Abstract
The MMPs and their inhibitors [tissue inhibitor of MMPs (TIMPs) ] form the mainstay of extracellular matrix homeostasis. They are expressed in response to numerous stimuli including cytokines and GPCR activation. This review highlights the importance of adrenoceptors and phosphoprotein phosphatases (PPP) in regulating MMPs in the cardiovascular system, which may help explain some of the beneficial effects of targeting the adrenoceptor system in tissue remodelling and will establish emerging crosstalk between these three systems. Although α- and β-adrenoceptor activation increases MMP but decreases TIMP expression, MMPs are implicated in the growth stimulatory effects of adrenoceptor activation through transactivation of epidermal growth factor receptor. Furthermore, they have recently been found to catalyse the proteolysis of β-adrenoceptors and modulate vascular tone. While the mechanisms underpinning these effects are not well defined, reversible protein phosphorylation by kinases and phosphatases may be key. In particular, PPP (Ser/Thr phosphatases) are not only critical in resensitization and internalization of adrenoceptors but also modulate MMP expression. The interrelationship is complex as isoprenaline (ISO) inhibits okadaic acid [phosphoprotein phosphatase type 1/phosphoprotein phosphatase type 2A (PP2A) inhibitor]-mediated MMP expression. While this may be simply due to its ability to transiently increase PP2A activity, there is evidence for MMP-9 that ISO prevents okadaic acid-mediated expression of MMP-9 through a β-arrestin, NF-κB-dependent pathway, which is abolished by knock-down of PP2A. It is essential that crosstalk between MMPs, adrenoceptors and PPP are investigated further as it will provide important insight into how adrenoceptors modulate cardiovascular remodelling, and may identify new targets for pharmacological manipulation of the MMP system.
Keywords: MMP, adrenoceptor, phosphoprotein phosphatase, β-arrestin, EGFR transactivation, cardiovascular, review
Introduction
MMPs belong to a large family of zinc- and calcium-dependent endopeptidases capable of degrading extracellular matrix (ECM) proteins. They are characterized by three conserved histidines (HEXXHXXGXXH) in the zinc-binding motif of their active centre, along with a conserved methionine, which forms a Met-turn (Bode et al., 1993). Of the 28 MMP genes currently identified, 24 are found in man (Sternlicht and Werb, 2001).
The classification of MMPs has moved towards a combined approach based on domain structure, cellular localization and activation mechanism (Ra and Parks, 2007). Under this system, MMPs are divided into two main groups, secreted and membrane-integrated/anchored. Secreted MMPs are sub-classified on the basis of having a minimal domain (MMP-7, -26), a gelatin-binding domain (MMP-2, -9) or a typical domain structure (MMP-1, -3, -8, -10, -11, -12, -13, -18, -19, -20, -22, -27, -28). The membrane-associated MMPs are also subdivided into type I membrane MMPs (MMP-14, -15, -16, and -24), type II membrane MMPs (MMP-23) and glycosyl-phosphatidyl inositol-anchored MMPs (MMP-17, -25).
As the ECM is both a space-filling material and a bioactive molecule modulating cell adhesion, migration, proliferation and survival (Werb, 1997; Bowers et al., 2010), the functional consequences of its degradation by MMPs under physiological and pathophysiological conditions is clear. However, their action is not limited to degradation of the ECM, as they are involved in activation and processing of TNF-α, Fas ligand and adhesion molecules (Gearing et al., 1994; Mitsiades et al., 2001; Vaisar et al., 2009), as well as activation/cleavage of cell surface receptors, including adrenoceptors (Prenzel et al., 1999; Rodrigues et al., 2010). As such, there has been great interest in developing MMP inhibitors. However, their clinical utility has been disappointing, and a growing body of evidence indicates that targeting signal transduction pathways may be a better approach to limit MMP-mediated tissue remodelling (Overall and Lopez-Otin, 2002).
In this review, we provide a brief overview of MMP regulation and go on to consider how adrenoceptors and protein phosphatases (PP) modulate MMP abundance, primarily in the cardiovascular system, and discuss potential crosstalk between these systems.
MMP regulation
MMP abundance is tightly controlled through multiple mechanisms including transcription/epigenetic, compartmentalization, zymogen activation and inhibition by endogenous tissue inhibitors of MMPs (TIMPs), and is briefly reviewed below.
Transcriptional regulation
MMPs frequently share commonality in their cis-acting elements. Several contain a TATA box, a proximal activator protein-1 (AP-1) binding motif and often a polyomavirus enhancer-binding activator-3 (PEA3) binding site (e.g. MMP-1, -3, -7, -9, -10, -12, -13, -19 and -26), while others have a TATA box but no proximal AP-1 binding site (e.g. MMP-8, MMP-11 and MMP-21), or have neither (e.g. MMP-2, MMP-14 and MMP-28) (Yan and Boyd, 2006; Vincenti and Brinckerhoff, 2007). While this is useful in stratifying MMPs, it is an oversimplification as most promoters contain binding motifs for multiple transcription factors. A case in hand is the MMP-9 promoter (Figure 1), which has a TATA box and several consensus sequences for NF-κB, AP-1, TGF-β inhibitory element, specificity protein 1 (Sp1)/GC and PEA3 (Sato and Seiki, 1993; Sato et al., 1993; Van den Steen et al., 2002). Indeed, there are five putative AP-1 binding sites and two conserved NF-κB binding sites, one for p65 and another for p50 (Han et al., 2001); these are key for regulation by cytokines. It also has a variable length microsatellite (CA)13–27 repeat, the length of which is directly related to promoter activity in diabetic and stroke patients (Peters et al., 1999; Maeda et al., 2001), although this has not been noted in healthy volunteers (Demacq et al., 2008). Nevertheless, an in vitro study clearly demonstrates that shortening of the microsatellite sequence inhibits MMP-9 expression in human lung adenocarcinoma cells (Huang et al., 2003).
Figure 1.
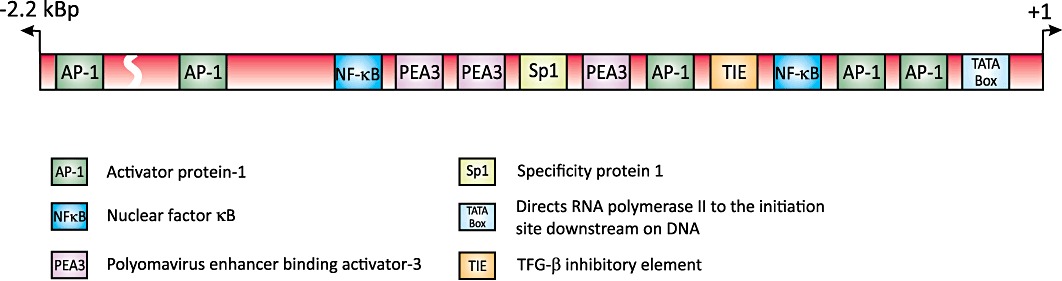
A schematic representation of the transcription factor binding sites in the human MMP-9 promoter (based upon data from Han et al., 2001; Huhtala et al., 1991; Sato et al., 1993; Song et al., 2008; Van den Steen et al., 2002).
While transcriptional regulation of MMP-9 may appear quite straightforward, many cis-regulatory elements function in a synergistic manner. Early studies found that the proximal AP-1 binding site cooperates with the NF-κB (−600 bp) and Sp1 binding sites to mediate 12-O-tetradecanoyl-phorbol-13-acetate (PMA) and TNF-α-induced MMP-9 promoter activity (Sato and Seiki, 1993; Bond et al., 1998). More recently, a ternary co-activator complex comprised of cAMP responsive element-binding protein (CREB)/p300, co-activator-associated arginine methyltransferase 1 and a p160 steroid receptor co-activator (SRC) family member; the glucocorticoid receptor interacting protein-1 has also been established to modulate MMP-9 gene expression following PMA stimulation (Zhao and Benveniste, 2008).
Compartmentalization
Targeted degradation by MMPs can be achieved through compartmentalization within the cell, at the cell surface or within the ECM. For instance, release of MMPs stored in neutrophils and macrophages (Belaaouaj et al., 1995; Ardi et al., 2007; Rosell et al., 2008) limits their action to sites of injury following cellular activation. Secreted MMPs may also be localized to the cell surface through binding to docking receptors, like CD44 (hyaluronan receptor), which facilitates MMP-9-mediated proteolysis and activation of latent TGF-β in keratinocytes (Yu and Stamenkovic, 2000). Additionally, co-localization of MMP-9 with CD44 and α4β1 integrin is implicated in leukaemic B cell migration and survival (Redondo-Munoz et al., 2008; 2010).
Zymogen activation
Once synthesized, MMPs are either constitutively released or secreted in response to a variety of signals as a latent or proMMP form (Sternlicht and Werb, 2001). ProMMPs are activated either intracellularly or extracellularly through proteolytic or oxidative cleavage of a ‘cysteine switch’ in their prodomain. MMP-11 (stromelysin-3) was the first MMP found to be activated intracellularly by furin, a Golgi-associated protease, as part of the secretory pathway (Pei and Weiss, 1995). Extracellular activation of zymogens is best documented for proMMP-2 (in vitro), which is activated at the cell surface through the cooperative action of MMP-14 [membrane type 1 (MT1)-MMP] and TIMP-2 in a 1:1:1 stoichiometry (Imai et al., 1996; Knauper et al., 1996; Sato et al., 1996). Although data pertaining to in vivo activation of MMPs are sparse, the in vitro data are consistent with the observation that only proMMP-2 is found in TIMP-2 knock out (KO) mice (Wang et al., 2000). The role of MMP-14 is less clear, as there is no change in proMMP-2 activation in MMP-14 KO mice (Ruangpanit et al., 2002). This phenomenon is not limited to MMP-14, as MMP-2 and MMP-3 can activate proMMP-9 (Ramos-DeSimone et al., 1999; Toth et al., 2003), as can elastase, trypsin and mast cell chymases (Ferry et al., 1997; Descamps et al., 2004; Tchougounova et al., 2005). ProMMPs can also be activated non-enzymatically through oxidation of thiol groups in their prodomain by reactive oxygen species (ROS) which promotes autolytic cleavage (Nelson and Melendez, 2004).
Endogenous inhibitors
MMP activity is fine-tuned by interaction with endogenous inhibitors such as α2-macroglobulin and TIMPs. α2-Macroglobulin is an endopeptidase inhibitor, which contains a bait region that is cleaved by proteases including metalloproteases. Once cleaved, it undergoes a conformational change trapping the MMP (Nagase et al., 1994). While this is the principal inhibitor, TIMPs likely have the greatest influence on MMP activity.
To date, four TIMPs have been identified (TIMP-1, -2, -3, -4), and inhibit MMP activity by binding in a 1:1 stoichiometry with varying affinity and specificity. TIMP-1 preferentially inhibits MMP-3, MMP-7 and MMP-9, while TIMP-2 inhibits MMP-2. TIMP-3 is bound to the ECM and has the broadest inhibitory spectrum, inhibiting not only MMP-2 and MMP-9, but also members of the A disintegrin and metalloproteinases (ADAMs), and ADAMs with thrombospondin motif (ADAMTS) families. Finally, TIMP-4 has a predilection for MMP-14 (MT1-MMP) and MMP-2. The TIMPs also exhibit differential binding to the latent isoforms of gelatinases, as TIMP-2, -3 and -4 can bind to proMMP-2, while TIMP-1 and -3 bind to proMMP-9 (Wang et al., 2000; Brew and Nagase, 2010). Mechanistically, the N-terminal domain of the TIMPs interact with the catalytic domain of the MMPs to inhibit their activity, while the C-terminal binds to proMMP-2 and -9 via their haemopexin domain, which stabilizes the inhibitor complex. Although many of the functional consequences of TIMPs are attributed to modulation of MMP activty, new evidence indicates that they can influence cell differentiation, growth, migration and angiogenesis in their own right (reviewed in Brew and Nagase, 2010).
Adrenoceptors and the MMP system
Since adrenoceptors were first classified into α- and β-adrenoceptor subtypes by Ahlquist in 1948 (Ahlquist, 1948), and shown to mediate the effects of sympathetic nerve stimulation, they have been further subdivided into α1-, α2- and β-adrenoceptors based on their pharmacological profile, physiological function and tissue location. Each of these adrenoceptor families currently contains three subtypes: the α1A-, α1B-, α1D-; α2A-, α2B-, α2C- and β1-, β2-, β3-adrenoceptors. Adrenoceptors underlie a plethora of physiological functions ranging from metabolic effects to vasoconstriction and immune modulation (Brodde, 1991; Civantos and Aleixandre de, 2001; Oberbeck, 2006). Nonetheless, it is well established that prolonged exposure to catecholamines contributes to vascular and cardiac remodelling in experimental models and in man. Moreover, evidence suggests that adrenoceptors may modulate this process via the MMP system.
