Abstract
BACKGROUND AND PURPOSE
ATP is a potent signalling molecule that regulates biological activities including increasing or decreasing proliferation in different types of cells. The aim of the present study was to investigate how ATP regulates the proliferation of human cardiac fibroblasts.
EXPERIMENTAL APPROACH
Reverse transcription (RT)-PCR, Western blot analysis, cell proliferation and migration assays were employed to investigate the effects of ATP on human adult ventricular fibroblasts.
KEY RESULTS
ATP increased cell proliferation in a concentration-dependent manner. Similarly, the P2X receptor agonist α,β-methylene ATP and P2Y receptor agonist ATP-γS also up-regulated cell proliferation. The P2 receptor antagonists suramin and reactive blue-2 prevented the ATP-induced increase in proliferation and RT-PCR and Western blot analysis revealed that mRNAs of P2X4/7 and P2Y2 are abundant in cardiac fibroblasts. ATP increased phosphorylated PKB (Akt) and ERK1/2 levels; an effect antagonized by suramin, reactive blue-2, the PI3-kinase inhibitor, wortmannin, PKB inhibitor, API-2, and MAPK inhibitor, PD98059. These kinase inhibitors also prevented the ATP-induced increase in proliferation. In addition, ATP enhanced the progression of cells from the G0/G1 phase to the S phase by increasing the expression of proteins for cyclin D1 and cyclin E. Silencing the P2X4/7 and P2Y2 receptors with siRNA targeting the corresponding receptor diminished ATP-stimulated proliferation and migration of the cardiac fibroblasts.
CONCLUSION AND IMPLICATION
ATP activates P2X4/7 and P2Y2 receptors and up-regulates the proliferation of human cardiac fibroblasts by promoting cell cycling progression. It also increases the migration of these cells. These effects of ATP may be involved in cardiac remodelling of injured hearts.
Keywords: ATP, cardiac fibroblasts, proliferation, cell cycling, cell migration
Introduction
Cardiac fibroblasts play an important role in the structural, mechanical, biochemical and electrical characteristics of the heart (Long and Brown, 2002; Sun et al., 2002; Camelliti et al., 2006; Souders et al., 2009). Generally, cardiac fibroblasts physiologically maintain extracellular matrix (ECM) homeostasis and generate related factors associated with the equilibrium between synthesis and degradation of connective tissue constituents, such as growth factors, cytokines and matrix metalloproteinases (Brilla et al., 1992; Long and Brown, 2002; Sun et al., 2002). During the pathological development and progression of cardiovascular diseases, cardiac fibroblasts participate in myocardial remodelling (Sun and Weber, 2000; Long and Brown, 2002; Porter and Turner, 2009). The unduly proliferative fibroblasts and increased protein content of the ECM are found to lead to myocardial stiffening, which is a major symptom in the pathology of cardiac dysfunction (Zannad et al., 2001; Long and Brown, 2002; Porter and Turner, 2009). Hence, understanding the mechanism of cell proliferation of cardiac fibroblasts is important in the development of new therapies to manage cardiac remodelling.
ATP is a multifunctional nucleotide serving not only as an intracellular energy source but also as an important extracellular signalling molecule, which acts by binding to purinoceptors on the cell membrane (Burnstock, 1997). Purinoceptors, including P2Y receptors and P2X receptors, are present in different tissues/organs including fetal and adult hearts (Bogdanov et al., 1998; Vassort, 2001; Wihlborg et al., 2006). ATP is secreted from cardiac myocytes, endothelial cells, platelets, red blood cells, as well as from damaged cells in the pathogenesis of cardiovascular disorders such as ischaemia and atherosclerosis, and has multiple actions, regulating myocardial and vascular remodelling, platelet aggregation and coagulation, and is involved in the development of heart failure (Ralevic and Burnstock, 1991; Burnstock and Ralevic, 1994; Vassort, 2001; Erlinge and Burnstock, 2008). It has been reported that ATP increases the proliferation of rat glial cells (Franke et al., 2009) and bovine corneal endothelial cells (Cha et al., 2000) and bovine adventitial fibroblasts (Gerasimovskaya et al., 2005); however, it inhibits the proliferation of human mesenchymal stem cells (Coppi et al., 2007), human endometrial stromal cells (Chang et al., 2008), human stomach cancer cells (Wang et al., 2005) and neonatal rat cardiac fibroblasts (Zheng et al., 1998). It is unclear whether these controversial results are related to the species differences and/or specific tissues/cell types. Little is known about the potential roles of ATP in the cellular physiology of human cardiac fibroblasts, and the present study was therefore designed to investigate how ATP regulates proliferation in human cardiac fibroblasts. Our results demonstrate that in addition to increasing their migration, ATP, by stimulating P2X4/7 and P2Y2 receptors, enhances the proliferation of human cardiac fibroblasts, in culture, by promoting the progression of G0/G1 cells to the S phase.
Methods
Culture of human cardiac fibroblasts
Human adult ventricular cardiac fibroblasts (Catalog # 6310) were purchased from ScienCell Research Laboratory (San Diego, CA, USA). The cells were primarily cultured as a monolayer in DMEM containing 10% fetal bovine serum (FBS, Invitrogen, Hong Kong) and antibiotics (100 U·mL−1 penicillin G and 100 µg·mL−1 streptomycin) at 37°C in a humidified atmosphere of 95% air, 5% CO2. Cells used in this study were from the passages 3–6 (Li et al., 2009; Chen et al., 2010; He et al., 2011).
Messenger RNA determination
The messenger RNA was examined using the reverse transcription (RT)-PCR technique as described previously (Li et al., 2009; Chen et al., 2010). Briefly, total RNA was extracted from human cardiac fibroblasts using Trizol reagent (Invitrogen), and further treated with DNase I (Invitrogen) for 30 min at 37°C, then heated to 75°C for 5 min and finally cooled to 4°C to remove genomic DNA. Reverse transcription was performed using a RT system (Promega, Madison, WI, USA) in a 20 µL reaction mixture. A total of 2 µg RNA was used in the reaction, and a random hexamer primer was used for the initiation of cDNA synthesis. After the RT procedure, the reaction mixture (cDNA) was used for PCR.
PCR was performed with thermal cycling conditions of 94°C for 2 min followed by 35 cycles at 94°C for 45 s, 55–58°C for 45 s and 72°C for 1 min using a Promega PCR kit and oligonucleotide primers as shown in Table 1. This was followed by a final extension at 72°C (10 min) to ensure complete product extension. The PCR products were electrophoresed through 1.5% agarose gels and visualized by ethidium bromide staining and imaged using Chemi-Genius Bio Imaging System (Syngene, Cambridge, UK).
Table 1.
