Abstract
BACKGROUND AND PURPOSE
The locus coeruleus (LC) is a major source of noradrenergic projections to the dorsal spinal cord, and thereby plays an important role in the modulation of nociceptive information. The LC receives inputs from substance P (SP)-containing fibres from other regions, and expresses the NK1 tachykinin receptor, a functional receptor for SP. In the present study, we investigated the roles of SP in the LC in neuropathic pain.
EXPERIMENTAL APPROACH
Chronic constriction injury (CCI) of the left sciatic nerve was performed in rats to induce neuropathic pain. After development of neuropathic pain, SP was injected into the LC and the nocifensive behaviours were assessed. The involvement of noradrenergic descending inhibition in SP-induced analgesia was examined by i.t. administration of yohimbine, an α2-adrenoceptor antagonist. NK1 receptor expression in the LC was examined by immunohistochemistry.
KEY RESULTS
In CCI rats, mechanical allodynia was alleviated by SP injection into the LC. These effects were abolished by prior injection of WIN 51708, an NK1 receptor antagonist, into the LC or i.t. treatment with yohimbine. NK1 receptor-like immunoreactivity was observed in noradrenergic neurons throughout the LC in intact rats, and remained unchanged after CCI.
CONCLUSION AND IMPLICATIONS
SP in the LC exerted analgesic effects on neuropathic pain through NK1 receptor activation and resulted in facilitation of spinal noradrenergic transmission. Accordingly, manipulation of the SP/NK1 receptor signalling pathway in the LC may be a promising strategy for effective treatment of neuropathic pain.
Keywords: locus coeruleus, NK1 receptor, substance P, neuropathic pain, noradrenaline, α2-adrenoceptor
Introduction
The locus coeruleus (LC) in the pons is a major source of noradrenergic projections to the dorsal horn of the spinal cord. These noradrenergic neurons play an important role in the modulation of nociceptive information at the level of the spinal cord (Millan, 2002; Pertovaara, 2006). Chemical or electrical stimulation of the LC produces antinociception that is attenuated by spinal administration of α2-adrenoceptor antagonists (Jones and Gebhart, 1986; West et al., 1993). Spinal administration of an α2-adrenoceptor agonist elicits antinociception in intact rats (Takano and Yaksh, 1992). In addition, noradrenergic neurons in the LC receive inputs from other pain-modulating systems, such as the periaqueductal grey (PAG) and rostral ventromedial medulla (RVM) (Clark and Proudfit, 1991a; Bajic and Proudfit, 1999). Despite a lack of noradrenergic neurons in the RVM or PAG, the antinociceptive effects induced by stimulation of the PAG or RVM are partially attenuated by spinal administration of α2-adrenoceptor antagonists, suggesting that these brain regions modulate nociception at least partially through indirect projections via noradrenergic neuronal nuclei including the LC (Barbaro et al., 1985; Aimone et al., 1987). The antinociceptive effects induced by stimulation of the lateral hypothalamus are also mediated by the LC (Safari et al., 2009). Taken together, the LC holds a key position in the endogenous descending modulation of pain by relaying inputs from several brain regions.
Neuropathic pain is an intractable chronic pain resulting from lesions or diseases of the sensory transmission pathways in the peripheral or central nervous system. In clinical practice, this pain syndrome remains a major issue because of the limited effectiveness of existing analgesics (Dworkin et al., 2007; Baron et al., 2010). Selective noradrenaline and 5-HT (serotonin) re-uptake inhibitors (O'Connor and Dworkin, 2009) and i.t. clonidine (Eisenach et al., 1995; Siddall et al., 2000; Ackerman et al., 2003), an α2-adrenoceptor agonist, have been shown to be effective in treating neuropathic pain in humans. However, their adverse side effects limit their use in clinical practice (Siddall et al., 2000; O'Connor and Dworkin, 2009). Gabapentin has also been reported to alleviate neuropathic pain through stimulation of the LC (Hayashida et al., 2008). Pregabalin also activates descending noradrenergic inhibition in a neuropathic pain state (Takeuchi et al., 2007). Thus, activation of the descending noradrenergic pathway is a potential strategy for treating neuropathic pain.
Substance P (SP) is one of principal neuropeptides involved in the modulation of pain in a variety of nociceptive systems, including peripheral tissues and the spinal cord (Zhang et al., 2007; Todd, 2010; Uematsu et al., 2011). In the LC, high levels of both the mRNA and protein of the NK1 receptor, a functional receptor for SP, are expressed in noradrenergic neurons (Nakaya et al., 1994; Chen et al., 2000; Caberlotto et al., 2003). SP-containing axon terminals are distributed in the vicinity of the dendrites of noradrenergic neurons in the LC (Pickel et al., 1979; Tamiya et al., 1994). Application of SP increases the LC neuronal firing rate in vivo (Guyenet and Aghajanian, 1977) and in vitro (Cheeseman et al., 1983). SP depolarizes the LC neurons by increasing an inward non-specific cation current and decreasing an inwardly rectifying potassium current (Shen and North, 1992; Koyano et al., 1993). Although there is anatomical and physiological evidence suggesting that SP directly activates the noradrenergic neurons in the LC, the roles of the SP/NK1 receptor system in the LC in the regulation of chronic pain, including neuropathic pain, remain unknown. In the present study, we examined the effects of SP in the LC in a rat model of neuropathic pain, and found that SP injected into the LC alleviated the neuropathic pain through activation of inhibitory noradrenergic descending pathways.
Methods
Animals
All animal care and experimental procedures were approved by the Institute's committee on laboratory animals (approval number 22–054) and performed under the guidelines recommended by the International Association for the Study of Pain (Zimmermann, 1983). A total of 128 adult male Sprague-Dawley rats (7 weeks of age and weighing between 210–250 g at the time of surgery) were used for all experiments. The rats were purchased from Japan SLC (Hamamatsu, Japan) and individually housed in plastic cages with soft bedding under a 14-h/10-h light/dark cycle at least 3 days before the experiments. Food and water were available ad libitum. Efforts were made to minimize animal suffering and reduce the number of animals used. All surgical procedures were performed under deep anaesthesia induced by i.p. administration of sodium pentobarbital (50 mg kg−1) with further pentobarbital administered as necessary. The level of anaesthesia was assessed visually by the lack of reflex responses, including withdrawal body movements and respiratory change.