α-Adrenoceptor activation (Table 1)
Table 1.
α-Adrenoceptors and the MMP system
| Receptor | Drug | MMP/TIMP | Tissue/Model | Notes | Authors |
|---|---|---|---|---|---|
| α1 | Bunazosin | MMP-2, -3, -9 ↔ | Monkey ciliary muscle cells | 6 h | (Akaishi et al., 2004) |
| α1 | Phenylephrine | MMP-9 ↑ | ECV304 cells | 24 h | (Song et al., 2006) |
| α1 | Noradrenaline; prazosin | MMP-2, TIMP-2 ↑ (NA); ↔ (NA + PRAZ) | Rat ventricular myocardium | 3 to 4 days | (Briest et al., 2004) |
| α1 | Doxazosin | MMP-2, -3, -9 ↔; TIMP-1 ↔ | Human mesangial cells + macrophage medium | 1, 3 days | (Pawluczyk et al., 2006) |
| α1 | Prazosin | MMP-9 ↑ | Rat skeletal muscle | 7 days | (Van Gieson and Skalak, 2001) |
| α1 | Bunazosin | MMP-3 activity ↑, MMP-3 mRNA ↔; MMP-1, -2 ↔; TIMP-1, -3 ↓ | Rat conjunctival tissue, human keratinocytes and fibroblasts | Daily, 2 weeks | (Ito et al., 2006) |
| α1 | 6-Hydroxydopamine; prazosin | TIMP-1 ↓; Liver fibrosis ↓ | CCl4-induced liver fibrosis in rats | 6 weeks | (Dubuisson et al., 2002) |
| α1 | Doxazosin | TIMP-2/MMP-2 ratio ↔ | Aortic-banded rats | 10 weeks | (Perlini et al., 2005) |
| α1 | Terazosin | proMMP-2, MMP-2 ↑; TIMP-1,-2 ↔ | Rat ventral prostate | 120 days | (Mitropoulos et al., 2007) |
| α1 | Anti- α1-adrenoceptor antibody | MMP-2 ↑ | Mouse heart | 1 year | (Zhou et al., 2005) |
| α2 | Brimonidine | proMMP-9 expression ↑, activity ↔; TIMP-4 ↓; proMMP-1, -2, -3, and -24 ↔ | Ciliary body smooth muscle cells | 1 and 3 days | (Ooi et al., 2009) |
| α2 | Brimonidine | MMP-3 ↑; MMP-1, -2 ↔; TIMP-1, -3 ↓ | Rat conjunctival tissue, human keratinocytes and fibroblasts | Daily, 2 weeks | (Ito et al., 2006) |
| α1/2 | Phenoxybenzamine | MMP-2, -9 ↓; TIMP-1 ↑ | Pig vein | 2 weeks | (Chung et al., 2005) |
| ND | Noradrenaline | TIMP-1, -3, MMP-2 ↑ | Mouse heart | 4 h; 1, 3 days | (Meier et al., 2007) |
| ND | Noradrenaline | MMP-2, TIMP-2 ↑ | Rat heart | 3, 4, 7 days | (Briest et al., 2001) |
NA, noradrenaline; ND, not determined; PRAZ, prazosin; ↑, up-regulation; ↔, no effect; ↓, down-regulation.
Several lines of evidence support the role of α-adrenoceptors in controlling MMP abundance (Figure 2). Firstly, noradrenaline (NA) increases cardiac MMP-2 activity and mRNA expression, and decreases TIMP-1 and TIMP-2 expression in rat and mouse models of NA-induced cardiac remodelling (Briest et al., 2001; 2004; Meier et al., 2007). This effect is attributed to its α-agonist activity as the response is attenuated by prazosin (PRAZ) but not metoprolol (Briest et al., 2004). Secondly, long-term exposure to α1-adrenoceptor antibodies, which activate the receptor, increases cardiac MMP-2 expression and interstitial collagen deposition in rat (Zhou et al., 2005). Data from our laboratory extend these studies to show that phenylephrine induces MMP-9 promoter activity in ECV304 cells, although the response is refractory to PRAZ (Song et al., 2006). This is interesting as it may indicate the presence of α1L-adrenoceptors, which have a low affinity for PRAZ (Ford et al., 1997), or may reflect the low potency of α-blockers in certain tissues due to a tissue-specific property of the receptors (Argyle and McGrath, 2000).
Figure 2.
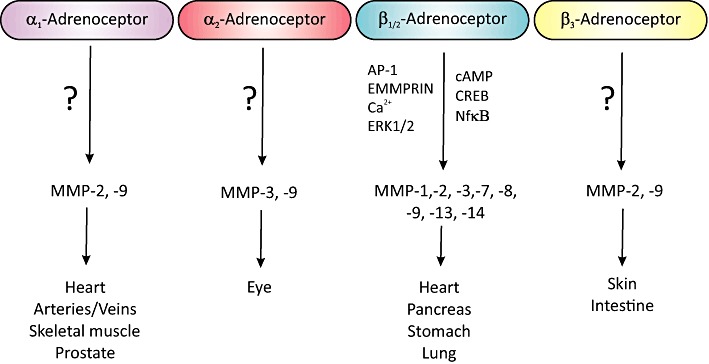
A diagrammatic summarizing the interrelationship between adrenoceptor subtypes and MMP expression, detailing tissues/organs where this has been noted along with specifics of the signal transduction pathways. EMMPRIN, extracellular matrix metalloproteinase inducer.
α2-Adrenoceptors are also implicated in modulation of the MMP system as brimonidine, an α2-adrenoceptor agonist, increases MMP-3 and proMMP-9 expression in rat conjunctival and human ciliary body smooth muscle cells, while attenuating TIMP-1, -3 and -4 expression (Ito et al., 2006; Ooi et al., 2009). However, it has no effect on MMP-1, MMP-2 or TIMP-2 expression in rat conjunctival tissue (Ito et al., 2006).
α-Adrenoceptor antagonists (Table 1)
Other studies have focussed on the effects of α-adrenoceptor antagonists on the MMP system, albeit with little consensus. For instance, when jugular vein grafts are pharmacologically relaxed with phenoxybenzamine (non-selective α-adrenoceptor antagonist), MMP-9 and pro/active MMP-2 expression are reduced compared to mechanically distended vessels (Chung et al., 2005). Juxtaposed to this, the α1-adrenoceptor antagonists, PRAZ and terazosin, increase MMP-9 and pro/active MMP-2 expression in rat skeletal muscle and rat ventral prostate respectively (Van Gieson and Skalak, 2001; Mitropoulos et al., 2007). Yet others report that doxazosin and bunazosin do not affect MMP-2, MMP-3 or MMP-9 abundance in human mesangial cells, monkey ciliary muscle cells or conjunctival tissue (Akaishi et al., 2004; Ito et al., 2006; Pawluczyk et al., 2006).
It is also unclear how α1-adrenoceptor antagonists alter TIMPs. While they have been found to not alter TIMP-1 or TIMP-2 abundance in rat ventral prostate (Mitropoulos et al., 2007) or in conditioned medium from human mesangial cells or monkey ciliary muscle cells (Akaishi et al., 2004; Pawluczyk et al., 2006), bunazosin decreases TIMP-1 and -3 expression in rat conjunctival tissue (Ito et al., 2006) and phenoxybenzamine increases TIMP-1 in porcine jugular vein (Chung et al., 2005).
Although these studies provide clear evidence that α-adrenoceptor agonists and antagonists modulate MMP and TIMP expression, clarity over the net effect remains to be established. The lack of consensus may reflect differences in the regulation of individual MMP and TIMP family members, species-/tissue-/cell line-specific properties, differences in the model employed (unstimulated vs. stimulated conditions) or duration of exposure to the α-adrenoceptor modulating agents and time point at which the effects are studied (Table 1).
β-adrenoceptor activation and the MMP system (Table 2)
Table 2.
β-Adrenoceptors and the MMP system
| Receptor | Drug | MMP/TIMP | Tissue/Model | Notes | Authors |
|---|---|---|---|---|---|
| β1 | Adrenaline; noradrenaline; phenoxybenzamine; doxazosin; yohimbine; propranolol; metoprolol; butoxamine | MMP-1, -9 ↑ (ADN/NA); ↔ (ADN/NA + PROP); ↔ (ADN/NA + MET); TIMP-1 ↑ (ADN/NA) | PBMC, macrophages, U937 cells | 48 h | (Speidl et al., 2004) |
| β1 | Noradrenaline; metoprolol | MMP-2, TIMP-2 ↑ (NA); ↔ (NA + MET) | Rat ventricular myocardium | 3 to 4 days | (Briest et al., 2004) |
| β1 | Metoprolol | MMP-1 ↓; MMP-13 ↔; TIMP-1 ↓; TIMP-2 ↔ | TGF-β1 TG mice | 6 weeks | (Seeland et al., 2009) |
| β1 | Metoprolol | MMP-1 ↓ | Rabbit vulnerable plaque model (local transfection of p53) | 12 weeks | (Liang et al., 2009) |
| β1 | – | 5 months: MMP-1, -13 and proMMP-2 ↑; 12 months: proMMP-2 and MMP-14, TIMP-2 ↑ | Transgenic mice over-expressing β1-adrenoceptors | 5, 12 months | (Seeland et al., 2007) |
| β2 | Clenbuterol (agonist) | MMP-9 ↓; proMMP-2 ↑ | Mouse heart | 20 h | (Patiyal and Katoch, 2005) |
| β2 | Formoterol (agonist) | MMP-9 ↓; MMP-2 ↔ | Cadmium induced acute pulmonary inflammation in rats | 24 h | (Zhang et al., 2010b) |
| β2 | Salbutamol (agonist) | MMP-9 ↑; TIMP-1, -2 (in vitro only) ↓ | Distal lung epithelial cells and bronchioalveolar lavage fluid | 4 days | (O'Kane et al., 2009) |
| β2 | – | MMP | β2-AR over-expressing transgenic mice | (Xu et al., 2011) | |
| β3 | SAR150640 (agonist) | MMP-2, -9 ↔; MMP-2, -9 ↓ (+ LPS) | LPS-stimulated human myometrial samples | 48 h | (Lirussi et al., 2010) |
| β3 | Adrenaline; propranolol; SR-59230A; phentolamine | MMP-2 ↓ (ADN); ↓↓ (ADN + PROP); ↔ (ADN + SR); ↓ (ADN + PHEN) | Murine dermal fibroblasts | 24 h | (Romana-Souza et al., 2011) |
| β1/2 | Isoprenaline | MMP-2 ↓ (1 nM); ↑ (1 µM) | Rat adrenal medulla endothelial cells | 3 h | (Papadimitriou et al., 2001) |
| β1/2 | Isoprenaline | MMP-9 ↑ | ECV304 cells | 4 h | (Song et al., 2006) |
| β1/2 | Isoprenaline | MMP-2 ↑; MMP-9 ↔; MMP-14 ↑ | Porcine left ventricular myocytes | 6 h | (Coker et al., 2001) |
| β1/2 | Noradrenaline; propranolol | MMP-2, -9 ↑ (NA); ↔ (NA + PROP) | Nasopharyngeal carcinoma tumour cell lines | 6 and 12 h | (Yang et al., 2006) |
| β1/2 | Isoprenaline; propranolol; ICI 118,551 | MMP-7 ↑ (ISO); ↔ (ISO + PROP/ICI) | Human gastric cancer cell lines | 12 and 24 h | (Shi et al., 2010) |
| β1/2 | Noradrenaline; propranolol | MMP-2 ↑ (NA); ↔ (NA + PROP) | Human umbilical vein endothelial cells (HUVECs) | 18 h | (Lamy et al., 2010) |
| β1/2 | Isoprenaline | MMP-2, -3, -7 and -9 ↔ | Human hepatic cancer cell lines | 24 h | (Lodewyks et al., 2011) |
| β1/2 | Isoprenaline | MMP-2, TIMP-1 ↑; TIMP-2 ↓; MMP-9 ↔ | Rat ventricular myocytes | 24 h | (Menon et al., 2005) |
| β1/2 | Isoprenaline | MMP-9 ↑ (okadaic acid); ↔ (okadaic acid +I SO) | Murine embryonic fibroblasts, adult human cardiac ventricular fibroblasts | 24 h | (Rietz et al., 2012) |
| β1/2 | Noradrenaline; propranolol; prazosin | MMP-2 ↑ (NA + PRAZ); EMMPRIN expression ↑ (NA + PRAZ), ↔ (NA + PROP) | Adult rat ventricular myocytes | 24 h | (Siwik et al., 2008) |
| β1/2 | Noradrenaline; propranolol | MMP-2, -9 ↑ (NA); ↔ (NA + PROP) | Human pancreatic cancer cell lines | 24 h | (Guo et al., 2009) |
| β1/2 | Isoprenaline | MMP-2 ↑; MMP-9 ↔ | Rat cardiac ventricles | 4 days | (Kizaki et al., 2005) |
| β1/2 | Isoprenaline | MMP-2 ↑; TIMP ↔ | Spontaneously hypertensive rats | 4–5 days | (Veliotes et al., 2010) |
| β1/2 | Isoprenaline | MMP-9 mRNA LV ↔, septum ↑, RV ↔; MMP-9 expression LV ↑, septum ↑, RV ↓ | Rat heart | 10 days | (Cheng et al., 2009) |
| β1/2 | Isoprenaline; spironolactone | MMP-2 ↑ (ISO); ↔ (ISO + spironolactone) | Rat myocardial ventricle | 14 days | (Hori et al., 2011) |
| β1/2 | Isoprenaline; doxycycline | MMP-2, -9 ↑ (ISO); ↔ (ISO + DOX) | Rat myocardial ventricles | 14 days | (Hori et al., 2009) |
| β1/2 | Propranolol | proMMP-9 ↓ | PMA stimulated-brain tumour- derived medulloblastoma cells | 18 h | (Annabi et al., 2010) |
| β1/2 | Propranolol | proMMP-9 ↓; proMMP-2 ↔ | PMA stimulated-human brain microvascular endothelial cells | 18 h | (Annabi et al., 2009) |
