Abstract
BACKGROUND AND PURPOSE
Although naturally occurring polyamines are indispensable for a variety of cellular events in eukaryotic cells, little attention has been paid to their physiological and pathological significance in bone remodelling to date. In this study, we evaluated the pharmacological properties of several natural polyamines on the functionality and integrity of bone in both in vitro and in vivo experiments.
EXPERIMENTAL APPROACH
Mice were subjected to ovariectomy (OVX) and subsequent oral supplementation with either spermidine or spermine for determination of the bone volume together with different parameters regarding bone formation and resorption by histomorphometric analyses in vivo. Pre-osteoclasts were cultured with receptor activator of NF-κB ligand (RANKL), with or without spermidine and spermine to determine cellular maturation by tartrate-resistant acid phosphatase (TRAP) staining and cellular viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide reduction in vitro.
KEY RESULTS
Spermidine or spermine, given in drinking water for 28 days, significantly prevented the increased osteoclast surface/bone surface ratio and the reduced bone volume following OVX in mice. Either spermidine or spermine significantly inhibited the increased number of multinucleated TRAP-positive cells in osteoclasts cultured with RANKL in a concentration-dependent manner without affecting cell survival.
CONCLUSIONS AND IMPLICATIONS
The natural polyamines spermidine and spermine prevented OVX-induced bone loss through the disruption of differentiation and maturation of osteoclasts, rather than affecting osteoblasts. The supplementation with these natural polyamines could be beneficial for the prophylaxis as well as therapy of metabolic bone diseases such as post-menopausal osteoporosis.
Keywords: osteoclast, osteoblast, ovariectomy, polyamine, spermidine, spermine, osteoporosis, bone resorption, NF-κB, p-65
Introduction
The prevailing view is that bone-resorbing osteoclasts and bone-forming osteoblasts carefully regulate bone homeostasis (Karsenty et al., 2009). Osteoclasts are multinucleated cells derived from hematopoietic stem cells shared with macrophage and dendritic cell lineages (Boyle et al., 2003; Teitelbaum and Ross, 2003), while the osteoblast lineage is derived from primitive multipotent mesenchymal stem cells with potential to differentiate into bone marrow stromal cells, chondrocytes, muscles and adipocytes (Ducy et al., 2000; Harada and Rodan, 2003). Imbalance between regulations by osteoclasts and osteoblasts leads to the pathogenesis as well as the aetiology of certain metabolic bone diseases including osteoporosis, Paget's disease and osteopetrosis (Feng and McDonald, 2011). Osteoporosis is a common disease characterized by a systemic impairment of bone mass, strength and microarchitecture, which leads to the increased fragility of bone integrity towards possible fractures (Rodan and Martin, 2000; Rachner et al., 2011). Fractures commonly occur in the spine, hip or wrist, thereby resulting in a loss of mobility and autonomy, and subsequently confer a major detrimental impact on the quality of life in patients with osteoporosis, in addition to increasing medical and socio-economic concerns (Center et al., 1999).
The naturally occurring polyamines such as putrescine (1,4-butane diamine), spermidine (N-(3-(aminopropyl)-1,4-butane diamine) and spermine (N, N′-bis (3-aminopropyl)-1,4-butane diamine) are well known to be essential regulators for diverse cellular processes including DNA stability, transcription, translation and apoptosis, besides modulating cellular growth and differentiation (Igarashi and Kashiwagi, 2010). Several independent lines of evidence indicate the possible relationship between particular polyamines and pathophysiology in some disorders. For example, both spermidine and spermine inhibited experimental inflammation in association with suppressed expression of pro-inflammatory cytokines (Zhang et al., 1997; Soda et al., 2005). A clear correlation is seen between polyamine levels and the incidence of symptoms of skeletal muscle hypertrophy (Turchanowa et al., 2000) and of neurodegenerative disorders such as Alzheimer's disease (Morrison and Kish, 1995) and ischaemia (Paschen et al., 1987). Exogenous supplementation with spermidine has recently been shown to prolong the lifespan of several model organisms including yeast, nematodes, flies and mice (Eisenberg et al., 2009). However, little attention has been paid to pharmacological properties of natural polyamines on bone remodelling mediated by osteoblasts and osteoclasts to date. In the present study, therefore, we have focused on two naturally occurring polyamines (spermidine and spermine), with regard to possible pharmacological properties beneficial for the prophylaxis as well as the therapy of post-menopausal osteoporosis using both in vitro and in vivo experimental analyses.
Methods
Ovariectomy (OVX)
All animal care and experimental protocols used complied with the guidelines of the Japanese Society for Pharmacology and was approved by the Committee for Ethical Use of Experimental Animals at Kanazawa University (approval number: AP-101806). All efforts were made to minimize animal suffering, to reduce the number of animals used (56 mice were used in this study), and to utilize alternatives to in vivo techniques. Female ddY mice were kept in cages under a standard 12:12 light/dark cycle, and allowed access to food and water ad libitum. Eight-week-old mice were anaesthetized by an i.p. injection of pentobarbital (50 mg·kg−1) and subjected to OVX or sham operation under aseptic environments as described previously (Uno et al., 2011). Ovariectomized mice were treated with daily oral supplementation with either spermidine or spermine, freshly dissolved in drinking water at concentrations of 0.3 or 3 mM every day. No significant change was seen in the amount of daily intake of drinking water by mice, regardless of the concentrations of polyamines used. Mice were killed by decapitation 28 days after operation, followed by dissection of femurs, tibiae and vertebrae, and subsequent fixation with either 10% formalin or 70% ethanol.
Bone histomorphometric analyses
Bone histomorphometric analyses were performed on undecalcified vertebrae as previously described (Kajimura et al., 2011). Briefly, vertebrae were fixed with 10% formalin, followed by dehydration in different concentrations of ethanol and subsequent embedding in methyl methacrylate resin according to standard protocols. The bone volume : tissue volume (BV/TV) ratio was measured by Von Kossa staining. Bone formation rate (BFR) was analysed by the calcein double-labelling method. Calcein was injected to mice twice with an interval of 3 days, and then mice were killed 2 days after the last injection. Osteoblast and osteoclast parameters were analysed by staining with toluidine blue or with tartrate-resistant acid phosphatase (TRAP) respectively. Analyses were performed using the Osteomeasure Analysis System (Osteometrics, Atlanta, GA, USA) according to standard protocols (Parfitt et al., 1987).
