Abstract
BACKGROUND AND PURPOSE
The 5-ht1E receptor is highly expressed in the human brain and its structure is conserved in humans, suggesting an important physiological role for 5-ht1E receptors. However, neither the function nor the distribution of this receptor has been characterized in the mammalian brain.
EXPERIMENTAL APPROACH
Rats and mice lack the 5-ht1E receptor gene; thus, we used guinea pig brain tissue and immunofluorescent staining techniques to provide the first specific localization of 5-ht1E receptors in the mammalian brain.
KEY RESULTS
High levels of 5-ht1E receptors are detected in olfactory bulb glomeruli as well as the molecular layer of the dentate gyrus (DG). In DG membranes, BRL54443, a 5-ht1E/5-HT1F agonist, selectively stimulated 5-ht1E receptors and potently inhibited forskolin-dependent cAMP production (IC50= 14 nM). The staining pattern of 5-ht1E receptors in brain tissue suggests that this receptor is expressed predominantly in neurons rather than in glia. Additionally, 5-ht1E receptors were detected in the adventitial layer of cerebral arteries but not in the microvasculature, venous tissue or other brain arteries.
CONCLUSIONS AND IMPLICATIONS
These observations should help to predict clinical effects of 5-ht1E-selective drugs. For example, the stimulation of 5-ht1E receptors and subsequent inhibition of adenylate cyclase activity in the DG suggests that 5-ht1E receptors may mediate regulation of hippocampal activity by 5-HT, making it a possible drug target for the treatment of neuropsychiatric disorders characterized by memory deficits (such as Alzheimer's disease) or as a target for the treatment of temporal lobe epilepsy.
Keywords: 5-ht1E, 5-HT receptor, guinea pig, dentate gyrus, olfactory glomeruli, cerebral arteries, adenylate cyclase activity
Introduction
The 5-ht1E receptor is one of 13 human receptor types for the neurotransmitter 5-HT. The 5-ht1E receptor was discovered by virtue of its unique pharmacology through the use of [3H]5-HT radioligand binding methodology in human frontal cortical tissue (Leonhardt et al., 1989). At the time of its discovery, only three 5-HT receptor families were known: the 5-HT1 receptors (high affinity for [3H]5-HT and coupled to the inhibition of adenylate cyclase), 5-HT2 receptors (lower affinity for [3H]5-HT and coupled to the accumulation of inositol phosphates and Ca2+ mobilization) and the 5-HT3 receptor (a ligand-gated ion channel) (Teitler and Herrick-Davis, 1994; receptor nomenclature follows Alexander et al., 2011). This new [3H]5-HT binding site was sensitive to guanylyl nucleotides, indicating it was a GPCR, and thus not related to the 5-HT3 receptor. The receptor's relatively high affinity for [3H]5-HT and low affinity for 5-HT2 receptor ligands such as mesulergine strongly suggested that it was a member of the 5-HT1 family. However, it also lacked high affinity for 5-carboxamidotryptamine (5-CT), the prototypical 5-HT1 receptor agonist. Detailed pharmacological characterization of the new receptor in human and bovine cortical membrane homogenates clearly identified this binding site as a new member of the 5-HT1 family of receptors and was given the next available name: 5-ht1E (Leonhardt et al., 1989).
Soon after the 5-ht1E receptor was pharmacologically characterized in human brain tissue, a GPCR gene, originally termed S31, was cloned. When expressed in recombinant cells, the pharmacological properties of the receptor encoded by S31 were very similar to the tissue-expressed 5-ht1E receptor binding site, thus, it was concluded that this gene encodes the receptor discovered by Leonhardt et al. (McAllister et al., 1992; Zgombick et al., 1992). However, the 5-HT1F receptor, discovered after these early reports on the 5-ht1E receptor, has a pharmacological profile very similar to the 5-ht1E reporter (Adham et al., 1993; Lovenberg et al., 1993), and a careful examination of the binding data presented in the original report for the 5-ht1E receptor (Leonhardt et al., 1989) suggests the binding site characterized by this group and termed the 5-ht1E receptor was likely a composite of both 5-ht1E and 5-HT1F receptor binding sites. Drugs that can discriminate between these receptor populations were, unfortunately, not identified until after a number of studies were published that attempted to identify the distribution of 5-ht1E receptors via radioligand binding and autoradiographic methodologies (Miller and Teitler, 1992; Beer et al., 1993; Barone et al., 1994; Stanton et al., 1996; Fugelli et al., 1997). This resulted in reports that incorrectly attributed [3H]5-HT radioligand binding to the 5-ht1E receptor in both rat and mouse brain tissue, species that were later identified as lacking the 5-ht1E receptor gene (Bai et al., 2004). Even reports that utilized tissue from species that do express a 5-ht1E receptor gene (e.g. humans, monkeys, guinea pigs, cows) were confounded by the labelling of 5-HT1F receptors, and thus present data reflecting a mixture of 5-ht1E and 5-HT1F receptor properties. Ultimately, this has prevented a clear identification of the distribution of 5-ht1E receptors in the mammalian brain.
Herein, we report the first specific localization of 5-ht1E receptors in the brain. Rats and mice, the animal species typically used for preclinical pharmacology lack a 5-ht1E receptor homologue; fortunately, guinea pigs do possess a homologous 5-ht1E receptor gene, which encodes a protein nearly identical to the human receptor, both structurally (95% amino acid sequence homology) and pharmacologically (Bai et al., 2004). Radioligand binding determined that the pharmacological profile of 5-ht1E receptors expressed in the guinea pig brain is nearly indistinguishable from human 5-ht1E receptors (Klein and Teitler, 2009). This indicates that the guinea pig is an appropriate species to investigate 5-ht1E functionality. Radioligands selective for the 5-ht1E receptor which are also suitable for autoradiographic localization of the receptor are not currently available (M. Teitler, unpubl. obs.). Thus we have used immunofluorescent labelling techniques to localize the 5-ht1E receptor in various guinea pig brain regions.
The 5-ht1E receptor has also never been shown to be functionally linked to a second messenger system in animal tissue; 5-ht1E receptors have only been shown to couple to Gi/o signalling pathways in recombinant cell lines (Adham et al., 1994; Klein et al., 2011). BRL54443 is the only drug known to be partly selective for the 5-ht1E receptor; it is also equipotent at 5-HT1F receptors (Brown et al., 1998). This drug has been used in several recent reports (McKune and Watts, 2001; Watts et al., 2001; Granados-Soto et al., 2010) to allegedly stimulate 5-ht1E-mediated behavioural effects in rodents and evoke 5-ht1E receptor-mediated signal transduction in rodent tissue, despite the lack of a 5-ht1E receptor gene in any rodent genome. These and other reports utilizing BRL54443 have yet to demonstrate 5-ht1E-specific activity in tissue or in vivo. The observed effects mediated by BRL54443 are likely attributable to its action at 5-HT1F receptors or other 5-HT receptors. In this report, we provide the first evidence of 5-ht1E receptor function in animal tissue, demonstrating that 5-ht1E receptors couple to the inhibition of adenylate cyclase in guinea pig dentate gyrus (DG)/CA1 membranes. These results and the 5-ht1E receptor distribution presented here provide a basis for addressing the physiological relevance of this receptor and may help to open new therapeutic avenues for the treatment of diseases related to the psychiatric and neurological roles of 5-ht1E receptors in humans.
Methods
Cell culture
Recombinant cell lines were cultured that heterologously express GPCRs. CHO-K1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA, USA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). CHO-K1 cells stably expressing the human 5-ht1E receptor (donated by Scripps Research Molecular Screening Center, Jupiter, FL, USA) were cultured in DMEM, containing 10% FBS, 1% penicillin/streptomycin/neomycin (Invitrogen), 0.1 mM non-essential amino acids (NEAA;Invitrogen), 25 mM HEPES (Invitrogen) and 1 mg·mL−1 G418 (Invitrogen). LM(tk-) cells stably expressing the human 5-HT1F receptor (donated by Dr David Nelson, Lilly Research Laboratories, Indianapolis, IN, USA) were cultured in DMEM containing 10% FBS, 1% penicillin/streptomycin and 400 µg·mL−1 G418. Cells were cultured on poly-D-lysine coated glass coverslips to 95% confluence, then serum starved for 12 h, washed with PBS solution, fixed in 4% paraformaldehyde for 20 min and washed 3 × 3 min with PBS.