Primers of specific human genes
| Gene | Accession number | Primer | Sequences (5′–3′) | Fragment Size (bp) |
|---|---|---|---|---|
| P2X1 | NM_002558 | Forward | GACCAACTACCGTCACCTCT | 213 |
| Reverse | TCAGCCTATTTGAACTTCTTCT | |||
| P2X2 | NM_016318 | Forward | GCTGCTCATCCTGCTCTACTT | 185 |
| Reverse | TGGCGTTGTGGACCCTTA | |||
| P2X3 | NM_002559 | Forward | ATTAAGATCGGCTGGGTG | 321 |
| Reverse | GAGGAAGTTGAGCAGGATG | |||
| P2X4 | NM_002560 | Forward | GAGATTCCAGATGCGACC | 296 |
| Reverse | GACTTGAGGTAAGTAGTGG | |||
| P2X5 | NM_002561 | Forward | TCCTGAAATCATGCCACTT | 203 |
| Reverse | ATTGTCCAGACGGCTAAAA | |||
| P2X6 | NM_005446.3 | Forward | AGTTCAACTTCTCTAAGTCCAATGC | 470 |
| Reverse | CTCTATCCACATACAGCAGTAGC | |||
| P2X7 | NM_002562 | Forward | CACTGCCGTCCCAAATAC | 223 |
| Reverse | AGAGGGTTGAGCCGATGT | |||
| P2Y1 | NM_002563 | Forward | CTATGGCAGCATCTTGTT | 234 |
| Reverse | CTTCGCAGGTACTCGTCT | |||
| P2Y2 | NM_002564 | Forward | GCCGCTGCTGGTCTATT | 269 |
| Reverse | CGCTGGTGGTGACAAAGT | |||
| GAPDH | J02642 | Forward | AACAGCGACACCCACTCCTC | 258 |
| Reverse | GGAGGGGAGATTCAGTGTGGT |
P2X, purinoceptor 2X; P2Y, purinoceptor 2Y; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell proliferation assays
The cell proliferation assays were conducted using [3H]-thymidine incorporation and 5, 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) methods. Cells were seeded in 96-well plates at approximately 4 × 104 to 6 × 104 per well in DMEM containing 10% FBS as described previously (Deng et al., 2007; Tao et al., 2008). Briefly, in cells incubated with ATP or other reagents, 10 µL per well of 5 mg·mL−1 MTT solution was added directly to the cell supernatant. After a 4 h incubation at 37°C, the cell culture medium was removed by decanting, and the supernatant was removed by tapping off suction on paper towels, and the formazan crystals in adherent cells were dissolved in DMSO, 100 µL per well. The plates were read (wavelengths: test, 570; reference, 630 nm) using a µQuant microplate spectrophotometer (Bio-Tek Instruments Inc., Winooski, VT, USA). Results were standardized using control group values.
The [3H]-thymidine incorporation assay was performed in human cardiac fibroblasts plated in 96-well plate, and subjected to different treatments. A total of 1 µCi (0.037 MBq) [3H]-thymidine (GE Healthcare, Hong Kong) was added to each well. The cells were harvested after 4 h incubation, and transferred to a nitrocellulose-coated 96-well plate via suction. Nitrocellulose membrane was washed with water, and the plate was air dried at 50°C overnight. Liquid scintilla (20 µL per well) was then added to each well. The counts per minute (cpm) for each well was read by a TopCount microplate scintillation and luminescence counter (PerkinElmer, Waltharn, MA, USA), and the data were normalized with control.
Western blot analysis
The Western blotting analysis was performed following the procedure described previously (Tao et al., 2008). Briefly, cell lysates were extracted via a modified radioimmunoprecipitation buffer, and protein concentrations were determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA) protein assay. Cell lysates (50 µg) were mixed with sample buffer and denatured by heating to 95°C for 5 min. Samples were resolved via SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween-20 (TTBS) buffer then probed with primary antibodies at 4°C overnight with agitation. After wash with TTBS, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or donkey anti-goat IgG antibody at 1:4000 dilutions in TTBS at room temperature for 1 h. Membranes were washed again with TTBS then developed on X-ray film using an enhanced chemiluminescence detection system (GE Healthcare). The relative band intensities were measured by a quantitative scanning densitometer and image analysis software (Bio-1D, version 97.04).
RNA interference
Short interference RNA (siRNA) molecules targeting human P2X4 (sc-42569), P2X7 (sc-42575) and P2Y2 (sc-42579) were purchased from Santa Cruz Biotech, Inc. (Santa Cruz, CA, USA). The siRNA is a pool of three target-specific 20–25 nucleotide siRNAs designed to knock-down the expression of the corresponding gene. Human cardiac fibroblasts at 40∼50% confluence were transfected with siRNA molecules at 10 and 40 nM using Lipofectamine 2000 reagent (Invitrogen) in accordance with the manufacturer's protocol. The silencer negative control #1 siRNA (Ambion, #AM4611, Austin, TX, USA), which contains no known target in mammalian genomes, was used as negative control. After 72 h of transfection, the cells were used for Western blot analysis, proliferation and migration assays.
Flow cytometry and cell cycle analysis
Cell cycle distribution of human cardiac fibroblasts was determined by flow cytometry (FC500, Beckman Coulter, Fullerton, CA, USA) as described previously (Deng et al., 2007; Tao et al., 2008). Briefly, the cells were synchronized at the early G0/G1 phase by culture in low FBS (0.5%) for 24 h; the cell cycle progression was resumed in normal culture medium (10% FBS), and the cells were treated with different interventions. The cells were removed from the plates with 0.25% trypsin, washed with PBS and fixed with ice-cold ethanol (70%). Ethanol was removed by centrifugation and cell pellets were washed with PBS again. The cells were then incubated in a propidium iodide/PBS staining buffer (propidium iodide: 20 µg·mL−1, RNase A 100 µg·mL−1 and 0.1% Triton-X 100) at 37°C for 30 min. Flow cytometry data were acquired using CellQuest software, and the percentage of cells in the G0/G1, S and G2/M phases were calculated with MODFIT software.
Cell migration assay
The migration of human cardiac fibroblasts was determined by a wound-healing assay. Confluent cultures of cardiac fibroblasts in six-well plates were damaged (wounded) with a sterile 200 µL plastic pipette tip as described previously (Su et al., 2011). The starting point was marked with a marker pen at the bottom of the plate. After incubation with the medium containing 1% FBS and 10 µM ATP for 20 h, the defined area of the wound was photographed under a phase contrast microscope (Olympus, Tokyo, Japan) and the number of migrated cells was counted.
A microchemotaxis assay was performed using a modified Boyden chamber with 8 µm-pore polycarbonate membranes (Corning Inc, Corning, NY, USA) following the manufacturer's instructions. After the membrane was incubated with 700 µL serum-free cell culture medium for 1 h, human cardiac fibroblasts were seeded in the upper chamber (2 × 104 cells per chamber) for 2 h. The cells were then incubated with a culture medium containing 1% FBS and 10 µM ATP for 6 h. Following removal of the medium and washing with PBS for three times, the cells were fixed with formaldehyde, and stained with crystal violet for 15 min. Non-migrated cells on the upper surface of the membrane were scraped off with cotton swabs after the stain had been removed and washed away with PBS. The migrated cells on the lower surface of the membrane were counted under a microscope.
