Abstract
BACKGROUND AND PURPOSE
Bradykinin, through the kinin B2 receptor, is involved in inflammatory processes related to arthropathies. B2 receptor antagonists inhibited carrageenan-induced arthritis in rats in synergy with anti-inflammatory steroids. The mechanism(s) underlying this drug interaction was investigated.
EXPERIMENTAL APPROACH
Drugs inhibiting inflammatory mediators released by carrageenan were injected, alone or in combination, into the knee joint of pentobarbital anaesthetized rats 30 min before intra-articular administration of carrageenan. Their effects on the carrageenan-induced inflammatory responses (joint pain, oedema and neutrophil recruitment) and release of inflammatory mediators (prostaglandins, IL-1β, IL-6 and the chemokine GRO/CINC-1), were assessed after 6 h.
KEY RESULTS
The combination of fasitibant chloride (MEN16132) and dexamethasone was more effective than each drug administered alone in inhibiting knee joint inflammation and release of inflammatory mediators. Fasitibant chloride, MK571, atenolol, des-Arg9-[Leu8]-bradykinin (B2 receptor, leukotriene, catecholamine and B1 receptor antagonists, respectively) and dexketoprofen (COX inhibitor), reduced joint pain and, except for the latter, also diminished joint oedema. A combination of drugs inhibiting joint pain (fasitibant chloride, des-Arg9-[Leu8]-bradykinin, dexketoprofen, MK571 and atenolol) and oedema (fasitibant chloride, des-Arg9-[Leu8]-bradykinin, MK571 and atenolol) abolished the respective inflammatory response, producing inhibition comparable with that achieved with the combination of fasitibant chloride and dexamethasone. MK571 alone was able to block neutrophil recruitment.
CONCLUSIONS AND IMPLICATIONS
Bradykinin-mediated inflammatory responses to intra-articular carrageenan were not controlled by steroids, which were not capable of preventing bradykinin effects either by direct activation of the B2 receptor, or through the indirect effects mediated by release of eicosanoids and cytokines.
Keywords: carrageenan-induced arthritis, bradykinin, drug interaction, fasitibant chloride (MEN16132), B2 receptor antagonist, dexamethasone, anti-inflammatory steroids
Introduction
Bradykinin is a nonapeptide released in plasma and tissues through the cleavage of kininogen precursors by kallikrein enzymes and which selectively binds to the kinin B2 receptor (Leeb-Lundberg et al., 2005; receptor nomenclature follows Alexander et al., 2011).
The kallikrein system is activated in several arthropathies such as osteoarthritis, rheumatoid arthritis, gout and psoriatic arthritis (Rahman et al., 1995; Nishimura et al., 2002; Meini and Maggi, 2008). Bradykinin can contribute to inflammation and structural alterations in the arthritic joints, as its intra-articular administration in animals causes excitation and sensitization of sensory nerves, evoking pain and hyperalgesia, leukocyte recruitment, and an increase in vascular permeability and vasodilation, producing local heating and oedema (Cambridge and Brain, 1995; Lo et al., 1999; Pawlak et al., 2008). Accordingly, joint inflammation can be reduced by blocking bradykinin receptors, as already observed in arthritis models with carrageenan, Freund's adjuvant, monosodium iodoacetate, lipopolysaccharides and urate crystals (Damas and Remacle-Volon, 1992; Sharma and Wirth, 1996; Cialdai et al., 2009; Valenti et al., 2010). Bradykinin has also been involved in endothelial cell proliferation, cartilage matrix homeostasis and bone resorption, thus potentially affecting the synovial angiogenesis, cartilage destruction and subchondral bone remodelling, that characterize arthritic joints (Colman, 2006; Brechter and Lerner, 2007; Meini and Maggi, 2008).
Therefore, bradykinin antagonists could represent a promising alternative to the conventional therapies for treating joint arthritis, such as anti-inflammatory steroids and nonsteroidal anti-inflammatory drugs (NSAIDs). The initial clinical trials indicated that intra-articular administration of a kinin B2 receptor antagonist (icatibant) was very well tolerated and reduced pain at rest and during activity in patients affected by osteoarthritis, although no anti-inflammatory effect was demonstrated (Sorbera et al., 2006; Song et al., 2008). We have recently observed an interesting synergistic drug interaction between kinin B2 receptor antagonists and anti-inflammatory steroids in controlling inflammatory symptoms in carrageenan-induced arthritis models in rats (Valenti et al., 2010). Intra-articular fasitibant chloride (MEN16132), a selective nonpeptide kinin B2 receptor antagonist, or dexamethasone, given separately exerted maximal inhibition of about 50% of knee joint incapacitation, swelling and myeloperoxidase activity measured 6 h after carrageenan administration, whereas their combination abolished these symptoms and showed a greater inhibitory effect than the sum of each drug acting alone. A similar outcome was achieved by combining the peptide kinin B2 receptor antagonist, icatibant and dexamethasone, as well as fasitibant chloride and hydrocortisone (Valenti et al., 2010). Therefore, a beneficial interaction between the kinin B2 receptor antagonists and the anti-inflammatory steroids seems to be a general characteristic of this combination that could be used to selectively amplify the anti-inflammatory activity of very low doses of glucocorticoids, thus reducing their adverse side effects. This approach has also been implemented successfully with the combination of prednisolone and the antithrombotic drug dipyridamole in rat models of acute inflammation and arthritis (Zimmermann et al., 2009).