Cannula implantation into the LC
The rats were deeply anaesthetized with pentobarbital and placed in a stereotaxic apparatus (Kopf, Tujunga, CA, USA) to permanently implant a guide cannula. A small burr hole was made in the skull and a stainless-steel guide cannula (8.2 mm in length, 26 gauge; AG-8.2, Eicom, Kyoto, Japan) was lowered in the left (ipsilateral) or right (contralateral) side to 1 mm above the LC (anteroposterior, −9.8 mm from the bregma; mediolateral, 1.3 mm from the midline; dorsoventral, 5.8 mm from the brain surface) (Paxinos and Watson, 1998). The guide cannula was secured to the skull with dental cement (UNIFAST 2, GC Corporation, Tokyo, Japan). A 33 gauge dummy cannula (AD-8.2, Eicom) was placed into the guide cannula to maintain the patency. The rats were allowed to recover from the surgery for 5–7 days.
I.t. catheter insertion
On the same day that the guide cannula was inserted, a polyethylene catheter (PE-10; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) filled with sterile saline was also inserted under anaesthesia with pentobarbital as previously described (Yaksh et al., 1985). The catheter was passed 7.5–8.0 cm in the subarachnoid space from the atlanto-occipital membrane to the level of lumbar enlargement. Only rats without apparent neurological dysfunction after the catheter insertion were used for the subsequent experiments.
Production of a neuropathic pain model
At 5–7 days after the guide cannula implantation, a chronic constriction injury (CCI) of the sciatic nerve was performed as described previously (Bennett and Xie, 1988), except that 4-0 silk thread was used instead of chromic gut. Briefly, the ipsilateral common sciatic nerve was exposed at the level of the middle thigh and loosely ligated with 4-0 silk thread in four regions at intervals of approximately 1 mm. The incision was closed with a 4-0 silk suture. The contralateral sciatic nerve was left intact as a control.
Behavioural tests
Two behavioural tests (von Frey and Plantar tests) were performed to assess the pain thresholds during the light cycle before and at 7 days after the CCI surgery. The rats were acclimatized to the testing environment for 15 min before the behavioural tests. A set of von Frey filaments (Muromachi Kikai, Tokyo, Japan) with bending forces ranging from 0.5 to 32.0 g was used to examine mechanical allodynia. Each rat was placed on a metallic mesh floor covered with a plastic box, and a von Frey monofilament was applied from underneath the mesh floor to the plantar surface of either the contralateral or ipsilateral hind paw. The weakest force (g) required to induce withdrawal of the stimulated paw at least three times in five trials was referred to as the paw withdrawal threshold. The Plantar Test (Ugo Basile, Varese, Italy) was used to examine thermal hyperalgesia. Each rat was placed on a glass plate with a radiant heat generator underneath, and each hindpaw was stimulated twice at 1 min intervals, alternating from side to side. The average time until withdrawal of the hindpaw from the thermal stimuli was recorded as the paw withdrawal latency. A 25 s cut-off was used to prevent tissue injury.
Injection of drugs into the LC
All injections were performed under light sedation with isoflurane (1.5–2%). SP (Peptide Institute, Osaka, Japan) was dissolved in saline at concentrations of 0.2, 0.5, 2, 5 and 20 pmol 0.5 µL−1 and saline was used as a control. WIN 51708 (5 pmol 0.5 µL−1; Sigma-Aldrich, St. Louis, MO, USA), a potent NK1 receptor antagonist (Venepalli et al., 1992), was dissolved in PBS (pH 7.4) containing 0.025% dimethyl sulfoxide and the solvent (vehicle) was used as a control for WIN 51708. The drugs were injected into the LC through a 33 gauge stainless-steel injection cannula (AMI-9.2, Eicom) that extended 1 mm below the tip of the guide cannula. Injections were performed using a 10 µL Hamilton syringe (Hamilton Company, Reno, NY, USA) connected to the injection cannula by a polytetrafluoroethylene tube (JT-10, Eicom). The drugs were slowly administered at a dose of 0.5 µL for 30 s and the injection cannula was left in place for at least 30 s to minimize any backflow of drug into the injector track. SP was injected twice with a 6-h interval on day 7 after CCI and either the von Frey or Plantar test was performed after a single SP injection. The order of these two tests was randomized. The behavioural test was conducted at 5 and 15 min after the injection of SP, when the rats had recovered from the sedation. WIN 51708 and vehicle for control was also injected into the LC in the same manner as SP. At 15 min after the WIN 51708 injection, SP (2 pmol 0.5 µL−1) was injected into the LC and the behavioural tests were performed as described earlier.
I.t. administration of the α2-adrenoceptor antagonist yohimbine
Yohimbine (Sigma-Aldrich), an α2-adrenoceptor antagonist, was dissolved in saline. The rats were administered i.t. 10 µL of yohimbine (20 µg; Sigma-Aldrich), or saline as a control, followed by 10 µL of saline. After 15 min, SP (2 pmol 0.5 µL−1) was injected into the LC and the behavioural tests were performed at 5 and 15 min after the SP injection as described earlier.
Histological examination of cannula placement
At the end of the experiments, the rats were deeply anaesthetized with pentobarbital and transcardially perfused with freshly prepared 4% paraformaldehyde in PBS. The pons was removed, post-fixed at 4°C overnight and cryoprotected in 20% sucrose in PBS at 4°C overnight. The pons was cut into serial transverse cryosections (20 µm in thickness) using a cryostat (Leica, Tokyo, Japan). The most ventral site of the cannula trail was examined under a microscope (Olympus, Tokyo, Japan). We used the behavioural data from the rats in which the guide cannulae were adequately positioned above the centre of LC. The following key landmarks were used to map the injection sites as described previously (Brightwell and Taylor, 2009): motor trigeminal nucleus; mesencephalic 5 nucleus; nucleus of the trapezoid body; middle cerebellar peduncle; and sensory root of the 7th cranial nerve.
