halt AR. Satry JM. Kanodia R. Schulz SC. Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Giniatullin RA. Sokolova EM. Di Angelantonio S. Skorinkin A. Talantova MV. Nistri A. Rapid relief of block by mecamylamine of neruronal nicotinic acetylcholine receptors of rat chromaffin cells in vitro: An electrophysiological and modeling study. Mol Pharmacol. 2000;58:778–787. doi: 10.1124/mol.58.4.778. [DOI] [PubMed] [Google Scholar]
- Handen BL. Johnson CR. McAuliffe-Bellin SJ. Hardan AY. Safety and efficacy of donepezil in children and adolescents with autism: Behavioral measures. Int. Publ. Health J. 2010;2:125–134. doi: 10.1089/cap.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL. Johnson CR. McAuliffe-Bellin S. Murray PJ. Hardan AY. Safety and efficacy of donepezil in children and adolescents with autism: Neuropsychological measures. J Child Adolesc Psychopharmacol. 2011;21:43–50. doi: 10.1089/cap.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY. Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2002;12:237–241. doi: 10.1089/104454602760386923. [DOI] [PubMed] [Google Scholar]
- Kassiou M. Eberl S. Meikle SR. Birrell A. Constable C. Fulham MJ. Wong DF. Musachio JL. In vivo imaging of nicotinic receptor upregulation following chronic (-)-nictonic treatment in baboon using SPECT. Nucl Med Biol. 2001;28:165–175. doi: 10.1016/s0969-8051(00)00206-7. [DOI] [PubMed] [Google Scholar]
- Lam KLS. Aman MG. Arnold LE. Neurochemical correlates of autistic disorder: A review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lee M. Martin-Ruiz C. Graham A. Court J. Jaros E. Perry R. Iversen P. Bauman M. Perry E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002;125:1483–1495. doi: 10.1093/brain/awf160. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED. Conners CK. Silva D. Hinton SC. Meck WH. March J. Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Lipiello PM. Nicotinic cholinergic antagonists: A novel approach for the treatment of autism. Med Hypotheses. 2006;66:985–990. doi: 10.1016/j.mehy.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM. Lee M. Perry RH. Baumann M. Court JA. Perry EK. Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Marubio LM. Changeux J. Nicotinic acetylcholine receptor knockout mice as animal models for studying receptor function. Eur J Pharmacol. 2000;393:113–121. doi: 10.1016/s0014-2999(00)00007-8. [DOI] [PubMed] [Google Scholar]
- McGehee DS. Nicotinic receptors and hippocampal synaptic plasticity…it's all in the timing. Trends Neurosci. 2002;25:171–172. doi: 10.1016/s0166-2236(00)02127-5. [DOI] [PubMed] [Google Scholar]
- Mihailescu S. Palomero-Rivero M. Meade-Huerta P. Maza-Flores A. Drucker-Colin R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur J Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Mochizuki T. Villemagne VL. Scheffel U. Dannals RF. Finley P. Zahn Y. Wagner HN., Jr. Musachio JL. Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: Gender differences. Synapse. 1998;30:116–118. doi: 10.1002/(SICI)1098-2396(199809)30:1<116::AID-SYN15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- OSU Research Unit on Pediatric Psychopharmacology (OSU RUPP): OSU Autism Rating Scale—DSM-IV (OARS-4) Columbus, Ohio: OSU Research Unit on Pediatric Psychopharmacology; 2005. [Google Scholar]
- Perry DC. Davila-Garcia MI. Stockmeier CA. Kellar KJ. Increased nicotinic receptors in brains from smokers; membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry EK. Lee ML. Martin Ruiz CM. Court JA. Volsen SG. Merrit J. Folly E. Iversen PE. Bauman ML. Perry RH. Wenk GL. Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;158:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Brunzell DH. Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Purcell AE. Jeon OH. Zimmerman AW. Blue ME. Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Seidl R. Tiefenthaler M. Hauser E. Lubec G. Effects of transdermal nicotine on cognitive performance in Downs's syndrome. Lancet. 2000;356:1409–1410. doi: 10.1016/S0140-6736(00)02848-8. [DOI] [PubMed] [Google Scholar]
- Shytle RD. Penny E. Silver AA. Goldman J. Sanberg PR. Mecamylamine (Inversine): An old antihypertensive with new research directions. J Human Hypertens. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- Silver AA. Shytle RD. Philipp MK. Wilkinson BJ. MCConville B. Sanberg PR. Transdermal nicotine and haloperidol in Tourette's disorder: A double-blind placebo-controlled study. J Clin Psychiatry. 2001a;62:707–714. doi: 10.4088/jcp.v62n0908. [DOI] [PubMed] [Google Scholar]
- Silver AA. Shytle RD. Sheehan KH. Sheehan DV. Ramos A. Sanberg PR. Multicenter, double-blind, placebo-controlled study of mecamylamine monotherapy for Tourette's disorder. J Am Acad Child Adolesc Psychiatry. 2001b;40:1103–1110. doi: 10.1097/00004583-200109000-00020. [DOI] [PubMed] [Google Scholar]
- Sparks JA. Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57B1/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Wesnes K. Parrott A. Smoking, nicotine, human performance. In: Smithe A., editor; Jones D., editor. Handbook of Human Performance. Vol. 2. London: Academic Press; 1992. pp. 127–167. [Google Scholar]
- Wesnes K. Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology (Berl) 1984;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Wesnes K. Warburton DM. Matz B. Effects of nicotine on stimulus sensitivity and response bias in a visual vigilance task. Neuropsychobiology. 1983;9:41–44. doi: 10.1159/000117935. [DOI] [PubMed] [Google Scholar]
- Williams KT, editor. Expressive Vocabulary Test Manual. Second. Minneapolis, MN: NCS Pearson, Inc.; 2007. [Google Scholar]