The aim of this study was to investigate the mechanism(s) underlying this drug interaction by using a range of pharmacological tools acting on different targets known to be involved in carrageenan-induced inflammation.
Methods
Animals
All animal care and experimental procedures were conducted in compliance with the principles and guidelines of the European Union (2010/63/UE) and the Italian government regulations, and approved by the ethical committee of Menarini Ricerche. Experiments were performed in male Wistar rats (Harlan Laboratories, Udine, Italy) weighing 250–300 g.
Induction of inflammatory arthritis
Under pentobarbital (40 mg·kg−1, i.p.) anaesthesia, rats received 25 µL of a sterile solution of drugs in saline solution (filtration through MillexGV 0.22 µm, Millipore, Billerica, MA, USA) via injection into the right knee joint and 25 µL of sterile saline solution into the contralateral (left) knee. Fasitibant chloride (100 µg per knee), dexamethasone (100 µg per knee) and dexketoprofen (300 µg per knee) were used at their maximally effective dose against the carrageenan-induced inflammatory responses in the rat knee joint (Valenti et al., 2010). Atenolol (50 µg per knee) and des-Arg9-[Leu8]-bradykinin (100 µg per knee) were administered at doses inhibiting the hyperalgesia evoked by intraplantar IL-8 in rats (Cunha et al., 1991) or by intra-articular carrageenan in rats (Tonussi and Ferreira, 1999) respectively. The dose selected for MK571 (100 µg per knee) was maximally effective in blocking the carrageenan-induced recruitment of neutrophils in the synovium of the rat knee. Thirty minutes after drug administration, 25 µL of 2% λ-carrageenan, previously dissolved in saline solution and autoclaved, were injected into the right knee, whereas the left knee of all animals and the right knee of the control group once again received 25 µL of sterile saline solution. After each injection, the knee was flexed and extended repeatedly to allow the diffusion of the drugs and carrageenan in the joint. Animals recovered from anaesthesia within 60–90 min.
In all the tests, the drugs were only administered in combination after each one had proven its efficacy in that particular test.
Incapacitance test, knee size measurement and sample collection
Carrageenan-induced pain and oedema were assessed by measuring the knee joint incapacitation and diameter, as described previously (Valenti et al., 2010). Briefly, the weight distribution on the two hind limbs of rats was recorded at 6 h after carrageenan administration, using the Incapacitance Tester MkV (Linton Instrumentation, Norfolk, UK). Rats were then anaesthetized with urethane, the diameter of the right knee measured with callipers, and the skin and patellar ligament removed to perform a synovial lavage via infusion of sterile saline solution into the joint cavity at a rate of 100 µL·min−1 through a peristaltic pump until 250–300 µL were collected. The synovial lavage fluid was centrifuged (10000×g for 5 min at 4°C) and the supernatant was collected for the assay of prostaglandins. Finally, the anterior side of the knee capsule was removed to assess the myeloperoxidase (MPO) activity and cytokine levels in the joint tissues. All samples were stored at −80°C until being tested.
MPO assay
The infiltration of neutrophils was evaluated by assessing the MPO activity in the knee joint capsule homogenates, as described previously (Valenti et al., 2010). The joint capsule was homogenized in cold lysis buffer (6 mM hexadecyltrimethylammonium chloride in 10 mM of citric acid/sodium citrate buffer, pH 5) and sonicated twice for 30 s. Homogenates were centrifuged at 25 000×g for 10 min at 4°C, and 75 µL of the supernatant, appropriately diluted in lysis buffer, were added to 75 µL of substrate solution (3 mM 3′,5,5′-tetramethylbenzidine, 120 µM resorcinol and 2.2 mM H2O2 in distilled water) in a 96-well plate. The change per minute of the absorbance at 620 nm in the linear range of the reaction kinetics was determined and the corresponding value of enzymatic activity in iu was obtained via interpolation with a standard curve (MPO from human leukocytes 0.02–5 iu·mL−1). Data were expressed as iu per mg of tissue (wet weight).
Prostaglandin assay in the synovial fluid
PGE2 is rapidly degraded in vivo; therefore we estimated its actual production through an assay of the prostaglandin E2 metabolites using an enzyme immunoassay kit according to the manufacturer's instructions (Cayman Chemical Company, Ann Arbor, MI, USA). Prostaglandin levels were expressed as total pg recovered in the synovial lavage fluid.
Cytokine (IL-1β, IL-6 and GRO/CINC-1) assay in the knee joint tissues
The joint capsule was dried by lyophilization, weighed and homogenized with a glass Dounce homogenizer in 1 mL of cold phosphate buffered saline solution containing 1% bovine serum albumin and 0.05% Tween 20. Homogenates were sonicated twice for 10 s, centrifuged (10 000× g, for 15 min at 4°C) and the supernatant collected. The levels of IL-1β, IL-6 and the chemokine GRO/CINC-1 (the rat orthologue of human IL-8) were assayed in the supernatant using an elisa kit according to the manufacturer's instructions (Immuno-Biological Laboratories, Gunma, Japan). Data for both IL-1β and GRO/CONC-1 (as IL-8) were expressed as pg per mg of tissue (dry weight), whereas data for IL-6 were expressed as arbitrary unit (AU, corresponding to the ED50 in a rat IL-6-dependent cell line growth test, as indicated in the assay kit) per mg of tissue.
