Abstract
Clinical studies of women from the United States demonstrate a sensitivity of the ovarian system to energetic stress. Even moderate exercise or caloric restriction can lead to lower progesterone levels and failure to ovulate. Yet women in many nonindustrial populations experience as many as a dozen pregnancies in a lifetime despite poor nutritional resources, heavy workloads, and typical progesterone levels only about two-thirds of those of U.S. women. Previous cross-sectional studies of progesterone may, however, suffer from inadvertent selection bias. In a noncontracepting population, the most fecund women, who might be expected to have the highest progesterone, are more likely to be pregnant or breastfeeding and hence unavailable for a cross-sectional study of the ovarian cycle. The present longitudinal study was designed to ascertain whether lower progesterone also characterizes conception, implantation, and gestation in women from nonindustrialized populations. We compared rural Bolivian Aymara women (n = 191) to women from Chicago (n = 29) and found that mean-peak-luteal progesterone in the ovulatory cycles of Bolivian women averaged ≈71% that of the women from Chicago. In conception cycles, progesterone levels in Bolivian women during the periovulatory period were ≈63%, and during the peri-implantation period were ≈50%, those of the U.S. women. These observations argue that lower progesterone levels typically characterize the reproductive process in Bolivian women and perhaps others from nonindustrialized populations. We discuss the possible proximate and evolutionary explanations for this variation and note the implications for developing suitable hormonal contraceptives and elucidating the etiology of cancers of the breast and reproductive tract.
In an evaluation of demographic data from populations world-wide, Bongaarts (1) concluded that, except in cases of famine, nutritional factors play a relatively minor role in determining human fecundity (capacity to conceive) or fertility (number of live births). Despite characteristically marginal nutritional status and the demands of arduous activities, women in less developed countries often average seven to eight pregnancies, some having 12 or more during a lifetime. Differences among populations, or reductions from a theoretical maximum, appear fully attributable to a limited set of behavioral and physiological proximate fertility determinants apparently little affected by nutritional factors. If neither fecundity nor fertility is thus significantly influenced, neither is fecundability (the monthly probability of conception). Yet clinical studies of women from the United States indicate a sensitivity of ovarian function to both relatively low energy intake and/or high energy expenditure. The most extreme response, failure to ovulate, may occur in the face of only moderate energetic stress (2-4) and, of course, reduces fecundity and fecundability to 0. Short of anovulation, milder disruptions of ovarian function may also reduce fecundability. Why should ovarian function respond to energetic stress in women from well-nourished populations yet, paradoxically, appear to be relatively impervious in women from populations typically experiencing energetic stress? This question has been the subject of considerable debate among demographers and physiologists (5, 6), but no clear answer has yet emerged.
Studies of variation in progesterone, which plays a pivotal role in preparing maternal tissues for implantation of a fertilized egg and in maintaining a pregnancy, also appear contradictory. During the ovarian cycle, progesterone is relatively low during the preovulatory (follicular) phase, rising subsequent to ovulation and continuing to do so in the event of conception and implantation. Increasing progesterone during gestation prevents new follicle development and ovulation, and by inhibiting the contractility of the smooth muscles of the myometrium, precludes premature expulsion of the fetus. In U.S. women, relatively lower progesterone is associated with subfecundity among patients at infertility clinics, perhaps signaling failure to ovulate or luteal insufficiency (7), and is also associated with a reduction in risk of conception (8). Yet studies of women from nonindustrialized populations have consistently observed progesterone levels averaging only about two-thirds of the average levels of U.S. women (9-13). Does the typically much lower progesterone observed among women in nonindustrialized populations signal a significant impairment of reproductive function relative to U.S. women? If not, then why not? In any case, why might progesterone levels vary among populations?
To investigate these and other questions, we implemented a longitudinal study to measure normal progesterone levels during the reproductive life cycle in rural Bolivian Aymara women native to high altitude. This population was chosen because of widespread chronic undernutrition, often accompanied by physically demanding labor, and a nearly universal lack of contraceptive use at the time of the study. Two prior cross-sectional studies of Bolivian high-altitude natives (9, 10) had observed progesterone levels comparable to other nonindustrialized populations (11-13), arguing against an effect of altitude on progesterone in these women. Also like these populations, progesterone was significantly lower than in samples of U.S. women (14, 15). However, unavoidable selection bias could explain the lower progesterone in these earlier studies of nonindustrialized, noncontracepting populations because the most fecund women, who might be expected to have the highest progesterone levels, are more likely to be pregnant or breastfeeding and hence unavailable for a cross-sectional study. The present longitudinal study was designed to ascertain whether the relatively lower progesterone levels observed in previous cross-sectional studies of women in nonindustrialized populations also characterize conception, implantation, and gestation.