Statistical analysis
Data are expressed as means ± SEM. Results were analysed by Student's t-test for two groups and anova for multiple group comparison. Values of P < 0.05 were considered to be statistically significant.
Results
ATP and cell proliferation
Figure 1 displays the effect of ATP on proliferation of human cardiac fibroblasts. The MTT assay showed that ATP (0.1–100 µM) increased cell proliferation in a concentration-dependent manner (Figure 1A). A significant effect was observed at 0.1 µM (P < 0.05 vs. control), and maximum effect was observed at 100 µM ATP. ATP also enhanced the rate of [3H]-thymidine incorporation in a concentration-dependent manner after a 24 h incubation (Figure 1B). The maximum effect on the proliferation of these cells, similar to that induced by basic fibroblast growth factor (50 ng·mL−1, Invitrogen), was observed with 100 µM ATP, in both the MTT and [3H]-thymidine incorporation assays; we therefore employed this concentration of ATP in the following biochemical experiments.
Figure 1.
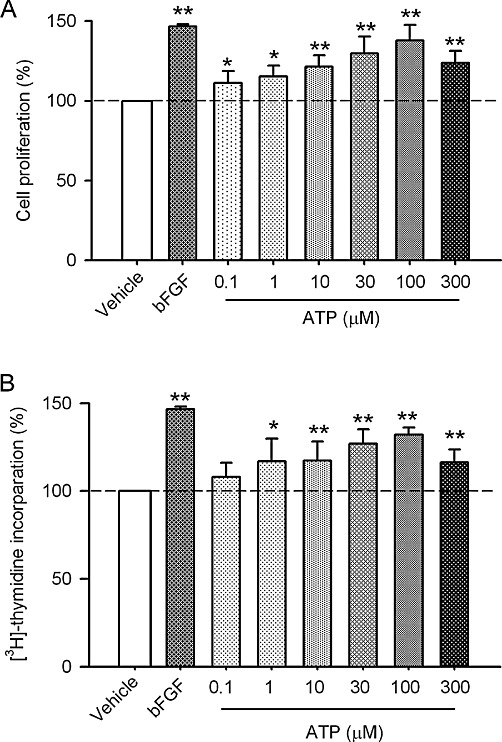
Stimulation of the proliferation of human cardiac fibroblasts by ATP. (A) Cell proliferation was assayed by MTT in cells incubated with 0.1–300 µM ATP for 24 h (n= 5, *P < 0.05, **P < 0.01 vs. control, 0 µM ATP). (B) [3H]-thymidine incorporation was determined in cells incubated with 0.1–300 µM ATP for 24 h (n= 5, *P < 0.05, **P < 0.01 vs. control, 0 µM ATP).
Relationship between P2 receptors and cell proliferation
Figure 2A and B illustrate the RT-PCR and Western blot results for P2 receptors. The levels of expression of mRNAs and proteins of P2X4/7 and P2Y2 were significant in human cardiac fibroblasts. This suggests that the increased proliferation of these cells induced by ATP is probably mediated by activating P2 receptors present in human cardiac fibroblasts. Figure 2B shows that the P2X receptor agonist α,β-methylene ATP (100 µM) and the P2Y agonist ATP-γS (100 µM), like ATP, increased [3H]-thymidine incorporation rate (P < 0.01 vs. vehicle). Further, Figure 2C shows that the P2Y receptor antagonist reactive blue-2 (1 µM) partially inhibited the proliferation increase induced by ATP (P < 0.05 vs. ATP), while suramin (10 µM, a non-selective antagonist of P2X and P2Y receptors) almost fully antagonized ATP-induced proliferation (P < 0.01 vs. ATP). These results indicate that ATP-induced increase in cell proliferation is related to the activation of both P2X and P2Y receptors in human cardiac fibroblasts.
Figure 2.
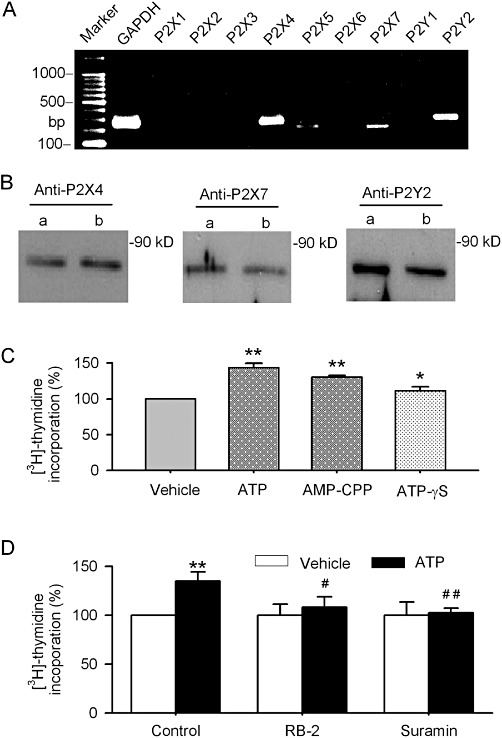
P2 receptors gene expression and effects of P2 receptor antagonists on [3H]-thymidine incorporation. (A, B) Images of RT-PCR and Western blots for detecting mRNAs and proteins of P2 receptors in human cardiac fibroblasts. (C) [3H]-thymidine incorporation was determined in cells incubated with ATP (100 µM), AMP-CPP (α,β-methylene ATP, 100 µM) or ATP-γS (100 µM) for 24 h. (D) Pre-incubation (for 30 min) with reactive blue-2 (RB-2, 1 µM) or suramin (10 µM) attenuated or abolished the increase in [3H]-thymidine incorporation rate induced by ATP. n= 4, *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. ATP alone.
Molecular mechanisms of the enhanced proliferation by ATP
To investigate the molecular mechanism by which ATP regulates cell growth in human cardiac fibroblasts, the phosphorylation levels of the proliferation-related enzymes (PKB, serine/threonine kinase and ERKs) were determined using Western blot analysis. Figure 3A shows that the phosphorylated level of PKB(308) was significantly increased after incubation of the cells with 100 µM ATP for 60 min, and this effect was abolished by suramin (10 µM) or reactive blue-2 (1 µM, 30 min pre-incubation). However, the level of phosphorylated PKB(473) was not affected by ATP, or the co-application of suramin or reactive blue-2 (Figure 3B). This suggests that ATP-induced PKB phosphorylation is site-dependent in human cardiac fibroblasts, similar to that observed in human bone marrow-derived mesenchymal stem cells (Tao et al., 2011).
Figure 3.