Culture of osteoclasts, TRAP staining, actin ring assay and pit formation assay
Primary osteoclasts were prepared from bone marrows of both tibia and femur isolated from male Std-ddY mice at 4 weeks old according to the procedures previously described (Hinoi et al., 2007). Primary osteoclasts were cultured in the presence of 20 ng·mL−1 macrophage colony-stimulating factor (M-CSF) and 20 ng·mL−1 receptor activator of NF-κB ligand (RANKL) for 5 consecutive days. Pre-osteoclastic RAW264.7 cells were also cultured with 20 ng·mL−1 RANKL for 4 consecutive days. For TRAP staining, cultured cells were fixed with 10% formalin in PBS, and subsequently with ethanol-acetone (50:50; v/v). Cells were then incubated in acetate buffer (pH 5.0) containing naphthol AS-MX phosphate as a substrate and fast red violet LB salt as a dye in the presence of 50 mM sodium tartrate. TRAP-positive cells with more than five nuclei were scored as TRAP-positive multinucleated cells (MNCs). For actin ring assay, cells were cultured on a bovine bone slice for 5 days, followed by fixation with 4% paraformaldehyde and subsequent treatment with 0.1% Triton X-100 for immunostaining by Alexa Fluor 488 phalloidin. For pit formation analysis, cells were removed from bone slices with a mechanical agitation. Bone slices were then incubated with peroxidase-conjugated wheat germ agglutinin for 1 h and stained with 3,3′-diaminobenzidine.
Determination of cellular viability
Cultured cells were washed with PBS and incubated with 0.5 mg·mL−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), followed by addition of 0.04 M HCl in isopropyl alcohol to the well and subsequent shaking of the mixture to dissolve the formazan. The dissolved suspension was subjected to an elisa reader and the absorbance at a wavelength of 550 nm was measured. In RAW264.7 cells exposed to 100 µM H2O2 for 24 h, a marked decrease of MTT reduction was always seen, as a result of cell death.
Real-time quantitative reverse transcription PCR
Total RNA was extracted from cells, followed by synthesis of cDNA with reverse transcriptase and oligo-dT primer. The cDNA samples were then used as templates for real-time PCR analysis, which was performed on an MX3005P instrument (Agilent Technologies, Santa Clara, CA, USA), by using specific primers for each gene. Expression levels of the genes examined were normalized by using the 36b4 expression levels as an internal control for each sample.
Determination of alkaline phosphatase (ALP) activity and Ca2+ accumulation in cultured osteoblasts
Primary osteoblasts were prepared from calvaria of 1- to 2-day-old mice by the sequential enzymatic digestion method as described previously (Uno et al., 2011). Pre-osteoblastic MC3T3-E1 cells were cultured in the presence of differentiation cocktails (50 µg·mL−1 ascorbic acid and 5 mM β-glycerophosphate). Determinations of ALP activity and Ca2+ accumulation were done as indices of cellular differentiation and maturation respectively. In brief, osteoblastic cells were solubilized with 0.1% Triton X-100, followed by determination of the ALP activity in lysates using p-nitrophenol phosphate as a substrate. The lysates solubilized with 0.1% Triton X-100 were also treated with hydrochloric acid for 16 to 24 h, followed by centrifugation at 20,000×g and subsequent determination of Ca2+ contents in the supernatant using a commercial kit.
Luciferase assay
Osteoclastic RAW264.7 cells were transfected with the luciferase reporter vector containing five tandem copies of NF-κB site (pNF-κB-Luc) as described previously (Hinoi et al., 2007), followed by culture with or without spermidine and spermine at different concentrations (0.1–10 µM) for 24 h and subsequent incubation with RANKL at 50 ng·mL−1 for an additional 24 h before determination of the luciferase activity using specific substrates in a luminometer (ATTO, Tokyo, Japan). Transfection efficiency was normalized by determining the activity of Renilla luciferase.
Immunoblotting analysis
Cultured cells were solubilized in lysis buffer containing 1% Nonidet P-40. Samples were then subjected to SDS-PAGE, followed by transfer to nitrocellulose membranes and subsequent immunoblotting assay as described previously (Uno et al., 2011). Quantification was performed by densitometry using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Data analysis
Results are expressed as means ± SE and the statistical significance was determined by two-tailed and unpaired Student's t-test or by one-way anova with Bonferroni/Dunnett's post hoc test.
Materials
Putrescine, spermidine, spermine, calcein, naphthol AS-MX phosphate and fast red violet Lurina-Bertani salt were obtained from Sigma Chemicals (St Louis, MO, USA). Recombinant mouse M-CSF and recombinant mouse RANKL were purchased from R&D Systems International (Minneapolis, MN, USA). Antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphorylated p65 and p65 were all from Cell Signaling Technology (Danvers, MA, USA). Dual luciferase assay system and RNase-free DNase were purchased from Promega (Madison, WI, USA). A pNF-κB-Luc was provided by Stratagene (La Jolla, CA, USA). M-MLV Reverse Transcriptase was supplied by Invitrogen (Carlsbad, CA, USA). THUNDERBIRD SYBR qPCR Mix was supplied by TOYOBO (Osaka, Japan). ISOGEN and calcium C-test kit was obtained from WAKO (Osaka, Japan). elisa kits for C-telopeptide of type I collagen (CTx), bone alkaline phosphatase (B-ALP) and tartrate-resistant acid phosphatase-5b (TRACP-5b) were obtained from Mybiosource (San Diego, CA, USA) and Immunodiagnosticsystems (Boldon, UK) respectively. Other chemicals used were all of the highest purity commercially available.
Results
Spermidine and spermine prevent OVX-induced bone loss and osteoclast activation
We first administered spermidine or spermine in drinking water to ovariectomized mice for 28 consecutive days. Although OVX drastically decreased the uterine weight, determined 28 days after operation, the OVX-induced loss of uterine weight was unaffected by either spermidine or spermine, irrespective of the concentrations used in the drinking water (Figure 1A). Under these experimental conditions, a marked reduction was observed in cancellous bone (Von Kossa staining) in vertebrae of ovariectomized mice compared with that of sham-operated mice (Figure 1B). In vertebrae of ovariectomized mice given either spermidine or spermine, however, this loss of cancellous bone was markedly decreased. Quantification of these data clearly revealed that the supplementation with either spermidine or spermine significantly suppressed the reduction of BV/TV ratios in cancellous bone of ovariectomized mice, without affecting those in sham-operated mice (Figure 1C).
