Abstract
Expression of the herpes simplex virus (HSV) immediate early (IE) genes is regulated by a multiprotein complex that is assembled on the TAATGARAT enhancer core element. The complex contains the cellular POU domain protein Oct-1, the viral transactivator VP16, and the cellular cofactor host cell factor 1. The current model suggests that the assembly depends on recognition of the core element by Oct-1. Here, HSV infection of Oct-1-deficient mouse embryonic fibroblast cells demonstrates that Oct-1 is critical for IE gene expression at low multiplicities of infection (moi). However, the protein is not essential for IE gene expression at high moi, indicating that VP16-mediated transcriptional induction through other IE regulatory elements is also important. This induction depends, at least in part, on the GA-binding protein binding elements that are present in each IE enhancer domain. Surprisingly, whereas the viral IE genes are expressed after high moi infection of Oct-1-deficient cells, the assembly of viral replication factories is severely impaired, revealing a second critical role for Oct-1 in HSV replication. The results have implications for both the HSV lytic and latency-reactivation cycles.
The transcriptional regulation of the immediate early (IE) genes of herpes simplex virus type 1 (HSV-1) is determined by a complex cis-regulatory domain consisting of reiterated enhancer core elements (TAATGARAT elements) flanked by binding sites for factors such as GA-binding protein (GABP) and Sp1 (1). The enhancer core elements are the primary regulatory units and are sites for the assembly of protein complexes containing the POU homeodomain protein Oct-1, the viral transactivator protein VP16 [infected cell protein (ICP) 25, α-trans-induction factor], and the coactivator host cell factor 1 (HCF-1; C1) (1–4). The IE genes have been a model system for delineating mechanisms of combinatorial control and multiprotein transcription assemblies.
Oct-1 recognizes the consensus octamer element (ATGCAAAT) by means of POU and homeo subdomains that contact the major groove on opposite sides of the helix (ATGC and AAAT, respectively). A flexible segment separating the two subdomains allows for the recognition of sequences and contexts that are divergent from the consensus (5). Regulation of transcription by Oct-1 depends on promoter element context and interactions with specific transcription factors (SNAPc, glucocorticoid receptor, androgen receptor, cAMP-response element-binding protein, VP16) (5) and coactivators such as OCAB/Bob-1/OBF-1 (6–8) and OCA-S (9).
In the HSV IE enhancer core, Oct-1 recognizes the 5′ portion of the core element and is hypothesized to initiate or nucleate the enhancer assembly. The second component, VP16, the viral transactivator protein, is encoded by HSV, packaged in the tegument structure of the virion, and released into the cell upon initial infection (1). This protein contributes two distinct specificities involved in selection of the Oct-1-bound HSV IE enhancer core: (i) recognition of the DNA core element sequences 3′ to the octamer (GARAT) (10, 11) and (ii) specific recognition of the Oct-1 homeodomain (12, 13). The VP16 structure shows a conserved structural core with tightly clustered interaction surfaces for Oct-1, DNA, and HCF-1 (11). The DNA binding domain of VP16 is a structure resembling a “seat” and containing a DNA recognition cleft. In addition to binding the 3′ core sequences, VP16 recognizes Oct-1 and discriminates between Oct-1 and the highly related POU domain protein Oct-2 by means of selective recognition of the Oct-1 homeobox helices 1 and 2, thus specifying the Oct-1-bound HSV TAATGARAT enhancer (10, 12, 13).
The final component of the enhancer core assembly, HCF-1, was identified by its requirement for the stable interaction of VP16 with Oct-1 (14–16). This protein is an unusual family of polypeptides derived from a 230-kDa precursor by site-specific proteolytic processing (15–17). The protein (i) functions as transcriptional coactivator for VP16 (18) and GABP (19), (ii) interacts with transcription factors (GABP, Sp1, VP16, L-ZIP, Zhangfei) (19–23) and coactivators (peroxisome proliferator-activated receptor γ coactivator) (24), (iii) is required for multiple cell cycle progression stages (25, 26), and (iv) appears to be involved in mRNA splicing (27). In its role in the regulation of the IE genes of HSV, the amino-terminal kelch domain of HCF-1 interacts with VP16 by means of a short core DHXY motif (20). This interaction is required for both the nuclear transport of VP16 (28) and the formation of the stable enhancer core complex. In addition, because HCF-1 interacts with other transcription factors such as GABP and Sp1, the contribution of these transcription factors to the expression of the IE genes may depend on the presence of HCF-1.