Immunohistochemistry
Immunohistochemistry was performed on free-floating sections as previously described (Kobayashi et al., 2010). Briefly, transverse cryosections (20 µm) of the pons were incubated with a rabbit anti-NK1 receptor polyclonal antibody (1:5000 dilution; AB5060; Chemicon, Temecula, CA, USA) and a sheep anti-TH polyclonal antibody (1:1000 dilution; AB1542, Chemicon) for 3 days at 4°C. The specificities of the anti-TH and anti-NK1 receptor antibodies were shown in previous studies (Vigna et al., 1994; Kaufling et al., 2009). The sections were then incubated with secondary antibodies labelled with Alexa Fluor 594 (1:2000 dilution; Life Technologies, Carlsbad, CA, USA) for NK1 receptors and Alexa Fluor 488 (1:2000 dilution; Life Technologies) for TH. Images were captured using a high-resolution digital camera equipped with a computer (Olympus). Densitometric quantification of the immunofluorescence was performed using a computerized image analysis system (ImageJ, NIH, Bethesda, MA, USA). The averaged fluorescence intensity [arbitrary units (a.u.) from 0 to 255 per pixel] was obtained from three serial LC sections at 9.80 mm caudal to the bregma. Regions of interest were positioned on the LC based on the TH immunoreactivity.
Statistical analysis
Values are expressed as means ± SEM. Tukey–Kramer's multiple comparison test was used to compare the immunofluorescence intensities of TH and NK1 receptors among intact and CCI rats. Two-way repeated-measures anova followed by Tukey–Kramer's post hoc test was used to compare values in the paw withdrawal thresholds and paw withdrawal latencies at successive time points between drug and vehicle treatments. Values for the paw withdrawal thresholds and paw withdrawal latencies, among several time points in the experiments examining the effects of SP injection into the contralateral LC or into the vicinity of the ipsilateral LC, were compared with the pre-drug values by one-way repeated-measures anova followed by Dunnett's post hoc comparisons. Values of P < 0.05 were considered to indicate statistical significance.
Nomenclature
The drug/molecular target nomenclature conforms with BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Results
NK1 receptor expression in the noradrenergic LC neurons
To investigate the expression of NK1 receptors in the LC, we performed immunostaining for NK1 receptors and TH, a marker for noradrenergic neurons. TH-like immunoreactivity was observed in the neuronal cell bodies in the LC and the dendrites of LC neurons in the peri-LC dendritic field (n= 5; Figure 1A), as previously described (Aston-Jones et al., 2004). NK1 receptor-like immunoreactivity was observed in both the cell bodies and dendrites of TH-like immunoreactive neurons throughout the LC in intact rats (n= 5; Figure 1A).
Figure 1.
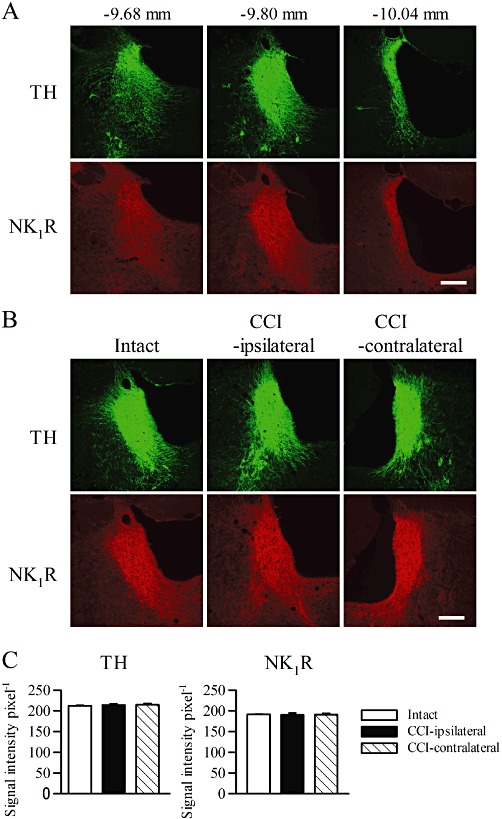
Representative images of immunohistochemical staining for TH and NK1 receptors (NK1 R) in the LC. (A) Coronal sections were obtained from the rostral to caudal regions of the LC. The numbers represent the distances from the bregma (n= 5). (B) Sections (−9.80 mm from the bregma) were obtained from intact rats (n= 5) and the contralateral and ipsilateral sides of CCI rats 7 days after the surgery (n= 6). Scar bars, 100 µm. (C) Densitometric quantification of TH and NK1 receptor immunoreactivities in the intact LC and the ipsilateral and contralateral LC of CCI rats (n= 6–10).
Next, we examined whether the TH and NK1 receptor expressions in the LC were affected by peripheral nerve injury. At 7 days after CCI, the TH-like immunoreactivity in the LC was unchanged on both the ipsilateral and contralateral sides (n= 5–6; Figure 1B and C). The NK1 receptor-like immunoreactivity in the LC was also unchanged on both the ipsilateral and contralateral sides (n= 5–6; Figure 1B and C).
Alleviation of mechanical allodynia in neuropathic pain by SP injected into the LC
To examine the effects of SP in the LC on neuropathic pain, SP was injected into the LC. As illustrated by the representative results shown in Figure 2A, most of the cannula tips were correctly positioned in the LC. The rats in which the cannula tips were incorrectly positioned were excluded from the analyses.
Figure 2.