Statistical analysis
The effect of carrageenan was compared with the control group response by the unpaired Student's t-test, whereas the differences with the drug-treated groups were analysed using the one-way anova followed by Dunnett's multiple comparison test. Differences were considered statistically significant at a level of P < 0.05.
Materials
Fasitibant chloride (MEN16132; 4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl}piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride) was synthesized at the Chemical Development Department of Menarini Ricerche in Pisa, Italy. Dexketoprofen was obtained from A. Menarini Manufacturing Logistics and Services, Florence, Italy. Des-Arg9-[Leu8]-bradykinin (Peninsula Laboratories Inc., San Carlos, CA, USA) and MK571 sodium salt (Calbiochem, San Diego, CA, USA) were purchased. Dexamethasone 21-phosphate disodium salt (dexamethasone), atenolol, λ-carrageenan and all the other reagents, if not specified, were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Results
Carrageenan-induced knee joint incapacitation
Carrageenan-treated rats only maintained 24.4 ± 1.2% (n= 12) of their weight on the inflamed (right) hind limb, shifting the remaining weight onto the contralateral leg, whereas rats of the control group evenly distributed their weight between both hind limbs.
Fasitibant chloride, dexamethasone (100 µg per knee) or dexketoprofen (300 µg per knee), reached a comparable maximal inhibitory effect of about 40–45% on the carrageenan-induced joint pain, whereas after treatment with the combination of fasitibant chloride and dexamethasone, full inhibition was achieved (Figure 1). The combination of fasitibant chloride and dexketoprofen, however, showed the same activity as the two drugs alone, with no signs of interaction between them (Figure 1).
Figure 1.
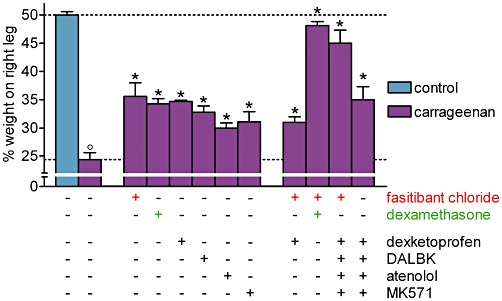
Carrageenan-induced knee joint incapacitation, 6 h after its intra-articular administration in rats. Right knee, 30 min before injection of saline (control group) or λ-carrageenan (2%, 25 µL), received, alone or in combination, fasitibant chloride (100 µg), dexamethasone (100 µg), dexketoprofen (300 µg), des-Arg9-[Leu8]-bradykinin (DALBK; 100 µg), atenolol (50 µg) or MK571 (100 µg). Data are the mean ± SEM of 6–12 rats. °P < 0.05, significantly different from the control group; unpaired Student's t-test and *P < 0.05, significantly different from the carrageenan-treated group; one-way anova followed by Dunnett's post-test. Dotted lines indicate the control value (upper) and the response after carrageenan treatment (lower).
Des-Arg9-[Leu8]-bradykinin (100 µg per knee), atenolol (50 µg per knee) and MK571 (100 µg per knee) were also partially effective against carrageenan-induced joint pain, indicating that in addition to bradykinin and prostaglandins, des-Arg9-BK, catecholamines and leukotrienes also contribute to the algesic effect of carrageenan (Figure 1).
Knee joint incapacitation was markedly reduced by the co-administration of fasitibant chloride, dexketoprofen, des-Arg9-[Leu8]-bradykinin, atenolol and MK571. The presence of the kinin B2 receptor antagonist in this cocktail of drugs was essential, as without fasitibant chloride, significantly less inhibition was observed.
Carrageenan-induced knee joint oedema
Intra-articular carrageenan increased the knee joint diameter and fasitibant chloride or dexamethasone (100 µg per knee) given separately inhibited this response by about 50% but abolished the increase when administered together (Figure 2).
Figure 2.
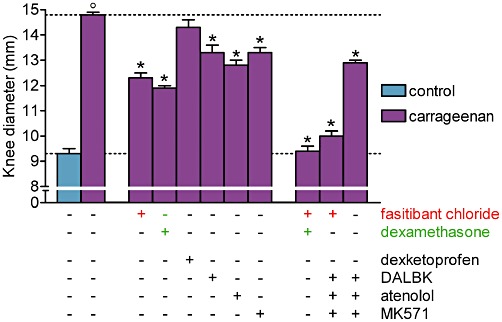
Carrageenan-induced knee joint oedema 6 h after its intra-articular administration in rats. Right knee, 30 min before injection of saline (control group) or λ-carrageenan (2%, 25 µL), received, alone or in combination, fasitibant chloride (100 µg), dexamethasone (100 µg), dexketoprofen (300 µg), des-Arg9-[Leu8]-bradykinin (DALBK; 100 µg), atenolol (50 µg) or MK571 (100 µg). Data are the mean ± SEM of 6–12 rats. °P < 0.05, significantly different from the control group; unpaired Student's t-test and *P < 0.05, significantly different from the carrageenan-treated group; one-way anova followed by Dunnett's post-test. Dotted lines indicate the control value (lower) and the response after carrageenan treatment (upper).