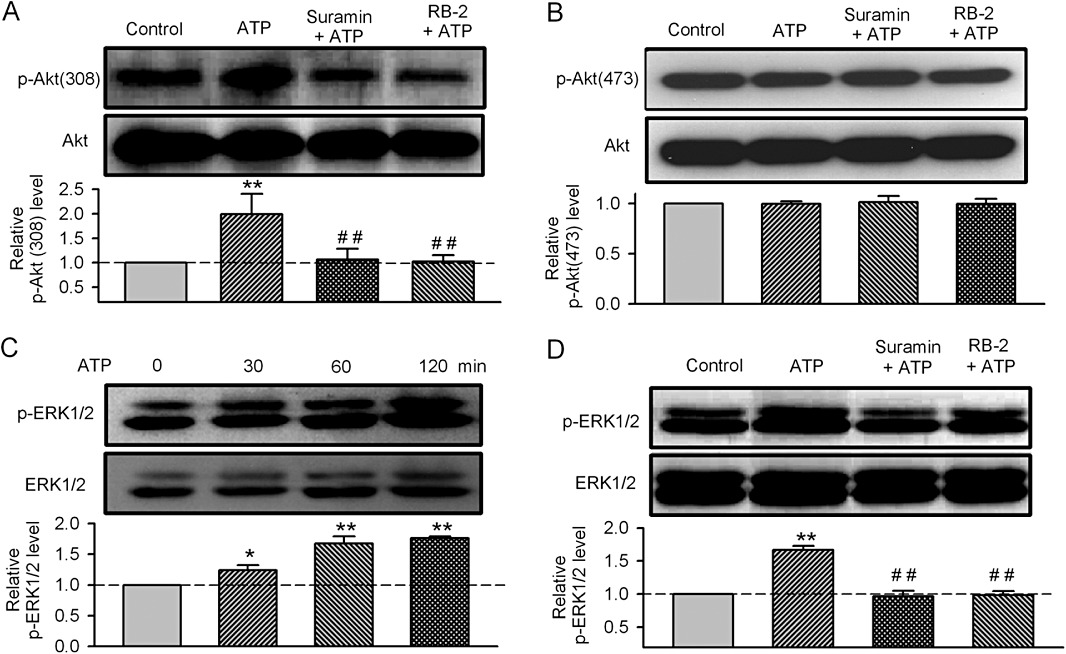
Effect of ATP on the phosphorylation of PKB and ERK1/2. (A, B) Images of total PKB (Akt) and phosphorylated PKB (P-Akt308, P-Akt407, upper panels) and relative mean values of phosphorylated PKB in cells treated with ATP (100 µM for 60 min incubation), alone or after pretreatment with either suramin (10 µM for 30 min) or reactive blue-2 (RB-2, 1 µM for 30 min) (n= 3, **P < 0.01 vs. vehicle control; ##P < 0.01 vs. ATP alone). (C) Time-dependent effect of ATP (100 µM) on P-ERK1/2 (n = 3, P < 0.05 or P < 0.01 vs. 0 min). (D) Images of total ERK1/2 and phosphorylated ERK1/2 (P-ERK1/2) and relative mean values of phosphorylated ERK1/2 in cells treated with ATP (100 µM for 60 min incubation), alone or after pretreatment with either suramin (10 µM for 30 min) or reactive blue-2 (RB-2, 1 µM for 30 min) (n= 3, **P < 0.01 vs. vehicle control; ##P < 0.01 vs. ATP alone).
Figure 3C shows that ATP (100 µM) also increased the level of phosphorylated ERK1/ERK2 after a 30 min incubation, and this effect was evident at 60 and 120 min. Suramin (10 µM) or reactive blue-2 (1 µM) prevented this ATP-induced increase in phosphorylated ERK1/ERK2 (Figure 3D). These results suggest that the phosphorylation of PKB and ERK1/2 is involved in the stimulant effect of ATP on the proliferation of cardiac fibroblasts.
To further determine whether activation of PKB/PI3-kinases (PI3Ks) and/or MAPKs increases phosphorylation of ERK1/2 by ATP in human cardiac fibroblasts, the cells were pre-incubated with the PKB inhibitor API2 (10 µM), the PI3K inhibitor wortmannin (1 µM) and the MAPKK/MEK 1 inhibitor PD98059 (1 µM) for 30 min. The ATP-enhanced ERK1/2 phosphorylation level was fully antagonized by API2, wortmannin or PD98059 (Figure 4A). In addition, API2, wortmannin or PD98059 slightly reduced cell proliferation and completely prevented the increase in proliferation and [3H]-thymidine incorporation induced by ATP (Figure 4B and C). These results suggest that activation of PKB/PI3K, MAPK or ERK1/2 is involved in ATP-induced increase in cell growth in human cardiac fibroblasts.
Figure 4.
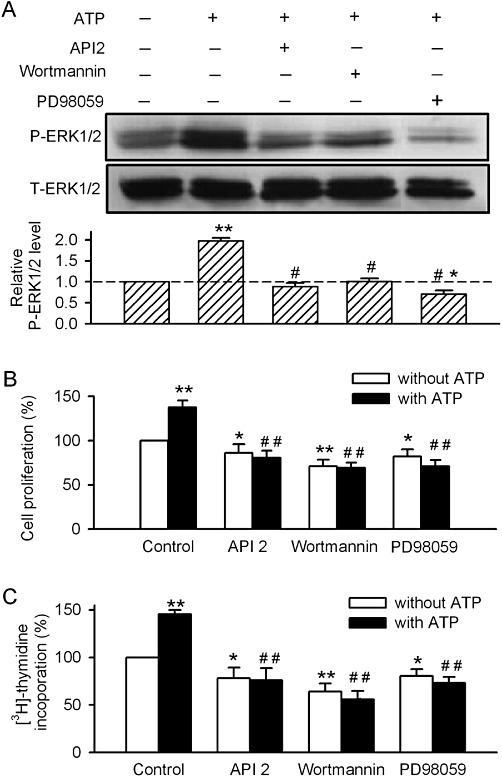
Effects of kinase inhibitors on the ATP-stimulated ERK1/2 phosphorylation or proliferation of human cardiac fibroblasts. (A) Images of total and phosphorylated ERK1/2 and mean values of phosphorylated ERK1/2 levels (relative to total ERK1/2) in cells treated with 100 µM ATP (60 min), alone or pretreated with the PKB inhibitor API2 (10 µM for 30 min), the PI3K inhibitor wortmannin (1 µM for 30 min) or the MAPK inhibitor PD98059 (1 µM for 30 min) (n= 3, *P < 0.05, **P < 0.01 vs. vehicle control; #P < 0.01 vs. ATP alone). (B, C) API2 (10 µM), wortmannin (1 µM) or PD98059 (1 µM) abolished the increase of cell proliferation and [3H]-thymidine incorporation induced by ATP (n= 4, *P < 0.05 **P < 0.01 vs. vehicle control; ##P < 0.01 vs. ATP alone.
