Abstract
BACKGROUND AND PURPOSE
B1 and B2 kinin receptors are involved in pain transmission but they may have different roles in the muscle pain induced by intense exercise or inflammation. We investigated the contribution of each of these receptors, and the intracellular pathways involved, in the initial development and maintenance of the muscle pain associated with inflammation-induced tissue damage.
EXPERIMENTAL APPROACH
Mechanical hyperalgesia was measured using the Randall–Selitto apparatus after injecting 5% formalin solution into the gastrocnemius muscle in mice treated with selective antagonists for B1 or B2 receptors. The expression of kinin receptors and cytokines and the activation of intracellular kinases were monitored by real-time PCR and immunohistochemistry.
KEY RESULTS
The i.m. injection of formalin induced an overexpression of B1 and B2 receptors. This overexpression was associated with the mechanical hyperalgesia induced by formalin because treatment with B1 receptor antagonists (des-Arg9[Leu8]-BK, DALBK, and SSR240612) or B2 receptor antagonists (HOE 140 and FR173657) prevented the hyperalgesia. Formalin increased myeloperoxidase activity, and up-regulated TNF-α, IL-1β and IL-6 in gastrocnemius. Myeloperoxidase activity and TNF-α mRNA expression were inhibited by either DALBK or HOE 140, whereas IL-6 was inhibited only by HOE 140. The hyperalgesia induced by i.m. formalin was dependent on the activation of intracellular MAPKs p38, JNK and PKC.
CONCLUSIONS AND IMPLICATIONS
Inflammatory muscle pain involves a cascade of events that is dependent on the activation of PKC, p38 and JNK, and the synthesis of IL-1β, TNF-α and IL-6 associated with the up-regulation of both B1 and B2 kinin receptors.
Keywords: kinin B1 and B2 receptors, muscle pain, inflammation, hyperalgesia, cytokines, intracellular kinases
Introduction
Muscle pain represents a serious clinical problem and frequently occurs in the population. However, the mechanisms of the origin and maintenance of muscle pain have not been completely elucidated. Therefore, studies in which the pathways triggered during this event might aid the design of appropriate therapies for an effective treatment. Currently, non-steroidal anti-inflammatory drugs are the first option for the treatment of muscle pain because this particular pain is usually associated with inflammatory process (Ziltener et al., 2010). Regrettably, these drugs present low efficacy in some patients and their prolonged use is frequently associated with numerous side effects (Rooks, 2007; Rainsford, 2009; Mackenzie and MacDonald, 2010; Khan and Lee, 2011).
The inflammatory events that result from muscle injury are characterized by the release/synthesis of inflammatory cytokines, calcitonin gene-related peptide, nerve growth factor, PGE2 and kinin peptides (Wallace et al., 2001; Schafers et al., 2003; Mense, 2009; Manjavachi et al., 2010). In this situation, kinin peptides, mainly bradykinin, directly activate Aδ and C-fibre primary sensory neurons, initiating pain transmission (Dray and Perkins, 1997; Ferreira et al., 2004). Bradykinin, kalidin and their fragments are a group of peptides formed in plasma and tissues by the action of the kallikrein-kinin enzyme system in response to either a physiological stimulus or during the inflammatory process. Their signalling in the vascular endothelium induces vasodilatation, plasma extravasation and increase in blood flow (Barnes, 1992; Lopes et al., 1993; Polosa, 1993; Regoli et al., 1998; Calixto et al., 2000; 2004).
Kinins act as agonists of two subtypes of GPCR, B1 and B2 receptors (Regoli and Barabe, 1980). The B2 receptor is constitutively expressed in both the central and peripheral nervous system and it has also been found in myocytes; whereas the B1 receptor is poorly expressed in healthy peripheral tissue, but is increased after tissue injury and in response to pro-inflammatory agents (Regoli and Barabe, 1980; Figueroa et al., 1996; Marceau and Bachvarov, 1998; Calixto et al., 2000; 2004; Couture et al., 2001). Several studies have shown that both receptors are involved in pain transmission (Dray and Perkins, 1997; Calixto et al., 2000; Pesquero et al., 2000; Couture et al., 2001; Ferreira et al., 2002) and the role of the B2 receptor in muscle hyperalgesia after exercise has been well-documented (Murase et al., 2010). An i.m. injection of bradykinin does not induce hyperalgesia in rats and induces only mild pain in humans (Jensen et al., 1990; Babenko et al., 1999; Boix et al., 2005; Murase et al., 2010). However, bradykinin is found in high concentrations in the trapezius interstitial fluid of patients suffering from chronic musculoskeletal pain (Gerdle et al., 2008). These findings suggest that B2 receptors may affect nociceptive transmission only after intense exercise or after muscle injury. In contrast to B2 receptors, the participation of the B1 receptor in muscle pain after strenuous exercise has been ruled out (Murase et al., 2010).
In this context, it seems that these receptors have distinct effects in muscle pain transmission and may have different roles in the muscle pain induced by intense exercise or inflammation. Taking this into account, the present study was undertaken to address the contribution of B1 and B2 kinin receptors to the initial development and maintenance of muscle pain after inflammation-induced tissue damage. The aim was to elucidate the mechanisms of pain after a muscle injury. For this purpose, we used an i.m. injection of formalin, which initially sensitizes neurons through activation of the transient receptor potential ankyrin 1 (Tian et al., 2009) and then induces the release of inflammatory mediators that mimic the inflammatory processes (Hunskaar and Hole, 1987; Tjolsen et al., 1992; Sawamura et al., 1999). The involvement of kinin receptors was evaluated by measuring their gene expression and the effect of systemic treatments with selective B1 and/or B2 receptor antagonists on mechanical hyperalgesia. Other parameters, such as myeloperoxidase activity, cytokine expression and kinase-dependent signalling, were also analysed.
Methods
Animals
This study was carried out using male Swiss mice (25–35 g) housed at a temperature of 22 ± 2°C, 60–80% humidity, under a 12:12 h light-dark cycle and with free access to food and water. Approximately 280 mice were used for all experimental procedures. The experiments were performed during the light phase of the cycle. All experiments were conducted in accordance with the ethical guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and the National Institutes of Health guide for the care and use of Laboratory animals (NHI Publication no. 85-23, revised in 1996). All experimental procedures were approved by the Committee on the Ethical Use of Animals of the Universidade Federal de Santa Catarina (protocol number 053/CEUA/PRPe/2008). The intensity of the nociceptive stimuli was the minimum needed to demonstrate a consistent effect of the treatments.
Formalin-induced muscle pain
The procedure was essentially similar to the method described previously (Schafers et al., 2003), with minor modifications. Briefly, the mice (n= 6 per group) were anaesthetized using a mixture of 3% isoflurane in oxygen before receiving a unilateral i.m. injection of 5% formalin solution (50 µL per site) or saline (0.9%) into the belly of the gastrocnemius muscle.
Mechanical hyperalgesia was evaluated at different time points (3, 6, 24, 48, 72 and 168 h) following the formalin injection and measured as the hind limb withdrawal threshold in response to linearly increasing mechanical forces (16 g·s−1) applied to the gastrocnemius muscle and quantified by means of Randall–Selitto apparatus (Ugo Basile, Comerio, VA, Italy). Briefly, the mice were lightly restrained in a vented tubular Plexiglas holder with openings that allow easy access to the hind limb, and each leg was successively positioned so that incremental pressure (maximum of 400 g) could be applied to the mid-gastrocnemius muscle. The pressure required to elicit hind limb withdrawal was determined, and measurements were taken three times at 5 min intervals; the mean value was taken as the hind limb withdrawal threshold. A baseline (∼300–400 g) measurement was taken before the treatments.
