Abstract
Monoclonal antibodies (mAbs) and fusion proteins directed towards cell surface targets make an important contribution to the treatment of disease. The purpose of this review was to correlate the clinical and preclinical data on the 15 currently approved mAbs and fusion proteins targeted to the cell surface. The principal sources used to gather data were: the peer reviewed Literature; European Medicines Agency ‘Scientific Discussions’; and the US Food and Drug Administration ‘Pharmacology/Toxicology Reviews’ and package inserts (United States Prescribing Information). Data on the 15 approved biopharmaceuticals were included: abatacept; abciximab; alefacept; alemtuzumab; basiliximab; cetuximab; daclizumab; efalizumab; ipilimumab; muromonab; natalizumab; panitumumab; rituximab; tocilizumab; and trastuzumab. For statistical analysis of concordance, data from these 15 were combined with data on the approved mAbs and fusion proteins directed towards soluble targets. Good concordance with human pharmacodynamics was found for mice receiving surrogates or non-human primates (NHPs) receiving the human pharmaceutical. In contrast, there was poor concordance for human pharmacodynamics in genetically deficient mice and for human adverse effects in all three test systems. No evidence that NHPs have superior predictive value was found.
Keywords: monoclonal antibody, biopharmaceutical, non-clinical safety, clinical safety, adverse effects, toxicology, surrogate, rodent, cynomolgus monkey, genetically deficient mice
Introduction
Monoclonal antibodies (mAb) and soluble receptors (fusion proteins) make an important contribution to the treatment of a variety of diseases. Over 30 mAb or fusion proteins have been approved for clinical use and more than 200 more are in various stages of clinical development (Dimitrov and Marks, 2009). Because species cross-reactivity is usually highly restricted to humans and non-human primates (NHPs), mAbs directed towards human targets are usually pharmacologically inactive in rodents (Cavagnaro, 2008). As a consequence, preclinical studies with many of the mAbs or mAb-like molecules need to be conducted in NHPs (ICH, 2011). However, there are some notable exceptions, for example abatacept (Platt et al., 2010), where the human therapeutic agent is also active in rodents. This is more often the case with fusion proteins than with mAbs (refer also to Martin and Bugelski, 2012).
For mAbs and fusion proteins directed towards cellular targets, exaggerated pharmacology is often the most important factor in determining their adverse effect profile (Cavagnaro, 2008). This can be as a direct extension of the pharmacologic effect, for example infections seen with abatacept due to suppression of T-cell function (Khraishi et al., 2010), as an indirect consequence of the pharmacologic effect, for example reactivation of cytomegalovirus (CMV) seen with alemtuzumab due to long-term depletion of T-cells (Laurenti et al., 2004), or as a consequence of expression of the target in undesired sites, for example cardiotoxicity of trastuzumab due to expression of Her2 by normal cardiac myocytes (Keefe, 2002). True ‘off-target’ toxicity for mAbs and fusion proteins directed towards cellular targets, for example glomerulonephritis associated with anti-drug antibodies in cynomolgus monkeys that received natalizumab (FDA, 2005b), are rare and are generally not clinically relevant.
An alternative to studying the human biopharmaceutical in NHP is to study the effects of a homologous (surrogate) mAb in rodents (Bussiere et al., 2009). As will be described in this review, there are data on surrogates for essentially all the approved biopharmaceuticals. Other alternatives are genetically deficient rodents or transgenic rodents that express the human target. Prior to the development of a human mAb for therapeutic use, there are often published data on surrogate mAbs that are directed towards the homologous target in rodents. Although rarely conducted in compliance with good laboratory practice regulations and usually intended to address the pathophysiologic role of the target in disease, studies with surrogate mAb often provide insights into safety aspects that may not be available from studies in NHP. In some cases, surrogate mAbs have been specifically developed to supplement the NHP safety data or to address specific safety concerns (Treacy, 2000; Clarke et al., 2004). Finally, in some cases, there are published data from humans or genetically modified rodents where the target of the mAb is inactive or knocked out. Because they represent an extreme case or because of undefined compensatory changes in phenotype, for example utilization of cluster of differentiation (CD)8-independent T-cell receptors (TCRs) in CD8 deficient mice (Angelov et al., 2009), genetic deficiencies may over- or under-predict the effects of a mAb. Thus, although they should be viewed with some caution, findings in genetically deficient humans or rodents may provide additional insights into the potential safety of mAbs.
The purpose of this review was to collate and correlate the clinical and preclinical data on the currently approved mAbs and fusion proteins targeted to cell surface antigens (mAb and fusion proteins that target soluble antigens will be covered in a companion manuscript (Martin and Bugelski, 2012). The review will cover mAbs approved for marketing in the European Union (EU) or the United States to determine the concordance of preclinical and clinical findings. Some mAbs that were marketed but then subsequently withdrawn are also included.
In this review, particular emphasis has been placed on the value of the rodent studies using surrogate molecules and use of genetically modified mice in the overall risk assessment for human safety. Three principal sources were used to gather the data: the peer reviewed literature; European Medicines Agency (EMA) ‘Scientific Discussions’; and the US Food and Drug Administration (FDA) ‘Pharmacology/Toxicology Reviews’. Other secondary sources of information include the United States Prescribing Information (USPI) and the EMA Summary of Product Characteristics (SPC). Because of the scope of the project, we have not attempted to provide an exhaustive catalogue of every adverse effect observed either clinically or preclinically. Similarly, no attempt was made to fully review the preclinical data on each mAb target (in some cases, there are hundreds of published papers on the effects of mAb directed towards the rodent homologue, for example anti-CD25). Rather, the review focuses on the most commonly observed adverse effects or those that were of critical importance in determining the risk-benefit assessment for each mAb included in the analysis.
Each individual section will describe the characteristics and function of the target, the findings from genetically deficient humans and rodents (if known), the characteristics of the marketed mAb or fusion protein, the adverse effects observed in humans, the adverse effects observed in NHP, the adverse effects observed in rodents (where available, data from other species are included) and a conclusion statement on the concordance of the preclinical and clinical findings for that target.
The individual sections will be followed by a summary section that will evaluate the overall concordance of preclinical and clinical pharmacology and adverse effects.
mAbs directed towards cellular targets
CD3
Structure and function
CD3 is a member of the TCR complex. CD3 is composed of three subunits. Assembly, expression and signal transduction by the pre-T cell receptor and TCR complex are critical for normal thymocyte development. Invariant CD3 polypeptide chains ensure correct intracellular assembly, surface expression and signal transduction by the TCR complex (Dave, 2009; Portoles and Rojo, 2009).
Genetic deficiency
CD3 chains play critical roles in thymocyte development. In humans, homozygous mutations in CD3δ and CD3ε genes lead to a complete block in T-cell development and to severe combined immunodeficiency (SCID). The defect in T-cell development occurs at the transition between CD4/CD8 ‘double-negative’ and ‘double-positive’ thymocytes. These results contrast with the partial T-cell immunodeficiency caused by a deficiency in CD3γ (Fischer et al., 2005).
In mice deficient in CD3γ, CD3ε or CD3ζ, the transition between CD4/CD8 ‘double-negative’ and ‘double-positive’ thymocytes is severely impaired. In contrast, CD3δ deficiency impairs maturation at the CD4(+)CD8(+) double-positive (DP) stage (Pan et al., 2006).
Marketed human therapeutic agents
Muromonab-CD3 (Orthoclone®, OKT3) is a murine IgG2a used to treat acute allograft rejection (Cosimi et al., 1981). A variety of humanized and human variants of anti-CD3 have also been tested clinically (Silva et al., 2009), and anti-CD3 mAbs are being explored in autoimmune disease (Chatenoud and Bluestone, 2007).
Human adverse effects
The most common and important adverse effect associated with muromonab-CD3 is cytokine release syndrome, a condition in which activated T-cells release their cytokines resulting in a systemic inflammatory response (hypotension, pyrexia and rigors) similar to that found in severe infection. Muromonab-induced toxicity is dose-dependent and is mediated not only by cytokine release but also by activation of complement and neutrophils (Parlevliet et al., 1995). Other toxicities of muromonab therapy include aseptic meningitis, seizures, acute respiratory distress, reactivation of CMV infection and post-transplant lymphoproliferative disorders (Thistlethwaite et al., 1988; Hibberd et al., 1992; Macdonald et al., 1993; Wasson et al., 2006; Orthoclone OKT3 USPI, 2011).
Pharmacology/toxicity of human therapeutic agent in animals
Muromonab-CD3 does not bind monkey or rodent CD3 and, thus, is not pharmacologically active in these species. Surrogate anti-CD3 mAbs bind CD3+ T-cells in rhesus monkeys, but are depleting and suppress allograft rejection only when administered as immunotoxin conjugates (Steinhoff et al., 1990; Thomas et al., 1997).
Pharmacology/toxicity of surrogate molecules
Anti-murine CD3 mAbs have been shown to deplete CD4-CD8 double-positive T-cells in the thymus of adult mice (Rueff-Juy et al., 1991), and to have beneficial effects in a model of systemic lupus erythematosus (SLE), reducing lymphadenopathy and mortality but having no effect on anti-DNA or IgG titres (Henrickson et al., 1994). Anti-murine CD3 mAb can also suppress graft-versus-host disease (Blazar et al., 1994). Neonatal mice are relatively resistant to anti-murine CD3 (Rueff-Juy et al., 1991). Anti-murine CD3 mAb can induce cytokine release syndrome in mice characterized by hypothermia, hypoglycaemia, acute renal tubular necrosis and fatty infiltration of the liver (Alegre et al., 1991).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD3 is immunosuppressive in mice and humans, but genetic deficiency does not mimic the adverse effects of anti-CD3 mAb. Similarly, anti-CD3 mAbs are immunosuppressive in mice and humans. NHPs appear to be resistant to the depleting effects of anti-CD3 mAb, but this may only reflect the specific mAb tested. Although anti-CD3 mAbs cause cytokine release in mice, mice do not fully mimic the adverse effect profile of anti-CD3 mAb seen in humans. There are insufficient data to draw conclusions regarding concordance of adverse effects in NHPs.
CD11a (LFA1)
Structure and function
CD11a is a subunit of lymphocyte function associated antigen-1 (LFA1), an αLβ3 integrin involved in cell–cell interactions important to immune responses and inflammation (Clarke et al., 2004; Simmons, 2005). CD11a is expressed on activated T-cells and platelets. The principal ligand for CD11a is intercellular adhesion molecule-1 (ICAM-1, CD54). Blocking the interaction of CD11a and ICAM-1 inhibits various T-cell processes including activation, adhesion to endothelial cells and migration (Cather et al., 2003).
Genetic deficiency
Deficiency of LFA1 in humans has not been described. In mice, deficiency of LFA1 was associated with leukocytosis and decreased emigration into a subcutaneous air pouch in response to TNF-α (Ding et al., 1999). LFA1-deficient mice did not show increased spontaneous infections (Ding et al., 1999) but developed aggravated carditis after exposure to Borrelia burgdorferi (Guerau-de-Arellano et al., 2005).
Marketed human therapeutic agent
Efalizumab (Raptiva®) is a humanized IgG1. Efalizumab was approved for treatment of moderate to severe plaque psoriasis (0.7–1 mg·kg−1·week−1) but was withdrawn from the market because of progressive multifocal leukoencephalopathy (PML) (EMA, 2004b).
Human adverse effects
The most common adverse reactions associated with efalizumab were a first-dose reaction complex that included headache, chills, fever, nausea and myalgia. The most serious adverse reactions were serious infections (0.4%, bacterial sepsis, viral meningitis, invasive fungal disease and other opportunistic infections1), malignancies (1.8/100 patient-years; mostly non-melanoma skin cancers, 0.7%), thrombocytopenia (0.3%), haemolytic anaemia, arthritis events and psoriasis worsening and variants (0.7%) (Hernandez Garcia, 2008; Prignano et al., 2008; Ashraf-Benson et al., 2009). Post-marketing, efalizumab was associated with PML, a demyelinating disease of the brain attributed to infection with human polyoma JC virus (JCV) (Pugashetti and Koo, 2009; Berger, 2010). Although very rare [three reports per 46 000 exposed patients (Kothary et al., 2011)], PML and other serious infections lead to withdrawal of efalizumab from the market in 2009. In a small study of patients with severe chronic plaque psoriasis and concomitant hepatitis C virus infection, efalizumab did not increase viral replication or progression of liver disease over an 8–20 month follow-up period (Gisondi et al., 2009).
Pharmacology/toxicity of the human therapeutic agent in animals
Efalizumab binds only to human and chimpanzee CD11a. Efalizumab was tested in non-terminal tolerability studies of up to 6 months duration in chimpanzees (EMA, 2004b). Single-dose and 14 day studies revealed no adverse effects. In the 6 month study, efalizumab was associated with paracortical atrophy and decreased CD4+ lymphocytes in biopsied lymph nodes, decreased CD4/CD8 cell ratio in peripheral blood and partial inhibition of the humoral immune response to immunization with tetanus toxoid. One animal died of an intestinal infection, possibly of viral origin.
Pharmacology/toxicity of surrogate molecules
Treatment of mice with a mAb to murine LFA-1 beginning 1 day before herpes simplex virus-1 corneal infection resulted in a delay in the onset of stromal inflammation and exacerbated periocular skin disease, but did not render mice susceptible to encephalitis (Dennis et al., 1995). Anti-LFA-1 promoted significant long-term survival of both cardiac and islet allografts (Grazia et al., 2005). A chimeric mouse/rat anti-mouse CD11a surrogate mAb (muM17) that was specifically designed to mimic efalizumab inhibited murine mixed lymphocyte reactions in vitro and delayed-type hypersensitivity reactions in vivo (Clarke et al., 2004). muM17 has also been reported to exacerbate experimental autoimmune encephalomyelitis (EAE) when administered 4 days after transfer of sensitized lymphocytes to naive SJL mice (Cannella et al., 1993).
muM17 was also tested in formal toxicology studies at doses up to 30 mg·kg−1·week−1 (EMA, 2004b). In a single-dose study, the only observation was leukocytosis. In multi-dose studies of up to 26 weeks duration in CD1 and TSG p53 wild type mice, decreased platelets, increased white blood cells (WBCs), lymphocytes, eosinophils, neutrophils and monocytes in peripheral blood, increased spleen weight, histological changes in the spleen and decreased cellularity in lymph nodes were observed. Partial inhibition of the IgM response (but not the subsequent IgG response) to sheep red blood cells (RBCs) decreased natural killer (NK) cell activity and increased splenic lymphocytes (CD4+ cells in males, and CD8+ in females) were also observed. In reproductive toxicity studies, muM17 had no effect on embryo-fetal development, but in a pre–post-natal study, muM17 transiently decreased the antibody-forming cell response to sheep RBCs in the F1 mice. There was no effect on reproductive parameters in the F0 or F1 mice and on the development of the F1 mice, and no visible effects in the F2 mice. Spleen weights were increased in the F1 mice, and there was an increase in the CD4/CD8 ratio in the F1 mice.
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD11a is not overtly immunosuppressive in mice and does not mimic the adverse effects of anti-CD11a mAb in humans. Anti-CD11a mAbs are immunosuppressive in mice, NHP and humans. However, neither NHP treated with efalizumab nor mice treated with surrogate anti-LFA1 mimic the adverse effect profile of anti-CD11a mAb seen in humans.
CD20
Structure and function
CD20 is a non-glycosylated phosphoprotein expressed on the surface of almost all normal and malignant B-cells (Cragg et al., 2005). Although it has no known natural ligand, CD20 is resident in lipid raft domains of the plasma membrane where it probably functions as a calcium channel following ligation of the B-cell receptor for antigen (Uchida et al., 2004). Anti-CD20 mAbs mediate depletion of B-cells by several mechanisms: antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), induction of apoptosis and sensitization to chemotherapy (Johnson and Glennie, 2003).
Genetic deficiency
In a patient with CD20 deficiency due to a homozygous mutation in a splice junction of the CD20 gene antigen-independent B-cells developed normally, but antibody formation, particularly after vaccination with T-cell-independent antigens, was strongly impaired (Kuijpers et al., 2010).
In CD20-deficient mice, expression of IgM by immature and mature B-cells was only ∼30% lower compared to B-cells from wild-type littermates, and T-cell-dependent antibody responses and affinity maturation were normal (Uchida et al., 2004). In contrast, antibody responses to T-cell-independent polysaccharide antigens were severely impaired in CD20-deficient mice (Kuijpers et al., 2010).
Marketed human therapeutic agent
Rituximab (Rituxan®, MabThera®) is a human–murine chimeric mAb approved for use in non-Hodgkin's lymphoma (375 mg·m−2), chronic lymphocytic leukaemia (500 mg·m−2·month−1), rheumatoid arthritis (1000 mg per patient every 16–24 weeks), Wegener's granulomatosis and microscopic polyangiitis (375 mg·m−2·week−1 for 1 month) (Coiffier, 2007; Fleischmann, 2009; Rituxan USPI, 2011).
Human adverse effects
The principal adverse effects of rituximab in humans are acute allergic and infusion reactions occurring in ≥25% of oncology patients (Kimby, 2005; Fleischmann, 2009). Boxed warnings for rituxumab include fatal infusion reactions, tumour lysis syndrome, severe mucocutaneous reactions and PML (Rituxan USPI 2011). Anti-CD20 mAbs are also associated with late-onset neutropenia, slow recovery of normal B-cells, infections, reactivation of hepatitis, intestinal perforation and interstitial pneumonia (Ram et al., 2009; Peerzada et al., 2010). Rituximab administration during pregnancy can result in a reversible depletion of B-cells in the neonates (Vinet et al., 2009).