Effect of ATP on cell cycle progression
The effect of ATP on cell cycle progression was determined with flow cytometry in human cardiac fibroblasts. Figure 5A illustrates the representative cell cycle distribution in cells without (control) and with 100 µM ATP treatment for 16 h; treatment with ATP caused a shift in the proportion of cells in the G0/G1 phase to the S phase. Figure 5B displays the mean values of cell cycle distribution in different phases in control cells and in cells treated with 100 µM ATP for 16 h (left panel) and 24 h (right panel). After an incubation in 100 µM ATP for 16 h, the % of cells in the G0/G1 phase was decreased from 65.9 ± 2.9% of control to 50.6 ± 2.8% (n= 4, P < 0.05 vs. control), while the % of cells in the S phase was increased from 30.3 ± 3.5% of control to 42.4 ± 3.3% with ATP treatment (P < 0.05 vs. control). No significant change was observed in the % of cells in the G2/M phase. Similar results were observed after incubating the cells for 24 h in 100 µM ATP. These results suggest that ATP stimulates the proliferation of cardiac fibroblasts by promoting the progression of cells from the G0/G1 phase to the S phase.
Figure 5.
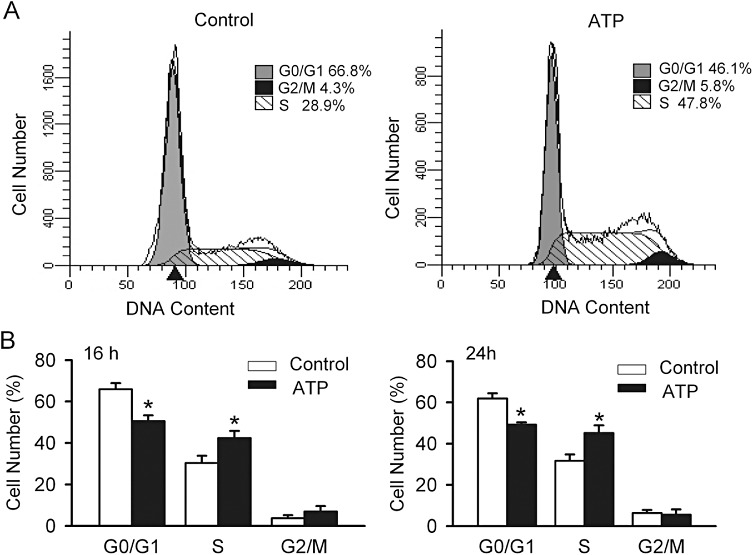
Effect of ATP on cell cycle progression. (A) Representative flow cytometry graphs in cells without (control) and with ATP (100 µM for 16 h) treatment. (B) Mean values of cell cycle distribution in control cells and in cells treated with ATP (100 µM) for 16 h (left panel) and 24 h (right panel) (n= 5, *P < 0.05 vs. control).
Effects of ATP on the expression of cell cycle regulatory proteins
It is generally believed that the cell cycle regulators cyclin D1 and cyclin E play an important role in early and late G1 progression. Therefore, whether the G0/G1 reduction induced by ATP is associated with the modulation of cyclin D1 and/or cyclin E modulation was examined in human cardiac fibroblasts. ATP (100 µM) significantly increased both cyclin D1 and cyclin E protein levels after the 12 h incubation (Figure 6). This effect was partially antagonized by a 30 min pre-incubation with the P2Y receptor antagonist reactive blue-2 (1 µM), and fully prevented by the non-selective P2 receptor antagonist suramin (10 µM). In addition, the PI3K inhibitor wortmannin (1 µM) and MAPK inhibitor PD98059 (1 µM) slightly reduced the level of cyclin D1 protein, completely inhibited the increase in cyclin D1, and partially prevented the increase in cyclin E induced by ATP. These results indicate that ATP participates in the regulation of cell cycle progression by activating P2 receptors, PKB/PI3K and MAPK, and modulating the expression of cyclin D1 and cyclin E proteins in human cardiac fibroblasts.
Figure 6.
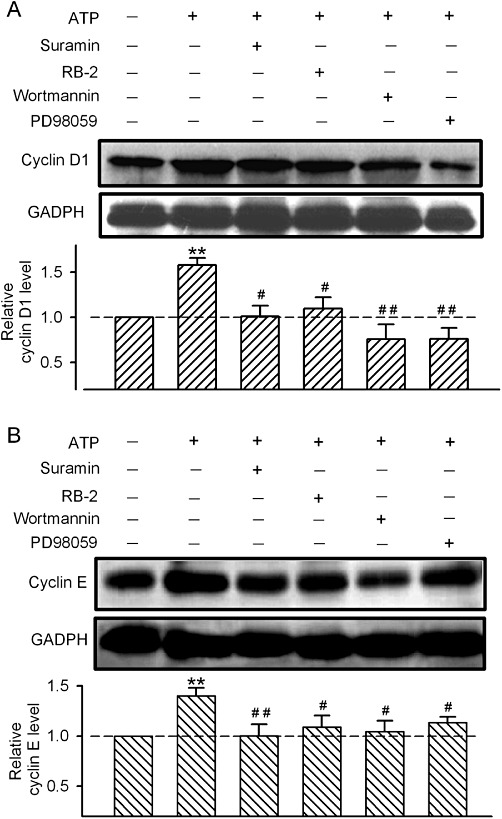
Effect of ATP on the expression of cyclin D1 and cyclin E. (A) Images of cyclin D1 protein and GAPDH protein and mean values of cyclin D1 protein level (relative to GAPDH) in cells incubated with 100 µM ATP, alone or in the presence of the P2 receptor antagonists suramin (10 µM) and RB2 (1 µM), the PI3K inhibitor wortmannin (1 µM) or the MAP inhibitor PD98059 (1 µM) (n= 3, **P < 0.01 vs. vehicle control; #P < 0.05, ##P < 0.01 vs. ATP alone). (B) Images of cyclin E protein and GAPDH protein and mean values of cyclin E level (relative to GAPDH) in cells incubated with 100 µM ATP, alone or in the presence of suramin (10 µM), RB2 (1 µM), wortmannin (1 µM), or PD98059 (1 µM) (n= 3, **P < 0.01 vs. vehicle control; #P < 0.05, ##P < 0.01 vs. ATP alone).
Effect of silencing P2 receptors on ATP-induced increase in cell proliferation
To determine which type(s) of P2 receptors mediate the ATP effect on cell proliferation, P2X4, P2X7 and P2Y2 were silenced, respectively, using siRNA molecules targeting the corresponding gene in human cardiac fibroblasts. Figure 7A shows that the protein expression of P2X4, P2X7 and P2Y2 was significantly reduced in cells transfected with 10 and 40 nM corresponding siRNA for 72 h (n= 5, P < 0.05 or P < 0.01 vs. control siRNA). Figure 7B and C show that although ATP (100 µM) significantly stimulated cell proliferation and [3H]-thymidine incorporation rate in cells transfected with control siRNA (n= 3, P < 0.01 vs. control), cell proliferation and [3H]-thymidine incorporation rate were reduced in cells transfected with P2X4 siRNA, P2X7 siRNA or P2Y2 siRNA (40 nM, n= 4, P < 0.01 vs. siRNA). ATP-induced increase of cell proliferation was attenuated in these cells (n= 3, P < 0.05 or P < 0.01 vs. control siRNA with ATP). These results indicate that ATP-induced stimulation of cell growth is mediated by P2X4, P2X7 and P2Y2 receptors.