Figure 1.

Oral supplementation with spermidine and spermine inhibits OVX-induced bone loss. Eight-week-old female ddY mice were subjected to OVX, followed by daily oral intake of spermidine (SPD) or spermine (SPM) in drinking water (0.3 mM or 3 mM) for 28 consecutive days (sham-none, n= 12; sham-SPD 3 mM, n= 4; sham-SPM 3 mM, n= 4; OVX-none, n= 12; OVX-SPD 0.3 mM, n= 6; OVX-SPD 3 mM, n= 6; OVX-SPM 0.3 mM, n= 6; OVX-SPM 3 mM, n= 6). Mice were decapitated for determination of (A) uterine weight and BV/TV ratios of vertebrae by Von Kossa staining. Typical pictures of Von Kossa staining are shown in the panel (B), while quantitative data are shown in the panel (C). **P < 0.01, significantly different from each control value obtained in sham-operated mice. #P < 0.05, ##P < 0.01, significantly different from the values obtained in ovariectomized mice.
To define the cellular basis of the protective effect of spermidine and spermine against bone loss, histomorphometric analyses were performed on vertebral sections. In vertebrae of ovariectomized mice, significant increases were seen in the extent of osteoclast surface/bone surface (Oc.S/BS) (Figure 2A), the number of osteoclast/bone perimeter (N.Oc/B.Pm) (Figure 2B), the extent of osteoblast surface/bone surface (Ob.S/BS) (Figure 2C), the number of osteoblast/bone perimeter (N.Ob/B.Pm) (Figure 2D) and BFR (Figure 2E). The administration of either spermidine or spermine significantly diminished the increased osteoclastic indices such as Oc.S/BS (Figure 2A) and N.Oc/B.Pm (Figure 2B) in vertebrae of ovariectomized mice to the level in sham-operated mice, but not of elevated osteoblastic indices including Ob.S/BS (Figure 2C), N.Ob/B.Pm (Figure 2D) and BFR (Figure 2E). In accordance with the results from the BV/TV ratios, neither spermidine nor spermine affected any histomorphometric parameters examined in sham-operated mice. A significant increase was seen in serum levels of CTx (Figure 2F), TRACP-5b (Supporting Information Figure S1A) and B-ALP (Supporting Information Figure S1B) in ovariectomized mice. Administration of spermine significantly normalized these increased serum levels of both CTx (Figure 2F) and TRACP-5b (Supporting Information Figure S1A). Although spermidine was similarly effective in significantly preventing the OVX-induced increase in serum CTx levels (Figure 2F), neither spermidine nor spermine significantly changed the increased serum level of B-ALP (Supporting Information Figure S1B).
Figure 2.
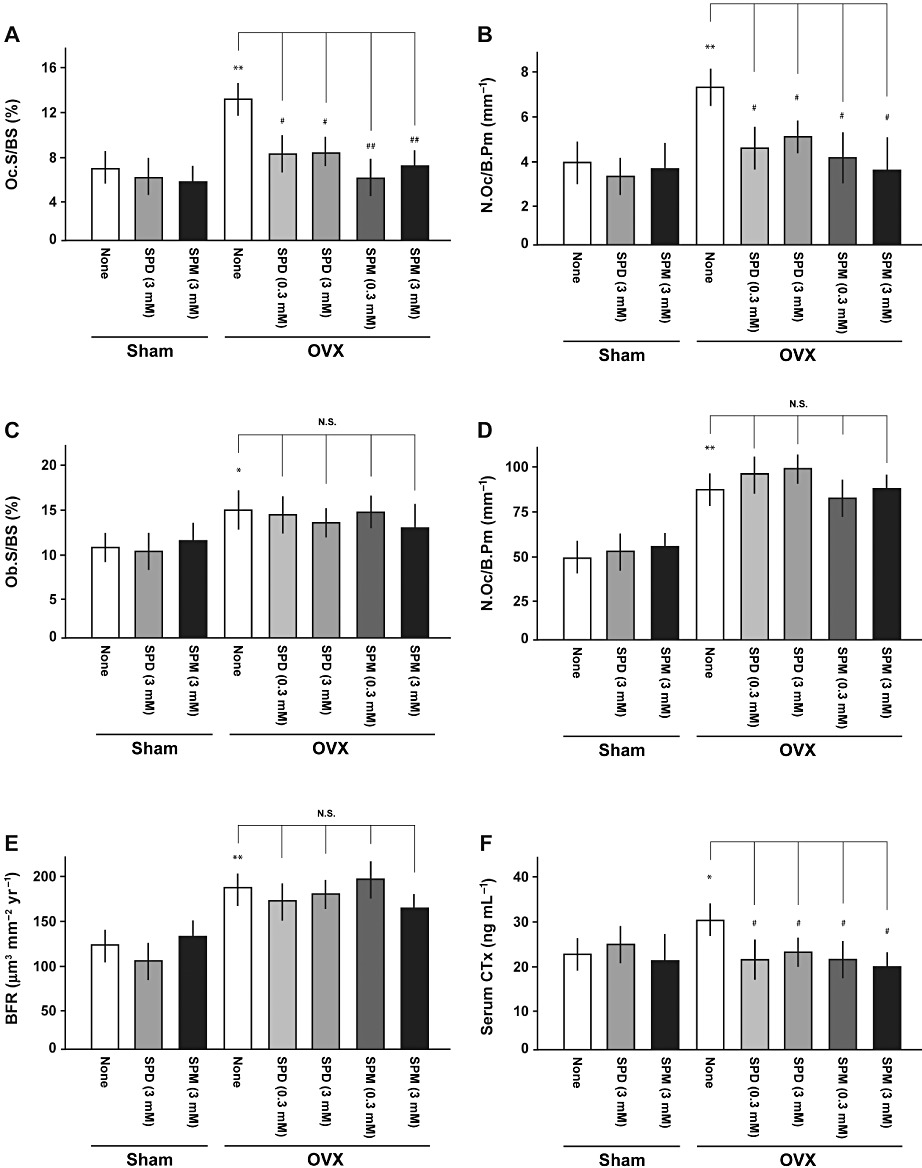
Oral supplementation with spermidine and spermine inhibits OVX-induced osteoclastic activation. Eight-week-old female ddY mice were subjected to OVX, followed by intake of spermidine (SPD) or spermine (SPM) in drinking water and subsequent determination of different parameters for bone formation and resorption at 28 days after operation (sham-none, n= 12; sham-SPD 3 mM, n= 4; sham-SPM 3 mM, n= 4; OVX-none, n= 12; OVX-SPD 0.3 mM, n= 6; OVX-SPD 3 mM, n= 6; OVX-SPM 0.3 mM, n= 6; OVX-SPM 3 mM, n= 6). Bone histomorphometric analyses were performed on vertebrae using the Osteomeasure Analysis System. Different analyses were done with determinations of (A) Oc.S/BS, (B) N.Oc/B.Pm, (C) Ob.S/BS, (D) N.Ob/B.Pm and (E) BFR respectively. (F) Serum CTx levels were also measured. *P < 0.05, **P < 0.01, significantly different from each control value obtained in sham-operated mice. #P < 0.05, ##P < 0.01, significantly different from the value obtained in ovariectomized mice. N.S., not significant.