Animal tissue sectioning
Brains from adult Sprague Dawley rats and adult Hartley guinea pigs were purchased from Bioreclamation (Westbury, NY, USA), where animals were asphyxiated with CO2, decapitated, brains were harvested, meninges were left intact and brains were shipped in ice-cold PBS. Brains were drop-fixed in ice-cold 4% paraformaldehyde (Invitrogen) in PBS containing 1% sucrose (pH 7.4) for 2 h. Brains were blocked into 3 mm thick coronal sections, then drop fixed for another 2 h. Sections were washed 3 × 10 min with PBS and incubated overnight in PBS containing 30% sucrose. Brain sections were frozen in OCT compound (Tissue-Tek, Sakura Finetek, Alphen aan den Rijn, the Netherlands), processed in a −20°C cryostat (Microm, Walldorf, Germany) and 16 µm thick coronal sections were mounted onto gelatin coated slides. Slides were stored in sealed containers at −80°C.
Immunostaining
Tissue slices mounted on slides were allowed to come to room temperature. Tissue slices or cultured cells were permeabilized with PBS containing 0.3% Triton-X 100 for 10 min, then blocking buffer (10% normalized donkey serum (NDS) and 0.3% Triton-X 100 in PBS, pH 7.4) was added and incubated for 30 min. Primary antibodies were diluted in blocking buffer: mouse anti-h5-ht1E (1:100, Abnova, cat# H00003354-M03, Taipei City, Taiwan), rabbit anti- glial fibrillary acidic protein (GFAP) (1:300, Millipore, cat#AB5804, Billerica, MA, USA), rabbit anti-MAP2 (1:200, Cell Signaling, cat#4542, Danvers, MA, USA) and/or rabbit anti- von Willebrand factor (vWF) (1:100, Invitrogen, cat#F3520). Slides were incubated with primary antibody for 20 h at 4°C, and then washed 3 × 15 min with PBS at room temperature. Secondary antibodies and nuclear stain (4′,6-diamidino-2-phenylindole; DAPI) were diluted in blocking buffer: Cy3-conjugated donkey anti-mouse (1:100, Millipore, cat#AP192C), Alexa488-conjugated donkey anti-rabbit (1:200, Invitrogen, cat#A21206) and DAPI nuclear stain (1:500, Invitrogen, cat#D3571). Slides were incubated with secondary antibody and DAPI for 2 h at room temperature, and then washed 3 × 15 min with PBS at room temperature. Cover-slips were applied with a 1:9 solution of PBS to glycerol, containing 0.05% NaN3.
Imaging
Slides were visualized on an Olympus Optical Provis AX70 microscope and fluorescent images were captured by a Sony DKC-ST5 camera interfaced with Northern Eclipse 6.0 (Empix Imaging, Mississauga, ON, Canada). Full colour images were captured for Cy3 (ex: 528–553 nm, em: 590–650 nm), Alexa488 (em: 460–500, em: 510–560) and DAPI (ex: 375–400, em:450–475), and colour channels were isolated: Cy3 (red), Alexa488 (green) and DAPI (green, pseudo-coloured blue). Channels were merged and contrast adjusted using Adobe Photoshop CS5. Images from corresponding guinea pig and rat brain regions were processed identically.
Western blots
Whole guinea pig or rat brains were homogenized with a Polytron homogenizer (Kinematica, Lucerne, Switzerland) on setting 5 for 5 s in 12 mL·mg−1 ice-cold cell lysis buffer: 50 mM Tris, 1 mM EDTA, 1× complete protease inhibitors, pH 7.4. Homogenates were centrifuged at 21 000× g for 5 min at 4°C. The pellet was resuspended in ice-cold cell solubilization buffer (cell lysis buffer containing 150 nM NaCl and 1% SDS) and sonicated for 30 s. Lysates were centrifuged at 1500× g for 5 min at 4°C; the supernatant was collected and centrifuged at 21 000× g for 30 min at 4°C. The pellet was resuspended in cell solubilization buffer, incubated on ice for 60 min, centrifuged once more at 21 000× g for 30 min at 4°C and the supernatant was collected. Protein was quantified by the bicinchoninic acid (BCA) method (Pierce, Thermo Scientific, Waltham, MA, USA). Samples were loaded onto 4–20% Tris-HCl SDS-polyacrylamide gels (Bio-Rad) and separated by electrophoresis, then transferred to nitrocellulose membranes and blocked for 12 h with blocking buffer [10% non-fat dry milk in tris buffered saline solution with Tween20 (TBST), pH 7.4]. Primary antibody was diluted in blocking buffer [1:300 mouse anti-5-ht1E or 1:2000 rabbit anti-ERK2 (Santa Cruz Biotechnology, cat# SC-154, Santa Cruz, CA, USA)] and incubated with membranes for 1 h at room temperature, and then washed 3 × 5 min with TBST. HRP-conjugated secondary antibodies were diluted in blocking buffer and incubated with membranes for 1 h, and then washed 3 × 5 min with TBST. Staining was visualized by ECL (Pierce, Thermo Scientific) in a Fujifilm LAS imaging chamber. Images were processed using Adobe Photoshop CS5.
Radioligand binding
Fresh, frozen guinea pig and rat brains were thawed in tissue buffer (50 mM Tris-HCl, 0.5 mM EDTA, 10 mM MgS04, pH 7.7). Whole brains or isolated brain regions were homogenized with a polytron homogenizer on setting 5 for 5 s in 12 mL·mg−1 ice-cold tissue buffer. Homogenates were centrifuged at 14 000× g for 30 min at 4°C, pellets were homogenized and incubated at 37°C for 15 min, and then centrifuged and resuspended twice more, as described previously. Membrane homogenates (15 mg wet weight) were added to the radioligand binding assay mixture in a final volume of 1 ml; final concentrations: 50 mM Tris-HCl, 0.5 mM EDTA, 10 mM MgS04, 0.1% L-ascorbic acid, 10 µM pargyline, 5 nM [3H]5-HT (specific activity = 28.1 Ci mmol–1), pH 7.4. Drugs were present at appropriate concentrations to mask [3H]5-HT binding to non-5-ht1E receptors: 100 nM 5-CT, 100 nM LY344864 and 30 nM ritanserin (see Klein and Teitler, 2009). To assess the availability of 5-ht1E receptors, binding was performed in the absence and presence of the 5-ht1E/5-HT1F-selective agonist BRL54443. Tubes were incubated at 37°C for 40 min and membranes were collected by filtration through PEI-soaked glass fibre filter pads. Radioactivity was measured in a Beckman LS 1801 scintillation counter (Brea, CA, USA). DPM values were converted to fmol of [3H]5-HT. Protein was quantified by BCA method. Data were analysed using GraphPad Prism 5.0.
cAMP assay
Fresh, frozen guinea pig and rat brains were thawed in PBS then dissected. Hippocampi were isolated, and CA2-CA3 regions were dissected and discarded, leaving mainly DG and CA1. Tissue was homogenized in PBS (12 mL·mg−1 wet weight tissue) with a Polytron homogenizer on setting 5 for 5 s. Homogenates were centrifuged at 14 000× g for 30 min at 4°C; pellets were homogenized and centrifuged two more times. The final pellet was resuspended in LANCE stimulation buffer containing an ATP regeneration system: 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich, St. Louis, MO, USA), and 0.1% bovine serum albumin (PerkinElmer, Waltham, MA, USA), 10 mM MgCl2, 10 mM phosphocreatine (Sigma-Aldrich), 10 units·mL−1 creatine phosphokinase (Sigma-Aldrich), 50 µM GTP (Sigma-Aldrich), 200 µM ATP (Sigma-Aldrich), in PBS, pH 7.4. Gi/o-coupled receptor activity was measured by the inhibition of forskolin-stimulated adenylate cyclase activity using the LANCE cAMP Detection Kit (PerkinElmer), as previously described (Klein and Teitler, 2011) with modifications. In brief, membrane homogenates were pre-incubated with concentrations of agonist in LANCE stimulation buffer containing the ATP regeneration system for 10 min at 37°C. Membrane homogenates were exposed to 10 µM forskolin with a maintenance concentration of agonist in LANCE stimulation buffer containing the LANCE anti-cAMP antibody and ATP regeneration system. This mixture was incubated for 20 min at 37°C. The reaction was stopped by the addition of LANCE detection buffer (50 mM HEPES, 10 mM CaCl2 and 0.35% Triton X-100, pH 7.4.), and assay plates were incubated for 2 h at room temperature. Time-resolved fluorescence resonance energy transfer was detected by a Victor3 1420 multilabel plate reader (PerkinElmer). All experiments were performed in the presence of the 5-HT7-selective antagonist SB269970 (1 µM).