Figure 7.
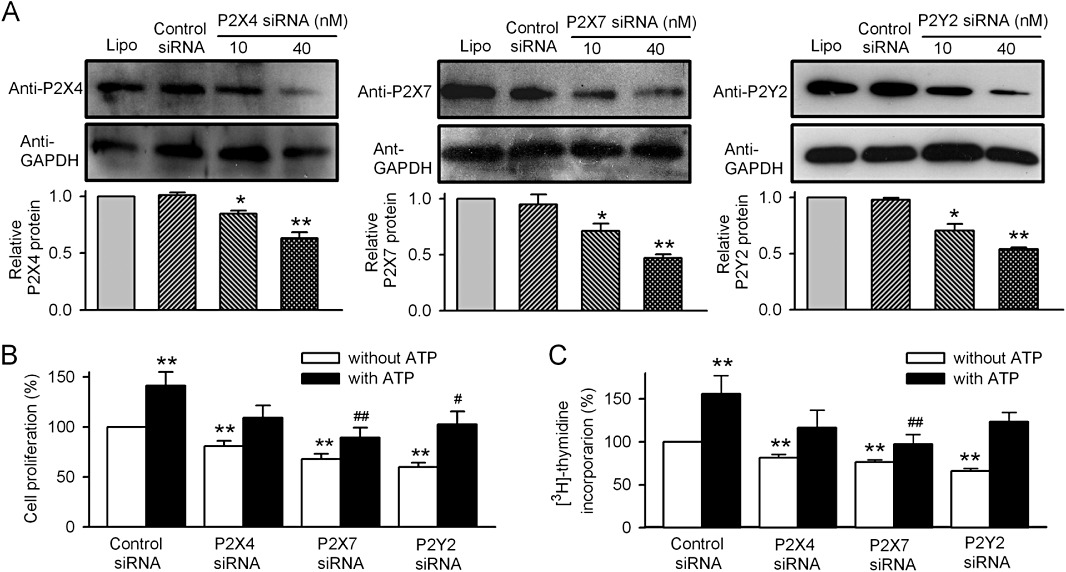
Effect of silencing the P2 receptors on ATP-stimulated proliferation of human cardiac fibroblasts. (A) Western blots and mean values of protein expression of P2X4, P2X7 and P2Y2 in cells treated with corresponding siRNA molecules (n= 4, *P < 0.05, **P < 0.01 vs. Lipofectamine or control siRNA). (B) Cell proliferation in cells treated with siRNA molecules (40 nM) targeting P2X4, P2X7 and P2Y2 (n= 3). (C) [3H]-thymidine incorporation in cells treated with siRNA molecules (40 nM) targeting P2X4, P2X7 and P2Y2, respectively (n= 3). **P < 0.01 vs. control siRNA without ATP; #P < 0.05, ##P < 0.01 vs. control siRNA with ATP.
Effects of ATP on cell migration in human cardiac fibroblasts
To investigate whether the migration of human cardiac fibroblasts is regulated by ATP, cell migration was determined in a wound-healing assay. Cells in culture were scraped off with a pipette tip, and a wide acellular area was produced (Figure 8A). Cardiac fibroblasts migrating into this acellular area were counted and expressed as number of migrated cells (Figure 8B). ATP (10 µM) significantly increased the migration of human cardiac fibroblasts after the 20 h incubation; this effect was reduced by the silencing of the P2X4, P2X7 and P2Y2 receptors with siRNAs (n= 4, P < 0.05 or P < 0.01 vs. Lipofectamine or control siRNA). Figure 8C shows that the cell migration assayed by a modified Boyden chamber also showed an increased cell migration after a 6-h incubation with 10 µM ATP (n= 3, P < 0.01 vs. control). These results suggest that in addition to stimulating proliferation, ATP enhances the migration of human cardiac fibroblasts by activating P2 receptors.
Figure 8.
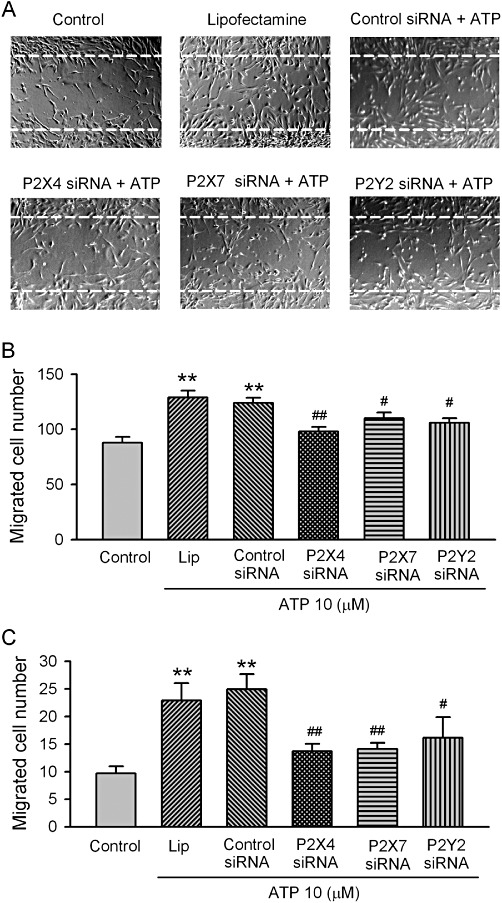
Effect of ATP on the migration of human cardiac fibroblasts. (A) Images of human cardiac fibroblasts in the wound-healing migration assay. Confluent cardiac fibroblasts were scraped off with a pipette tip to induce acellular areas, then treated 10 µM ATP in cells transfected with Lipofectamine, control siRNA, P2X4, P2X7 and P2Y2 siRNAs, respectively (40 nM each). Images were taken after 20 h incubation with 10 µM ATP. Broken white lines indicate the initial acellular wound regions. (B) Mean values for number of migrated human cardiac fibroblasts counted in areas as marked in (A) (n= 4, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. control siRNA). (C) Mean values for number of migrated human cardiac fibroblasts counted on lower surface of the Transwell membrane (microchemotaxis assay with 10 µM ATP incubation for 6 h, n= 3, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. control siRNA).
Discussion
The effect of extracellular ATP on cell proliferation has been reported in numerous types of cells; however, conflicting results were obtained in different types of cells and/or species (Wang et al., 1992; 2005; Yang and Krauss, 1997; Wilden et al., 1998; Coppi et al., 2007). Although the proliferative cardiac fibroblasts play an important role in the maintenance of matrix in normal hearts and pathogenic remodelling in diseased heart, little is known about the effect of ATP on growth in human cardiac fibroblasts. The present study provides novel information indicating that ATP promotes cell proliferation by activating PI3K/PKB and MAPKs; effects mediated by P2 receptors in human cardiac fibroblasts.