Effect of kinin antagonists on formalin-induced muscle pain in mice
The involvement of kinin B1 and B2 receptors in the muscle nociception induced by formalin was evaluated after systemic administration of the selective B1 and/or B2 receptor antagonists. The antagonists to the B1 receptor, SSR240612 (a non-peptide antagonist) and des-Arg9-[Leu8]-bradykinin (DALBK, a peptide antagonist), were administered i.p., 30 min before formalin, both at doses of 1 mg·kg−1. The non-peptide antagonist to the B2 receptor, FR173657, and the peptide antagonist, HOE 140, were administered i.p. at doses of 10 mg·kg−1 and 0.057 mg·kg−1, respectively, 30 min before the formalin injection (Cloutier and Couture, 2000; Ferreira et al., 2002; Werner et al., 2007; Paszcuk et al., 2008; Quintao et al., 2008). The withdrawal threshold was analysed 3, 6 and 24 h after formalin injection.
In order to evaluate the therapeutic effect of B1 and B2 receptor antagonists, mice received the peptidergic antagonists DALBK (1 mg·kg−1) or HOE 140 (0.057 mg·kg−1) 24 h after an injection of formalin (post-treatment). The withdrawal threshold was evaluated 0.5, 2 and 4 h after injection of the antagonists. To verify any additive effect of B1 and B2 antagonists, mice were treated with sub-active doses of DALBK (0.3 mg·kg−1, i.p) and HOE 140 (0.017 mg·kg−1, i.p.) 30 min before or 24 h after formalin for the pre- or post-treatment schedule respectively.
For comparison, other groups of mice were treated with the standard analgesic and anti-inflammatory drugs dipyrone (120 mg·kg−1, i.p.), celecoxib (30 mg·kg−1, i.p.), morphine (10 mg·kg−1, s.c.) and gabapentin (70 mg·kg−1, p.o.), 30 min before formalin, and evaluated against the hyperalgesic effects of formalin. Hyperalgesia was evaluated 3, 6 and 24 h after formalin. The total inhibition caused by these drugs was obtained by calculating the area under the curve (AUC) for each individual treatment.
Effect of p38, JNK and PKC inhibitors on formalin-induced muscle pain in mice
In order to determine the cell signalling pathway activated in the gastrocnemius muscle by formalin, mice were treated with inhibitors of PKC (GF109203X 0.4 µg per site, i.m.), p38 (SB203580 11 µg per site, i.m.) or JNK (SP600125 7 µg per site, i.m.) 5 min before formalin injection (Motta et al., 2006). Mechanical hyperalgesia was evaluated 1, 3, 6 and 24 h after formalin through the application of a crescent force using the Randall–Selitto apparatus, as described earlier.
Effect of kinin receptor antagonists on the mechanical hyperalgesia induced by i.m. injection of octapeptide ψεRACK
In order to evaluate the role of the PKCε on the mechanical hyperalgesia in muscle pain, mice were treated with the PKC isoform using octapeptide ψεRACK (1 µg per site, 50 µL). Mechanical hyperalgesia was evaluated by the Randall–Selitto apparatus from 15 min and up to 6 h after peptide administration. In order to investigate the kinin-dependent mechanism in the mechanical hyperalgesia induced by PKCε, the B1 receptor antagonist (DALBK 1 mg·kg−1, i.p.) or the B2 receptor antagonist (HOE 140; 0.057 mg·kg−1, i.p.) was administered 30 min before i.m. injection of the octapeptide ψεRACK (1 µg per site, 50 µL).
Immunohistochemical assay
The mice (n= 5) were pretreated with DALBK (1 mg·kg−1, i.p.), HOE 140 (0.057 mg·kg−1, i.p.) or vehicle 30 min before the 5% formalin i.m. injection. They were then anaesthetized with 7% chloral hydrate 1 h after the formalin injection. The mice were transcardially perfused with heparin (1000 U·mL−1) in physiological saline, followed by 4% paraformaldehyde in 0.9% saline solution. The gastrocnemius muscles were extracted and post-fixed overnight at room temperature in 4% paraformaldehyde. The immunohistochemistry technique was carried out on paraffin-embedded muscle sections. The primary antibodies were prepared at a specific concentration for each protein: phosphorylated JNK (p-JNK, 1:200) and phosphorylated p38 MAPK (p-p38 1:60) antibodies (Cell Signaling Technology, Danvers, MA, USA). The quenching of endogenous peroxidase was performed by incubating the segments with 3% hydrogen peroxide in methanol (v v-1) for 20 min. High-temperature antigen retrieval was performed by immersion of the slides in 10 mM trisodium citrate buffer pH 6.0 in a water bath at 95–98°C for 45 min. The slides were then processed using the Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. The sections were covered with the appropriate biotinylated secondary antibody and then developed with 3,3'-diaminobenzidine (Dako Cytomation, Carpinteria, CA, USA) in chromogen solution and counterstained with Harris's haematoxylin. Control and experimental tissues were placed on the same slide and processed under the same conditions. Images of the gastrocnemius muscles were acquired using a Sight DS-5 M-L1 digital camera (Nikon, Melville, NY, USA) connected to an Eclipse 50i light microscope (Nikon). An OD threshold that best discriminated staining from the background was obtained using NIH ImageJ 1.36b imaging software (NIH, Bethesda, MD, USA). The total pixel intensity was determined, and data were expressed as OD.
Expression of B1, B2, TNF-α, IL-1β and IL-6 mRNA by Real-Time-PCR
The RNA was extracted using TRIzol reagent following the manufacturer's instructions (Invitrogen, São Paulo, Brazil). The concentration of total RNA was determined by measuring the absorbance at 260 nm. One microgram of the total RNA was reverse transcribed to cDNA using 500 µg·mL−1 oligo(dT), 10 mM deoxynucleotide, 100 mM dithiothreitol, 40 mU·mL−1 RNaseOUT and 200 U of SuperScript reverse transcriptase (Invitrogen) in a reaction buffer (2.5 mM MgCl2, 50 mM KCl, and 20 mM Tris–HCl, pH 8.4), with a final volume of 12.5 µL. The samples were incubated for 5 min at 65°C, 5 min at 4°C, 50 min at 42°C, 5 min at 70°C and 5 min at 4°C. The cDNA (100 ng) was amplified in duplicate using TaqMan-based chemistry with specific primers and 6-Carboxyfluorescein-labelled probes for mouse B1 (Mm00432059_s1) and B2 (Mm01339907_m1), or 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein-labelled probes for mouse TNF-α (Mm00443258_m1), IL-1β (Mm01336189_m1), IL-6 (Mm99999064_m1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Mm01843776_s1) as an endogenous control for normalization (Applied Biosystems, Warrington, UK). Amplifications were carried out in a thermal cycler (StepOne Plus, Applied Biosystems) for 50 cycles; the fluorescence was collected for each amplification cycle and the data analysed using the 2-ΔΔCt method for the relative quantification of expression. Expression of the target genes was calibrated against the conditions found in saline or naive animals, i.e. without treatment.
The mice received 5% formalin or saline i.m., as described earlier. The gastrocnemius was harvested 3, 6 and 24 h after formalin injection for quantification of B1 and B2 mRNA. Distinct groups of mice were pretreated (30 min before formalin) with DALBK (1 mg·kg−1, i.p.) or HOE 140 (0.057 mg·kg−1, i.p.). One hour after formalin injection, the tissues were collected, and mRNA for TNF-α, IL-1β and IL-6 was quantified.
Myeloperoxidase activity assay
Neutrophil activation and recruitment into the gastrocnemius muscle was indirectly estimated by measuring the activity of myeloperoxidase. The activity of the enzyme was assayed as previously described (Bradley et al., 1982). Animals (n= 5) were treated with DALBK (1 mg·kg−1, i.p.), HOE 140 (0.057 mg·kg−1, i.p.) or saline (0.9%) 30 min before the i.m. injection of formalin (5%). The gastrocnemius muscle was removed 3 h after formalin injection. The muscles were homogenized at 20% w v-1 in EDTA/NaCl buffer (pH 4.7) and centrifuged at 11200 g for 15 min at 4°C. The pellet was resuspended in 0.5% hexadecyltrimethyl ammonium bromide buffer (pH 5.4), and the samples were frozen and thawed three times in liquid nitrogen. The samples were centrifuged (11200 g, 15 min, 4°C) and 25 µL of the supernatant was used for the myeloperoxidase assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM sodium phosphate buffer pH 7.2 and 0.3 mM hydrogen peroxide. The absorbance was measured at 650 nm. The results are expressed as the OD mg-1 tissue.