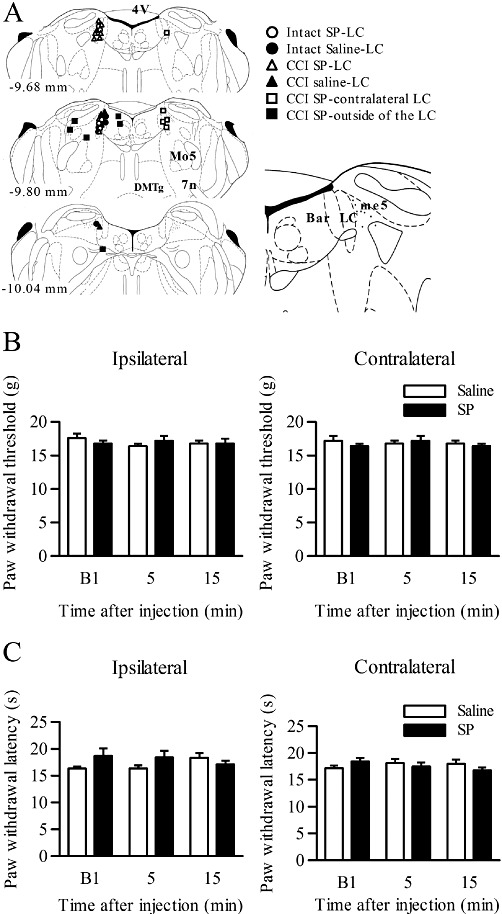
Effects of SP injection into the LC in intact rats. (A) Schematic depiction of the microinjection sites. The numbers on the left of the coronal sections represent the distances from the bregma. The different symbols represent the tip sites in intact or CCI rats injected with SP or saline, in CCI rats with contralateral SP injection and in CCI rats injected with SP outside the LC. Abbreviations: 4V, fourth ventricle; Bar, Barrington's nucleus; me5, mesencephalic 5 tract; DMTg, dorsomedial tegmental area; Mo5, motor trigeminal nucleus; 7n, facial nucleus or its root. (B, C) Paw withdrawal thresholds (B) and paw withdrawal latencies (C) on the ipsilateral and contralateral sides in response to mechanical and thermal stimuli in intact rats (n= 5). SP (2 pmol) or saline was injected into the ipsilateral LC. B1 indicates the values before drug injection.
In intact rats, injection of SP (2 pmol) into the ipsilateral LC did not affect the paw withdrawal thresholds in response to mechanical or thermal stimuli at 5 and 15 min after the injection, on both the ipsilateral and contralateral sides, compared with saline injection (n= 5; Figure 2B and C).
Next, we examined the effects of injection of SP (2 pmol) into the ipsilateral LC on mechanical allodynia in CCI rats, because most descending LC neurons predominantly innervate the ipsilateral spinal cord (Clark and Proudfit, 1991b; Howorth et al., 2009). Before the CCI operation, the ipsilateral paw withdrawal thresholds to mechanical stimuli in rats assigned to the SP and saline treatments were comparable (n= 5–8; Figure 3A). At 7 days after the CCI operation, the ipsilateral paw withdrawal thresholds to mechanical stimuli were significantly decreased compared with the values before the CCI operation (F(3,33)= 29.9, P= 0.000 by two-way repeated-measures anova and P= 0.000 by Tukey–Kramer's post hoc test; n= 5–8; Figure 3A). The ipsilateral paw withdrawal latencies to thermal stimuli were also significantly decreased (F(3,33)= 39.6, P= 0.000 by two-way repeated-measures anova and P= 0.000 by Tukey–Kramer's post hoc test; n= 5–8; Figure 3B). On the other hand, the contralateral paw withdrawal thresholds and latencies to mechanical and thermal stimuli, respectively, were unchanged in both groups at day 7 (n= 5–8; Figure 3A and B). At 5 min after SP injection into the ipsilateral LC, the paw withdrawal thresholds to mechanical stimuli were significantly increased compared with saline injection (F(1,11)= 5.8, P= 0.034 by two-way repeated-measures anova and P= 0.002 by Tukey–Kramer's post hoc test; n= 5–8; Figure 3A). The analgesic effect had worn off 15 min after the SP injection. The paw withdrawal thresholds to mechanical stimuli on the contralateral side were unchanged by SP injection at any time point (Figure 3A). Although SP showed a tendency to reduce thermal hyperalgesia at 5 min after the injection, there were no significant differences during the subsequent time course between the CCI rats with SP and saline injections (n= 5–8; Figure 3B).
Figure 3.
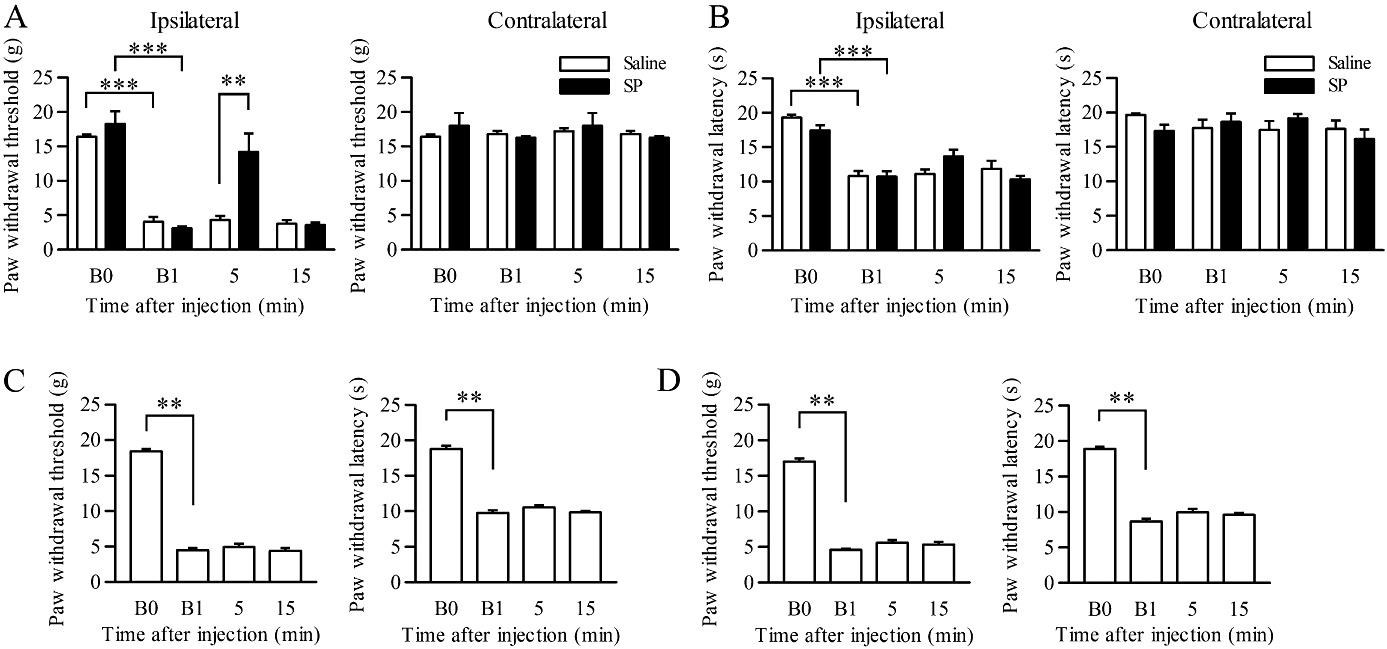
Analgesic effects of SP injected into the LC on mechanical allodynia. (A, B) Paw withdrawal thresholds (A) and paw withdrawal latencies (B) on the ipsilateral and contralateral sides in response to mechanical and thermal stimuli, respectively, in CCI rats at day 7. SP (2 pmol; n= 8) or saline (n= 5) was injected into the ipsilateral LC. **P < 0.01 and ***P < 0.001 by two-way repeated-measures anova followed by Tukey–Kramer's post hoc test. (C, D) Paw withdrawal thresholds and paw withdrawal latencies on the ipsilateral side in response to mechanical and thermal stimuli, respectively, in CCI rats at day 7. SP was injected into the contralateral LC (C; n= 6) or in the vicinity of the ipsilateral LC (D; n= 6). **P < 0.01, versus the value before drug injection by one-way repeated-measures anova followed by Dunnett's post hoc comparisons. B0 and B1 indicate the values before CCI and before drug injection at day 7 after the CCI operation, respectively.