It is generally believed that extracellular ATP concentrations are not only determined by the balance between energy production and expenditure, but also rely on the balance between the rates of AMP synthesis and degradation (Ataullakhanov and Vitvitsky, 2002). The extracellular ATP concentrations vary from nanomolar to micromolar level in different circumstances (Schwiebert, 2000). In the present study, ATP at concentrations ≥1 µM increased cell proliferation in human cardiac fibroblasts (Figure 1). This observation differs from those in human bone marrow mesenchymal stem cells (Coppi et al., 2007), human endometrial stromal cells (Chang et al., 2008), human stomach cancers (Wang et al., 2005) and also in neonatal rat cardiac fibroblasts (Zheng et al., 1998), in which extracellular ATP reduces cell proliferation. However, the increased proliferation is consistent with reports in bovine corneal endothelial cells (Cha et al., 2000) and adventitial fibroblasts (Gerasimovskaya et al., 2005), and in rat glial cells (Franke et al., 2009) and adult rat cardiac fibroblasts (Braun et al., 2010).
In an earlier study, Zheng and colleagues reported that ATP and UTP had no significant effect on proliferation; these authors showed that ATP inhibited noradrenaline-induced cell growth in neonatal rat cardiac fibroblasts through the stimulation of P2Y receptors (Zheng et al., 1998). Interestingly, a recent report demonstrated that UTP and ATP increase both proliferation and migration in adult rat cardiac fibroblasts by activating the P2Y2 receptor, and mediate the nucleotide-induced profibrotic responses (Braun et al., 2010). It is not known whether the different responses to ATP and UTP in rat cardiac fibroblasts from those in neonatal and adult hearts are related to the age of animals. The results from the present study support the notion that P2 receptor activation mediates proliferation and migration in cardiac fibroblasts from adults. We found that silencing the P2X4, P2X7 or P2Y2 reduced the stimulation of cell proliferation and migration induced by ATP in the cultured human cardiac fibroblasts.
The results of the present study demonstrated that stimulation of the P2 receptors is linked to the activation of the PKB/PI3K and MAPK/ERK1/2 pathways. As a downstream PI3K target, PKB signalling modulates a variety of biological effects (Dugourd et al., 2003; Stabile et al., 2003). PKB phosphorylates and/or interacts with other intracellular molecules (e.g. RAS, Src, etc.) to play an important role in cell proliferation, survival and differentiation in normal and malignant cells (Cheng et al., 2005). The PI3K/PKB pathway mediates the growth and proliferation of NIH 3T3 fibroblasts (Kim et al., 2007). We found that extracellular ATP-induced increase in proliferation was associated with PI3K/PKB phosphorylation in human cardiac fibroblasts, and the effect was fully prevented by P2 receptor antagonists.
It is well known that the downstream signal (MAPK/ERKs) of PI3K/PKB plays a cardinal role in the mitogen-generated growth response in a number of cell types. ERKs are believed to be the ‘end’ of the MAPK cascade. It was reported that ERK functioned as one of the most important protein kinases in modulating proliferation in neonatal rat cardiac fibroblasts (Du et al., 2008). The present study demonstrated that the increase in phosphorylated ERK1/2 was mediated by the activation of P2 receptors, PI3K/PKB and MAPKs, and the effect correlated with the proliferation of human cardiac fibroblasts. This observation is consistent with the reports in human monocytic cells (Santiago-Perez et al., 2001) and mouse embryonic stem cells (Heo and Han, 2006).
Extracellular ATP was found to inhibit cell proliferation in human gastric carcinoma cells by increasing G0/G1 cell population and reducing the proportion of cells in the S phase and G2/M phase (Wang et al., 2005). However, we found that ATP increased cell proliferation in human cardiac fibroblasts by reducing the G0/G1 cell population and increasing proportion of cells in the S phase. The cell cycle regulators cyclin D1 and cyclin E modulate activity of the cyclin-dependent kinase complex (Kato, 1999; Buolamwini, 2000; Vermeulen et al., 2003), and cyclin d-associated kinase activity is crucial for the progression of cells from the G1 to S phase (Sherr and Roberts, 1999). Our results demonstrate that ATP increases the expression of cyclins D1 and E, which probably accounts for its promotion of G0/G1 cells to the S phase in human cardiac fibroblasts. The inhibition of P2 receptors, PI3K/PKB or MAPKs prevented or attenuated the expression of cyclin D1 and cyclin E. Hence, it is likely that the increase in cyclin D1 and cyclin E expression by ATP is mediated by the activation of P2 receptors, PI3K/PKB and MAPK/ERK1/2 signal pathways (Figure 4). This is consistent with the observations in lung fibroblast (Cordes and van, 2004), and in tumour cells (Yang et al., 2006; Liu et al., 2007).
In summary, the present study provides novel information indicating that multiple P2 receptors are expressed in human cardiac fibroblasts and ATP increases cell proliferation by promoting cell cycling progression. These effects of ATP are mediated by activating P2 receptors, increasing phosphorylated PI3K/PKB and MAPK/ERK1/2 signal pathways and enhancing cyclin D1 and cyclin E expression. These effects may be involved in the cardiac remodelling of injured hearts.
Acknowledgments
The study was supported by a General Research Fund (HKU 770108 M) from Research Grant Council of Hong Kong. J-BC was supported by a postgraduate studentship from the University of Hong Kong.
Glossary
- AMP-CPP
α,β-methylene ATP
- API-2
1,5-dihydro-5-methyl-1-β-D-ribofuranosyl-1,4,5,6,8-pentaazaacenaphthylen-3-amine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PD98059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- MTT
5, 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
- RT-PCR
reverse transcription-PCR
Conflicts of interest
None is declared.