Methods
Population and Samples. Data collection was conducted within the framework of Project REPA (Reproduction and Ecology in Provincía Aroma), a multidisciplinary longitudinal study of reproductive functioning and health among rural Aymara families indigenous to the Bolivian altiplano. Preliminary work began in 1989, followed by >2 years of continuous fieldwork from 1995 to 1997. All study protocols were approved by the Institutional Review Board of the University of California, Riverside. Volunteers, recruited during 12 months beginning in November 1995, represented >80% of the eligible women (aged 19-40 years, currently in stable sexual unions, and not using contraception) in 30 communities scattered over 200 km2 situated about midway between La Paz and Oruro. Of 316 adult female participants, 125 were pregnant and/or lactating and noncycling throughout the study's duration, 98 were lactating at the time of the first observed menstrual segment, and 93 were menstruating/not breastfeeding at recruitment. A sample of women from Chicago (n = 29) attempting to conceive and having no known fertility problems provided comparative data; recruitment details and sample characteristics are published elsewhere (15).
Measurements. Adapting Wood's suggestion (16) and work by Wilcox et al. (17), we implemented a protocol that monitored levels of reproductive steroids from before conceiving through to delivery. Throughout participation, menstruating women (n = 191) were visited every other day by a bilingual (Aymara-Spanish) female member of the research team to record menstrual status and collect a 5-ml saliva sample according to a previously established procedure (18). Beginning at 24-25 days after cycle initiation (the first day of menstrual bleeding), a urine sample was collected to detect human chorionic gonadotropin (hCG), evidence of conception and implantation, by using a commercially available pregnancy test (QuPID, StanBio, Boerne, TX, sensitive at 25 milliunits/ml hCG). Urine collection continued every other day until the next menses or, if the hCG test was positive, until evidence of a fetal loss (two sequential negative tests) or the sixth month of gestation.
Of 853 menstrual cycle initiations (one to eight per woman), 47 verified conceptions (two sequential positive human chorionic gonadotropin tests over 3 days) were detected of which 23 were observed to term (13 conceptions were lost to follow-up, principally caused by waning participant interest; 1 was medically aborted; 10 ended in fetal loss). Samples from conceptions to full-term births were assayed for progesterone (n = 19; samples for four women were lost to laboratory handling/storage failure) at Northwestern University (Chicago) following previously published methods (15). For 10 of these 19 conceptions, samples collected during ovulatory cycles before the conception cycle were also available and similarly assayed.
Of 29 women from Chicago attempting to conceive, 18 reported a conception and provided daily saliva samples, assayed for progesterone in the same laboratory according to identical protocols, for up to 26-43 days of the conception cycle. Ten of these conceivers and 10 nonconceiving women also provided samples, assayed similarly, from ovulatory cycles. Because follow-up did not continue beyond the woman's report of a conception, the rate of fetal loss in this sample is unknown.
Analyses. Because the luteal (postovulatory) phase is typically 12-14 days long and much less variable than the follicular (preovulatory) phase [ovulation in “normal” cycles may occur, with varying probability, at any time from 11 to 21 days after cycle initiation (19)], completed cycles were aligned on the first day of menstrual bleeding of the subsequent cycle and reverse-numbered (i.e., final cycle day = -1). To summarize the changing level of progesterone over the course of an ovarian cycle, indices (Table 1) were defined as (∫ of P from x to y)/(y - x), where x to y is any span of days and P at any time is defined by linear interpolation of the observed progesterone data (10). Three progesterone indices suitable for analyzing completed cycles are mean-follicular (mF), mean-luteal, and mean-peak-luteal (mPL).
Table 1. Progesterone indices in ovulatory cycles.
| Index | x | y | Sample‡ | n | Median | Mean | SEM |
|---|---|---|---|---|---|---|---|
| mF* | First observed day + 2 | -15.5 | Bolivia rural: all | 118 | 74 | 77 | 3.3 |
| Bol rural: conceiving | 10 | 74 | 72 | 7.5 | |||
| Chicago: all | 20 | 92 | 100 | 10.1 | |||
| Chicago: conceiving | 10 | 122 | 108 | 14.6 | |||
| Mean luteal† | -0.5 | -14.5 | Bolivia rural: all | 118 | 152 | 163 | 5.0 |
| Bol rural: conceiving | 10 | 143 | 152 | 11.6 | |||
| Chicago: all | 20 | 268 | 242 | 21.5 | |||
| Chicago: conceiving | 10 | 311 | 264 | 31.6 | |||
| mPL† | Day of peak -2.5 | Day of peak +2.5 | Bolivia rural: all | 118 | 222 | 237 | 7.5 |
| Bol rural: conceiving | 10 | 217 | 209 | 17.7 | |||
| Chicago: all | 20 | 355 | 336 | 29.1 | |||
| Chicago: conceiving | 10 | 388 | 363 | 42.6 |
Index is defined as (integral of P from x to y)/(y - x); cycles aligned on first day of subsequent cycle; units are pmol/liter.