Inhibitory effect of spermidine and spermine on RANKL-induced osteoclast differentiation and maturation
Pre-osteoclastic RAW264.7 cells were cultured with RANKL with or without spermidine, spermine or putrescine at concentrations of 1 nM to 1 µM for 4 consecutive days, followed by TRAP staining for counting the number of TRAP-positive MNCs. Both spermidine (Figure 3A and Supporting Information Figure S2A) and spermine (Figure 3B and Supporting Information Figure S2B) significantly decreased the number of TRAP-positive MNCs in a concentration-dependent manner at concentrations over 100 nM and 10 nM, respectively, while both spermidine and spermine failed to significantly affect MTT reduction as an index of cellular survival at concentrations examined. Moreover, propidium iodide-positive cells were not observed at all in RAW264.7 cells cultured with either spermidine or spermine at the highest concentration used (data not shown). By contrast, putrescine did not significantly alter MTT reduction or the number of TRAP-positive MNCs at concentrations of 1 nM to 1 µM (Figure 3C and Supporting Information Figure S2C). To confirm the pharmacological profiles of polyamines, RAW264.7 cells were cultured in the presence of RANKL with either spermidine or spermine at 1 µM for 4 consecutive days for determination of osteoclastic marker gene expression. This sustained exposure markedly decreased mRNA expression of all osteoclastic marker genes examined in RAW264.7 cells (Figure 3D). These were Ctsk, Calcr, Acp5, Mmp9 or Tm7sf4. Moreover, both spermidine and spermine significantly prevented RANKL-induced osteoclastic differentiation without affecting cell survival at the concentrations used in primary cultured murine osteoclasts (Figure 3E).
Figure 3.
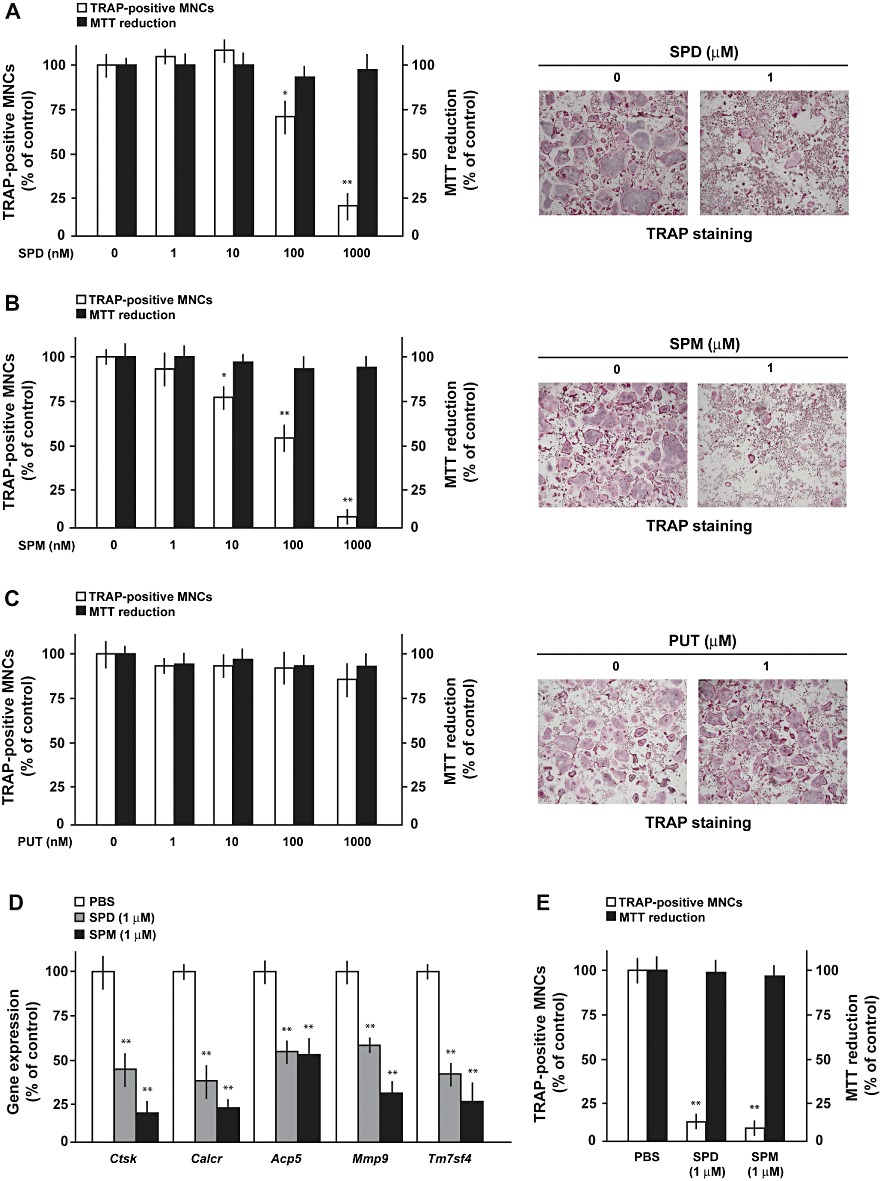
Spermidine and spermine inhibit osteoclastic differentiation without affecting cell survival. Pre-osteoclastic RAW264.7 cells were cultured with RANKL in either the presence or absence of (A) spermidine (SPD), (B) spermine (SPM) and (C) putrescine (PUT) at a concentration range from 1 nM to 1 µM, followed by determination of the number of TRAP-positive MNCs and MTT reduction. Quantitative data are shown on the left, while typicalmicrophotographs are shown on the right. (D) RAW264.7 cells were cultured with RANKL in either the presence or absence of spermidine and spermine, followed by real-time PCR for determination of mRNA expression of osteoclastic marker genes. (E) Primary osteoclasts were cultured with RANKL with or without 1 µM spermidine and spermine, followed by determination of the number of TRAP-positive MNCs and MTT reduction. *P < 0.05, **P < 0.01, significantly different from each control value obtained in the absence of polyamines added.