References
- Ataullakhanov FI, Vitvitsky VM. What determines the intracellular ATP concentration. Biosci Rep. 2002;22:501–511. doi: 10.1023/a:1022069718709. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci. 1998;62:697–703. doi: 10.1016/s0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]
- Braun OO, Lu D, Aroonsakool N, Insel PA. Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J Mol Cell Cardiol. 2010;49:362–389. doi: 10.1016/j.yjmcc.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilla CG, Maisch B, Weber KT. Myocardial collagen matrix remodelling in arterial hypertension. Eur Heart J. 1992;13(Suppl. D):24–32. doi: 10.1093/eurheartj/13.suppl_d.24. [DOI] [PubMed] [Google Scholar]
- Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527–543. doi: 10.1016/0007-1226(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Green CR, Kohl P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv Cardiol. 2006;42:132–149. doi: 10.1159/000092566. [DOI] [PubMed] [Google Scholar]
- Cha SH, Hahn TW, Sekine T, Lee KH, Endou H. Purinoceptor-mediated calcium mobilization and cellular proliferation in cultured bovine corneal endothelial cells. Jpn J Pharmacol. 2000;82:181–187. doi: 10.1254/jjp.82.181. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Tzeng CR, Lee YH, Tai CJ. Extracellular ATP activates the PLC/PKC/ERK signaling pathway through the P2Y2 purinergic receptor leading to the induction of early growth response 1 expression and the inhibition of viability in human endometrial stromal cells. Cell Signal. 2008;20:1248–1255. doi: 10.1016/j.cellsig.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Chen JB, Tao R, Sun HY, Tse HF, Lau CP, Li GR. Multiple Ca(2+) signaling pathways regulate intracellular Ca(2+) activity in human cardiac fibroblasts. J Cell Physiol. 2010;223:68–75. doi: 10.1002/jcp.22010. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, et al. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25:1840–1849. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- Cordes N, van BD. Arrest of human lung fibroblasts in G2 phase after irradiation is regulated by converging phosphatidylinositol-3 kinase and beta1-integrin signaling in vitro. Int J Radiat Oncol Biol Phys. 2004;58:453–462. doi: 10.1016/j.ijrobp.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 2007;40:656–670. doi: 10.1111/j.1365-2184.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun. 2008;368:402–407. doi: 10.1016/j.bbrc.2008.01.099. [DOI] [PubMed] [Google Scholar]
- Dugourd C, Gervais M, Corvol P, Monnot C. Akt is a major downstream target of PI3-kinase involved in angiotensin II-induced proliferation. Hypertension. 2003;41:882–890. doi: 10.1161/01.HYP.0000060821.62417.35. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Sauer C, Rudolph C, Krugel U, Hengstler JG, Illes P. P2 receptor-mediated stimulation of the PI3-K/Akt-pathway in vivo. Glia. 2009;57:1031–1045. doi: 10.1002/glia.20827. [DOI] [PubMed] [Google Scholar]
- Gerasimovskaya EV, Tucker DA, Weiser-Evans M, Wenzlau JM, Klemm DJ, Banks M, et al. Extracellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin, and p70 S6 kinase signaling pathways. J Biol Chem. 2005;280:1838–1848. doi: 10.1074/jbc.M409466200. [DOI] [PubMed] [Google Scholar]
- He ML, Liu WJ, Sun HY, Wu W, Liu J, Tse HF, et al. Effects of ion channels on proliferation in cultured human cardiac fibroblasts. J Mol Cell Cardiol. 2011;51:198–206. doi: 10.1016/j.yjmcc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Heo JS, Han HJ. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells. 2006;24:2637–2648. doi: 10.1634/stemcells.2005-0588. [DOI] [PubMed] [Google Scholar]
- Kato J. Induction of S phase by G1 regulatory factors. Front Biosci. 1999;4:D787–D792. doi: 10.2741/kato. [DOI] [PubMed] [Google Scholar]
- Kim SE, Lee WJ, Choi KY. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell Signal. 2007;19:511–518. doi: 10.1016/j.cellsig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS ONE. 2009;4:e7307. doi: 10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Mao Z, LaFortune TA, Alonso MM, Gallick GE, Fueyo J, et al. Cell cycle-dependent nuclear export of phosphatase and tensin homologue tumor suppressor is regulated by the phosphoinositide-3-kinase signaling cascade. Cancer Res. 2007;67:11054–11063. doi: 10.1158/0008-5472.CAN-07-1263. [DOI] [PubMed] [Google Scholar]
- Long CS, Brown RD. The cardiac fibroblast, another therapeutic target for mending the broken heart? J Mol Cell Cardiol. 2002;34:1273–1278. doi: 10.1006/jmcc.2002.2090. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Santiago-Perez LI, Flores RV, Santos-Berrios C, Chorna NE, Krugh B, Garrad RC, et al. P2Y(2) nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases. J Cell Physiol. 2001;187:196–208. doi: 10.1002/jcp.1063. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM. Extracellular ATP-mediated propagation of Ca(2+) waves. Focus on ‘mechanical strain-induced Ca(2+) waves are propagated via ATP release and purinergic receptor activation’. Am J Physiol Cell Physiol. 2000;279:C281–C283. doi: 10.1152/ajpcell.2000.279.2.C281. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, et al. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- Su XL, Wang Y, Zhang W, Zhao LM, Li GR, Deng XL. Insulin-mediated upregulation of K(Ca)3.1 channels promotes cell migration and proliferation in rat vascular smooth muscle. J Mol Cell Cardiol. 2011;51:51–57. doi: 10.1016/j.yjmcc.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. doi: 10.1007/s00395-002-0365-8. [DOI] [PubMed] [Google Scholar]
- Tao R, Lau CP, Tse HF, Li GR. Regulation of cell proliferation by intermediate-conductance Ca2+-activated potassium and volume-sensitive chloride channels in mouse mesenchymal stem cells. Am J Physiol Cell Physiol. 2008;295:C1409–C1416. doi: 10.1152/ajpcell.00268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Sun HY, Lau CP, Tse HF, Lee HC, Li GR. Cyclic ADP ribose is a novel regulator of intracellular Ca(2+) oscillations in human bone marrow mesenchymal stem cells. J Cell Mol Med. 2011;15:2684–2696. doi: 10.1111/j.1582-4934.2011.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DJ, Huang NN, Heppel LA. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- Wang MX, Ren LM, Shan BE. Inhibitory effects of extracellular adenosine triphosphate on growth of esophageal carcinoma cells. World J Gastroenterol. 2005;11:5915–5919. doi: 10.3748/wjg.v11.i38.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wihlborg AK, Balogh J, Wang L, Borna C, Dou Y, Joshi BV, et al. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ Res. 2006;98:970–976. doi: 10.1161/01.RES.0000217402.73402.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Krauss RS. Extracellular ATP induces anchorage-independent expression of cyclin A and rescues the transformed phenotype of a ras-resistant mutant cell line. J Biol Chem. 1997;272:3103–3108. doi: 10.1074/jbc.272.5.3103. [DOI] [PubMed] [Google Scholar]
- Yang K, Guo Y, Stacey WC, Harwalkar J, Fretthold J, Hitomi M, et al. Glycogen synthase kinase 3 has a limited role in cell cycle regulation of cyclin D1 levels. BMC Cell Biol. 2006;7:33. doi: 10.1186/1471-2121-7-33. doi: 10.1186/1471-2121-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannad F, Dousset B, Alla F. Treatment of congestive heart failure: interfering the aldosterone–cardiac extracellular matrix relationship. Hypertension. 2001;38:1227–1232. doi: 10.1161/hy1101.099484. [DOI] [PubMed] [Google Scholar]
- Zheng JS, O'Neill L, Long X, Webb TE, Barnard EA, Lakatta EG, et al. Stimulation of P2Y receptors activates c-fos gene expression and inhibits DNA synthesis in cultured cardiac fibroblasts. Cardiovasc Res. 1998;37:718–728. doi: 10.1016/s0008-6363(97)00245-9. [DOI] [PubMed] [Google Scholar]


