Abstract
BACKGROUND AND PURPOSE
We have tested the hypothesis that calcitonin gene-related peptide (CGRP) is a mediator of capsaicin-induced angiogenesis in vivo.
EXPERIMENTAL APPROACH
In a series of experiments, the knee joints of rats were injected with CGRP, capsaicin or vehicle control. Groups of animals (n = 6) were treated with the CGRP receptor antagonist BIBN4096BS and/or the NK1 receptor antagonist SR140333. Endothelium, proliferating endothelial cell nuclei and macrophages were identified 24 h later in the synovium by immunohistochemistry and quantified by image analysis. mRNA for the receptors for CGRP and adrenomedullin were sought in normal and inflamed rat and human synovia using RT-PCR.
KEY RESULTS
Intra-articular CGRP injection increased the endothelial cell proliferation index, whereas macrophage infiltration and knee joint diameters were similar to saline-injected controls. CGRP-induced endothelial cell proliferation was dose-dependently inhibited by BIBN4096BS. mRNA for adrenomedullin and the CGRP receptor subunits were detected in normal and inflamed human and rat synovia. In capsaicin-induced synovitis, the increased endothelial cell proliferation index was partially blocked by administration of NK1 or CGRP antagonists individually and was reduced to the level of saline controls by coadministration of both receptor antagonists.
CONCLUSIONS AND IMPLICATIONS
These data support the hypothesis that CGRP stimulates angiogenesis in vivo directly by activating CGRP receptors. Capsaicin-induced endothelial cell proliferation was completely blocked by coadministration of CGRP and NK1 receptor antagonists, indicating that both CGRP and substance P may contribute to angiogenesis in this model of synovitis.
Keywords: angiogenesis, endothelial cell proliferation, calcitonin gene-related peptide, capsaicin
Introduction
Angiogenesis, the growth of new blood vessels from a preexisting vasculature, is a common feature of chronic inflammation in most tissues (Walsh and Haywood, 2001), including the synovium (Walsh et al., 1998). Angiogenesis is tightly regulated during normal growth, wound healing and the female reproductive cycle (Walsh, 1999), whereas persistent angiogenesis is characteristic of chronic inflammatory disease. An example of this is the blood vessel growth seen in rheumatoid arthritis (Klareskog et al., 2009). Angiogenesis is also a key component in the transition from acute to persistent inflammation (Ashraf et al., 2010). Thus, an understanding of the factors that can be involved in early angiogenesis may identify targets for treating arthritis.
We have previously shown that exogenous substance P (SP) can increase endothelial cell proliferation in the rat knee joint, and that this can be blocked completely by the competitive nonpeptide NK1 receptor antagonist SR140333 (nolpitantium) (Malcangio and Bowery, 1994; receptor nomenclature follows Alexander et al., 2011). The specificity of SR140333 cann be summarized by its inhibition constants (Ki) at various receptors. Ki at the NK1 receptor in rat brain was 0.027 nM, in pig brain was 0.039 nM and in cultured human cells IM9 (lymphoblast), U373 and STTG1 (astrocytoma) cells were 0.019, 0.7 and 0.24 nM respectively. Ki values for binding to NK2 and NK3 receptors were >1000 and 500 nM respectively (Emonds-Alt et al., 1993). Endothelial cell proliferation was neither blocked by the inactive enantiomer SR140603 nor by the NK2 receptor antagonist SR144190 (Seegers et al., 2003). This is consistent with the hypothesis that the proangiogenic action of SP is mediated via the NK1 receptor. NK1 receptors have been localized to the vascular endothelium in both rat and human synovium (Walsh et al., 1992; 1993).
Capsaicin, the pungent component in chilli peppers (Szallasi and Blumberg, 1999), can be used to stimulate the release of neuropeptides from sensory nerve fibres, giving rise to neurogenic inflammation. We have shown by immunohistochemistry that intra-articular injection of capsaicin results in the depletion of neuropeptides from nerves within the synovium (Mapp et al., 1996). Thus, this model was chosen as a means to the release of endogenous neuropeptides SP and CGRP.
Enhanced angiogenesis also occurs during this capsaicin-induced synovitis, but this effect is not wholly attributable to actions of SP on NK1 receptors (Seegers et al., 2003). A dose of 1 µmol SR140333, which completely inhibited the effects of exogenous SP, was found to block only 65% of the capsaicin-induced enhanced endothelial cell proliferation (Seegers et al., 2003). We therefore hypothesized that another mediator may be playing a part in enhanced angiogenesis during capsaicin-induced synovitis.
Calcitonin gene-related peptide (CGRP) is a neuropeptide colocalized with SP in secretory granules at the peripheral terminals of perivascular sensory nerves. It is a potent vasodilator and can enhance endothelial cell proliferation and migration in vitro (Haegerstrand et al., 1990). Thus, CGRP is a candidate for a further neuropeptide involved in capsaicin-enhanced angiogenesis.
The CGRP receptor is a heterodimer consisting of the calcitonin receptor–like receptor (CRLR) and one of three receptor activity modifying proteins (RAMPs). RAMP1 confers specificity for CGRP (Hay et al., 2004), whilst RAMPs 2 and 3 confer specificity for adrenomedullin (McLatchie et al., 1998; see Walker et al. (2010: Recober and Russo (2009).
We used the nonpeptide, competitive, CGRP receptor antagonist BIBN4096BS (Olcegepant) (Doods et al., 2000) to test the contribution of CGRP to in vivo endothelial cell proliferation. The Kivalues for BIBN4096BS are 0.018 nM at the human CGRP receptor and 2.1 nM at the rat CGRP receptor. The major determinant of the species selectivity is the amino acid at position 74 in RAMP1 (Mallee et al., 2002). Human RAMP 1 contains tryptophan at this position, whereas this is lysine in rodent RAMP1. Over and above species selectivity, BIBN4096BS shows selectivity for CGRP receptors over adrenomedullin receptors of at least 1000-fold (Hay et al., 2006). BIBN4096BS shows no antagonistic effects on cells expressing CRLR/RAMP2 and CRLR/RAMP3 at concentrations up to 10 µM.
The experiments we have performed have investigated the effects of exogenous CGRP and adrenomedullin on angiogenesis in the rat knee and tested the ability of a CGRP receptor antagonist, both alone and in combination with a NK1 receptor antagonist to inhibit angiogenesis in capsaicin-induced synovitis. In order to assess the potential relevance of our rat models to human synovitis, we have looked for the presence of mRNAs for CRLR and RAMPs in both normal and inflamed rat and human synovia.