Pharmacology/toxicity of the human therapeutic agent in animals
Rituximab binds to human and NHP CD20 but does not bind to rodent CD20. Rituximab has anti-tumour activity against human CD20 transgenic murine B-cell lymphoma cells (Mineo et al., 2008). Rituximab depletes B-cells in cynomolgus monkeys (Reff et al., 1994; EMA, 2005c; Goldenberg et al., 2010) and African green monkeys (Gaufin et al., 2009). Rituximab also blocked seroconversion in three of six cynomolgus monkeys inoculated with SIVmac 239 and these monkeys, but not the other three, progressed to AIDS by 16 weeks post-infection (Miller et al., 2007).
Formal toxicology studies were conducted in cynomolgus monkeys with rituximab (FDA, 1997). In single-dose toxicity studies in cynomolgus monkeys at doses up to 100 mg·kg−1 and multiple-dose toxicity studies at doses up to 20 mg·kg−1·week−1 (276 mg·m−2) B-cells were depleted, and effacement of the splenic pulp and lymphoid atrophy were observed. There were no other signs of toxicity. The B-cells slowly recovered following cessation of treatment.
In embryo-fetal development and pre- and post-natal developmental toxicity studies (Vaidyanathan et al., 2011), female cynomolgus monkeys were administered rituximab from gestation day (GD) 20 to 50 for the embryo-fetal development study and GD 20 to post-partum day 28 for the pre- and post-natal study. Placental transfer of rituximab was demonstrated at GD 100 and fetuses demonstrated B-cell depletion in lymphoid tissues at maternal exposures similar to human therapeutic exposures (Rituxan USPI, 2011). There was no significant effect on T-cell-dependent antibody responses following vaccination or antigenic challenge, and recovery of B-cells in the infants was demonstrated.
Pharmacology/toxicity of surrogate molecules
In human CD20 transgenic mice, anti-human CD20 mAbs have been shown to decrease arthritis (Huang et al., 2010).
Human and murine CD20 have similar patterns of distribution and function (Uchida et al., 2004). Anti-murine CD20 mAbs have been shown to deplete B-cells and to have beneficial activity in a murine model of systemic lupus erythematosus (Bekar et al., 2010) and proteoglycan-induced arthritis (Hamel et al., 2008). Anti-murine CD20 mAbs have also been shown to have anti-tumour activity in a syngeneic murine B-cell lymphoma (Gadri et al., 2009) and to augment anti-tumour immune responses in non-hematopoietic tumours, including tumours that do not express CD20 (Kim et al., 2008).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD20 is immunosuppressive in mice and humans, particularly for T-cell-independent antigens, but genetic deficiency does not mimic the adverse effects of anti-CD20 mAb. Anti-CD20 mAb produce the expected pharmacological response, that is, depletion of B-cells, in humans, NHP and mice, and all species show selective immunosuppression. However, neither mice dosed with anti-mouse CD20 mAbs nor NHP dosed with anti-human CD20 mAbs mimic the adverse effect profile of anti-human CD20 mAbs seen in humans.
CD25
Structure and function
CD25 is the alpha subunit of the IL-2 receptor. CD25 is a transmembrane glycoprotein that activates signal transduction pathways involved in the generation of proliferative and anti-apoptotic signals in T-cells (Boggi et al., 2004). CD25 is expressed on activated effector T-cells and is also constitutively expressed on regulatory T-cells (TREG).
Genetic deficiency
CD25 deficiency in humans is associated with immunodysregulation, polyendocrinopathy enteropathy X-linked (IPEX)-like syndrome and defective IL-10 expression from CD4 lymphocytes (Caudy et al., 2007). CD25-deficient mice develop a lethal autoimmune syndrome believed to be due to the inability to generate TREG cells (Furtado et al., 2002) and unregulated expansion of memory CD8+ T-cells (Sharma et al., 2007).
Marketed human therapeutics agents
Basiliximab (Simulect®) is a murine/human chimeric IgG1, and daclizumab (Zenapax®) is a humanized IgG1 mAb that binds CD25. Basiliximab is approved for the prevention of organ allograft rejection (20 mg per adult patient once before and once after transplant surgery) (EMA, 2005d; Simulect USPI, 2005; McKeage and McCormack, 2010). Daclizumab was approved for the prevention of organ allograft rejection (Pescovitz, 2005) but is no longer authorized in the EU (EMA, 2005e).
Human adverse effects
The most common adverse effects of basiliximab (≥10% of treated patients, Simulect USPI, 2005) are constipation, infections, pain, nausea, peripheral oedema, hypertension, anaemia, headache, hyperkalemia, hypercholesterolemia, increase in serum creatinine and hypophosphataemia. There were no increased malignancies or post-transplant lymphoproliferative disorders after treatment with basiliximab at either 12 months or 5 years post-transplantation. Rare cases of hypersensitivity reactions to basiliximab have been reported but not cytokine release syndrome. Compared to other immunosuppressive regimens, anti-CD25 mAb caused no greater increase in the incidence of infection, including CMV infection (Chapman and Keating, 2003; Boggi et al., 2004; Kapic et al., 2004).
Pharmacology/toxicity of the human therapeutic agent in animals
Basiliximab is pharmacologically active in rhesus and cynomolgus monkeys and showed beneficial effects in models of lung and islet allografts (Hausen et al., 2000; Wijkstrom et al., 2004). The toxicity of basiliximab was reviewed in the EMA Scientific Discussion (EMA, 2005d). In a toxicity study of 39 weeks duration in rhesus monkeys, there were no adverse effects at doses up to 24 mg·kg−1·week−1 (1000 times the clinical exposure) (Simulect SPC, 2011). In a study in pregnant cynomolgus monkeys, at exposures up to 13-fold greater than in humans (doses up to 5 mg·kg−1 twice per week) no maternal toxicity, embryotoxicity or teratogenicity was observed (EMA, 2005c; Simulect USPI, 2005).
Daclizumab is pharmacologically active in cynomolgus and rhesus monkeys and showed beneficial effects in models of cardiac and renal allografts, autoimmune uveoretinitis and collagen-induced arthritis (Guex-Crosier et al., 1997; Brok et al., 2001; Montgomery et al., 2002). The toxicity of daclizumab was reviewed in the EMA Scientific Discussion (EMA, 2005e). In a 28 day toxicology study, where cynomolgus monkeys received doses of up to 15 mg·kg−1·day−1 of daclizumab, two animals ‘died from pulmonary inflammation, possibly due to pneumonia’. There were decreased platelet and leukocyte counts and an increase in serum glucose in males. In general, ‘daclizumab displayed a toxicological profile similar to that, which might be expected of an immunoglobulin’(EMA, 2005e). An embryo-fetal development study showed an increase in prenatal loss (doses not specified, Zenapax USPI, 2005).
Pharmacology/toxicity of surrogate molecules
Neither basiliximab nor daclizumab is pharmacologically active in rodents. There is an extensive literature on the effects of anti-murine CD25 mAb in mice. Anti-CD25 mAbs are beneficial in murine models of collagen-induced arthritis (Banerjee et al., 1988), allergic conjunctivitis (Fukushima et al., 2007) and silica-induced pulmonary fibrosis (Liu et al., 2010). In contrast, depletion of CD25+ TREG cells with anti-CD25 mAb leads to the expansion of memory CD8+ T-cells (Murakami et al., 2002), impaired induction of tolerance to ovalbumin in pulmonary challenge model (Boudousquie et al., 2009) and blocked spontaneous tolerance in hepatic transplants (Li et al., 2006), exacerbated models of systemic lupus erythematosus (Hsu et al., 2006) and autoimmune haemolytic anaemia (Mqadmi et al., 2005).
In models of infection, anti-CD25 mAb had no effect on infections with pseudomonas (Carrigan et al., 2009) and increased clearance of herpes simplex virus in neonates (Fernandez et al., 2008), but increased susceptibility to toxoplasma (Couper et al., 2009), helminths (D'Elia et al., 2009) and trypanosomes (Mariano et al., 2008). In models of neoplasia, anti-CD25 mAb suppressed tumour growth in murine models of glioma (El Andaloussi et al., 2006), neuroblastoma (Johnson et al., 2007), melanoma (Jones et al., 2002) and osteosarcoma (Kozawa et al., 2010).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD25 in mice and humans is associated with autoimmune disease. Thus, genetic deficiency does not mimic either the pharmacologic or adverse effects of anti-CD25 mAb. Anti-CD25 mAbs are immunosuppressive in mice, NHP and humans. However, in mice, anti-CD25 can potentiate immune responses by depletion of CD25+ TREG cells. Neither mice nor NHP mimic the adverse effect profile of anti-CD25 mAb seen in humans.
CD49d (very late antigen-4)
Structure and function
CD49d is the α4 subunit of very late antigen-4 (VLA-4). VLA-4 is a member of the family of integrins, cell adhesion molecules that are important in leukocyte trafficking and extravasation by binding any of a number of cell surface ligands, for example vascular cell adhesion molecule-1 (VCAM-1), mucosal addressin cell adhesion molecule-1, fibronectin, or osteopontin at sites of inflammation (Yusuf-Makagiansar et al., 2002; Dedrick et al., 2003; Khan et al., 2003). VLA-4 is also believed to play a role in metastases and haematopoiesis (Holzmann et al., 1998; Imai et al., 2010).
Genetic deficiency
Deficiency of CD49d in humans has not been described. Deficiency of CD49d in mice results in failure of the allantois and chorion to fuse leading to interrupted placentation and cardiac development and embryo lethality (Crofts et al., 2004).
Marketed human therapeutic agent
Natalizumab (Tysabri®) is a humanized IgG4 mAb. Natalizumab is approved as monotherapy for patients with relapsing multiple sclerosis and for treatment of moderately to severely active Crohn's disease in patients who have failed or are intolerant to immunosuppressants and/or tumour necrosis factor inhibitors (300 mg per patient every 4 weeks) (Rudick and Panzara, 2008; Edula and Picco, 2009; Tysabri USPI, 2011).
Human adverse effects
The common adverse events associated with natalizumab were generally mild and included headache, fatigue, urinary tract infections and arthralgias (Rudick and Panzara, 2008). Other adverse effects include hypersensitivity and infusion reactions (Calabresi et al., 2007; Camacho-Halili et al., 2011), infections and liver injury (Bezabeh et al., 2010; Tysabri USPI, 2011).
Natalizumab is also associated with PML. Initially, three cases of PML were described, and natalizumab was withdrawn from the market (Keeley et al., 2005). Subsequently, additional cases of PML have been described (Piehl et al., 2011). The incidence of PML with natalizumab is 0.4/1000 patients treated for up to 24 months and 1.3–1.9/1000 patients treated for 25 to 48 months (Tysabri USPI, 2011). Natalizumab is also associated with asymptomatic reactivation of polyomavirus JCV in some but not in all studies (Chen et al., 2009; Rinaldi et al., 2010), and with reactivation of human herpesvirus-6 (Yao et al., 2008).
Pharmacology/toxicity of the human therapeutic agent in animals
Natalizumab binds to the α4 integrin and inhibits cell adhesion in monkeys and guinea pigs but not in rats or mice. In guinea pigs, natalizumab decreased clinical signs and inflammatory cell infiltrates in brain and spinal cord in the experimental autoimmune encephalomyelitis (EAE) model (FDA, 2005b).
In a 28 day toxicity study in cynomolgus monkeys, increases in WBC, lymphocyte and reticulocyte counts and increased spleen weights were observed (FDA, 2005b). Natalizumab had no effect on NK cell activity or lymphocyte blastogenesis ex vivo. Flow cytometry revealed no effect on the relative numbers among WBC subsets. There were no test article-related microscopic findings.
Similar effects were observed in a 28 day toxicity study in rhesus monkeys with the addition of lymphoid hyperplasia in the spleen and lymph nodes and lymphoid cell aggregates in the liver (FDA, 2005b).
In a 6 month toxicity study in cynomolgus monkeys, natalizumab at doses of 3, 10, 30 and 60 mg·kg−1·week−1 caused dose-related increases in WBC counts, lymphocyte, monocytes and eosinophils and spleen weights but had no effect on the percentages of circulating B-cells, T-cells, T-cell subsets (CD4, CD8) or hematopoietic stem cells (CD34) (FDA, 2005b; Wehner et al., 2009a). Natalizumab caused a modest and highly variable delay in the primary humoral response to T-cell-dependent antigens. Ex vivo, natalizumab did not alter immune regulatory or effector cell functions in blood lymphocytes or spleen cells. Microscopic findings included inflammation of the intestinal mucosa, follicular hyperplasia in the spleen and glomerulonephritis. The glomerulonephritis was attributed to immune complex deposition related to the immunogenicity of the test article.
In a 6 month juvenile toxicity study in cynomolgus monkeys, natalizumab at doses of 10, 30 and 60 mg·kg−1·week−1 was associated with increases in WBC and lymphocytes, decreases in neutrophils counts and a decrease in complement activity (FDA, 2005b). Spleen weights were increased and flow cytometry showed that total WBC, CD3+ and CD20+ counts were elevated. A single animal showed an acute infusion reaction attributable to anti-drug antibodies and complement.
In embryo-fetal developmental toxicity studies in cynomolgus monkeys, natalizumab (10 or 30 mg·kg−1 every other day) was associated with slight thymic atrophy, increased extramedullary haematopoiesis in the spleen, increases in WBC and nucleated RBC, decreases in RBC parameters and decreases in lymphoid CD20 staining in the fetuses (Wehner et al., 2009b). Natalizumab was not associated with increased abortions or external, visceral or skeletal abnormalities at doses up to 30 mg·kg−1. In a post-natal development study, natalizumab at a dose of 30 mg·kg−1 every other day resulted in reversible increases in lymphocyte and nucleated RBC counts, and reductions in platelet counts were observed in dams and infants (Wehner et al., 2009c). In developmental and reproductive toxicity studies in guinea pigs, natalizumab caused decreased fertility in females attributed to decreased successful implantation and decreased pup survival but had no teratogenic effect (FDA, 2005b). Natalizumab had no effect on male fertility (Wehner et al., 2009d,e).
To evaluate the potential of natalizumab to affect human tumour growth, studies were conducted in immune-deficient mice implanted with human tumours. Natalizumab did not potentiate the growth of human leukaemia or melanoma cells that expressed the α4 subunit of VLA-4 and bound natalizumab (FDA, 2005b).
Pharmacology/toxicity of surrogate molecules
Studies with another anti-human (IgG1) VLA4 mAb (HP1/2) showed a reduction in colitis in the cotton-top tamarin (Podolsky et al., 1993). Studies with anti-murine VLA4 mAb have shown that blocking VLA4 can decrease Toxarcara canis-mediated cardiac eosinophil infiltration (Hokibara et al., 1998), decrease collagen-arthritis paw inflammation and histological scores, normalize matrix metalloprotease expression (Raychaudhuri et al., 2003), and reduce cellular infiltrates and demyelinization within the CNS but did not affect the clearance of virus in Semliki Forest virus-exacerbated EAE (Smith et al., 2000).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD49d is not immunosuppressive in mice. Thus, genetic deficiency does not mimic either the pharmacologic or the adverse effects of anti-CD49d mAb. Anti-CD49 mAbs are immunosuppressive in mice, NHP and humans. However, neither mice nor NHP mimic the adverse effect profile of anti-CD49d mAb seen in humans.
CD52
Structure and function
CD52 is a 21–28 kDa glycoprotein expressed on the surface of all B- and T-cells and by epithelial cells in the epididymis, vas deferens and seminal vesicle (Alinari et al., 2007) and cumulus oophorus (Hasegawa et al., 2008). CD52 is also expressed in a number of lymphomas and leukaemias. The physiologic function of CD52 is unclear, but among a variety of suggested functions, for example signal transduction and cell adhesion, it may be involved in activation of TREG cells (Watanabe et al., 2006). Anti-CD52 mAbs are believed to mediate depletion of B- and T-cells by several mechanisms: ADCC, CDC and induction of apoptosis (Demko et al., 2008).
Genetic deficiency
Deficiency of CD52 in humans has not been described. Mice deficient in CD52 show no abnormality (Yamaguchi et al., 2008).
Marketed human therapeutic agent
Alemtuzumab (Campath 1H® MabCampath) is a humanized anti-CD52 mAb. It is approved for chronic B-cell lymphocytic leukaemia 30 mg per patient per day three times per week for 12 weeks (Demko et al., 2008; Campath USPI, 2009; Cheson, 2010). Alemtuzumab also has clinical activity in mature T-cell diseases such as T-cell-prolymphocytic leukaemia and cutaneous T-cell lymphoma (Dearden and Matutes, 2006) and has been tested in multiple sclerosis (Lim and Constantinescu, 2010) and graft-versus-host disease (Martinez et al., 2009).
Human adverse effects
Alemtuzumab is associated with cytopenias, infusion reactions (cytokine release syndrome) and infections (including fatal, bacterial, viral, fungal and protozoan infections) (≥10%) (Boxed warnings; Damaj et al., 2002; Osterborg et al., 2006; Creelan and Ferber, 2008; Demko et al., 2008; Sachdeva and Matuschak, 2008; Campath USPI, 2009). It is also associated with reactivation of latent CMV infection (Laurenti et al., 2004) and herpes zoster (Alcaide et al., 2008).
Pharmacology/toxicity of the human therapeutic in animals
In vitro, alemtuzumab plus complement depleted T-cells from the bone marrow from sabaeus and rhesus monkeys (Phan et al., 1987; Gerritsen et al., 1988). The affinity of alemtuzumab for cynomolgus CD52 is 16-fold lower than the affinity for human CD52 (EMA, 2005a). In vivo, in cynomolgus monkeys, alemtuzumab at doses of 1 mg·kg−1 and above caused reversible lymphopenia and, at high repeated doses, neutropenia (EMA, 2005b). Alemtuzumab also caused slight to moderate hypotension and mild to moderate fever but not cytokine release syndrome. Alemtuzumab was immunogenic in cynomolgus monkeys, but there was no apparent consequence of seroconversion. In addition, alemtuzumab also binds an antigen on RBC in some but not all cynomolgus monkeys (de Giorgi et al., 1987; van der Windt et al., 2010) and causes haemolysis in monkeys that express CD52.