Bolivia rural vs. Chicago ovulatory cycles, Mann-Whitney test, P < 0.02
Bolivia rural vs. Chicago ovulatory cycles, Mann-Whitney test, P < 0.002
Within population, ovulatory cycles of conceiving vs. nonconceiving women, Mann-Whitney, not significant
Cycles in which a conception occurred cannot be aligned as described above. Instead, we took advantage of the well-established fact that progesterone, having been relatively low and flat during the follicular phase, rises sharply after ovulation. Graphed profiles of the serial progesterone values were inspected for a change in slope, and this point was designated as the putative day of ovulation on which to align cycles. Defined progesterone indices for cycles aligned thusly (Table 2) are mean preovulatory, mean-periovulatory, mean-postovulatory-week-1 (approximating the first half of the luteal phase), mean-postovulatory-week-2 (approximating the early postimplantation period), and mean-postovulatory-week-3. An index for a given cycle was only calculated if data spanned the entire defined duration of that index. One Bolivian conception that had led to a live birth was not included in these analyses because it had been preceded by a conception and fetal loss. In this case, neither onset of the follicular phase nor a clear change in the slope of progesterone could be reasonably determined. To compare progesterone levels in conception and ovulatory cycles of the same woman, the latter were also aligned and indices were determined in the same manner as had been done for the conception cycles.
Table 2. Progesterone indices in conceiving women.
| Index | x | y | Sample | Cycle type | n | Median | Mean | SEM |
|---|---|---|---|---|---|---|---|---|
| Mean-preovulatory* | 2.5 | Ovulation day -1.5 | Bolivia rural | Ovulatory∥ | 10 | 70 | 74 | 7.8 |
| Conception | 15 | 88 | 70 | 11.1 | ||||
| Chicago | Ovulatory∥ | 10 | 115 | 102 | 14.7 | |||
| Conception | 14 | 72 | 90 | 14.2 | ||||
| Mean-periovulatory† | Ovulation day -1 | Ovulation day +2 | Bolivia rural | Ovulatory∥ | 10 | 69 | 79 | 9.2 |
| Conception | 17 | 72 | 79 | 8.7 | ||||
| Chicago | Ovulatory∥ | 10 | 133 | 128 | 19.2 | |||
| Conception | 17 | 120 | 125 | 15.0 | ||||
| Mean-postovulatory 1‡ | Ovulation day +0.5 | Ovulation day +7.5 | Bolivia rural | Ovulatory∥ | 10 | 131 | 140 | 13.1 |
| Conception | 17 | 140 | 146 | 11.9 | ||||
| Chicago | Ovulatory∥ | 10 | 287 | 261 | 33.4 | |||
| Conception | 18 | 283 | 286 | 28.8 | ||||
| Mean-postovulatory2§ | Ovulation day +7.5 | Ovulation day +14.5 | Bolivia rural | Conception | 17 | 205 | 226 | 19.5 |
| Chicago | Conception | 15 | 499 | 546 | 52.1 | |||
| Mean-postovulatory3¶ | Ovulation day +14.5 | Ovulation day +21.5 | Bolivia rural | Conception | 17 | 232 | 250 | 32.2 |
| Chicago | Conception | 7 | 836 | 656 | 113.9 |
Index is defined as (integral of P from x to y)/(y - x); cycles aligned on putative day of ovulation; units are pmol/liter.
Bolivia rural vs. Chicago conception cycles, F = 0.835, not significant
Bolivia rural vs. Chicago conception cycles, F = 4.27, P = 0.023
Bolivia rural vs. Chicago conception cycles, F = 10.92, P < 0.001
Bolivia rural vs. Chicago conception cycles, F = 18.39, P < 0.001
Bolivia rural vs. Chicago conception cycles, F = 10.41, P < 0.001
Within population, conception cycles vs. prior ovulatory cycles, paired t tests, not significant
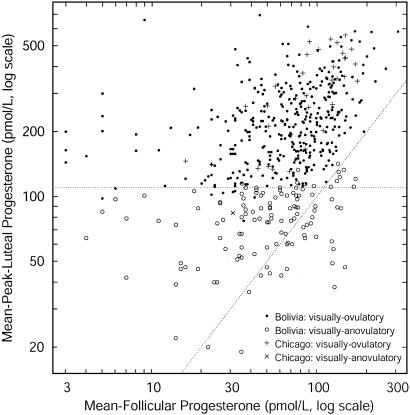
Ascribing Ovulation. Although a luteal rise in progesterone is characteristic of normal ovulation, there is no definitive threshold of salivary progesterone below which ovulation can be said not to have occurred. In a previous study of urban Bolivian women we developed an algorithm to distinguish ovulatory from nonovulatory cycles and ascribed ovulation to any cycle having a mPL >110 pmol/liter (10). In the present study, the progesterone profile of each cycle was first visually inspected for an apparent luteal rise relative to the previous follicular phase. If present, the cycle was designated visually ovulatory, and if absent, visually anovulatory. A scatterplot of mPL progesterone against mF progesterone for each cycle distinguishes those cycles that are visually ovulatory from visually anovulatory (Fig. 1). As was the case in the study of urban Bolivian women, a cut-off of 110 pmol/liter discriminates well between visually ovulatory and visually anovulatory cycles, and hence is a reasonable compromise between the probabilities of false positives vs. false negatives. Additional discrimination was achieved by requiring that mPL progesterone > mF progesterone for a cycle to be designated ovulatory. Only those cycles that were visually ovulatory with a mPL progesterone >110 pmol/liter and mPL progesterone > mF progesterone were included in the analyses reported here.