Furthermore, we investigated the dose-dependency of the antinociceptive effects of SP. The analgesic effects on mechanical allodynia at 5 min after injection of SP into the LC were obvious at a dose of 2 pmol (F(5,30)= 4.5, P= 0.003 by one-way repeated-measures anova and P= 0.004 by Dunnett's post hoc test; n= 5–8; Table 1), whereas higher or lower doses of SP were ineffective.
Table 1.
Dose-response relationships for the analgesic effects of SP on mechanical allodynia and thermal hyperalgesia
| Paw withdrawal threshold (g) | Paw withdrawal latency (s) | |||
|---|---|---|---|---|
| Drug | Before injection | After injection | Before injection | After injection |
| Saline | 4.1 ± 0.8 | 4.3 ± 0.7 | 10.8 ± 0.9 | 11.1 ± 0.8 |
| SP (pmol) 0.2 | 4.9 ± 0.5 | 5.4 ± 0.5 | 10.8 ± 1.1 | 11.7 ± 0.9 |
| 0.5 | 3.5 ± 0.2 | 4.7 ± 0.6 | 10.5 ± 0.9 | 13.2 ± 1.3 |
| 2 | 3.1 ± 0.3 | 14.2 ± 2.9** | 10.7 ± 0.8 | 13.6 ± 1.1 |
| 5 | 3.8 ± 0.6 | 9.5 ± 1.5 | 10.2 ± 1.0 | 14.0 ± 1.8 |
| 20 | 4.3 ± 0.2 | 6.0 ± 2.0 | 10.3 ± 0.8 | 11.6 ± 0.9 |
The data show the paw withdrawal thresholds and paw withdrawal latencies on the ipsilateral side in response to mechanical and thermal stimuli, respectively, in CCI rats before and at 5 min after the injection of various doses of SP (n= 5–8).
P < 0.01, versus saline group at each time point.
We also examined the effects of SP injection into the contralateral LC on the ipsilateral paw withdrawal thresholds in CCI rats. Injection of SP (2 pmol) into the contralateral LC did not alleviate the mechanical allodynia and the thermal hyperalgesia at any time point (n= 5; Figure 3C). To verify that the analgesic effect was attributed to an LC-specific action, SP was injected into the vicinity of the LC (Figure 2A). Injection of SP (2 pmol) into the vicinity of LC did not affect the mechanical allodynia and the thermal hyperalgesia at any time point (n= 6; Figure 3D).
SP alleviates mechanical allodynia through activation of NK1 receptors in the LC
We investigated whether the analgesic effects of SP in the LC were caused by activation of the actual NK1 receptor. Injection of WIN 51708 alone at 5 pmol into the ipsilateral LC affected neither the mechanical allodynia nor thermal hyperalgesia at any time point in CCI rats as compared with vehicle alone (n= 5, Figure 4A). When WIN 51708 was injected 15 min before the SP injection, the analgesic effect of SP (2 pmol) on mechanical allodynia was abolished at 5 min after SP injection in CCI rats compared with vehicle injection (F(1,10)= 177.4, P= 0.000 by two-way repeated-measures anova and P= 0.000 by Tukey–Kramer's post hoc test; n= 6; Figure 4B). The effect of SP on thermal hyperalgesia was unchanged, although the paw withdrawal latency in response to the thermal stimuli was slightly prolonged in the vehicle-treated group compared with the WIN 51708-treated group (n= 6; Figure 4B).
Figure 4.
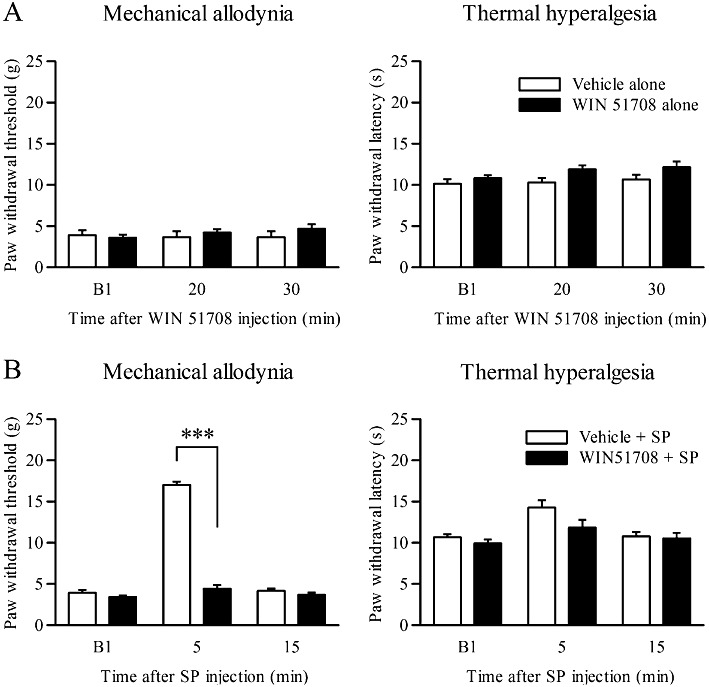
Effects of an NK1 receptor antagonist on the analgesic effects of SP. Drugs were injected at day 7 after the CCI operation. (A) Paw withdrawal thresholds and paw withdrawal latencies on the ipsilateral side at 20 and 30 min after WIN 51708 or vehicle injection into the LC (n= 5). (B) Paw withdrawal thresholds and paw withdrawal latencies on the ipsilateral side at 5 and 15 min after SP (2 pmol) injection into the LC following injection of WIN 51708 (5 pmol; n= 6) or vehicle (n= 6) into the LC. ***P < 0.001 by two-way repeated-measures anova followed by Tukey–Kramer's post hoc test. B1 indicates values before drug injection.