The interactions and specificities involved in assembling the IE enhancer regulatory complexes have critical implications for HSV biology, because viral IE proteins are essential for the progression of the lytic cycle. In addition, HSV establishes latent infections in sensory neurons that can be punctuated by reactivation leading to recurrent lytic infection (1). Although the molecular details of the establishment of latency and the reactivation processes are unclear, the prevalent models indicate that blocks to IE gene expression may be important for the establishment of latency, whereas induction of IE gene transcription would be essential for the reactivation process. Thus, regulation of the HSV IE enhancer by the Oct-1-dependent assemblies has implications for lytic and latent infections.
Recently a genetic model of Oct-1 deficiency has been described in which the third exon of Oct-1 was replaced by a neomycin resistance cassette in the antisense transcriptional orientation (29). Mice harboring this severely hypomorphic allele of Oct-1 die in utero between embryonic day 13.5 and embryonic day 18.5 and display erythropoietic defects. Primary and immortalized mouse embryonic fibroblast (MEF) lines derived from these embryos are viable, appear normal, and replicate at normal rates. Therefore, this system was ideal to test the effect of Oct-1 ablation in HSV infection.
In this study, the requirement for Oct-1 in HSV infection was investigated by using MEF cell lines derived from Oct-1-deficient (dOct) mice (29). The results demonstrate that Oct-1 is critical for HSV IE gene expression at low multiplicity of infection (moi) but is not essential for the expression of these genes at high moi. At high moi, the expression of the IE genes is VP16-dependent, indicating that alternative VP16-mediated stimulation of the IE genes can occur in the absence of Oct-1. In addition, an essential requirement for Oct-1 in later stages of HSV infection was revealed.
Materials and Methods
Cell Lines and Virus. WT and dOct MEF cells were derived from 13.5-day embryos and were immortalized by using a serial 3T3 protocol (29). Two independent lines derived from each genotype gave similar results in all experiments described.
The control (C) and restored Oct-1 (rOct) lines were produced by infecting the dOct line with the retrovirus vector pBABE or pBABE expressing the human Oct-1, respectively. HSV-1(F) stocks were produced and titered by infection of Vero cells according to standard procedures.
DNA Protein Gel-Shift Assay. Electrophoretic mobility-shift assays were done essentially as described (10).
Western Blots. Equivalent amounts of soluble protein extracts were probed with antibodies to ICP4 (HIA021, Virusys, Sykesville, MD), ICP0 (HIA027, Virusys), gG (HIA020, Virusys), gC (HIA022, Virusys), GAPDH (TRK5G4-6C5, Research Diagnostic), or anti-UL9 sera (R249, gift of M. Challberg, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda). The blots were developed by using the appropriate horseradish peroxidase-conjugated secondary antibodies and SuperSignal West Pico (Pierce) or ECL Plus Western Blotting Detection System (Amersham Pharmacia Biosciences) chemiluminescent reagents. Protein bands were quantitated by using the Kodak Scientific Imaging System (version 6.3.1) and normalized to the levels of endogenous GAPDH.
Microscopy. Cells were infected at an moi of 10. Six hours after infection, the cells were fixed with 4% paraformaldehyde and permeabilized with 1.0% Triton X-100. The cells were stained with R249 anti-UL9 serum followed by anti-rabbit Alexa 488 secondary antibody (Molecular Probes). 4′,6-diamidino-2-phenylindole was added during mounting. Channel images (488 and 364 nm) were collected separately on a Leica TCS-SP2 confocal microscope with a ×63 oil immersion objective, and the images were processed with Leica software. The contrast phase images were collected by using a ×40 objective in a Leica DMIRBE microscope fitted with a Cooke Sensican QE digital camera (Cooke, Auburn Hills, MI) and image pro 4.0 software (Media Cybernetics, Silver Spring, MD).
Reporter Assays. The ICP4 IE enhancer (-330 to -110), TAATGARAT, and GABP elements (-292 to -275) were cloned upstream of the SV40 promoter in pGL3 promoter (Promega). pCMV-VP16, containing the HSV trans-induction factor expressed from the cytomegalovirus IE promoter, was constructed by subcloning the VP16 gene from p3722 (gift of J. L. C. McKnight). WT and dOct cells (1 × 105) were transfected with promoter reporter plasmids or cotransfected with increasing amounts of pCMV-VP16. phRL-null was included in all transfections as an internal control. Transfections were done by using FuGENE 6 (Roche) according to the manufacturer's protocol, and the reporter activity was measured 24 h posttransfection by using the Promega Dual-Luciferase Assay in a Berthold luminometer.