Data analysis
All radioligand binding and cAMP accumulation data are shown as the means ± SEM of three independent experiments performed in triplicate. Nonlinear regression analysis of concentration response data and all other statistical analyses, including one-way ANOVA with Dunnett's post tests and two-way ANOVA with Bonferroni pair-wise comparisons, were performed by GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 was taken as indicating significant differences between means.
Materials
5-CT, 5-HT, BRL54443, forskolin, L-ascorbic acid, pargyline, ritanserin, SB269970, and spiperone were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). LY344864 was purchased from Tocris Bioscience (Bristol, UK). [3H]5-HT (28.1 Ci mmol-1) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA, USA).
Results
No previous reports exist for immunolabelling 5-ht1E receptors, and thus the selectivity of available anti-5-ht1E antibodies remained unverified. We assessed the selectivity of several antibodies via Western blot analysis of guinea pig and rat whole brain lysates. Rats lack the 5-ht1E receptor gene, thus rat brains were used in this experiment and in subsequent experiments as a negative control for 5-ht1E receptor expression and immunoreactivity. Figure 1A demonstrates the expression of 5-ht1E receptors in guinea pig brain homogenates and the lack of expression in rat brain homogenates using radioligand binding methodology. Figure 1B shows the representative staining pattern of guinea pig and rat whole brain lysates with a mouse monoclonal antibody directed against intracellular loop 3 (IL3) of the human 5-ht1E receptor. Minimal background and non-specific staining is observed; only one prominent band is stained in guinea pig brain lysates. This band corresponds to the predicted molecular weight of the unmodified 5-ht1E receptor (43 kDa), and a band of similar size and intensity was not observed in rat whole brain lysates. All other anti-5-ht1E antibodies that were tested produced multiple non-specific bands, high background staining and/or prominent bands in rat whole brain lysates (data not shown). While Western blot analysis is useful for detecting non-selective labelling of proteins with molecular weights different from the 5-ht1E receptor, this methodology only confirms that this primary antibody can label the SDS-denatured 5-ht1E receptor protein. Thus, in Figure 2A, we demonstrate that the anti-5-ht1E antibody used in Figure 1B also stains human 5-ht1E receptors stably expressed in fixed, permeabilized CHO-K1 cells but does not label the parental (untransfected) CHO-K1 cell line (Figure 2B). This antibody failed to stain cells that express human 5-HT1F receptors (Figure 2C), a receptor that is closely related to the 5-ht1E receptor, sharing 42% and 43% amino acid sequence homology with IL3 of the 5-ht1E receptor (the epitope of the antibody) for human and guinea pig receptors respectively. This homology makes the 5-HT1F receptor one of the most likely off-target proteins to cross-react with the anti-5-ht1E antibody and provides a stringent test of the specificity of the antibody. Taken together, these data indicate that the mouse monoclonal anti-5-ht1E antibody is highly selective for the 5-ht1E receptor and should provide highly specific staining of 5-ht1E receptors in brain sections.
Figure 1.
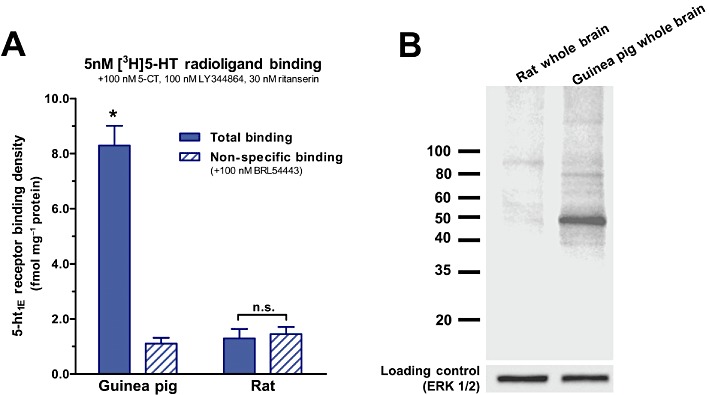
Specificity of the anti-5-ht1E antibody assessed by Western blot. (A) [3H]5-HT radioligand binding demonstrates that 5-ht1E receptors are expressed in guinea pig brain tissue (*P < 0.001, two-way anova with Bonferroni corrected pair-wise comparison) but not in rat brain tissue. Drugs are present at concentrations to mask [3H]5-HT binding to non-5-ht1E receptor sites (Klein and Teitler, 2009). Data are the means ± SEM of three independent experiments performed in triplicate. (B) Western blot analysis of guinea pig and rat whole brain lysates agrees with radioligand binding: the anti-5-ht1E antibody intensely stains a single ∼45 kDa band in guinea pig but not rat brain lysates. The predicted molecular weight of the 5-ht1E receptor is 43 kDa. The immunoblot is representative of three independent experiments.
Figure 2.
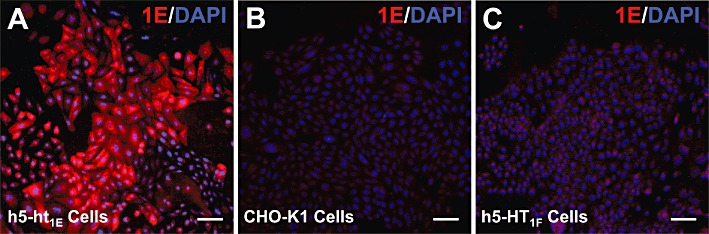
Specificity of the anti-5-ht1E antibody assessed in recombinant cell lines. Significant 5-ht1E staining is observed in paraformaldehyde-fixed and permeabilized CHO-K1 cells that stably express h5-ht1E receptors (A) but not in untransfected CHO-K1 cells (B) or in LM(tk-) cells that stably express h5-HT1F receptors (C). Scale bars = 10 µm. Images are representative of at least three independent experiments.
To guide our investigation of 5-ht1E distribution in the guinea pig brain, we first identified brain regions with high levels of 5-ht1E receptor expression. This was accomplished by radiolabelling 5-ht1E receptors in membrane homogenates prepared from various guinea pig brain regions with 5nM [3H]5-HT (Figure 3). 5-ht1E receptor densities in both the olfactory bulb and the hippocampus were approximately 2.5-fold greater than the average 5-ht1E receptor density of the whole guinea pig brain. The frontal cortex also displayed significant levels of 5-ht1E receptor expression that were comparable with the whole brain. We did not detect statistically significant levels of 5-ht1E receptors expressed in the other brain regions. Despite the lack of significant radioligand binding in these other brain regions, these data alone do not preclude the possibility of highly localized, punctate 5-ht1E receptor expression in various brain sub-regions, as radioligand binding methodology may not be sensitive enough to detect these receptors. These data indicate that the hippocampus and olfactory bulb contained substantial levels of 5-ht1E receptors and we therefore examined 5-ht1E receptor distribution in these regions first.
Figure 3.
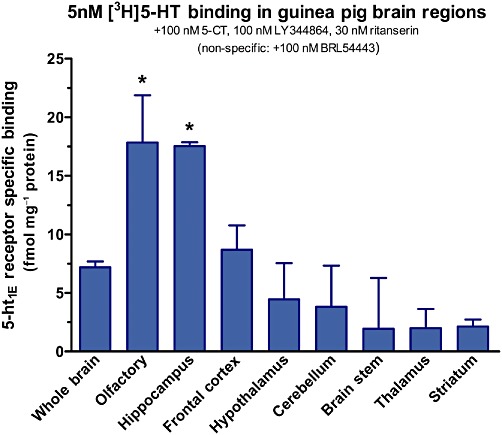
5-ht1E receptor densities in guinea pig brain regions. Membrane homogenates of guinea pig brain regions are probed for 5-ht1E receptor binding using conditions identical to Figure 1A. High levels of 5-ht1E receptors are detected in the hippocampus and olfactory bulb (*P < 0.05 compared with ‘Whole Brain’, one-way anova with Dunnett's post-test). Data are the means ± SEM of three independent experiments performed in triplicate.
Guinea pig olfactory bulbs were transversely sectioned and stained for 5-ht1E receptors, and in agreement with radioligand binding data, revealed the presence of high receptor expression levels (Figure 4A). 5-ht1E receptors appear to be localized to olfactory glomeruli (spheroid regions indicated by arrows, Figure 4B) with some staining in juxtaglomerular cells and more diffuse staining elsewhere in the olfactory bulb. Glomeruli are known to possess few cell bodies and glial projections are largely absent from glomeruli; this is evident in Figure 4C by the respective lack of DAPI and GFAP staining in the glomeruli. Thus, the identity of the cell types expressing 5-ht1E receptors within the olfactory glomeruli is limited to neuronal dendrites or terminals. Rat olfactory bulbs served as negative controls for 5-ht1E receptor staining and no significant staining was detected (Figure 4D).