Statistical analysis
The results are presented as the mean ± SEM of six animals for the behavioural tests and of three to five animals for the RT-PCR analyses. The data were analysed by one-way anova with repeated measures for mechanical hyperalgesia, followed by Bonferroni's test when appropriate. The AUC was calculated using the GraphPad Software (San Diego, CA, USA). The % inhibition was calculated from AUC data in the behavioural tests. Thus, the effect of the drug treatment was obtained throughout the entire experiment. A P < 0.05 was considered to be indicative of significance.
Reagents
The B2 receptor antagonist FR173657 was kindly donated by Fujisawa Pharmaceutical Co. (Osaka, Japan); the B1 receptor antagonist SSR240612 was kindly donated by ©Sanofi-Aventis (Bridgewater, NJ, USA); the B2 receptor antagonist HOE 140 was kindly donated by Hoechst (Frankfurt Main, Germany). The B1 receptor antagonist des-Arg9-[Leu8]-bradykinin (DALBK) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The kinase protein inhibitors GF109203X, SB203580 and SP60015 were purchased from Tocris Bioscience (Ellisville, MO, USA). The primers and probes for mouse B1, B2, TNF-α, IL-1β, IL-6 and GAPDH were obtained from Applied Biosystems. Hydrogen peroxide (H2O2), Tween 20 and PBS tablets were purchased from Sigma Chemical Co. The B1 receptor, B2 receptor, JNK and p38 MAPKs antibodies were acquired from Cell Signaling Technology. The peptide agonist of PKCε, the octapeptide ΨεRACK, was obtained from EZBiolab (Westfield, IN 46074, USA). The secondary antibody Envision Plus, the streptavidin-horseradish peroxidase reagent and 3,3-diaminobenzidine chromogen were purchased from Dako Cytomation. The drugs were prepared in saline solution (0.9% NaCl).
Results
The i.m. injection of formalin up-regulates mRNA expression of B1 and B2 receptors in gastrocnemius muscle
The involvement of B1 and B2 receptors in muscle pain was first demonstrated by the increase in mRNA expression of these receptors (Figure 1). Quantification of the mRNA revealed that both B1 and B2 receptors are continuously synthesized in the muscle from naive mice, demonstrating that, even in the absence of injury, there is a constant turnover of these receptors in muscle (Figure 1). An increase in the synthesis of both B1 and B2 receptors was identified as early as 3 h after formalin injection in the gastrocnemius muscle. This up-regulation was present at all of the time points investigated (3, 6 and 24 h).
Figure 1.
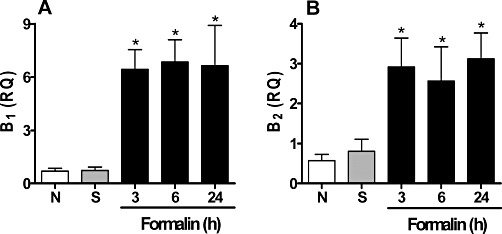
Expression of mRNA of B1 and B2 receptors in formalin-induced inflammatory muscle pain. The quantification of mRNA for (A) B1 and (B) B2 was performed by RT-PCR. Data were normalized to mRNA levels for GAPDH. ‘N’ represents the naive group and ‘S’ represents non-inflamed mice that received an i.m. injection of 0.9% saline solution (50 µL per site). Data represent the mean ± SEM (n= 5). The symbols denote a significant difference: *P < 0.05 compared to the saline group. Statistical analyses were performed using one-way anova followed by Bonferroni's test.
The B1 and B2 receptors contribute to mechanical hyperalgesia in inflammatory muscle pain
The next step was to investigate whether or not the overexpressed B1 and B2 receptors were really functional and whether they would in fact contribute to inflammatory muscle pain or not. The i.m. injection of formalin induced mechanical hyperalgesia from the third hour after its administration. This hyperalgesia was significant for up to 72 h (3 days) and disappeared 7 days after formalin injection (data not shown). From these results, we selected three time points, 3, 6 and 24 h, to evaluate the behaviour in mice treated with kinin receptor antagonists.
Pretreatment with the B1 receptor antagonist DALBK (1 mg·kg−1, i.p.) significantly reduced the mechanical hyperalgesia induced by formalin (Figure 2A). The inhibition calculated from the AUC was 44 ± 9%. Likewise, pretreatment with the non-peptide selective antagonist SSR240612 (1 mg·kg−1, i.p.) significantly inhibited formalin-induced hyperalgesia (Figure 2B), with an inhibition percentage of 43 ± 15%. In addition, the systemic post-treatment with DALBK significantly decreased the hyperalgesia induced by formalin. The calculated inhibition was 41 ± 1% (Figure 2C).
Figure 2.
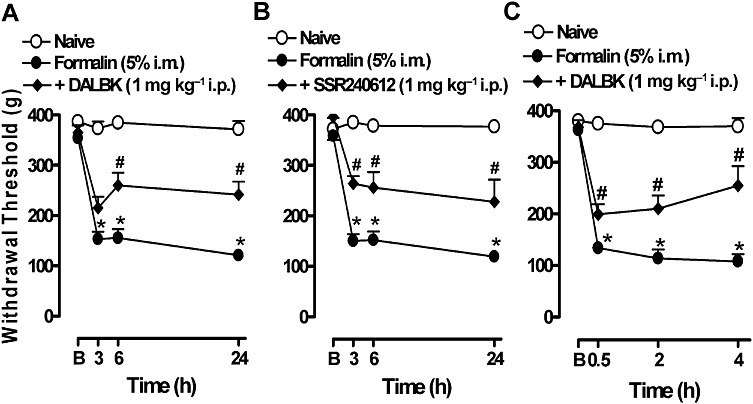
Effect of pre- and post-treatment with the kinin B1 receptor antagonists on the mechanical hyperalgesia induced by formalin in gastrocnemius muscle. (A) DALBK or (B) SSR240612 was administered i.p. 30 min before formalin injection and hyperalgesia was assessed 3, 6 and 24 h after the formalin injection. (C) DALBK was administered i.p. 24 h after the formalin injection (post-treatment). The hyperalgesia was assessed 0.5, 2 and 4 h after the DALBK injection. B, represents the baseline measurement (immediately before formalin injection). Data represent the mean ± SEM (n= 5–7). The symbols denote a significant difference: *P < 0.05 compared to the naive group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed using one-way anova (repeated measures) followed by Bonferroni's test.
Similar to the B1 receptor antagonists, pretreatment with the peptide B2 receptor antagonist HOE 140 (0.057 mg·kg−1, i.p.) significantly inhibited the hyperalgesia induced by formalin (Figure 3A). The % inhibition revealed that the antagonism of B1 and B2 receptors had a similar effect in decreasing muscle pain. The calculated value was 38 ± 6%. Systemic pretreatment with the non-peptide B2 receptor antagonist FR173657 (10 mg·kg−1, i.p.) inhibited the hyperalgesia only 6 h after formalin injection (Figure 3B), with a total inhibition of 32 ± 6%. Systemic post-treatment with HOE 140 was also effective at decreasing hyperalgesia (Figure 3C), with an inhibition of 34 ± 9%.
Figure 3.