Results
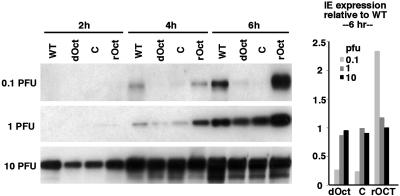
Oct-1 Is Critical for HSV IE Gene Expression at Low moi but Not at High moi. Oct-1 has been defined in vitro as an essential component of an HSV IE gene enhancer complex assembly. However, the actual contribution of this protein to HSV biology has not been established. Therefore, immortalized MEF cell lines, derived from WT and dOct embryos and control MEF cell lines carrying a retrovirus expressing the human Oct-1 (rOct) or the retrovirus vector alone (C), were infected with HSV-1 (F) at various plaque-forming unit (pfu)/cell ratios. The expression of the viral IE ICP4 and ICP0 was assessed by Western blot at various times postinfection. As shown in Fig. 1 Left, expression of ICP4 was detectable but significantly impaired in dOct-cells at both 0.1 and 1 pfu per cell (Fig. 1, 4- and 6-h time points) relative to the WT cells. Expression of ICP4 was restored to at least WT levels in cells expressing the human Oct-1 (rOct) relative to the retroviral vector alone (C). In contrast, infection with 10 pfu per cell resulted in ICP4 expression in both the dOct and retroviral control cells that was nearly equivalent to the level detected in WT cells at all time points (Fig. 1). Similar data and quantitation were obtained from Western blots monitoring the expression of a second IE gene product (ICP0, data not shown). The results indicate that, although Oct-1 is critical for IE gene expression at low moi, it is not required for efficient viral IE expression at high multiplicities.
Fig. 1.
HSV IE gene expression in WT and dOct cells. (Left) WT, dOct, retrovirus control (C), and retrovirus rOct cells were infected at 0.1, 1, and 10 pfu per cell, and protein extracts were prepared at 2, 4, and 6 h postinfection. Extracts were resolved in 4–20% Tris-glycine gels, transferred to membrane, and probed with anti-ICP4 sera. (Right) The levels of ICP4 expression were quantitated, normalized to endogenous GAPDH levels, and graphically represented relative to WT cell levels.
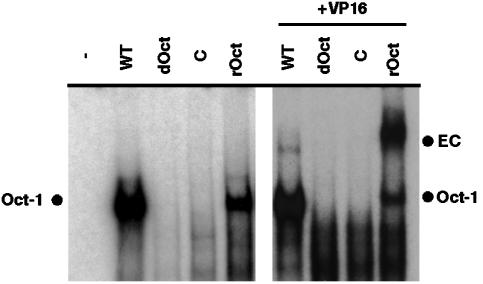
dOct Cells Are Deficient in Enhancer Core Assembly. The formation of the Oct-1/VP16/HCF-1 stable enhancer core complex on the TAATGARAT element can be monitored by electrophoretic mobility-shift assay (EMSA). To investigate the ability to form the enhancer core complex in the absence of Oct-1, extracts were prepared from each cell line and EMSA reactions were performed in the presence and absence of exogenously added purified VP16 protein. As shown in Fig. 2 Left, Oct-1 binding activity was detected in extracts derived from WT and rOct cells. In contrast, Oct-1 binding activity was not detected in extracts of dOct or retroviral control cells, supporting the previous finding that Oct-1 binding activity in cell lines derived from dOct embryos is significantly reduced (1/40th of WT levels; ref. 29). The addition of purified VP16 protein to the reactions (Fig. 2 Right) generated the stable Oct-1/VP16/HCF-1 enhancer core complex in extracts of WT and rOct cells. However, in the absence of Oct-1, no enhancer core complex was formed (dOct and C) and no alternative complexes were detected. It should be noted that the enhanced efficiency of complex formation in extracts of rOct cells is likely to be due to the higher affinity of VP16 for the human Oct-1 relative to the mouse protein.
Fig. 2.
Electrophoretic mobility-shift assay complexes in extracts of WT and dOct cells. Nuclear extracts (10 μg) from WT, dOct, retrovirus control (C), and retrovirus rOct cells were mixed with HSV TAATGARAT probe DNA (ICP0, pRB608) in the absence (Left) or presence (Right) of exogenously added VP16 protein (50 ng). Complexes were resolved in 4% native acrylamide gels and visualized by autoradiography. Oct-1, Oct-1/DNA complex; EC, HSV IE enhancer core complex containing Oct-1, VP16, and HCF-1.