Figure 4.
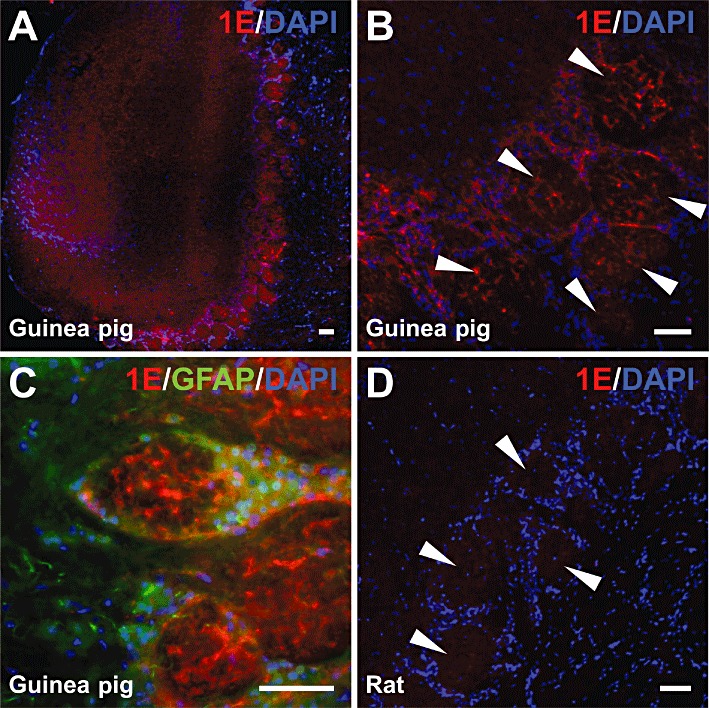
5-ht1E receptor staining in the olfactory bulb. (A) Low magnification image of 5-ht1E receptor staining in guinea pig olfactory bulb showing robust staining in glomerular layer cells; minimal staining is observed elsewhere. (B) 5-ht1E staining is largely confined to olfactory glomeruli (arrows). (C) High magnification image of 5-ht1E staining in guinea pig olfactory glomeruli; most 5-ht1E receptor staining is found within glomeruli with minimal staining observed in juxtaglomerular cells. (D) No 5-ht1E receptor staining is observed in rat olfactory bulb; rat olfactory glomeruli (arrows) are devoid of specific 5-ht1E staining. Scale bars = 50 µm. All images are representative of brain sections from at least three animals.
Consistent with the radioligand binding results, the hippocampal structure displayed one of the highest levels of 5-ht1E receptor staining in the guinea pig brain (Figure 5). Guinea pig coronal sections reveal the organization of the caudal hippocampus along the septal-temporal axis: 5-ht1E receptor staining is localized within the DG (Figure 5A) with little to no staining in the CA1 region (Figure 5B). Rostral hippocampal sections also displayed a lack of 5-ht1E staining in CA3-CA2 regions (Figure 5C). 5-ht1E staining was most intense in the middle third of the molecular layer of the DG in both the suprapyramidal and infrapyramidal blades (Figure 5A), with limited staining in the polymorphic layer and no staining in the granular layer. This expression pattern is generally consistent throughout the septal-temporal axis, evident in Figure 5B, which displays a coronal section near the temporal pole of the hippocampus. While staining of 5-ht1E receptors on individual dendritic or axonal processes cannot be discriminated, 5-ht1E receptor staining does not resemble glial staining and the focused localization of 5-ht1E expression in the DG molecular layer suggests that this receptor is expressed on neurons rather than glial cells. Co-staining for the astrocytic marker GFAP and the neuronal marker MAP2 could not clearly label either cell type in this tissue. Because the anti-5-ht1E receptor antibody was produced in mouse, only non-mouse-derived cell type markers could be used for co-staining: none of the tested non-mouse-derived anti-GFAP and anti-MAP2 antibodies produced clear and reproducible staining patterns in hippocampal sections (data not shown). Figure 5D displays the lack of 5-ht1E staining in rat DG coronal sections, and 5-ht1E staining was not observed in any other rat hippocampal sections; this supports the staining patterns observed in guinea pig hippocampal sections as specific to the 5-ht1E receptor.
Figure 5.
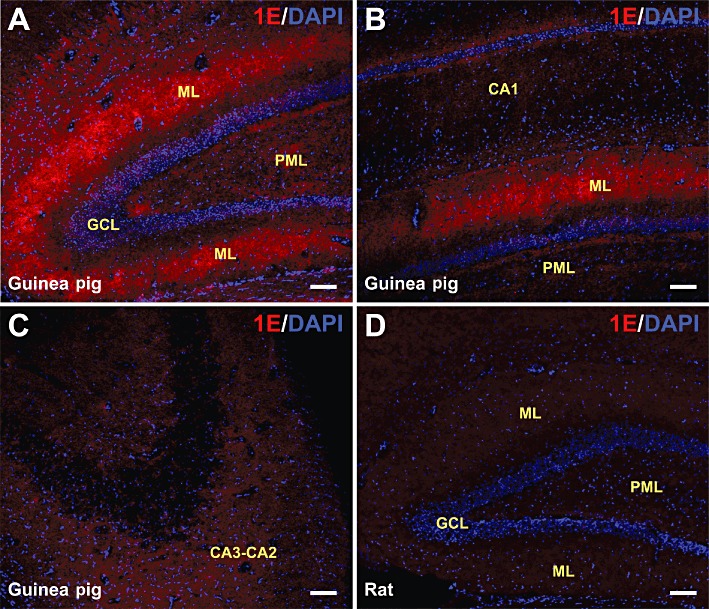
5-ht1E receptor staining in the hippocampus. (A) The septal pole of the DG from caudal guinea pig hippocampal coronal sections is shown. Significant 5-ht1E staining is observed in the molecular layer (ML) but not in the granular cell layer (GCL), and minimal 5-ht1E staining is found in the polymorphic layer (PML). (B) A region near the temporal pole of the DG from guinea pig hippocampal coronal sections is shown. No significant 5-ht1E receptor staining is found in the CA1 region; significant staining is present in the DG molecular layer. (C) Coronal section of rostral guinea pig hippocampus shows the lack of 5-ht1E receptor staining in the CA3-CA2 region. (D) Rat hippocampus (coronal section of DG septal pole shown) does not stain for 5-ht1E receptors. Scale bars = 100 µm. All images are representative of brain sections from at least three animals.
To confirm the expression of functional 5-ht1E receptors in guinea pig DG, we probed for Gi/o-mediated receptor activity in membrane homogenates prepared from this tissue. Guinea pig and rat hippocampi were isolated and the CA3-CA2 regions were dissected and discarded, leaving the DG and CA1 regions largely intact. The CA1 region, similar to the CA3-CA2 region, displayed a low level of 5-ht1E receptor expression but could not be easily dissected from the DG and membrane homogenates were prepared from a combination of DG and CA1, referred to as DG/CA1 membrane homogenates. Basal and 10 µM forskolin-stimulated cAMP production were respectively 3.90 ± 0.02 and 8.23 ± 1.61 pmol·mg−1 protein for guinea pig DG/CA1 membranes and 5.68 ± 0.89 and 10.03 ± 2.07 pmol·mg−1 protein for rat DG/CA1 membranes. Figure 6A demonstrates the ability of 1 µM 5-HT and 1 µM of the 5-ht1E/5-HT1F-selective agonist BRL54443 (Brown et al., 1998) to mediate the inhibition of forskolin-stimulated cAMP production in guinea pig DG/CA1 membranes. The 5-HT1F-selective agonist LY344864 (1 µM) (Phebus et al., 1997), having similar potency and efficacy at the 5-HT1F receptor as BRL54443, did not affect forskolin-mediated activity, demonstrating the lack of 5-HT1F receptor activity in guinea DG/CA1 membranes, a finding consistent with a previous report showing insignificant levels of 5-HT1F receptor expression in the guinea pig hippocampus (Lucaites et al., 2005). The lack of effect of LY344864 suggests that the inhibition of forskolin's activity by BRL54443 is not mediated by 5-HT1F receptors.
Figure 6.