Des-Arg9-[Leu8]-bradykinin (100 µg per knee), atenolol (50 µg per knee) and MK571 (100 µg per knee) also exerted a partial but significant inhibitory effect, therefore bradykinin, des-Arg9-BK, catecholamines and leukotrienes all contribute to carrageenan-induced knee joint oedema, whereas dexketoprofen was ineffective as previously described (Valenti et al., 2010), indicating that prostaglandins are not involved in this inflammatory response (Figure 2).
The combination of fasitibant chloride, des-Arg9-[Leu8]-bradykinin, atenolol and MK571 almost achieved complete inhibition and this effect was significantly diminished by omitting fasitibant chloride from this combination of drugs (Figure 2).
Carrageenan-induced neutrophil infiltration in the synovium
Intra-articular carrageenan markedly increased the MPO activity, reflecting the infiltration of neutrophils, in the knee joint capsule homogenates (Figure 3). Given separately, either fasitibant chloride or dexamethasone (100 µg per knee) reduced the neutrophil infiltration in the synovium by about 60% and inhibited it more strongly when administered in combination (Figure 3) MK571 (100 µg per knee), given alone, exerted an almost full inhibitory effect, whereas dexketoprofen, des-Arg9-[Leu8]-bradykinin and atenolol had no effect on this response, indicating that the action of cysteinyl leukotrienes was essential for neutrophil infiltration induced by carrageenan (Figure 3).
Figure 3.
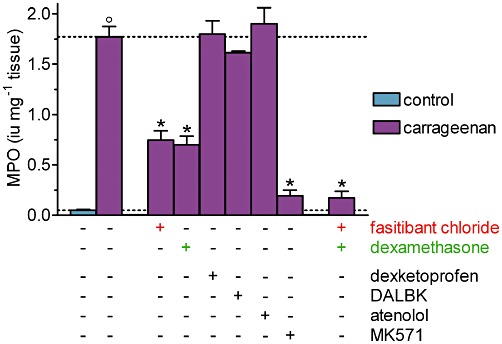
Carrageenan-induced neutrophil infiltration, measured as MPO activity in the synovial capsule homogenates, 6 h after its intra-articular administration in rats. Right knee, 30 min before injection of saline (control group) or λ-carrageenan (2%, 25 µL), received, alone or in combination, fasitibant chloride (100 µg), dexamethasone (100 µg), dexketoprofen (300 µg), des-Arg9-[Leu8]-bradykinin (DALBK; 100 µg), atenolol (50 µg) or MK571 (100 µg). Data are the mean ± SEM of 6–12 rats. °P < 0.05, significantly different from the control group; unpaired Student's t-test and *P < 0.05, significantly different from the carrageenan-treated group; one-way anova followed by Dunnett's post-test. Dotted lines indicate the control value (lower) and the response after carrageenan treatment (upper).
Carrageenan-induced release of prostaglandins in the synovial fluid
Intra-articular carrageenan increased the level of PGE metabolites in the synovial fluid by about 10-fold to 7.5 ± 0.9 pg (Figure 4). Fasitibant chloride and dexamethasone (100 µg per knee) reduced the release of prostaglandins by about 30%, when administered alone. When administered in combination, these two drugs achieved nearly the same inhibitory effect (about 80%) as that obtained with dexketoprofen alone (300 µg per knee) (Figure 4).
Figure 4.
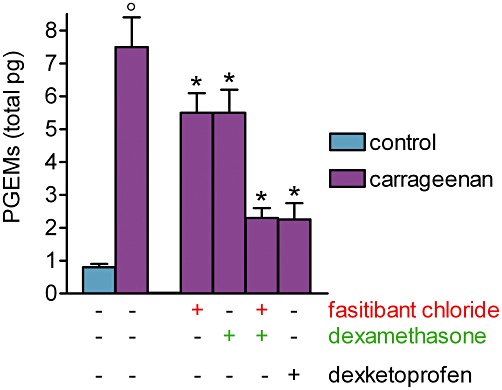
Carrageenan-induced prostaglandin release in the synovial fluid. Carrageenan (2%, 25 µL) or saline (control group) were injected into the right knee of rats. After 6 h, a joint lavage was performed by saline perfusion and synovial levels of prostaglandin E metabolites (PGEMs) were measured. Fasitibant chloride (100 µg), dexamethasone (100 µg) or dexketoprofen (300 µg) were given alone or in combination in the right knee, 30 min before carrageenan administration. Data are the mean ± SEM of 10 rats. °P < 0.05, significantly different from the control group; unpaired Student's t-test and *P < 0.05, significantly different from the carrageenan-treated group; one-way anova followed by Dunnett's post-test.
Carrageenan-induced release of cytokines in the knee joint tissues
The levels of IL-1β, IL-6 and GRO/CINC-1 (the rat orthologue of human IL-8) in the knee joint capsule were markedly increased following the carrageenan–treatment (Figure 5). Both fasitibant chloride and dexamethasone (100 µg per knee) were equipotent in inhibiting the release of IL-1β (Figure 5A), IL-6 (Figure 5B) and GRO/CINC-1 (Figure 5C). The release of IL-1β and GRO/CINC-1 were more sensitive than that of IL-6 to treatment with these drugs. The combination of fasitibant chloride and dexamethasone was more effective than the drugs administered alone, in reducing the levels of all three cytokines, IL-1β, IL-6 and GRO/CINC-1.