To further investigate the effects of polyamines on osteoclastic maturation, pre-osteoclastic RAW264.7 cells were cultured with RANKL with or without spermidine and spermine at 1 µM for 5 consecutive days, followed by determination of actin ring and pit formations. Both polyamines significantly decreased the number of actin rings (Figure 4A) and the area of pit formation (Figure 4B) in RANKL-treated RAW264.7 cells. Moreover, cathepsin K protein expression was markedly decreased in RAW264.7 cells cultured with RANKL in the presence of spermidine or spermine at 1 µM (Figure 4C).
Figure 4.

Spermidine and spermine inhibit osteoclastic maturation. Pre-osteoclastic RAW264.7 cells were cultured with RANKL in either the presence or absence of spermidine (SPD) and spermine (SPM) at 1 µM, followed by determination of (A) actin ring and (B) pit formations. Typical microphotographs are shown on left, while quantitative data are shown on the right. **P < 0.01, significantly different from each control value obtained in the absence of any polyamines added. (C) RAW264.7 cells were cultured with RANKL with or without spermidine and spermine, followed by immunoblotting for determination of cathepsin K expression.
Natural polyamines do not alter cell viability, differentiation and maturation in cultured osteoblasts
Pre-osteoblastic MC3T3-E1 cells were next cultured with differentiation cocktails in either the presence or absence of three different natural polyamines, followed by determination of cell survival with MTT reduction, cell differentiation with ALP activity and cell maturation with Ca2+ accumulation as an individual index. As shown in Figure 5A, ALP activity was gradually increased from 7 to 21 days with a slight decline thereafter by 28 days, while Ca2+ accumulation was not detected up to 14 days but markedly increased from 21 to 28 days. Under these experimental conditions, however, spermidine (Figure 5B), spermine (Figure 5C) and putrescine (Figure 5D) did not affect ALP activity and MTT reduction at concentrations of 10 nM to 1 µM in MC3T3-E1 cells cultured for 21 days. Moreover, sustained exposure to these polyamines did not lead to significant alterations of Ca2+ accumulation in osteoblastic MC3T3-E1 cells cultured for 21 days (Figure 5E). No significant alterations were observed in ALP activity determined at 7, 14 and 28 days, or Ca2+ accumulation determined at 28 days, in MC3T3-E1 cells cultured with these three polyamines examined (data not shown). Similar negative results were also seen in ALP activity and Ca2+ contents in primary murine osteoblasts cultured for 21 days (data not shown). Furthermore, neither spermidine, spermine nor putrescine altered mRNA expression of genes involved in osteoclast differentiation, such as Tnfsf11, Tnfrsf11b, Csf1 and Il6, in MC3T3-E1 cells (Figure 5F).
Figure 5.

SPD and spermine do not alter osteoblastic differentiation and maturation. (A) Pre-osteoblastic MC3T3-E1 cells were cultured with differentiation cocktails, followed by determination of ALP activity and Ca2+ contents. **P < 0.01, significantly different from value obtained in cells cultured for 7 days. ##P < 0.01, significantly different from value obtained in cells cultured for 21 days. MC3T3-E1 cells were cultured with differentiation cocktails in either the presence or absence of (B) spermidine (SPD), (C) spermine (SPM) or (D) putrescine (PUT) at a concentration range from 10 nM to 1 µM, followed by determination of ALP activity and MTT reduction. (E) MC3T3-E1 cells were cultured with differentiation cocktails with or without thethree polyamines at 1 µM, followed by determination of Ca2+ contents. (F) MC3T3-E1 cells were cultured with or without spermidine, spermine and putrescine, followed by real-time PCR for determination of mRNA expression of genes involved in osteoclast differentiation. N.D., not detectable.
Spermidine and spermine inhibit transcriptional activity of NF-κB in osteoclasts
An attempt was next made to elucidate the underlying mechanism for the inhibition by spermidine and spermine of osteoclastic differentiation. Pre-osteoclastic RAW264.7 cells were cultured with RANKL for 4 consecutive days, while polyamines were added to culture medium on different days after the initiation of culture with RANKL for TRAP staining on day 4. The number of TRAP-positive MNCs was significantly decreased in cells exposed to either spermidine or spermine for 4 days from day 0 to day 4, whereas both polyamines failed to significantly decrease the number of TRAP-positive MNCs when added to cells later than 1 day after the initiation of culture with RANKL (Figure 6A).
Figure 6.

Spermidine and spermine inhibit transcriptional activity of NF-κB. (A) RAW264.7 cells were cultured with RANKL for 4 consecutive days, while polyamines were added on different days after the initiation of culture at 1 µM, followed by TRAP staining on Day 4. **P < 0.01, significantly different from each control value obtained in cells cultured in the absence of polyamines. (B) RAW264.7 cells were transiently transfected with the reporter plasmid of NF-κB, followed by culture with or without spermidine (SPD) and spermine (SPM) at 0.1 to 10 µM and subsequent further culture with RANKL for 24 h. **P < 0.01, significantly different from each control value obtained in cells cultured in the absence of both RANKL and polyamines. #P < 0.05, ##P < 0.01, significantly different from the value obtained cells cultured in the presence of RANKL alone. (C) RAW264.7 cells were exposed to spermidine or spermine at 1 µM for 24 h, followed by further treatment with RANKL at 50 ng·mL−1 for 5 min and subsequent preparation of cell lysates for immunoblotting analysis. A typical result is shown in this panel, with similar results in three independent determinations.