These data are consistent with the compromised expression of the IE genes in dOct cells at low moi infection. However, the data also suggest that alternative mechanisms (non-TAATGARAT) may exist for the expression of the IE genes at high moi in the absence of Oct-1. Because VP16 is packaged within the tegument structure of the virion, increased levels of VP16 might be responsible for the IE gene expression under these conditions.
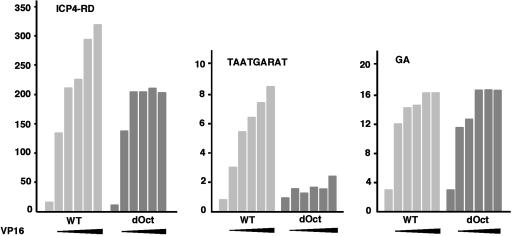
Alternative VP16-Mediated Induction of the HSV IE Enhancer in the Absence of Oct-1. The ability of the regulatory domain and enhancer core element to respond to VP16 in the absence of Oct-1 was assessed in reporter assays. Constructs containing the complete ICP4 IE regulatory domain (ICP4-RD) and the TAATGARAT enhancer core fused to the SV40 minimal promoter-luciferase gene were transfected into WT and dOct cells in the absence or presence of increasing amounts of a VP16 expression plasmid. As shown in Fig. 3 Left, the expression of the IE regulatory domain construct was nearly equivalent in WT and dOct cells. Surprisingly, in the presence of increasing amounts of cotransfected VP16, both cell lines supported the induction of the IE gene reporter expression. However, whereas the response of the reporter in WT cells continued to increase with increasing VP16, the ability of the reporter to respond to VP16 in dOct cells plateaued more rapidly. The results suggest that VP16-mediated induction of the IE genes can proceed in the absence of Oct-1.
Fig. 3.
VP16-mediated induction of HSV reporter constructs in WT and dOct cells. WT and dOct cells (1 × 105) were transfected with the ICP regulatory domain (ICP4-RD; 25 ng), IE enhancer core element (TAATGARAT, ICP0, pRB608; 200 ng), or GA-binding element (-292 to -275, ICP4; 100 ng) derived from the ICP4-RD in the absence or presence of increasing amounts of a construct expressing VP16 (2.5, 5, 10, 20, and 75 ng). The results of the luciferase assay, normalized to the baseline of the promoter alone, are graphically represented. The first bar in each set represents that basal level in the absence of VP16, and expanded arrows indicate increasing amounts of cotransfected VP16 expression plasmid. Each reporter result is representative of three independent transfection reporter experiments. All assays were done 48 h posttransfection.
As shown in Fig. 3 Center, the isolated TAATGARAT element contributes little to the basal level expression of the reporter and responds to increasing VP16-mediated transactivation in WT cells. Interestingly, in contrast to the response of the full ICP4-RD, the TAATGARAT core element did not significantly respond to VP16 in dOct cells, indicating that stimulation through this element depends primarily on Oct-1.
As noted, the IE regulatory domain consists of reiterated enhancer core elements (TAATGARAT) flanked by binding sites for cellular factors such as Sp1 and GABP. Previously, deletion mutants of the ICP4-RD suggested that the GA element might contribute to VP16-mediated induction of the IE genes (30). Therefore, reporter constructs containing the GA-binding element from the ICP4 RD were transfected or cotransfected with VP16 in WT and dOct cells. As shown in Fig. 3 Right, the GA reporters responded in a dose-dependent manner in both WT and dOct cells, thus identifying an Oct-1- and TAATGARAT-independent, VP16-mediated mechanism for the induction of the viral IE genes.