Figure 5.
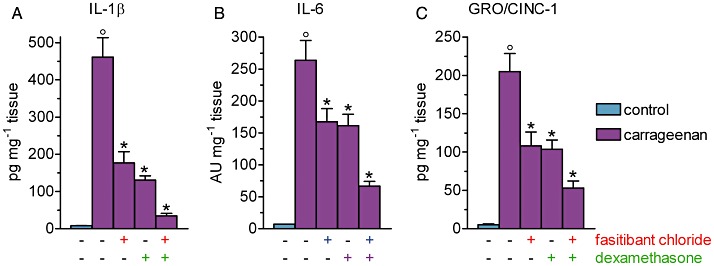
Carrageenan-induced cytokine release in the synovium. Carrageenan (2%, 25 µL) or saline (control group) were injected into the right knee of rats. After 6 h, the levels of IL-1β, IL-6 and GRO/CINC-1 (rat orthologue of human IL-8) were measured in the synovial capsule homogenates. Fasitibant chloride (100 µg) or dexamethasone (100 µg) were given alone or in combination in the right knee, 30 min before carrageenan administration. Data are the mean ± SEM of 10 rats. °P < 0.05, significantly different from the control group; unpaired Student's t-test and *P < 0.05, significantly different from the carrageenan-treated group; one-way anova followed by Dunnett's post-test).
Discussion and conclusions
The negatively charged macromolecular surface of carrageenan initiates the contact activation of plasma kallikrein, generating bradykinin, which exerts a pivotal role in eliciting knee joint inflammation (Ferreira et al., 1993). Bradykinin acts through stimulation of the kinin B2 receptor either directly (Cambridge and Brain, 1995; Cheng and Ji, 2008) or indirectly, releasing other mediators such as prostaglandins, leukotrienes, catecholamines and cytokines (Green et al., 1993; Sharma and Buchanan, 1994). Ferreira et al. (1993) reported that intra-articular carrageenan stimulates the bradykinin-mediated release of TNF-α, which in turn induces IL-1β, IL-6 and IL-8 production. However, carrageenan can also initiate this cascade of cytokines independently of bradykinin, if the inflammatory stimulus is of sufficient magnitude (Valenti et al., 2010). Both IL-1β and IL-6 evoke joint pain through the synthesis of prostaglandins, whereas the nociceptive effects of IL-8 are mainly mediated by the release of sympathetic amines (Cunha et al., 1992). Des-Arg9-bradykinin, deriving from the metabolic cleavage of bradykinin, is also involved in carrageenan-induced inflammation through stimulation of inducible kinin B1 receptors (Sharma and Buchanan, 1994).
Figure 6 summarizes the carrageenan-activated inflammatory cascade and illustrates the main mediators involved in the carrageenan-induced inflammatory response in the synovium of the rat knee joint as demonstrated in our experimental conditions and according to the pharmacological tools we used. In fact, our findings have shown that by blocking the effects of the inflammatory mediators investigated, complete inhibition of the inflammation was achieved at the 6 h time-point. Nevertheless, the role and relevance of the mediators involved in the carrageenan effects may vary when measured in other tissues or at different time-points. In addition to these mediators, carrageenan can also induce the activation of the complement system (Capasso et al., 1975; Miyama et al., 2002; Ting et al., 2008) and the release of histamine, 5-HT, tachykinins and platelet activating factors (Di Rosa and Sorrentino, 1970; Di Rosa et al., 1971a; Hwang et al., 1986; Gilligan et al., 1994). However, we cannot rule out the possibility that if the model had been followed for longer than 6 h, when the leukocyte infiltration and the prostaglandin synthesis would have been higher (Di Rosa et al., 1971b), the effect of the bradykinin receptor antagonists might have been less prominent than those of the anti-inflammatory steroids and NSAIDs.
Figure 6.
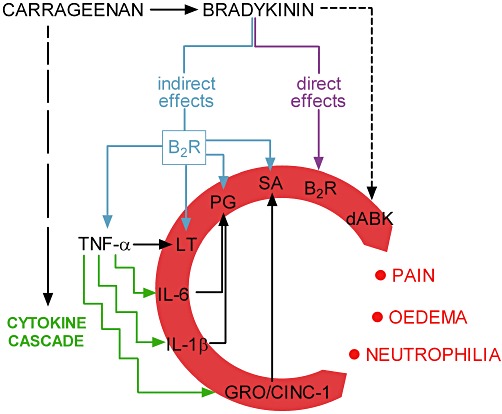
Diagram representing the inflammatory pathways activated by intra-articular carrageenan. B2R, kinin B2 receptor; dABK, des-Arg9-bradykinin; LT, leukotrienes; PG, prostaglandins; SA, sympathetic amines.