| β1/2 | Propranolol; ICI 118,551; Metoprolol | MMP-2, -9 ↓ (PROP; ICI; MET) | Human pancreatic cancer cells | 24 h | (Zhang et al., 2010a) |
| β1/2 | Propranolol | MMP-8, -9 mRNA ↑ (MI), ↑↑ (MI + PROP); MMP-9 activity ↑ (MI), ↔ (MI + PROP); MMP-2 ↓ (MI), ↓↓ (MI + PROP) | Rat model of myocardial infarction (MI) | 6–72 h | (Deten et al., 2003) |
| β1/2 | Propranolol | MMP-2, -9 ↑ (day 14; stress); ↑ (day 7, 14 stress + PROP) | Chronically stressed mice, wound healing | 7 and 14 days | (Romana-Souza et al., 2010) |
| β1/2 | Timolol; betaxolol; carteolol | TIMP-1, -2, -3 ↑ (TIMO); MMP-3, -2, -11↓(TIMO); TIMP-2 ↑ (BET); MMP-3 ↑ (CART) | Rat conjunctival tissue | Daily, 2 weeks | (Ito et al., 2006) |
| β1/2 | Propranolol | MMP-2, -9 ↓ | Human gastric cancer cells | (Liao et al., 2010) | |
| β1/2 | Carvedilol; metoprolol; metoprolol + bunazosin | MMP-8, -13 ↑ (all); TIMP-1 ↔ (all) | Rat ligated heart model after myocardial infarction | 4 weeks | (Zhuang et al., 2009) |
| β1/2 | Propranolol | TIMP-2/MMP-2 ratio ↑ | Aortic-banded rats | 10 weeks | (Perlini et al., 2005) |
| α/β | Carvedilol; probucol; propranolol; prazosin | MMP-2, -9 ↑ (ATH); ↓ (ATH + CARV/PROB); MMP-2, -9 ↑ (TNF-α); MMP-2, -9 ↓ (TNF-α+ CARV/PROB but not PROP/PRAZ) | Rabbit model of atherosclerosis (ATH); human aortic smooth muscle cells. | 21 days; 18 h drugs prior TNF-α (24 h) | (Wu et al., 2007) |
| ND | Carvedilol; metoprolol | MMP-3, -8, -9, -14 ↑, TIMP-4; MMP-8 ↓ (+ CARV) | Murine coxsackie virus myocarditis | 9 days | (Pauschinger et al., 2005) |
| ND | Carvedilol | MMP-2 ↑; ↔ (+ CARV); TIMP-1 ↓; ↑ (+ CARV) | Daunorubicin-induced cardiotoxicity and nephrotoxicity in rats | 41 days | (Arozal et al., 2010) |
| ND | Carvedilol | proMMP-9 ↓; TIMP-1 ↔ | Heart failure patients plasma | 4, 12 weeks | (Song et al., 2006) |
| ND | Carvedilol | MMP-9 ↑; ↓ (+ CARV) | Dilated cardiomyopathic rat | 90 days | (Chua et al., 2008) |
| ND | Carvedilol | MMP-1, -3, -9, TNF-α↑, NA ↑; MMP-1, -3, -9, TNF-α↓ (+ CARV) | Idiopathic dilated cardiomyopathy patients plasma | 6 months | (Ohtsuka et al., 2003) |
ADN, adrenaline; ATH, atherosclerosis; BET, betaxolol; CARV, Carvedilol; CART, carteolol; DOX, doxycycline; EMMPRIN, extracellular matrix metalloproteinase inducer; ICI, ICI-118 551; ISO, isoprenaline; LV, left ventricle; MET, metoprolol; NA, noradrenaline; ND, not determined; PBMC, peripheral blood mononuclear cells PHEN, phentolamine; PRAZ, prazosin; PROB, probucol; PROP, propranolol; RV, right ventricle; SR, SR-59230A; TIMO, timolol; ↑, up-regulation; ↑↑, augmented up-regulation; ↔, no effect; ↓, down-regulation; ↓↓ augmented down-regulation.
Non-selective β-adrenoceptor activation (isoprenaline)
Chronic administration of isoprenaline (ISO) to rats not only causes extensive tissue remodelling but also increases cardiac-specific expression of MMP-1, MMP-2 and MMP-9 (Miura et al., 2003; Hori et al., 2009; 2011). Kizaki et al. report that ISO increases MMP-2 but not MMP-9 mRNA expression in rats (Kizaki et al., 2005). While the reason for the lack of effect on MMP-9 in the latter study is not clear, it may reflect differences in exposure time to ISO (Table 2) or may represent its effects on the spatial dispersion of MMP-9 in the left ventricle and septum, but not the right ventricle (Cheng et al., 2009).
These effects could be mediated through a plethora of cell types present in the complex milieu of the heart, such as inflammatory cell infiltrates, fibroblasts and cardiomyocytes. Indeed, ISO potentiates LPS-induced MMP-1 and MMP-9 expression in human monocytes and U937 cells, an effect which is abrogated by β1- but not β2- or α-adrenoceptor antagonism (Speidl et al., 2004). Nevertheless, Coker et al. demonstrated that ISO can increase MMP-2 and MMP-14 (MT1-MMP) abundance in conditioned media from isolated ventricular cardiomyocytes (Coker et al., 2001), a finding corroborated by Menon et al. who showed that ISO increases MMP-2 and TIMP-1 but decreases TIMP-2 mRNA expression in isolated adult ventricular cardiomyocytes (Menon et al., 2005).
β1-Adrenoceptors clearly have a role in this, as cardiac over-expression of β1-adrenoceptors in mice is associated with development of cardiac hypertrophy and increased expression of MMP-1, proMMP-2 and MMP-13 in the short term (5 months). By 12 months of age, the animals have overt cardiac dilation and fibrosis, and MMP-2 and MMP-14 (MT1-MMP) expression is increased (Seeland et al., 2007). Moreover, MMP-14 (MT1-MMP) was found to co-localize with MMP-2, which is consistent with its proposed role in activating proMMP-2 (Imai et al., 1996; Knauper et al., 1996; Sato et al., 1996).
It is worth noting that β-adrenoceptor modulation of MMPs is not restricted to the cardiovascular system (Figure 2), as recent studies indicate that NA and ISO increase MMP-2, MMP-7 and MMP-9 abundance in a β-adrenoceptor-dependent manner in human pancreatic and gastric cancer cells (Guo et al., 2009; Shi et al., 2010).
β2- and β3-adrenoceptor agonists
The role of β2-adrenoceptors in MMP modulation has only been reported in a select number of studies (Table 2). In patients with acute respiratory distress syndrome, salbutamol (β2-agonist) increases MMP-9 activity in bronchioalveolar lavage fluid, and increases MMP-9 but decreases TIMP-1 and -2 expression in distal lung epithelial cells (O'Kane et al., 2009). Similar findings are reported for formoterol (β2-adrenoceptor agonist) in a rat model of pulmonary inflammation (Zhang et al., 2010b). However, in the mouse heart, clenbuterol (β2-adrenoceptor agonist) decreases MMP-9 activity (Patiyal and Katoch, 2005). More recently, over-expression of β2-adrenoceptors has been associated with increased MMP-2 activity and progressive development of ventricular dysfunction in mice. Surprisingly, these effects were largely abrogated by N-acetylcysteine, indicating regulation of MMP-2 by ROS (Xu et al., 2011).
It is only in the last two years that a couple of studies have emerged implicating β3-adrenoceptor in MMP regulation (Figure 2). SAR150640 (β3-adrenoceptor agonist) increases MMP-2 and -9 abundance in human myometrial strips (Lirussi et al., 2010), while SR-59230A, a β3-adrenoceptor antagonist, inhibits adrenaline-induced MMP-2 abundance in skin fibroblasts (Romana-Souza et al., 2011).
β-adrenoceptor antagonists and the MMP system
The clinical benefits of β-adrenoceptor antagonists in heart failure (Packer et al., 1996; Goldstein and Hjalmarson, 1998) are well documented. However, a seminal study by McDonald et al. revealed that metoprolol (β1-adrenoceptor antagonist) could regress established ventricular remodelling in a canine model of myocardial damage, independent of haemodynamic changes (McDonald et al., 1994). This raised the possibility that β-blockers may affect remodelling through effects on the MMP system. Senzaki et al. published the first report supporting this hypothesis in 2000, in which they showed that supra-therapeutic doses of atenolol (β1-adrenoceptor antagonist; 2.8 g·day−1 vs. clinical maximum dose of 200 mg·day−1) attenuates the increase in MMP-9 abundance associated with heart failure in pigs (Senzaki et al., 2000). Since this report, several groups have demonstrated that metoprolol abrogates the increase in MMP-2 abundance in an experimental model of myocardial infarction (MI; Table 2) (Bernstein and Tyagi, 2001; Cimmino et al., 2011), and reduces MMP-1 abundance and vessel remodelling in a rabbit model of the vulnerable plaque (Liang et al., 2009). In addition, timolol (non-selective β-antagonist) increases TIMP-1, -2 and -3 expression in conjunctival tissue (Ito et al., 2006). While these effects may represent an anti-fibrotic process, metoprolol increases (normalized) expression of MMP-1 and decreases TIMP-1 abundance in the myocardium, while reducing cardiomyocyte size consistent with an anti-hypertrophic rather than an anti-fibrotic effect in TGF-β1 over-expressing mice (Seeland et al., 2009).
Clinically, in patients with idiopathic dilated cardiomyopathy, 6 months treatment with carvedilol (CARV; α1- and β1/2-adrenoceptor antagonist) reduces plasma levels of MMP-1 and -9, but not MMP-3 abundance (Ohtsuka et al., 2003). In keeping with this, short-term (12 weeks) treatment with CARV attenuates the increase in plasma proMMP-9 activity observed in non-CARV-treated heart failure patients (Song et al., 2006). Although not studied, data from an animal study would indicate that the decrease in MMP-9 abundance may be manifest as a decrease in myocardial fibrosis and apoptosis (Chua et al., 2008). CARV also prevents MMP-8 up-regulation in a murine model of viral myocarditis (Pauschinger et al., 2005), and reduces MMP-2 and MMP-9 expression in a rabbit model of atherosclerosis and following exposure of human aortic smooth muscle cells to TNF-α (Wu et al., 2007). Interestingly, in the latter study, effects on MMP-2 and MMP-9 were unaffected by propranolol or PRAZ (Wu et al., 2007). This raises the possibility that the inhibitory effects of CARV on MMP abundance may be related to its antioxidant rather than adrenoceptor blocking properties (Yue et al., 1992); oxidative stress is a potent regulator of MMPs (Siwik et al., 2001; Kameda et al., 2003). In support of this, CARV decreases cardiac expression of MMP-2 and reduces oxidative stress in a rat model of daunorubicin-induced cardiotoxicity (Arozal et al., 2010). While the antioxidant properties of CARV may be important, propranolol, which is not renowned for its antioxidant properties (some of its metabolites are antioxidants, Mak and Weglicki, 2004) decreases MMP-9 and/or MMP-2 abundance in a rat model of MI (Deten et al., 2003), in human gastric cancer cells (Liao et al., 2010) and in human umbilical vein endothelial cells (Lamy et al., 2010). This aside, propranolol differentially abrogates PMA-mediated MMP-9 and MMP-2 expression in human brain endothelial cells (Annabi et al., 2009) and medulloblastoma cells (Annabi et al., 2010), supporting receptor-dependent modulation.