Accordingly, RAW264.7 cells were transiently transfected with pNF-κB-Luc, followed by culture in either the presence or absence of spermidine and spermine at 0.1 to 10 µM for 24 h and subsequent addition of RANKL 24 h before determination of the luciferase activity. The addition of RANKL increased, more than three-fold, the luciferase activity in RAW264.7 cells transfected with NF-κB reporter plasmid, while the further addition of either spermidine or spermine prevented this increase by RANKL in a concentration-dependent manner at concentrations over 1 µM (Figure 6B). However, both polyamines did not affect the luciferase activity in cells cultured in the absence of RANKL even at the highest concentration used.
We next investigated the effect of polyamines on the RANKL-initiated signalling cascade in osteoclasts. RAW264.7 cells were exposed to 1 µM spermidine or spermine for 2 days, followed by further incubation with RANKL for 5 min and subsequent determination of the phosphorylation of p65 on immunoblotting analysis. Both spermidine and spermine markedly inhibited the phosphorylation of p65, without affecting p65 levels in cells cultured with RANKL, while both polyamines did not affect the phosphorylation in cells cultured in the absence of RANKL (Figure 6C).
Discussion
The essential message of the present findings is that daily oral supplementation with the natural polyamines spermidine and spermine significantly prevented the decreased bone volume and the increased osteoclastic parameters observed in ovariectomized mice in vivo. Moreover, both spermidine and spermine markedly prevented the RANKL-induced osteoclastic differentiation in association with the inhibition of phosphorylation and transcriptional activity of the transcription factor NF-κB without affecting cell survival in vitro. In previous studies, natural polyamines (putrescine, spermidine and spermine) inhibited the calcium mobilization induced by parathyroid hormone (Ljunggren et al., 1991) and calcitriol (Stern et al., 1991) in a concentration-dependent manner in cultured calvarial bone. In addition, the Snyder–Robinson syndrome associated with splice mutation in spermine synthase is a rare X-linked syndrome characterized by skeletal defects and osteoporosis, in addition to mental retardation (Cason et al., 2003). Although these previous studies are suggestive of the modulating effects of natural polyamines on osteoclastic functions essential for bone resorption, no direct evidence is available for the prevention by natural polyamines in vivo against OVX-induced bone loss, in conjunction with the in vitro inhibition of cellular differentiation and maturation in osteoclasts.
However, the reason why both spermidine and spermine failed to affect different bone histomorphometric parameters in sham-operated mice in vivo in spite of their strong inhibitory properties on in vitro osteoclastogenesis is not clarified so far. This paradox could be resolved by the fact that the in vitro analysis involved the differentiation and maturation of osteoclasts cultured with RANKL alone, in contrast to the in vivo situation where other cells are present alongside osteoclasts in bone, together with the expression of a range of other cytokines apart from RANKL. For example, several cytokines such as IL-4 and IL-13 are shown to promote the production of the decoy receptor for RANKL osteoprotegerin (OPG) in osteoblasts, in addition to directly inhibiting differentiation of osteoclastic precursors, towards diminished osteoclastogenesis (Yamada et al., 2007). By contrast, the anti-RANKL antibody prevented or reversed the loss of bone mass, density and strength in ovariectomized monkeys (Ominsky et al., 2011), suggesting a pivotal role of RANKL up-regulation in the underlying mechanism for bone loss after OVX. Considering all these data, one possibility is that either spermidine or spermine may depress osteoclastic differentiation and maturation through a mechanism related to the preferential interference with signalling processes mediated by RANKL in osteoclasts. The present findings that both spermidine and spermine inhibited NF-κB-Luc activity and p65 phosphorylation, only in the presence of RANKL in pre-osteoclastic RAW264.7 cells give support to this idea. Unidentified paracrine and/or autocrine factors from cells other than osteoclasts may mediate the marked inhibition by polyamines of osteoclastogenesis in bones of sham-operated mice. Although the exact mechanism underlying the inhibition by several natural polyamines of the RANKL/NF-κB pathway required for osteoclastogenesis remains to be elucidated in future studies, these polyamines could be beneficial for patients suffering from post-menopausal osteoporosis without any severe untoward side effects. In this study, moreover, we employed female mice at 8 weeks old for the OVX operation in order to follow, in vivo, the experimental conditions used earlier (Nakamura et al., 2007). As mice are still developing a skeleton at this age, however, it is possible that the use of a skeletally immature model may confound the results in the present study. This could be one reason why the overall BV/TV ratio was relatively higher in sham-operated animals with less bone loss after OVX, than the corresponding values reported earlier in this model . At any rate, bone morphometric analysis as well as BV/TV ratio determination undoubtedly validates our experimental protocols used for a post-menopausal osteoporosis model.
It should also be noted that both spermidine and spermine did not prevent the increases in BFR and osteoblast numbers, along with significant amelioration of the increased histomorphometric osteoclast parameters, in ovariectomized mice. These findings suggest that osteoblastogenesis could be, at least in part, activated by a mechanism not related to the compensation for activated osteoclastogenesis after oestrogen deficiency in ovariectomized mice. Indeed, oestrogen deficiency after OVX increased plasma levels of systemic factors promoting both proliferation and differentiation of osteoblasts in rats (Yokose et al., 1996). The T-cell costimulatory molecule CD40 ligand (CD40L) is responsible for the expanded stromal cells and promoted osteoblastogenesis after OVX by the increased production of the osteoclastogenic factors M-CSF, RANKL and OPG, whereas OVX failed to induce bone loss and resorption in mice defective of CD40L (Li et al., 2011). Osteoblastogenesis could be directly activated as a consequence of this altered expression of endogenous factors after OVX, in addition to a compensation for the stimulated osteoclastogenesis. Accordingly, it is possible that polyamines may ameliorate the increased osteoclastic parameters through a mechanism involving interference with the RANKL/NF-κB signalling pathway in osteoclasts, without affecting the increased osteoblastic indices, after oestrogen deficiency in ovariectomized mice. By taking into consideration the present data on in vivo bone morphometric parameters, it is unlikely that those active polyamines affect ALP activity and/or Ca2+ accumulation in immature osteoblastic cells at an early differentiation stage in vitro.