The potential immunosuppressive effect was investigated in cynomolgus monkeys vaccinated with live attenuated simian immunodeficiency virus (SIV). About 14 months after vaccination, the monkeys received alemtuzumab (cumulative dose 23 mg·kg−1 over 7 days) and were challenged with wild-type SIV. Despite profound lymphopenia (99% depletion of T lymphocytes), the vaccinated animals did not develop viraemia (EMA, 2005b).
Alemtuzumab is not active in normal mice but is active in human-CD52 transgenic mice (Hu et al., 2009). In these mice, treatment with alemtuzumab caused a transient increase in serum cytokines and depletion of peripheral blood lymphocytes but did not cause cytokine release syndrome. Lymphocyte depletion was not as profound in lymphoid organs. Both lymphocyte depletion and cytokine induction by alemtuzumab appeared to be mediated by neutrophils and NK cells. Alemtuzumab is also active against human tumour xenografts (Siders et al., 2010).
Pharmacology/toxicity of surrogate molecules
In normal mice, an anti-murine-CD52 depletes lymphocytes (Qu et al., 2009).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD52 is not immunosuppressive in mice. Thus, genetic deficiency of CD52 does not mimic either the pharmacologic or the adverse effects of anti-CD52 mAb. Anti-CD52 mAb deplete lymphocytes and are immunosuppressive in mice, NHP and humans. However, neither mice nor NHP mimic the adverse effect profile of anti-CD52 mAb seen in humans.
CD126 (IL-6 receptor)
Structure and function
CD126 is the alpha subunit of the IL-6 receptor. CD126 is expressed on a variety of normal and cancer cells (Rose-John et al., 2007). IL-6 is a pleiotropic cytokine secreted from T-cells and macrophages that is a co-stimulator for T-cells, induces acute phase responses, enhances B-cell replication, differentiation and immunoglobulin production and promotes haematopoiesis and thrombopoiesis.
Genetic deficiency
Deficiency of CD126 in humans has not been reported. Mice that are genetically deficient in CD126 are viable, fertile and morphologically normal at birth, have reduced acute phase responses and exhibit a reduction in wound healing (McFarland-Mancini et al., 2010).
Marketed human therapeutic
Tocilizumab (atlizumab, Actemra®, RoActemra®) is a humanized IgG1 anti-CD126 mAb approved for the treatment of rheumatoid arthritis in the United States, Europe and Japan (8 mg·kg−1 every 4 weeks in adults), and for the treatment of Castleman's disease in Japan (Oldfield et al., 2009). Tocilizumab binds specifically to both soluble and membrane-bound IL-6 receptors and has been shown to inhibit IL-6-mediated signalling through these receptors (Mihara et al., 2005).
Human adverse effects
The most common adverse reactions associated with tocilizumab treatment (incidence of at least 5%) are upper respiratory tract infections, nasopharyngitis, headache, hypertension and increased alanine aminotransferase (Tanaka et al., 2010; Actemra USPI, 2011; Bannwarth and Richez, 2011; Campbell et al., 2011). Other less frequent, possible, treatment-related adverse events include serious and opportunistic infections (boxed warning) (pneumonia, urinary tract infection, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis and bacterial arthritis, tuberculosis, cryptococcus, aspergillosis, candidiasis, pneumocystosis), gastrointestinal perforation (primarily as complications of diverticulitis), decreased neutrophils and platelets and elevated lipids and liver enzymes.
Pharmacology/toxicity of the human therapeutic in animals
Tocilizumab binds to CD126 and inhibits IL-6 signalling in NHPs but not in rodents. In mice, tocilizumab suppressed the growth of human tumour xenografts of squamous cell carcinoma (Shinriki et al., 2009) and multiple myeloma (Tsunenari et al., 1996). In cynomolgus monkeys, tocilizumab showed a reduction in disease severity and anaemia in collagen-induced arthritis (Uchiyama et al., 2008; Hashizume et al., 2010).
The non-clinical safety program to support the clinical use of tocilizumab included repeated dose toxicology studies at dose of up to 100 mg·kg−1·week−1 for up to 6 months duration and an embryo-fetal development study in cynomolgus monkeys at doses up to 50 mg·kg−1·day−1 (EMA, 2009a; 2010a; FDA, 2010). No noteworthy toxicity was observed in the 6 month study. In the embryo-fetal development study an increased incidence of abortions was noted in the 10 and 50 mg·kg−1·day−1 groups relative to the control and 2 mg·kg−1·day−1 groups. The cardiovascular safety of tocilizumab was studied in cynomolgus monkeys (EMA, 2009b). Tocilizumab showed no effect on the cardiac electrophysiological performance, cardiac tissue integrity or systemic pro-thrombotic activity.
Pharmacology/toxicity of surrogate molecules
Tocilizumab does not bind to rodent IL-6R (FDA, 2010). Pharmacology studies conducted in mice using a surrogate rat anti-mouse IL-6R mAb (MR16-1) showed a reduction in the T-cell-independent antibody response following antigenic challenge and a reduction in the DTH responses (Mihara et al., 2002) and a reduction in inflammation and disease severity in a number of disease models including models of arthritis (Takagi et al., 1998) and nephritis (Mihara et al., 1998; Tomiyama-Hanayama et al., 2009). MR16-1 also showed a reduction in Castleman's disease-like symptoms in IL-6 over-expressing mice (Katsume et al., 2002), and showed a reduction in cancer-induced anaemia. In a murine methionine choline-deficient diet-induced model of non-alcoholic steatohepatitis, MR16-1 increased liver injury, hepatocyte apoptosis and liver fibrosis (Yamaguchi et al., 2011).
MR16-1 was studied in fertility and pre-/post-natal development studies in mice. Deaths attributable to the immune response to the rat immunoglobulin were observed in all studies. Peripheral cell blood phenotyping, primary IgM or IgG antibody responses, clinical signs, body weight, food consumption, and gross necropsy revealed no toxicologically relevant impairment of reproductive and developmental functions including immune system development at doses up to 50 mg·kg−1 every 3 days (EMA, 2009a; 2010a).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD126 is not overtly immunosuppressive in mice. Thus, genetic deficiency of CD126 does not mimic either the pharmacologic or the adverse effects of anti-CD126 mAb. Anti-CD126 mAbs are immunosuppressive in mice, NHP and humans. However, neither mice nor NHP mimic the adverse effect profile of anti-CD126 mAb seen in humans.
CD152 (cytotoxic T lymphocyte antigen 4)
Structure and function
CD152 [also known as cytotoxic T lymphocyte antigen 4 (CTLA4)] is a transmembrane protein that serves as a negative regulator of T-cell activation (Rudd, 2008). The ligands for CD152 are CD86 and CD28. CD152-mediated signal transduction leads to activation of the ubiquitin ligase and enhanced ubiquitination of JunB (Hoff et al., 2010).
Genetic deficiency
Deficiency of CD152 in humans has not been described. CD152-deficient mice express a pronounced autoimmune syndrome characterized by polyclonal hypergammaglobulinaemia with increased levels of SLE-associated IgG autoantibodies, glomerular IgG and C3 deposition, and interstitial nephritis (Stohl et al., 2008). T-cells from CD152-deficient mice are resistant to gamma-irradiation-induced apoptosis (Bergman et al., 2001) and to the induction of a stop signal by anti-CD3 ligation (Downey et al., 2008) but expression of CD152 is not required for the development or function of TREG cells (Boden et al., 2003).
Marketed human therapeutic
Ipilimumab (Yervoy®) is a fully human IgG1 mAb approved for treatment of late-stage metastatic melanoma (3 mg·kg−1 every 3 weeks for a total of four doses, Yervoy USPI, 2011). Blockade of CD152 with ipilimumab can potentiate anti-tumour immunity (Boasberg et al., 2010). In a phase III trial, ipilimumab demonstrated an improvement in overall survival in patients with previously treated, advanced melanoma (Hoos et al., 2010). Ipilimumab has also shown activity in non-Hodgkin's lymphoma (Ansell et al., 2009) and non-small cell lung cancer, renal cell cancer and castrate-resistant prostate cancer (Tarhini et al., 2010).
Human adverse effects
The major drug-related adverse side effects associated with ipilimumab are immune related and may involve any organ system (Boxed warning) (Berman et al., 2010; Boasberg et al., 2010; Di Giacomo et al., 2010). Most commonly see are colitis/diarrhoea, hypophysitis, thyroiditis, dermatitis (including toxic epidermal necrolysis), neuropathy and hepatitis. Endocrinopathies, uveitis, nephritis and inflammatory myopathy have also been reported. Colonic perforation can occur.
Pharmacology/toxicity of the human therapeutic in animals
Ipilimumab suppressed the growth of murine tumours in mice transgenic for human CD152 (FDA, 2011). Ipilimumab also increased T-cell activation in SIV-infected monkeys (Cecchinato et al., 2008).
Ipilimumab is active in NHPs. In monkeys immunized with various T-cell-independent antigens, ipilimumab produced enhanced immune responses (EMA, 2011). Ipilimumab was well tolerated in cynomolgus monkeys at doses up to 10 mg·kg−1·week−1 for 1 month or 10 mg·kg−1·month−1 for 6 months (FDA, 2011). It was specifically noted in the FDA review that in monkeys, ipilimumab did not show any of the serious adverse effects seen in the clinical trials. In a reproductive toxicity test in cynomolgus monkeys, there were no treatment-related adverse effects on reproduction in the first two trimesters of pregnancy at dose levels 2.6 or 7.2 times the clinical dose (Yervoy USPI, 2011). Beginning in the third trimester, a dose-related increased incidence of abortion, stillbirth, premature delivery (with corresponding lower birth weight) and infant mortality were observed.
Pharmacology/toxicity of surrogate molecules
Ipilimumab is not active in rodents. Surrogate CTLA4 antagonist antibodies in combination with a cellular vaccine were capable of inducing regression of established B16 melanoma. Poorly immunogenic tumours did not respond to therapy with anti-CTLA4 alone, although in combination with a granulocyte-macrophage colony-stimulating factor-secreting cell vaccine, regression of tumour was associated with autoimmune vitiligo (Weber, 2010).
Mice are unusually tolerant of hepatic transplantation across major histocompatibility barriers. Anti-CD152 antagonist mAb induce acute rejection of hepatic allografts and promote donor-specific T-cell activation, cytotoxicity, Th1 polarization and protect alloreactive T-cells from apoptosis (Li et al., 2005). Similarly, anti-CD152 antagonist mAb exacerbate a recoverin peptide-induced murine model of cancer-associated retinopathy (Maeda et al., 2006).
In mice, administration of an agonist anti-CD152 mAb decreased LPS-induced bronchoalveolar lavage fluid albumin and IL-17A, while increasing the number of CD4(+)Foxp3(+) cells and Foxp3 expression by these cells (Nakajima et al., 2010). Similarly, in murine models of cutaneous leishmaniasis, anti-CD152 mAb caused a more rapid progression of disease in sensitive BALB/c but not in normally resistant C57BL/6 mice (Heinzel and Maier, 1999).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD152 in mice is associated with autoimmune disease. Thus, genetic deficiency mimics the pharmacologic or adverse effects of anti-CD152 mAb. Blocking CD152 signalling with antagonist mAb potentiates immune responses in mice and humans and can cause autoimmune-mediated adverse effects. There are insufficient data to draw conclusions regarding concordance of adverse effects in NHPs.
Epidermal Growth Factor Receptor
Structure and function
Epidermal growth factor receptor (EGFR) erbB1, a member of the EGFR family, is a 170 kDa transmembrane glycoprotein with tyrosine kinase activity. EGFR is expressed constitutively throughout the body and found on many epithelial tissues (Jean and Shah, 2008). EGFR is important for normal homeostasis in a variety of tissues and, when abnormally expressed or mutated, contributes to the development of many diseases, particularly neoplasia (Lee and Threadgill, 2009).
Genetic deficiency
Deficiency of EGFR in humans has not been described. Deficiency of EGFR in mice is associated with pre-/post-natal mortality (Zhang et al., 2010). A conditional deficiency expressed after weaning is associated with a wavy coat (Lee and Threadgill, 2009).
Marketed human therapeutics agents
Cetuximab (Erbitux®) is a chimeric murine/human IgG1 (Luo et al., 2005), and panitumumab (Vectibix®) is a fully human IgG2 (Peeters et al., 2008). Cetuximab (400 mg·m−2 then 250 mg·m−2 weekly) and panitumumab (6 mg·kg−1 every 14 days) are active against EGFR-expressing, colorectal carcinoma (Jean and Shah, 2008; Giusti et al., 2009; Erbitux USPI, 2011; Vectibix USPI, 2011) and a number of other tumour types with high expression of EGFR (Hoag et al., 2009).
Human adverse effects
The most common adverse events of EGFR mAb therapy are dermatologic toxicity (sometimes severe), diarrhoea and electrolyte depletion (Jean and Shah, 2008; Erbitux USPI, 2011; Vectibix USPI, 2011). Skin toxicity is typically manifest as a papulopustular rash in the majority (45–100%) of patients receiving EGFR inhibitors (Li and Perez-Soler, 2009). Other serious, but less common adverse events include infusion reactions (3% with cetuximab, 1% with panitumumb), cardiopulmonary arrest with cetuximab (2%), pulmonary fibrosis with panitumumab and ocular toxicity (Erbitux USPI, 2011; Vectibix USPI, 2011) (Jean and Shah, 2008; Hoag et al., 2009; Ouwerkerk and Boers-Doets, 2010). Lengthening of the eyelashes has also been reported (Cohen et al., 2011). While trivial as toxicity, this is of interest in the context of this review because of the changes in coat condition in the conditional knockout mice (Lee and Threadgill, 2009).
Pharmacology/toxicity of the human therapeutic in animals
Cetuximab is active against a variety of human tumour xenografts in immunocompromised mice including: colon, gastric and squamous cell carcinoma (EMA, 2004a; Luo et al., 2005; Patel et al., 2009; Helman et al., 2010). Similarly, panitumumab has been shown to have activity against a variety of tumour xenografts (Yang et al., 2001; Foon et al., 2004).
Cetuximab and panitumumab are pharmacologically active in cynomolgus monkeys. The toxicity of cetuximab was evaluated in cynomolgus monkeys in single-dose and repeat-dose studies of 39 weeks duration (EMA, 2004a). Repeated doses of cetuximab (7.5, 24 and 75 mg·kg−1·week−1, 0.4–4 times the human dose) were associated with severe, dose-related skin toxicity as well as lesions of the tongue, nasal cavity and oesophagus. At 75 mg·kg−1·week−1, mortality occurred on 50% of the animals due to sepsis (Erbitux USPI, 2011). Eye lesions, diarrhoea and changes in a number of haematologic and clinical biochemistry parameters were also observed. There were no effects on safety pharmacology parameters. An embryo-fetal development study conducted in cynomolgus monkeys showed no malformations but resulted in an increase in abortions and/or embryo-fetal deaths at doses of 1.6–4 times the clinical dose.
The toxicity of panitumumab has been studied in cynomolgus monkeys (EMA, 2010b). In repeat-dose studies at doses ranging from 7.5 to 30 mg·kg−1·week−1 (1.25–5 times the human dose) for up to 6 months duration, skin rash and diarrhoea were observed. Microscopic evaluation of male reproductive organs revealed no abnormality. In reproductive toxicity studies at doses up to 30 mg·kg−1·week−1, panitumumab produced no malformations but was shown to cause abortions and/or fetal deaths, and, in a fertility study, panitumumab prolonged the menstrual cycle and/or amenorrhoea and reduced pregnancy rate.
Pharmacology/toxicity of surrogate molecules
An anti-murine EGFR mAb had no effect in a mouse model of hepatic regeneration (Van Buren et al., 2008).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of EGFR is lethal in mice. Thus, genetic deficiency of EGFR does not mimic either the pharmacologic or the adverse effects of anti-EGFR mAb. Anti-EGFR mAb cause skin toxicity in NHP and humans. Thus, NHP mimic the most common adverse effect of anti-EGFR seen in humans. There are insufficient data to draw conclusions regarding concordance of adverse effects in mice.
Glycoprotein (GP) IIb/IIIa
Structure and function
GP IIb/IIIa (αIIβ3) is an integrin receptor and the most abundant adhesion/aggregation receptor on the surface of platelets (Smyth et al., 2000). Integrins mediate cell–cell adhesion and adhesion of cells to the extracellular matrix during development, immunity, metastasis, thrombosis and wound healing (Fang et al., 2005). On platelets, GP IIb/IIIa mediates activation and aggregation.
Genetic deficiency
Deficiency of GP IIb/IIIa in humans is the cause of Glanzmann thrombasthaenia (GT), an autosomal recessive bleeding disorder (Tomiyama, 2000). Deficiency of β3 integrin in mice results in a phenotype that resembles GT (Smyth et al., 2000) but expresses additional defects consequent upon the absence of integrins αVβ 3 and αIIB β3 not seen in GT, including defects in placental development and in bone resorption (Hynes and Hodivala-Dilke, 1999). Forced expression of human β3 in (β3-/-) mice restored platelet function and improved bleeding times (Fang et al., 2005).
Marketed human therapeutic agent
Abciximab (ReoPro®) is a Fab fragment of a chimeric murine–human antibody (c7E3) that inhibits activity of GP IIb/IIIa and αVβ3 integrins (Trikha et al., 2002). Abciximab is approved for the prevention of thrombotic complications of percutaneous coronary intervention and for medical treatment of patients with acute coronary ischaemic syndromes (0.25 mg·kg−1 followed by 0.125 µg·kg−1·h−1 for up to 12 h, Reopro USPI, 2005) (Brown, 2003).