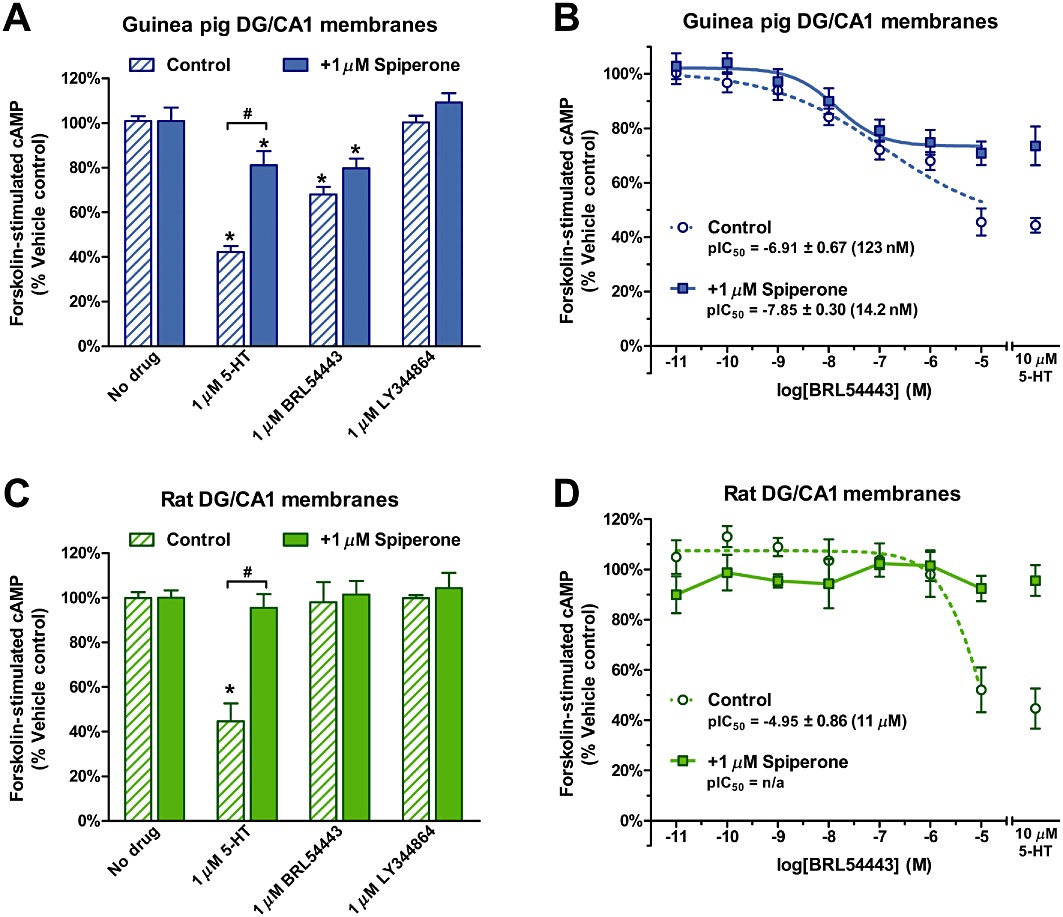
5-ht1E receptor activity in DG and CA1 (DG/CA1) membranes. (A) Guinea pig DG/CA1 membrane homogenates were stimulated with 10 µM forskolin subsequent to exposure to 1 µM 5-HT, 1 µM of the 5-ht1E/5-HT1F-selective agonist BRL54443, or 1 µM of the 5-HT1F-selective agonist LY344864 in the absence or presence of 1 µM spiperone. In the absence of spiperone, 5-HT and BRL54443 significantly inhibited forskolin-stimulated cAMP accumulation, but LY344864 had no effect.. *P < 0.01, significantly different from ‘No drug’, one-way anova with Dunnett's post-test. Spiperone (1 µM) partially inhibited 5-HT-mediated activity, but not BRL54443-mediated activity. #P < 0.001, two-way anova with Bonferroni corrected pair-wise comparison. (B) Concentration-response curves for BRL54443 in guinea pig DG/CA1 membranes. In the absence and presence of 1 µM spiperone, BRL54443 maximally inhibited forskolin-mediated activity by 54 ± 5% and 27 ± 3%, respectively, compared with 56 ± 3% and 26 ± 7% for 10 µM 5-HT. (C) Rat DG/CA1 membrane homogenates were stimulated with forskolin and agonists in the same manner as (A). In the absence of spiperone, only 5-HT significantly inhibited forskolin-stimulated cAMP accumulation. *P < 0.001, significantly different from ‘No drug’, one-way anova with Dunnett's post-test); BRL54443 and LY344864 had no effect. Spiperone (1 µM) completely inhibited 5-HT-mediated activity. #P < 0.001, two-way anova with Bonferroni corrected pair-wise comparison). (D) A partial BRL54443 concentration-response curve is observed in rat DG/CA1 membranes only in the absence of 1 µM spiperone. BRL54443 maximally inhibited forskolin-stimulated activity by 47 ± 9% in the absence of spiperone, compared with 55 ± 8% 10 µM 5-HT; this activity is abolished by 1 µM spiperone. All experiments were performed in the presence of 1 µM SB269970, a 5-HT7-selective antagonist. Data are the means ± SEM of three independent experiments performed in triplicate.
The 5-HT1A receptor is the predominant 5-HT receptor expressed in the hippocampus. To assess the contribution of 5-HT1A receptors to the activity of BRL54443, guinea pig DG/CA1 membranes were also exposed to 1 µM spiperone, an antagonist with low affinity for 5-ht1E receptors (Ki= 5051 nM, Zgombick et al., 1992) but much higher affinity for 5-HT1A receptors (Ki= 33 nM, Corradetti et al., 2005). Spiperone blocked much, but not all, of 5-HT-mediated inhibition of forskolin-stimulated cAMP, reducing 5-HT-mediated activity to levels comparable with those of 1 µM BRL54443. Spiperone did not significantly block 1 µM BRL54443 activity, suggesting that BRL54443's activity and the remaining 5-HT-mediated activity were likely mediated by 5-ht1E receptors. Full BRL54443 concentration-response curves were determined in the absence and presence of 1 µM spiperone (Figure 6B); BRL54443 inhibited forskolin-stimulated activity with a potency of 123 nM and 14.2 nM respectively. In the absence of spiperone, a Hill coefficient of 0.39 was observed, a value significantly less than unity (P < 0.005, F-test) and indicative of multiple receptors mediating the effect of BRL54443. In the presence of spiperone, BRL54443 inhibited forskolin activity with a potency consistent with BRL54443's high affinity for 5-ht1E receptors and produced a concentration-response curve with a Hill coefficient that did not significantly deviate from unity (P= 0.464, F-test). Taken together, these results indicate that, in the presence of 1 µM spiperone, BRL54443 selectively revealed the activity of 5-ht1E receptors, maximally inhibiting 27% of forskolin-stimulated cAMP accumulation in guinea pig DG/CA1 membranes. In comparison, 1 µM BRL54443 failed to stimulate the inhibition of forskolin activity in rat DG/CA1 membranes (Figure 6C) and only inhibited forskolin activity at the highest concentration (IC50 > 10 µM), which was blocked by 1 µM spiperone (Figure 6D), suggesting that only at high concentrations was BRL54443's activity mediated by non-5HT1E receptors. The lack of BRL54443-mediated activity in rat membranes was consistent with the lack of expression of 5-ht1E receptors in rat tissue and further supported the observation of BRL54443's activity in guinea pig DG/CA1 members as 5-ht1E specific.
Other regions of the guinea pig brain were examined for a 5-ht1E immunofluorescent signal. The guinea pig frontal cortex expressed significant levels of 5-ht1E receptor binding sites (see Figure 3) and 5-ht1E mRNA (Bai et al., 2004) but did not produce a substantial 5-ht1E receptor immunofluorescent signal (Figure 7A). Immunofluorescence was comparable with that of the striatum (Figure 7B), a brain region shown to have little 5-ht1E receptor binding (Figure 3), insignificant 5-ht1E receptor activity (data not shown) and insignificant 5-ht1E mRNA expression (Bai et al., 2004). Other cortical regions were examined, but no other regions produced reproducible and substantial 5-ht1E receptor staining. Likewise, rat brain sections showed no significant 5-ht1E receptor staining in frontal cortex (Figure 7C) and striatum (Figure 7D), consistent with the lack of 5-ht1E gene expression in this species.
Figure 7.