We have previously observed that the kinin B2 receptor antagonists and the anti-inflammatory steroids act on distinct pathways in the network of inflammatory mediators that are active at 6 h after intra-articular carrageenan, so that their combination yields full inhibition of the inflammatory responses such as joint pain, oedema and neutrophil infiltration (Valenti et al., 2010). In order to explore the mechanisms responsible for this drug interaction, pharmacological tools able to block the effects of bradykinin, prostaglandins, cysteinyl leukotrienes, sympathetic amines and des-Arg9-bradykinin, were used. In particular, tests were conducted with fasitibant chloride, a potent and selective non-peptide kinin B2 receptor antagonist (Cucchi et al., 2005; Valenti et al., 2005), dexketoprofen, a non-selective COX-inhibitor, MK571, a CysLT1 receptor antagonist, atenolol, a β-adrenoceptor antagonist, and des-Arg9-[Leu8]-bradykinin, a kinin B1 receptor antagonist, alone or in combination.
Each drug, when administered alone, affects joint pain indicating that in addition to bradykinin, prostaglandins, leukotrienes, catecholamines and des-Arg9-bradykinin also contribute to the nociceptive effects of carrageenan. These mediators also play a role in carrageenan-induced joint oedema, apart from the prostaglandins, which did not affect this inflammatory response, in agreement with previous findings obtained with other COX-inhibitors (Francischi et al., 2002).
When drugs that are effective against joint pain (fasitibant chloride, des-Arg9-[Leu8]-bradykinin, dexketoprofen, MK571 and atenolol) and joint oedema (fasitibant chloride, des-Arg9-[Leu8]-bradykinin, MK571 and atenolol) were administered in combination, almost full inhibition of the respective inflammatory response was observed, similar to the inhibition obtained from co-administration of fasitibant chloride and dexamethasone. The same combination of drugs, without the bradykinin B2 receptor antagonist, exerted a lower inhibitory effect, which was comparable with that obtained with dexamethasone alone, indicating that among the inflammatory mediators investigated, bradykinin was the only one not controlled by dexamethasone, in agreement with the finding that dexamethasone did not antagonize paw oedema induced by intraplantar bradykinin in rats (Campos and Calixto, 1995). Conversely, dexamethasone was able to block the effects of prostaglandins, leukotrienes, catecholamines and des-Arg9-bradykinin. In fact, anti-inflammatory steroids do not prevent the release of bradykinin produced by the carrageenan-induced kallikrein contact activation (Valenti et al., 2010); however, they can control the synthesis of eicosanoids through multiple actions such as inhibition of COX-2 expression, induction of the formation of annexin A1 and prevention of the migration of leukocytes (Angel et al., 1994; Yang et al., 1997; Perretti and Dalli, 2009; Coutinho and Chapman, 2011). Anti-inflammatory steroids also inhibit the effect of des-Arg9-bradykinin, preventing expression of kinin B1 receptors (Zhang et al., 2005), as well as the effects of IL-1β, IL-6 and IL-8 through the repression of their encoding genes and the trans-repression of the transcriptional regulators of pro-inflammatory genes (Coutinho and Chapman, 2011). On the contrary, steroids are able to control the effects of bradykinin when it is released by cytokines, activated neutrophils or complement activation (Tonussi and Ferreira, 1999; Cassim et al., 2009; Kaplan and Ghebrehiwet, 2010), by blocking the primary inflammatory events which lead to bradykinin release, as observed in LPS-induced arthritis in rats (Valenti et al., 2010).
As demonstrated, the combination of a steroid with a kinin B2 receptor antagonist should be capable of yielding the block of all carrageenan-induced inflammatory stimuli, at least in our experimental conditions. Therefore, in the cascade of mediators released by carrageenan, as described in Figure 6, the anti-inflammatory steroids potentially act by blocking the effects not mediated by bradykinin and the indirect effects of bradykinin, whereas they do not affect direct stimulation of the kinin B2 receptor by bradykinin. However, this hypothesis does not explain why fasitibant chloride and dexketoprofen exerted a comparable analgesic effect when administered alone or in combination, indicating either that both drugs act on the same prostaglandin-mediated pain pathways or that bradykinin does not directly induce pain, but indirectly through the release of prostaglandins. Therefore, dexamethasone could not completely control the bradykinin-mediated algesic effect of carrageenan because it is unable to inhibit the release of prostaglandins induced by bradykinin.
This evidence was confirmed by the assay of PGE metabolites in the synovial fluid, which indicated that fasitibant chloride and dexamethasone only partially reduced the release of prostaglandins when administered alone at their maximally effective doses. The combination of these drugs exerted a greater inhibition of PGE metabolites, comparable with that obtained by blocking the synthesis of prostaglandins with the COX inhibitor, dexketoprofen. Cyclooxygenases are the source of prostaglandins in the inflamed joint tissue, either in constitutive (COX-1) or inducible (COX-2) isoforms, and dexamethasone can interfere with their activity as described earlier. However, in vitro studies carried out in cultured human airway smooth muscle cells, showed that dexamethasone did not affect the bradykinin-induced short-term release of PGE2 due to constitutive COX-1, whereas it reduced the bradykinin-stimulated long-term release of PGE2 preventing COX-2 expression (Pang and Knox, 1997). On the contrary, dexamethasone abolished the release of prostaglandins stimulated by cytokines, such as IL-1β, which act by inducing COX-2 expression in these cells (Belvisi et al., 1997; Pang et al., 1998). Accordingly, we can hypothesize that intra-articular carrageenan generates a pool of prostaglandins, whose formation was sensitive to treatment with anti-inflammatory steroids, deriving from the direct activation of the cytokine cascade by carrageenan, together with another pool of prostaglandins stimulated by bradykinin through kinin B2 receptor activation, which is resistant to anti-inflammatory steroids.