Human adverse effects
The principal adverse effects of abciximab are major and minor bleeding and thrombocytopenia (Trivedi et al., 2002; Tamhane and Gurm, 2008). The central nervous system is the most common site of fatal bleeding (Brown, 2003).
Pharmacology/toxicity of the human therapeutic in animals
Abciximab is active in NHPs. In an in vitro model of heparin-induced thrombocytopenia, abciximab inhibited platelet aggregation in cynomolgus monkeys (Untch et al., 2002). In vivo, in a femoral thrombosis model co-administration of abciximab and reteplase (a tissue plasminogen activator analogue) decreased the time to reperfusion and resulted in more consistent and sustained vessel patency. In the case of systemic intravenous reteplase, the co-administration of abciximab resulted in effective reperfusion of thrombosed vessels at decreased doses of the lytic agent (Nakada et al., 2004). Similarly, in a carotid artery thrombosis model in monkeys, the F(ab')2 fragment of 7E3 abolished thrombus formation (Coller et al., 1989). In monkeys, ReoPro bolus doses of 0.25 mg·kg−1 blocked at least 80% of platelet receptors and inhibited platelet aggregation. Inhibition of platelet function was temporary following a bolus dose but could be sustained by continuous intravenous infusion (Reopro USPI, 2005).
Abciximab does not bind GP IIb/IIIa in mice (Trikha et al., 2002) but has some activity in rats (Kuo et al., 2002). The bivalent F(ab')2 fragment of c7E3 is more active in rats than the Fab fragment (Sassoli et al., 2001).
In rats, abciximab decreased platelet activation/aggregation, decreased activated platelet deposition on the vascular endothelium and promoted skin flap survival (Kuo et al., 2002). Abciximab also reduced macromolecular efflux during leukocyte-independent endotoxaemia (Walther et al., 2004). In a partial limb amputation ischemia and reperfusion model, abciximab improved limb salvage but did not improve mortality rate (Cunha et al., 2009).
In rat in vitro assays, the bivalent F(ab')2 fragment of 7E3 binds GP IIb/IIIa and αVβ3, blocks platelet aggregation and inhibits microvascular sprout formation in an aortic ring assay (Sassoli et al., 2001). In vivo, 7E3 F(ab')2 blocks platelet aggregation and thrombus formation in response to abdominal aorta double crush injury. Also, in rats, the full mAb 7E3 induced a reproducible, severe thrombocytopenia and extended bleeding times in a manner consistent with human ITP (Hansen and Balthasar, 2001).
Pharmacology/toxicity of surrogate molecules
Administration of a surrogate anti-murine GP IIb/IIIa antibody (MWReg30) to mice resulted in FcγRIII and TNF-α-dependent hypothermia, engorgement of alveolar septal vessels and acute lung injury (Nieswandt et al., 1999). In vitro, MWReg30 inhibited platelet aggregation and did not induce morphological signs of platelet activation.
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency in of GP IIb/IIIa in mice and humans is associated with bleeding dyscrasias. Thus, genetic deficiency mimics the pharmacologic and adverse effects of anti-GP IIb/IIIa mAb. Anti-GP IIb/IIIa mAb produce the expected pharmacologic effect in mice and NHP and mimic the adverse effect profile of anti-GP IIb/IIIa mAb seen in humans.
Her2 (ErbB2)
Structure and function
Her2, the human form of erbB2, is a member of the EGF receptor family. Her2 is a transmembrane tyrosine kinase that plays a critical role in cardiac and neuromuscular junction development and function (Lin et al., 2000; Negro et al., 2004). Her2 is encoded by the proto-oncogene c-erbB-2 and is over-expressed by a number of neoplastic cell types, particularly breast, and high expression is associated with aggressive tumours (Mendelsohn and Baselga, 2000).
Genetic deficiency
Her2 deficiency in humans has not been described. ErbB2-deficient mice die of a cardiac trabeculation defect by embryonic day 11. ErbB2 conditional mutants exhibit dilated cardiomyopathy characterized by chamber dilation, wall thinning and decreased contractility (Negro et al., 2004). ErbB2-deficient embryos also show pre- and post-synaptic defects of developing neuromuscular junctions. The pre-synaptic defects include defasciculation and degeneration of the motor nerves and an absence of Schwann cells (Lin et al., 2000).
Marketed human therapeutic agent
Trastuzumab (Herceptin®) is a humanized IgG1 mAb directed against the extracellular domain of Her2 (Agus et al., 2005; Garnock-Jones et al., 2010). Trastuzumab is approved for the treatment of HER2 over-expressing breast and gastric tumours (4–8 mg·kg−1 then 2–6 mg·kg−1 every 1 to 3 weeks) (Scholl et al., 2001; Yeon and Pegram, 2005; Herceptin USPI, 2010).
Human adverse effects
The principal adverse effect of trastuzumab is cardiotoxicity (boxed warning Herceptin USPI, 2010) (Herbst et al., 2007; Perez, 2008). The signs and symptoms include tachycardia, palpitations and exertional dyspnoea, which may progress to congestive heart failure (Keefe, 2002). Although these signs are similar to those associated with anthracycline-induced cardiac toxicity, trastuzumab-related cardiac dysfunction does not increase with cumulative dose and is generally reversible (Perez, 2008). In the adjuvant breast cancer studies, the incidence of congestive heart failure was 0.4–2% in trastuzumab-treated patients versus 0.3–0.4% in control patients. In the metastatic breast cancer studies, the incidence of cardiac dysfunction in trastuzumab-treated patients was 5–28% versus 1–7% in control patients with the highest incidence being in the groups receiving trastuzumab in combination with anthracyclines (Herceptin USPI, 2010). Trastuzumab has also been associated with rare pulmonary toxicity (Boxed warning Herceptin USPI, 2010) (Peerzada et al., 2010). Infusion reactions and exacerbations of chemotherapy-induced neutropenia can also occur with trastuzumab (Herceptin USPI, 2010).
Exposure to trastuzumab during pregnancy has been associated with oligohydramnios (low amniotic fluid), in some cases, complicated by pulmonary hypoplasia and neonatal death (Boxed warning Herceptin USPI, 2010). This is of particular relevance as pertuzumab, a HER2 dimerization inhibitor mAb that is currently in clinical trials, was associated with delayed kidney development, oligohydramnios and embryo/fetal losses in an embryo-fetal development study in cynomolgus monkeys at doses ranging from 10 to 100 mg·kg−1 administered twice per week (Ortega et al., 2009). As will be described below, these effects were not observed in a similar study with trastuzumab when administered at doses up to 50 mg·kg−1 once per week (Herceptin USPI, 2010).
Pharmacology/toxicity of the human therapeutic agent in animals
Trastuzumab was evaluated in single-dose and repeat-dose toxicity studies in cynomolgus monkeys (of up to 26 weeks duration) and in a 4 week study in rhesus monkeys (EMA, 2005a). Other than injection site trauma in the 4 week study, no adverse effects were found. Trastuzumab was also tested in reproductive toxicity studies in cynomolgus monkeys. No effect on menstrual cycles, sex hormone profiles, maternal toxicity, embryotoxicity, teratogenicity, fetal or neonatal toxicity was observed.
Trastuzumab has an anti-tumour activity against human xenografts (Klos et al., 2003), and treatment of transgenic mice expressing constitutively active erbB-2/neuT with an anti-erbB2 mAb significantly delayed growth of established tumours (Stagg et al., 2008).
Pharmacology/toxicity of surrogate molecules
An anti-rodent erbB2 (Neu) has been shown to be cardiotoxic in mice, impairing contractility and causing fibrosis (Riccio et al., 2009). However, an anti-rat c-erbB2 surrogate mAb did not exacerbate doxorubicin-induced cardiotoxicity in rats (EMA, 2005a).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of erbB2 in mice is associated with cardiopathy. Thus, genetic deficiency mimics the adverse effects of anti-Her2. Anti-erbB2 mAb produce the expected pharmacological response, that is, anti-tumour activity in humans and mice. Mice, but not NHP, mimic the cardiotoxicity of anti-Her2.
Fusion proteins directed towards cellular targets
CD58 (lymphocyte function associated antigen-3)
Structure and function
CD58, lymphocyte function-associated antigen-3 (LFA-3), is expressed on a variety of cells (Bierer and Hahn, 1993) and is a ligand for CD2 (LFA-2). CD58 fused to the Fc portion of human IgG (CD58-Ig, soluble CD58) competes with cell surface CD58 for binding to CD2 and blocks signalling by CD2. In addition, by virtue of its Fc functionality, CD58-Ig mediates CD16-dependent NK cell-mediated apoptosis of activated memory T-cells (Jenneck and Novak, 2007). CD2 is a 63 kDa glycoprotein member of the immunoglobulin gene superfamily expressed on T-lymphocytes and NK cells and acts as a co-receptor for the TCR complex (Bromberg, 1993; Wilkins et al., 2003). CD2 plays a major role in mediating adhesive interactions via CD58 and in transducing signals for lymphocyte activation. CD2 is up-regulated on activated T-cells and is internalized on activated but not resting T-cells (Abraham et al., 1991).
Genetic deficiency
CD2 deficiency in humans has not been reported. CD2-deficient mice showed a normal response to infection with lymphocytic choriomeningitis virus (LCMV) (Evans et al., 1993). Mice infected intracerebrally with LCMV died as a consequence of cytotoxic lymphocyte (CTL)-mediated choriomeningitis. When infected i.p., they mounted a virus-specific MHC-restricted CTL response and cleared the infection by 2 weeks post-infection and generated normal memory CTL responses. In contrast, compared to wild-type mice, CD2-deficient mice infected with Toxoplasma gondii survived longer, showed less weight loss and decreased ileal inflammation and had a lower number of parasites in the ileum (Pawlowski et al., 2007).
Marketed human therapeutic agent
Alefacept (Amevive® USPI, 2011) is a fully human CD58/IgG1 fusion protein approved for the treatment of moderate to severe chronic plaque psoriasis (15 mg per patient per week) (Jenneck and Novak, 2007).
Human adverse effects
The principal adverse effect of alefacept is a dramatic decrease in CD4+ T-cell counts, sometimes below 250 cells·µL−1 (Scheinfeld, 2007). Other adverse effects include headache, nasopharyngitis, influenza, upper respiratory tract infection, pruritus, arthralgias, fatigue, nausea and increases in liver enzymes. Serious infections and malignancies do not appear linked to the use of alefacept (Goffe et al., 2005; Scheinfeld, 2005).
Pharmacology/toxicity of the human therapeutic agent in animals
Alefacept binds to CD2 on human, baboon, chimpanzee and cynomolgus monkey lymphocytes. In rhesus monkeys, alefacept selectively eliminated memory T-cells and, when combined with a co-stimulation blockade-based regimen using CTLA4-Ig, a CD80- and CD86-specific fusion protein, prevented renal allograft rejection and alloantibody formation. (Weaver et al., 2009).
Formal pharmacokinetic and toxicity studies were conducted with alefacept and BG9712 (LFA3TIP), a product closely related to alefacept (Chisholm et al., 1994). In single-dose studies in cynomolgus monkeys, CD2, CD3, CD4 and CD8 cell were depleted from peripheral blood. Maximum depletions were noted at day 2, were similar across the subpopulations and did not recover by day 8 study termination. Unexpectedly, CD20 cells were also depleted on day 2. Similarly, in a single-dose study in baboons, alefacept and BG9712 decreased peripheral blood lymphocytes by ∼50%.
Single- and repeated-dose PK/PD and toxicology studies in cynomolgus monkeys (up to 9 months) and baboons (up to 3 months) showed a reversible reduction in CD2, CD4 and CD8 lymphocytes in the circulation and in the lymphoid tissues. T-cell-independent antibody response to keyhole limpet haemocyanin (KLH) was unaffected by treatment. In a 52 week study, one cynomolgus monkey dosed with alefacept at 20 mg·kg−1·week−1 was diagnosed as having lymphoma at week 28 of the study (Hutto, 2010). This animal and all of the other animals in the cohort were seropositive for lymphocryptovirus, an oncogenic gamma herpes virus homologous to Epstein–Barr virus.
In reproductive toxicity studies in pregnant cynomolgus monkeys, alefacept at doses up to 5 mg·kg−1 (62 times the clinical dose) produced no maternal toxicity and no adverse developmental defects in the fetuses/infants. Peripheral lymphocyte counts and T-lymphocyte subsets in the Caesarean and full-term groups were decreased. One monkey infant developed reticulocytosis and thymic aplasia but the relationship of this finding to alefacept treatment is unknown.
Pharmacology/toxicity of surrogate molecules
Alefacept does not bind murine CD2, and CD2 function in mice likely differs from CD2 function in humans. However, human CD2 is functional in mice (Monostori et al., 1991), and BG9712 depleted peripheral T-cells in human CD2 transgenic mice (Chisholm et al., 1994).
A murine anti-CD2 mAb did not modulate expression of CD2, deplete lymphocytes or prolong skin allograft survival in cynomolgus monkeys (Berlin et al., 1992). In contrast, an anti-baboon CD2 mAb depleted human and cynomolgus monkey T-cells in chimeric NOD-SCID IL2rgamma(null) mice (Brady et al., 2009).
The murine homologue of CD58 is CD48 (Wilkins et al., 2003). There is a very limited literature on the murine homologue of alefacept, that is, a CD48-Ig fusion protein. Transfecting an insulinoma cell line expressing SV40 T antigen with CD48-Ig promoted tumour cell survival in immunocompetent NOD mice (Brady et al., 2001).
There is a literature on the effects of anti-murine CD2 mAb in rats and mice. In new-born mice, repeated injections of anti-CD2 mAb inhibited CD2 expression on thymocytes and peripheral lymphocytes without altering expression of CD3, CD4, CD8 and TCR V beta 8 (Kyewski et al., 1989).
In rats, anti-CD2 treatment inhibited TCR-driven as well as CD2-mediated proliferation (Sido et al., 1997). Similarly, in mice, anti-CD2 mAb down-modulates CD2 on T lymphocytes but without affecting CD3, TCRαβ, CD4 or CD8 counts, and delayed deletion of superantigen-responsive T-cells following the administration of staphylococcal enterotoxin B (Fortner et al., 1998). Anti-murine CD2 mAb have also been shown to prolong allograft and xenograft survival in mice (Chavin et al., 1992).
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD2 is not overtly immunosuppressive in mice and does not mimic the adverse effects of sCD58 in humans. Blocking CD58-CD2 binding is immunosuppressive in mice, NHP and humans. However, neither NHP treated with alefacept nor mice treated with surrogate constructs or anti-CD2 mimic the adverse effect profile of alefacept seen in humans.
CD152 (cytotoxic lymphocyte antigen 4)
Structure and function
CD152 (also known as CTLA4) is a transmembrane protein that serves as a regulator of T-cell activation (Rudd, 2008). The ligands for CD152 are CD86 and CD80. Engagement of CD152 and CD28 on T-cells with CD86/80 on dendritic cells (DC) provides a pivotal co-stimulatory signal. The lack of co-stimulation after engagement of the T-cell receptor by antigen can result in tolerance (Najafian and Sayegh, 2000). CTLA4-Ig binds CD86/80 and inhibits co-stimulation.
Genetic deficiency
The consequences of genetic deficiency of CD152 have been described earlier (see section on anti-CD152). Forced expression of a non-signalling mutant CD152 in mice resulted in T-cells that were unable to secrete IL-2 or proliferate in response to stimulation with antigen/DC in vitro. In adoptive transfer studies in RAG-/- mice, naive mutant CTLA4 T-cells exhibited compromised capability to expand while memory T-cells showed reduced survival (Mao et al., 2004).
Marketed human therapeutic
Abatacept (Orencia®) is a soluble homodimeric fusion protein composed of two identical subunits covalently linked by one disulphide bond. Each subunit consists of the modified amino acid sequences of the extracellular domain of human CTLA4 and human immunoglobulin IgG1 hinge, CH2 and CH3 regions (EMA, 2007). Abatacept is approved for the treatment of rheumatoid arthritis (500–1000 mg per patient IV followed by 0.125 mg per patient per week SC, Orencia USPI, 2011).
Human adverse effects
The most common adverse events (≥10%) with abatacept are headache, upper respiratory tract infection, nasopharyngitis and nausea (Orencia USPI, 2011). Abatacept has been associated with infusion reactions, serious infections (e.g. tuberculosis), cardiovascular events, gastrointestinal bleeding and basal cell carcinoma (Sibilia and Westhovens, 2007; Khraishi et al., 2010; Maxwell and Singh, 2010; Storage et al., 2010). Patients receiving concomitant abatacept and TNF antagonist therapy experienced more infections (63%) and serious infections (4.4%) compared to patients treated with only TNF antagonists (43% and 0.8%, respectively) (Orencia USPI, 2011). In a small study, abatacept did not exacerbate chronic hepatitis C infection (Mahajan et al., 2010).
Pharmacology/toxicity of the human therapeutic in animals
Abatacept is pharmacologically active across multiple species including monkey, rat, mouse and rabbit. General toxicity studies with abatacept were conducted in cynomolgus monkeys and mice (FDA, 2005a; EMA, 2007). In monkeys, there were no adverse effects noted in a single-dose study at a dose up to 100 mg·kg−1. In 6 month and 1 year monkey toxicity studies, abatacept at doses up to 50 mg·kg−1·week−1 (9.2 times the clinical exposure) was associated with minimal transient decreases in serum IgG and minimal to severe lymphoid depletion of germinal centres in the spleen and lymph nodes and transient suppression of T-cell-independent antibody responses. There was no evidence of lymphomas or pre-neoplastic change (FDA, 2005a).