Fig. 1.
Scatter plot of mPL vs. mF progesterone. Visually ovulatory (• and +) and visually anovulatory (○ and ×) cycles are well separated by a mPL threshold of 110 pmol/liter (dashed horizontal line). Above the dashed diagonal line, mPL > mF. Only those cycles that are visually ovulatory and fall above both dashed lines were included in these analyses.
To ensure independence of data points for statistical analyses, samples of nonconception cycles comprised a single data point from each woman (i.e., the mean of progesterone indices for that woman's ovulatory cycles). Only those ovulatory cycles that did not result in a conception followed by fetal loss, nor which followed a fetal loss, were included in these analyses (final sample of cycles meeting all criteria represent 118 women; median number of cycles per woman = 3).
Progesterone indices for conceptions from Bolivia and Chicago samples were compared by univariate ANOVA (spss for Windows, version 10.0); fixed factors were population and a dummy dichotomous variable distinguishing samples assayed before 2000 and those not assayed until late 2000, to control for any variation in sample degradation over this extended period. Because of the large differences in sample size, indices for ovulatory cycles from Bolivia and Chicago were compared by using the Mann-Whitney test. Within each sample of conceiving women, indices from conception and ovulatory cycles were compared with a paired t test.
Results
The average age of the Bolivian women who had been observed through delivery was significantly (Mann-Whitney test, P < 0.01) younger (27.2 ± 1.1 years) than the women from Chicago who had conceived (30.8 ± 0.8 years). However, progesterone [known to be relatively constant during the age range of these women (14)] did not vary with age in either sample nor in analyses of a pooled sample.
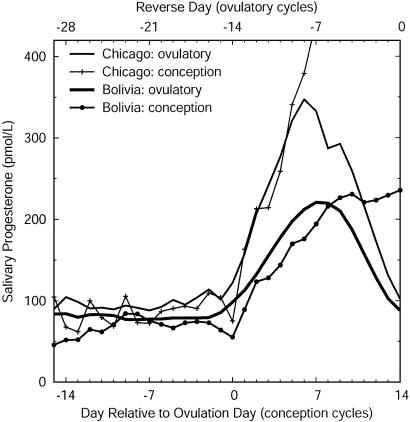
Progesterone was significantly lower in rural Bolivian women than in women from Chicago during both the follicular (mF-Bolivia = 77% mF-Chicago) and luteal (mean-luteal-Bolivia = 67% mean-luteal-Chicago; mPL-Bolivia = 71% mPL-Chicago) phases of ovulatory cycles (Table 1 and Fig. 2). The similarity of these progesterone levels to those previously reported for Bolivia (10) and the United States (14) argues that they are not spurious. For example, mPL progesterone averaged 232 ± 14.0 pmol/liter in a sample of ovulatory cycles from urban “middle-class” Bolivian women (10) compared to 237 ± 7.5 pmol/liter in these rural women.
Fig. 2.
Salivary progesterone profiles in conception and ovulatory nonconception cycles in women from Chicago and rural Bolivia (days 1-28). Ovulatory cycles are aligned on the first day of the subsequent cycle, and days are numbered backward; conception cycles are aligned on the putative day of ovulation and numbered accordingly. Progesterone levels in ovulatory cycles are significantly lower in women from Bolivia than in women from Chicago throughout the ovarian cycle, and also lower during and subsequent to ovulation in conception cycles.
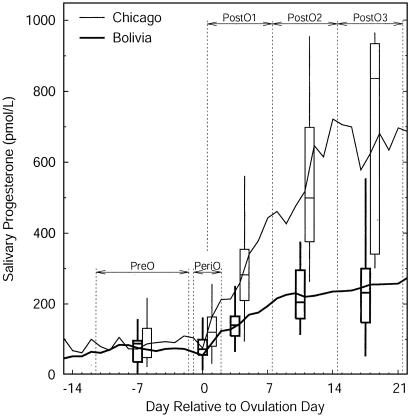
In the conception cycles of Bolivian women, progesterone during the follicular phase (mean-preovulatory) averaged 78% that of women from Chicago, but this difference was not statistically significant, most likely because of the small sample sizes available for calculating this index. These samples were significantly different during the periods before and after ovulation (mean-periovulatory-Bolivia = 63% mean-periovulatory-Chicago) and thereafter (Table 1 and Fig. 2). During the first week after ovulation, progesterone rose at a greater rate in the women from Chicago than in the Bolivian women, and the divergence increased throughout the second and third weeks after ovulation, periods approximately corresponding to the first 2 weeks after implantation of the conceptus (Fig. 3). At the time of implantation (8-10 days after ovulation), progesterone in the Bolivian women was only about half that of the women from Chicago.