A similar conclusion can be advanced for the combination of fasitibant chloride and dexamethasone on carrageenan-induced neutrophil infiltration. In fact, the CysLT1 receptor antagonist, MK571, blocked the increase of MPO activity in joint tissues, confirming that leukotrienes were the main mediators responsible for neutrophil recruitment (Kim et al., 2006). The combination of fasitibant chloride and dexamethasone blocked this response; however, these drugs were only partially effective when administered separately. It follows that dexamethasone failed to control the release of leukotrienes stimulated by bradykinin as already observed for the release of prostaglandins. Interaction between fasitibant chloride and dexamethasone was also observed in inhibiting the release of cytokines in the synovium because their combination was more effective than single drugs in reducing the levels of IL-1β, IL-6 and IL-8.
In conclusion, we have demonstrated that bradykinin, released by carrageenan-induced contact activation of plasma kallikrein, exerts both direct and indirect inflammatory effects in the rat knee joint that cannot be controlled by anti-inflammatory steroids, and that the interaction between fasitibant and dexamethasone mainly involves inflammatory mediators such as prostaglandins, des-Arg9-bradykinin, leukotrienes, catecholamines and cytokines. Consequently, the kinin B2 receptor antagonists could represent a new class of anti-inflammatory drugs that may act synergistically with steroids in acute arthritis, suggesting the possibility of reducing their effective doses and side effects.
Acknowledgments
None.
Glossary
- AU
arbitrary unit
- MPO
myeloperoxidase
- NSAID
nonsteroidal anti-inflammatory drug
Conflicts of interest
Authors are employees of Menarini Ricerche S.p.A.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel J, Berenbaum F, Le Denmat C, Nevalainen T, Masliah J, Fournier C. Interleukin-1-induced prostaglandin E2 biosynthesis in human synovial cells involves the activation of cytosolic phospholipase A2 and cyclooxygenase-2. Eur J Biochem. 1994;226:125–131. doi: 10.1111/j.1432-1033.1994.tb20033.x. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Saunders MA, Haddad EB, Hirst SJ, Yacoub MH, Mitchell JA. Induction of cyclooxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of this cell type. Br J Pharmacol. 1997;120:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–923. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]
- Cambridge H, Brain SD. Mechanism of bradykinin-induced plasma extravasation in the rat knee joint. Br J Pharmacol. 1995;115:641–647. doi: 10.1111/j.1476-5381.1995.tb14980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MM, Calixto JB. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br J Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso F, Dunn CJ, Yamamoto S, Willoughby DA, Giroud JP. Further studies on carrageenan-induced pleurisy in rats. J Pathol. 1975;116:117–124. doi: 10.1002/path.1711160208. [DOI] [PubMed] [Google Scholar]
- Cassim B, Shaw OM, Mazur M, Misso NL, Naran A, Laglands DR, et al. Kallikreins, kininogens and kinin receptors on circulating and synovial neutrophils: role in kinin generation in rheumatoid arthritis. Rheumatology. 2009;48:490–496. doi: 10.1093/rheumatology/kep016. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of intra-articular MEN16132, a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009;331:1025–1032. doi: 10.1124/jpet.109.159657. [DOI] [PubMed] [Google Scholar]
- Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599–2607. doi: 10.2174/138161206777698710. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosoppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi P, Meini S, Bressan A, Catalani C, Bellucci F, Santicioli P, et al. MEN16132, a novel potent and selective nonpeptide antagonist for the human bradykinin B2 receptor. In vitro pharmacology and molecular characterization. Eur J Pharmacol. 2005;528:7–16. doi: 10.1016/j.ejphar.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH. Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol. 1991;104:765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J, Remacle-Volon G. Influence of a long-acting bradykinin antagonist, Hoe 140, on some acute inflammatory reactions in the rat. Eur J Pharmacol. 1992;211:81–86. doi: 10.1016/0014-2999(92)90266-7. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Sorrentino L. Some pharmacodynamic properties of carrageenan in the rat. Br J Pharmacol. 1970;38:214–220. doi: 10.1111/j.1476-5381.1970.tb10350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, Giroud JP, Willoughby DA. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971a;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Papadimitriou JM, Willoughby DA. A histopathological and pharmacological analysis of the mode of action of non-steroidal anti-inflammatory drugs. J Pathol. 1971b;105:239–256. doi: 10.1002/path.1711050403. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischi JN, Chaves CT, Moura ACL, Lima AS, Rocha OA, Ferreira-Alves DL, et al. Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation. Br J Pharmacol. 2002;137:837–844. doi: 10.1038/sj.bjp.0704937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan JP, Lovato SJ, Erion MD, Jeng AY. Modulation of carrageenan-induced hind paw edema by substance P. Inflammation. 1994;18:285–292. doi: 10.1007/BF01534269. [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller PH, Levine JD. Further substantiation of a significant role for the sympathetic nervous system in inflammation. Neuroscience. 1993;55:1037–1043. doi: 10.1016/0306-4522(93)90317-9. [DOI] [PubMed] [Google Scholar]