In a murine model of rheumatoid arthritis associated with breach of self-tolerance to self-antigens, abatacept prevented the emergence of self-reactivity and arthritis (Platt et al., 2010). Similarly, in a rat collagen model of rheumatoid arthritis, abatacept decreased inflammation scores, anti-collagen antibody and cytokine production and bone erosion (FDA, 2005a).
Treatment of mice chronically infected with Mycobacterium tuberculosis did not result in reactivation or disease progression (Bigbee et al., 2007). Moreover, in a murine model of pneumococcal sepsis, treatment with abatacept led to increased survival (LeMessurier et al., 2010).
In repeat-dose toxicity studies in mice (6 months at doses up to 200 mg·kg−1·week−1 (4.7-fold the clinical exposure) and rats (7 days) abatacept caused decreases in IgG, decreases in the percentage of splenic B-cells. Abatacept also caused inhibition of ex vivo B- and T-cell mitogen responses in mice and suppression of antibody response to KLH in mice in vivo. In a carcinogenicity study in mice, abatacept caused an increase in lymphomas and mammary adenocarcinomas at 65 and 200 mg·kg−1·week−1 (no observed adverse effect level 20 mg·kg−1·week−1). This was considered to be the result of failure to control infections by murine leukaemia virus and murine mammary tumour virus as a consequence of long-term immunosuppression. Karyomegaly in the renal tubular epithelium was also reported in mice.
Reproductive toxicity studies were conducted in mice at doses up to 300 mg·kg−1·day−1 and in rats and rabbits at doses up to 200 mg·kg−1·day−1 (29 times human exposure) (FDA, 2005a). Abatacept had no effect on fertility, reproductive function, gestation, parturition or lactation in F0 rats and had no effect on embryo-fetal development in mice, rats or rabbits. In F1-generation rats, abatacept had no effect on reproductive performance and, although immune function was generally unaffected, an increase in T-cell-independent antibody response was observed.
Concordance of preclinical and clinical pharmacology/toxicity
Genetic deficiency of CD152 in mice is associated with autoimmune disease. Thus, genetic deficiency does not mimic the pharmacologic or adverse effects of abatacept. Abatacept produces the expected pharmacologic effect in mice and NHP. However, neither mice nor NHP mimic the adverse effect profile of abatacept seen in humans.
Summary analysis of the concordance of the preclinical and clinical pharmacodynamics and adverse effects and conclusions
The data on concordance of genetic deficiency, pharmacodynamics and adverse effects are summarized in Figures 1 and 2, and the results of Fisher's exact tests are summarized in Table 1. The ‘raw’ data for cellular targets are contained in this paper, while the data for soluble targets are contained in the companion manuscript (Martin and Bugelski, 2012). Concordance of pharmacodynamics was determined categorically by comparing the phenotype of genetic-deficient mice with human pharmacodynamic effects described in the literature. Similarly, the pharmacodynamic effects in rodents or NHPs were compared with human pharmacodynamics. Concordance of adverse effects was also determined categorically, in this case by comparing the occurrence of serious adverse effects in humans as identified from the product prescribing information with the occurrence of these effects in preclinical studies.
Figure 1.
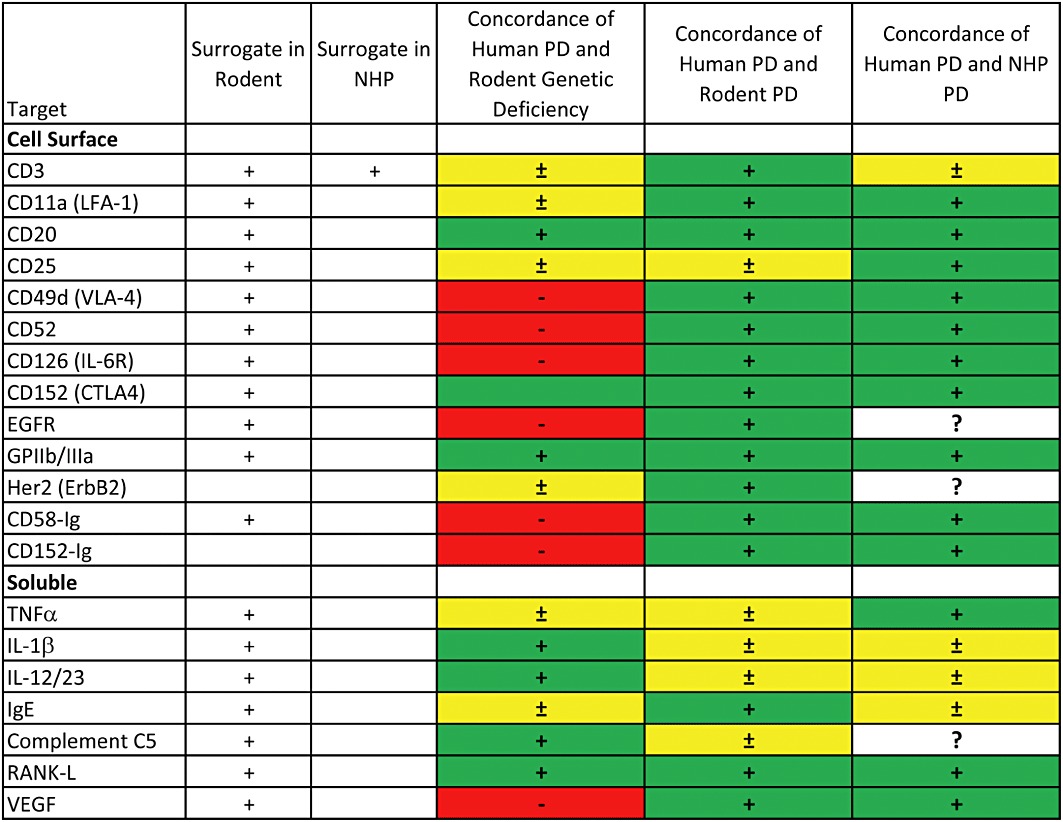
Summary data on concordance of human pharmacodynamics (PD) with genetically deficient mice, mice receiving a surrogate construct* or cynomolgus monkeys receiving the human biopharmaceutical. Green indicates an accurate reflection of the major effects of the biopharmaceutical in humans. Yellow indicates that some of the major effects in humans are not reflected in the preclinical data. Red indicates that major effects in humans are not reflected in the preclinical data. *In the cases of sCD58 (alefacept) and sCD152 (abatacept), the human biopharmaceutical was tested in mice. In the case of CD3, a surrogate was tested in NHP.
Figure 2.
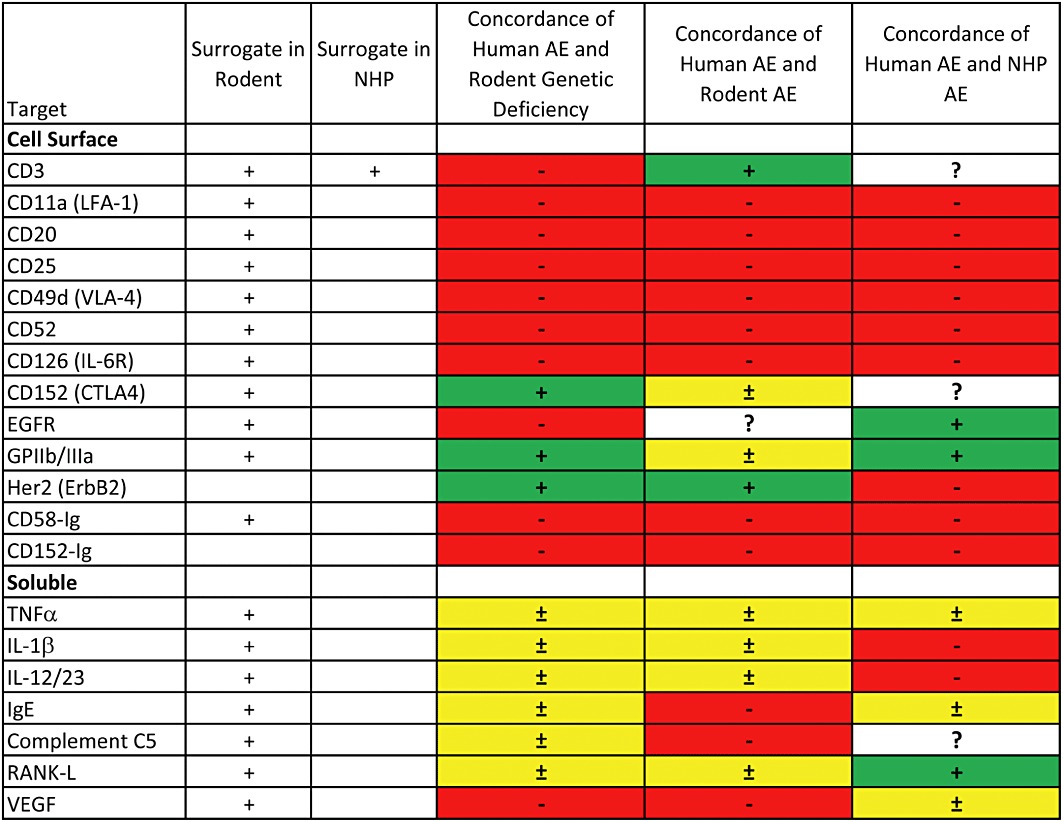
Summary data on concordance of human adverse effects (AEs) with genetically deficient mice, mice receiving a surrogate construct* or cynomolgus monkeys receiving the human biopharmaceutical. Green indicates an accurate reflection of the major effects of the biopharmaceutical in humans. Yellow indicates that some of the major effects in humans are not reflected in the preclinical data. Red indicates that major effects in humans are not reflected in the preclinical data. *In the cases of sCD58 (alefacept) and sCD152 (abatacept), the human biopharmaceutical was tested in mice. In the case of CD3, a surrogate was tested in NHP.
Table 1.
Statistical analysis of concordance/non-concordance
| Concordance of pharmacodynamics | Concordance of adverse effects | ||||||
|---|---|---|---|---|---|---|---|
| Genetic deficiency | Rodent | NHP | Genetic deficiency | Rodent | NHP | ||
| % Concordant with human | 65 | 100 | 100 | 45 | 42 | 35 | |
| P-value | Human | 0.008* | 1.000 | 1.000 | <0.001* | <0.001* | <0.001* |
| Genetic deficiency | 0.008* | 0.009* | 1.000 | 0.738 | |||
| Rodent | 1.000 | 0.742 | |||||
Statistically significant difference in concordance. A P-value ≤0.05 indicates non-concordance between the groups (Fisher's exact test).
To be concordant, the preclinical data had to reflect the full range of pharmacologic or adverse effects observed in humans. To be partially concordant, the preclinical data had to reflect most but not all the pharmacologic or adverse effects. And, to be non-concordant, the preclinical data had to miss important human pharmacologic or serious adverse effects.
Statistically significant differences between the various categorical data were determined by a Fisher's exact test. For the statistical analysis, instances of partial concordance were counted as concordant. P-values less than 0.05 were accepted as significant.
The concordance of preclinical and human pharmacodynamics is shown in Figure 1 and Table 1. A Fisher's exact test showed a statistically significant difference in the incidence of non-concordance between genetic deficiency and human pharmacodynamics. In contrast, both surrogate mAbs in rodents and the human biopharmaceuticals in monkeys showed 100% concordance with human pharmacodynamics. A Fisher's exact test showed that genetically deficient mice are inferior in predicting the effects of either surrogate mAbs in rodents or the human biopharmaceuticals in monkeys.
The concordance of preclinical and human adverse effects is shown in Figure 2 and Table 1. A Fisher's exact test showed a statistically significant difference in the incidence of non-concordance for human adverse effects and all three test systems. The test also showed no difference in the predictive value for human adverse effects among genetically deficient mice, surrogate mAbs in rodents or the human biopharmaceutical in monkeys.
Conclusion
In conclusion, review of the peer-reviewed literature and the documents available from regulatory agencies on mAb and fusion proteins directed towards cellular targets has shown good concordance with human pharmacodynamics for mice receiving surrogates or NHPs receiving the human pharmaceutical. In contrast, there was poor concordance for human pharmacodynamics in genetically deficient mice and for human adverse effects in all three test systems. No evidence that NHP have superior predictive value was found.
Acknowledgments
The authors are grateful to Dr Jesse Berlin for helpful discussions on statistical analysis and Dr David Shealy who reviewed the concordance data in great detail.
Glossary
- ADCC
antibody-dependent cellular cytotoxicity
- CD
cluster of differentiation
- CDC
complement-dependent cytotoxicity
- CTL
cytotoxic T lymphocyte
- CTLA4
cytotoxic T lymphocyte antigen 4
- CMV
cytomegalovirus
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- EGFR
epidermal growth factor receptor
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- ICAM-1
intercellular adhesion molecule-1
- LFA
lymphocyte function associated antigen
- JCV
human polyoma JC virus
- mAb
monoclonal antibody
- NHP
non-human primate
- NK
natural killer
- PML
progressive multifocal leukoencephalopathy
- RBC
red blood cell
- SCID
severe combined immunodeficiency
- SIV
simian immunodeficiency virus
- SLE
systemic lupus erythematosus
- TCR
T-cell receptor
- USPI
United States Prescribing Information
- VLA-4
very late antigen-4
- WBC
white blood cell
Footnotes
The highest level of warning issued by the US FDA.
Conflict of interest
The authors are employees of The Biotechnology Center of Excellence, Janssen R&D, LLC, a subsidiary of Johnson and Johnson, Inc., who markets abciximab, golimumab, infliximab, muromonab and ustekinumab. No other sources of funding were provided.