Fig. 3.
Salivary progesterone profiles in conception cycles in women from Chicago and rural Bolivia (days 1-35). Box plots display median, quartiles, and range of progesterone indices corresponding to the range of days delimited by vertical dashed lines to the respective left and right of box plot. Progesterone levels do not significantly differ in women from Bolivia and Chicago during the follicular phase but are significantly different during and subsequent to ovulation and through implantation. PostO, postovulatory; PreO, preovulatory; PeriO, periovulatory.
Within each sample of conceiving women, progesterone during the follicular (mean-preovulatory) and early luteal (mean-postovulatory-week-1) phases of conception cycles is not significantly different from those levels observed in the nonconception ovulatory cycles of the same woman as assessed by paired t tests (Table 2). In addition, within the respective Chicago and Bolivia samples, all indices of ovulatory cycles of women who went on to conceive are not different from the ovulatory cycles of those not conceiving (Table 1). These similarities suggest that the relatively low progesterone of nonconception ovulatory cycles and progesterone levels at the time of, and after, conception appear to be the norm for Bolivian women. The sufficiency of these progesterone levels for successful gestation is borne out by the choice for this analysis of only those Bolivian conceptions that went to full term.
Discussion
This study finds that progesterone levels in the ovulatory cycles of rural Bolivian women average ≈70% of those in women from Chicago and that such relatively lower levels also typically accompany conception and implantation. However, it is unknown whether the probability of conception and/or implantation¶ among Bolivian women is comparable to women from Chicago (despite lower progesterone) or is lower.
Indirect evidence is available to support either possibility. In a study of U.S. women (20), conception cycles had higher midluteal progesterone. Similarly, another found a lower probability of conception associated with lower midluteal progesterone (8). Among Japanese women, a single midluteal salivary progesterone sample of <189 pmol/liter served to distinguish luteally insufficient cycles, a condition associated with subfecundity, from sufficient cycles (21). Thus, because of their low progesterone levels, one might expect fecundability to be reduced in these Bolivian women, and perhaps also to be lower in similar nonindustrialized populations and/or in other populations in which progesterone is typically low.
However, there is little, if any, evidence of interpopulational differences in fecundability attributable to natural variation in ovarian functioning (excluding the known effects of female aging). In lieu of direct biological measurements, difficult to obtain, one indirect indicator is interpopulational variation in fertility that is not attributable to differences in other proximate determinants of fertility. Bongaarts (1) found no such evidence, but it has been suggested (5, 6) that his observation may be due to the level of data aggregation (e.g., individual vs. population). Perhaps, but the original question remains open: how and to what extent do energy intake and expenditure affect human reproductive functioning?
The fertility levels of the studied Bolivian women provide additional, albeit indirect, evidence regarding the effect of relatively lower progesterone on fecundability in this sample. Between the ages of 20 and 30 years, these women had, on average, four live births. For each live birth, they breastfed on demand for 1-2 years, with a typical period of postpartum amenorrhea lasting ≈1 year. Adding time for gestation, the live birth rate does not appear to be either particularly low, and hence suggestive of a reduction in fecundability, nor so high as to rule out some reduction. Thus, although it may be the case that fecundability is somewhat lower, progesterone levels ≈70% that of the women from Chicago do not appear to have caused a substantial reduction in fecundability in these Bolivian women.
In either case, this study has demonstrated significant interpopulational variation in progesterone levels that cannot be attributed to the methodological limitations of previous studies. To elucidate the possible causes of this observed variation necessitates distinguishing proximate from ultimate explanations. With respect to biological phenomena, the latter are derived from currently known principles regarding evolutionary processes and, in some sense, answer “why” questions. Proximate explanations deal with mechanisms and “how” questions. In general, proximate explanations are empirically formulated, whereas ultimate explanations are rarely directly observed and are difficult to test. Ideally, ultimate explanations rise or fall on their ability to predict observed phenomena, that is, to explain why the proximate mechanisms work as they do.
Interpopulational comparison of studies of salivary progesterone provides some insight into possible proximate causes of its variation. In samples from several nonindustrialized populations, Bolivia, Nepal, Poland, and the Democratic Republic of the Congo (formerly Zaire), progesterone levels are typically low relative to U.S. women and, when adjusted for age and season of collection, roughly comparable despite numerous environmental and lifestyle differences (9-13). For example, progesterone in Bolivian women at 4,000 m is no lower than that observed in Polish and Congolese women, living within a few hundred meters of sea level. Other dissimilarities among the population samples include average body size, age at menarche, dietary composition, caloric intake, and activity patterns. The one feature clearly shared by these four populations, in contrast to the U.S. samples, is seasonal energetic stress from food shortages and/or increased workloads.