Cellular polyamine levels are regulated by a balance between biosynthesis, catabolism, metabolism, uptake and excretion (Pegg, 1988; Wallace et al., 2003). There are two rate-limiting enzymes in the polyamine biosynthesis pathway; ornithine decarboxylase (Pendeville et al., 2001) and S-adenosylmethionine decarboxylase (Nishimura et al., 2002). Spermidine/spermine N1-acetyltransferase-1 is the rate-limiting enzyme in the polyamine catabolic pathway (Casero and Pegg, 1993). Moreover, polyamines are metabolized by serum amine oxidases such as polyamine oxidase and spermine oxidase (Vujcic et al., 2002), generating reactive oxygen species (ROS) including hydrogen peroxide as well as cytotoxic aldehydes including acrolein (Higgins et al., 1969; Sharmin et al., 2001). There is accumulating evidence that both ROS and aldehydes regulate osteoclast functions in terms of cellular viability, differentiation and maturation (Bai et al., 2005; Tsuji-Naito, 2008). The present findings that treatment with either spermidine or spermine inhibited the RANKL-induced formation of TRAP-positive MNCs without affecting cell survival in osteoclasts cultured in the presence of fetal bovine serum suggests that the inhibition by polyamines would be at least in part mediated by cytotoxic polyamine metabolites, such as acrolein and hydrogen peroxide, formed during the culture with fetal bovine serum. In our preliminary experiments, however, neither catalase nor aldehyde dehydrogenase affected the inhibition by polyamines of the formation of TRAP-positive MNCs in RAW264.7 cells (data not shown). Although these findings argue in favour of the possible intracellular action site of polyamines extracellularly added, the effective concentrations (µM) are considerably lower than the endogenous intracellular polyamine levels (mM) (Igarashi and Kashiwagi, 2000). One possibility is that particular natural polyamines added extracellularly would interfere with the interaction between RANKL and RANK at cell surfaces in a structure-selective manner in osteoclastic cells. An alternative possibility that exogenous polyamines would play a pivotal role different from that of endogenous polyamines in the regulation of osteoclastogenesis, however, is not ruled out so far.
Osteoclastogenesis is a multistep process dependent on the cellular interaction of myeloid pre-osteoclastic precursors with either osteoblasts or stromal cells under the influence of a wide range of local autocrine and/or paracrine factors such as M-CSF (Yoshida et al., 1990) and RANKL (Lacey et al., 1998). The NF-κB signalling pathway is widely accepted as being critical for osteoclastogenesis after the interaction between RANKL and RANK (Novack, 2011). Mice defective in both p50 and p52 NF-κB subunits display severe osteopetrosis due to the impairment of osteoclast differentiation (Iotsova et al., 1997), indeed, while pre-osteoclast specific IκB kinase-β conditional knockout mice show increased bone mass (Otero et al., 2008). In addition to the NF-κB signalling pathway, several transcription factors such as nuclear factor of activated T cells (NFATc1) and activator protein-1, also play a critical and essential role in osteoclastogenesis downstream of RANKL signalling (Takayanagi et al., 2002; Teitelbaum, 2004). Considering our preliminary findings that spermidine and spermine failed to modify the transcriptional activity of NFAT and activator protein-1 in RAW264.7 cells (data not shown). It is possible that both spermidine and spermine would elicit a preventive effect on osteoclast differentiation through a mechanism highly relevant to the selective inhibition of the RANKL-initiated NF-κB signalling pathway.
Soybean and various types of soy products, such as natto, tofu and miso, have long been a part of human diet in Japan (Kubota and Shimizu, 2009). Soybean, a rich source of plant proteins, contains a relatively high amount of calcium in addition to isoflavones which are diphenolic compounds with a chemical structure similar to oestrogen that bind to both types of oestrogen receptors (Lagari and Levis, 2010). Natto (fermented soybeans) contains vitamin K, which is involved in the activation of osteocalcin (Yamaguchi et al., 1999). In addition, soybeans have the highest amount of polyamines (spermidine and spermine) among different natural foods (Bardócz, 1993; Okamoto et al., 1997). As both spermidine and spermine are not enzymically degraded in the alimentary tract, these two polyamines taken orally should be quickly absorbed from the intestinal lumen and distributed to all organs and tissues (Bardócz et al., 1995). Indeed, a long-term intake of polyamine-rich foods gradually increased blood polyamine levels in humans and animals (Soda et al., 2009). On the basis of the present findings, we would propose that the appropriate consumption of soybean and soy products could be beneficial for the maintenance of bone health and the prophylaxis of menopausal osteoporosis through a mechanism linked to the preventive profiles of particular polyamines, in addition to isoflavones, calcium and vitamin K, against osteoclast differentiation and maturation without affecting bone volume in healthy subjects.
In conclusion, some natural polyamines, such as spermidine and spermine, prevent OVX-induced bone loss through inhibiting osteoclast activation. The tetraamine spermine elicited more effective prevention of RANKL-induced osteoclast differentiation in vitro as well as OVX-induced bone loss in vivo than the triamine spermidine in this study. Evaluation of the structure–activity relationship would provide clues to the discovery and development of novel drugs useful for the treatment of a variety of bone disorders related to osteoclastic overactivation such as osteoporosis, rheumatoid arthritis and/or bone metastasis of tumours.
Acknowledgments
This work was supported in part by Grants-in-Aids for Scientific Research to E.H. (No. 22659065) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and in part by research grants to E.H. from the Nakajima Foundation and the Kanzawa Medical Research Foundation, Japan.
Glossary
- ALP
alkaline phosphatase
- B-ALP
bone alkaline phosphatase
- BFR
bone formation rate
- BV/TV
bone volume : tissue volume ratio
- CD40L
CD40 ligand
- CTx
C-telopeptide of type I collagen
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IκB
inhibitory-κB
- IL
interleukin
- M-CSF
macrophage colony-stimulating factor
- MNCs
multinucleated cells
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- NFAT
nuclear factor of activated T cells
- N.Ob/B.Pm
number of osteoblast/bone perimeter
- N.Oc/B.Pm
number of osteoclast/bone perimeter
- Ob.S/BS
osteoblast surface/bone surface
- Oc.S/BS
osteoclast surface/bone surface
- OPG
osteoprotegerin
- OVX
ovariectomy
- PBS
phosphate-buffered saline
- p-p65
phosphorylated p65
- RANK
receptor activator of nuclear factor-κB
- RANKL
receptor activator of nuclear factor-κB ligand
- ROS
reactive oxygen species
- RT-PCR
reverse transcription polymerase chain reaction
- TRAP
tartrate-resistant acid phosphatase
- TRACP-5b
tartrate-resistant acid phosphatase-5b
Conflicts of interest
No authors have any conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effect of spermidine (SPD) and spermine (SPM) on serum bone remodelling markers. Eight-week-old female ddY mice were subjected to OVX, followed by oral supplementation with spermidine or spermine, and subsequent determination of serum levels of (A) TRACP-5b and (B) B-ALP. *P < 0.05, significantly different from each control value obtained in sham-operated mice. #P < 0.05, significantly different from the value obtained in ovariectomized mice. N.S., not significant.