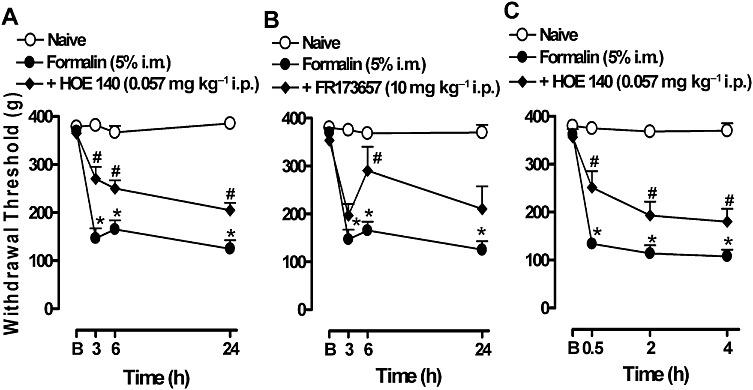
Effect of pre- and post-treatment with the kinin B2 receptor antagonists on the mechanical hyperalgesia induced by formalin in gastrocnemius muscle. (A) HOE 140 or (B) FR173657 was administered i.p. 30 min before the formalin injection and hyperalgesia was assessed 3, 6 and 24 h after the formalin injection. (C) HOE 140 was administered i.p. 24 h after the formalin injection (post-treatment). The hyperalgesia was assessed 0.5, 2 and 4 h after DALBK injection. B, represents the baseline measurement (immediately before formalin injection). Data represent the mean ± SEM (n= 5–7). The symbols denote a significant difference: *P < 0.05 compared to the naive group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed using one-way anova (repeated measures) followed by Bonferroni's test.
Low doses of DALBK (0.3 mg·kg−1) or HOE 140 (0.017 mg·kg−1) were ineffective when the antagonists were given alone. However, the combination of both antagonists significantly prevented the hyperalgesia induced by formalin. Pretreatment with DALBK plus HOE 140, 30 min before formalin, prevented the hyperalgesia at all time points tested (3–24 h, Figure 4A), whereas post-treatment with the mixture of the antagonists was active only at the second hour after treatment (Figure 4B). The calculated inhibitions were 61 ± 5% and 83 ± 4% for the pre- and post-treatment, respectively.
Figure 4.
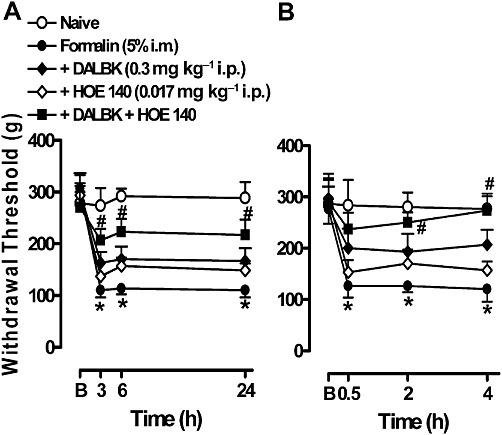
Effect of the co-treatment with the kinin B1 and B2 receptor antagonists on the mechanical hyperalgesia induced by formalin in gastrocnemius muscle. DALBK (0.3 mg·kg−1 i.p.) or HOE 140 (0.017 mg·kg−1) or both were administered 30 min before formalin in (A) or 24 h after formalin in (B). The assessment of hyperalgesia was performed as described in Figures 2 and 3. B, the baseline measurement (immediately before formalin injection). Data represent the mean ± SEM (n= 5–7). The symbols denote a significant difference: *P < 0.05 compared to the naive group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed using one-way anova (repeated measures) followed by Bonferroni's test.
Systemic treatment of mice with standard analgesic and anti-inflammatory drugs significantly inhibited the hyperalgesia induced by formalin injected in the gastrocnemius muscle. The effect of these drugs was comparable to the effects observed for the B1 and B2 receptor antagonists. The inhibition values were obtained by calculating the AUC in each individual treatment. The results are presented in Table 1.
Table 1.
Effect of B1 and B2 antagonists and standard analgesic drugs on the hyperalgesia induced by formalin
| Treatment | AUC (mean ± SEM) | Supported weight (% of naive group) |
|---|---|---|
| Naive | 7916 ± 212 | 100 ± 2.6 |
| Formalin | 3015 ± 286* | 38 ± 3.6* |
| Formalin + DALBK (1 mg·kg−1 i.p.) | 5227 ± 470# | 66 ± 5.9# |
| Formalin + HOE 140 (0.057 mg·kg−1 i.p.) | 4875 ± 298# | 61 ± 3.8# |
| Formalin + Dipyrone (120 mg /kg i.p.) | 6384 ± 892# | 80 ± 11# |
| Formalin + Celecoxib (30 mg·kg−1 i.p.) | 7985 ± 412# | 100 ± 5.2# |
| Formalin + Morphine (10 mg·kg−1 s.c.) | 5925 ± 338# | 75 ± 4.2# |
| Formalin + Gabapentin (70 mg·kg−1 p.o.) | 5482 ± 403# | 69 ± 5# |
Data represent the mean ± SEM (n= 6). Mechanical hyperalgesia was evaluated at 3, 6 and 24 h after formalin injection. The drugs were administered 30 min before formalin via i.p., s.c. or p.o. The area was calculated as the AUC for each individual treatment. Statistical analysis was performed using one-way anova followed by Bonferroni's test. The symbols denote a significant difference, *P < 0.05 compared to the naive group and #P < 0.05 compared to the formalin group.
The treatment with B1 and B2 receptor antagonists decreased myeloperoxidase activity and TNF-α synthesis
The injection of formalin in the gastrocnemius muscle resulted 3 h later in a significant increase in myeloperoxidase activity (Figure 5A). The systemic pretreatment with either the B1 or B2 receptor antagonists, DALBK and HOE 140, significantly prevented myeloperoxidase activity (Figure 5A). One hour after formalin injection, we detected a significant increase in the mRNA for TNF-α, IL-1β and IL-6 (Figure 5B, C and D). Interestingly, both kinin antagonists inhibited TNF-α mRNA synthesis (Figure 5B) without affecting the synthesis of IL-1β (Figure 5C). Furthermore, the B2 antagonist HOE 140 significantly inhibited the synthesis of new IL-6 (Figure 5D).
Figure 5.
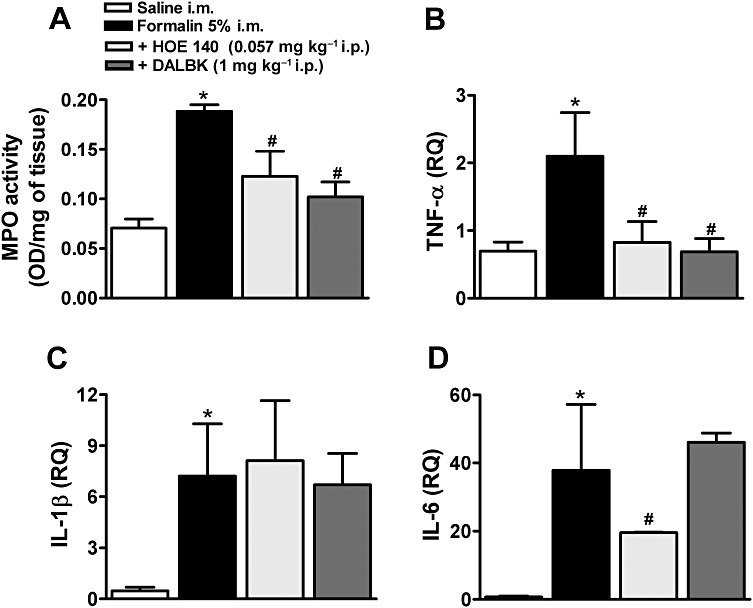
Myeloperoxidase (MPO) activity and expression of mRNA for TNF-α, IL-1β and IL-6 in formalin-induced inflammatory muscle pain. (A) Myeloperoxidase activity and mRNA quantification for (B) TNF-α, (C) IL-1β and (D) IL-6 in the muscle of formalin-injected mice. Mice were pretreated, i.p., with saline, DALBK or HOE 140. Data were normalized to mRNA levels for GAPDH. Control mice received an i.m. injection of 0.9% saline solution (50 µL per site). Data represent the mean ± SEM (n= 3–5). The symbols denote a significant difference: *P < 0.05 compared to the control (saline i.m.) group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed by one-way anova followed by Bonferroni's test.