- Hwang SB, Lam MH, Li CL, Shen TY. Release of platelet activating factor and its involvement in the first phase of carrageenin-induced rat foot edema. Eur J Pharmacol. 1986;120:33–41. doi: 10.1016/0014-2999(86)90636-9. [DOI] [PubMed] [Google Scholar]
- Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47:2161–2169. doi: 10.1016/j.molimm.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lo EJ, Green PG, Miao FJ, Relchling DB, Levine JD. Bradykinin-induced neurogenic migration of neutrophils into the rat knee joint. Neuroreport. 1999;10:3821–3824. doi: 10.1097/00001756-199912160-00018. [DOI] [PubMed] [Google Scholar]
- Meini S, Maggi CA. Knee osteoarthritis: a role for bradykinin? Inflamm Res. 2008;57:351–361. doi: 10.1007/s00011-007-7204-1. [DOI] [PubMed] [Google Scholar]
- Miyama K, Takano K, Atsumi I, Nakagawa H. Identification of C3a and N-truncated C3a as vascular permeability-enhancing factors from the exudate of chronic phase of carrageenan-induced inflammation in rats. Biol Pharm Bull. 2002;25:648–651. doi: 10.1248/bpb.25.648. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Segami N, Kaneyama K, Suzuki T, Miyamaru M. Relationships between pain-related mediators and both synovitis and joint pain in patients with internal derangements and osteoarthritis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:328–332. doi: 10.1067/moe.2002.124106. [DOI] [PubMed] [Google Scholar]
- Pang L, Knox AJ. PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am J Physiol. 1997;273:L1132–L1140. doi: 10.1152/ajplung.1997.273.6.L1132. [DOI] [PubMed] [Google Scholar]
- Pang L, Holland E, Knox AJ. Role of cyclo-oxygenase-2 induction in interleukin-1β induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br J Pharmacol. 1998;125:1320–1328. doi: 10.1038/sj.bjp.0702193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M, Borkiewicz P, Podgòrski T, Schmidt RF. The activity of fine afferent nerve fibres of the rat knee joint and their modulation by inflammatory mediators. Ortop Traumatol Rehabil. 2008;10:63–74. [PubMed] [Google Scholar]
- Perretti M, Dalli J. Exploiting the annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Bhoola KD, Elson CJ, Lemon M, Dieppe PA. Identification and functional importance of plasma kallikrein in the synovial fluids of patients with rheumatoid, psoriatic, and osteoarthritis. Ann Rheum Dis. 1995;54:345–350. doi: 10.1136/ard.54.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JN, Buchanan WW. Pathogenic responses of bradykinin system in chronic inflammatory rheumatoid disease. Exp Toxicol Pathol. 1994;46:421–433. doi: 10.1016/S0940-2993(11)80053-9. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Wirth KJ. Inhibition of rats adjuvant arthritis by a bradykinin antagonist Hoe 140 and its influence on kallikreins. Gen Pharmacol. 1996;27:133–136. doi: 10.1016/0306-3623(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Song IH, Althoff CE, Hermann K, Scheel AK, Knetsch T, Burmester G, et al. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor-2 antagonist in painful knee osteoarthritis compared to magnetic resonance imaging. Ann Rheum Dis. 2008;68:75–83. doi: 10.1136/ard.2007.080382. [DOI] [PubMed] [Google Scholar]
- Sorbera LA, Fernandez-Forner D, Bayes M. Icatibant acetate. Drugs Future. 2006;31:101–106. [Google Scholar]
- Ting E, Guerrero ATG, Cunha TM, Verri WA, Jr, Taylor SM, Woodruff TM, et al. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol. 2008;153:1043–1053. doi: 10.1038/sj.bjp.0707640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonussi CR, Ferreira SH. Tumour necrosis factor-α mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82:81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Valenti C, Cialdai C, Giuliani S, Lecci A, Tramontana M, Meini S, et al. MEN16132, a novel potent and selective nonpeptide kinin B2 receptor antagonist: in vivo activity on bradykinin-induced bronchoconstriction and nasal mucosa microvascular leakage in anesthetized guinea pigs. J Pharmacol Exp Ther. 2005;315:616–623. doi: 10.1124/jpet.105.088252. [DOI] [PubMed] [Google Scholar]
- Valenti C, Giuliani S, Cialdai C, Tramontana M, Maggi CA. Synergistic anti-inflammatory effect of MEN16132, a kinin B2 receptor antagonist, and dexamethasone on carrageenan-induced knee joint arthritis in rats. Br J Pharmacol. 2010;161:1616–1627. doi: 10.1111/j.1476-5381.2010.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation. 1997;21:583–596. doi: 10.1023/a:1027330021479. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adner M, Cardell LO. Glucocorticoids suppress transcriptional up-regulation of bradykinin receptors in a murine in vitro model of chronic airway inflammation. Clin Exp Allergy. 2005;35:531–538. doi: 10.1111/j.1365-2222.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann GR, William A, Finelli AL, Farwell M, Fraser CC, Borisy AA. Selective amplification of glucocorticoid anti-inflammatory activity through synergistic multi-target action of a combination drug. Arthritis Res Ther. 2009;11:R12. doi: 10.1186/ar2602. [DOI] [PMC free article] [PubMed] [Google Scholar]