Within populations, midluteal progesterone in ovulatory cycles is positively correlated with stature in Bolivian women (10). In U.S. women, associations of measures of body size and levels of hormones and sex-hormone-binding globulin have also been observed (22, 23). These statistical relationships may exist if hormonal variables, like anthropometric variables, are influenced by energy intake and expenditure. This hypothesis would explain, for example, the observation that increasing height, presumably as a proxy for hormone levels, is a risk factor for breast cancer (24). Adult stature is, itself, a sensitive reflection of energetic stress during growth (25): it has been dubbed a bioassay of environmental conditions (26) and is used as “the biological standard of living” by historical economists (27).
Studies within populations have also found that either low or high normative levels of progesterone can be induced to be relatively lower by a short-term increase in energetic stress. For example, average progesterone was lower in Nepalese, Polish, and Congolese women during seasons of food shortage and/or increased work demands compared to relatively less stressful seasons in the respective populations (11-13), and progesterone was lowered by moderate weight loss in a study of U.S. women (28).
In sum, the empirical findings of several studies suggest that progesterone levels in ovulatory cycles differ substantially among populations, being relatively lower in nonindustrialized populations characterized by seasonal energetic stress; that within Bolivia, and perhaps other populations, progesterone levels in ovulatory cycles are positively correlated with body size (a proxy for preadulthood energetic stress); and that acute energetic stress lowers progesterone levels. The present study finds that successful conception, implantation, and gestation typically occur in Bolivian women at progesterone levels much lower than those of women from Chicago, but we cannot definitely ascertain whether or not fecundability is lower as a result of the lower progesterone.
Why might progesterone function in this way? That is, based on what is currently understood of evolutionary processes, is there an ultimate explanation for the above observations? It is a fundamental principle of evolutionary ecology that all organisms must partition energy to growth, maintenance/survival, and reproduction over a life span (29). The myriad ways in which this goal is addressed are referred to as life history strategies, the varying success of which is dictated by the process of natural selection. Thus, from the perspective of life history theory, one is led to ask, “What sort of reproductive strategy would have the greatest selective advantage in a slow-maturing, long-lived species investing heavily in single successive births?” Developed in response to this question and based on known mammalian physiology, the flexible response model (FRM) (30-32) posits that natural selection favors a human reproductive system that reflects developmental conditions and responds to current ecological circumstances. In particular, these two predictions of the FRM may help to understand interpopulational variation in progesterone.
Several aspects of adult physiology are known to depend on conditions experienced during development. For example, because of exposure during growth and development, Andean high-altitude natives function normally under hypoxic conditions, but adult migrants from sea level rarely do as well even after substantial physiological adjustments to the stress of hypoxia (33, 34). The FRM argues that, in like manner, in a given set of environmental conditions, the reproductive system is under selection to function normally. Because of the high energetic expense of reproduction, energetic stress is a major feature of the environment to which the reproductive system can be expected to be responsive (35). Thus, the FRM predicts that although progesterone levels will be relatively lower in women experiencing chronic energetic stress, their reproductive function will be normal at these lower levels. Although it remains to be determined whether there has been any reduction in fecundability, the lack of evidence for substantial impairment in the studied Bolivian women is consistent with this prediction of the FRM. Support for the hypothesis that dietary patterns during growth influence adult reproductive steroid levels comes from a recently published longitudinal study (36) of U.S. adolescent girls (ages 9-16 years). After an intervention promoting a low-fat diet, luteal progesterone was 52.9% lower than in the control group. It remains to be determined whether their adult reproductive functioning is, as the FRM predicts, normal.
Like some other models (26, 37, 38), the FRM also predicts that an unfavorable change in the conditions necessary for successful reproduction will prompt an organism to suspend reproductive effort in favor of somatic maintenance. The finding that poor urban Bolivian women experiencing seasonal food stress during the winter months had a significantly higher rate of anovulation than either better-off Bolivian women or women from Chicago (10) supports this hypothesis.
Whatever the ultimate explanation for interpopulational variation in progesterone levels, such variation may also have relevance for understanding the etiology of certain cancers. Changes in reproductive patterns that result in more total menstrual cycles (including earlier age at menarche, fewer births, and reduced breastfeeding) have been linked to increased risks of breast, endometrial, and ovarian cancer (39). The relatively elevated reproductive steroid levels (if nonindustrialized populations better approximate a specieswide norm) among U.S. women may also contribute to their very high breast cancer rates. Explanations for these high levels include dietary and activity patterns, but definitive tests of these hypotheses have remained elusive (40).
The present study also reaffirms the conclusion of others (41, 42) that hormonal contraceptive dosages designed for U.S. women and other industrialized countries may be excessively high for women in developing countries, resulting in severe side-effects leading to discontinuation and, potentially, unplanned pregnancy. We have often heard Bolivian women and health workers express concern about negative experiences with hormonal contraceptives. Contrary to arguments that noncompliance is more a matter of education than biology, these data succinctly support the reports of these women that negative sequelae of hormonal contraceptives are more than an imagined problem.