How are β-adrenoceptors coupled to MMPs?
Delineation of the signalling mechanisms coupling β-adrenoceptors to MMPs is not well investigated. However, a cAMP/PKA axis appears to be a key component (Figure 2). For instance, activation of cAMP-dependent protein kinase by dibutyryl-cAMP or forskolin (adenylate cyclase activator) increases MMP-9 and MMP-2 abundance (Liu et al., 2004; Staun-Ram et al., 2004; Pavlovic et al., 2006), while ISO increases MMP-2 expression via cAMP-dependent phosphorylation of CREB in cardiomyocytes and cardiofibroblasts from a phosphoinositide 3 kinase γ (PI3Kγ) knockout model of heart failure (Guo et al., 2010). Likewise, expression of other cAMP-responsive MMPs (MMP-14 and MMP-13) are also increased by ∼3- and 12-fold, respectively (Guo et al., 2010), as is TIMP-1 mRNA expression (Liu et al., 2004).
Juxtaposed to this, ISO, forskolin, dibutyryl-cAMP and intracellular Ca2+ chelation decrease MMP-2 expression in rat adrenal medullary endothelial cells (Papadimitriou et al., 2001). Furthermore, forskolin decreases MMP-9 activity in keratinocytes following exposure to EGF (McCawley et al., 2000), while dibutyryl-cAMP, IBMX and rolipram (phosphodiesterase inhibitors) attenuate LPS-induced transcription of MMP-9 in rat primary astrocytes (Lee et al., 2008). The latter appears to occur independent of PKA involvement, as the response is refractory to H89 and Rp-cAMP (Lee et al., 2008). In keeping with the inhibitory effects of ISO, it decreases okadaic acid-induced MMP-9 expression through a pathway, which is dependent upon an intact NF-κB binding motif, but is not associated with altered inhibitor of κB (IκB) expression in fibroblasts (Rietz et al., 2012). Furthermore, in the absence of okadaic acid, ISO increases MMP-9 expression via a 762-bp region (from 1285-523) of its promoter, which contains consensus sequences for NF-κB and AP-1 (Song et al., 2006). Why this disparity exists requires further investigation. Crosstalk between β-adrenoceptors and MMPs may be indirect, as over-expression of a dominant negative extracellular matrix metalloproteinase inducer prevents β-adrenoceptor-mediated induction of MMP-2 (Siwik et al., 2008). Moreover, aldosterone receptor antagonism also prevents ISO-induced MMP-2 expression, but not that of TIMP in spontaneously hypertensive rats or a rat model of ISO-induced ventricular fibrosis (Veliotes et al., 2010; Hori et al., 2011).
The effects of β-adrenoceptor antagonists on MMP modulation may simply represent blockade of the agonist response, as ICI 118,551 (β2-adrenoceptor selective antagonist) abrogates ISO-induced MMP-7 expression in an AP-1 dependent manner, via c-Jun interacting with signal transducer and activator of transcription 3 at an AP-1 binding motif in gastric cancer cells (Shi et al., 2010). Alternatively, ICI 118,551 can inhibit MMP-2 and MMP-9 expression in the absence of agonist in human pancreatic cancer cells, which may be mediated through NF-κB, AP-1 and CREB, as their expression is decreased (Zhang et al., 2010a). In support of this, propranolol in addition to decreasing MMP abundance (Deten et al., 2003; Annabi et al., 2010; Liao et al., 2010) also inhibits NF-κB expression (Liao et al., 2010), reduces phosphorylation of IκB (Annabi et al., 2010), inhibits ERK-1/2 signalling (Lamy et al., 2010) and decreases nucleocytoplasmic export of the mRNA-stabilizing factor HuR (Annabi et al., 2009); many of which are well established in regulating the MMP system (Yan and Boyd, 2006). In contrast, CARV, metoprolol and co-administration of metoprolol and bunazosin increase MMP-8 and MMP-13 expression and NF-κB expression in non-infarcted tissue from a rat model of MI (Zhuang et al., 2009). This apparent inconsistency likely reflects complexity and diversity in the regulation of different MMP family members (Yan and Boyd, 2006; Vincenti and Brinckerhoff, 2007).
Other synergism between MMPs and adrenoceptors
MMPs and adrenoceptor cleavage
Although the preceding sections highlight the role of adrenoceptors in modulating MMP abundance, emerging reports now indicate that MMPs are involved in catalysing proteolysis of β1- and β2-adrenoceptors (Figure 3). Utilizing an in vitro and in vivo approach, Hakalahti et al. eloquently demonstrated that GM6001 (non-specific MMP and ADAM inhibitor) prevented cleavage of the N-terminus of the β1-adrenoceptor at Arg31 and Leu32 and Pro52 and Leu53 (Hakalahti et al., 2010). In addition, ISO induces proteolysis of the receptor in a time- and concentration-dependent manner, an effect which is mimicked by activation of PKC and adenylate cyclase (Hakalahti et al., 2010). Around the same time, Rodrigues et al. discovered that doxycycline (MMP inhibitor) and EDTA prevented the loss of β2-adrenoceptors from the plasma membrane of aortic endothelial cells and cardiac micro-vessels from control vessels exposed to plasma from spontaneously hypertensive rats (Rodrigues et al., 2010). Although neither of these studies identified the MMP(s) involved, it could be MMP-2 as its activity is ∼4-fold higher in the aorta from spontaneously hypertensive rats compared to normotensive controls; MMP-9 activity is virtually undetectable (Spiers et al., 2005). This paradigm is strengthened by a recent study showing that MMP-2 and NF-κB mediate proteolysis of the extracellular domain of β2-adrenoceptors in kidney from spontaneously hypertensive rats (Wu and Schmid-Shonbein, 2011). Nevertheless, other MMPs such as MMP-7 and MMP-9 could also be involved as they attenuate vascular tone following intravenous administration in spontaneously hypertensive rats (Rodrigues et al., 2010).
Figure 3.
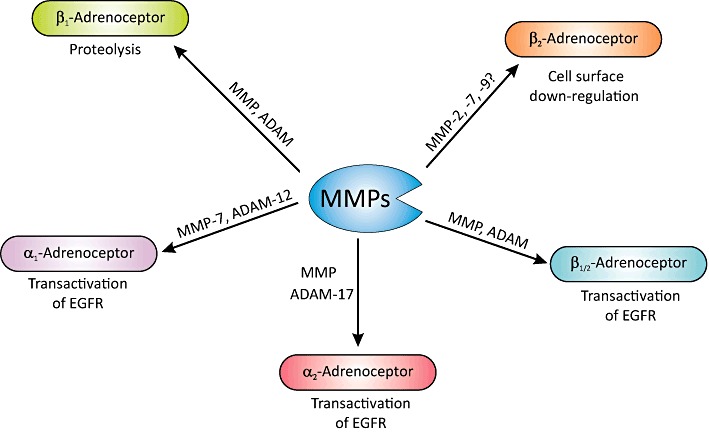
A graphic representation of the central role that MMPs and ADAMs have in the proteolysis of β-adrenoceptor, and in mediating transactivation of EGFR following α- and β-adrenoceptor stimulation via release of HB-EGF (see text for details).
Adrenoceptors, MMPs and transactivation
Catecholamines have important growth regulatory and remodelling effects, which are mediated through activation of the MAPK signalling cascade. Several paradigms have been proffered to explain this link, including canonical GPCR signalling pathway involving activation of ERK1/2 MAPK and adrenoceptor-mediated transactivation of epidermal growth factor receptor (EGFR). The latter is thought to occur via MMP-dependent shedding of heparin-binding EGF-like growth factor (HB-EGF) and subsequent activation of the EGFR (Prenzel et al., 1999), or it may involve intracellular activation of Src and agonist-independent phosphorylation of EGFR (Luttrell et al., 1997). In the case of MMP-dependent transactivation of EGFR, it is both receptor and cell type specific, and involves multiple intermediaries including Gi switching, β-arrestin, free radicals, Src, phospholipase A2 (PLA2), PLC and arachidonic acid metabolites.
Both α- and β-adrenoceptors are associated with transactivation of EGFR (Figure 3). α1-Adrenoceptor-mediated transactivation has been found to involve ROS generation and proteolytic cleavage by MMPs (e.g. MMP-7) in smooth muscle cells, rat mesenteric arteries and cardiomyocytes (Hao et al., 2004; 2006; Zhang et al., 2004; Li et al., 2011). Interstingly, Hao et al. found that doxycyclin (MMP inhibitor) reduced systolic blood pressure and HB-EGFR shedding in spontaneously hypertensive rats, implicating EGFR transactivation in α1-adrenoceptor-mediated regulation of vascular tone and hypertrophy (Hao et al., 2004). The effects on vascular tone may be mediated through either regulation of mitochondrial ATP synthesis by PI3K (Nagareddy et al., 2009) or via Src-independent but PI3K- and ERK1/2 MAPK-dependent signalling (Ulu et al., 2010). In keeping with these studies, MMP-mediated transactivation of EGFR following α1-adrenoceptor activation in a murine hypothalamic neuronal cell line involves PKC, ERK1/2 MAPK, ROS and Src (Shah et al., 2006). α2-Adrenoceptors can also initiate ERK1/2 signalling via MMP-dependent transactivation of EGFR through PLA2 and arachidonic in a renal tubular cell line (Cussac et al., 2002). However, in α2-adrenoceptor transfected PC12 cells, arachidonic release and PLC, but not PLA2, is involved in transactivation of EGFR (Karkoulias et al., 2006).
With regard to β1-adrenoceptors, ERK1/2 signalling is instigated via a cascade involving GPCR kinase (GRK) (GRK5 and GRK6), β-arrestin-dependent activation of Src and MMP/HB-EGF-mediated EGFR transactivation (Noma et al., 2007). Interestingly, this does not require the participation of G-proteins. The role of β-arrestin is interesting as it maintains formation of a β1-adrenoceptor/EGFR complex that retains ERK1/2 in the cytosol, while activation of EGFR by EGF directs ERK1/2 to the nucleus (Tilley et al., 2009). As the authors point out, this may represent an innovative mechanism by which β1-adrenoceptors may educe diverse cellular responses through modulation of EGFR signalling. Surprisingly, when a library of β-adrenoceptor antagonists was studied, alprenolol and CARV also elicited G-protein-independent but β-arrestin-dependent β1-adrenoceptor transactivation of EGFR (Kim et al., 2008), which may account for their effects on the MMP system.
The process may be more complex as β-adrenoceptors can switch from being coupled to Gs to Gi in response to PKA-mediated receptor phosphorylation (Daaka et al., 1997; Martin et al., 2004). In particular, at low β-agonist (ISO) concentrations, β2-adrenoceptors are activated resulting in β-arrestin-2-dependent activation of Src, which in turn activates the EGFR. However, at high agonist concentrations, β1-adrenoceptors transactivate EGFR via a Gi-protein-mediated pathway involving β-arrestin, MMP and HB-EGF in astrocytes (Du et al., 2010). In addition, β2-adrenoceptors can transactivate EGFR through a pathway similar to that of β1-adrenoceptors, as it involves Src, MMP and HB-EGF in cardiac fibroblasts (Kim et al., 2002), but is independent of MMP/HB-EGF in COS7 cells (Drube et al., 2006). Other members of the metzincin superfamily, to which MMPs belong, such as ADAM-10, -12 and -17 (Figure 3), are also implicated in α- and β-adrenoceptor-mediated transactivation of EGFR via cleavage of EGF precursors (Prenzel et al., 1999; Asakura et al., 2002; Yan et al., 2002; Schafer et al., 2004; Chen et al., 2006; Karkoulias et al., 2006; Hakalahti et al., 2010; Peng et al., 2010).
Interrelationship between phosphoprotein phosphatases, MMPs and adrenoceptors
Reversible protein phosphorylation mediated by protein kinases and phosphatases is probably the single most important event regulating cell function. While the role of kinases has been extensively studied, that of PP is less well investigated. Nonetheless, it is now apparent that they are equally important in mediating cell signalling, apoptosis, growth, migration and adhesion. Within this group of enzymes, the serine/threonine PP form a major subfamily, which can be subdivided into phosphoprotein phosphatases (PPP), metal-dependent protein phosphatases (PPM) and aspartate-based PP. The PPP subfamily has seven family members, PP1, PP2A, PP2B (calcineurin), PP4, PP5, PP6 and PP7. Of these, phosphoprotein phosphatase type I (PP1) and type 2A (PP2A) catalyse approximately 90% of all serine/threonine dephosphorylation reactions (Haystead et al., 1989). Their activity is tightly regulated by several endogenous inhibitors including inhibitor-1 (I-1), inhibitor-2 (I-2) and dopamine- and cAMP-regulated phosphoprotein of 32 kDa, which inhibit PP1, while I-1 of PP2A and I-2 of PP2A (I2PP2A) modulate PP2A (for review, see Shi, 2009).