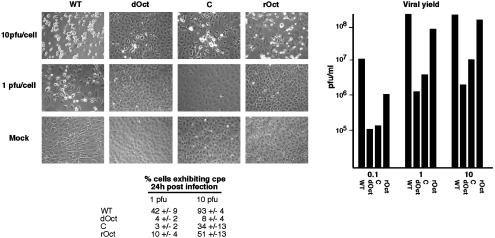
dOct Cells Do Not Support Efficient Productive Viral Infection. The deficiency of viral IE gene expression in dOct cells at low pfu and the apparent Oct-1-independent expression at higher pfu suggested that infection of Oct-1 cells at high pfu should result in no discernable defect in viral replication. Surprisingly, however, a significant reduction in cytopathic effects (CPE) was evident after infection of either dOct or the retroviral control cells at either 1 or 10 pfu per cell relative to WT or rOct cells (Fig. 4 Left). This reduction in CPE was also reflected in a significant reduction in viral yields derived from infection of the dOct cells (Fig. 4 Right), suggesting that, although Oct-1 was dispensable for viral IE expression at high pfu, the protein was critically required in a later stage of productive viral infection.
Fig. 4.
CPE and viral yields from HSV infection of WT and dOct cells. (Left) WT, dOct, retrovirus control (C), and retrovirus rOct cells were infected with 1 and 10 pfu and visualized 24 h after infection by contrast phase microscopy. Each panel is a representative field of cells exhibiting the average CPE in each infection and cell line. The percent cells exhibiting CPE were counted in at least five fields and are represented ±SD. (Right) The indicated cell lines were infected with 0.1, 1, and 10 pfu per cell for 24 h, and the resulting viral yields were determined by titration and are graphically represented.
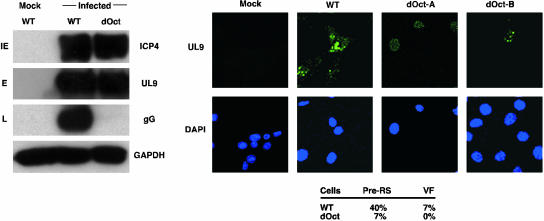
Oct-1 Is Required for the Formation of HSV Replication Factories and Late Gene Expression. The results suggested that a late stage of viral infection was critically dependent on Oct-1. Therefore, cells were infected at high pfu (10 pfu), and protein extracts were probed by Western blot with antiserum to IE (ICP4), E (UL9), and L (gG) protein expression. As shown in Fig. 5 Left, WT and dOct cells infected with high pfu expressed WT levels of ICP4 and UL9. Strikingly, however, the expression of the late gene (gG) was severely compromised in the dOct cells. Identical results were obtained by using antiserum to a second late gene, gC (data not shown).
Fig. 5.
Expression of late genes and formation of viral replication factories in HSV-infected WT and dOct cells. (Left) WT and dOct cells were infected at 10 pfu per cell, and protein extracts were prepared at 6 h postinfection. Equivalent amounts of extracts were resolved in a 4–20% Tris-glycine gel, transferred to membrane, and probed with antibodies to an IE protein (IE, ICP4), an early protein (E, UL9), and a late protein (L, gG). GADPH was used as an internal control. (Right) Cell lines were infected at 10 pfu per cell and fixed at 6 h postinfection. Cells were stained with antiserum to UL9 and 4′,6-diamidino-2-phenylindole and visualized by using a Leica confocal microscope. The percent of cells exhibiting HSV prereplicative sites (PreRS) and replication factories (VF) is shown. dOct-A, dOct cells illustrating the predominant diffuse UL9 nuclear staining and the absence of prereplicative sites and viral factories; dOct-B, dOct cells illustrating the low frequency formation of prereplicative sites but not replication factories.
Because the expression of the late gene products depends on viral DNA replication (1), WT and dOct cells were infected with 10 pfu per cell, fixed, and stained with antiserum to UL9 to visualize the formation of viral prereplicative and replicative factories. In contrast to the WT cells (Fig. 5 Right), dOct cells exhibit primarily diffuse UL9 nuclear localization (Fig. 5, dOct-A) and do not efficiently support the formation of prereplicative sites (dOct-B) or replication factories. In an HSV infection, prereplicative sites are formed by the colocalization of viral replication proteins, and a subpopulation of these progresses to viral replication factories and DNA synthesis (31, 32). Therefore, the data indicate that the block to efficient viral productive infection in dOct cells was before viral DNA synthesis and identifies a second critical requirement for Oct-1 in the replication of HSV.
Discussion
The transcription factor Oct-1 is a versatile regulator of gene expression. Although ubiquitously expressed, it has been implicated in the regulation of tissue-specific as well as ubiquitous cellular genes. In several viral systems, the protein is a critical transcriptional regulatory component and can also play a significant role in DNA replication (5). In HSV, the expression of HSV IE genes depends on the VP16-mediated induction by means of the IE regulatory domain. Within the domains, reiterated TAATGARAT core elements nucleate the assembly of a core complex containing Oct-1, VP16, and HCF-1. The model dictates that Oct-1 is required for the initial recognition of the TAATGARAT element and subsequent complex assembly. The requirement for this assembly as the critical mechanism for transcriptional induction of the IE genes of HSV has implications for the HSV lytic cycle as well as the establishment and reactivation from the latent state.