Systemic treatment with the B1 and B2 receptor antagonists prevented formalin-induced MAPK phosphorylation in gastrocnemius muscle
The p38 and JNK MAPKs were slightly activated (phosphorylated) after the i.m. injection of saline. However, the i.m. injection of formalin caused a marked activation of both MAPKs. As depicted in Figure 6, JNK was clearly more sensitive to formalin-induced muscle injury than p38. The peak of phosphorylation detected for these enzymes was 1 h after formalin injection. The activation of p38 and JNK declined 6 and 3 h after formalin injection, respectively (Figure 6A and B). It was noteworthy that the activation of both p38 and JNK MAPKs was significantly prevented by the treatment with DALBK and HOE 140 (Figure 6C and D). The phosphorylated forms of these MAPKs mostly surrounded the nuclei of the cells (Figure 6D).
Figure 6.
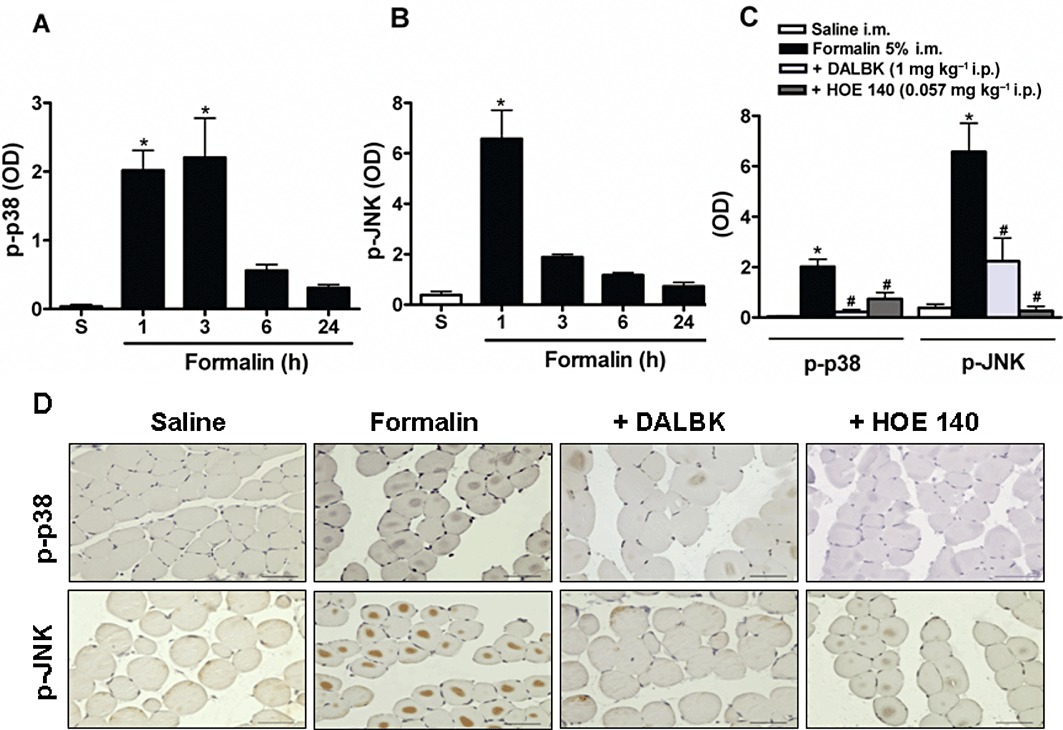
Activation of MAPKs in formalin-induced inflammatory muscle pain. Mice received 5% formalin via the i.m. route. The tissues were obtained at different time points after formalin injection for immunohistochemical analyses of (A) p-p38 or (B) p-JNK. Two other groups of mice were pretreated with DALBK (1 mg·kg−1. i.p.) or HOE 140 (0.057 mg·kg−1, i.p.) 30 min before formalin. One hour after formalin injection, the tissues were obtained for immunohistochemical analyses for (C) p-p38 and p-JNK. (D) Representative photomicrography of (C). Control mice received an i.m. injection of 0.9% saline solution (50 µL per site) (S). Data represent the mean ± SEM (n= 5). The symbols denote a significant difference: *P < 0.05 compared to the control group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed using one-way anova followed by Bonferroni's test.
The mechanical hyperalgesia induced by an i.m. injection of formalin is dependent on the activation of intracellular kinases
In accordance with the above results, the hyperalgesia induced by formalin was clearly dependent on the activation of intracellular kinases, p38, JNK and PKC (Figure 7). In fact, treatment with the p38 inhibitor, SB203580, or the JNK inhibitor, SP60015, prevented the early hyperalgesia induced by formalin. However, they were unable to provide protection in the later periods of formalin administration (Figure 7A and B), which is in agreement with the time course for the activation of these proteins (Figure 6A and B). Local administration of the PKC inhibitor, GF109203X, also prevented the hyperalgesia induced by formalin. The protective effect was evident up to 6 h after formalin injection (Figure 7C).
Figure 7.
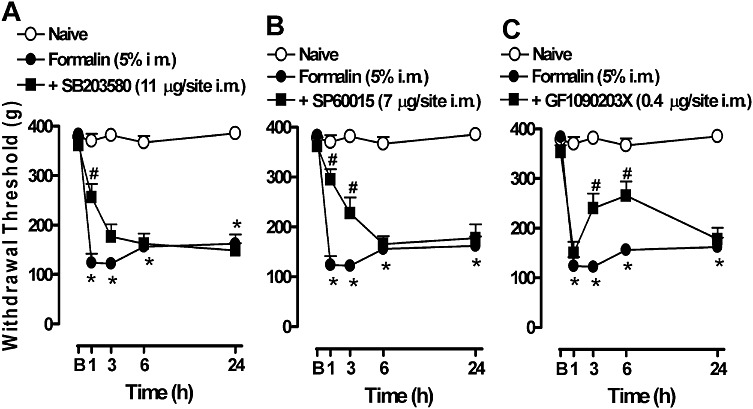
Involvement of MAPK kinases and PKC in the mechanical hyperalgesia induced by formalin in gastrocnemius muscle. Mice were treated with the protein kinase inhibitors (A) p38 (SB203580 11 µg per site, i.m.), (B) JNK (SP60015 7 µg per site, i.m.) or (C) PKC (GF109203X 0.4 µg per site, i.m.) 5 min before the i.m. injection of formalin. The mechanical hyperalgesia was evaluated from 1 h after formalin injection. B, baseline measurement (before formalin injection). Data represent the mean ± SEM (n= 5). The symbols denote a significant difference: *P < 0.05 compared to the naive group and #P < 0.05 compared to the formalin i.m. group. Statistical analyses were performed using one-way anova (repeated measures) followed by Bonferroni's test.
Effect of B1 and B2 receptor antagonists on the PKCε-dependent hyperalgesia
In order to further explore the role of PKC in mechanical hyperalgesia, we investigated whether direct activation of the PKCε isoform would reproduce such a hyperalgesic response. Interestingly, i.m. injection of the octapeptide ψεRACK, a specific activator of PKCε, induced nociceptive behaviour to the mechanical pressure as early as 15 min. This hyperalgesia remained for 4 h following peptide administration (Figure 8). Interestingly, treatment with the B2 antagonist HOE 140 did not prevent the hyperalgesia induced by the PKCε activator, demonstrating that the activation of PKCε occurs downstream to the B2 receptor and, therefore, antagonism of the receptor was not sufficient to prevent hyperalgesia to this stimulus. On the other hand, the B1 receptor antagonist DALBK significantly prevented the hyperalgesia induced by PKCε activation from only the second hour after the administration of octapeptide ψεRACK. This time-dependent effect on DALBK is consistent with the up-regulation of the B1 receptor promoted by PKC (Figure 8).
Figure 8.