Neuropathic pain is alleviated by SP through spinal α2-adrenoceptor activation
It is well known that the noradrenergic neurons in the LC suppress nociception via activation of α2-adrenoceptors in the spinal cord. Therefore, we determined whether the analgesic effect of SP in the LC was mediated by spinal noradrenergic transmission. In rats pretreated with the α2-adrenoceptor antagonist yohimbine i.t. 15 min before the SP injection, the analgesic effect of SP on mechanical allodynia was abolished in CCI rats at 5 min after SP injection compared with those pretreated with i.t. saline (F(1,9)= 27.3, P= 0.001 by two-way repeated-measures anova and P= 0.000 by Tukey–Kramer's post hoc test; n= 5–6; Figure 5A). The latency in response to thermal stimuli was significantly shortened by in the CCI rats pretreated with yohimbine at 5 min after SP injection (F(1,9)= 11.3, P= 0.008 by two-way repeated-measures anova and P= 0.000 by Tukey–Kramer's post hoc test; n= 5–6; Figure 5B). Injection of yohimbine alone did not affect either the mechanical allodynia or the thermal hyperalgesia at any time point (data not shown).
Figure 5.
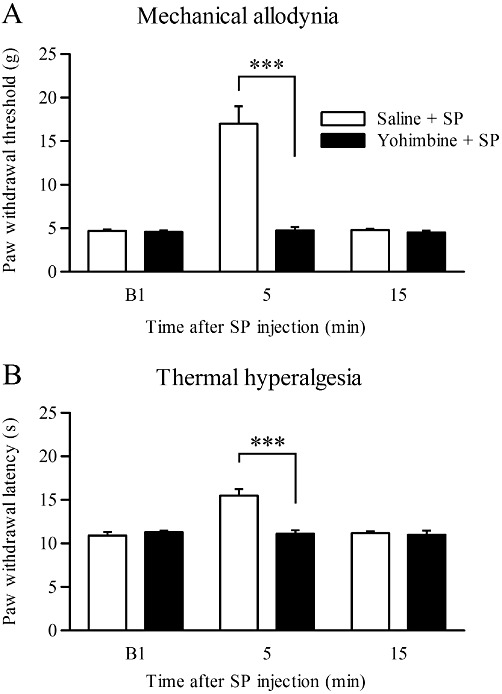
Effects of i.t. administration of an α2-adrenoceptor antagonist (yohimbine) on the analgesic effects of SP. (A, B) Paw withdrawal thresholds and paw withdrawal latencies on the ipsilateral side in response to mechanical stimuli (A) and thermal stimuli (B) at 5 and 15 min after SP (2 pmol) injection into the LC following i.t. administration of yohimbine (20 µg; n= 6) or saline (n= 5). ***P < 0.001 by two-way repeated-measures anova followed by Tukey–Kramer's post hoc test.
Discussion and conclusions
NK1 receptor activation by SP in the LC induces analgesia in a neuropathic pain state
In the present study, SP applied to the LC exerted analgesic effects on mechanical allodynia in a rat CCI model of neuropathic pain. The analgesic effects of SP were considered to be mediated by NK1 receptor activation, because SP-induced analgesia was blocked by prior treatment with an NK1 receptor antagonist, and NK1 receptor immunoreactivity was abundantly expressed in almost all of the TH-positive noradrenergic neurons in the LC, consistent with previous reports (Hahn and Bannon, 1999; Chen et al., 2000). SP-containing nerve terminals innervate noradrenergic LC neurons (Pickel et al., 1979; Tamiya et al., 1994). SP was reported to depolarize LC neurons (Shen and North, 1992; Koyano et al., 1993) and increase the firing rate of LC neurons (Guyenet and Aghajanian, 1977; Cheeseman et al., 1983). However, NK1 receptor antagonists do not modify the firing rate of NA neurons in the LC in vivo (Haddjeri and Blier, 2008), suggesting that SP is not tonically released onto the LC neurons in physiological conditions. On the other hand, the release of SP is considerably enhanced by stress exposure (Ebner and Singewald, 2007). The expression of c-fos in the LC induced by restraint stress and s.c. formalin can be attenuated by i.c.v. administration of an NK1 receptor antagonist (Hahn and Bannon, 1999; Baulmann et al., 2000). Since WIN 51708 did not affect the nociceptive threshold in the present study, SP inputs into the LC do not seem to be persistently activated in the neuropathic pain state in this model. Also, in the present study, the analgesic effects of SP injection on thermal hyperalgesia were not statistically significant. Although the mechanisms underlying this discrepancy between our results and previous findings are not known, previous studies have indicated that peripheral sensitization may play a role in thermal hyperalgesia, whereas central sensitization plays a role in mechanical allodynia (Woolf, 2011). Since SP-evoked noradrenaline release into the spinal cord is expected to suppress central sensitization, mechanical allodynia is more likely to be affected by SP injection than thermal hyperalgesia.