Methods
Animal models
All animal care and experimental proceduress were approved by the University of Nottingham Local Ethics Review Committee and performed under United Kingdom Home Office licence. We used male Wistar rats of approximately 180 g. Animals were given food and water ad libitum and maintained on a 12 h light/dark cycle. CGRP (dose range 0.025–2.5 nmol), adrenomedullin (at the highest practicable dose of 8 nmol), capsaicin (0.5% w/v) or control vehicle was each injected into the right knee (n = 6 per group). All the above reagents were obtained from Sigma Aldrich Ltd. (Dorset, UK). Left knees were injected with normal sterile saline alone, which does not increase indices of angiogenesis, macrophage infiltration or knee diameter at 24 h compared with naïve animal knees (Walsh et al., 1998). CGRP and adrenomedullin were dissolved in normal sterile saline. Capsaicin was dissolved in 10% ethanol, 10% Tween 80 and 80% normal sterile saline (v/v/v) (Mapp et al., 1996). Volumes for all intra-articular injections were 100 µL, performed under isofluorane (2%) anaesthesia (Schering Plough, Middlesex, UK). Anaesthesia was assessed by absence of paw withdrawal following foot pinching.
Administration of receptor antagonists
The CGRP receptor antagonist BIBN4096BS (6 mg) (Doods et al., 2000) was dissolved in 200 µL 0.1 M HCl and then diluted to 1 mL with normal sterile saline. The pH was then adjusted to neutral by the stepwise addition of 0.1 M NaOH. The receptor antagonist was then administered i.v. at a dose of 0.7 or 0.07 µmol per rat immediately before intra-articular injection of 2.5 nmol of CGRP or 0.5% capsaicin. The NK1 receptor antagonist SR140333 was dissolved in ethanol to a stock concentration of 100 mM and then diluted with normal sterile saline to give a final ethanol concentration of ≤10%. SR140333 was administered by i.p. injection at a dose of 1 µmol per animal (Seegers et al., 2003).
Tissue collection and preparation
Twenty-four hours after intra-articular injection, rats were killed by asphyxiation in carbon dioxide, and the right and left knee synovia with patellae were immediately removed. Synovia for histomorphometry were embedded in Tissue Tek™ frozen in melting isopentane onto cork block mounts and stored at −80°C until use. Synovia for RNA extraction were collected in RNA Later™ (Ambion Inc., Austin, TX, USA) and stored at −20°C before analysis.
Staining procedures
Double sequential immunohistochemistry was used to identify endothelial proliferation using previously published methods (Walsh et al., 1998; Seegers et al., 2003; 2004). Briefly, sections (5 µm) were first immunostained for PCNA using monoclonal antibody clone PC10 and then for endothelium using a monoclonal antibody directed against CD31. PC10 in this setting gives comparable results to 5-bromo-2′-deoxyuridine (BrdU) (Seegers et al., 2004) but does not require tissue fixation, allowing other markers such as ED1 (see below) to be localized in the same tissues. Nuclei were counterstained by immersion of sections in the fluorescent DNA ligand, 4′-6′-diamidino-2-phenylindole hydrochloride (DAPI). Macrophage infiltration was identified by immunoreactivity for the monoclonal antibody clone ED1 (Waseem and Lane, 1990; Male et al., 1995; Seegers et al., 2003; 2004). Proliferation markers and ED1 were developed with diaminobenzidine using the glucose oxidase/nickel-enhanced method (Walsh et al., 1998). Endothelium markers were developed using Fast Red according to manufacturer's instructions. Haematoxylin and eosin stains were performed on sections, after those used for immunohistochemistry, to assist in identification of tissue structures.
Human synovium
Protocols for the use of human tissues had local Research Ethics Committee approval (NNHA/420, NNHA/544, NNHA/673 and 05/Q2403/24). Human synovia were obtained, with informed consent, at total joint replacement surgery from patients fulfilling American College of Rheumatology criteria for rheumatoid arthritis (RA; n = 5) (Arnett et al., 1988) or osteoarthritis (OA; n = 12) (Altman et al., 1991) or from the knees of patients without known arthritis collected post mortem (PM; n = 7). OA samples were selected according to the extent of inflammation displayed on a haematoxylin and eosin stained index section from a formalin-fixed tissue block of adjacent synovium to that used for RNA extraction. Inflammation was graded as previously described (Haywood et al., 2003). Samples were graded as inflamed [grade 3 inflammation, OA(i)] or not inflamed [grade 0 inflammation, OA(n)]. Samples from patients with RA (median age 68; range 53–80 years; 2 male) were from knee (n = 2), hip (n = 2) or elbow (n = 1). OA(i) samples were from patients (median age 60; range 41–82 years; 4 male) undergoing arthroplasty of the knee (n = 5) or hip (n = 1), and OA(n) samples were from patients (median age 65; range 62–81 years; 5 male) undergoing arthroplasty of the knee (n = 3) or hip (n = 3). Human synovium samples were snap-frozen in liquid nitrogen then stored at −80°C until use.
Measurements made
Quantification was performed by an observer unaware of experimental details, using an Axioskop-50 microscope (Carl Zeiss Ltd., Welwyn Garden City, UK) with a 20× objective lens. Transmitted light and fluorescence images of the same field were each captured using a 3-CCD camera and analysed using a KS300 image analysis system (Imaging Associates Ltd., Abingdon, UK).
Synovium was delineated according to morphology and synovial area was measured. Within this synovial region, a mask of the endothelial area was created that included CD31-positive blood vessels. This endothelial mask was applied over corresponding images of PCNA-positive and DAPI-positive nuclei. Nuclei falling within endothelium were counted as PCNA-positive endothelial nuclei and total endothelial nuclei respectively. Endothelial PCNA index was defined as the percentage of endothelial nuclei positive for PCNA. Vascular density was defined as the percentage of synovial area immunoreactive for endothelium within the frame area measured. Macrophage fractional area was defined as the fraction of synovial area (from 0 to 1) that was ED1-positive. We examined four fields per section and one section per rat to give a minimum coefficient of variation and a standard error of ±12.5% of the mean for the endothelial PCNA index (Walsh et al., 1998) and the same for macrophage fractional area.
Knee diameters were measured (mm) with a digital electronic caliper (Mitutoyo UK Ltd., Andover, UK).
RT-PCR
Total RNA was extracted using Trizol reagent as per manufacturer's instructions. Briefly, synovia were homogenized and shaken with 110 µL chloroform. After centrifugation (12 000× g, 30 min) at 4°C, the upper, aqueous phase was eluted into 700 µL isopropanol and pelleted and washed in 75% ethanol and air-dried.
RNA was suspended in water and split into two halves for rt(+) and rt(–), and reverse transcription reactions similar to previously published methods were performed (Seegers et al., 2004).
A GAPDH PCR was performed on rt(+) and rt(–) samples from each preparation to determine successful RNA extraction and the quality of cDNA produced. Each sample's rt(+) GPADH PCR product was compared with its rt(–) product to ensure amplification of cDNA and not genomic DNA. The best-quality cDNA from each sample was then chosen for further analysis. The 50 µL PCR reaction was the same as previous reported, using 1 unit Taq polymerase, 40 nmol PCR Nucleotide Mix, 10 nmol each of sense and antisense GAPDH primers and an annealing temperature of 54°C for 40 cycles (McWilliams et al., 1998). Samples were then selected for further analysis for the human groups RA (n = 5), OA(i) (n = 6), OA(n) (n = 6) and PM (n = 7) and rat groups (n = 6 per group).