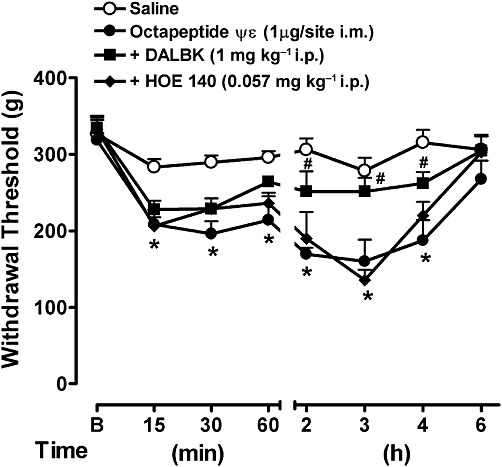
Effect of the B1 and B2 antagonists on the mechanical hyperalgesia induced by the PKCε activator octapeptide ψεRACK. Mice received DALBK or HOE 140 i.p. 30 min before an i.m. injection of the octapeptide ψεRACK. The mechanical hyperalgesia was evaluated from 15 min after the injection of octapeptide ψεRACK. Data represent the mean ± SEM (n= 5). The symbols denote a significant difference: *P < 0.05 compared to the naive group and #P < 0.05 compared to the octapeptide ψεRACK i.m. group. Statistical analyses were performed using one-way anova (repeated measures) followed by Bonferroni's test.
Discussion
The results presented in this study demonstrate the critical role exerted by both B1 and B2 kinin receptors in muscle hyperalgesia after a harmful inflammatory stimulus induced by formalin. Previous investigations reported that the i.m. injection of formalin is a reliable model for evaluating muscle pain in rodents (Schafers et al., 2003; Vadakkan et al., 2006). Our results expanded these previous findings and revealed that an i.m. injection of formalin induced a marked hyperalgesia within 24 h after its administration, an effect that remained for up 72 h (data not shown). The mechanisms of formalin-induced pain are closely related to the activation of both B1 and B2 receptors (Correa and Calixto, 1993), and the role of these receptors in pain and inflammation has been broadly demonstrated (Regoli and Barabe, 1980; Calixto et al., 2000; 2004; Couture et al., 2001; Marceau and Regoli, 2004; Mizumura et al., 2009).
The putative participation of bradykinin in chronic muscle pain in humans was recently reported. In this study, the concentration of the peptide was elevated in the trapezius interstitial fluid of patients suffering from myalgia compared to control patients (Gerdle et al., 2008). It is of interest that the effect of bradykinin in muscle pain seems to be significant only in the presence of injury or after strenuous exercise (Murase et al., 2010). In agreement with this, a direct infusion of bradykinin induced hyperalgesia only when administered in combination with other mediators like 5-HT, prostaglandin or histamine (Babenko et al., 1999; Mork et al., 2003). This suggests that bradykinin evokes a mild hyperalgesia in muscle and its effects depend on the presence of inflammatory mediators. In contrast to the B2 receptor, the role of the B1 receptor in muscle pain has been less well-researched; as this receptor is not usually expressed in peripheral healthy tissue, it may comprise a negligible effect in muscle pain processes that are not related to inflammation. In fact, antagonism of the B1 receptor did not ameliorate hyperalgesia in a model of strenuous exercise (Murase et al., 2010).
Our results demonstrated that both B1 and B2 receptors are up-regulated after inflammation induced by formalin and the antagonism of these receptors effectively reduced mechanical hyperalgesia in this state. Interestingly, pretreatment with either the B1- or B2-selective receptor antagonists presented a long-lasting anti-hyperalgesic action. In agreement with our results, the vascular and anti-oedema effects of HOE 140 were shown to extend to all time points measured (up to 5 h) (Wirth et al., 1991). However, in previous studies the B1 antagonist SSR240612 was found to have a shorter inhibitory effect on hyperalgesia and inflammation induced by different agents (Gougat et al., 2004). The main difference between our results and those obtained previously may due to differences in the treatment schedule; we administered the antagonists before the formalin injection (in the pretreatment schedule), which resulted in the hyperalgesia being inhibited even 24 h after the treatment. This suggests that the antagonism of the receptor at an early stage in the onset of an inflammatory response would prevent any further damage and would prevent the complete development of inflammation.
The signal events triggered by the activation of kinin receptors include the production of other inflammatory and pain mediators, such as prostanoids, tachykinins, cytokines and nitric oxide (Marceau and Bachvarov, 1998; Brechter and Lerner, 2002; Calixto et al., 2004; Costa-Neto et al., 2008; Meini and Maggi, 2008). In this study, we observed an increase in the activity of myeloperoxidase in the gastrocnemius, indicating the migration of inflammatory cells to the site of injury. The myeloperoxidase is mostly expressed in neutrophils, but can also be expressed in resident stimulated macrophages (Klebanoff, 1991; Sugiyama et al., 2001). No evidence has been presented showing that myeloperoxidase is expressed in fibroblasts, so we assumed that the increase in myeloperoxidase activity is mostly due to the migration and activation of neutrophils and/or macrophages. Interestingly, myeloperoxidase activity was significantly inhibited by systemic treatment with both B1 and B2 receptor antagonists, demonstrating that neutrophil and/or macrophage chemotaxis and activation is partially dependent on the functionality of these receptors.
Further, we demonstrated that, after an initial burst of mediators, a rapid up-regulation of mRNA for TNF-α, IL-1β and IL-6 occurred. In this context, the continuous overexpression of these cytokines is likely to be responsible for the maintenance of a more persistent hyperalgesia. In accord with these findings, previous studies have demonstrated that TNF-α, IL-1β and IL-6 mediate muscle pain (Schafers et al., 2003; Rosendal et al., 2005; Manjavachi et al., 2010), and that IL-6 is the main contributor to chronic latent pain in inflammatory muscle conditions (Dina et al., 2008a). Interestingly, antagonism of the B1 receptor was only effective in preventing the mRNA transcription of TNF-α, whereas blockade of the B2 receptor prevented the transcription of both TNF-α and IL-6 without affecting IL-1β mRNA. The different effects of the various kinin receptor antagonists on the transcriptional regulation of cytokines may be attributed to a cell-type specific response, but this hypothesis clearly requires further investigation.
The B1 and B2 receptors belong to the classic seven transmembrane GPCR family, and their activation releases inositol 1,4,5-triphosphate from membranes via a PLC-mediated mechanism. The signal transduction followed by bradykinin binding to receptor B2 activates the formation of cAMP, the phosphorylation of the intracellular kinases tyrosine kinase, PKC, MAPKs, the ribosomal protein S6 kinase and phosphatidylinositol-3-kinase, and the activation of transcriptional factors such as the cAMP-response element binding (Pyne et al., 1997; Pan et al., 1999; Ritchie et al., 1999; Zhao et al., 2002). In this context, it has been proposed that B2 receptor activation takes place at the beginning of the inflammatory process and displays some auto-regulatory mechanisms. An interplay between B1 and B2 receptors has been proposed from results obtained in in vitro and in vivo studies where the desensitization of B2 receptors was followed by the up-regulation of B1 receptors (Campos and Calixto, 1995; Phagoo et al., 1999; Calixto et al., 2004). In support of this proposal, we found that subactive concentrations of both antagonists presented an additive effect against hyperalgesia.
Since MAPKs are strictly involved in signal transduction after the stimulation of kinin receptors, and because these proteins can directly contribute to the translational and post-translational sensitization of B1 and B2 receptors (Schanstra et al., 1998; Angers et al., 2000; Zhao et al., 2002; Calixto et al., 2004; Medeiros et al., 2004; Ferreira et al., 2005), we investigated the activation of the MAPKS p38 and JNK in our formalin-induced muscle pain model. The two MAPKs were activated in the first minutes following formalin injection, showing that these proteins are important in the initiation of the inflammatory process. In line with this, pretreatment with DALBK or HOE 140 significantly reduced the activation of p38 and JNK, suggesting that the activation of both MAPKs occurred secondarily to the activation of B1 and B2 receptors. Noteworthy, the inhibition of MAPKs phosphorylation cascade could explain, at least in part, the long-lasting effects of the B1 and B2 antagonists against hyperalgesia. The role of MAPKs in the formalin-induced hyperalgesia was further confirmed by the treatment with the p38 and JNK inhibitors, SB203580 and SP60015. The maximum level of inhibition was achieved 1 h after formalin injection, which is in accordance with the peak of phosphorylation of these proteins.