PPP and MMPs
PPP have an emerging role in tissue remodelling through modulation of the MMP system, and via regulation of adrenoceptor signalling. It was first noted in 1997 by Neumann et al. that PP1 activity is increased in patients with end-stage heart failure, and it was proposed that this may well be causally related to disease development, or may exacerbate ‘loss of function’ to inotropic agents (Neumann et al., 1997). This premise was strengthened by data from murine models in which over-expression of PP1 or PP2A depressed cardiac function and caused ventricular remodelling (Carr et al., 2002; Gergs et al., 2004). However, this may be PPP subtype specific, as no change in total calcineurin activity (protein phosphatase 3; PP2B) is found in tissue from patients with compensated left ventricular hypertrophy (Grammer et al., 2006). While the authors did not note any change in MMP-2/MMP29 or TIMP-1–4 abundance, this may be because calcineurin Aβ is up-regulated while calcineurin Bα is down-regulated.
Despite this, the consensus from other studies utilizing pharmacological agents to inhibit PPP supports their involvement in MMP and TIMP regulation. Holladay et al. published one of the earliest reports showing that okadaic acid, a protein phosphatase 1 and 2A inhibitor (Millward et al., 1999), increases MMP-3 (stromelysin-1) expression in murine keratinocytes (Holladay et al., 1992). This has been confirmed and extended by others to include collagenase, interstitial collagenase (rat homologue of collagenase-3/MMP-13), MMP-13 and MMP-9 in fibroblasts and chondrocytes (Westermarck et al., 1994; 2000; Grumbles et al., 1996; Rietz et al., 2012). Interestingly, these MMPs have a TATA box, proximal AP-1 and PEA3 binding sites in their promoters (Yan and Boyd, 2006). The pharmacological studies are strengthened by data from a knock-down study demonstrating that silencing of PPM1A (PPM family member) increases proMMP-9 but decreases TIMP-2 expression in extravillous trophoblasts (Zhang et al., 2009). There is little evidence implicating calcineurin or PP2B in regulation of the MMP system as cyclosporine but not tacrolimus increases TIMP-1 expression, and neither affect MMP-9 expression (Esposito et al., 2004).
So what mechanism(s) contribute to the increase in MMP abundance following PPP inhibition? While this question remains largely unexplored, it may simply reflect maintenance of the phosphorylation and hence activation state of signalling pathways upstream of transcriptional regulation of the MMPs. For instance, PP2A mediates dephosphorylation of MAPK kinase 1 and ERK family kinases (Gomez and Cohen, 1991; Garcia et al., 2002; Zhou et al., 2002), which are associated with MMP and TIMP regulation (Yan and Boyd, 2006). More specifically, Westermarck's group found that okadaic acid-induced expression of MMP-3 is mediated through transactivation of AP-1 complexes containing c-Jun and JunB in HT-1080 cells (Westermarck et al., 1994). However, the process may be more complex than this as recent work from our group shows that okadaic acid increases MMP-9 abundance in fibroblasts through p38-MAPK, and requires intact AP-1 transcription factor binding sites, albeit independently of PP2A inhibition, as knockout of PP2A by siRNA did not alter the response (Rietz et al., 2012). This does not rule out the involvement of other PPP, as okadaic acid also inhibits phosphoprotein phosphatase 1 and 5, and recombinant PP4, leading to increased phosphorylation of numerous proteins including Cdc25, histone H1, phosphorylase kinase, PKA, PKB, PKC, IκB kinases and cyclin-dependent kinases (Fujiki et al., 1991; Hastie and Cohen, 1998; Dean et al., 2001; Janssens and Goris, 2001). This aside, data from transgenic mice over-expressing dominant negative c-Jun (TAM-67) would indicate that c-Jun is not involved in regulating MMP-3 expression (Thompson et al., 2002).
PP and adrenoceptors
Receptor phosphorylation and dephosphorylation play a key role in the regulation of GPCR function. This is best exemplified by the β2-adrenoceptor, which is phosphorylated by GRK2 and PKA, resulting in β-arrestin recruitment to the receptor complex. Binding of β-arrestin sterically prevents the re-coupling of the G-protein to the receptor, and marks the receptor for internalization via clathrin-coated pits (Rockman et al., 2002; Delom and Fessart, 2011). Once internalized, receptors are either dephosphorylated (resensitization) by protein phosphatase 2A in the early endosome (Krueger et al., 1997) prior to recycling back to the plasma membrane, or undergo ubiquitin-mediated degradation (Figure 4A) (Shenoy et al., 2001). Therefore, it is not surprising that ISO increases serine/threonine PP activity in rat heart (Boknik et al., 2000), and PP2A activity in ventricular cardiomyocytes, keratinocytes and mouse fibroblasts (Pullar et al., 2003; De Arcangelis et al., 2008; Rietz et al., 2012). A functional consequence of this increase in PP2A activity is enhanced cardiac contractility due to altered myofilament sensitivity to Ca2+ (Wijnker et al., 2011).
Figure 4.
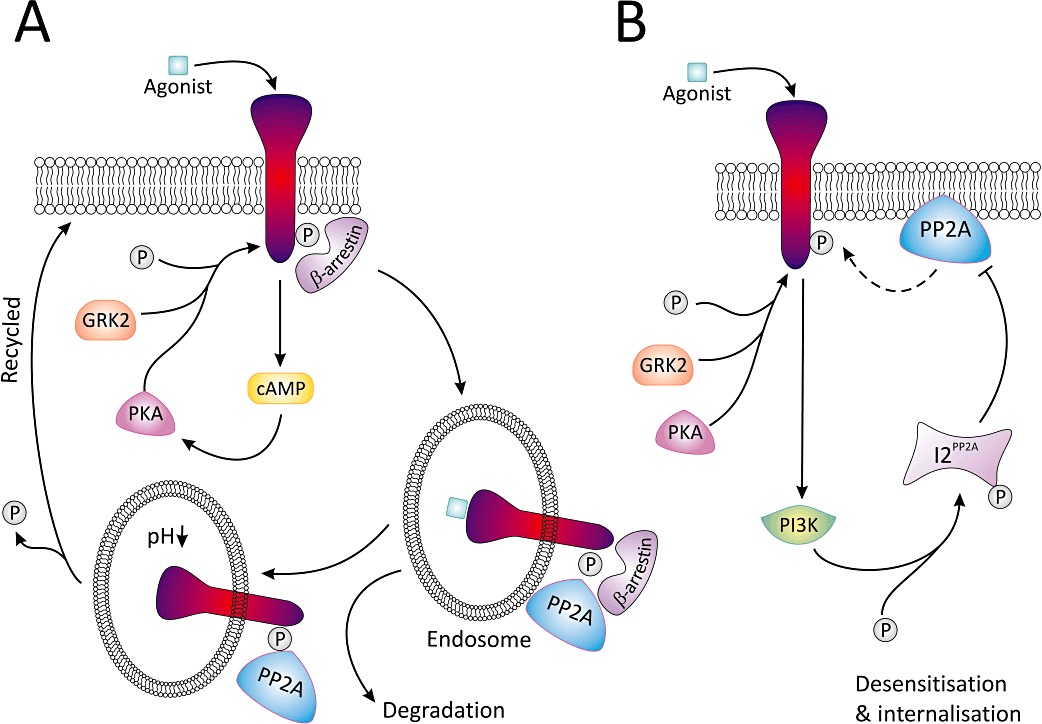
A summary of the role PP2A and its inhibitor I2PP2A have in β-adrenoceptor resensitization (A) and desensitization (B). (A) Following agonist, cAMP levels are elevated and either PKA or GRK2 phosphorylate the receptor permitting binding of β-arrestin. The receptor β-arrestin complex undergoes sequestration into the endosome; following acidification, β-arrestin is released and PP2A dephosphorylates the receptor. As a result of this, the receptor is resensitized and may be recycled back to the plasma membrane. (B) Alternatively, agonist binding and receptor phosphorylation can lead to PI3K activation, which phosphorylates I2PP2A thereby inhibiting PP2A-mediated dephosphorylation of the receptor at the plasma membrane, thus driving the system towards desensitization and internalization of the receptor.
PPP have other effects on adrenoceptor function that are probably independent of endosomal dephosphorylation. In Rat-1 fibroblasts expressing α1b-adrenoceptors, okadaic acid increases receptor phosphorylation and attenuates G-protein coupling, independent of effects on intracellular calcium and inositol trisphosphate formation (Alcantara-Hernandez et al., 2000). While this initial report indicates that PKC is responsible for the increase in receptor phosphorylation, this is now questionable, as a follow-up study found the response to be refractory to staurosporine, a PKC inhibitor (Alcantara-Hernandez and Garcia-Sainz, 2005). Nevertheless, the latter study established that receptor phosphorylation and desensitization involve recrutiment of PI3K and Akt/PKB to the receptor. In a similar vein, β2-adrenoceptors are desensitized via PI3Kγ-dependent activation of I2PP2A, thereby inhibiting PP2A-mediated dephosphorylation of the receptor at the plasma membrane, thus driving the system towards desensitization and internalization of the receptor (Figure 4B) (Vasudevan et al., 2011). PP2A is also involved in a feedback mechanism to protect against or limit sympathetic overdrive. In this scenario, high concentrations of ISO activate PP2A, and limit PKA-dependent contraction of ventricular cardiomyocytes from β2-adrenoceptor knockout mice. This is associated with recruitment of PP2A to a PKA/A kinase-anchoring complex (De Arcangelis et al., 2008).
Several other studies show that PKA and β-adrenoceptor activation inhibit PP1 activity, albeit through activation/phosphorylation of I-1 (Neumann et al., 1991; Carr et al., 2002; Gupta et al., 2002; El-Armouche et al., 2003; El-Armouche and Eschenhagen, 2009). However, it is unclear if this and the decrease in expression of I-1 in cardiac tissue from patients with heart failure (El-Armouche et al., 2008) and following exposure to ISO in hearts from rats (Boknik et al., 2000; El-Armouche et al., 2007) are beneficial. For example, cardiac over-expression of a constitutively active form of I-1 clearly protects against ISO-induced apoptosis by increasing B-cell lymphoma 2 protein (Bcl-2, anti-apoptotic protein) and decreasing Bcl-2-associated X protein (pro-apoptotic protein) expression (Chen et al., 2010). On the other hand, two studies argue against this, as knockout of I-1 attenuates ISO-induced myocardial hypertrophy and arrhythmogenesis in mice (El-Armouche et al., 2008), and over-expression of I-1 caused hyperphosphorylation of phospholamban and ryanodine receptors, resulting in generation of catecholamine-induced Ca2+ sparks (Wittkopper et al., 2010).
Is there a relationship between adrenoceptors, MMPs and PPP?
From the data presented above, it is evident that there is an interrelationship between adrenoceptors and MMPs, and that PPP can control both. Therefore, it is reasonable to suggest that there may be crosstalk between the systems. Several pieces of circumstantial evidence support this paradigm. Firstly, okadaic acid abrogates the inhibitory effects of β2-adrenoceptor stimulation on keratinocytes through recruitment of PP2A to the receptor, and increases the association between PP2A and ERK2 (Pullar et al., 2003), both of which modulate the MMP system. Secondly, PKC, which is associated with adrenoceptor signalling, decreases MMP-2 activity through phosphorylation of serine/threonine residues, while dephosphorylation by alkaline phosphatase increases its activity (Sariahmetoglu et al., 2007).
More convincingly, Rietz et al. recently demonstrated that the inhibitory effect of ISO on okadaic acid-mediated MMP-9 expression is blocked by siRNA knock-down of PP2A in NIH3T3 fibroblasts, indeed indicating communication between all three signalling components. Mechanistically, this involves β-arrestin-2 and a consensus sequence for NF-κB on the MMP-9 promoter, but not NF-κB itself (Rietz et al., 2012). Although the mechanism by which these components interact remains to be determined, we postulate that β-arrestin-2 forms a complex with PP2A and NF-κB upon β-adrenoceptor activation, which inhibits MMP-9 gene expression in a non-classical way (Figure 5). Consistent with this, β-arrestin can form a scaffolding complex resulting in activation of ERK1/2 (Luttrell et al., 1999; Defea et al., 2000), and associates with PP2A following exposure to insulin to disrupt G-protein-mediated MAPK signalling (Hupfeld et al., 2005). Finally, β-arrestin can inhibit NF-κB activity (Witherow et al., 2004), an important transcription factor implicated in transcriptional regulation of several MMPs and TIMPs (Yan and Boyd, 2006).