Recently, a targeted mutation was introduced into the mouse germ-line Oct-1 locus, resulting in a severe hypomorph with ≈40-fold reduced octamer–DNA binding activity (29). The availability of cell lines derived from Oct-1 mutant embryos allows the various functions ascribed to Oct-1 to be tested in vivo.
Oct-1 Is a Critical Component of the HSV IE Regulatory Complex. dOct cells were used to assess the role and significance of Oct-1 in HSV IE gene expression. Infection at low moi clearly resulted in significantly compromised expression of the IE genes in the absence of Oct-1, suggesting that the protein is critical for expression of these genes under these conditions. This finding clearly complements previous results that indicated that Oct-1 mediated nucleation of the enhancer complex assembly and that the TAATGARAT enhancer core is the critical regulatory element for IE expression during productive infection.
While Oct-1 is critical for HSV lytic infection, it may also be significant for the establishment of and reactivation from the latent state. After a primary infection, HSV establishes a quiescent latent infection of sensory neurons that may persist throughout an individual's life or may be interrupted by recurrent reactivation events leading to episodes of replication (1). Although the mechanisms involved in the establishment of latency and reactivation remain unclear, prevalent models suggest that latency establishment should involve a block to IE gene expression. In sensory neurons, Oct-1 is expressed at relatively low levels, and other TAATGARAT-binding POU family members such as Oct-2 and Brn family members predominate (33–35). The demonstrated requirement for Oct-1 in IE expression would support the hypothesis that repression of IE genes by blocking the assembly of the Oct-1/VP16/HCF-1 enhancer complex could be a contributing factor in the establishment of HSV latency. Conversely, reactivation of HSV from the latent state in animal models occurs in response to a variety of stimuli including UV irradiation, heat shock, tissue damage, neuronal explant, and hormonal alterations (1). Interestingly, the expression of Oct-1 is induced in sensory neurons after neuronal explant, and the activity of the protein has been shown to increase after UV irradiation (36–38). Although induction of Oct-1 may not be an initiating factor, it could contribute significantly to the reactivation process.
VP16-Mediated Induction of IE Genes in the Absence of Oct-1. Surprisingly, infection of dOct cells at a high pfu/cell ratio resulted in the expression of WT levels of IE proteins. This result suggested the presence of a VP16-dependent but Oct-1-independent mechanism for the induction of IE expression. This induction depends, at least partially, on the conserved GA elements that are present in each IE gene and are recognized by the ets transcription factor GABP.
The VP16 regulation of the GA element may be direct by means of a bridging of VP16 and GABP through the common coactivator HCF-1 (19) or may be an indirect effect of VP16 that results in the activation of GABP. Regardless, the VP16 regulation of the GA elements provides an alternative mechanism for VP16 regulation of the IE genes and challenges the exclusivity of the Oct-1-dependent paradigm of viral IE expression. These results emphasize the ability of the viral IE regulatory domain to respond to multiple combinations of factors that may be very significant in conditions where Oct-1 is limiting or unavailable, such as in sensory neurons.
Deletion of Oct-1 Reveals a Second Role for Oct-1 in HSV Infection. The genetic depletion of Oct-1 revealed a second critical function of Oct-1 in productive HSV infection. In the absence of Oct-1, infection at high moi resulted in nearly WT levels of the IE proteins. However, viral yields, late gene expression, and the formation of viral replication factories were severely compromised. This strongly indicates that there is an unanticipated role for Oct-1 late in the viral lytic cycle. The lack of viral prereplication sites and replication factories in the dOct cells suggests that the deficiency may be at a point before significant DNA synthesis. The requirement for Oct-1 may reflect a direct interaction between Oct-1 and viral proteins for the assembly of replication complexes, or Oct-1 may have an indirect involvement such as in the expression of a required cellular cofactor.