Our results also indicated that the intracellular second messenger PKC exerts a relevant role in mediating the hyperalgesia induced by formalin in muscle pain. Within the PKC family, the PKCε isoform has been reported to have a particular contribution towards nociception and it can act as a second messenger in the activation of kinin receptors (Cesare et al., 1999; Ferreira et al., 2005; 2008). Furthermore, PKCε is only involved in inflammatory muscle pain (Dina et al., 2008b). Here, we found that the activation of PKCε mimics the hyperalgesia induced by formalin. The hyperalgesia caused by the octapeptide ψεRACK was probably due to a direct activation of intracellular PKCε, an event downstream of the B1 and B2 receptors. This conclusion is supported by the absence of protective effect by the B2 receptor antagonist against PKCε-induced hyperalgesia. In addition, antagonism of the B1 receptor disrupted the hyperalgesia induced by the octapeptide ψεRACK only 2 h after its injection. This latter effect of DALBK might be as a result of direct antagonism of the newly synthesized B1 receptors, assuming that the synthesis of new receptors was stimulated by PKCε; as hypothesized by Ferreira et al. (2008).
Together, these results demonstrate that the inflammatory process triggered by formalin injection into the muscle induces a cascade of events that is responsible for the hyperalgesia. In this context, the activation of PKC, p38 and JNK, the synthesis of new IL-1β, TNF-α and IL-6 and the up-regulation of B1 and B2 receptors might bring about mechanical hyperalgesia. In our model of inflammatory hyperalgesia, the mRNA synthesis of IL-1β and IL-6 was unrelated to the p38 and JNK cascade because the inhibition of these kinases by B1 and B2 receptor antagonists was unable to prevent IL-1β and IL-6 mRNA transcription. In agreement with previous data, this may occur because MAPKs affect the expression of cytokines at the post-translational level without affecting mRNA expression (Young et al., 1993; Prichett et al., 1995).
In summary, this study extends previous findings by reporting a direct role of the B1 and B2 receptors in the inflammatory muscle pain. As reported before, bradykinin injection into the muscle induced only mild hyperalgesia, and the B1 receptor did not seem to play an important role for pain transmission in muscle (Murase et al., 2010). However, after an initial inflammatory stimulus, both B1 and B2 receptors play a major role in the resulting hyperalgesia, as could occur after a mechanical wound, tissue infection or surgery. The finding that the intracellular kinase PKC, p38 and JNK pathways and inflammatory cytokines were intimately involved in this inflammatory response in muscle is of particular interest. Hence, this study contributes to the elucidation of the mechanisms underlying muscle pain and supports the use of kinin receptor antagonists in the management of pain in injured muscle.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Apoio a Pesquisa do Estado de Santa Catarina, all from Brazil. R. Campos is an undergraduate student and holds a fellowship support from CNPq. R. Costa and K.A.B.S.S. are PhD students in Pharmacology; they receive grants from CNPq. F.C.M. holds postdoctoral fellowships from CNPq. The authors thank Fujisawa Pharmaceutical Co., Hoechst and ©Sanofi-Aventis by the kind donation of the B2 receptor antagonists FR173657, HOE 140 and the B1 receptor antagonist SSR240612 respectively.
Glossary
- DALBK
des-Arg9-[Leu8]-bradykinin
- FR173657
(E)-3-(6- acetamido- 3-pyridyl)- N-[N- [2,4- dichloro- 3-[(2-methyl-8- quinolinyl)oxymethyl]phenyl]-N-methylaminocarbonylmethyl]acrylamide
- GF109203X
3-[1-[3-(dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride
- HOE
140,D-Arg-L-Arg-L-Pro-L-Hyp-Gly-L-(2-thienyl)Ala-L-Ser-D-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-L-(2alpha; 3 beta; 7 alpha beta)-octahydro-1H-indole-2-carbonyl-L-Arg (Icatibantacetate)
- SSR240612
[(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-[[(6-methoxy-2-naphthyl)sulfonyl]amino]propanoyl)amino]-3-(4-[[2R,6S)-2,6-dimethylpiperidinyl]methyl]phenyl)-N-isopropyl-N-methylpropanamide hydrochloride]
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole
- SP600125
anthra[1-9-cd]pyrazol-6(2H)-one
Conflicts of interest
All authors declare that there are no conflicts of interest.
References
- Angers M, Drouin R, Bachvarova M, Paradis I, Marceau F, Bachvarov DR. In vivo protein-DNA interactions at the kinin B(1) receptor gene promoter: no modification on interleukin-1 beta or lipopolysaccharide induction. J Cell Biochem. 2000;78:278–296. doi: 10.1002/(sici)1097-4644(20000801)78:2<278::aid-jcb10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Babenko V, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain and muscular hyperalgesia induced by combinations of serotonin and bradykinin. Pain. 1999;82:1–8. doi: 10.1016/S0304-3959(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Bradykinin and asthma. Thorax. 1992;47:979–983. doi: 10.1136/thx.47.11.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix F, Roe C, Rosenborg L, Knardahl S. Kinin peptides in human trapezius muscle during sustained isometric contraction and their relation to pain. J Appl Physiol. 2005;98:534–540. doi: 10.1152/japplphysiol.01340.2003. [DOI] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Characterization of bradykinin receptors in a human osteoblastic cell line. Regul Pept. 2002;103:39–51. doi: 10.1016/s0167-0115(01)00325-1. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MM, Calixto JB. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br J Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Cloutier F, Couture R. Pharmacological characterization of the cardiovascular responses elicited by kinin B(1) and B(2) receptor agonists in the spinal cord of streptozotocin-diabetic rats. Br J Pharmacol. 2000;130:375–385. doi: 10.1038/sj.bjp.0703319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa CR, Calixto JB. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Neto CM, Dillenburg-Pilla P, Heinrich TA, Parreiras-e-Silva LT, Pereira MG, Reis RI, et al. Participation of kallikrein-kinin system in different pathologies. Int Immunopharmacol. 2008;8:135–142. doi: 10.1016/j.intimp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008a;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase C epsilon-dependent chronic-latent muscle pain. J Pain. 2008b;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A, Perkins M. Kinins and pain. In: Farmer SG, editor. The Handbook of Immunopharmacology: The Kinin System. London: Academic Press; 1997. pp. 157–172. [Google Scholar]
- Ferreira J, Campos MM, Araujo R, Bader M, Pesquero JB, Calixto JB. The use of kinin B1 and B2 receptor knockout mice and selective antagonists to characterize the nociceptive responses caused by kinins at the spinal level. Neuropharmacology. 2002;43:1188–1197. doi: 10.1016/s0028-3908(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain. 2005;117:171–181. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Triches KM, Medeiros R, Cabrini DA, Mori MA, Pesquero JB, et al. The role of kinin B1 receptors in the nociception produced by peripheral protein kinase C activation in mice. Neuropharmacology. 2008;54:597–604. doi: 10.1016/j.neuropharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Figueroa CD, Dietze G, Muller-Esterl W. Immunolocalization of bradykinin B2 receptors on skeletal muscle cells. Diabetes. 1996;45(Suppl. 1):S24–S28. doi: 10.2337/diab.45.1.s24. [DOI] [PubMed] [Google Scholar]