Forty cycles of PCR amplification was performed with an annealing temperature of 54°C for CRLR, RAMPs-1, -2, and -3, and adrenomedullin in a volume of 50 µL. The PCR reaction mix consisted of 1 unit of Amplitaq Gold in manufacturer's buffer with 1.5 mM MgCl2 (the human CRLR PCR used 2.5 mM MgCl2, and the rat RAMP-1 used 2.0 mM MgCl2), 40 nmol PCR Nucleotide Mix and 10 pmol sense and antisense primers (the rat CRLR and RAMP-1 PCRs used 100 pmol primers). Primer sequences for each reaction are shown in Tables 1 and 2. Each PCR reaction was characterized to ensure that no rt(–) PCR products appeared at similar molecular weights to the rt(+) expected products, and no PCR carry-over contamination was detected in any reaction performed during this study. Rat brain and DU145 (a gift from Dr Neil Cross, Academic Urology, University of Sheffield, UK) or HUVEC (a gift from Julie Corfield, Clinical Oncology, University of Nottingham, UK) cDNAs were used as positive controls for rat and human PCR reactions respectively. The PCR reactions were not tested for sensitivity. PCR products were separated on 2% (w/v) agarose/TBE gels and visualized using ethidium bromide.
Table 1.
Human PCR primer sequences and product sizes
| Human | Primer sequence (5′–3′) | Product (bp) |
|---|---|---|
| GAPDH sense | GGTGAAGGTCGGAGTCAACGGA | 240 (McWilliams et al., 1998) |
| GAPDH antisense | GAGGGATCTCGCTCCTGGAAGA | |
| hAdM sense | AAGAAGTGGAATAAGTGGGCT | 291 (Matsushita et al., 2004) |
| hAdM antisense | TGTGAACTGGTAGATCTGGT | |
| CRLR sense | ACCAGGCCTTAGTAGCCACA | 296 (Linscheid et al., 2005) |
| CRLR antisense | ACAAATTGGGCCATGGATAA | |
| RAMP1 sense | CTGCCAGGAGGCTAACTACG | 298 (Linscheid et al., 2005) |
| RAMP1 antisense | GACCACGATGAAGGGGTAGA | |
| RAMP2 sense | GGGGGACGGTGAAGAACTAT | 227 (Linscheid et al., 2005) |
| RAMP2 antisense | GTTGGCAAAGTGGATCTGGT | |
| RAMP3 sense | AACTTCTCCCGTTGCTGCT | 165 (Linscheid et al., 2005) |
| RAMP3 antisense | GACGGGTATAACGATCAGCG |
Table 2.
Rat PCR primer sequences and product sizes
| Rat | Primer sequence (5′–3′) | Product (bp) |
|---|---|---|
| Rat AdM sense | CTCGACACTTCCTCGCAGTT | 446 (Pan et al., 2005) |
| Rat AdM antisense | GCTGGAGCTGAGTGTGTCTG | |
| Rat CRLR sense | CAACTGCTGGATCAGCTCAG | 446 (Pan et al., 2005) |
| Rat CRLR antisense | CATCGCTGATTGTTGACACC | |
| Rat RAMP1 sense | GCTGCTGGCTCATCATCTCT | 402 (Pan et al., 2005) |
| Rat RAMP1 antisense | TACACGATGCCCTCTGTGC | |
| Rat RAMP2 sense | TGAGGACAGCCTTCTGTCA | 371 (Pan et al., 2005) |
| Rat RAMP2 antisense | CATCGCCGTCTTTACTCCTC | |
| Rat RAMP3 (CRLR-RAMP3-400 sense) | AAGGTGGACGTCTGGAAGTG | 352 (Pan et al., 2005) |
| Rat RAMP3 (CRLR-RAMP3-400 antisense) | CTGGGCGACATTCTTCTAGC |
Data analyisis
The optimum number of fields per section and sections per case were determined in previous experiments (Walsh et al., 1998). Six cases per group were required to have a 90% chance of detecting a 10% difference in macrophage fractional area and PCNA index. Data (means ± SD unless otherwise stated) are presented on six knees per experimental group and were log-transformed prior to statistical analysis by one-way anova with Bonferroni corrections using GraphPad Prism 4.0 (San Diego, CA, USA).
Materials
Trizol reagent, Supercript II, agarose was from Invitrogen (Paisley, UK). Isopropanol was from Fisher Scientific (Leicestershire, UK). Random hexamer primers, Taq polymerase (Go Taq™) and PCR nucleotide mix were from Promega (Southampton, UK). Amplitaq Gold was from Applera Biosciences (Warrington, UK). Primers were synthesized by Thermo Fisher (Ulm, Germany). Haematoxylin, eosin and Tissue Tek™ were from RA Lamb (Eastbourne, UK). Anti-PCNA antibody clone PC10 was from Dako (Ely, UK). Anti-CD31 (clone TLD-3A12) antibody was from AbD Serotec (Oxford, UK). Horse antimouse-biotinylated antibody was from Vector Labs (Peterborough, UK).
Results
Intra-articular CGRP-injection, synovial angiogenesis and inflammation
The purpose of this experiment was to determine if injection of exogenous CGRP could increase endothelial cell proliferation. Macrophages and joint swelling were monitored to see if CGRP-enhanced endothelial PCNA index was an indirect effect via induction of inflammation. There were no significant differences in joint swelling (t = 0.55, P > 0.05) between CGRP-treated knees [mean = 11.6 mm; 95% confidence interval (95% CI) 11.5–11.8 mm] or vehicle-treated knees (mean = 11.7 mm; 95% CI 11.5–11.8 mm). Macrophage fractional areas were also not significantly different (t = −1.2, P > 0.05) between CGRP-treated knees (mean = 3.19%; 95% CI 1.32–3.72%) and vehicle-treated knees (mean = 2.09%; 95% CI 1.26–3.51%).
Intra-articular injection of recombinant CGRP induced an increase over vehicle injection in endothelial PCNA index in rat synovia, which was significant at doses of 0.25 nmol (t = 4.1, P < 0.01) and 2.5 nmol (t = 17.3, P < 0.001) (Figure 1A). Figure 1B shows immunohistochemical staining for PCNA and an example of a negative control. Adrenomedullin (8 nmol) did not induce a significant change in endothelial PCNA index over vehicle (P > 0.05) [means 2.04 (95% CI1.68-2.60) and 2.11 (95% CI 1.42-3.12) respectively].
Figure 1.