It is intriguing that the analgesic effects of SP were more marked at 2 pmol than at 20 pmol. Similar dose-response relationships for the effects of SP were demonstrated in previous reports (Kubo and Kihara, 1987; Yeomans and Proudfit, 1992), although the underlying mechanisms are not known. One possible mechanism is receptor desensitization. SP-induced NK1 receptor internalization is markedly influenced by the concentration of SP (Mantyh et al., 1995; Roosterman et al., 2004). Alternatively, diffusion of SP to distinct neuronal nuclei adjacent to the injection site might facilitate the nociception and thereby antagonize the antinociceptive effects in the LC. In fact, another descending pathway originating from the RVM expresses NK1 receptors (Budai et al., 2007; Pinto et al., 2008) and facilitates nociception in the neuropathic pain state (Zhang et al., 2009; Wei et al., 2010; Mase et al., 2011). However, this possibility seems less likely, because injection of SP into regions adjacent to the LC did not induce analgesic effects. The third possibility is that SP fragments counteract the effects of SP. SP is metabolized to SP (1–7), a major bioactive N-terminal fragment of SP, in the rat, and SP (1–7) reportedly exerts opposite effects to SP (Nyberg and Hallberg, 2007).
Noradrenergic descending pathways and spinal α2-adrenoceptor activation mediate the analgesic effects of SP injected into the LC
The importance of NK1-mediated tachykininergic functions in the LC has been shown in NK1 knockout mice. The development of some types of analgesia, which are thought to be mediated by descending inhibitory pathways, is markedly reduced in NK1 knockout mice (De Felipe et al., 1998). Fisher et al. (2007) showed that somatodendritic α2A-adrenoeceptors are desensitized in the LC in NK1 knockout mice. In the present study, injection of SP into the LC ipsilateral, but not contralateral, to the injured peripheral nerve induced analgesic effects, which were abolished by i.t. application of an α2-adrenoceptor antagonist. Consistently, LC activation has been reported to exert analgesic effects on neuropathic pain (Pertovaara, 2006; Viisanen and Pertovaara, 2007), although it has also been shown that inhibition of LC neuronal activity suppresses neuropathic pain (Brightwell and Taylor, 2009). Stimulation of the LC induces release of noradrenaline in the spinal cord (Hentall et al., 2003). Anatomical studies have shown that noradrenergic descending projections are bilateral, but predominantly innervate the ipsilateral spinal dorsal horn (Clark and Proudfit, 1991b; Howorth et al., 2009). Electrical stimulation of the LC produces antinociceptive effects only in the ipsilateral hind limb in rats with neuropathic pain (Viisanen and Pertovaara, 2007). Therefore, SP may activate the noradrenergic LC neurons and thereby facilitate descending inhibition mainly on the ipsilateral side in the spinal cord. In addition, the antinociceptive efficacy of α2-adrenoceptor agonists has been reported to be increased in the neuropathic pain state (Obata et al., 2005). The efficacy of G protein coupling from spinal α2-adrenoceptors was also increased, whereas no significant change in the total number or affinity of α2-adrenoceptors was observed (Bantel et al., 2005). Therefore, a functional upregulation of spinal α2-adrenoceptors may also contribute to the analgesic effects of SP on neuropathic pain.
However, in the present study in intact rats, injection of SP into the LC did not affect the normal nociceptive behaviour. SP can depolarize and increase the firing rate of LC neurons (Guyenet and Aghajanian, 1977; Cheeseman et al., 1983; Shen and North, 1992; Koyano et al., 1993), and electrical stimulation of the LC induces spinal release of noradrenaline, resulting in changes in the nociceptive threshold in intact rats (Hentall et al., 2003). On the other hand, lesions of the bilateral LC, pharmacological inhibition of α-adrenoceptors in the spinal cord and noradrenergic denervation, induced by i.t. injection of dopamine β-hydroxylase antibodies conjugated to saporin, do not change the basal withdrawal responses to nociceptive stimuli (Takano and Yaksh, 1992; Tsuruoka and Willis, 1996; Jasmin et al., 2003). Therefore, noradrenergic descending pathways may not be activated in the intact state, and application of SP alone into the LC may be not sufficient to change the nociceptive threshold through an effect on noradrenaline release in the spinal cord.
In conclusion, SP in the LC exerted antinociceptive effects in the neuropathic pain, but not normal, condition through NK1 receptor activation, and resulted in facilitation of spinal noradrenergic transmission. These findings suggest that detailed analysis of the NK1 receptor signalling pathway in the control of nociception in the LC neurons and their descending fibres may assist in the development of an effective medication for neuropathic pain.
Acknowledgments
This study was supported by a Grant-in-Aid for Encouragement of Young Scientists (B) (22791457 to A.S.) from the Japan Society for the Promotion of Science, a Grant-in-Aid for Science Research (20591820 to A.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a grant (S0801035 to H.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
- CCI
chronic constriction injury
- LC
locus coeruleus
- PAG
periaqueductal grey
- RVM
rostral ventromedial medulla
- SP
substance P
- WIN 51708
17-β-hydroxy-17-α-ethynyl-5-α-androstano[3,2-β]pyrimido[1,2-α]benzimidazole
Conflict of interest
None.