Interestingly, the HSV short component viral origins of replication are flanked by the IE enhancer elements. Studies have indicated that these elements enhance DNA replication and have identified Sp1 sites as important components (39, 40). Because Oct-1 and Sp1 can physically interact (41), it is possible that Oct-1 contributes to the Sp1-dependent stimulation. However, this model is unlikely to account for the striking requirement for Oct-1 at this stage of infection, because the IE elements have only a minor stimulatory effect on DNA replication. Interestingly, Oct-1 is modified by HSV infection at later stages of infection. This modification negatively regulates Oct-1 DNA-binding activity and may contribute to the shutoff of IE gene expression (42). The modification may also be important for function of the protein in viral DNA replication, and the data would favor a non-DNA-binding mechanism.
Finally, the critical function of Oct-1 in productive HSV infection may again contribute to the establishment or maintenance of HSV latency. As noted above, a block to IE gene expression in sensory neurons is likely to be important in this process. In fact, there are likely to be multiple blocks to productive infection including: (i) competitive binding of POU proteins other than Oct-1 to the TAATGARAT element (33–35), (ii) cytoplasmic sequestering of the coactivator HCF-1 (43), (iii) rapid targeted turnover of the HSV UL9 origin binding protein (44), and (iv) inhibition of the Oct-1-dependent late-stage replication. Because the Oct-1 hypomorphic allele tested here is embryonic lethal, it is not possible to directly test the role of Oct-1 in HSV latency. However, the use of conditional mutation schemes for deleting Oct-1 in sensory neurons could be valuable in addressing this question.
Acknowledgments
We thank J. L. C. McKnight for VP16 plasmids, M. Challberg for UL9 antiserum, J. Vogel and A. McBride for critical reading of this manuscript, A. Pierce for technical assistance, and members of the Laboratory of Viral Diseases and Center for Cancer Research for helpful discussions. These studies were supported by the Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (T.M.K.) and U.S. Public Health Service Grant PO1-CA42063 (to P.A.S.) and partially supported by National Institutes of Health Cancer Center Core Grant P30-CA14051 (to P.A.S.).
Abbreviations: HSV, herpes simplex virus; IE, immediate early; MEF, mouse embryonic fibroblast; HCF-1, host cell factor 1; dOct, Oct-1-deficient; rOct, restored Oct-1; ICP, infected cell protein; GABP, GA-binding protein; pfu, plaque-forming unit; CPE, cytopathic effects.
References
- 1.Roizman, B. & Sears, A. E. (1996) in Fundamental Virology, eds. Fields, B., Knipe, D. M. & Howley, P. M. (Lippincott-Raven, Philadelphia), pp. 1043-1107.
- 2.Wilson, A. C., Cleary, M. A., Lai, J. S., LaMarco, K., Peterson, M. G. & Herr, W. (1993) Cold Spring Harbor Symp. Quant. Biol. 58, 167-178. [DOI] [PubMed] [Google Scholar]
- 3.Vogel, J. L. & Kristie, T. M. (2001) in The Encyclopedia of Molecular Medicine, ed. Creighton, T. E. (Wiley, New York), Vol. 1, pp. 732-735. [Google Scholar]
- 4.Wysocka, J. & Herr, W. (2003) Trends Biochem. Sci. 28, 294-304. [DOI] [PubMed] [Google Scholar]
- 5.Phillips, K. & Luisi, B. (2000) J. Mol. Biol. 302, 1023-1039. [DOI] [PubMed] [Google Scholar]
- 6.Luo, Y. & Roeder, R. G. (1995) Mol. Cell. Biol. 15, 4115-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gstaiger, M., Knoepfel, L., Georgiev, O., Schaffner, W. & Hovens, C. M. (1995) Nature 373, 360-362. [DOI] [PubMed] [Google Scholar]
- 8.Strubin, M., Newell, J. W. & Matthias, P. (1995) Cell 80, 497-506. [DOI] [PubMed] [Google Scholar]
- 9.Zheng, L., Roeder, R. G. & Luo, Y. (2003) Cell 114, 255-266. [DOI] [PubMed] [Google Scholar]
- 10.Kristie, T. M. & Sharp, P. A. (1990) Genes Dev. 4, 2383-2396. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y., Gong, W., Huang, C. C., Herr, W. & Cheng, X. (1999) Genes Dev. 13, 1692-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomerantz, J. L., Kristie, T. M. & Sharp, P. A. (1992) Genes Dev. 6, 2047-2057. [DOI] [PubMed] [Google Scholar]