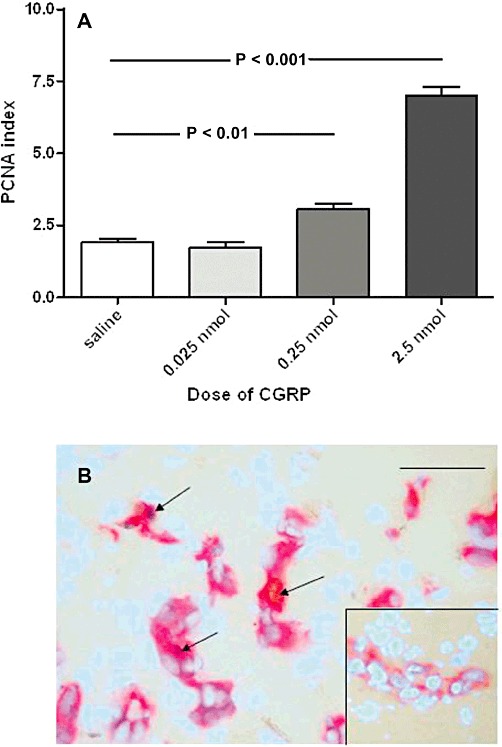
Intra-articular administration of exogenous CGRP causes a dose-dependent increase in the endothelial cell proliferation index in the synovium. (A). PCNA indices in saline-injected controls and 24 h after intra-articular injection of CGRP from 0.025 to 2.5 nmol per knee. The two higher doses of CGRP caused an increase in the PCNA index (P < 0.01 and P < 0.001, respectively) compared with saline-injected controls. Data shown are means ± SD; n = 6 rats per group. (B). Main panel: Synovium from a rat 24 h after intra-articular injection of 2.5 nmol CGRP, showing CD31-positive vascular cells and PCNA-positive proliferating nuclei. Counterstaining with DAPI shows all other nonstained nuclei. Arrows indicate PCNA-positive endothelial cells. This allows determination of the PCNA index as the percentage of vascular nuclei, which were positive for the proliferation marker. Inset shows a blood vessel from a control saline-injected synovium not displaying PCNA-positive endothelial cell nuclei. Bar = 100 µm.
To further investigate if exogenous CGRP administration was responsible for the increase in endothelial PCNA index, we employed the specific CGRP receptor antagonist BIBN4096BS. The increase in endothelial PCNA index in synovia that followed intra-articular injection of 2.5 nmol CGRP was reduced to a similar level to naïve knees after i.v. injection of 0.7 µmol per rat of the CGRP receptor antagonist BIBN4096BS (t = 10.2, P < 0.001 compared with CGRP alone). Administration of 0.07 µmol per rat BIBN4086BS also inhibited the CGRP-induced endothelial PCNA index (t = 5.4, P < 0.001; Figure 2.
Figure 2.
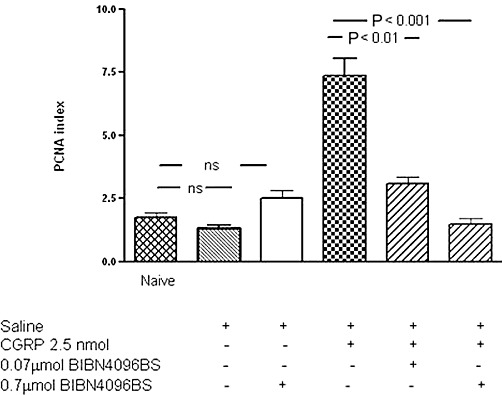
Blockade of the effect of exogenous intra-articular CGRP administration on the endothelial cell proliferation index using the specific CGRP receptor antagonist BIBN4096BS. Bar 1 shows the baseline PCNA index in naïve animals, negative control. Bar 2 shows the response to saline alone injection, which was not significantly different from naïve control. Bar 3 shows the PCNA index in animals intra-articularly injected with saline and i.v. injected with the CGRP receptor antagonist BIBN 4096BS. Intra-articular saline did not significantly increase the PCNA index, negative control. Bar 4 shows the effect of intra-articular administration of 2.5 nmol CGRP (positive control) on the PCNA index. The effect of CGRP on the PCNA index was diminished by the CGRP receptor antagonist BIBN406BS (bars 5 and 6) when compared with CGRP injection alone. Rats were given CGRP 2.5 nmol alone or together with BIBN406BS at either 0.07 µmol per rat (bar 5) 0.7 µmol per rat (bar 6). P < 0.001 for both treatments. Data shown are means ± SD; n = 6 rats per group.
Effects of BIBN4096BS and SR140333 on capsaicin-induced angiogenesis and inflammation
We used 0.5% capsaicin to release the endogenous neuropeptides SP and CGRP from storage granules in the sensory nerves of the synovium. Intra-articular injection of capsaicin (0.5%) was followed at 24 h by increased joint swelling and endothelial PCNA index within the synovium compared with controls The increased synovial angiogenesis was partially inhibited by the NK1 receptor antagonist SR140333, 1 µmol per rat (t = 11.0, P < 0.001), or by the CGRP receptor antagonist BIBN4096BS, 0.7 µmol per rat (t = 12.6, P < 0.001) (Figure 3A). Coadministration of BIBN4096BS and SR140333 at the same doses completely blocked capsaicin-induced angiogenesis in the rat synovium (t = 16.7, P < 0.001) (Figure 3A). This effect was greater than each antagonist alone; SR140333 (t = 5.2, P < 0.01) and BIBN 4096BS (t = 4.2, P < 0.001).
Figure 3.
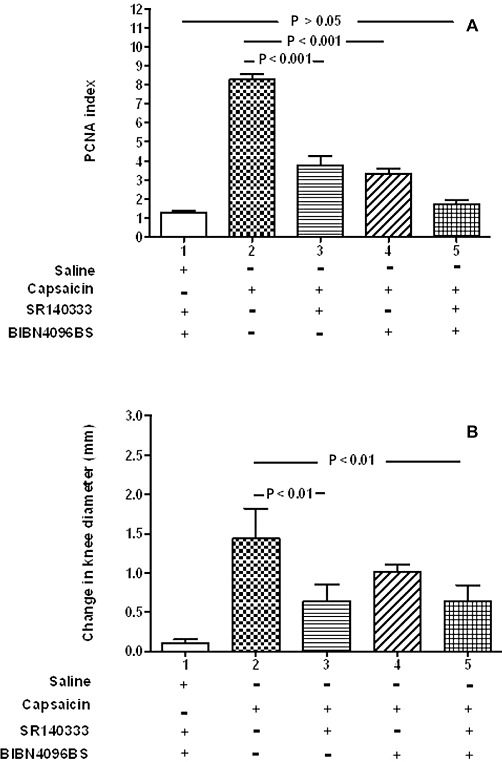
Capsaicin-induced angiogenesis was partially inhibited by the administration of each receptor antagonist, and coadministration of BIBN1096BS and SR140333 completely blocked capsaicin-enhanced angiogenesis. (A) Endothelial PCNA indices of rat synovia from knees injected with 0.5% capsaicin were significantly reduced by either the SP receptor antagonist SR140333 (bar 3) or by the CGRP receptor antagonist BIBN4096BS (bar 4) when compared with the positive control capsaicin alone, bar 2; P < 0.001 for both treatments. When given in combination, the two receptor antagonists (bar 5) reduced the rise in PCNA index attributable to capsaicin to the same level as saline controls; P > 0.05. PCNA index was defined as the percentage of endothelial cell nuclei positive for PCNA. Data shown are means ± SD; n = 6 rats per group. (B). Knee joint diameters corresponding to the same groups in (A), showing significant inhibition of joint swelling by SR140333, either alone (bar 2, P < 0.01) or in combination with BIBN4096BS (bar 5, P < 0.01). The change in knee diameter from baseline to 24 h is shown in mm, as means ± SD; n = 6 rats per group.. In each panel, the negative control (bar 1) represents data from synovia 24 h after intra-articular saline injection accompanied by i.v. injection of the CGRP receptor antagonist BIBN4096BS and i.p. injection of the SP receptor antagonist SR140333. The positive control (bar 2) represents data from synovia 24 h after intra-articular injection of capsaicin in the absence of either antagonist. Capsaicin is believed to release endogenous CGRP and SP from sensory nerves in the synovium. All groups of rats (bars 2–5), with the exception of saline controls (bar 1), were treated with capsaicin.