Figure S2 Spermidine (SPD) and spermine (SPM) inhibit osteoclastic differentiation. Pre-osteoclastic RAW264.7 cells were cultured with RANKL in either the presence or absence of (A) spermidine, (B) spermine and (C) putrescine at a concentration range from 1 to 100 nM, followed by TRAP staining.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- Bardócz S. The role of dietary polyamines. Eur J Clin Nutr. 1993;47:683–690. [PubMed] [Google Scholar]
- Bardócz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, et al. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase–the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- Cason AL, Ikeguchi Y, Skinner C, Wood TC, Holden KR, Lubs HA. X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur J Hum Genet. 2003;11:937–944. doi: 10.1038/sj.ejhg.5201072. [DOI] [PubMed] [Google Scholar]
- Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- Higgins ML, Tillman MC, Rupp JP, Leach FR. The effect of polyamines on cell culture cells. J Cell Physiol. 1969;74:149–154. doi: 10.1002/jcp.1040740206. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Takarada T, Uno K, Inoue M, Murafuji Y, Yoneda Y. Glutamate suppresses osteoclastogenesis through the cystine/glutamate antiporter. Am J Pathol. 2007;170:1277–1290. doi: 10.2353/ajpath.2007.061039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000;42:39–51. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- Kubota M, Shimizu H. Nutrition and bone health. Soybean and soy foods, and bone health. Clin Calcium. 2009;19:1514–1519. [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lagari VS, Levis S. Phytoestrogens and bone health. Curr Opin Endocrinol Diabetes Obes. 2010;17:546–553. doi: 10.1097/MED.0b013e32833f4867. [DOI] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren O, Fredholm BB, Lerner UH. On the role of polyamines in bone resorption induced by parathyroid hormone. Acta Physiol Scand. 1991;142:267–273. doi: 10.1111/j.1748-1716.1991.tb09156.x. [DOI] [PubMed] [Google Scholar]
- Morrison LD, Kish SJ. Brain polyamine levels are altered in Alzheimer's disease. Neurosci Lett. 1995;197:5–8. doi: 10.1016/0304-3940(95)11881-v. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nakatsu F, Kashiwagi K, Ohno H, Saito T, Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- Novack DV. Role of NF-κB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Sugi E, Koizumi Y, Yanagida F, Udaka S. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci Biotechnol Biochem. 1997;61:1582–1584. doi: 10.1271/bbb.61.1582. [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Stouch B, Schroeder J, Pyrah I, Stolina M, Smith SY, et al. Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone. 2011;49:162–173. doi: 10.1016/j.bone.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, et al. Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 2008;283:24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Paschen W, Schmidt-Kastner R, Djuricic B, Meese C, Linn F, Hossmann KA. Polyamine changes in reversible cerebral ischemia. J Neurochem. 1987;49:35–37. doi: 10.1111/j.1471-4159.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, et al. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Sharmin S, Sakata K, Kashiwagi K, Ueda S, Iwasaki S, Shirahata A, et al. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun. 2001;282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol. 2005;175:237–245. doi: 10.4049/jimmunol.175.1.237. [DOI] [PubMed] [Google Scholar]
- Soda K, Kano Y, Sakuragi M, Takao K, Lefor A, Konishi F. Long-term oral polyamine intake increases blood polyamine concentrations. J Nutr Sci Vitaminol (Tokyo) 2009;55:361–366. doi: 10.3177/jnsv.55.361. [DOI] [PubMed] [Google Scholar]
- Stern PH, Lucas RC, Seidenfeld J. Alpha-difluoromethylornithine inhibits bone resorption in vitro without decreasing beta-glucuronidase release. Mol Pharmacol. 1991;39:557–562. [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. RANKing c-Jun in osteoclast development. J Clin Invest. 2004;114:463–465. doi: 10.1172/JCI22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Tsuji-Naito K. Aldehydic components of cinnamon bark extract suppresses RANKL-induced osteoclastogenesis through NFATc1 downregulation. Bioorg Med Chem. 2008;16:9176–9183. doi: 10.1016/j.bmc.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Turchanowa L, Rogozkin VA, Milovic V, Feldkoren BI, Caspary WF, Stein J. Influence of physical exercise on polyamine synthesis in the rat skeletal muscle. Eur J Clin Invest. 2000;30:72–78. doi: 10.1046/j.1365-2362.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- Uno K, Takarada T, Nakamura Y, Fujita H, Hinoi E, Yoneda Y. A negative correlation between expression profiles of runt-related transcription factor-2 and cystine/glutamate antiporter xCT subunit in ovariectomized mouse bone. J Pharmacol Sci. 2011;115:309–319. doi: 10.1254/jphs.10310fp. [DOI] [PubMed] [Google Scholar]
- Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367:665–675. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Takami M, Kawawa T, Yasuhara R, Zhao B, Michizuki A, et al. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Taguchi H, Gao YH, Igarashi A, Tsukamoto Y. Effect of vitamin K2 (menaquinone-7) in fermented soybean (natto) on bone loss in ovariectomized rats. J Bone Miner Metab. 1999;17:23–29. doi: 10.1007/s007740050059. [DOI] [PubMed] [Google Scholar]
- Yokose S, Ishizuya T, Ikeda T, Nakamura T, Tsurukami H, Kawasaki K, et al. An estrogen deficiency caused by ovariectomy increases plasma levels of systemic factors that stimulate proliferation and differentiation of osteoblasts in rats. Endocrinology. 1996;137:469–478. doi: 10.1210/endo.137.2.8593791. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counter regulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