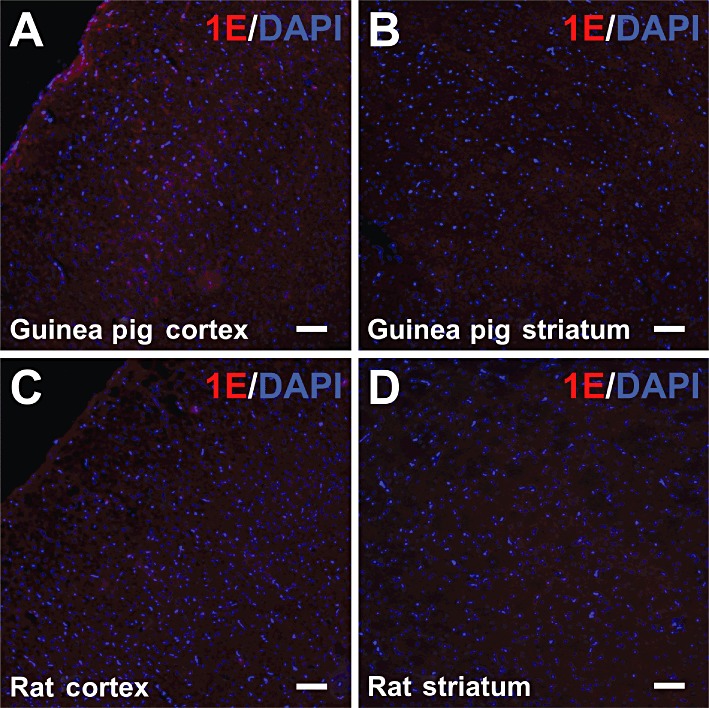
5-ht1E receptor staining in the frontal cortex and striatum. Significant 5-ht1E receptor staining was not observed in guinea pig frontal cortex (A) or striatum (B). Rat frontal cortex (C) and striatum (D) also lacked significant 5-ht1E receptor staining. Scale bars = 100 µm. All images are representative of brain sections from at least three animals.
Staining of 5-ht1E receptors was particularly intense in guinea pig cerebral arteries. The vasculature was identified by co-staining for von Willebrand factor (vWF), which labels the endothelium of major arteries, veins and the microvasculature. Figure 8A,B displays cross-sections of guinea pig anterior and posterior cerebral arteries respectively. 5-ht1E receptor staining was confined to the tunica adventitia (outermost layer of arteries composed of fibroblasts, perivascular nerve endings and connective tissue), with no significant staining found in the tunica intima (innermost endothelial layer that stains positive for vWF) or the tunica media (layer between the adventitia and intima composed of smooth muscles cells and elastic tissue). Interestingly, other guinea pig arteries, like the basilar artery (Figure 8C) and vertebral arteries (Figure 8D) did not display 5-ht1E receptor staining, nor did 5-ht1E receptor staining co-localize with the microvasculature and venous tissue of the guinea pig brain (data not shown). 5-ht1E receptor staining was not observed in any rat neurovasculature sections; this is exemplified by the lack of staining in rat anterior (Figure 8E) and posterior (Figure 8F) cerebral arteries. Lack of 5-ht1E receptor staining in rat neurovasculature supports the staining observed in guinea pig arteries as specific to the 5-ht1E receptor and suggests some role for 5-ht1E receptors unique to cerebral arteries.
Figure 8.
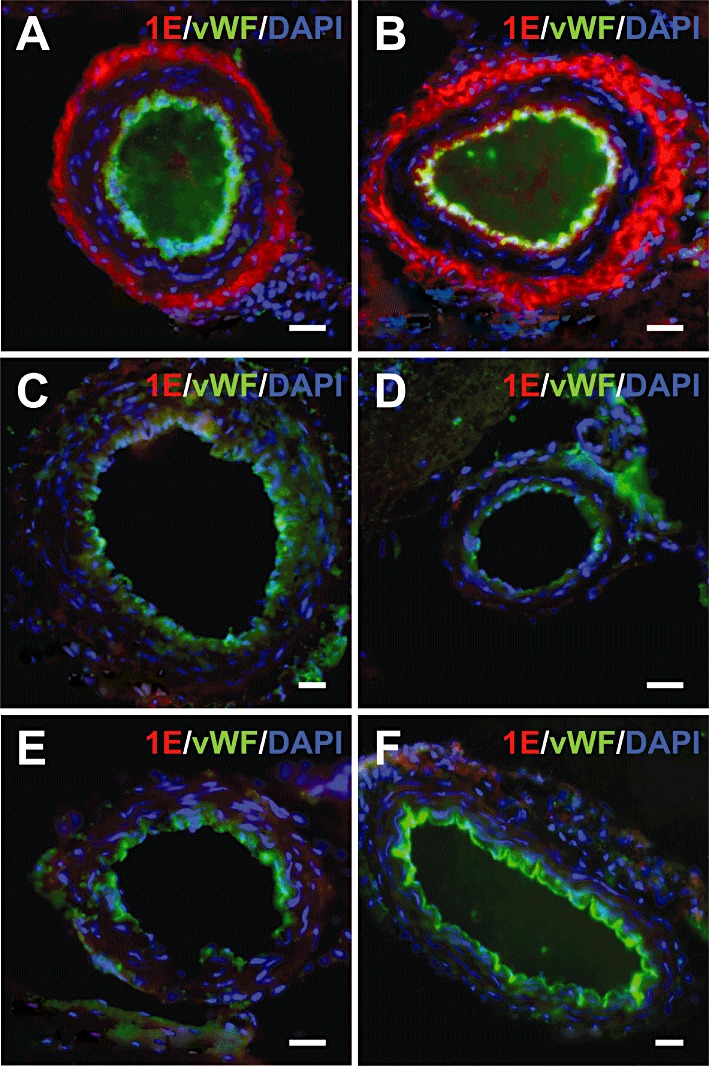
5-ht1E receptor staining in the neurovasculature. Robust 5-ht1E receptor staining is observed in the adventitial layer of guinea pig anterior (A) and posterior (B) cerebral arteries, whereas smooth muscle tissue and endothelial tissue lack 5-ht1E receptor staining. Other guinea pig arteries, including the basilar artery (C) and vertebral arteries (D), are devoid of 5-ht1E receptor staining. Rat arteries, including the anterior (E) and posterior (F) cerebral arteries, also lack significant 5-ht1E receptor staining. Scale bars = 25 µm. All images are representative of brain sections from at least three animals.
Discussion and conclusions
The immunofluorescent labelling of 5-ht1E receptors presented in this investigation represents the first specific localization of 5-ht1E receptors in the brain. Previous attempts to localize 5-ht1E receptors using autoradiographic techniques have been either unsuccessful (M. Teitler, pers. obs.), confounded by the labelling of 5-HT1F receptors (Miller and Teitler, 1992; Beer et al., 1993; Barone et al., 1994; Castro et al., 1997), or resulted in the erroneous labelling of 5-ht1E receptors in tissue from species that lack the 5-ht1E receptor gene (Stanton et al., 1996; Fugelli et al., 1997), leading aberrant or inaccurate assessments of 5-ht1E receptor distribution in the brain. Two previous studies attempted to shed light on the expression of 5-ht1E receptor mRNA in the non-rodent brain. Bai et al., (2004) isolated 5-ht1E receptor mRNA from gross guinea pig brain regions while Bruinvels et al., (1994) labelled 5-ht1E receptor mRNA via in situ hybridization in guinea pig and primate brain sections. However, the main focus of both studies was not on 5-ht1E mRNA expression, thus little information was presented on the subject. More importantly, neither of these reports could provide information about the expression of the receptor protein, thus making interpretations of 5-ht1E receptor function and distribution difficult. Some studies (Lowther et al., 1992; Klein and Teitler, 2009), including the original report of the 5-ht1E receptor (Leonhardt et al., 1989) and the current investigation have used radioligand binding methodology to identify 5-ht1E receptor binding in homogenates prepared from gross brain regions. Apart from those of Klein and Teitler,(2009), all previous reports used conditions that labelled both 5-ht1E and 5-HT1F receptors, yet when comparing these findings with results obtained in species that lack 5-ht1E receptors (i.e. rats and mice), it seems likely that substantial levels of 5-ht1E receptors are expressed in the frontal cortex, hippocampus and olfactory bulb of humans, non-human primates, and other non-rodent species. Unfortunately, radioligand binding methodology lacks the ability to visualize receptors in the intact anatomy of the brain and is prone to overlook small punctate areas of receptor expression, such as expression on brain blood vessels (see Figure 8).