Figure 5.
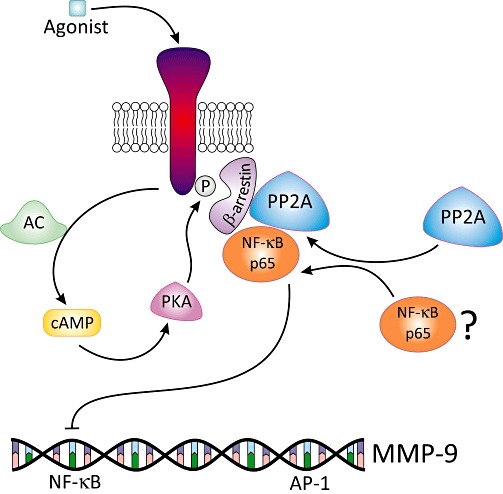
A model of the proposed mechanism by which β-adrenoceptor activation may down-regulate MMP-9 expression. Following binding of an agonist to the β-adrenoceptor, there is an increase in intracellular cAMP levels and phosphorylation of the receptor by PKA or GRK. As a result of this, β-arrestin is recruited to the receptor. Agonist stimulation also activates PP2A and initiates formation of a tri-molecular complex with β-arrestin and the p65 subunit of NF-κB. The trimolecular complex then interacts with the NF-κB binding motif on the MMP-9 promoter to inhibit transcription in a non-classical manner.
Conclusion and outlook
While these recent studies support crosstalk between adrenoceptors, MMPs and PPP, the implications of this paradigm, along with delineation of the signalling mechanisms, remain to be fully understood. Moreover, it will be particularly relevant to determine how this communication is altered in disease, since several diseases, including heart failure and cancer, are associated with alterations in adrenoceptor function, MMP expression and PPP activity. Indeed data from current and future studies may have broad implication for our understanding of the immunomodulatory effects of targeting the adrenergic system and may identify new targets for pharmacological manipulation of the MMP system. However, the communication between these three pivotal signalling elements does lead us to conclude exciting prospects in future investigation of disease remodelling.
Acknowledgments
Work from the author's laboratory included in this review was funded by a project grant from the Health Research Board (grant number RP/2006/51). The authors thank Dr Elizabeth Kelso for critically reading the manuscript.
Glossary
- ADAM
A disintegrin and metalloproteinase
- AP-1
activator protein-1
- CARV
carvedilol
- CREB
cAMP responsive element-binding protein
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- GRK
GPCR kinase
- HB-EGF
heparin-binding EGF-like growth factor
- IκB
inhibitor of κB
- I-1
inhibitor-1
- I-2
inhibitor-2
- ISO
isoprenaline
- MI
myocardial infarction
- NA
noradrenaline
- PEA3
polyomavirus enhancer-binding activator-3
- PI3K
phosphoinositide 3 kinase
- PLA2
phospholipase A2
- PP
protein phosphatase
- PPM
metal-dependent phosphatase
- PPP
phosphoprotein phosphatase
- PRAZ
prazosin
- ROS
reactive oxygen species
- Sp1
specificity protein 1
Conflict of interest
None.
References
- Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Takagi Y, Matsugi T, Ishida N, Hara H, Kashiwagi K. Effects of bunazosin hydrochloride on ciliary muscle constriction and matrix metalloproteinase activities. J Glaucoma. 2004;13:312–318. doi: 10.1097/00061198-200408000-00009. [DOI] [PubMed] [Google Scholar]
- Alcantara-Hernandez R, Garcia-Sainz JA. Okadaic acid increases the phoshorylation state of α1A-adrenoceptors and induces receptor desensitization. Eur J Pharmacol. 2005;525:18–23. doi: 10.1016/j.ejphar.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Alcantara-Hernandez R, Vazquesz-Prado J, Garcia-Sainz JA. Protein phosphatase-protein kinase interplay modulates α1b-adrenoceptor phosphorylation: effects of okadaic acid. Br J Pharmacol. 2000;129:724–730. doi: 10.1038/sj.bjp.0703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Lachambre MP, Plouffe K, Moumdjian R, Beliveau R. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res. 2009;60:438–445. doi: 10.1016/j.phrs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Annabi B, Vaillanourt-Jean E, Weil AG, Beliveau R. Pharmacological targeting of β-adrenergic receptor functions abrogates NF-κB signaling and MMP-9 secretion in medulloblastoma cells. Onco Targets Ther. 2010;3:219–226. doi: 10.2147/OTT.S14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyle SA, McGrath JC. An αA/α1L-adrenoceptor mediates contraction of canine subcutaneous resistance arteries. J Pharmacol Exp Ther. 2000;295:627–633. [PubMed] [Google Scholar]
- Arozal W, Watanabe K, Veeraveedu PT, Ma M, Thandavarayan RA, Sukumaran V, et al. Protective effect of carvedilol on daunorubicin-induced cardiotoxcity and nephrotoxicity. Toxicology. 2010;274:18–26. doi: 10.1016/j.tox.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- Belaaouaj A, Shipley JM, Kobayashi DK, Zimonjic DB, Popescu N, Silverman GA, et al. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem. 1995;270:14568–14575. doi: 10.1074/jbc.270.24.14568. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Tyagi SC. β-Blocker improves cardiac function by reducing oxidative stress and metalloproteinase activity after myocardial infarction. J Appl Res Clin Exp Ther. 2001;1:149–157. [Google Scholar]
- Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331:134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Boknik P, Fockenbrock M, Herzig S, Knapp J, Linck B, Luss H, et al. Protein phosphatase activity is increased in a rat model of long-term β-adrenergic stimulation. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:222–231. doi: 10.1007/s002100000283. [DOI] [PubMed] [Google Scholar]
- Bond M, Rosalind P, Fabunmi P, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol. 2010;48:474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briest W, Holzl A, Rabler B, Deten A, Leicht M, Baba HA, et al. Cardiac remodeling after long term norepinephrine treatment in rats. Cardiovasc Res. 2001;52:265–273. doi: 10.1016/s0008-6363(01)00398-4. [DOI] [PubMed] [Google Scholar]
- Briest W, Homagk L, Rassler B, Ziegelhoffer-Mihalovicova B, Meier H, Tannapfel A, et al. Norepinephrine-induced changes in cardiac transforming growth factor-beta isoform expression pattern of female and male rats. Hypertension. 2004;44:410–418. doi: 10.1161/01.HYP.0000141414.87026.4d. [DOI] [PubMed] [Google Scholar]
- Brodde OE. β1- and β2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hodges RR, Funaki C, Zoukhri D, Gaivin RJ, Perez DM, et al. Effects of α1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am J Physiol Cell Physiol. 2006;291:C946–C956. doi: 10.1152/ajpcell.00014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhou X, Florea S, Qian J, Cai W, Zhang Z, et al. Expression of active protein phosphatase 1 inhibitor-1 attenuates chronic beta-agonist-induced cardiac apoptosis. Basic Res Cardiol. 2010;105:573–581. doi: 10.1007/s00395-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Isoproterenol disperses distribution of NADPH oxidase, MMP-9, and pPKCε in the heart, which are mitigated by endothelin receptor antagonist CPU0213. Acta Pharmacol Sin. 2009;30:1099–1106. doi: 10.1038/aps.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua S, Sheu JJ, Chang LT, Lee FY, Wu CJ, Sun CK, et al. Comparison of losartan and carvedilol on attenuating inflammatory and oxidative response and preserving energy transcription factors and left ventricular function in dilated cardiomyopathy rats. Int Heart J. 2008;49:605–619. doi: 10.1536/ihj.49.605. [DOI] [PubMed] [Google Scholar]
- Chung AW, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pressure distention compared with pharmacologic relaxation in vein grafting upregulates matrix metalloproteinase-2 and -9. J Vasc Surg. 2005;42:747–746. doi: 10.1016/j.jvs.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Cimmino G, Ibanez B, Giannarelli C, Prat-Gonzalez S, Hutter R, Garcia M, et al. Carvedilol administration in acute myocardial infarction results in stronger inhibition of early markers of left ventricular remodeling than metoprolol. Int J Cardiol. 2011;153:256–261. doi: 10.1016/j.ijcard.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Civantos CB, Aleixandre de AA. Alpha-adrenoceptor subtypes. Pharmacol Res. 2001;44:195–208. doi: 10.1006/phrs.2001.0857. [DOI] [PubMed] [Google Scholar]
- Coker ML, Jolly JR, Joffs C, Etoh T, Holder JR, Bond BR, et al. Matrix metalloproteinase expression and activity in isolated myocytes after neurohormonal stimulation. Am J Physiol. 2001;281:H543–H551. doi: 10.1152/ajpheart.2001.281.2.H543. [DOI] [PubMed] [Google Scholar]
- Cussac D, Schaak S, Denis C, Paris H. α2B-Adrenergic receptor activates MAPK via a pathway involving arachidonic acid metabolism, matrix metalloproteinases, and epidermal growth factor receptor transactivation. J Biol Chem. 2002;277:19882–19888. doi: 10.1074/jbc.M110142200. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- De Arcangelis V, Soto D, Xiang Y. Phosphodiesterase 4 and phosphatase 2A differentially regulate cAMP/protein kinase a signaling for cardiac myocyte contraction under stimulation of β1 adrenergic receptor. Mol Pharmacol. 2008;74:1453–1462. doi: 10.1124/mol.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, et al. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2:1–9. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Sci. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delom F, Fessart D. Role of phosphorylation in the control of clathrin-mediated internalization of GPCR. Int J Cell Biol. 2011;2011:246954. doi: 10.1155/2011/246954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demacq C, Vasconcellos VB, Marcaccini AM, Gerlach RF, Silva WA, Jr, Tanus-Santos JE. Functional polymorphisms in the promoter of the matrix metalloproteinase-9 (MMP-9) gene are not linked with significant plasma MMP-9 variations in healthy subjects. Clin Chem Lab Med. 2008;46:57–63. doi: 10.1515/CCLM.2008.014. [DOI] [PubMed] [Google Scholar]
- Descamps FJ, Martens E, Ballaux F, Geboes K, Opdenakker G. In vivo activation of gelatinase B/MMP-9 by trypsin in acute pancreatitis is a permissive factor in streptozotocin-induced diabetes. J Pathol. 2004;204:555–561. doi: 10.1002/path.1669. [DOI] [PubMed] [Google Scholar]
- Deten A, Volz HC, Holzl A, Briest W, Zimmer HG. Effect of propranolol on cardiac cytokine expression after myocardial infarction in rats. Mol Cell Biochem. 2003;251:127–137. [PubMed] [Google Scholar]
- Drube S, Stirnweiss J, Valkova C, Liebmann C. Ligand-independent and EGF receptor-supported transactivation: lessons from beta2-adrenergic receptor signalling. Cell Signal. 2006;18:1633–1646. doi: 10.1016/j.cellsig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Du T, Li B, Li H, Lim M, Hertz L, Peng L. Signaling pathways of isoproterenol-induced ERK 1/2 phosphorylation in primary cultures of astrocytes are concentration dependent. J Neurochem. 2010;115:1007–1023. doi: 10.1111/j.1471-4159.2010.06995.x. [DOI] [PubMed] [Google Scholar]
- Dubuisson L, Desmouliere A, Decourt B, Evade L, Bedin C, Boussarie L, et al. Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology. 2002;35:325–331. doi: 10.1053/jhep.2002.31166. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann WH, et al. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;17:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Gocht F, Jaeckel E, Wittkopper K, Peeck M, Eschenhagen T. Long-term beta-adrenergic stimulation leads to downregulation of protein phosphatase inhibitor-1 in the heart. Eur J Heart Fail. 2007;9:1077–1080. doi: 10.1016/j.ejheart.2007.09.006. [DOI] [PubMed] [Google Scholar]
- El-Armouche A, Wittkopper K, Degenhardt F, Weinberger F, Didie M, Melnychenko I, et al. Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy. Cardiovasc Res. 2008;80:396–406. doi: 10.1093/cvr/cvn208. [DOI] [PubMed] [Google Scholar]
- Esposito C, Foschi A, Parrilla B, Cornacchia F, Fasoli G, Plati AR, et al. Effect of calcineurin inhibitors on extracellular matrix turnover in isolated human glomeruli. Transplant Proc. 2004;36:695–697. doi: 10.1016/j.transproceed.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ferry G, Lonchampt M, Pennel L, de Nanteuil G, Canet E, Tucker GC. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 1997;402:111–115. doi: 10.1016/s0014-5793(96)01508-6. [DOI] [PubMed] [Google Scholar]
- Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, et al. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Suganuma M, Yoshizawa S, Nishiwaki S, Winyar B, Sugimura T. Mechanisms of action of okadaic acid class tumour promoters on mouse skin. Health Perspect. 1991;93:211–214. doi: 10.1289/ehp.9193211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L, Garcia F, Llorens F, Unzeta M, Itarte E, Gomez N. PP1/PP2A phosphatase inhibitors okadaic acid and calyculin A block ERK5 activation by growth factors and oxidative stress. FEBS Lett. 2002;523:90–94. doi: 10.1016/s0014-5793(02)02950-2. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, et al. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem. 2004;279:40827–40834. doi: 10.1074/jbc.M405770200. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Hjalmarson A. Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Circulation. 1998;98(Suppl I):I-364. [Google Scholar]