References
- Abraham DJ, Bou-Gharios G, Beauchamp JR, Plater-Zyberk C, Maini RN, Olsen I. Function and regulation of the murine lymphocyte CD2 receptor. J Leukoc Biol. 1991;49:329–341. doi: 10.1002/jlb.49.4.329. [DOI] [PubMed] [Google Scholar]
- Actemra USPI. 2011. Available at http://www.gene.com/gene/products/information/actemra/pdf/pi.pdf (accessed 15 February 2012)
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- Alcaide ML, Abbo L, Pano JR, Gaynor JJ, Tryphonopoulos P, Weppler D, et al. Herpes zoster infection after liver transplantation in patients receiving induction therapy with alemtuzumab. Clin Transplant. 2008;22:502–507. doi: 10.1111/j.1399-0012.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- Alegre ML, Vandenabeele P, Depierreux M, Florquin S, Deschodt-Lanckman M, Flamand V, et al. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: prevention by high doses of methylprednisolone. J Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644–3653. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- Amevive® USPI. 2011. Available at http://www.astellas.us/docs/amevive.pdf (accessed 15 February 2012)
- Angelov GS, Guillaume P, Luescher IF. CD8beta knockout mice mount normal anti-viral CD8+ T cell responses – but why? Int Immunol. 2009;21:123–135. doi: 10.1093/intimm/dxn130. [DOI] [PubMed] [Google Scholar]
- Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf-Benson S, Wall GC, Veach LA. Serum sickness-like reaction associated with efalizumab. Ann Pharmacother. 2009;43:383–386. doi: 10.1345/aph.1L495. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Wei BY, Hillman K, Luthra HS, David CS. Immunosuppression of collagen-induced arthritis in mice with an anti-IL-2 receptor antibody. J Immunol. 1988;141:1150–1154. [PubMed] [Google Scholar]
- Bannwarth B, Richez C. Clinical safety of tocilizumab in rheumatoid arthritis. Expert Opin Drug Saf. 2011;10:123–131. doi: 10.1517/14740338.2011.537256. [DOI] [PubMed] [Google Scholar]
- Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 2010;33:969–983. doi: 10.2165/11537510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bergman ML, Cilio CM, Penha-Goncalves C, Lamhamedi-Cherradi SE, Lofgren A, Colucci F, et al. CTLA-4-/- mice display T cell-apoptosis resistance resembling that ascribed to autoimmune-prone non-obese diabetic (NOD) mice. J Autoimmun. 2001;16:105–113. doi: 10.1006/jaut.2000.0474. [DOI] [PubMed] [Google Scholar]
- Berlin PJ, Bacher JD, Sharrow SO, Gonzalez C, Gress RE. Monoclonal antibodies against human T cell adhesion molecules – modulation of immune function in nonhuman primates. Transplantation. 1992;53:840–849. doi: 10.1097/00007890-199204000-00026. [DOI] [PubMed] [Google Scholar]
- Berman D, Parker SM, Siegel J, Chasalow SD, Weber J, Galbraith S, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- Bezabeh S, Flowers CM, Kortepeter C, Avigan M. Clinically significant liver injury in patients treated with natalizumab. Aliment Pharmacol Ther. 2010;31:1028–1035. doi: 10.1111/j.1365-2036.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- Bierer BE, Hahn WC. T cell adhesion, avidity regulation and signaling: a molecular analysis of CD2. Semin Immunol. 1993;5:249–261. doi: 10.1006/smim.1993.1029. [DOI] [PubMed] [Google Scholar]
- Bigbee CL, Gonchoroff DG, Vratsanos G, Nadler SG, Haggerty HG, Flynn JL. Abatacept treatment does not exacerbate chronic Mycobacterium tuberculosis infection in mice. Arthritis Rheum. 2007;56:2557–2565. doi: 10.1002/art.22750. [DOI] [PubMed] [Google Scholar]
- Blazar BR, Taylor PA, Vallera DA. In vivo or in vitro anti-CD3 epsilon chain monoclonal antibody therapy for the prevention of lethal murine graft-versus-host disease across the major histocompatibility barrier in mice. J Immunol. 1994;152:3665–3674. [PubMed] [Google Scholar]
- Boasberg P, Hamid O, O'Day S. Ipilimumab: unleashing the power of the immune system through CTLA-4 blockade. Semin Oncol. 2010;37:440–449. doi: 10.1053/j.seminoncol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Boden E, Tang Q, Bour-Jordan H, Bluestone JA. The role of CD28 and CTLA4 in the function and homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp. 2003;252:55–66. doi: 10.1002/0470871628.ch5. [DOI] [PubMed] [Google Scholar]
- Boggi U, Danesi R, Vistoli F, Del Chiaro M, Signori S, Marchetti P, et al. A benefit-risk assessment of basiliximab in renal transplantation. Drug Saf. 2004;27:91–106. doi: 10.2165/00002018-200427020-00002. [DOI] [PubMed] [Google Scholar]
- Boudousquie C, Pellaton C, Barbier N, Spertini F. CD4+CD25+ T cell depletion impairs tolerance induction in a murine model of asthma. Clin Exp Allergy. 2009;39:1415–1426. doi: 10.1111/j.1365-2222.2009.03314.x. [DOI] [PubMed] [Google Scholar]
- Brady JL, Yamashita K, Lew AM. Enhanced survival of grafts genetically endowed with the ability to block CD2 and B7. Cell Transplant. 2001;10:175–181. [PubMed] [Google Scholar]
- Brady JL, Mannering SI, Kireta S, Coates PT, Proietto AI, Cowan PJ, et al. Monoclonal antibodies generated by DNA immunization recognize CD2 from a broad range of primates. Immunol Cell Biol. 2009;87:413–418. doi: 10.1038/icb.2009.4. [DOI] [PubMed] [Google Scholar]
- Brok HP, Tekoppele JM, Hakimi J, Kerwin JA, Nijenhuis EM, De Groot CW, et al. Prophylactic and therapeutic effects of a humanized monoclonal antibody against the IL-2 receptor (DACLIZUMAB) on collagen-induced arthritis (CIA) in rhesus monkeys. Clin Exp Immunol. 2001;124:134–141. doi: 10.1046/j.1365-2249.2001.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JS. The biology of CD2: adhesion, transmembrane signal, and regulatory receptor of immunity. J Surg Res. 1993;54:258–267. doi: 10.1006/jsre.1993.1041. [DOI] [PubMed] [Google Scholar]
- Brown DL. Deaths associated with platelet glycoprotein IIb/IIIa inhibitor treatment. Heart. 2003;89:535–537. doi: 10.1136/heart.89.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere J, Martin P, Horner M, Couch J, Flaherty M, Andrews L, et al. Alternative strategies for toxicity testing of species-specific biopharmaceuticals. Int J Toxicol. 2009;28:230–253. doi: 10.1177/1091581809337262. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology. 2007;69:1391–1403. doi: 10.1212/01.wnl.0000277457.17420.b5. [DOI] [PubMed] [Google Scholar]
- Camacho-Halili M, George R, Gottesman M, Davis-Lorton M. An approach to natalizumab hypersensitivity: a case series of induction of tolerance. Mult Scler. 2011;17:250–253. doi: 10.1177/1352458510388966. [DOI] [PubMed] [Google Scholar]
- Campath USPI. 2009. Available at http://www.campath.com/pdfs/2009-08-Campath%20US%20PI.pdf (accessed 15 February 2012)
- Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJK. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology. 2011;50:552–562. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- Cannella B, Cross AH, Raine CS. Anti-adhesion molecule therapy in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1993;46:43–55. doi: 10.1016/0165-5728(93)90232-n. [DOI] [PubMed] [Google Scholar]
- Carrigan SO, Yang YJ, Issekutz T, Forward N, Hoskin D, Johnston B, et al. Depletion of natural CD4+CD25+ T regulatory cells with anti-CD25 antibody does not change the course of Pseudomonas aeruginosa-induced acute lung infection in mice. Immunobiology. 2009;214:211–222. doi: 10.1016/j.imbio.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Cather JC, Cather JC, Menter A. Modulating T cell responses for the treatment of psoriasis: a focus on efalizumab. Expert Opin Biol Ther. 2003;3:361–370. doi: 10.1517/14712598.3.2.361. [DOI] [PubMed] [Google Scholar]
- Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Cavagnaro J. Preclinical Safety Evaluation of Biopharmaceuticals: A Science-Based Approach to Facilitating Clinical Trials. Hoboken, NJ: John A. Wiley and Sons; 2008. [Google Scholar]
- Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai W-P, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63:2803–2835. doi: 10.2165/00003495-200363240-00009. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- Chavin KD, Lau HT, Bromberg JS. Prolongation of allograft and xenograft survival in mice by anti-CD2 monoclonal antibodies. Transplantation. 1992;54:286–291. doi: 10.1097/00007890-199208000-00018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. NEJM. 2009;361:1067–1074. doi: 10.1056/NEJMoa0904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD. Monoclonal antibody therapy of chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2010;23:133–143. doi: 10.1016/j.beha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Chisholm PL, Williams CA, Jones WE, Majeau GR, Oleson FB, Burrus-Fischer B, et al. The effects of an immunomodulatory LFA3-IgG1 fusion protein on nonhuman primates. Ther Immunol. 1994;1:205–216. [PubMed] [Google Scholar]
- Clarke J, Leach W, Pippig S, Joshi A, Wu B, House R, et al. Evaluation of a surrogate antibody for preclinical safety testing of an anti-CD11a monoclonal antibody. Regul Toxicol Pharmacol. 2004;40:219–226. doi: 10.1016/j.yrtph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen PR, Escudier SM, Kurzrock R. Cetuximab-associated elongation of the eyelashes: case report and review of eyelash trichomegaly secondary to epidermal growth factor receptor inhibitors. Am J Clin Dermatol. 2011;12:63–67. doi: 10.2165/11531920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26:3603–3613. doi: 10.1038/sj.onc.1210376. [DOI] [PubMed] [Google Scholar]
- Coller BS, Folts JD, Smith SR, Scudder LE, Jordan R. Abolition of in vivo platelet thrombus formation in primates with monoclonal antibodies to the platelet GPIIb/IIIa receptor. Correlation with bleeding time, platelet aggregation, and blockade of GPIIb/IIIa receptors. Circulation. 1989;80:1766–1774. doi: 10.1161/01.cir.80.6.1766. [DOI] [PubMed] [Google Scholar]
- Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, et al. Treatment of acute renal allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- Couper KN, Lanthier PA, Perona-Wright G, Kummer LW, Chen W, Smiley ST, et al. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. J Immunol. 2009;182:3985–3994. doi: 10.4049/jimmunol.0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- Creelan B, Ferber A. A fatal case of alemtuzumab-associated interstitial pneumonitis. Am J Ther. 2008;15:82–84. doi: 10.1097/MJT.0b013e3181400395. [DOI] [PubMed] [Google Scholar]
- Crofts F, Rohatagi S, Pino M, DeLise B, Zhang J, Nguyen M, et al. Critical period for a teratogenic VLA-4 antagonist: developmental effects and comparison of embryo drug concentrations of teratogenic and non-teratogenic VLA-4 antagonists. Birth Defects Res B Dev Reprod Toxicol. 2004;71:69–79. doi: 10.1002/bdrb.20000. [DOI] [PubMed] [Google Scholar]
- Cunha MS, da Silva JCF, Nakamoto HA, Machado FA, Ferreira MC. Effect of IIb-IIIa glycoprotein inhibitors in a partial limb amputation model submitted to warm ischemia in rats. J Reconstr Microsurg. 2009;25:283–288. doi: 10.1055/s-0029-1202552. [DOI] [PubMed] [Google Scholar]
- D'Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj G, Rubio MT, Audard V, Hermine O. Severe cardiac toxicity after monoclonal antibody therapy. Eur J Haematol. 2002;68:324–329. doi: 10.1034/j.1600-0609.2002.00712.x. [DOI] [PubMed] [Google Scholar]
- Dave VP. Hierarchical role of CD3 chains in thymocyte development. Immunol Rev. 2009;232:22–33. doi: 10.1111/j.1600-065X.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- Dearden CE, Matutes E. Alemtuzumab in T-cell lymphoproliferative disorders. Best Pract Res Clin Haematol. 2006;19:795–810. doi: 10.1016/j.beha.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Dedrick RL, Bodary S, Garovoy MR. Adhesion molecules as therapeutic targets for autoimmune diseases and transplant rejection. Expert Opin Biol Ther. 2003;3:85–95. doi: 10.1517/14712598.3.1.85. [DOI] [PubMed] [Google Scholar]
- Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist. 2008;13:167–174. doi: 10.1634/theoncologist.2007-0218. [DOI] [PubMed] [Google Scholar]
- Dennis RF, Siemasko KF, Tang Q, Hendricks RL, Finnegan A. Involvement of LFA-1 and ICAM-1 in the herpetic disease resulting from HSV-1 corneal infection. Curr Eye Res. 1995;14:55–62. doi: 10.3109/02713689508999914. [DOI] [PubMed] [Google Scholar]
- Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends – is a paradigm change coming soon? Methods Mol Biol. 2009;525:1–27. doi: 10.1007/978-1-59745-554-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- Downey J, Smith A, Schneider H, Hogg N, Rudd CE. TCR/CD3 mediated stop-signal is decoupled in T-cells from Ctla4 deficient mice. Immunol Lett. 2008;115:70–72. doi: 10.1016/j.imlet.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edula RG, Picco MF. An evidence-based review of natalizumab therapy in the management of Crohn's disease. Ther Clin Risk Manag. 2009;5:935–942. doi: 10.2147/tcrm.s5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–437. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- EMA. Erbitux (Cetuximab) Scientific Discussion. 2004a. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000558/WC500029113.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Raptiva (Efalizimab) Scientific Discussion. 2004b. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000542/WC500057849.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Herceptin (trastuzumab) Scientific Discussion. 2005a. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000278/WC500049816.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. MabCampath (alemtuzumab) Scientific Discussion. 2005b. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000353/WC500025264.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. MabThera (rituximab) Scientific Discussion. 2005c;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000165/WC500025817.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Simulect (basiliximab) Scientific Discussion. 2005d;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000207/WC500053538.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. 2005e. Zenapax (dacilizumab). Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000198/WC500057572.pdf (accessed 15 February 2012)
- EMA. Orencia (Abatacept) Scientific Discussion. 2007;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000701/WC500048938.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Roactemra (Tocilizumab) Assessment Report. 2009a;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000955/WC500054888.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. RoActemra (tocilizumab) Assessment Report. 2009b;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000955/WC500111086.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Roactemra (Tocilizumab) Scientific Discussion. 2010a;2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion_-_Variation/human/000955/WC500094604.pdf (accessed 15 February 2012) [Google Scholar]
- EMA. Vectabix: (Panitumumab) Summary of Product. 2010b. Characteristics, Vol. 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000741/WC500047707.pdf (accessed 15 February 2012)
- EMA. Yervoy (Ipilimumab) 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002213/WC500109302.pdf (accessed 15 February 2012) [Google Scholar]
- Erbitux USPI. 2011. Available at http://packageinserts.bms.com/pi/pi_erbitux.pdf (accessed 15 February 2012)
- Evans CF, Rall GF, Killeen N, Littman D, Oldstone MB. CD2-deficient mice generate virus-specific cytotoxic T lymphocytes upon infection with lymphocytic choriomeningitis virus. J Immunol. 1993;151:6259–6264. [PubMed] [Google Scholar]
- Fang J, Hodivala-Dilke K, Johnson BD, Du LM, Hynes RO, White GC, et al. Therapeutic expression of the platelet-specific integrin, alphaIIbbeta3, in a murine model for Glanzmann thrombasthenia. Blood. 2005;106:2671–2679. doi: 10.1182/blood-2004-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Rituximab (Rituxan) Preclinical and Clinical Pharmacology Review. 1997;2011 Available at http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm113334.pdf (accessed 15 February 2012) [Google Scholar]
- FDA. Orencia (abatacept) Pharmacology/Toxicology Review and Evaluation. 2005a;2011 Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/125118_S0000_PharmR.pdf (accessed 15 February 2012) [Google Scholar]
- FDA. Tysabri (natalizumab) Pharmacology/Toxicology Review and Evaluation. 2005b. Parts 1, 2 and 3, Vol. 2011. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/125104s000_Natalizumab_Pharmr_P1.pdf (accessed 15 February 2012)
- FDA. Actemra (Tocilizumab) Pharmacology Review(S) 2010;2011 Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/125276s000PharmR.pdf (accessed 15 February 2012) [Google Scholar]
- FDA. Yervoy (Ipilimumab) Pharmacology Review. 2011. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125377Orig1s000PharmR.pdf (accessed 15 February 2012) [Google Scholar]
- Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, Jones CA. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J Immunol. 2008;180:1556–1564. doi: 10.4049/jimmunol.180.3.1556. [DOI] [PubMed] [Google Scholar]
- Fischer A, de Saint Basile G, Le Deist F. CD3 deficiencies. Curr Opin Allergy Clin Immunol. 2005;5:491–495. doi: 10.1097/01.all.0000191886.12645.79. [DOI] [PubMed] [Google Scholar]
- Fleischmann RM. Safety of biologic therapy in rheumatoid arthritis and other autoimmune diseases: focus on rituximab. Semin Arthritis Rheum. 2009;38:265–280. doi: 10.1016/j.semarthrit.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Foon KA, Yang X-D, Weiner LM, Belldegrun AS, Figlin RA, Crawford J, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58:984–990. doi: 10.1016/j.ijrobp.2003.09.098. [DOI] [PubMed] [Google Scholar]
- Fortner KA, Russell JQ, Budd RC. Down-modulation of CD2 delays deletion of superantigen-responsive T cells. Eur J Immunol. 1998;28:70–79. doi: 10.1002/(SICI)1521-4141(199801)28:01<70::AID-IMMU70>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Yamaguchi T, Sumi T, Fukuda K, Kumagai N, Nishida T, et al. Roles of CD4+CD25+ T cells in the development of experimental murine allergic conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2007;245:705–714. doi: 10.1007/s00417-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadri Z, Kukulansky T, Bar-Or E, Haimovich J, Hollander N. Synergistic effect of dendritic cell vaccination and anti-CD20 antibody treatment in the therapy of murine lymphoma. J Immunother. 2009;32:333–340. doi: 10.1097/CJI.0b013e31819b7c17. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010;70:215–239. doi: 10.2165/11203700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gaufin T, Pattison M, Gautam R, Stoulig C, Dufour J, MacFarland J, et al. Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host. J Virol. 2009;83:10347–10357. doi: 10.1128/JVI.00880-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen WR, Wagemaker G, Jonker M, Kenter MJ, Wielenga JJ, Hale G, et al. The repopulation capacity of bone marrow grafts following pretreatment with monoclonal antibodies against T lymphocytes in rhesus monkeys. Transplantation. 1988;45:301–307. doi: 10.1097/00007890-198802000-00010. [DOI] [PubMed] [Google Scholar]