In sum, the present study was designed to ascertain whether the relatively lower progesterone levels observed in previous cross-sectional studies of women in nonindustrialized populations are due to selection bias or also characterize conception, implantation, and gestation in these populations. For the rural Bolivian women studied here, we find that lower progesterone levels typically characterize the entire reproductive process.
Acknowledgments
This work is dedicated to the Andean women whose participation were acts of courage undertaken in the hopes of improving the lives of themselves and their families. We thank E. Caceres for invaluable assistance in the field and the laboratory; several assistants for help with data collection and preparation; G. James, S. Lisman, F. Montemurro (all at Binghamton University, Binghamton, NY), K. Benson (National Science Foundation), and three anonymous reviewers for generously sharing their expertise; and the Instituto Boliviano de Biología de Altura for facilities and logistical support. Financial support for the collection of Bolivian data and analyses was provided by the University of California (Riverside), the State University of New York (Binghamton), and National Science Foundation Grant SBR 9506107 (to V.J.V.). Funding for progesterone assays and collection of samples from Chicago was provided by National Science Foundation Grant SBR 9507320 to G. Bentley and R. Chatterton of Northwestern University.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: mF, mean-follicular; mPL, mean-peak-luteal; FRM, flexible response model.
Footnotes
Detected conceptions in this and similar studies (8) are actually implanted conceptions. Because of progesterone's role in uterine wall development, it is possible that lower progesterone has no effect on conception per se but rather hampers successful implantation. Definitive evaluation of this possibility awaits a technology that allows detection of preimplantation concepti.
References
- 1.Bongaarts, J. (1980) Science 208, 564-569. [DOI] [PubMed] [Google Scholar]
- 2.Pirke, K. M., Schweiger, U., Lemmel, W., Krieg, J. C. & Berger, M. (1985) J. Clin. Endocrinol. Metabol. 60, 1174-1179. [DOI] [PubMed] [Google Scholar]
- 3.Pirke, K. M., Wuttke, W. & Schweiger, U., eds. (1989) The Menstrual Cycle and Its Disorders: Influences of Nutrition, Exercise, and Neurotransmitters (Springer, Berlin).
- 4.Schweiger, U., Laessle, R., Pfister, H., Hoehl, C., Schwingenschloegel, M. M., Schweiger, M. & Pirke, K. M. (1987) Fertil. Steril. 48, 746-751. [DOI] [PubMed] [Google Scholar]
- 5.Wood, J. W. (1994) Ann. N.Y. Acad. Sci. 709, 101-116. [DOI] [PubMed] [Google Scholar]
- 6.Hobcraft, J. N. (1994) Ann. N.Y. Acad. Sci. 709, 408-415. [DOI] [PubMed] [Google Scholar]
- 7.Riad-Fahmy, D. (1984) Front. Oral Physiol. 5, 110-123. [Google Scholar]
- 8.Baird, D. D., Weinberg, C. R., Zhou, H., Kamel, F., McConnaughet, D. R., Kesner, J. S. & Wilcox, A. J. (1999) Fertil. Steril. 71, 40-49. [DOI] [PubMed] [Google Scholar]
- 9.Vitzthum, V. J., Ellison, P. T., Sukalich, S., Caceres, E. & Spielvogel, H. (2000) High Altitude Med. Biol. 1, 39-49. [DOI] [PubMed] [Google Scholar]
- 10.Vitzthum, V. J., Bentley, G. R., Spielvogel, H., Caceres, E., Thornburg, J., Jones, L., Shore, S., Hodges, K. R. & Chatterton, R. T., et al. (2002) Hum. Reprod. 17, 1906-1913. [DOI] [PubMed] [Google Scholar]
- 11.Panter-Brick, C., Lotstein, D. S. & Ellison, P. T. (1993) Hum. Reprod. 8, 684-690. [DOI] [PubMed] [Google Scholar]
- 12.Jasienska, G. & Ellison, P. T. (1998) Proc. R. Soc. London Ser. B 265, 1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison, P. T., Peacock, N. R. & Lager, C. (1989) Am. J. Phys. Anthropol. 78, 519-526. [DOI] [PubMed] [Google Scholar]
- 14.Lipson, S. F. & Ellison, P. T. (1992) J. Biosoc. Sci. 24, 233-244. [DOI] [PubMed] [Google Scholar]
- 15.Lu, Y.-C., Bentley, G. R., Gann, P. H., Hodges, K. R. & Chatterton, R. T. (1999) Fertil. Steril. 71, 863-868. [DOI] [PubMed] [Google Scholar]
- 16.Wood, J. W. (1994) Dynamics of Human Reproduction (Aldine, NY), p. 243.