We monitored knee swelling and macrophage infiltration as measures of inflammation to investigate possible indirect effects of inflammatory mediators on the endothelial cells. Joint swelling 24 h after capsaicin injection was also significantly reduced by administration of SR140333 (t = 3.6, P < 0.01) and the coadministration of SR140333 and BIBN1096BS (t = 3.1, P < 0.01), but not by administration of BIBN4096BS alone (Figure 3B). Macrophage fractional area in the synovium was not statistically significantly different between the groups (Figure 4A). Immunocytochemical demonstration of macrophages is shown in capsaicin-treated animals (Figure 4B) and animals treated with both SR140333 and BIBN4096BS (Figure 4C); the quantity and distribution of positive cells appear similar.
Figure 4.
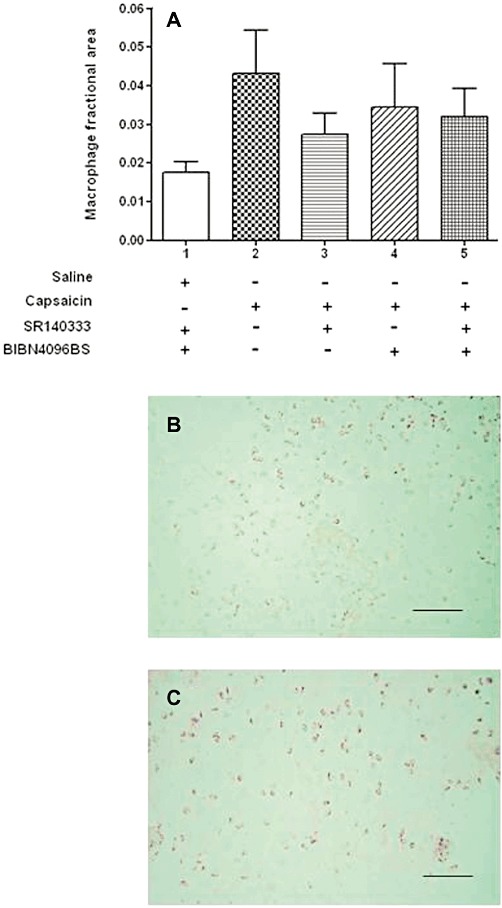
Detection of macrophages in the synovium. (A). Low levels of macrophage infiltration in all groups. There were no statistically significant differences in macrophage fractional areas for each group of rats, indicating that any small cellular inflammatory response present was below the detection threshold. Data shown are means ± SD; n = 6 rats per group Macrophages in the synovium were detected by immunohistochemistry using the primary antibody ED1 and were stained with nickel/DAB and appear as black. (B) Synovium from a capsaicin-treated animal. (C) Synovium from a saline-injected knee of an animal treated with the CGRP receptor antagonist BIBN4096BS i.v. and the SP receptor antagonist SR140333 i.p. The number of positively staining cells in each panel appears similar. When quantified, there were no statistically significant differences in the macrophage fractional area between any of the groups. Bar = 100 µm.
RT-PCR
In capsaicin-induced synovitis and naïve rat knees, mRNAs for adrenomedullin and RAMPs-1, -2 and -3 were detected in all samples. mRNA for CRLR was detected in synovia from three out of six capsaicin-injected and four out of six naïve knees (Figure 5A). In human synovia, RAMPs, CRLR and adrenomedullin were detectable in samples from all disease groups (Figure 5B,C).
Figure 5.
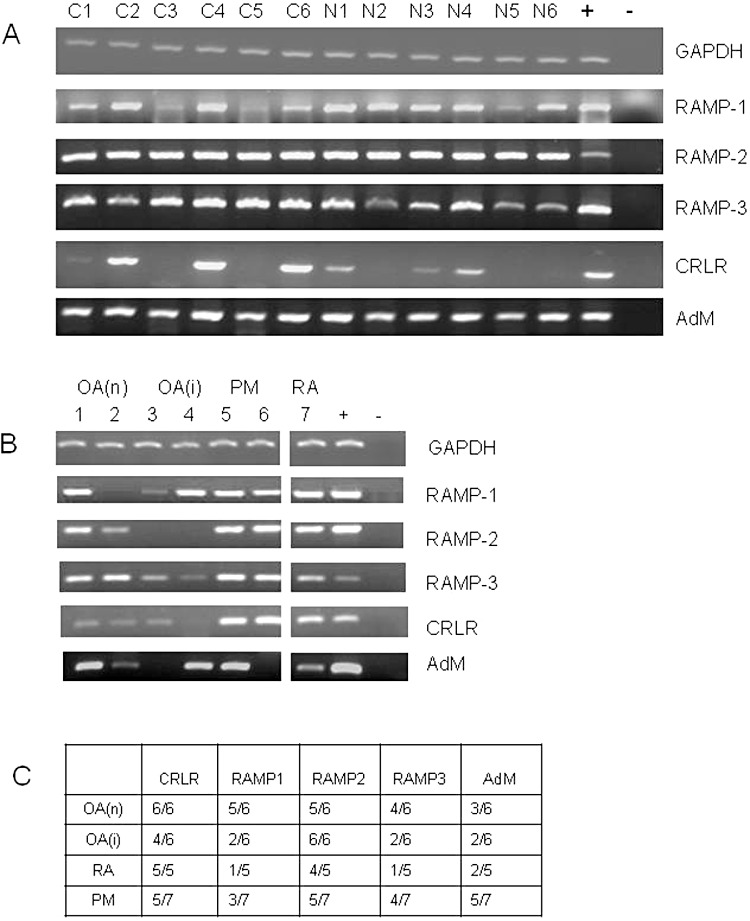
Components of the CGRP and adrenomedullin receptor-ligand complex are expressed in rat and human synovia. (A). Products of RT-PCR for GAPDH, adrenomedullin (AdM), CRLR, RAMP-1, RAMP-2 and RAMP-3 are shown after separation on agarose gels. Samples from synovia taken 24 h after intra-articular injection with capsaicin (C1–C6, lanes 1–6) and naïve knees (N1–N6, lanes 7–12) were analysed. Positive controls (+, lane 13) were rat brain cDNA. Negative controls (–, lane 14) were PCR's without any template DNA. (B). Examples of PCR products from RT-PCR on human synovia for CRLR, RAMP-1, RAMP-2, RAMP-3 and adrenomedullin are shown after separation on agarose gels. Samples from OA without inflammation (OA(n), lanes 1, 2), severely inflamed OA (OA(i), lanes 3, 4), normal post mortem (PM; lanes 5, 6) and RA (lane 7) are shown. Positive controls (+, lane 9) were HUVEC cells for CRLR and DU145 cells for RAMPs-1, -2 and -3. Negative controls (–, lane 10) were PCRs without any template DNA. (C). Table showing detection rates for PCR products in human synovia.