- de Giorgi L, Brent L, Linch D, Nicolaides K, Rodeck CH. The inheritance of a Macaca fascicularis red cell antigen detected by CAMPATH-1 antibody. Immunol Lett. 1987;15:101–103. doi: 10.1016/0165-2478(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Prignano F, Grossi P, Lotti T, Girolomoni G. Treatment of psoriasis with efalizumab in patients with hepatitis C viral infection: report of five cases. Dermatology. 2009;219:158–161. doi: 10.1159/000224433. [DOI] [PubMed] [Google Scholar]
- Giusti RM, Cohen MH, Keegan P, Pazdur R. FDA review of a panitumumab (Vectibix) clinical trial for first-line treatment of metastatic colorectal cancer. Oncologist. 2009;14:284–290. doi: 10.1634/theoncologist.2008-0254. [DOI] [PubMed] [Google Scholar]
- Goffe B, Papp K, Gratton D, Krueger GG, Darif M, Lee S, et al. An integrated analysis of thirteen trials summarizing the long-term safety of alefacept in psoriasis patients who have received up to nine courses of therapy. Clin Ther. 2005;27:1912–1921. doi: 10.1016/j.clinthera.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM, Morschhauser F, Wegener WA. Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk Lymphoma. 2010;51:747–755. doi: 10.3109/10428191003672123. [DOI] [PubMed] [Google Scholar]
- Grazia TJ, Gill RG, Gelhaus HC, Jr, Doan AN, Sleater ML, Pietra BA. Perturbation of leukocyte function-associated antigen-1/intercellular adhesion molecule-1 results in differential outcomes in cardiac vs islet allograft survival. J Heart Lung Transplant. 2005;24:1410–1414. doi: 10.1016/j.healun.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Guerau-de-Arellano M, Alroy J, Bullard D, Huber BT. Aggravated Lyme carditis in CD11a-/- and CD11c-/- mice. Infect Immun. 2005;73:7637–7643. doi: 10.1128/IAI.73.11.7637-7643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex-Crosier Y, Raber J, Chan CC, Kriete MS, Benichou J, Pilson RS, et al. Humanized antibodies against the alpha-chain of the IL-2 receptor and against the beta-chain shared by the IL-2 and IL-15 receptors in a monkey uveitis model of autoimmune diseases. J Immunol. 1997;158:452–458. [PubMed] [Google Scholar]
- Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- Hansen RJ, Balthasar JP. Pharmacokinetics, pharmacodynamics, and platelet binding of an anti-glycoprotein IIb/IIIa monoclonal antibody (7E3) in the rat: a quantitative rat model of immune thrombocytopenic purpura. J Pharmacol Exp Ther. 2001;298:165–171. [PubMed] [Google Scholar]
- Hasegawa A, Takenobu T, Kasumi H, Komori S, Koyama K. CD52 is synthesized in cumulus cells and secreted into the cumulus matrix during ovulation. Am J Reprod Immunol. 2008;60:187–191. doi: 10.1111/j.1600-0897.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30:917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- Hausen B, Gummert J, Berry GJ, Christians U, Serkova N, Ikonen T, et al. Prevention of acute allograft rejection in nonhuman primate lung transplant recipients: induction with chimeric anti-interleukin-2 receptor monoclonal antibody improves the tolerability and potentiates the immunosuppressive activity of a regimen using low doses of both microemulsion cyclosporine and 40-O-(2-hydroxyethyl)-rapamycin. Transplantation. 2000;69:488–496. doi: 10.1097/00007890-200002270-00005. [DOI] [PubMed] [Google Scholar]
- Heinzel FP, Maier RA., Jr Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA4 antibody. Infect Immun. 1999;67:6454–6460. doi: 10.1128/iai.67.12.6454-6460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman EE, Newman JR, Dean NR, Zhang W, Zinn KR, Rosenthal EL. Optical imaging predicts tumor response to anti-EGFR therapy. Cancer Biol Ther. 2010;10:166–171. doi: 10.4161/cbt.10.2.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson M, Giannini EH, Hirsch R. Reduction of mortality and lymphadenopathy in MRL-lpr/lpr mice treated with nonmitogenic anti-CD3 monoclonal antibody. Arthritis Rheum. 1994;37:587–594. doi: 10.1002/art.1780370422. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Davies AM, Natale RB, Dang TP, Schiller JH, Garland LL, et al. Efficacy and safety of single-agent pertuzumab, a human epidermal receptor dimerization inhibitor, in patients with non small cell lung cancer. Clin Cancer Res. 2007;13:6175–6181. doi: 10.1158/1078-0432.CCR-07-0460. [DOI] [PubMed] [Google Scholar]
- Herceptin USPI. 2010. Available at http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf (accessed 15 February 2012)
- Hernandez Garcia I. Severe erythroderma in a patient under intermittent therapy with efalizumab. J Drugs Dermatol. 2008;7:987–989. [PubMed] [Google Scholar]
- Hibberd PL, Tolkoff-Rubin NE, Cosimi AB, Schooley RT, Isaacson D, Doran M, et al. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation. 1992;53:68–72. doi: 10.1097/00007890-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Hoag JB, Azizi A, Doherty TJ, Lu J, Willis RE, Lund ME. Association of cetuximab with adverse pulmonary events in cancer patients: a comprehensive review. J Exp Clin Cancer Res. 2009;28:113. doi: 10.1186/1756-9966-28-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H, Kolar P, Ambach A, Radbruch A, Brunner-Weinzierl MC. CTLA-4 (CD152) inhibits T cell function by activating the ubiquitin ligase Itch. Mol Immunol. 2010;47:1875–1881. doi: 10.1016/j.molimm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Hokibara S, Takamoto M, Isobe M, Sugane K. Effects of monoclonal antibodies to adhesion molecules on eosinophilic myocarditis in Toxocara canis-infected CBA/J mice. Clin Exp Immunol. 1998;114:236–244. doi: 10.1046/j.1365-2249.1998.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B, Gosslar U, Bittner M. Alpha 4 integrins and tumor metastasis. Curr Top Microbiol Immunol. 1998;231:125–141. doi: 10.1007/978-3-642-71987-5_8. [DOI] [PubMed] [Google Scholar]
- Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Hsu WT, Suen JL, Chiang BL. The role of CD4CD25 T cells in autoantibody production in murine lupus. Clin Exp Immunol. 2006;145:513–519. doi: 10.1111/j.1365-2249.2006.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128:260–270. doi: 10.1111/j.1365-2567.2009.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutto D. Opportunistic infections in non-human primates exposed to immunomodulatory biotherapeutics: considerations and case examples. J Immunotoxicol. 2010;7:120–127. doi: 10.3109/15476910903258252. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Hodivala-Dilke KM. Insights and questions arising from studies of a mouse model of Glanzmann thrombasthenia. Thromb Haemost. 1999;82:481–485. [PubMed] [Google Scholar]
- ICH. S6 (R1). Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals. 2011;2011 doi: 10.1038/nrd822. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S6_R1/Step4/S6_R1_Guideline.pdf (accessed 15 February 2012) [DOI] [PubMed] [Google Scholar]
- Imai Y, Shimaoka M, Kurokawa M. Essential roles of VLA-4 in the hematopoietic system. Int J Hematol. 2010;91:569–575. doi: 10.1007/s12185-010-0555-3. [DOI] [PubMed] [Google Scholar]
- Jean GW, Shah SR. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy. 2008;28:742–754. doi: 10.1592/phco.28.6.742. [DOI] [PubMed] [Google Scholar]
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411–420. [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Jing W, Orentas RJ. CD25+ regulatory T cell inhibition enhances vaccine-induced immunity to neuroblastoma. J Immunother. 2007;30:203–214. doi: 10.1097/01.cji.0000211336.91513.dd. [DOI] [PubMed] [Google Scholar]
- Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- Kapic E, Becic F, Kusturica J. Basiliximab, mechanism of action and pharmacological properties. Med Arh. 2004;58:373–376. [PubMed] [Google Scholar]
- Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, et al. Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman's disease like symptoms emerged in IL-6 transgenic mice. Cytokine. 2002;20:304–311. doi: 10.1006/cyto.2002.2012. [DOI] [PubMed] [Google Scholar]
- Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn's disease. Ann Pharmacother. 2005;39:1833–1843. doi: 10.1345/aph.1G134. [DOI] [PubMed] [Google Scholar]
- Khan SB, Allen AR, Bhangal G, Smith J, Lobb RR, Cook HT, et al. Blocking VLA-4 prevents progression of experimental crescentic glomerulonephritis. Nephron Exp Nephrol. 2003;95:e100–e110. doi: 10.1159/000074326. [DOI] [PubMed] [Google Scholar]
- Khraishi M, Russell A, Olszynski WP. Safety profile of abatacept in rheumatoid arthritis: a review. Clin Ther. 2010;32:1855–1870. doi: 10.1016/j.clinthera.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Kim S, Fridlender ZG, Dunn R, Kehry MR, Kapoor V, Blouin A, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31:446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- Kimby E. Tolerability and safety of rituximab (MabThera) Cancer Treat Rev. 2005;31:456–473. doi: 10.1016/j.ctrv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Klos KS, Zhou X, Lee S, Zhang L, Yang W, Nagata Y, et al. Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer. 2003;98:1377–1385. doi: 10.1002/cncr.11656. [DOI] [PubMed] [Google Scholar]
- Kothary N, Diak I, Brinker A, Bezabeth S, Avigan M, Pan DG. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546–551. doi: 10.1016/j.jaad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Kozawa E, Sugiura H, Wasa J, Kohyama K, Yamada K, Nishioka A, et al. Suppression of tumour metastasis in a murine osteosarcoma model with anti-CD25 monoclonal antibody treatment. Anticancer Res. 2010;30:5019–5022. [PubMed] [Google Scholar]
- Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IAM, Dolman KM, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-R, Jeng S-F, Wang F-S, Huang H-C, Wei F-C, Yang KD. Platelet glycoprotein IIb/IIIa receptor antagonist (abciximab) inhibited platelet activation and promoted skin flap survival after ischemia/reperfusion injury. J Surg Res. 2002;107:50–55. doi: 10.1006/jsre.2002.6500. [DOI] [PubMed] [Google Scholar]
- Kyewski BA, Jenkinson EJ, Kingston R, Altevogt P, Owen MJ, Owen JJ. The effects of anti-CD2 antibodies on the differentiation of mouse thymocytes. Eur J Immunol. 1989;19:951–954. doi: 10.1002/eji.1830190526. [DOI] [PubMed] [Google Scholar]
- Laurenti L, Piccioni P, Cattani P, Cingolani A, Efremov D, Chiusolo P, et al. Cytomegalovirus reactivation during alemtuzumab therapy for chronic lymphocytic leukemia: incidence and treatment with oral ganciclovir. Haematologica. 2004;89:1248–1252. [PubMed] [Google Scholar]
- Lee T-C, Threadgill DW. Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis. 2009;47:85–92. doi: 10.1002/dvg.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMessurier K, Hacker H, Tuomanen E, Redecke V. Inhibition of T cells provides protection against early invasive pneumococcal disease. Infect Immun. 2010;78:5287–5294. doi: 10.1128/IAI.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107–119. doi: 10.1007/s11523-009-0114-0. [DOI] [PubMed] [Google Scholar]
- Li W, Zheng XX, Kuhr CS, Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. 2005;5:978–986. doi: 10.1111/j.1600-6143.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- Li W, Carper K, Liang Y, Zheng XX, Kuhr CS, Reyes JD, et al. Anti-CD25 mAb administration prevents spontaneous liver transplant tolerance. Transplant Proc. 2006;38:3207–3208. doi: 10.1016/j.transproceed.2006.10.094. [DOI] [PubMed] [Google Scholar]
- Lim SY, Constantinescu CS. Current and future disease-modifying therapies in multiple sclerosis. Int J Clin Pract. 2010;64:637–650. doi: 10.1111/j.1742-1241.2009.02261.x. [DOI] [PubMed] [Google Scholar]
- Lin W, Sanchez HB, Deerinck T, Morris JK, Ellisman M, Lee KF. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc Natl Acad Sci U S A. 2000;97:1299–1304. doi: 10.1073/pnas.97.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu J, Weng D, Chen Y, Song L, He Q, et al. CD4+CD25+Foxp3+ regulatory T cells depletion may attenuate the development of silica-induced lung fibrosis in mice. PLoS ONE. 2010;5:e15404. doi: 10.1371/journal.pone.0015404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K, et al. Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemother Pharmacol. 2005;56:455–464. doi: 10.1007/s00280-005-1022-3. [DOI] [PubMed] [Google Scholar]
- Macdonald PS, Mundy J, Keogh AM, Chang VP, Spratt PM. A prospective randomized study of prophylactic OKT3 versus equine antithymocyte globulin after heart transplantation – increased morbidity with OKT3. Transplantation. 1993;55:110–116. doi: 10.1097/00007890-199301000-00021. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Liang Y, Yenerel M, Saperstein DA. Effects of cytotoxic T lymphocyte antigen 4 (CTLA4) signaling and locally applied steroid on retinal dysfunction by recoverin, cancer-associated retinopathy antigen. Mol Vis. 2006;12:885–891. [PubMed] [Google Scholar]
- Mahajan TD, Hooker R, Maher L, Brown G, Reimold A. Abatacept therapy for rheumatoid arthritis in the setting of hepatitis C infection. J Clin Rheumatol. 2010;16:332–334. doi: 10.1097/RHU.0b013e3181f4cd92. [DOI] [PubMed] [Google Scholar]
- Mao Y, Brigham D, Chen D. Overexpression of a mutant CTLA4 inhibits T-cell activation and homeostasis-driven expansion. Exp Hematol. 2004;32:735–747. doi: 10.1016/j.exphem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Mariano FS, Gutierrez FRS, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, et al. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10:825–833. doi: 10.1016/j.micinf.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Martin PL, Bugelski PJ. Concordance of preclinical and clinical pharmacology and toxicology of monoclonal antibodies and fusion proteins: soluble targets. Br J Pharmacol. 2012;166:806–822. doi: 10.1111/j.1476-5381.2011.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Solano C, Ferra C, Sampol A, Valcarcel D, Perez-Simon JA, et al. Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol of Blood Marrow Transplant. 2009;15:639–642. doi: 10.1016/j.bbmt.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Maxwell LJ, Singh JA. Abatacept for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2010;37:234–245. doi: 10.3899/jrheum.091066. [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- McKeage K, McCormack PL. Basiliximab: a review of its use as induction therapy in renal transplantation. Biodrugs. 2010;24:55–76. doi: 10.2165/11203990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Nishimoto N, Yoshizaki K, Suzuki T. Influences of anti-mouse interleukin-6 receptor antibody on immune response in mice. Immunol Lett. 2002;84:223–229. doi: 10.1016/s0165-2478(02)00201-8. [DOI] [PubMed] [Google Scholar]
- Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Genesca M, Abel K, Montefiori D, Forthal D, Bost K, et al. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J Virol. 2007;81:5024–5035. doi: 10.1128/JVI.02444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo J-F, Scheffer A, Karkoutly C, Nouvel L, Kerdraon O, Trauet J, et al. Using human CD20-transfected murine lymphomatous B cells to evaluate the efficacy of intravitreal and intracerebral rituximab injections in mice. Invest Ophthalmol Vis Sci. 2008;49:4738–4745. doi: 10.1167/iovs.07-1494. [DOI] [PubMed] [Google Scholar]
- Monostori E, Lang G, Kioussis D, Cantrell DA, Zamoyska R, Brown MH, et al. Human CD2 is functional in CD2 transgenic mice. Immunology. 1991;74:369–372. [PMC free article] [PubMed] [Google Scholar]
- Montgomery SP, Mog SR, Xu H, Tadaki DK, Hirshberg B, Berning JD, et al. Efficacy and toxicity of a protocol using sirolimus, tacrolimus and daclizumab in a nonhuman primate renal allotransplant model. Am J Transplant. 2002;2:381–385. doi: 10.1034/j.1600-6143.2002.20415.x. [DOI] [PubMed] [Google Scholar]
- Mqadmi A, Zheng X, Yazdanbakhsh K. CD4+CD25+ regulatory T cells control induction of autoimmune hemolytic anemia. Blood. 2005;105:3746–3748. doi: 10.1182/blood-2004-12-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafian N, Sayegh MH. CTLA4-Ig: a novel immunosuppressive agent. Expert Opin Investig Drugs. 2000;9:2147–2157. doi: 10.1517/13543784.9.9.2147. [DOI] [PubMed] [Google Scholar]
- Nakada MT, Montgomery MO, Nedelman MA, Guerrero JL, Cohen SA, Barnathan ES, et al. Clot lysis in a primate model of peripheral arterial occlusive disease with use of systemic or intraarterial reteplase: addition of abciximab results in improved vessel reperfusion. J Vasc Interv Radiol. 2004;15:169–176. doi: 10.1097/01.rvi.0000109395.74740.1f. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Suarez CJ, Lin K-W, Jen KY, Schnitzer JE, Makani SS, et al. T cell pathways involving CTLA4 contribute to a model of acute lung injury. J Immunol. 2010;184:5835–5841. doi: 10.4049/jimmunol.0903238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro A, Brar BK, Lee K-F. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Echtenacher B, Wachs FP, Schroder J, Gessner JE, Schmidt RE, et al. Acute systemic reaction and lung alterations induced by an antiplatelet integrin gpIIb/IIIa antibody in mice. Blood. 1999;94:684–693. [PubMed] [Google Scholar]
- Oldfield V, Dhillon S, Plosker GL. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs. 2009;69:609–632. doi: 10.2165/00003495-200969050-00007. [DOI] [PubMed] [Google Scholar]
- Orencia USPI. 2011. Available at http://packageinserts.bms.com/pi/pi_orencia.pdf (accessed 15 February 2012)
- Ortega S, Arima A, Chihaya Y, Allison D, Braen A, Lauriault V, et al. Embryo-fetal development study of pertuzumab administered by intravenous injection to pregnant cynomolgus monkeys. Int J Toxicol. 2009;29:89–90. [Google Scholar]
- Orthoclone OKT3 USPI. 2011. Available at http://www.janssenbiotech.com/assets/OKT3_PI.pdf (accessed 15 February 2012)