- 17.Wilcox, A. J., Weinberg, C. R., O'Connor, J. F., Baird, D. D., Schlatterer, J. P., Canfield, R. E., Armstrong, E. G. & Nisula, B. C. (1988) N. Engl. J. Med. 319, 189-194. [DOI] [PubMed] [Google Scholar]
- 18.Vitzthum, V. J., von Dornum, M. & Ellison, P. T. (1993) Am. J. Phys. Anthropol. 92, 539-544. [DOI] [PubMed] [Google Scholar]
- 19.Wood, J. W. (1994) Dynamics of Human Reproduction (Aldine, NY), p. 172.
- 20.Stewart, D. R., Overstreet, J. W., Nakajima, S. T. & Lasley, B. L. (1993) J. Clin. Endocrinol. Metab. 76, 1470-1476. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa, M., Sengoku, K., Tamate, K., Takaoka, Y., Kane, M. & Fottrell, P. F. (2002) Gynecol. Obstet. Invest. 53, 32-37. [DOI] [PubMed] [Google Scholar]
- 22.Dorgon, J. F., Reichman, M. E., Judd, J. T., Brown, C., Longcope, C., Schatzkin, A., Albanes, D., Campbell, W. S., Franz, C., Kahle, L., et al. (1995) Cancer Causes Control 6, 3-8. [DOI] [PubMed] [Google Scholar]
- 23.Kaye, S. A., Folsom, A. R., Soler, J. T., Prineas, R. J. & Potter, J. D. (1991) Int. J. Epidemiol. 20, 151-156. [DOI] [PubMed] [Google Scholar]
- 24.Vatten, L. J. & Kvinnsland, S. (1990) Br. J. Cancer 61, 881-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogin, B. (1999) Patterns of Human Growth (Cambridge Univ. Press, Cambridge, U.K.).
- 26.Ellison, P. T. (1990) Am. Anthropol. 92, 933-952. [Google Scholar]
- 27.Komlos, J. & Baten, J., eds. (1998) The Biological Standard of Living in Comparative Perspectives (Verlag, Stuttgart, Germany).
- 28.Lager, C. & Ellison, P. T. (1990) Am. J. Hum. Biol. 2, 303-312. [DOI] [PubMed] [Google Scholar]
- 29.Pianka, E. R. (1988) Evolutionary Ecology (Harper & Row, New York).
- 30.Vitzthum, V. J. (1990) An Adaptational Model of Ovarian Function, Research Report No. 90-200, Population Studies Center (University of Michigan, Ann Arbor).
- 31.Vitzthum, V. J. (1997) in The Evolving Female: A Life History Perspective, eds. Morbeck, M. E., Galloway, A. & Zihlman, A. (Princeton Univ. Press, Princeton), pp. 242-258.
- 32.Vitzthum, V. J. (2001) in Reproductive Ecology and Human Evolution, ed. Ellison, P. T. (Aldine, New York), pp. 179-202.
- 33.Baker, P. T. & Little, M. A., eds. (1976) Man in the Andes (Dowden, Hutchinson, & Ross, Stroudsburg, PA).
- 34.Beall, C. M., Decker, M. J., Brittenham, G. M., Kushner, I., Gebremedhin, A. & Strohl, K. P. (2002) Proc. Natl. Acad. Sci. USA 99, 17215-17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronson, F. H. (1989) Mammalian Reproductive Biology (Univ. Chicago Press, Chicago)
- 36.Dorgan, J. F., Hunsberger, S. A., McMahon, R. P., Kwiterovich, P. O., Jr., Laver, R. M., Van Horn, L., Lasser, N. L., Stevens, V. J., Friedman, L. A., Yanovski, J. A., et al. (2003) J. Natl. Cancer Inst. 95, 132-141. [DOI] [PubMed] [Google Scholar]
- 37.Wasser, S. K. & Barash, D. P. (1983) Q. Rev. Biol. 58, 513-538. [DOI] [PubMed] [Google Scholar]
- 38.Peacock, N. (1990) in Primate Life History and Evolution, ed. DeRousseau, C. J. (Wiley, New York), pp. 195-220.
- 39.Eaton, S. B., Pike, M. C., Short, R. V., Lee, N. C., Trussell, J., Hatcher, R. A., Wood, J. W., Worthman, C. M., Jones, N. G., Konner, M. J., et al. (1994) Q. Rev. Biol. 69, 353-367. [DOI] [PubMed] [Google Scholar]
- 40.Jamison, D. T., Mosley, W. H., Measham, A. R. & Bobadilla, J. L. (1993) Disease Control Priorities in Developing Countries (Oxford Univ. Press, Oxford).
- 41.Bassol, S., Garza-Flores, J., Cravioto, M. C., Diaz-Sanchez, V., Fotherby, K., Lichtenberg, R. & Perez-Palacids, G., et al. (1984) Fert. Steril. 42, 216-222. [PubMed] [Google Scholar]
- 42.Fotherby, K., Koetsawang, S. & Mathrubutham, M. (1980) Contraception 22, 527-536. [DOI] [PubMed] [Google Scholar]