- Gomez N, Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991;353:170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Grammer JB, Bleiziffer S, Monticelli F, Lange R, Bauernschmitt R. Calcineurin and matrix protein expression in cardiac hypertrophy. Evidence for calcineurin B to control excessive hypertrophic signalling. Basic Res Cardiol. 2006;101:292–300. doi: 10.1007/s00395-006-0598-z. [DOI] [PubMed] [Google Scholar]
- Grumbles RM, Shao L, Jeffrey JJ, Howell DS. Regulation of rat interstitial collagenase gene expression in growth cartilage and chondrocytes by vitamin D3, interleukin-1 beta, and okadaic acid. J Cell Biochem. 1996;63:395–409. doi: 10.1002/(SICI)1097-4644(19961215)63:4%3C395::AID-JCB2%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Guo K, Ma Q, Wang L, Hu H, Li J, Zhag D, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22:825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- Guo D, Kassiri Z, Basu R, Chow FL, Kandalam V, Damilano F, et al. Loss of PI3Kγ enhances cAMP-dependent MMP remodeling of the myocardial N-cadherin adhesion complexes and extracellular matrix in response to early biomechanical stress. Circ Res. 2010;107:1275–1289. doi: 10.1161/CIRCRESAHA.110.229054. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Neumann J, Watanabe AM, Sabbah HN. Inhibition of type 1 protein phosphatase activity by activation of beta-adrenoceptors in ventricular myocardium. Biochem Pharmacol. 2002;63:1069–1076. doi: 10.1016/s0006-2952(02)00851-1. [DOI] [PubMed] [Google Scholar]
- Hakalahti AE, Vierimaa MM, Lilja MK, Kumpula EP, Tuusa JT, Petaja-Repo UE. Human β1-adrenergic receptor is subject to constitutive and regulated N-termnal cleavage. J Biol Chem. 2010;285:28850–28861. doi: 10.1074/jbc.M110.149989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-β- and tumor necrosis factor-α-mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem. 2001;276:22341–22350. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vacular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol. 2006;26:819–825. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- Hastie CJ, Cohen PT. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumour drug fostriecin and other tumour suppressors and promoters. FEBS Lett. 1998;431:357–361. doi: 10.1016/s0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- Haystead TA, Sim AT, Carling D, Honnor RC, Tsukitani Y, Cohen P, et al. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Holladay K, Fujiki H, Bowden GT. Okadaic acid induces the expression of both early and secondary response genes in mouse keratinocytes. Mol Carcinog. 1992;5:16–24. doi: 10.1002/mc.2940050106. [DOI] [PubMed] [Google Scholar]
- Hori Y, Kunihiro SI, Sato A, Yoshioka K, Hara Y, Kanai K, et al. Doxycycline attenuates isoproterenol-induced myocardial fibrosis and matrix metalloproteinase activity in rats. Biol Pharm Bull. 2009;32:1678–1682. doi: 10.1248/bpb.32.1678. [DOI] [PubMed] [Google Scholar]
- Hori Y, Yoshioka K, Kanai K, Hoshi F, Itoh N, Higuchi SI. Spironolactone decreases isoproterenol-induced ventricular fibrosis and matrix metalloproteinase-2 in rats. Biol Pharm Bull. 2011;34:61–65. doi: 10.1248/bpb.34.61. [DOI] [PubMed] [Google Scholar]
- Huang TS, Lee CC, Chang AC, Lin S, Chao CC, Jou YS, et al. Shorterning of microsatellite deoxy(CA) repeats involved in GL331-induced down-regulation of matrix metalloproteinase-9 gene expression. Biochem Biophys Res Commun. 2003;300:901–907. doi: 10.1016/s0006-291x(02)02962-5. [DOI] [PubMed] [Google Scholar]
- Huhtala P, Tuuttila A, Chow LT, Lohi J, Keski-Oja J, Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991;266:16485–16490. [PubMed] [Google Scholar]
- Hupfeld CJ, Resnik JL, Ugi S, Olefsky JM. Insulin-induced β-arrestin1 Ser-412 phosphorylation is a mechanism for desensitization of ERK activation by Gαi-coupled receptors. J Biol Chem. 2005;280:1016–1023. doi: 10.1074/jbc.M403674200. [DOI] [PubMed] [Google Scholar]
- Imai K, Ohuchi E, Aoki T, Nomura H, Fujii Y, Sato H, et al. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- Ito T, Ohguro H, Mamiya K, Ohguro I, Nakazawa M. Effects of antiglaucoma drops on MMP and TIMP balance in conjunctival and subconjunctival tissue. Invest Ophthalmol Vis Sci. 2006;47:823–830. doi: 10.1167/iovs.05-0902. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, et al. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J. 2003;24:2180–2185. doi: 10.1016/j.ehj.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Karkoulias G, Mastrogianni O, Lymperopoulos A, Paris H, Flordellis C. α2-Adrenergic receptors activate MAPK and Akt through a pathway involving arachidonic acid metabolism by cytochrome P450-dependent epoxygenase, matrix metalloproteinase activation and subtype-specific transactivation of EGFR. Cell Signal. 2006;18:729–739. doi: 10.1016/j.cellsig.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, et al. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Eckhart AD, Eguchi S, Koch WJ. Beta-adrenergic receptor-mediated DNA synthesis in cardiac fibroblasts is dependent on transactivation of the epidermal growth factor receptor and subsequent activation of extracellular signal-regulated kinases. J Biol Chem. 2002;277:32116–32123. doi: 10.1074/jbc.M204895200. [DOI] [PubMed] [Google Scholar]
- Kizaki K, Okada M, Ito R, Yoshioka K, Hashizume K, Mutoh KI, et al. Induction of heparanase gene expression in ventricular myocardium of rats with isoproterenol-induced cardiac hypertrophy. Biol Pharm Bull. 2005;28:2331–2334. doi: 10.1248/bpb.28.2331. [DOI] [PubMed] [Google Scholar]
- Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitisation: regulation of β2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Lamy S, Lachambre MP, Lord-Dufour S, Beliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vasc Pharmacol. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim HJ, Lee WJ, Joo SH, Jeon SJ, Kim JW, et al. Differential regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by the cyclic-AMP system in lipopolysaccharide-stimulated rat primary astrocytes. Neurochem Res. 2008;33:2324–2334. doi: 10.1007/s11064-008-9737-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Liao W, Song Y, Ma X, Chen C, et al. Transactivated EGFR mediates alpha-AR-induced STAT3 activation and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2011;301:H1941–H1951. doi: 10.1152/ajpheart.00338.2011. [DOI] [PubMed] [Google Scholar]
- Liang C, Xiaonan L, Xiaojun C, Changjang L, Xinsheng X, Guihua J, et al. Effect of metoprolol on vulnerable plaque in rabbits by changing shear stress around plaque and reducing inflammation. Eur J Pharmacol. 2009;613:79–85. doi: 10.1016/j.ejphar.2009.03.075. [DOI] [PubMed] [Google Scholar]
- Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep. 2010;24:1669–1676. doi: 10.3892/or_00001032. [DOI] [PubMed] [Google Scholar]
- Lirussi F, O'Brien M, Wendremaire M, Goirand F, Sagot P, Dumas M, et al. SAR150640, a selective β3-adrenoceptor agonist, prevents human myometrial remodelling and activation of matrix metalloproteinase in an in vitro model of chorioamnionitis. Br J Pharmacol. 2010;159:1354–1366. doi: 10.1111/j.1476-5381.2009.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ostrom RA, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol. 2004;286:C1089–C1099. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- Lodewyks C, Rodriguez JF, Yan J, Lerner B, Sun D, Rempel JD, et al. Activation of β-adrenergic receptors increases the in vitro migration of malignant hepatocytes. Hepatol Res. 2011;41:1000–1008. doi: 10.1111/j.1872-034X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta 2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Maeda S, Haneda M, Guo B, Koya D, Hayashi K, Sugimoto T, et al. Dinucleotide repeat polymorphism of matrix metalloproteinase-9 gene is associated with diabetic nephropathy. Kidney Int. 2001;60:1428–1434. doi: 10.1046/j.1523-1755.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- Mak IT, Weglicki WB. Potent antioxidant properties of 4-hydroxyl-propranolol. J Pharmacol Exp Ther. 2004;308:85–90. doi: 10.1124/jpet.103.058032. [DOI] [PubMed] [Google Scholar]
- Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell Signal. 2004;16:1397–1403. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Li S, Benavidez M, Halbleid J, Wattenberg EV, Hudson LG. Elevation of intracellular cAMP inhibits growth factor-mediated matrix metalloproteinase-9 induction and keratinocyte migration. Mol Pharmacol. 2000;58:145–151. doi: 10.1124/mol.58.1.145. [DOI] [PubMed] [Google Scholar]
- McDonald KM, Rector T, Carlyle PF, Francis GS, Cohn JN. Angiotensin-converting enzyme inhibition and beta-adrenoceptor blockade regress established ventricular remodeling in a canine model of discrete myocardial damage. J Am Coll Cardiol. 1994;24:1762–1768. doi: 10.1016/0735-1097(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Meier H, Bullinger J, Deten A, Marx G, Rabald S, Zimmer HG, et al. Tissue inhibitor of matrix metalloproteinase-1 in norepinephrine-induced remodeling of the mouse heart. Cell Physiol Biochem. 2007;20:825–836. doi: 10.1159/000110442. [DOI] [PubMed] [Google Scholar]
- Menon B, Singh M, Singh K. Matrix metalloproteinases mediate β-adrenergic receptor stimulated apoptosis in adult rat ventricular myocytes. Am J Physiol. 2005;289:C169–C176. doi: 10.1152/ajpcell.00606.2004. [DOI] [PubMed] [Google Scholar]
- Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- Mitropoulos D, Papakonstantinou E, Aletras AJ, Kalinderis N, Zervas A, Hatzichristou D, et al. Terazosin modifies the content of glycosaminoglycans and the activity of matrix metalloproteinase 2 in the rat ventral prostate. Eur Urol. 2007;51:456. doi: 10.1016/j.eururo.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Miura S, Ohno I, Suzuki J, Suzuki K, Okada S, Okuyama A, et al. Inhibition of matrix metalloproteinases prevents cardiac hypertrophy induced by beta-adrenergic stimulation in rats. J Cardiovasc Pharmacol. 2003;42:174–181. doi: 10.1097/00005344-200308000-00004. [DOI] [PubMed] [Google Scholar]
- Nagareddy PR, Chow FL, Hao L, Wang X, Nishimura T, MacLeod KM, et al. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc Res. 2009;84:368–377. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- Nagase H, Itoh Y, Binner S. Interaction of alpha 2-macroglobulin with matrix metalloproteinases and its use for identification of their active forms. Ann N Y Acad Sci. 1994;732:294–302. doi: 10.1111/j.1749-6632.1994.tb24744.x. [DOI] [PubMed] [Google Scholar]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Neumann J, Gupta RC, Schmitz W, Scholz H, Nairn AC, Watanabe AM. Evidence for isoproterenol-induced phosphorylation of phosphatase inhibitor-1 in the intact heart. Circ Res. 1991;69:1450–1457. doi: 10.1161/01.res.69.6.1450. [DOI] [PubMed] [Google Scholar]
- Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane CM, KcKeown SW, Perkins GD, Bassford CR, Gao F, Thichett DR, et al. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med. 2009;37:2242–2249. doi: 10.1097/CCM.0b013e3181a5506c. [DOI] [PubMed] [Google Scholar]
- Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem. 2006;13:1979–1989. doi: 10.2174/092986706777584997. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Hamada M, Saeki H, Ogimoto A, Hara Y, Shigematsu Y, et al. Serum levels of matrix metalloproteinases and tumour necrosis factor-α in patients with idiopathic dilated cardiomyopathy and effect of carvedilol on these levels. Am J Cardiol. 2003;91:1024–1027. doi: 10.1016/s0002-9149(03)00133-4. [DOI] [PubMed] [Google Scholar]
- Ooi YH, Oh DJ, Rhee DJ. Analysis of alpha2-adrenergic receptors and effect of brimonidine on matrix metalloproteinases and their inhibitors in human ciliary body. Invest Ophthalmol Vis Sci. 2009;50:4237–4243. doi: 10.1167/iovs.08-2312. [DOI] [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Papadimitriou E, Waters CR, Manolopoulos VG, Unsworth BR, Maragoudakis ME, Lelkes PI. Regulation of extracelular matrix remodeling and MMP-2 activation in cultured rat adrenal medullary endothelial cells. Endothelium. 2001;8:243–253. doi: 10.3109/10623320109090801. [DOI] [PubMed] [Google Scholar]