Discussion
We have previously shown (Seegers et al., 2003) that the angiogenesis induced by intra-articular injection of 0.5% capsaicin could be incompletely inhibited by an NK1 receptor antagonist, SR140333 (nolpitantium). Capsaicin stimulates the release of both SP and CGRP from storage granules in the peripheral terminals of fine unmyelinated sensory nerves (Mapp et al., 1996; Szallasi and Blumberg, 1999). SP can stimulate synovial angiogenesis (Seegers et al., 2003), and we now show that CGRP is also able to stimulate angiogenesis in the rat knee. Until relatively recently, CGRP receptor antagonists have been small peptides (e.g. CGRP 8–37) with short half-lives, which has made the contribution of CGRP to arthritis difficult to determine. Our study is one of the first in this field to employ a nonpeptide CGRP receptor antagonist BIBN4096BS. Inhibition of the angiogenic effect of CGRP by administration of BIBN4096BS indicates mediation by the CGRP receptor. Inhibition of capsaicin-induced endothelial cell proliferation by either the NK1 receptor antagonist SR140333, or the CGRP receptor antagonist BIBN4096BS suggests that both these neuropeptides mediate capsaicin-induced angiogenesis in the rat knee. Complete inhibition of capsaicin-induced endothelial cell proliferation by a combination of these two receptor antagonists suggests that neuropeptide release is a prerequisite for capsaicin-induced angiogenesis. This supports the concept of ‘neurogenic angiogenesis’.
Capsaicin (Min et al., 2004) and a related compound capsiate (Pyun et al., 2008) can inhibit angiogenesis both in vivo and in vitro. In vivo, this is true of test systems such as the Matrigel plug assay or the chick chorioallantoic membrane assay. Both of these test systems and in vitro tests do not contain sensory nerve fibres, which we believe to be the source of the angiogenic stimulus.
Capsaicin acts through the transient receptor potential vanilloid (TRPV1) receptor on the peripheral nerve. TRPV1 receptors have also been localized to mast cells and other cell types that may be present within the synovium (Stander et al., 2004), raising the possibility that there may be a small contribution of other TRPV1-expressing cells to the angiogenic effect. However, immunoreactivities for SP and CGRP are localized to sensory nerves in both human and rat synovia (Mapp et al., 1990; 1994), and blockade of capsaicin-induced angiogenesis by neuropeptide receptor antagonists suggests that this effect is due to neuropeptide release rather than actions on other cell types. The low levels of macrophage infiltration in the current study contrast with observations 24 h after intra-articular injection of carrageenan, when macrophage infiltration can reach levels of 20% of the synovial area (Walsh et al., 1998). Thus, whilst we cannot rule out the possibility of a contribution of low levels of inflammation to the endothelial cell proliferation induced by CGRP or capsaicin, direct effects of neuropeptides on their receptors appear to predominate.
Adrenomedullin is another angiogenic peptide that may interact with the CRLR receptor (Zhao et al., 1998). We detected mRNAs for adrenomedullin in rat and human synovia. Adrenomedullin has been shown to cause endothelial cell proliferation in culture at concentrations of 1 × 10−9 M (Miyashita et al., 2003) and may stimulate proliferation of vascular smooth muscle cells (Shichiri et al., 2003). However, we were unable to detect angiogenic or pro-inflammatory effects of recombinant adrenomedullin in the rat knee. We used the maximum economical dose of adrenomedullin (8 nmol per animal) and saw no response. The dose used was four times higher than the highest dose of CGRP that we used. There are a variety of reasons why adrenomedullin may have been ineffective perhaps through a lack of potency or synovial fluid protein/mucopolysaccharide binding. However, adrenomedullin in this context has served as a control showing that CGRP has not induced synovial angiogenesis through nonselective irritant properties. However, possible roles of adrenomedullin in synovitis, particularly through non-angiogenic pathways, deserve further study.
A neurogenic component to inflammation has been extensively described (see Walsh and McWilliams, 2006). Intra-articular injection of capsaicin induces synovitis, as indicated by joint swelling and infiltration of the synovium by inflammatory cells demonstrable at later time points (Mapp et al., 1996). NK1 receptor antagonists have been shown to attenuate carrageenan-induced plasma extravasation in the rat knee joint (Lam and Ferrell, 1991). The involvement of the nervous system in this model was demonstrated by prior denervation, which caused a 37% reduction in plasma extravasation, and pretreatment with 1% capsaicin, which produced a 44% decrease in plasma extravasation. Pretreatment of the knee with a peptide antagonist of SP, d-Pro4,d-Trp7,9,10-SP(4–11), resulted in a 93% reduction in plasma extravasation. SP stimulates plasma extravasation in the synovium, acting through the NK1 receptor (Lam and Ferrell, 1991). Our finding that the NK1 receptor antagonist SR401333 inhibited joint swelling after intra-articular capsaicin injection is consistent with this being a model of neurogenic inflammation.
CGRP is a potent vasodilator, although, consistent with previous reports (Buckley et al., 1991), the lack of joint swelling after intra-articular injection of CGRP in our study suggests that it does not on its own induce oedema formation. CGRP can potentiate IL-1α-induced oedema formation in the skin (Buckley et al., 1991) and also potentiates SP-induced oedema in the synovium (Cruwys et al., 1992). However, the lack of significant effect of BIBN4096BS on capsaicin-induced joint swelling suggests that CGRP is not a major facilitator of oedema 24 h after intra-articular injection of capsaicin.
This study, plus others (Seegers et al., 2003; 2004), shows that neuropeptides stimulate angiogenesis as well as synovitis. SP (Ziche et al., 1990) and CGRP (Haegerstrand et al., 1990, Zheng et al., 2010) can stimulate angiogenic activities in cultured endothelial cells. Administration of SP caused a concentration-dependent increase in proliferation of cultured human endothelial cells, with 0.1 nM SP giving the maximal response. An NK1 agonist induced similar proliferation, and two different NK1 antagonists blocked the response to SP. Administration of 10 nM CGRP to human endothelial cells in culture caused a rise in intracellular cAMP, a 40% increase in tritiated thymidine incorporation and a 50% increase in cell numbers, all as compared with controls, over a stimulation period of 24 h. More recently (Toda et al., 2008), CGRP has been shown to increase tube formation by endothelial cells in vitro and to enhance sponge-induced angiogenesis in vivo. Furthermore, growth of implanted tumours and tumour-associated angiogenesis were decreased in CGRP knockout mice compared with wild-type mice. The peptide CGRP 8–37, a CGRP receptor antagonist, was found to block tumour growth and tumour-associated angiogenesis in wild-type mice. Taken together, these data indicate that CGRP facilitates tumour-associated angiogenesis.