References
- Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668–677. doi: 10.1016/s0885-3924(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Zhu Y, Card JP. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J Neurosci. 2004;24:2313–2321. doi: 10.1523/JNEUROSCI.5339-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic D, Proudfit HK. Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J Comp Neurol. 1999;405:359–379. [PubMed] [Google Scholar]
- Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases α2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Res. 1985;343:223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Baulmann J, Spitznagel H, Herdegen T, Unger T, Culman J. Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience. 2000;95:813–820. doi: 10.1016/s0306-4522(99)00478-9. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160:174–185. doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M, et al. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- Cheeseman HJ, Pinnock RD, Henderson G. Substance P excitation of rat locus coeruleus neurones. Eur J Pharmacol. 1983;94:93–99. doi: 10.1016/0014-2999(83)90445-4. [DOI] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Liu HL, Rao ZR. Noradrenergic neurons expressing substance P receptor (NK1) in the locus coeruleus complex: a double immunofluorescence study in the rat. Brain Res. 2000;873:155–159. doi: 10.1016/s0006-8993(00)02494-x. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Res. 1991a;540:105–115. doi: 10.1016/0006-8993(91)90496-i. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991b;538:231–245. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJH, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. Stress-induced release of substance P in the locus coeruleus modulates cortical noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:73–82. doi: 10.1007/s00210-007-0185-3. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D. Epidural clonidine analgesia for intractable cancer pain. Pain. 1995;61:391–399. doi: 10.1016/0304-3959(94)00209-W. [DOI] [PubMed] [Google Scholar]
- Fisher AS, Stewart RJ, Yan T, Hunt SP, Stanford SC. Disruption of noradrenergic transmission and the behavioural response to a novel environment in NK1R-/- mice. Eur J Neurosci. 2007;25:1195–1204. doi: 10.1111/j.1460-9568.2007.05369.x. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Aghajanian GK. Excitation of neurons in the nucleus locus coeruleus by substance P and related peptides. Brain Res. 1977;136:178–184. doi: 10.1016/0006-8993(77)90144-5. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P. Neurokinin-1 receptor antagonists modulate brain noradrenaline and serotonin interactions. Eur J Pharmacol. 2008;600:64–70. doi: 10.1016/j.ejphar.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Bannon MJ. Stress-induced c-fos expression in the rat locus coeruleus is dependent on neurokinin 1 receptor activation. Neuroscience. 1999;94:1183–1188. doi: 10.1016/s0306-4522(99)00319-x. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Pain Med. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall ID, Mesigil R, Pinzon A, Noga BR. Temporal and spatial profiles of pontine-evoked monoamine release in the rat's spinal cord. J Neurophysiol. 2003;89:2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsive pontospinal noradrenergic neurons in the rat in vivo. J Comp Neurol. 2009;512:141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Comp Neurol. 2003;460:38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Characterization of coeruleospinal inhibition of the nociceptive tail-flick reflex in the rat: mediation by spinal α2-adrenoceptors. Brain Res. 1986;364:315–330. doi: 10.1016/0006-8993(86)90844-9. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T, et al. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci U S A. 2010;107:8434–8439. doi: 10.1073/pnas.0912690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K, Velimirović BM, Grigg JJ, Nakajima S, Nakajima Y. Two signal transduction mechanisms of substance P-induced depolarization in locus coeruleus neurons. Eur J Neurosci. 1993;5:1189–1197. doi: 10.1111/j.1460-9568.1993.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Blood pressure modulation by substance P in the rat nucleus tractus solitarius. Brain Res. 1987;413:379–383. doi: 10.1016/0006-8993(87)91033-x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, et al. Rapid endocytosis of a G protein-coupled receptor: substance P-evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase H, Sakai A, Sakamoto A, Suzuki H. A subset of µ-opioid receptor-expressing cells in the rostral ventromedial medulla contribute to thermal hyperalgesia in experimental neuropathic pain. Neurosci Res. 2011;70:35–43. doi: 10.1016/j.neures.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Nyberg F, Hallberg M. Peptide conversion – a potential pathway modulating G-protein signaling. Curr Drug Targets. 2007;8:147–154. doi: 10.2174/138945007779315597. [DOI] [PubMed] [Google Scholar]
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Obata H, Li X, Eisenach JC. α2-Adrenoceptor activation by clonidine enhances stimulation-evoked acetylcholine release from spinal cord tissue after nerve ligation in rats. Anesthesiology. 2005;102:657–662. doi: 10.1097/00000542-200503000-00027. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth edn. San Diego: Academic Press; 1998. [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Reis DJ, Leeman SE, Miller RJ. Electron microscopic localization of substance P and enkephalin in axon terminals related to dendrites of catecholaminergic neurons. Brain Res. 1979;160:387–400. doi: 10.1016/0006-8993(79)91068-0. [DOI] [PubMed] [Google Scholar]
- Pinto M, Sousa M, Lima D, Tavares I. Participation of µ-opioid, GABAB, and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: implications for descending modulation of nociceptive transmission. J Comp Neurol. 2008;510:175–187. doi: 10.1002/cne.21793. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. J Biol Chem. 2004;279:30670–30679. doi: 10.1074/jbc.M402479200. [DOI] [PubMed] [Google Scholar]
- Safari MS, Haghparast A, Semnanian S. Effect of lidocaine administration at the nucleus locus coeruleus level on lateral hypothalamus-induced antinociception in the rat. Pharmacol Biochem Behav. 2009;92:629–634. doi: 10.1016/j.pbb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Molloy AR, Walker S, Mather LE, Rutkowski SB, Cousins MJ. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493–1498. doi: 10.1097/00000539-200012000-00037. [DOI] [PubMed] [Google Scholar]
- Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J Pharmacol Exp Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin, S-(+)-3-isobutylgaba, activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology. 2007;53:842–853. doi: 10.1016/j.neuropharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Tamiya R, Inoue K, Takagi H. GABA-ergic and substance P-ergic double-innervation to noradrenergic neurons in the rat locus coeruleus. Osaka City Med J. 1994;40:1–11. [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruoka M, Willis WD. Bilateral lesions in the area of the nucleus locus coeruleus affect the development of hyperalgesia during carrageenan-induced inflammation. Brain Res. 1996;726:233–236. [PubMed] [Google Scholar]
- Uematsu T, Sakai A, Ito H, Suzuki H. Intra-articular administration of tachykinin NK1 receptor antagonists reduces hyperalgesia and cartilage destruction in the inflammatory joint in rats with adjuvant-induced arthritis. Eur J Pharmacol. 2011;668:163–168. doi: 10.1016/j.ejphar.2011.06.037. [DOI] [PubMed] [Google Scholar]
- Venepalli BR, Aimone LD, Appell KC, Bell MR, Dority JA, Goswami R, et al. Synthesis and substance P receptor binding activity of androstano[3,2-b]pyrimido[1,2-a]benzimidazoles. J Med Chem. 1992;35:374–378. doi: 10.1021/jm00080a025. [DOI] [PubMed] [Google Scholar]
- Vigna SR, Bowdenn JJ, McDonald DM, Fisher J, Okamoto A, McVey DC, et al. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994;14:834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viisanen H, Pertovaara A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience. 2007;146:1785–1794. doi: 10.1016/j.neuroscience.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WL, Yeomans DC, Proudfit HK. The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague-Dawley rats. Brain Res. 1993;626:127–135. doi: 10.1016/0006-8993(93)90571-4. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience. 1992;49:681–691. doi: 10.1016/0306-4522(92)90236-u. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCε: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gardell S, Zhang D, Xie JY, Agnes RS, Badghisi H, et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132:778–787. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