We attempted to map 5-ht1E receptors in guinea pig brain sections autoradiographically, using [3H]5-HT, the only available radioligand for the 5-ht1E receptor (Kd≍ 6 nM), under conditions previously established to mask labelling of non-5-ht1E receptors (Klein and Teitler, 2009). Despite the ability to conduct 5-ht1E-selective [3H]5-HT radioligand binding in homogenized guinea pig brain membranes, these conditions produced autoradiograms with minimal specific binding (data not shown). Quantitative autoradiographic localization of 5-ht1E receptors will probably not be possible until higher affinity and/or higher energy radioligands become available for this receptor. Thus, immunolabelling techniques appear to be the only feasible means of determining the distribution of 5-ht1E receptors in the brain. After examining putative 5-ht1E receptor antibodies, we identified only one highly selective probe for this receptor: a mouse monoclonal antibody targeted against the IL3 of the human 5-ht1E receptor. With this antibody, we assessed the expression pattern of 5-ht1E receptors in guinea pig brain sections.
Significant expression of 5-ht1E receptors was detected in olfactory glomeruli (Figure 4), which suggests that this receptor may have a role in regulating sensitivity to odorants. The cyto-architecture of olfactory glomeruli is well studied, and the staining pattern observed for 5-ht1E receptors was not found to resemble olfactory receptor neuron axons and terminals (see Detje et al., 2009 for comparison), astrocytes, or periglomerular interneurons (see Petzold et al., 2009 for comparison). Rather, the staining pattern of 5-ht1E receptors, best evident in Figure 4C, resembles the staining of mitral/tuft cell dendrites (see Petzold et al., 2009 for comparison), and, if 5-ht1E receptors are coupled to Gi/o or other inhibitory signalling pathways in these cells, stimulation of this receptor could result in hyperpolarization and decreased signalling of these olfactory efferent neurons, thus reducing olfactory bulb output. The role of 5-ht1E receptors in the olfactory bulb might be pharmacologically assessed in guinea pigs by local administration of the 5-ht1E/5-HT1F-selective agonist BRL54443 and monitoring the animal's sensitivity to odorants or monitoring olfactory bulb activity, strategies similar to those used previously to study 5-HT receptor activity in the olfactory bulbs of rats (Petzold et al., 2008; 2009). The expression of 5-HT1F receptors is insignificant in the guinea pig olfactory bulb (Lucaites et al., 2005) and thus BRL54443 could be used as a relatively selective agonist for 5-ht1E receptors in the olfactory bulb.
The robust expression of 5-ht1E receptors detected in the DG (Figure 5) along with the functional link between these receptors and the inhibition of adenylate cyclase activity (Figure 6) represents a discovery with potentially significant therapeutic relevance. Hippocampal activity is associated with learning and memory (Scoville and Milner, 1957; see Morgado-Bernal, 2011) as well as alertness and mood (Campbell and Macqueen, 2004); and hyperactivity in the hippocampus has been linked to temporal lobe epilepsy (Chang and Lowenstein, 2003; Sloviter, 2005). The expression of 5-ht1E receptors in the DG may suggest an important role for this receptor in regulating these hippocampal functions. In general, the classical tri-synaptic circuit of the hippocampal structure is comprised of three interconnected regions: the DG, the CA3-CA2 region and the CA1 region. Afferent, excitatory projections from the enterorhinal cortex (the perforant pathway) cross the hippocampal fissure to synapse on the apical dendrites of granule cells in the external two thirds of the DG molecular layer (Amaral and Lavenex, 2007): the molecular layer of the DG is where 5-ht1E receptors are found to be most densely expressed. Being upstream of the CA regions, 5-ht1E receptor activity in the DG may influence overall hippocampal activity. Figure 6 demonstrates that 5-ht1E receptors mediate the inhibition of adenylate cyclase activity in DG membranes. Theoretically, stimulation of this receptor should decrease activity of perforant pathway-granule cell synapses through the inhibition of cAMP accumulation and subsequent decrease in the cAMP-dependent responses, such as PKA activity, CREB-mediated gene transcription and neurite outgrowth. If this is the case, the 5-ht1E receptor may function as an important negative modulator of long-term potentiation or promote long-term depression in the DG by this means. Hence, selective inhibitors of 5-ht1E receptors might counter this effect and enhance hippocampal activity, which could be an extremely useful modality in the treatment of clinical depression or mental disorders characterized by memory deficits, such as Alzheimer's disease. The hippocampus is one of the first regions of the brain that suffers damage in the pathogenesis of Alzheimer's disease (Burke and Barnes, 2006; Hampel et al., 2008), and none of the currently available therapeutics aimed at ameliorating the cognitive deficits associated with Alzheimer's disease focuses specifically on enhancing hippocampal activity (Blennow, 2011; Delrieu et al., 2011; Herrmann et al., 2011). If 5-ht1E receptors function as we speculate, selective 5-ht1E inhibitors might represent a new drug development avenue for the treatment of Alzheimer's disease. Conversely, 5-ht1E-selective agonists may be effective in the treatment of temporal lobe epilepsy by reducing hippocampal hyperexcitability. These hypotheses can be assessed in future electrophysiological studies using currently available pharmacological tools. The expression of 5-HT1F receptors is insignificant in the guinea pig hippocampus (Lucaites et al., 2005), and no 5-HT1F receptor activity was observed in the DG (Figure 6C). Thus, as shown in the olfactory bulb, the 5-ht1E/5-HT1F-selective agonist BRL54443 could be used to selectively assess the neurophysiological functions of the 5-ht1E receptor in the hippocampus. Ultimately, the identification of highly selective pharmacological tools will be needed to fully explore the functions of 5-ht1E receptors in the brain.
Apart from the olfactory bulb and hippocampus, the only tissue found to express 5-ht1E receptors at levels high enough to be detected were cerebral arteries (Figure 8A,B). This was an unexpected observation that suggests a role for 5-ht1E receptors in modulating vascular tone or other arterial events. The staining for 5-ht1E receptors appears exclusive to the adventitial layer, the outermost layer of arteries, populated by fibroblasts and containing perivascular nerve endings. This suggests that 5-ht1E receptors may function as autoreceptors on perivascular nerve terminals (Silberstein, 2004) or may be expressed on fibroblasts and indirectly regulate vascular tone via fibroblast signalling to the smooth muscle cells (Majesky et al., 2011). In this way, 5-ht1E receptors might play a role in the pathogenesis of migraine, and like the 5-HT1B, 5-HT1D and 5-HT1F receptors, may be a potential target for new anti-migraine drugs (Classey et al., 2010). If 5-ht1E receptors are involved in regulating vascular tone, migraine or other vascular events, it appears the role of 5-ht1E receptors would be limited to the cerebral arteries, as 5-ht1E receptors staining was not identified on other large calibre arteries (Figure 8C,D).
In summary, the identification of 5-ht1E receptors in the olfactory bulb glomeruli and the DG molecular layer suggests critical roles for the 5-ht1E receptors in the functions associated with these brain regions. Olfactory bulb activity is tightly associated with the limbic system, of which the hippocampus is a central part. This interconnectedness of brain regions expressing 5-ht1E receptors may reflect an evolutionary importance of the 5-ht1E receptor, and the lack of 5-ht1E receptors in rodent species (Bai et al., 2004) and high degree of conservation of the receptor's structure in humans (Shimron-Abarbanell et al., 1995) makes the question of this receptor's evolutionary importance all the more pertinent. Drugs that can modulate the activity of 5-ht1E receptors may have significant effects on limbic-associated brain functions; including memory and learning, and may represent a class of drugs with new and potentially powerful clinical benefits in the treatment of cognitive disorders and memory deficits.
Acknowledgments
This work was supported by PHS grant no. MH56650 (M.T.).
Glossary
- 5-CT
5-carboxamidotryptamine
- BCA
bicinchoninic acid
- BRL54443
5-hydroxy-3-(1-methylpiperidin-4-yl)-1H-indole
- CA
cornu ammonis
- CREB
cAMP response element binding
- DAPI
4′,6-diamidino-2-phenylindole
- DG
dentate gyrus
- DMEM
Dulbecco's modified Eagle's medium
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- GCL
granular cell layer
- GFAP
glial fibrillary acidic protein
- HRP
horseradish peroxidase
- IL
intracellular loop
- LY344864
N-[(3R)-3-(dimethylamino)-2,3,4,9-tetrahydro-1H-carbazol-6-yl]-4-fluorobenzamide hydrochloride
- MAP2
microtubule-associated protein 2
- ML
molecular layer
- NDS
normalized donkey serum
- NEAA
non-essential amino acids
- PML
polymorphic layer
- SB269970
(2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride
- TBST
tris buffered saline solution Tween20
- vWF
von Willebrand factor
Conflict of interest
None.