Increased synovial endothelial cell proliferation was detected early (24 h) after intra-articular injection of capsaicin, SP (Seegers et al., 2003), or CGRP. These data suggest induction of angiogenesis through direct actions of neuropeptides on the vasculature. Infiltration of the synovium and production of other angiogenic factors by macrophages may help maintain angiogenesis and synovitis at later time points in chronic synovitis, whereas neuropeptides may play key roles in the initiation of angiogenesis in acute synovitis. The current studies were designed to investigate neuropeptide effects on endothelial cell proliferation and further work, for example, studying later time points in models of chronic synovitis would be required to investigate possible consequences of neurogenic angiogenesis on persistent synovitis.
Human synovia, like rat synovia, express NK1 receptors for SP, localized to vascular endothelium (Walsh et al., 1992). Human and rat synovia have also been found to express CRLR (Uzan et al., 2006) or receptors for CGRP (McMurdo et al., 1997). We now show expression of both CRLR and RAMP1 mRNA in both human and rat synovia. The PCRs show widespread expression of CRLR/RAMPs across two species in diseased and nondiseased states. They support our hypothesis that CRLR/RAMP1 complexes contribute to the angiogenesis but do not rule out other receptors or CRLR/RAMP complexes. Although mRNA for CRLR and RAMPs were not detected in all samples, this may be due to limited sensitivity, or to samples containing variable proportions of synovial lining and sublining fatty tissues. Other studies of human joint tissue (Uzan et al., 2006) have failed to find mRNA for CRLR in all samples. In a study of cultured joint tissue from osteoarthritic and rheumatoid patients, CRLR mRNA was detected in five of five RA sample and two of four OA samples. Western blot analysis for the CRLR protein showed protein in two RA samples and a faint band in one of two OA samples tested. Although densitometric comparisons may show some differences in the levels of mRNA expression, for instance, in RAMP3 or adrenomedullin, further studies of larger numbers of cases using quantitative real-time PCR would be required to determine whether expression of these genes is associated with inflammation.
CRLR and RAMP1 combine to form specific cell surface receptors through which CGRP mediates its biological effects. BIBN4096BS is a competitive antagonist of CGRP at its receptor (Verheggen et al., 2002). Inhibition of synovial angiogenesis by BIBN4096BS therefore implicates CGRP and its receptor in blood vessel growth. The presence in human synovia of CGRP-immunoreactive nerves (Mapp et al., 1990) and mRNA for CGRP receptors raises the possibility that, as in rats, CGRP receptor antagonists may have potential to inhibit synovial angiogenesis in man. The presence of CGRP receptors, as well as CGRP-immunoreactive nerves (Mapp et al., 1990) in both normal and diseased synovium, indicates potential roles of neurogenic angiogenesis and inflammation in very early synovitis. In a broader context, the nervous system has been implicated in human arthritis. Neurological lesions such as poliomyelitis and strokes are protective of both RA and OA. The nervous system clearly regulates the classic signs of acute inflammation: calor, rubor, tumor and dolor. These signs can be induced in man by the i.d. injection of capsaicin (Shenker et al., 2008). Thus, the mechanisms for neurogenic inflammation are in place, but the overall contribution is not known.
In conclusion, our data indicate that CGRP-induced endothelial cell proliferation within the synovium was both dose-dependent and sensitive to a specific CGRP receptor antagonist. The data are consistent with the hypothesis that CGRP can stimulate angiogenesis in vivo, and that this is a direct effect mediated by CGRP receptors. Capsaicin-induced endothelial cell proliferation was completely blocked by coadministration of both NK1 and CGRP receptor antagonists, an indication that both peptides may contribute to angiogenesis in this model of synovitis. Initiation of angiogenesis by neuropeptides released from the peripheral terminals of sensory nerves (neurogenic angiogenesis) occurred in parallel to neurogenic inflammation. The contribution of neurogenic angiogenesis to human synovitis and the therapeutic potential of combined NK1 and CGRP-receptor antagonism deserve further study.
Acknowledgments
The authors are grateful to: Dr Henri Doods, Boehringer Ingelheim, for the provision of the CGRP receptor antagonist BIBN4096BS. Dr Xavier Emunds-Alt, Sanofi Aventis, for the provision of the SP receptor (NK1) antagonist SR140333. DF McWilliams and PI Mapp were supported by grants W584 and 17064, respectively, from Arthritis Research UK, Copeman House, St. Mary's Gate Chesterfield, UK.
Glossary
- BIBN4096BS
1-piperidinecarboxamide-N-[2-[[5-amino-1-[[4-(4-pyridinyl)-1-piperazinyl] carbonyl] pentyl]amino]-1-[(3,5dibromo-4-hydroxyphenyl) methyl]-2-oxyethyl]-4-(1,4-dihydro-2-oxo(2H)-quinazolinyl)-, [[R,(R*.S*)])
- BrdU
5-bromo-2′-deoxyuridine
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- CRLR
calcitonin receptor-like receptor
- DAPI
4′-6′-diamidino-2-phenylindole hydrochloride
- NK1
neurokinin 1
- RAMP
receptor activity modifying protein
- SP
substance P
- SR140333
1-[2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidi-n-3yl]-4-phenyl-1-azoniabicyclo[2.2.2] octane, chloride
- SR140603
R enantiomer of SR14033
Conflict of interest
The collection of human material used in this study was funded by Astra Zeneca U.K.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Ashraf S, Mapp PI, Walsh DA. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 2010;62:1890–1898. doi: 10.1002/art.27462. [DOI] [PubMed] [Google Scholar]
- Buckley TL, Brain SD, Collins PD, Williams TJ. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol. 1991;146:3424–3430. [PubMed] [Google Scholar]
- Cruwys SC, Kidd BL, Mapp PI, Walsh DA, Blake DR. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992;107:116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonds-Alt X, Doutremepuich JD, Heaulme M, Neliat G, Santucci V, Steinberg R, et al. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur J Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- Haegerstrand A, Dalsgaard CJ, Jonzon B, Larsson O, Nilsson J. Calcitonin gene-related peptide stimulates proliferation of human endothelial cells. Proc Natl Acad Sci U S A. 1990;87:3299–3303. doi: 10.1073/pnas.87.9.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Conner AC, Howitt SG, Takhshid MA, Simms J, Mahmoud K, et al. The pharmacology of CGRP-responsive receptors in cultured and transfected cells. Peptides. 2004;25:2019–2026. doi: 10.1016/j.peptides.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1- [(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2 H)-quinazolinyl) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors–the role of receptor activity modifying protein 1. Mol Pharmacol. 2006;70:1984–1991. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- Lam FY, Ferrell WR. Specific neurokinin receptors mediate plasma extravasation in the rat knee joint. Br J Pharmacol. 1991;103:1263–1267. doi: 10.1111/j.1476-5381.1991.tb12334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–2708. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. Effect of the tachykinin NK1 receptor antagonists, RP 67580 and SR 140333, on electrically-evoked substance P release from rat spinal cord. Br J Pharmacol. 1994;113:635–641. doi: 10.1111/j.1476-5381.1994.tb17037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D, Rahman J, Linke A, Zhao W, Hickey W. An interferon-inducible molecule on brain endothelium which controls lymphocyte adhesion mediated by integrins. Immunology. 1995;84:453–460. [PMC free article] [PubMed] [Google Scholar]