- Osterborg A, Karlsson C, Lundin J, Kimby E, Mellstedt H. Strategies in the management of alemtuzumab-related side effects. Semin Oncol. 2006;33(Suppl. 5):S29–S35. doi: 10.1053/j.seminoncol.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk J, Boers-Doets C. Best practices in the management of toxicities related to anti-EGFR agents for metastatic colorectal cancer. Eur J Oncol Nurs. 2010;14:337–349. doi: 10.1016/j.ejon.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Pan Q, Brodeur J-F, Drbal K, Dave VP. Different role for mouse and human CD3delta/epsilon heterodimer in preT cell receptor (preTCR) function: human CD3delta/epsilon heterodimer restores the defective preTCR function in CD3gamma- and CD3gammadelta-deficient mice. Mol Immunol. 2006;43:1741–1750. doi: 10.1016/j.molimm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Parlevliet KJ, Bemelman FJ, Yong SL, Hack CE, Surachno J, Wilmink JM, et al. Toxicity of OKT3 increases with dosage: a controlled study in renal transplant recipients. Transpl Int. 1995;8:141–146. doi: 10.1007/BF00344424. [DOI] [PubMed] [Google Scholar]
- Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol. 2009;34:25–32. [PubMed] [Google Scholar]
- Pawlowski N-N, Struck D, Grollich K, Kuhl A-A, Zeitz M, Liesenfeld O, et al. CD2 deficiency partially prevents small bowel inflammation and improves parasite control in murine Toxoplasma gondii infection. World J Gastroenterol. 2007;13:4207–4213. doi: 10.3748/wjg.v13.i31.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerzada MM, Spiro TP, Daw HA. Pulmonary toxicities of biologics: a review. Anticancer Drugs. 2010;21:131–139. doi: 10.1097/CAD.0b013e328333d662. [DOI] [PubMed] [Google Scholar]
- Peeters M, Balfour J, Arnold D. Review article: panitumumab – a fully human anti-EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther. 2008;28:269–281. doi: 10.1111/j.1365-2036.2008.03717.x. [DOI] [PubMed] [Google Scholar]
- Perez EA. Cardiac toxicity of ErbB2-targeted therapies: what do we know? Clin Breast Cancer. 2008;8(Suppl. 3):S114–S120. doi: 10.3816/cbc.2008.s.007. [DOI] [PubMed] [Google Scholar]
- Pescovitz MD. Daclizumab: humanized monoclonal antibody to the interleukin-2 receptor. Expert Rev Clin Immunol. 2005;1:337–344. doi: 10.1586/1744666X.1.3.337. [DOI] [PubMed] [Google Scholar]
- Phan DT, Barta E, Gidali J, Feher I, Harsanyi V, Petranyi GG, et al. T-cell depletion of Cercopithecus aethiops monkey bone marrow with Campath-1 monoclonal antibody and complement. Haematologia. 1987;20:155–163. [PubMed] [Google Scholar]
- Piehl F, Holmen C, Hillert J, Olsson T. Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci. 2011;31(Suppl. 3):289–293. doi: 10.1007/s10072-010-0345-y. [DOI] [PubMed] [Google Scholar]
- Platt AM, Gibson VB, Patakas A, Benson RA, Nadler SG, Brewer JM, et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol. 2010;185:1558–1567. doi: 10.4049/jimmunol.1001311. [DOI] [PubMed] [Google Scholar]
- Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonla antibody. J Clin Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoles P, Rojo JM. The TCR/CD3 complex: opening the gate to successful vaccination. Curr Pharm Des. 2009;15:3290–3300. doi: 10.2174/138161209789105199. [DOI] [PubMed] [Google Scholar]
- Prignano F, Zanieri F, Mokhtarzadeh S, Lotti T. Efalizumab-induced severe thrombocytopenia can be resolved. Biologics. 2008;2:923–927. doi: 10.2147/btt.s4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugashetti R, Koo J. Efalizumab discontinuation: a practical strategy. J Dermatolog Treat. 2009;20:132–136. doi: 10.1080/09546630902984596. [DOI] [PubMed] [Google Scholar]
- Qu L, Li Q, Jiang H, Gu L, Zhang Q, Wang C, et al. Effect of anti-mouse CD52 monoclonal antibody on mouse intestinal intraepithelial lymphocytes. Transplantation. 2009;88:766–772. doi: 10.1097/TP.0b013e3181b47c61. [DOI] [PubMed] [Google Scholar]
- Ram R, Ben-Bassat I, Shpilberg O, Polliack A, Raanani P. The late adverse events of rituximab therapy – rare but there! Leuk Lymphoma. 2009;50:1083–1095. doi: 10.1080/10428190902934944. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri A, Chou M, Weetall M, Jeng AY. Blockade of integrin VLA-4 prevents inflammation and matrix metalloproteinase expression in a murine model of accelerated collagen-induced arthritis. Inflammation. 2003;27:107–113. doi: 10.1023/a:1023282701505. [DOI] [PubMed] [Google Scholar]
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- Reopro USPI. 2005. Available at http://www.janssenbiotech.com/assets/reopro.pdf (accessed 15 February 2012)
- Riccio G, Esposito G, Leoncini E, Contu R, Condorelli G, Chiariello M, et al. Cardiotoxic effects, or lack thereof, of anti-ErbB2 immunoagents. FASEB J. 2009;23:3171–3178. doi: 10.1096/fj.09-131383. [DOI] [PubMed] [Google Scholar]
- Rinaldi L, Rinaldi F, Perini P, Calabrese M, Seppi D, Grossi P, et al. No evidence of JC virus reactivation in natalizumab treated multiple sclerosis patients: an 18 month follow-up study. J Neurol Neurosurg Psychiatry. 2010;81:1345–1350. doi: 10.1136/jnnp.2009.201079. [DOI] [PubMed] [Google Scholar]
- Rituxan USPI. 2011. Available at http://www.gene.com/gene/products/information/pdf/rituxan-prescribing.pdf (accessed 15 February 2012)
- Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8:153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Panzara MA. Natalizumab for the treatment of relapsing multiple sclerosis. Biologics. 2008;2:189–199. doi: 10.2147/btt.s1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueff-Juy D, Liberman I, Drapier AM, Guillon JC, Leclerc C, Cazenave PA. Cellular basis of the resistance of newborn mice to the pathogenic effects of anti-CD3 treatment. Int Immunol. 1991;3:683–690. doi: 10.1093/intimm/3.7.683. [DOI] [PubMed] [Google Scholar]
- Sachdeva A, Matuschak GM. Diffuse alveolar hemorrhage following alemtuzumab. Chest. 2008;133:1476–1478. doi: 10.1378/chest.07-2354. [DOI] [PubMed] [Google Scholar]
- Sassoli PM, Emmell EL, Tam SH, Trikha M, Zhou Z, Jordan RE, et al. 7E3 F(ab')2, an effective antagonist of rat alphaIIbbeta3 and alphavbeta3, blocks in vivo thrombus formation and in vitro angiogenesis. Thromb Haemost. 2001;85:896–902. [PubMed] [Google Scholar]
- Scheinfeld N. Alefacept: a safety profile. Expert Opin Drug Saf. 2005;4:975–985. doi: 10.1517/14740338.4.6.975. [DOI] [PubMed] [Google Scholar]
- Scheinfeld N. Alefacept: its safety profile, off-label uses, and potential as part of combination therapies for psoriasis. J Dermatolog Treat. 2007;18:197–208. doi: 10.1080/09546630701247955. [DOI] [PubMed] [Google Scholar]
- Scholl S, Beuzeboc P, Pouillart P. Targeting HER2 in other tumor types. Ann Oncol. 2001;12(Suppl. 1):S81–S87. doi: 10.1093/annonc/12.suppl_1.s81. [DOI] [PubMed] [Google Scholar]
- Sharma R, Zheng L, Deshmukh US, Jarjour WN, Sung S-SJ, Fu SM, et al. A regulatory T cell-dependent novel function of CD25 (IL-2Ralpha) controlling memory CD8(+) T cell homeostasis. J Immunol. 2007;178:1251–1255. doi: 10.4049/jimmunol.178.3.1251. [DOI] [PubMed] [Google Scholar]
- Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. 2009;15:5426–5434. doi: 10.1158/1078-0432.CCR-09-0287. [DOI] [PubMed] [Google Scholar]
- Sibilia J, Westhovens R. Safety of T-cell co-stimulation modulation with abatacept in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(Suppl. 46):S46–S56. [PubMed] [Google Scholar]
- Siders WM, Shields J, Garron C, Hu Y, Boutin P, Shankara S, et al. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leuk Lymphoma. 2010;51:1293–1304. doi: 10.3109/10428191003777963. [DOI] [PubMed] [Google Scholar]
- Sido B, Otto G, Zimmermann R, Muller P, Meuer SC, Dengler TJ. Modulation of the CD2 receptor and not disruption of the CD2/CD48 interaction is the principal action of CD2-mediated immunosuppression in the rat. Cell Immunol. 1997;182:57–67. doi: 10.1006/cimm.1997.1204. [DOI] [PubMed] [Google Scholar]
- Silva HM, Vieira PMMM, Costa PLN, Pimentel BMS, Moro AM, Kalil J, et al. Novel humanized anti-CD3 antibodies induce a predominantly immunoregulatory profile in human peripheral blood mononuclear cells. Immunol Lett. 2009;125:129–136. doi: 10.1016/j.imlet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Simmons DL. Anti-adhesion therapies. Curr Opin Pharmacol. 2005;5:398–404. doi: 10.1016/j.coph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Simulect SPC. 2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000207/WC500053543.pdf (accessed 15 February 2012)
- Simulect USPI. 2005. Available at http://www.pharma.us.novartis.com/product/pi/pdf/simulect.pdf (accessed 15 February 2012)
- Smith JP, Morris-Downes M, Brennan FR, Wallace GJ, Amor S. A role for alpha4-integrin in the pathology following Semliki Forest virus infection. J Neuroimmunol. 2000;106:60–68. doi: 10.1016/s0165-5728(99)00235-0. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Tsakiris DA, Scudder LE, Coller BS. Structure and function of murine alphaIIbbeta3 (GPIIb/IIIa): studies using monoclonal antibodies and beta3-null mice. Thromb Haemost. 2000;84:1103–1108. [PubMed] [Google Scholar]
- Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci U S A. 2008;105:16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff G, Jonker M, Gubernatis G, Wonigeit K, Lauchart W, Bornscheuer A, et al. The course of untreated acute rejection and effect of repeated anti-CD3 monoclonal antibody treatment in rhesus monkey liver transplantation. Transplantation. 1990;49:669–674. doi: 10.1097/00007890-199004000-00002. [DOI] [PubMed] [Google Scholar]
- Stohl W, Jacob N, Quinn WJ, 3rd, Cancro MP, Gao H, Putterman C, et al. Global T cell dysregulation in non-autoimmune-prone mice promotes rapid development of BAFF-independent, systemic lupus erythematosus-like autoimmunity. J Immunol. 2008;181:833–841. doi: 10.4049/jimmunol.181.1.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storage SS, Agrawal H, Furst DE. Description of the efficacy and safety of three new biologics in the treatment of rheumatoid arthritis. Korean J Intern Med. 2010;25:1–17. doi: 10.3904/kjim.2010.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T, et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tamhane UU, Gurm HS. The chimeric monoclonal antibody abciximab: a systematic review of its safety in contemporary practice. Expert Opin Drug Saf. 2008;7:809–819. doi: 10.1517/14740330802500353. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2010;6:843–854. doi: 10.1586/eci.10.70. [DOI] [PubMed] [Google Scholar]
- Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25:601–613. doi: 10.1089/cbr.2010.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistlethwaite JR, Jr, Stuart JK, Mayes JT, Gaber AO, Woodle S, Buckingham MR, et al. Complications and monitoring of OKT3 therapy. Am J Kidney Dis. 1988;11:112–119. doi: 10.1016/s0272-6386(88)80192-6. [DOI] [PubMed] [Google Scholar]
- Thomas JM, Neville DM, Contreras JL, Eckhoff DE, Meng G, Lobashevsky AL, et al. Preclinical studies of allograft tolerance in rhesus monkeys: a novel anti-CD3-immunotoxin given peritransplant with donor bone marrow induces operational tolerance to kidney allografts. Transplantation. 1997;64:124–135. doi: 10.1097/00007890-199707150-00022. [DOI] [PubMed] [Google Scholar]
- Tomiyama Y. Glanzmann thrombasthenia: integrin alpha IIb beta 3 deficiency. Int J Hematol. 2000;72:448–454. [PubMed] [Google Scholar]
- Tomiyama-Hanayama M, Rakugi H, Kohara M, Mima T, Adachi Y, Ohishi M, et al. Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. Am J Physiol Renal Physiol. 2009;297:F679–F684. doi: 10.1152/ajprenal.90680.2008. [DOI] [PubMed] [Google Scholar]
- Treacy G. Using and analogous monoclonal antibody to evalaute the reproductive and chronic toxicity potential for a humanized anti-TNFalpha monoclonal anatibody. Hum Exp Toxciol. 2000;19:226–228. doi: 10.1191/096032700678815765. [DOI] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Timar J, Raso E, Kennel M, Emmell E, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62:2824–2833. [PubMed] [Google Scholar]
- Trivedi SM, Shani J, Hollander G. Bleeding complications of platelet glycoprotein IIb/IIIa inhibitor abciximab (ReoPro) J Invasive Cardiol. 2002;14:423–425. [PubMed] [Google Scholar]
- Tsunenari T, Akamatsu K, Kaiho S, Sato K, Tsuchiya M, Koishihara Y, et al. Therapeutic potential of humanized anti-interleukin-6 receptor antibody: antitumor activity in xenograft model of multiple myeloma. Anticancer Res. 1996;16:2537–2544. [PubMed] [Google Scholar]
- Tysabri USPI. 2011. Available at http://www.tysabri.com/en_US/tysb/site/pdfs/TYSABRI-pi.pdf (accessed 15 February 2012)
- Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, et al. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- Uchiyama Y, Yorozu K, Hashizume M, Moriya Y, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorates joint swelling in established monkey collagen-induced arthritis. Biol Pharm Bull. 2008;31:1159–1163. doi: 10.1248/bpb.31.1159. [DOI] [PubMed] [Google Scholar]
- Untch B, Ahmad S, Messmore HL, Schultz CL, Ma Q, Hoppensteadt DA, et al. Development of a non-human primate sub-clinical model of heparin-induced thrombocytopenia: platelet responses to human anti-heparin-platelet factor 4 antibodies. Thromb Res. 2002;106:149–156. doi: 10.1016/s0049-3848(02)00081-6. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan A, McKeever K, Anand B, Eppler S, Weinbauer GF, Beyer JC. Developmental immunotoxicology assessment of rituximab in cynomolgus monkeys. Toxicol Sci. 2011;119:116–125. doi: 10.1093/toxsci/kfq316. [DOI] [PubMed] [Google Scholar]
- Van Buren G, 2nd, Yang AD, Dallas NA, Gray MJ, Lim SJ, Xia L, et al. Effect of molecular therapeutics on liver regeneration in a murine model. J Clin Oncol. 2008;26:1836–1842. doi: 10.1200/JCO.2007.11.6566. [DOI] [PubMed] [Google Scholar]
- Vectibix USPI. 2011. Available at http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf (accessed 15 February 2012)
- Vinet E, Pineau C, Gordon C, Clarke AE, Bernatsky S. Biologic therapy and pregnancy outcomes in women with rheumatic diseases. Arthritis Rheum. 2009;61:587–592. doi: 10.1002/art.24462. [DOI] [PubMed] [Google Scholar]
- Walther A, Czabanka M, Gebhard MM, Martin E. Glycoprotein IIB/IIIA-inhibition and microcirculatory alterations during experimental endotoxemia – an intravital microscopic study in the rat. Microcirculation. 2004;11:79–88. doi: 10.1080/10739680490266216. [DOI] [PubMed] [Google Scholar]
- Wasson S, Zafar MN, Best J, Reddy HK. Post-transplantation lymphoproliferative disorder in heart and kidney transplant patients: a single-center experience. J Cardiovasc Pharmacol Ther. 2006;11:77–83. doi: 10.1177/107424840601100107. [DOI] [PubMed] [Google Scholar]
- Watanabe T, J-i M, Sohma Y, Inazawa H, Horie K, Kojima K, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120:247–259. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer – preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Wehner NG, Gasper C, Shopp G, Nelson J, Draper K, Parker S, et al. Immunotoxicity profile of natalizumab. J Immunotoxicol. 2009a;6:115–129. doi: 10.1080/15476910902977381. [DOI] [PubMed] [Google Scholar]
- Wehner NG, Shopp G, Oneda S, Clarke J. Embryo/fetal development in cynomolgus monkeys exposed to natalizumab, an alpha4 integrin inhibitor. Birth Defects Res B Dev Reprod Toxicol. 2009b;86:117–130. doi: 10.1002/bdrb.20190. [DOI] [PubMed] [Google Scholar]
- Wehner NG, Shopp G, Osterburg I, Fuchs A, Buse E, Clarke J. Postnatal development in cynomolgus monkeys following prenatal exposure to natalizumab, an alpha4 integrin inhibitor. Birth Defects Res B Dev Reprod Toxicol. 2009c;86:144–156. doi: 10.1002/bdrb.20193. [DOI] [PubMed] [Google Scholar]
- Wehner NG, Shopp G, Roccca MS. Effects of natalizumab, an α4 integrin inhibitor, on the development of Hartley guinea pigs. Birth Defects Res B Dev Reprod Toxicol. 2009d;86:98–107. doi: 10.1002/bdrb.20189. [DOI] [PubMed] [Google Scholar]
- Wehner NG, Skov M, Shopp G, Rocca MS, Clarke J. Effects of natalizumab, an alpha4 integrin inhibiutor, on fertility in male and female guinae pigs. Birth Defects Res B Dev Reprod Toxicol. 2009e;86:108–116. doi: 10.1002/bdrb.20191. [DOI] [PubMed] [Google Scholar]
- Wijkstrom M, Kenyon NS, Kirchhof N, Kenyon NM, Mullon C, Lake P, et al. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation. 2004;77:827–835. doi: 10.1097/01.tp.0000116390.76425.20. [DOI] [PubMed] [Google Scholar]
- Wilkins AL, Yang W, Yang JJ. Structural biology of the cell adhesion protein CD2: from molecular recognition to protein folding and design. Curr Protein Pept Sci. 2003;4:367–373. doi: 10.2174/1389203033487063. [DOI] [PubMed] [Google Scholar]
- van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant. 2010;10:773–783. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Itoh Y, Yokomizo C, Nishimura T, Niimi T, Umemura A, et al. Blockade of IL-6 signaling exacerbates liver injury and suppresses antiapoptotic gene expression in methionine choline-deficient diet-Fed db/db mice. Lab Invest. 2011;91:609–618. doi: 10.1038/labinvest.2011.2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Yamagata K, Hasuwa H, Inano E, Ikawa M, Okabe M. Cd52, known as a major maturation-associated sperm membrane antigen secreted from the epididymis, is not required for fertilization in the mouse. Genes Cells. 2008;13:851–861. doi: 10.1111/j.1365-2443.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17–23. doi: 10.1016/s1040-8428(00)00134-7. [DOI] [PubMed] [Google Scholar]
- Yao K, Gagnon S, Akhyani N, Williams E, Fotheringham J, Frohman E, et al. Reactivation of human herpesvirus-6 in natalizumab treated multiple sclerosis patients. PLoS ONE. 2008;3:e2028. doi: 10.1371/journal.pone.0002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon CH, Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest New Drugs. 2005;23:391–409. doi: 10.1007/s10637-005-2899-8. [DOI] [PubMed] [Google Scholar]
- Yervoy USPI. 2011. Available at http://packageinserts.bms.com/pi/pi_yervoy.pdf (accessed 15 February 2012)
- Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- Zenapax USPI. 2005. Available at http://www.gene.com/gene/products/information/zenapax/pdf/pi.pdf (accessed 15 February 2012)
- Zhang Z, Pascuet E, Hueber P-A, Chu L, Bichet DG, Lee T-C, et al. Targeted inactivation of EGF receptor inhibits renal collecting duct development and function. J Am Soc Nephrol. 2010;21:573–578. doi: 10.1681/ASN.2009070719. [DOI] [PMC free article] [PubMed] [Google Scholar]