- Patiyal SN, Katoch SS. β-adrenoceptor agonist clenbuterol down-regulates matrix metalloproteinase (MMP-9) and results in an impairment of collagen turnover in mice left ventricle. Jpn J Physiol. 2005;55:165–172. doi: 10.2170/jjphysiol.R2118. [DOI] [PubMed] [Google Scholar]
- Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Peter SL, et al. Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis association with reduced myocardial interleukin-1beta and MMP-8 expression and a modulated immune response. Eur J Heart Fail. 2005;7:444–452. doi: 10.1016/j.ejheart.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, et al. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- Pawluczyk IZA, Patel SR, Harris PG. The role of the α1-adrenoceptor in modulating human mesangial cell matrix production. Nephrol Dial Transplant. 2006;21:2417–2424. doi: 10.1093/ndt/gfl230. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- Peng L, Li B, Du T, Kong EK, Hu X, Zhang S, et al. Astrocytic transactivation by alpha2A-adrenergic and 5-HT2B serotonergic signaling. Neurochem Int. 2010;57:421–431. doi: 10.1016/j.neuint.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Perlini S, Palladini G, Ferrero I, Tozzi R, Fallarini S, Facoetti A, et al. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension. 2005;46:1213–1218. doi: 10.1161/01.HYP.0000185689.65045.4c. [DOI] [PubMed] [Google Scholar]
- Peters DG, Kassam A, Jean PL, Yonas H, Ferrell RE. Functional polymorphism in the matrix metaloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke. 1999;30:2612–2616. doi: 10.1161/01.str.30.12.2612. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555–22562. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- Redondo-Munoz J, Ugarte-Berzal E, Garcia-Marco JA, del Cerro MH, Van den Steen PE, Opdenakker G, et al. α4β1 integrin and 190-kDa CD44v constitute a cell surface docking complex for gelatinase B/MMP-9 in chronic leukemic but not in normal B cells. Blood. 2008;112:169–178. doi: 10.1182/blood-2007-08-109249. [DOI] [PubMed] [Google Scholar]
- Redondo-Munoz J, Ugarte-Berzal E, Terol MJ, Van den Steen PE, Hernandez del CM, Roderfeld M, et al. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia B cell survival through its hemopexin domain. Cancer Cell. 2010;17:160–172. doi: 10.1016/j.ccr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Rietz A, Volkov Y, Davies A, Hennessy M, Spiers J. Okadaic acid induces MMP-9 expression in fibroblast: crosstalk between protein phosphatase inhibition and β-adrenoceptor signalling. Br J Pharmacol. 2012;165:274–288. doi: 10.1111/j.1476-5381.2011.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Rodrigues SF, Tran ED, Tortes ZB, Schmid-Shonbein GW. Matrix metalloproteinases cleave the β2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–H35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamines. Exp Dermatol. 2010;19:821–829. doi: 10.1111/j.1600-0625.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- Romana-Souza B, Otranto M, Almeida TF, Porto LC, Monte-Alto-Costa A. Stress-induced epinephrine levels compromise murine dermal fibroblast activity through β-adrenoceptors. Exp Dermatol. 2011;20:413–419. doi: 10.1111/j.1600-0625.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Ruangpanit N, Price JT, Holmbeck K, Birkedal-Hansen H, Guenzler V, Huang X, et al. MT1-MMP-dependent and -independent regulation of gelatinase A activation in long-term, ascorbate-treated fibroblast cultures: regulation by fibrillar collagen. Exp Cell Res. 2002;272:109–118. doi: 10.1006/excr.2001.5403. [DOI] [PubMed] [Google Scholar]
- Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- Sato H, Seiki M. Regulatory mechanisms of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumour cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- Sato H, Kita M, Seiki M. v-Src activates the expression of the 92-kDa type IV collagenase gene through the AP-1 site and the GT box homologous to retinoblastoma control elements: a mechanism regulating gene expression independent of that by inflammatory cytokines. J Biol Chem. 1993;268:23460–23468. [PubMed] [Google Scholar]
- Sato H, Takino T, Kinoshita T, Imai K, Okada Y, Stetler Stevenson WG, et al. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Lett. 1996;385:238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- Schafer B, Marg B, Gschwind A, Ullrich A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J Biol Chem. 2004;279:47929–47938. doi: 10.1074/jbc.M400129200. [DOI] [PubMed] [Google Scholar]
- Seeland U, Selejan S, Engelhardt S, Muller P, Lohse MJ, Bohm M. Interstitial remodeling in β-adrenergic receptor transgenic mice. Basic Res Cardiol. 2007;102:183–193. doi: 10.1007/s00395-006-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeland U, Schaffer A, Selejan S, Hohl M, Reil JC, Muller P, et al. Effects of AT1- and beta-adrenergic receptor antagonists on TGF-beta1-induced fibrosis in transgenic mice. Eur J Clin Invest. 2009;39:851–859. doi: 10.1111/j.1365-2362.2009.02183.x. [DOI] [PubMed] [Google Scholar]
- Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT, et al. β-blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res. 2000;86:807–815. doi: 10.1161/01.res.86.7.807. [DOI] [PubMed] [Google Scholar]
- Shah BH, Shah FB, Catt KJ. Role of metalloproteinase-dependent EGF receptor activation in alpha-adrenoceptor-stimulated MAP kinase phosphorylation in GT1-7 neurons. J Neurochem. 2006;96:520–532. doi: 10.1111/j.1471-4159.2005.03585.x. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu D, Duan H, Han C, Wei B, Qian L, et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol Cancer. 2010;9:1–14. doi: 10.1186/1476-4598-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- Siwik DA, Kuster GM, Brahmbhatt JV, Zaidi Z, Malik J, Ooi H, et al. EMMPRIN mediates beta-adrenergic receptor-stimulated matrix metallproteinase activity in cardiac myocytes. J Mol Cell Cardiol. 2008;44:210–217. doi: 10.1016/j.yjmcc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Song G, Hennessy M, Zhao YL, Li Q, Han WD, Qi Y, et al. Effects of adrenoceptor blockade on plasma gelatinase activity in patients with heart failure and MMP-9 promoter activity in a human cell line (ECV304) Pharmacol Res. 2006;54:57–64. doi: 10.1016/j.phrs.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G, et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol Immunol. 2008;45:137–143. doi: 10.1016/j.molimm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Speidl WS, Toller WG, Kaun C, Weiss TW, Pfaffenberger S, Kastl SP, et al. Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. FASEB J. 2004;18:603–605. doi: 10.1096/fj.03-0454fje. [DOI] [PubMed] [Google Scholar]
- Spiers JP, Kelso EJ, Siah WF, Edge G, Song G, McDermott BJ, et al. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behaviour. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- Thompson EJ, MacGowan J, Young MR, Colburn N, Bowden GT. A dominant negative c-jun specifically blocks okadaic acid-induced skin tumour promotion. Cancer Res. 2002;62:3044–3047. [PubMed] [Google Scholar]
- Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. Beta-arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284:20375–20386. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun. 2003;308:386–395. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- Ulu N, Gurdal H, Landheer SW, Duin M, Guc MO, Buikema H, et al. α1-Adrenoceptor-mediated contraction of rat aorta is partly mediated via transactivation of the epidermal growth factor receptor. Br J Pharmacol. 2010;161:1301–1310. doi: 10.1111/j.1476-5381.2010.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisar T, Kassim SY, Gomez IG, Green PS, Hargarten S, Gough PJ, et al. MMP-9 sheds the β2 integrin subunit (CD18) from macrophages. Mol Cell Proteomics. 2009;8:1044–1060. doi: 10.1074/mcp.M800449-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- Van Gieson EJ, Skalak TC. Chronic vasodilation induces matrix metalloproteinase 9 (MMP-9) expression during microvascular remodeling in rat skeletal muscle. Microcirculation. 2001;8:25–31. [PubMed] [Google Scholar]
- Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Prasad SVN. Inhibition of protein phosphatase 2A activity by PI3Kγ regulates β-adrenergic receptor function. Mol Cell. 2011;41:636–648. doi: 10.1016/j.molcel.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliotes DG, Norton GR, Correia RJ, Strijdom H, Badenhorst D, Brooksbank R, et al. Impact of aldosterone receptor blockade on the deleterious cardiac effects of adrenergic activation in hypertensive rats. J Cardiovasc Pharmacol. 2010;56:203–211. doi: 10.1097/FJC.0b013e3181e92a01. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Lohi J, Keski-Oja J, Kahari VM. Okadaic acid-elicited transcriptional activation of collagenase gene expression in HT-1080 fibrosarcoma cells is mediated by JunB. Cell Growth Differ. 1994;5:1205–1213. [PubMed] [Google Scholar]
- Westermarck J, Li S, Jaakkola P, Kallunki T, Grenman R, Kahari VM. Activation of fibroblast collagenase-1 expression by tumor cells of squamous cell carcinomas is mediated by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase-2. Cancer Res. 2000;60:7156–7162. [PubMed] [Google Scholar]
- Wijnker PJ, Boknik P, Gergs U, Muller FU, Neumann J, Dos Remedios C, et al. Protein phosphatase 2A affects myofilament contractility in non-failing but not in failing human myocardium. J Muscle Res Cell Motil. 2011;32:221–233. doi: 10.1007/s10974-011-9261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. β-Arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc Natl Acad Sci U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkopper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsold B, et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KI, Schmid-Shonbein G. Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011;57:261–268. doi: 10.1161/HYPERTENSIONAHA.110.158709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Chen YH, Leu HB, Chen YL, Lin FY, Lin SJ, et al. Carvedilol, a pharmacological antioxidant, inhibits neointimal matrix metalloproteinase-2 and -9 in experimental atherosclerosis. Free Radic Biol Med. 2007;43:1508–1522. doi: 10.1016/j.freeradbiomed.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Xu Q, Dalic A, Fang L, Kiriazis H, Ritchie RH, Sim K, et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol. 2011;162:1012–1028. doi: 10.1111/j.1476-5381.2010.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2006;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol. 2002;158:221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Yue TL, Cheng HY, Lysko PG, McKenna PJ, Feuerstein R, Gu JL, et al. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J Pharmacol Exp Ther. 1992;263:92–98. [PubMed] [Google Scholar]
- Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res. 2004;95:989–997. doi: 10.1161/01.RES.0000147962.01036.bb. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhou Z, Lin H, Lv X, Fu J, Lin P, et al. Protein phosphatase 1A (PPM1A) is involved in human cytotrophoblast cell invasion and migration. Histochem Cell Biol. 2009;132:169–179. doi: 10.1007/s00418-009-0601-5. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma QY, Hu HT, Zhang M. β2-Adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 2010a;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fievez L, Cheu E, Bureau F, Rong W, Zhang F, et al. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur J Pharmacol. 2010b;628:171–178. doi: 10.1016/j.ejphar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Zhao X, Benveniste EN. Transcriptional activation of human matrix metalloproteinase-9 gene expression by multiple co-activators. J Mol Biol. 2008;383:945–956. doi: 10.1016/j.jmb.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. The specificity of extracellular signal-related kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277:31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liao YH, Wei Y, Wei F, Wang B, Li L, et al. Cardiac remodeling after long-term stimulation by antibodies against the α1-adrenergic receptor in rats. Clin Immunol. 2005;114:164–173. doi: 10.1016/j.clim.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Zhuang XF, Yin CQ, Wang HY, Sun NL. Distinctive effects of carvedilol in the non-infarct zone: remodelling of the ligated rat heart linked to oxidative. J Int Med Res. 2009;37:1354–1364. doi: 10.1177/147323000903700510. [DOI] [PubMed] [Google Scholar]