- Mallee JJ, Salvatore CA, Lebourdelles B, Oliver KR, Longmore J, Koblan KS, et al. Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem. 2002;277:14294–14298. doi: 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Kidd BL, Gibson SJ, Terry JM, Revell PA, Ibrahim NB, et al. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37:143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Walsh DA, Garrett NE, Kidd BL, Cruwys SC, Polak JM, et al. Effect of three animal models of inflammation on nerve fibres in the synovium. Ann Rheum Dis. 1994;53:240–246. doi: 10.1136/ard.53.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI, Kerslake S, Brain SD, Blake DR, Cambridge H. The effect of intra-articular capsaicin on nerve fibres within the synovium of the rat knee joint. J Chem Neuroanat. 1996;10:11–18. doi: 10.1016/0891-0618(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Matsui N, Yoshiya S, Fujioka H, Kurosaka M. Production of adrenomedullin from synovial cells in rheumatoid arthritis patients. Rheumatol Int. 2004;24:20–24. doi: 10.1007/s00296-003-0315-2. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- McMurdo L, Lockhart JC, Ferrell WR. Modulation of synovial blood flow by the calcitonin gene-related peptide (CGRP) receptor antagonist, CGRP(8-37) Br J Pharmacol. 1997;121:1075–1080. doi: 10.1038/sj.bjp.0701237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams DF, Watson SA, Crosbee DM, Michaeli D, Seth R. Coexpression of gastrin and gastrin receptors (CCK-B and delta CCK-B) in gastrointestinal tumour cell lines. Gut. 1998;42:795–798. doi: 10.1136/gut.42.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, et al. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004;64:644–651. doi: 10.1158/0008-5472.can-03-3250. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Sawada N, Fukunaga Y, Sone M, Yamahara K, et al. Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res. 2003;26(Suppl.):S93–S98. doi: 10.1291/hypres.26.s93. [DOI] [PubMed] [Google Scholar]
- Pan CS, Jiang W, Zhong GZ, Zhao J, Pang YZ, Tang CS, et al. Hypertension induced by nitric oxide synthase inhibitor increases responsiveness of ventricular myocardium and aorta of rat tissue to adrenomedullin stimulation in vitro. Life Sci. 2005;78:398–405. doi: 10.1016/j.lfs.2005.04.082. [DOI] [PubMed] [Google Scholar]
- Pyun BJ, Choi S, Lee Y, Kim TW, Min JK, Kim Y, et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008;68:227–235. doi: 10.1158/0008-5472.CAN-07-2799. [DOI] [PubMed] [Google Scholar]
- Recober A, Russo AF. Calcitonin gene-related peptide: an update on the biology. Curr Opin Neurol. 2009;22:241–246. doi: 10.1097/wco.0b013e32832b2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegers HC, Hood VC, Kidd BL, Cruwys SC, Walsh DA. Enhancement of angiogenesis by endogenous substance P release and neurokinin-1 receptors during neurogenic inflammation. J Pharmacol Exp Ther. 2003;306:8–12. doi: 10.1124/jpet.103.050013. [DOI] [PubMed] [Google Scholar]
- Seegers HC, Avery PS, McWilliams DF, Haywood L, Walsh DA. Combined effect of bradykinin B2 and neurokinin-1 receptor activation on endothelial cell proliferation in acute synovitis. FASEB J. 2004;18:762–764. doi: 10.1096/fj.03-0727fje. [DOI] [PubMed] [Google Scholar]
- Shenker NG, Haigh RC, Mapp PI, Harris N, Blake DR. Contralateral hyperalgesia and allodynia following intradermal capsaicin injection in man. Rheumatology (Oxford) 2008;47:1417–1421. doi: 10.1093/rheumatology/ken251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichiri M, Fukai N, Ozawa N, Iwasaki H, Hirata Y. Adrenomedullin is an autocrine/paracrine growth factor for rat vascular smooth muscle cells. Regul Pept. 2003;112:167–173. doi: 10.1016/s0167-0115(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Stander S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci U S A. 2008;105:13550–13555. doi: 10.1073/pnas.0800767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan B, Ea HK, Launay JM, Garel JM, Champy R, Cressent M, et al. A critical role for adrenomedullin-calcitonin receptor-like receptor in regulating rheumatoid fibroblast-like synoviocyte apoptosis. J Immunol. 2006;176:5548–5558. doi: 10.4049/jimmunol.176.9.5548. [DOI] [PubMed] [Google Scholar]
- Verheggen R, Bumann K, Kaumann AJ. BIBN4096BS is a potent competitive antagonist of the relaxant effects of alpha-CGRP on human temporal artery: comparison with CGRP(8-37) Br J Pharmacol. 2002;136:120–126. doi: 10.1038/sj.bjp.0704682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Conner AC, Poyner DR, Hay DL. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends Pharmacol Sci. 2010;31:476–483. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Walsh DA. Angiogenesis and arthritis. Rheumatology (Oxford) 1999;38:103–112. doi: 10.1093/rheumatology/38.2.103. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Haywood L. Angiogenesis: a therapeutic target in arthritis. Curr Opin Investig Drugs. 2001;2:1054–1063. [PubMed] [Google Scholar]
- Walsh DA, McWilliams DF. Tachykinins and the cardiovascular system. Curr Drug Targets. 2006;7:1031–1042. doi: 10.2174/138945006778019291. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Mapp PI, Wharton J, Rutherford RA, Kidd BL, Revell PA, et al. Localisation and characterisation of substance P binding to human synovial tissue in rheumatoid arthritis. Ann Rheum Dis. 1992;51:313–317. doi: 10.1136/ard.51.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Salmon M, Mapp PI, Wharton J, Garrett N, Blake DR, et al. Microvascular substance P binding to normal and inflamed rat and human synovium. J Pharmacol Exp Ther. 1993;267:951–960. [PubMed] [Google Scholar]
- Walsh DA, Rodway HA, Claxson A. Vascular turnover during carrageenan synovitis in the rat. Lab Invest. 1998;78:1513–1521. [PubMed] [Google Scholar]
- Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96((Pt 1)):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hague S, Manek S, Zhang L, Bicknell R, Rees MC. PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non estrogenic mechanism in the human endometrium. Oncogene. 1998;16:409–415. doi: 10.1038/sj.onc.1201768. [DOI] [PubMed] [Google Scholar]
- Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J, et al. Calcitonin gene-related peptide promotes angiogenesis via AMP-activated protein kinase. Am J Physiol Cell Physiol. 2010;299:C1485–C1492. doi: 10.1152/ajpcell.00173.2010. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–278. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]