References
- Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci USA. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham N, Vaysse PJ, Weinshank RL, Branchek TA. The cloned human 5-HT1E receptor couples to inhibition and activation of adenylyl cyclase via two distinct pathways in transfected BS-C-1 cells. Neuropharmacology. 1994;33:403–410. doi: 10.1016/0028-3908(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Ch. 3 Hippocampal Neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- Bai F, Yin T, Johnstone EM, Su C, Varga G, Little SP, et al. Molecular cloning and pharmacological characterization of the guinea pig 5-HT1E receptor. Eur J Pharmacol. 2004;484:127–139. doi: 10.1016/j.ejphar.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Barone P, Jordan D, Atger F, Kopp N, Fillion G. Quantitative autoradiography of 5-HT1D and 5-HT1E binding sites labelled by [3H]5-HT, in frontal cortex and the hippocampal region of the human brain. Brain Res. 1994;638:85–94. doi: 10.1016/0006-8993(94)90636-x. [DOI] [PubMed] [Google Scholar]
- Beer MS, Stanton JA, Hawkins LM, Middlemiss DN. 5-Carboxamidotryptamine-insensitive 5-HT1-like receptors are concentrated in guinea pig but not rat, claustrum. Eur J Pharmacol. 1993;236:167–169. doi: 10.1016/0014-2999(93)90243-b. [DOI] [PubMed] [Google Scholar]
- Blennow K. Dementia in 2010: paving the way for Alzheimer disease drug development. Nat Rev Neurol. 2011;7:65–66. doi: 10.1038/nrneurol.2010.214. [DOI] [PubMed] [Google Scholar]
- Brown AM, Avenell K, Young TJ, Ho M, Porter RA, Vimal M, et al. BRL 54443, a potent agonist with selectivity for human cloned 5-ht1E and 5-ht1F receptors. Br J Pharmacol. 1998;123:233. [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, et al. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Castro ME, Romon T, Castillo MJ, del Olmo E, Pazos A, del Arco C. Identification and characterization of a new serotonergic recognition site with high affinity for 5-carboxamidotryptamine in mammalian brain. J Neurochem. 1997;69:2123–2131. doi: 10.1046/j.1471-4159.1997.69052123.x. [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res. 2010;1361:76–85. doi: 10.1016/j.brainres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Mlinar B, Falsini C, Pugliese AM, Cilia A, Destefani C, et al. Differential effects of the 5-hydroxytryptamine (5-HT)1A receptor inverse agonists Rec 27/0224 and Rec 27/0074 on electrophysiological responses to 5-HT1A receptor activation in rat dorsal raphe nucleus and hippocampus in vitro. J Pharmacol Exp Ther. 2005;315:109–117. doi: 10.1124/jpet.105.087809. [DOI] [PubMed] [Google Scholar]
- Delrieu J, Piau A, Caillaud C, Voisin T, Vellas B. Managing cognitive dysfunction through the continuum of Alzheimer's disease: role of pharmacotherapy. CNS Drugs. 2011;25:213–226. doi: 10.2165/11539810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, et al. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- Fugelli A, Moret C, Fillion G. Autoradiographic localization of 5-HT1E and 5-HT1F binding sites in rat brain: effect of serotonergic lesioning. J Recept Signal Transduct Res. 1997;17:631–645. doi: 10.3109/10799899709039154. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V, Arguelles CF, Rocha-Gonzalez HI, Godinez-Chaparro B, Flores-Murrieta FJ, Villalon CM. The role of peripheral 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E and 5-HT1F serotonergic receptors in the reduction of nociception in rats. Neuroscience. 2010;165:561–568. doi: 10.1016/j.neuroscience.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Li A, Lanctot K. Memantine in dementia: a review of the current evidence. Expert Opin Pharmacother. 2011;12:787–800. doi: 10.1517/14656566.2011.558006. [DOI] [PubMed] [Google Scholar]
- Klein MT, Teitler M. Guinea pig hippocampal 5-HTIE receptors: a tool for selective drug development. J Neurochem. 2009;109:268–274. doi: 10.1111/j.1471-4159.2009.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MT, Teitler M. Antagonist interaction with the human 5-HT(7) receptor mediates the rapid and potent inhibition of non-G-protein-stimulated adenylate cyclase activity: a novel GPCR effect. Br J Pharmacol. 2011;162:1843–1854. doi: 10.1111/j.1476-5381.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MT, Dukat M, Glennon RA, Teitler M. Toward selective drug development for the human 5-hydroxytryptamine 1E receptor: a comparison of 5-hydroxytryptamine 1E and 1F receptor structure-affinity relationships. J Pharmacol Exp Ther. 2011;337:860–867. doi: 10.1124/jpet.111.179606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt S, Herrick-Davis K, Titeler M. Detection of a novel serotonin receptor subtype (5-HT1E) in human brain: interaction with a GTP-binding protein. J Neurochem. 1989;53:465–471. doi: 10.1111/j.1471-4159.1989.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Erlander MG, Baron BM, Racke M, Slone AL, Siegel BW, et al. Molecular cloning and functional expression of 5-HT1E-like rat and human 5-hydroxytryptamine receptor genes. Proc Natl Acad Sci USA. 1993;90:2184–2188. doi: 10.1073/pnas.90.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther S, De Paermentier F, Crompton MR, Horton RW. The distribution of 5-HT1D and 5-HT1E binding sites in human brain. Eur J Pharmacol. 1992;222:137–142. doi: 10.1016/0014-2999(92)90473-h. [DOI] [PubMed] [Google Scholar]
- Lucaites VL, Krushinski JH, Schaus JM, Audia JE, Nelson DL. [3H]LY334370, a novel radioligand for the 5-HT1F receptor. II. Autoradiographic localization in rat, guinea pig, monkey and human brain. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:178–184. doi: 10.1007/s00210-005-1036-8. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G, Charlesworth A, Snodin C, Beer MS, Noble AJ, Middlemiss DN, et al. Molecular cloning of a serotonin receptor from human brain (5HT1E): a fifth 5HT1-like subtype. Proc Natl Acad Sci USA. 1992;89:5517–5521. doi: 10.1073/pnas.89.12.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKune CM, Watts SW. Characterization of the serotonin receptor mediating contraction in the mouse thoracic aorta and signal pathway coupling. J Pharmacol Exp Ther. 2001;297:88–95. [PubMed] [Google Scholar]
- Miller KJ, Teitler M. Quantitative autoradiography of 5-CT-sensitive (5-HT1D) and 5-CT-insensitive (5-HT1E) serotonin receptors in human brain. Neurosci Lett. 1992;136:223–226. doi: 10.1016/0304-3940(92)90054-b. [DOI] [PubMed] [Google Scholar]
- Morgado-Bernal I. Learning and memory consolidation: linking molecular and behavioral data. Neuroscience. 2011;176:12–19. doi: 10.1016/j.neuroscience.2010.12.056. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12:784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, et al. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 1997;61:2117–2126. doi: 10.1016/s0024-3205(97)00885-0. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimron-Abarbanell D, Nothen MM, Erdmann J, Propping P. Lack of genetically determined structural variants of the human serotonin-1E (5-HT1E) receptor protein points to its evolutionary conservation. Brain Res Mol Brain Res. 1995;29:387–390. doi: 10.1016/0169-328x(95)00003-b. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl. 2):2–7. doi: 10.1111/j.1468-2982.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328:143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Stanton JA, Middlemiss DN, Beer MS. Autoradiographic localization of 5-CT-insensitive 5-HT1-like recognition sites in guinea pig and rat brain. Neuropharmacology. 1996;35:223–229. doi: 10.1016/0028-3908(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Teitler M, Herrick-Davis K. Multiple serotonin receptor subtypes: molecular cloning and functional expression. Crit Rev Neurobiol. 1994;8:175–188. [PubMed] [Google Scholar]
- Watts SW, Yang P, Banes AK, Baez M. Activation of Erk mitogen-activated protein kinase proteins by vascular serotonin receptors. J Cardiovasc Pharmacol. 2001;38:539–551. doi: 10.1097/00005344-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Zgombick JM, Schechter LE, Macchi M, Hartig PR, Branchek TA, Weinshank RL. Human gene S31 encodes the pharmacologically defined serotonin 5-hydroxytryptamine1E receptor. Mol Pharmacol. 1992;42:180–185. [PubMed] [Google Scholar]