- Gerdle B, Hilgenfeldt U, Larsson B, Kristiansen J, Sogaard K, Rosendal L. Bradykinin and kallidin levels in the trapezius muscle in patients with work-related trapezius myalgia, in patients with whiplash associated pain, and in healthy controls – a microdialysis study of women. Pain. 2008;139:578–587. doi: 10.1016/j.pain.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Gougat J, Ferrari B, Sarran L, Planchenault C, Poncelet M, Maruani J, et al. SSR240612 [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-[[(6-methoxy-2-naphthyl)sulfonyl]amino]propanoyl)amino]-3-(4-[[2R,6S)-2,6-dimethylpiperidinyl]methyl]pheny l)-N-isopropyl-N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;309:661–669. doi: 10.1124/jpet.103.059527. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Jensen K, Tuxen C, Pedersen-Bjergaard U, Jansen I, Edvinsson L, Olesen J. Pain and tenderness in human temporal muscle induced by bradykinin and 5-hydroxytryptamine. Peptides. 1990;11:1127–1132. doi: 10.1016/0196-9781(90)90141-q. [DOI] [PubMed] [Google Scholar]
- Khan MN, Lee YS. Cyclooxygenase inhibitors: scope of their use and development in cancer chemotherapy. Med Res Rev. 2011;31:161–201. doi: 10.1002/med.20182. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. Myeloperoxidase: occurence and biological function. In: Everse J, Everse KE, Grisham MB, editors. Peroxidases in Chemistry and Biology. Boca Raton: CRC Press; 1991. pp. 1–35. [Google Scholar]
- Lopes P, Regoli D, Couture R. Cardiovascular effects of intrathecally administered bradykinin in the rat: characterization of receptors with antagonists. Br J Pharmacol. 1993;110:1369–1374. doi: 10.1111/j.1476-5381.1993.tb13971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IS, MacDonald TM. Treatment of osteoarthritis in hypertensive patients. Expert Opin Pharmacother. 2010;11:393–403. doi: 10.1517/14656560903496422. [DOI] [PubMed] [Google Scholar]
- Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain. 2010;151:345–355. doi: 10.1016/j.pain.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Marceau F, Bachvarov DR. Kinin receptors. Clin Rev Allergy Immunol. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Cabrini DA, Ferreira J, Fernandes ES, Mori MA, Pesquero JB, et al. Bradykinin B1 receptor expression induced by tissue damage in the rat portal vein: a critical role for mitogen-activated protein kinase and nuclear factor-kappaB signaling pathways. Circ Res. 2004;94:1375–1382. doi: 10.1161/01.RES.0000128404.65887.08. [DOI] [PubMed] [Google Scholar]
- Meini S, Maggi CA. Knee osteoarthritis: a role for bradykinin? Inflamm Res. 2008;57:351–361. doi: 10.1007/s00011-007-7204-1. [DOI] [PubMed] [Google Scholar]
- Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Excitation and sensitization of nociceptors by bradykinin: what do we know? Exp Brain Res. 2009;196:53–65. doi: 10.1007/s00221-009-1814-5. [DOI] [PubMed] [Google Scholar]
- Mork H, Ashina M, Bendtsen L, Olesen J, Jensen R. Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur J Pain. 2003;7:145–153. doi: 10.1016/S1090-3801(02)00096-4. [DOI] [PubMed] [Google Scholar]
- Motta EM, Calixto JB, Rae GA. Mechanical hyperalgesia induced by endothelin-1 in rats is mediated via phospholipase C, protein kinase C, and MAP kinases. Exp Biol Med. 2006;231:1141–1145. [PubMed] [Google Scholar]
- Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZK, Christiansen SC, Ptasznik A, Zuraw BL. Requirement of phosphatidylinositol 3-kinase activity for bradykinin stimulation of NF-kappaB activation in cultured human epithelial cells. J Biol Chem. 1999;274:9918–9922. doi: 10.1074/jbc.274.15.9918. [DOI] [PubMed] [Google Scholar]
- Paszcuk AF, Quintao NL, Fernandes ES, Juliano L, Chapman K, Andrade-Gordon P, et al. Mechanisms underlying the nociceptive and inflammatory responses induced by trypsin in the mouse paw. Eur J Pharmacol. 2008;581:204–215. doi: 10.1016/j.ejphar.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr, Walther T, et al. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci U S A. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phagoo SB, Poole S, Leeb-Lundberg LM. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1beta shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol Pharmacol. 1999;56:325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- Polosa R. Role of the kinin-kallikrein pathway in allergic diseases. Allergy. 1993;48:217–225. doi: 10.1111/j.1398-9995.1993.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Prichett W, Hand A, Sheilds J, Dunnington D. Mechanism of action of bicyclic imidazoles defines a translational regulatory pathway for tumor necrosis factor alpha. J Inflamm. 1995;45:97–105. [PubMed] [Google Scholar]
- Pyne NJ, Tolan D, Pyne S. Bradykinin stimulates cAMP synthesis via mitogen-activated protein kinase-dependent regulation of cytosolic phospholipase A2 and prostaglandin E2 release in airway smooth muscle. Biochem J. 1997;328((Pt 2)):689–694. doi: 10.1042/bj3280689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintao NL, Passos GF, Medeiros R, Paszcuk AF, Motta FL, Pesquero JB, et al. Neuropathic pain-like behavior after brachial plexus avulsion in mice: the relevance of kinin B1 and B2 receptors. J Neurosci. 2008;28:2856–2863. doi: 10.1523/JNEUROSCI.4389-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- Regoli D, Nsa Allogho S, Rizzi A, Gobeil FJ. Bradykinin receptors and their antagonists. Eur J Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- Ritchie RH, Marsh JD, Schiebinger RJ. Bradykinin-stimulated protein synthesis by myocytes is dependent on the MAP kinase pathway and p70(S6K) Am J Physiol. 1999;276:H1393–H1398. doi: 10.1152/ajpheart.1999.276.4.H1393. [DOI] [PubMed] [Google Scholar]
- Rooks DS. Fibromyalgia treatment update. Curr Opin Rheumatol. 2007;19:111–117. doi: 10.1097/BOR.0b013e328040bffa. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2005;98:477–481. doi: 10.1152/japplphysiol.00130.2004. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Fujinaga M, Kingery WS, Belanger N, Davies MF, Maze M. Opioidergic and adrenergic modulation of formalin-evoked spinal c-fos mRNA expression and nocifensive behavior in the rat. Eur J Pharmacol. 1999;379:141–149. doi: 10.1016/s0014-2999(99)00463-x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Schanstra JP, Bataille E, Marin Castano ME, Barascud Y, Hirtz C, Pesquero JB, et al. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian LJ, Du YR, Xiao Y, Lv ZM, Yu YQ, Cui XY, et al. Mediating roles of the vanilloid receptor TRPV1 in activation of rat primary afferent nociceptive neurons by formaldehyde. Sheng Li Xue Bao. 2009;61:404–416. [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Vadakkan KI, Wang H, Ko SW, Zastepa E, Petrovic MJ, Sluka KA, et al. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Mol Pain. 2006;2:7–17. doi: 10.1186/1744-8069-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40:743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- Werner MF, Kassuya CA, Ferreira J, Zampronio AR, Calixto JB, Rae GA. Peripheral kinin B(1) and B(2) receptor-operated mechanisms are implicated in neuropathic nociception induced by spinal nerve ligation in rats. Neuropharmacology. 2007;53:48–57. doi: 10.1016/j.neuropharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Wirth K, Hock FJ, Albus U, Linz W, Alpermann HG, Anagnostopoulos H, et al. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, McDonnell P, Dunnington D, Hand A, Laydon J, Lee J. Pyridinyl imidazoles inhibit IL-1 and TNF production at the protein level. Agents Actions. 1993;39 doi: 10.1007/BF01972723. Spec No: C67–C69. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Ravindranath L, Mohamed AS, Zohar O, Chen GH, Lyketsos CG, et al. MAP kinase signaling cascade dysfunction specific to Alzheimer's disease in fibroblasts. Neurobiol Dis. 2002;11:166–183. doi: 10.1006/nbdi.2002.0520. [DOI] [PubMed] [Google Scholar]
- Ziltener JL, Leal S, Fournier PE. Non-steroidal anti-inflammatory drugs for athletes: an update. Ann Phys Rehabil Med. 2010;53:278–282. doi: 10.1016/j.rehab.2010.03.001. 282–288. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