- 13.Lai, J. S., Cleary, M. A. & Herr, W. (1992) Genes Dev. 6, 2058-2065. [DOI] [PubMed] [Google Scholar]
- 14.Kristie, T. M. & Sharp, P. A. (1993) J. Biol. Chem. 268, 6525-6534. [PubMed] [Google Scholar]
- 15.Kristie, T. M., Pomerantz, J. L., Twomey, T. C., Parent, S. A. & Sharp, P. A. (1995) J. Biol. Chem. 270, 4387-4394. [DOI] [PubMed] [Google Scholar]
- 16.Wilson, A. C., LaMarco, K., Peterson, M. G. & Herr, W. (1993) Cell 74, 115-125. [DOI] [PubMed] [Google Scholar]
- 17.Vogel, J. L. & Kristie, T. M. (2000) Proc. Natl. Acad. Sci. USA 97, 9425-9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luciano, R. L. & Wilson, A. C. (2002) Proc. Natl. Acad. Sci. USA 99, 13403-13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel, J. L. & Kristie, T. M. (2000) EMBO J. 19, 683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freiman, R. N. & Herr, W. (1997) Genes Dev. 11, 3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunther, M., Laithier, M. & Brison, O. (2000) Mol. Cell. Biochem. 210, 131-142. [DOI] [PubMed] [Google Scholar]
- 22.Lu, R. & Misra, V. (2000) Nucleic Acids Res. 28, 2446-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, R., Yang, P., Padmakumar, S. & Misra, V. (1998) J. Virol. 72, 6291-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, J., Puigserver, P., Donovan, J., Tarr, P. & Spiegelman, B. M. (2002) J. Biol. Chem. 277, 1645-1648. [DOI] [PubMed] [Google Scholar]
- 25.Goto, H., Motomura, S., Wilson, A. C., Freiman, R. N., Nakabeppu, Y., Fukushima, K., Fujishima, M., Herr, W. & Nishimoto, T. (1997) Genes Dev. 11, 726-737. [DOI] [PubMed] [Google Scholar]
- 26.Julien, E. & Herr, W. (2003) EMBO J. 22, 2360-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ajuh, P., Chusainow, J., Ryder, U. & Lamond, A. I. (2002) EMBO J. 21, 6590-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Boissiere, S., Hughes, T. & O'Hare, P. (1999) EMBO J. 18, 480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, V. E. H., Schmidt, T., Chen, J., Sharp, P. A. & Tantin, D. (2004) Mol. Cell. Biol. 24, 1022-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triezenberg, S. J., LaMarco, K. L. & McKnight, S. L. (1988) Genes Dev. 2, 730-742. [DOI] [PubMed] [Google Scholar]
- 31.Burkham, J., Coen, D. M. & Weller, S. K. (1998) J. Virol. 72, 10100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liptak, L. M., Uprichard, S. L. & Knipe, D. M. (1996) J. Virol. 70, 1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latchman, D. S. (1996) Int. J. Biochem. Cell Biol. 28, 1081-1083. [DOI] [PubMed] [Google Scholar]
- 34.Latchman, D. S. (1999) J. Cell. Physiol. 179, 126-133. [DOI] [PubMed] [Google Scholar]
- 35.Hagmann, M., Georgiev, O., Schaffner, W. & Douville, P. (1995) Nucleic Acids Res. 23, 4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin, S., Fan, F., Fan, W., Zhao, H., Tong, T., Blanck, P., Alomo, I., Rajasekaran, B. & Zhan, Q. (2001) Oncogene 20, 2683-2690. [DOI] [PubMed] [Google Scholar]
- 37.Zhao, H., Jin, S., Fan, F., Fan, W., Tong, T. & Zhan, Q. (2000) Cancer Res. 60, 6276-6280. [PubMed] [Google Scholar]
- 38.Valyi-Nagy, T., Deshmane, S., Dillner, A. & Fraser, N. W. (1991) J. Virol. 65, 4142-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong, S. W. & Schaffer, P. A. (1991) J. Virol. 65, 2601-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen-Huynh, A. & Schaffer, P. A. (1998) J. Virol. 72, 3635-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strom, A. C., Forsberg, M., Lillhager, P. & Westin, G. (1996) Nucleic Acids Res. 24, 1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Advani, S. J., Durand, L. O., Weichselbaum, R. R. & Roizman, B. (2003) J. Virol. 77, 11927-11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristie, T. M., Vogel, J. L. & Sears, A. E. (1999) Proc. Natl. Acad. Sci. USA 96, 1229-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eom, C. Y. & Lehman, I. R. (2003) Proc. Natl. Acad. Sci. USA 100, 9803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]